Abstract
Purpose
To investigate the morphology of the ciliary muscle during the act of accommodation in a population of children.
Methods
Thirty children ages 6 to 12 years were enrolled. Accommodative response was measured through habitual correction. Height was measured as a control variable. Central axial length was measured with the IOLMaster. Four images of the temporal ciliary muscle were taken with the Visante™ Optical Coherence Tomographer at three different stimulus levels (0D, 4D, 6D) while accommodative response was monitored concurrently with the PowerRefractor. Accommodative response monitoring was time- matched to ciliary muscle image capture, and the mean was calculated for five seconds surrounding this time point. Four cycloplegic images of the temporal ciliary muscle were also taken. Ciliary muscle thickness measurements were made at the point of maximum thickness (CMTMAX), and at 1 mm (CMT1), 2 mm (CMT2) and 3 mm (CMT3) posterior to the sclera spur.
Results
Increasing accommodative response was correlated with increases in the thickness of CMTMAX (p=<0.001) and CMT1 (p=<0.001), and decreases in the thickness of CMT3 (p=<0.001). Thicker values of CMTMAX under cycloplegic conditions were significantly correlated with values of CMTMAX (p=<0.001) and CMT1 (p=0.001) while accommodating, and approached significance in modeling CMT3 (p=0.06). Mean axial length was correlated with the amount of thinning at CMT3 with accommodation (p=0.002). Axial length was not significantly correlated with thickness values at CMTMAX (p=0.7) or CMT1 (p=0.6).
Conclusions
In a manner similar to previous adult studies ciliary muscle thickness at CMTMAX and CMT1 increased with accommodation, and CMT3 thinned with accommodation. Further investigation is necessary to determine if CMT2 is a “fulcrum” point along the length of the ciliary muscle where the net change with accommodation is always zero, or if that point varies across subjects or with varying levels of accommodative effort.
Keywords: accommodation, ciliary muscle, children
The ciliary muscle is a smooth muscle that contracts to generate accommodation of the crystalline lens in the human and non-human primate eye.1 Although we have a general understanding of the anatomy of the ciliary muscle, the exact structural changes that occur during the act of accommodation are only vaguely understood. This vague understanding is not due to a lack of interest in the scientific community. Accommodation has been investigated in several fields in order to better understand phenomena such as presbyopia,2–8 and even myopia progression.9–12
Historically, information about the ciliary muscle was derived from histological studies13 and imaging techniques such as magnetic resonance imaging (MRI)5 and ultrasound biomicroscopy (UBM).7 Within the last several years, anterior segment optical coherence tomography (OCT) has been used to evaluate ciliary muscle morphology and contraction.3, 14 OCT has advantages over UBM and MRI in that it is non-contact, it can capture images very quickly, and the subject can be oriented in a natural head position.
Stachs and co-workers (2002) used UBM imaging to study changes in the ciliary muscle morphology in pharmacologically induced accommodative states.7
A study by Sheppard and Davies (2010) analyzed ciliary muscle morphology in pre-presbyopic adults using OCT imaging.3 They found that most of the shape change occurred in the anterior portion of the ciliary muscle with accommodation, and interestingly, the temporal ciliary muscle appeared to shorten more than the nasal ciliary muscle.3 Strenk and co-workers (2006) conducted a study of ciliary muscle morphology in pre-presbyopic and presbyopic adults using MRI. Their data determined that the ciliary muscle continues to contract equally with age, even into presbyopia.5 These studies, however, were all completed in adult subjects.
Imaging of the ciliary muscle has proven difficult in the past due to the position of the iris, and this is the primary reason that ciliary muscle action has not been more fully evaluated in children. OCT seems to be the most feasible option for measuring ciliary muscle morphology in a pediatric population.15 More information regarding ciliary muscle contraction in children would be beneficial for several reasons. First, some binocular vision abnormalities, such as accommodative insufficiency, might be attributed to abnormal contraction of the ciliary muscle, but there are no published studies evaluating this relationship. Second, hyperopia is known to lead to variable and higher accommodative lag values,16 and both hyperopia and accommodative function are related to school performance.17–18 Future studies of contraction of the ciliary muscle in hyperopic or other children who perform poorly in school would require a comparison to the normal state of ciliary muscle contraction in children. Third, our laboratory has already shown that the ciliary muscle is thicker in children with myopia,14 and we intend to continue to investigate whether or not the ciliary muscle is the source of accommodative lag in progressing myopia.
The goals of the present study were to determine if the measurements of ciliary muscle morphology during accommodation were feasible in children, to determine if there were changes in ciliary muscle function related to age in children, and to determine if the change in ciliary muscle thickness with accommodation was linear throughout a range of accommodative responses. The data collected in the study described below provide insight into the structure of the ciliary muscle while the exact accommodative response was measured in school-age children, and they also provide insight into how accurately children accommodate to near stimuli.
METHODS
Subjects
Subjects were recruited within the Ohio State University College of Optometry. Children between the ages of 6 to 12 years with all types of refractive error were eligible for inclusion. Thirty subjects participated in the study. During study testing, 2 children wore contact lenses for myopia correction, 2 children wore spectacles for myopia correction, 3 children wore spectacles for hyperopia (>+2.00 D), and no hyperopes (>+2.00 D) were uncorrected. Exclusion criteria consisted of any ocular disease, history of strabismus or eye surgery, and the use of medications affecting the ciliary body. After a presentation and discussion of the procedures, all subjects and their parents provided written informed consent. The study was approved by the Institutional Review Board of The Ohio State University and was conducted in accordance with the tenets of the Declaration of Helsinki.
Measurements
Children were tested with their habitual correction. Their correction status was determined by what they wore to the testing session. Children who had been prescribed lenses but did not wear them to the study visit were treated as habitually uncorrected during study testing, i.e., no correction was worn during testing. The height of each child was recorded using a stadiometer, and this measurement was used as a control variable only.
High-contrast distance visual acuity was measured monocularly at 4 meters through habitual correction in normal examination room illumination using logMAR charts. The total number of letters correct was recorded. Guessing was encouraged. Visual acuity testing was stopped after 3 or more errors were made on a line.
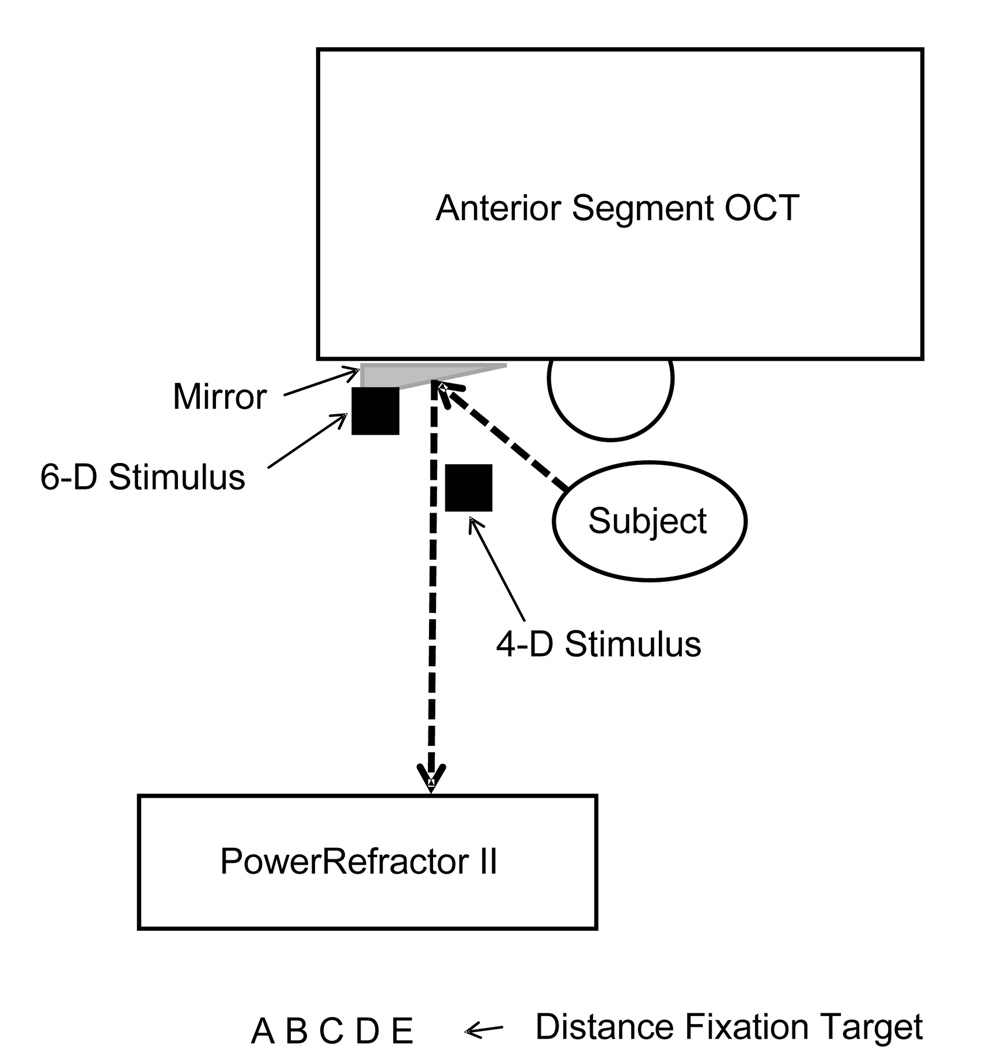
The ciliary muscle of the right eye was imaged during natural accommodation, i.e., without the use of any pharmaceuticals. The temporal ciliary muscle of the right eye was imaged with the Zeiss Visante Anterior Segment OCT while the subjects viewed targets at a 0-D, 4-D, and 6-D stimulus levels. The scanning beam of the Visante was aligned at the mid-point of the pupil, and images were captured in Enhanced High Resolution Corneal Mode. Targets consisted of a row of large letters (approximately 20/100) for the 0-D stimulus, which could easily be spotted through the mirror and used for fixation as the children looked across the room, and a Maltese cross was used at the 4-D and 6-D stimulus levels to elicit an accommodative response. To determine the actual accommodative state of the eye, simultaneous measurements of accommodative response were taken using an infrared optometer PowerRefractor II (MultiChannelSystems, Reutlingen, Germany). The PowerRefractor took accommodative measurements and pupil size measurements continuously while four images of the ciliary muscle were obtained using the Zeiss Visante Anterior Segment OCT at each accommodative stimulus level. Figure 1 is a schematic diagram of the setup that was used to capture ciliary muscle images while monitoring the power of the eye with an autorefractor.
Figure 1.
A diagram of the set up used to simultaneously monitor accommodation during imaging of the ciliary muscle. While the subject was positioned in the head rest for the Visante™ Anterior Segment Optical Coherence Tomographer, he or she viewed a fixation target at distance, or accommodative stimuli at either 0.25 m (4-D) or 0.17 m (6-D) through a mirror that was positioned on the left side of the Visante. The PowerRefractor II was positioned so that it could read the power of the eye through the mirror. The dashed line shows the optical path of the eye viewing the fixation targets or accommodative stimuli as well as the optical path of the PowerRefractor.
If the child wore spectacle correction, a special trial frame was utilized for obtaining ciliary muscle and simultaneous accommodative response data. The spherical equivalent correction was placed over the left eye so that the left eye could fixate and respond to the accommodative target. If a correction was placed in front of the right eye during imaging, it would have distorted the Visante images, so the right eye was covered with a gel filter (Wratten 89B; Kodak, Rochester, New York) that only transmitted light with wavelengths longer than 680 nm. This filter effectively occluded vision of the right eye so that subjects would fixate and accommodate with the left eye, but still allowed imaging of the ciliary muscle of the right eye without distorting the images. PowerRefractor data were then obtained for the left eye through the spectacle correction, so that the PowerRefractor data were reflective of accommodative response and not refractive error.
After the above testing, one drop of 0.5% proparacaine was instilled in the right eye followed by an intraocular pressure measurement with Tonopen. One subject was unable to hold the eye open for Tonopen, so palpation pressures were taken. Then, two drops of 1% tropicamide were placed in the right eye. The two drops of 1% tropicamide were instilled five minutes apart.19
The central axial length of the eye was determined by using the IOLMaster (Carl Zeiss Meditec, Dublin, CA). Five axial length measurements of high confidence were recorded. High-confidence measurements were defined as those with a signal to noise ratio greater than 2.0. The mean of the five, high-confidence measurements was used in data analysis.
Cycloplegic measurements were made 25 minutes after the last drop of tropicamide was instilled. Cycloplegic refractive error of the right eye was measured using the Grand Seiko (WR-5100K) autorefractor and the mean of 10 measurements was used in analysis. Cycloplegic images of the temporal ciliary muscle of the right eye were taken with the Zeiss Visante Anterior Segment OCT by obtaining 4 images.
Determination of Accommodative Response during Ciliary Muscle Imaging
PowerRefractor data were matched in time as closely to the ciliary muscle image capture as was possible for the 0D, 4D, and 6D stimulus levels. Before each imaging session, the clock in the Visante operating system and the clock in the PowerRefractor were synchronized. One examiner operated the PowerRefractor (MDB) and another examiner operated the Visante (HAL). Prior to capture of any images, the PowerRefractor began recording data. At the exact time the last image was acquired by the Visante, the operator of the PowerRefractor stopped the recording of the accommodative response. When the raw image files of the Visante are exported, a time stamp, including two-digits for the hour, minute and second the file was captured, is automatically generated for each ciliary muscle image in the file name. These time stamps were recorded, and then matched to the time in seconds from the PowerRefractor spreadsheet, assuming that the last image file was captured within a second of the last reading in the PowerRefractor spreadsheet. Five seconds of accommodative data were analyzed for each ciliary muscle image. The data were selected from the five seconds surrounding (2.5 seconds before and 2.5 seconds after) the ciliary muscle image time recorded from the image timestamp. The mean of each five-second interval was determined for both pupil size and accommodative response. According to the recommendations of Gabriel and co-workers (2009), PowerRefractor data were not calibrated prior to analysis.20
Because PowerRefractor alignment was sometimes difficult to achieve through the mirror, it was not always possible to record the accommodative response from both eyes in all subjects. For uncorrected subjects, the right eye’s data were used to determine the accommodative response. Left eye’s data were used for all subjects corrected with spectacles during ciliary muscle imaging, because only the left eye was corrected with a spectacle lens. In the rare case that data were exceptionally sparse, the mean of right and left eyes’ data was used to calculate the accommodative response to the 4-D and 6-D stimuli; however, most of the recordings only captured one of the two eyes, and the eye with the majority of data points was used to evaluate the mean pupil size and accommodative response.
Ciliary Muscle Image Analysis
All ciliary muscle images were analyzed using a semi-automatic algorithm that has been evaluated extensively in a previous publication.15 In that publication,15 we presented details regarding the validity and repeatability of our methods, as well as general information regarding the aspects of the ciliary muscle that are visible in this imaging technique. Briefly, the location of the scleral spur was manually selected, the ocular structures were identified from the background in the images, and an outline of the ciliary muscle was generated. Refractive index corrections were used to obtain accurate measurements. A refractive index of 1.41 was used for the sclera, and a refractive index of 1.38 was used for the ciliary muscle based on best estimates in the literature.21–22 Once the outline was generated, the thickest portion of the ciliary muscle (CMTMAX) was measured under non-cycloplegic and cycloplegic conditions. CMTMAX measurements under cycloplegic conditions were used as a baseline reference, and were referred to as “cycloplegic ciliary muscle thickness” in our data analyses. Thickness measurements were also obtained 1 mm, 2 mm, and 3 mm posterior to the scleral spur (CMT1, CMT2, and CMT3). The scleral spur was used as an anatomical landmark because it is the anterior insertion point of the ciliary muscle. An illustration of the outline and thickness measurements is provided in Figure 1 of our companion paper23 in this issue of the journal describing these measurements in young adults.
Data Analyses
When plots of ciliary muscle thickness versus accommodative response were reviewed, the data suggested that it was reasonable to assume a linear relationship between ciliary muscle thickness and accommodative response. For each subject and location of ciliary muscle thickness measurement (CMTMAX, CMT1, CMT2, and CMT3) there were up to 12 measures of ciliary muscle thickness taken at varying levels of accommodation. The study objective was to estimate the across-subject mean relationship between ciliary muscle thickness and accommodative response. A standard way to estimate a mean value of this sort when repeated, within-subject observations are available is to fit a multilevel model.24 The multilevel model fitted to the ciliary muscle thickness (CMT) versus accommodative response (ACC) data had the following form:
In the model, i indexes the subject, and j his or her repeated measures. ACCij is the jth measure of ACC for subject i. The two γ terms are constants providing the across-subject mean intercept and slope coefficients. The two u terms are random effects that correct the average intercept and slope for subject-level variation.
The model was expanded to include the following control variables: axial length, height, age, and overall ciliary muscle thickness. Height and axial length were considered because both are related to the age of the child,25 and it was thought that their inclusion would allow for a more direct evaluation of the effects of age alone. The effect of each of these variables on the mean intercept and slope was evaluated. Control variables that were not statistically significant were removed with the goal of achieving parsimonious models of each of the ciliary muscle thickness measurements versus accommodative response. All models were fitted using the MIXED procedure in SAS version 9.2.
Accommodative response was computed for each subject with the following equation:
where ACC is the accommodative response, Powerref is the mean reading of the PowerRefractor at a particular stimulus level, M is the cycloplegic, spherical-equivalent refractive error, and Specsph is the spherical equivalent of the subject’s habitual spectacle correction. Zero was used for the Specsph term if no correction was worn. This calculation was used to better compare accommodative response between subjects.
Thirty subjects were initially enrolled in the study. Three subjects did not have accommodative response data from the PowerRefractor due to an equipment malfunction, and were excluded from analysis. One subject did not have usable ciliary muscle images under cycloplegic conditions, but did have usable ciliary muscle images under accommodative conditions. Data from 26 subjects were analyzed to generate our regression models.
RESULTS
General characteristics of the study sample are summarized in Table 1. None of the interactions between accommodative response and other model variables were statistically significant. All interaction terms were removed, leaving just main effects models. The parameter estimates for these main effects models are provided in Table 2. The statistically significant variables retained after further pruning for parsimony were accommodative response, mean axial length, and cycloplegic ciliary muscle thickness. Age and height were not found to be statistically significant predictors in any of the thickness models. Table 3 summarizes the final regression models for CMTMAX, CMT1 and CMT3. The relationship between CMT2 and accommodative response was not statistically significant (p = 0.8) so its final model is not presented. The final regression models are represented by the following equations:
Table 1.
Characteristics of the sample used in modeling ciliary muscle thickness as a function of accommodative response.
| Measurement | Mean | Standard Deviation |
Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 9.0 | 2.1 | 6.0 | 13.00 |
| Refractive Error (D)† | +0.36 | 2.41 | −7.41 | +5.44 |
| Height (cm) | 135.2 | 14.0 | 106.7 | 161.3 |
| Mean Axial Length (mm) | 23.12 | 1.16 | 20.13 | 24.76 |
| Maximum Cycloplegic Ciliary Muscle Thickness (µm) | 788.0 | 81.0 | 660.0 | 990.0 |
cycloplegic, spherical equivalent
Table 2.
Models of ciliary muscle thickness as a function of accommodative response while including all control variables: age, axial length, height, and the cycloplegic ciliary muscle thickness.
| Variable | CMTMAX | CMT1 | CMT2 | CMT3 | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Intercept | 756.7 | 730.2 | 525.1 | 315.0 | ||||
| Accommodative Response (D) | −14.4 | <0.001 | −10.5 | <0.001 | 0.33 | 0.8 | 5.3 | <0.001 |
| Age (years) | −8.8 | 0.3 | −9.0 | 0.3 | −14.3 | 0.2 | −14.7 | 0.1 |
| Mean Axial Length (mm) | −4.9 | 0.6 | 3.1 | 0.7 | 34.1 | 0.003 | 29.2 | 0.003 |
| Height (cm) | 2.2 | 0.1 | 2.1 | 0.1 | 3.4 | 0.05 | 2.7 | 0.06 |
| Cycloplegic ciliary muscle thickness (µm) | 0.45 | <0.001 | 0.39 | 0.002 | 0.23 | 0.1 | 0.24 | 0.05 |
CMTMAX = maximum ciliary muscle thickness
CMT1, CMT2, and CMT3 = ciliary muscle thickness 1 mm, 2 mm, and 3 mm posterior to scleral spur respectively
Table 3.
Best models of ciliary muscle thickness as a function of accommodative response after controlling for axial length and the cycloplegic ciliary muscle thickness.
| Variables | CMTMAX | CMT1 | CMT3 | |||
|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Intercept | 756.3 | 730.6 | 315.4 | |||
| Accommodative Response (D) | −14.2 | <0.001 | −10.4 | <0.001 | 5.4 | <0.001 |
| Mean Axial Length (mm) | 30.2 | 0.002 | ||||
| Cycloplegic Ciliary Muscle Thickness (µm) | 0.45 | <0.001 | 0.41 | 0.001 | 0.23 | 0.06 |
CMTMAX=maximum ciliary muscle thickness
CMT1 and CMT3 = ciliary muscle thickness 1 mm and 3 mm posterior to scleral spur, respectively
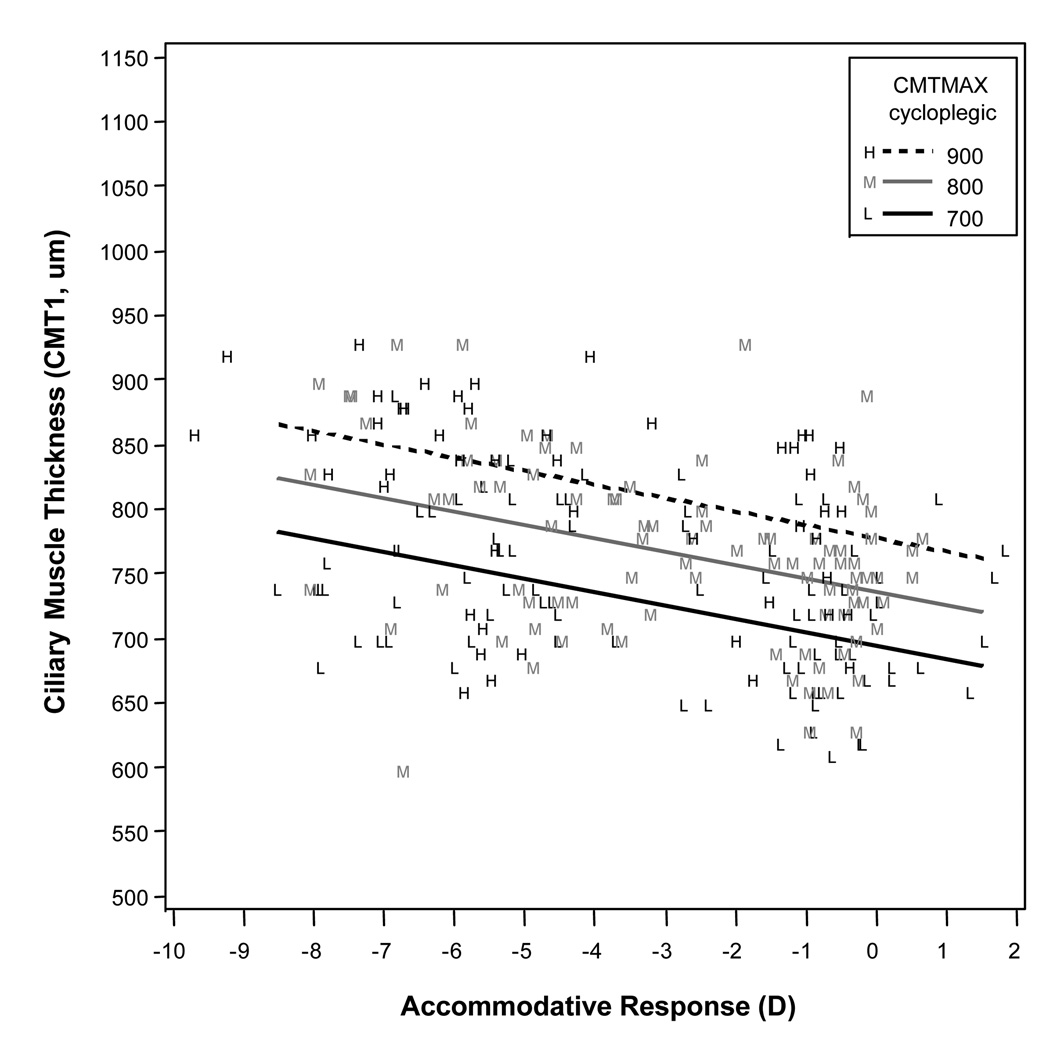
In Figures 2–4, predicted thickness is graphed relative to accommodative response for selected values of cycloplegic CMTMAX. The model lines are superimposed on the observed data, with the observed data coded according to whether they were closest to the low, middle, or high value of the thicknesses given in the legend. Increased accommodative response (more negative readings on the PowerRefractor) was correlated with increases in the thickness of CMTMAX (p ≤ 0.001) and CMT1 (p ≤ 0.001), and decreases in the thickness of CMT3 (p ≤ 0.001). Accommodative response was not associated with changes in thickness of CMT2 (p = 0.8). Thicker values of cycloplegic ciliary muscle thickness were correlated with thicker readings for CMTMAX (p ≤ 0.001) and CMT1 (p = 0.001). Cycloplegic ciliary muscle thickness approached significance in modeling CMT3 (p = 0.06). Note that an interaction term for cycloplegic ciliary muscle thickness and accommodative response was not included in the final models because it was not statistically significant.
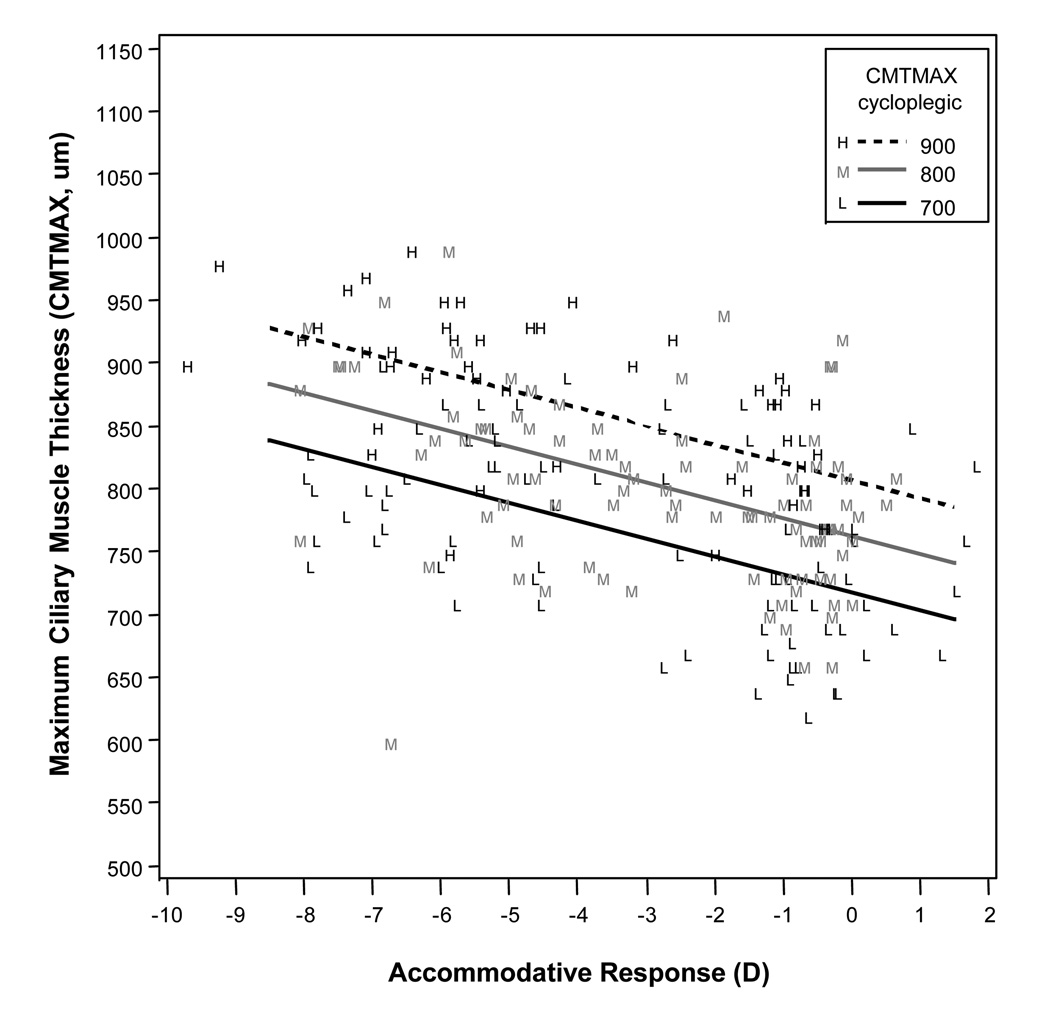
Figure 2.
Modeled results for maximum ciliary muscle thickness (CMTMAX) for a range of accommodative responses, showing that higher levels of accommodative response (more negative values) were associated with a thicker measurement at CMTMAX. Cycloplegic maximum ciliary muscle thickness (CMTMAX) was a statistically significant variable in the model, so the three lines represent three different cycloplegic ciliary muscle thicknesses across the range of our sample.
Figure 4.
Modeled results for ciliary muscle thickness 3 mm posterior to the scleral spur (CMT3) for a range of accommodative responses, showing that higher levels of accommodative response (more negative values) were associated with a thinner measurement at CMT3. Cycloplegic maximum ciliary muscle thickness (CMTMAX) was a statistically significant variable in the model, so the three lines represent three different cycloplegic ciliary muscle thicknesses across the range of our sample.
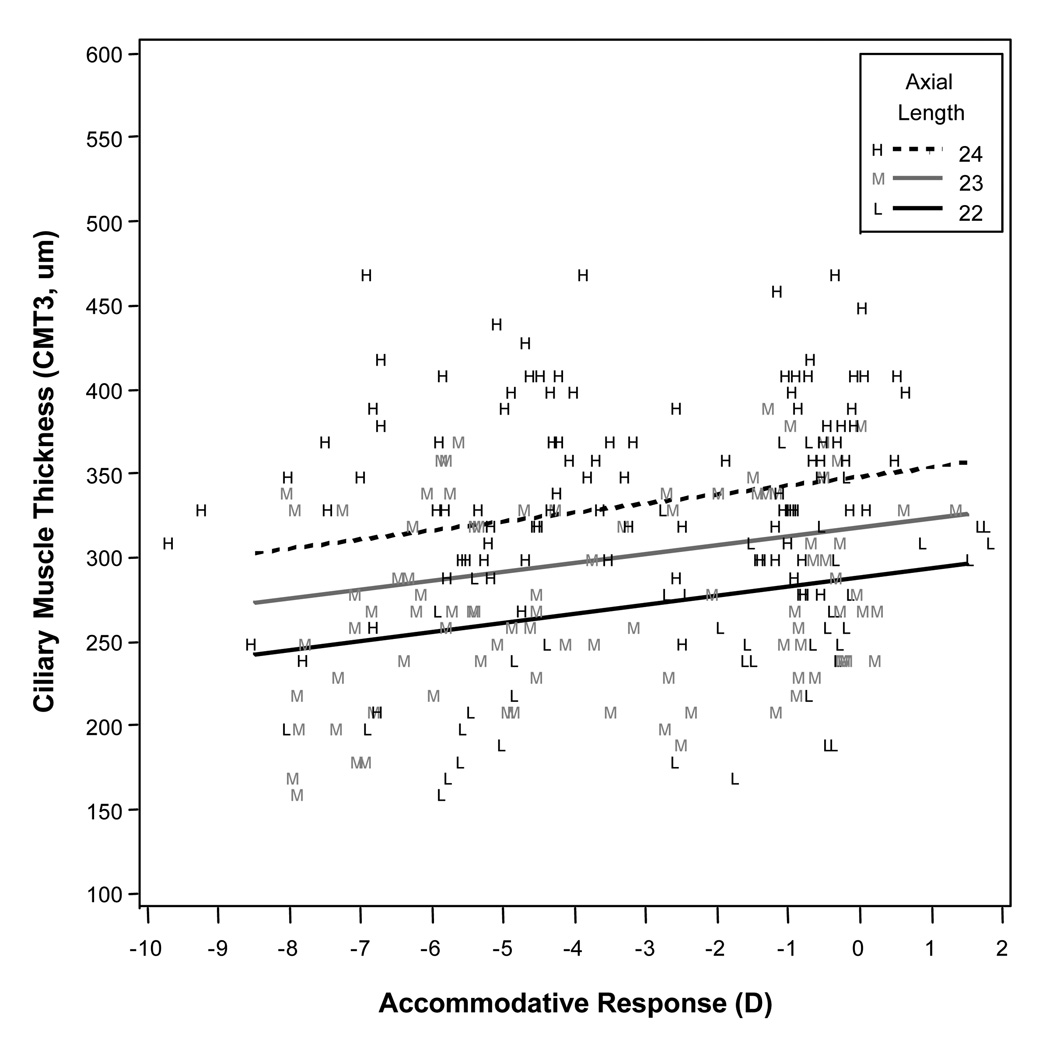
Mean axial length was correlated with the amount of thinning at CMT3 (p = 0.002). This relationship is illustrated in Figure 5. Mean axial length was not significantly correlated with thickness values at CMTMAX (p = 0.7) or CMT1 (p = 0.6). In the best models, there was a mean increase in thickness at CMTMAX of 14.2 µm per diopter of accommodation, a mean increase of thickness at CMT1 of 10.4 µm per diopter of accommodation, and a mean decrease of thickness at CMT3 of 5.4 µm per diopter of accommodation.
Figure 5.
Modeled results for ciliary muscle thickness 3 mm posterior to the scleral spur (CMT3) for a range of accommodative responses, showing that higher levels of accommodative response (more negative values) were associated with a thinner measurement at CMT3. Axial length was a statistically significant variable in the model, so the three lines represent three different axial lengths across the range of our sample.
DISCUSSION
Our study found that accommodative response in children was correlated with ciliary muscle thickness, a result that was predicted based on previous studies of accommodation,3, 5, 7, 26–29 but also serves as proof of concept for our method of ciliary muscle measurement. Mean axial length, and the maximum ciliary muscle thickness under cycloplegic conditions were also correlated with ciliary muscle thickness during accommodation. Anatomically, it is intuitive that eyes with thicker ciliary muscles without accommodation would generate thicker readings while accommodation was occurring. Because axial length and refractive error were correlated, our findings that eyes with longer axial lengths also seem to have thicker ciliary muscle readings with accommodation support earlier findings of myopes having thicker ciliary muscles than emmetropes or hyperopes.14, 30
Overall, we consistently found thickening of the anterior portion of the ciliary muscle (CMTMAX and CMT1) and thinning of the posterior portion of the ciliary muscle (CMT3) with greater accommodative response. These findings are consistent with Helmholtz’s theory of accommodation,26 and support previous findings by Sheppard and Davies (2010) that the ciliary muscle contracts longitudinally as well as radially with accommodation.3 There are two other studies to which we can compare our results. In the present study, we found a 10.4 µm change in CMT1 per diopter of accommodation and another adult study from our laboratory23 reported a similar value at 12.3 µm per diopter of accommodation. Similarly, in the present study we found 5.4 µm per diopter thinning of the ciliary muscle at CMT3, while we reported a 12.0 µm per diopter of thinning in our other adult study.23 Although due to methodological differences,31 there is not an exact comparison to the previous young adult study by Sheppard and Davies (2010), we have previously discussed that the results of another young adult study from our laboratory23 are similar to that of Sheppard and Davies (2010).3 In general, the three studies using anterior segment OCT to evaluate accommodation in non-presbyopic patients are in agreement.
Accommodative response was not a significant factor for modeling the behavior of CMT2, as CMT2 measurements were relatively flat over a spectrum of accommodative responses. Our data set showed that some subjects have thickening in this area with accommodation while other subjects have thinning in this area. Sheppard and Davies (2010) hypothesized that anchoring measurements to the sclera spur, such as a measurement 2.0 mm posterior to the sclera spur (CMT2), would lead to measurements in different anatomical areas in the ciliary muscle in different subjects.3 They reported thinning of the ciliary muscle at CMT2 with accommodation, but their standard deviations were large compared to the mean amount of thinning and included zero (−2.2 ± 11 µm), suggesting they too observed both thickening and thinning at this point in the muscle.3 The lack of interaction between accommodative response and ciliary muscle thickness, i.e., thinner muscles behaved in a manner that was identical to thicker muscles, suggests that CMT2 is not a fundamentally different anatomical position depending on its size. Rather, the ciliary muscle may thicken at CMT2 for certain subjects and thin for others. Unfortunately, there were no obvious trends in our data set to predict which muscles would thicken versus thin at CMT2.
We do wonder, however, if we have uncovered an approximate position for a “fulcrum” in the ciliary muscle where the muscle transitions from thickening behavior to thinning with accommodation. In some subjects, this position may be slightly ahead of CMT2, while in other subjects it may be slightly behind this location. It is also possible that this location varies depending on the level of accommodative effort that is exerted. Further investigation into the morphological behavior of the ciliary muscle in the act of accommodation is warranted.
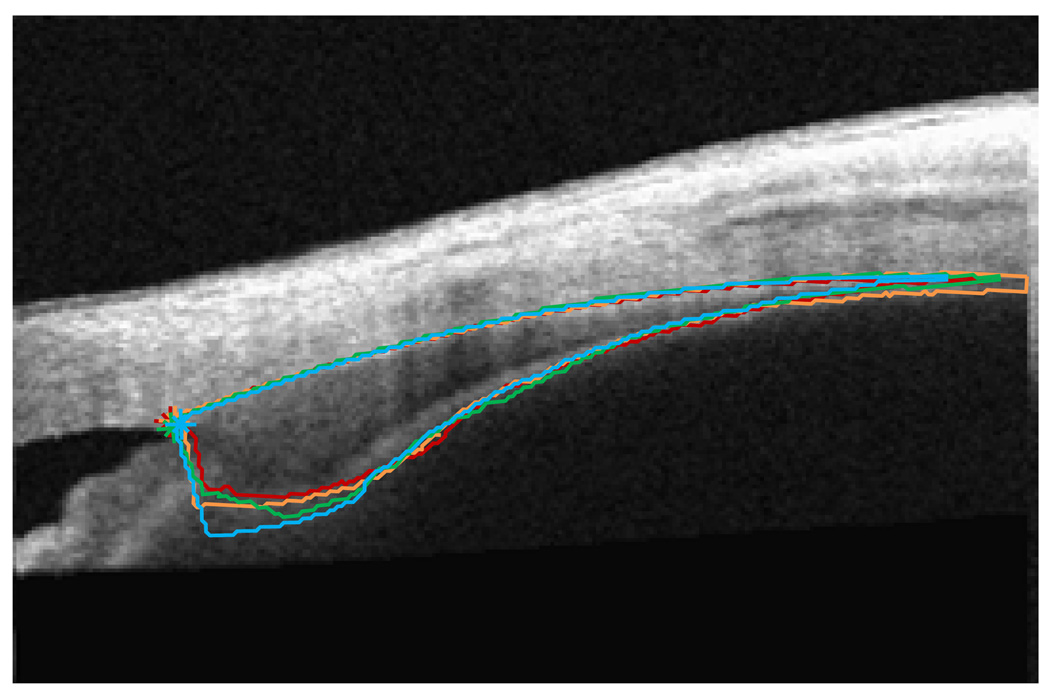
Because the semi-automatic algorithm used to analyze images in this study outlines the entire ciliary muscle,15 it would be possible to determine the thickness of the ciliary muscle at any point along the length of the muscle (Figure 6). In the future, we plan to obtain thickness measurements at smaller intervals along the length of the muscle. Use of this strategy may help us to uncover an exact “fulcrum” of the ciliary muscle in individual patients and perhaps determine predictive trends in the anatomy of the muscle. We will also determine if this location changes within an individual as the level of accommodative effort increases.
Figure 6.
Ciliary muscle morphology changes in an emmetropic, pre-presbyopic adult with regulated accommodative response, i.e. when the subject was producing 0-D (red), 2-D (orange), 4-D (green), and 6-D (blue) accommodative responses. Because subjects produce a wide variety of accommodative responses to a given stimulus, this strategy of imaging during a precise accommodative response may be a better protocol for determining the action of the ciliary muscle during accommodation.
Although we have found that accommodative response is correlated with ciliary muscle thickness, our subjects were not always reliable when accommodating to a particular stimulus. In other words, just because our target was a 4.00-D stimulus does not mean that subjects reliably produced a 4.00-D accommodative response. Future studies will be able to make better assessments of how ciliary muscle thickness changes with accommodation if we regulate and produce the desired accommodative response instead of assuming accommodative effort based on stimulus distance. Figure 6 illustrates ciliary muscle changes with regulated accommodative responses from the PowerRefractor in an emmetropic, pre-presbyopic adult. In this case, imaging the ciliary muscle only when the subject had produced a precise accommodative response allowed for more direct comparisons of the ciliary muscle morphology for a known accommodative output, and a future study using this strategy may provide better morphological information about the ciliary muscle during accommodative tasks. This will be especially important if the change in ciliary muscle thickness does not increase linearly with increasingly larger accommodative responses.
There is some suggestion that the ciliary muscle contraction required to produce a diopter of accommodative response may not be linear, and will increase with increasing accommodative responses.3 Whether or not the change in ciliary muscle thickness is linear over a range of accommodative responses may be an important, and not adequately investigated, aspect of accommodative function. If this aspect is not considered, vision scientists and clinicians might assume that individuals have accommodative lag because of factors such as depth of focus, a simple lack of effort or laziness in focusing, or binocular vision conditions that force the individual to choose between a blurry image and one that is single. There is an additional possibility. It may be that individuals prefer to accommodate at a level such that the response of the ciliary muscle can remain in a linear range. For example, if generating a 6-D response required a much larger change in thickness per diopter of accommodation generated over that which is required to produce a 4-D response, the subject may be less willing or able to produce the 6-D response. Thus, in this scenario, the examiner might observe what he or she thought was a lack of effort or laziness. Again, protocols that measure the change in ciliary muscle thickness per diopter of accommodation after specific accommodative responses are generated may allow us to learn whether or not the relationships depicted in Figures 2–4 are actually linear for an individual. In the present study, we cannot rule out the fact that when subjects lagged in their accommodative effort, they did so in order to remain in their own range of linear responses.
We see determining the linearity of ciliary muscle contraction in children as an important future application of ciliary muscle measurements in children. Candy and co-workers (2012) have shown previously that hyperopic children have larger/more variable accommodative lags.16 It may be possible that some children with hyperopia have an amplitude of accommodation that would allow them to accommodate through their hyperopia and to a near target, but that this puts the child in a range of accommodative response that requires a great deal of effort, i.e., the non-linear portion of his or her ciliary muscle contraction curve. Thus, variable accommodation and accommodative lag are observed in these hyperopic children. This might also explain why children with developing myopia may have larger accommodative lags. Perhaps the linear range of contraction in a child with developing myopia is shorter than that of the average child. In order to investigate these two possibilities further, or to investigate any other accommodative dysfunction in children, more data regarding the response of the ciliary muscle during accommodation will be needed in children. We suspect that the linear range will be shorter in adults because the loss of crystalline lens elasticity could theoretically shorten the linear range with age. Again, the experimental protocol must move the target such that each subject achieves a given accommodative response, 2-D, 4-D, 6-D, etc., in order to study this further. If subjects do indeed lag once they reach their non-linear range, studies that use a fixed stimulus, rather than a fixed response, will not detect the non-linear range as easily.
We may have found no difference between thinner and thicker ciliary muscles during accommodation in this study due to the flexibility of the crystalline lens in childhood. Studies have shown, however, that the crystalline lens increases in thickness and does not respond as readily to stretching forces with increasing age in adulthood.4,32–34 Perhaps a thicker ciliary muscle only becomes advantageous during accommodation after the lens becomes stiffer in older individuals, creating a higher workload for the muscle. Further study in adults is warranted.
Interestingly, age and height were not good predictors of ciliary muscle thickness changes with accommodation. This may imply that the ciliary muscle behaves very similarly during accommodation from ages 6 to 12 years, or we may have missed an age effect because that would have been noted in the point where the curve became non-linear. Because there were no obvious age effects, these data may indicate that the ciliary muscle is fully developed by the age of 6 years (at least in most children), which could be suggested in the one histological study of childhood ciliary muscle development,35 and age-related changes in ciliary muscle function should not be expected in this age range of subjects. There is still some debate over how the function of the ciliary muscle changes in adults approaching presbyopia, but the present study did not include adult data.
No interactions were found among accommodative response and our other model variables. As the accommodative response increased, thinner ciliary muscles did not behave radically differently than thicker ciliary muscles. This aspect of the model is captured in Figures 2–4 by the parallel slopes of the regression lines. In future studies, we will investigate how muscle thickness affects the point where contraction enters the non-linear range of accommodation.
Although imaging the ciliary muscle with anterior segment OCT is perhaps the only feasible method for imaging the ciliary muscle in large-scale studies of children, it is not without limitations. As we have mentioned previously,15, 31 it is not possible to determine how the length of the muscle is changing with accommodative effort. It is also not possible to determine if we are imaging in exactly the same image plane each time an image is captured. With each image capture, we do our best to align the scanning beam of the Visante with the midpoint of the pupil, but unlike images of the cornea or the crystalline lens, where a corneal reflex is present to assist in alignment,36 there is no easily identifiable landmark in ciliary muscle images to assist with alignment. Since these data were collected, Version 3.0 of the Visante software was released. In this software, the examiner’s view of the scanning beam position on the surface of the globe is also captured. In the future, we plan to use this feature to determine how systematic misalignment impacts thickness measurements, and also to complete post-data capture evaluation of image alignment during capture to further improve quality control. For the present study, however, we did capture multiple images and use the mean of multiple measurements from those images in analysis, as we have extensively described in a publication of our methods,15 so that should have eliminated much within-subject variability.
Additional limitations should also be considered. Matching the time of image acquisition to the accommodative response was as accurate as possible, but could certainly have contained errors. Also, at the time of collecting these data, we used a cycloplegic state to assist us in determining the precise accommodative response a child exerted during testing. This, of course, is not a natural state of the ciliary muscle, and might not be the best baseline to use as the “relaxed” power of the eye or position of the ciliary muscle. In young children, however, verifying that they are not accommodating while viewing the distance fixation target is also a challenge. This is an issue we intend to explore and improve upon as we make future protocol adjustments.
A study in an adult sample controlling precise accommodative response with ciliary muscle imaging as described above may provide more salient data regarding ciliary muscle morphology with accommodation (Figure 6). Adult accommodative responses tend to be more accurate and stable than children, and attention confounds can be minimized in this population. Once a protocol is established, a pediatric sample can be tested to generate normative data.
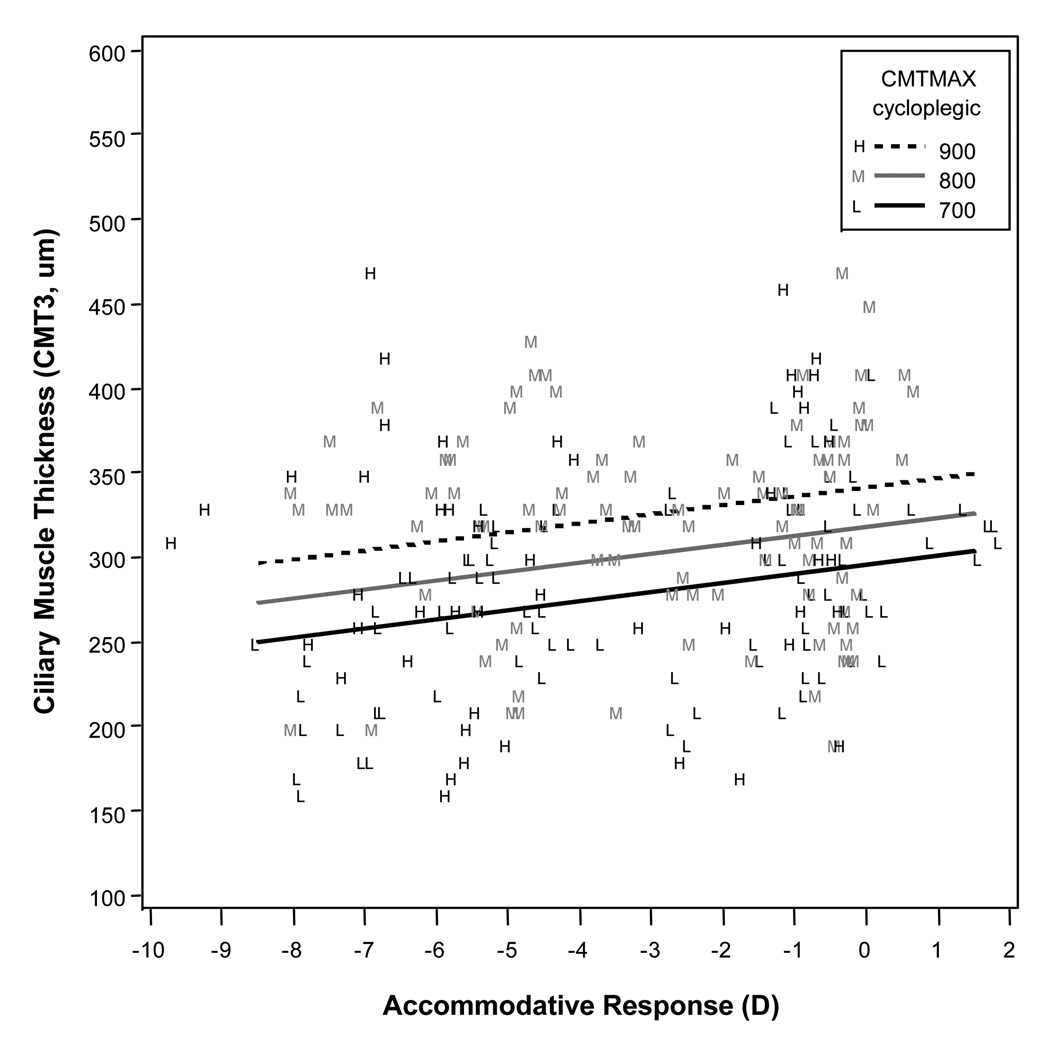
Figure 3.
Modeled results for ciliary muscle thickness 1 mm posterior to the scleral spur (CMT1) for a range of accommodative responses, showing that higher levels of accommodative response (more negative values) were associated with a thicker measurement at CMT1. Cycloplegic maximum ciliary muscle thickness (CMTMAX) was a statistically significant variable in the model, so the three lines represent three different cycloplegic ciliary muscle thicknesses across the range of our sample.
ACKNOWLEDGMENTS
NSF DMS 0811003 and Sloan Fellowship (Kao) and NEI Grants R24-EY014792 (Sinnott)
The project described was supported by Award Number KL2 RR025754 (Bailey) from the National Center for Research Resources, funded by the Office of the Director, National Institutes of Health (OD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The Ohio State University has filed a provisional patent application on the behalf of the authors: U.S. Provisional Patent Application No. 61/594,027, filed February 2, 2012, entitled: “Semiautomatic Extraction of Algorithm for Images of the Ciliary Muscle”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rohen JW. Scanning electron microscopic studies of the zonular apparatus in human and monkey eyes. Invest Ophthalmol Vis Sci. 1979;18:133–144. [PubMed] [Google Scholar]
- 2.Lutjen-Drecoll E, Tamm E, Kaufman PL. Age changes in rhesus monkey ciliary muscle: light and electron microscopy. Exp Eye Res. 1988;47:885–899. doi: 10.1016/0014-4835(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard AL, Davies LN. In vivo analysis of ciliary muscle morphologic changes with accommodation and axial ametropia. Invest Ophthalmol Vis Sci. 2010;51:6882–6889. doi: 10.1167/iovs.10-5787. [DOI] [PubMed] [Google Scholar]
- 4.Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 5.Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006;32:1792–1798. doi: 10.1016/j.jcrs.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle. A quantitative morphometric study. Mech Ageing Dev. 1992;62:209–221. doi: 10.1016/0047-6374(92)90057-k. [DOI] [PubMed] [Google Scholar]
- 7.Stachs O, Martin H, Kirchhoff A, Stave J, Terwee T, Guthoff R. Monitoring accommodative ciliary muscle function using three-dimensional ultrasound. Graefes Arch Clin Exp Ophthalmol. 2002;240:906–912. doi: 10.1007/s00417-002-0551-2. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard AL, Davies LN. The effect of ageing on in vivo human ciliary muscle morphology and contractility. Invest Ophthalmol Vis Sci. 2011;52:1809–1816. doi: 10.1167/iovs.10-6447. [DOI] [PubMed] [Google Scholar]
- 9.Charman WN, Radhakrishnan H. Accommodation, pupil diameter and myopia. Ophthalmic Physiol Opt. 2009;29:72–79. doi: 10.1111/j.1475-1313.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vision Res. 1995;35:1299–1304. doi: 10.1016/0042-6989(94)00238-h. [DOI] [PubMed] [Google Scholar]
- 11.Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82:273–278. doi: 10.1097/01.opx.0000159363.07082.7d. [DOI] [PubMed] [Google Scholar]
- 12.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–846. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 13.Tamm ER, Lutjen-Drecoll E. Ciliary body. Microsc Res Tech. 1996;33:390–439. doi: 10.1002/(SICI)1097-0029(19960401)33:5<390::AID-JEMT2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008;49:4353–4360. doi: 10.1167/iovs.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao CY, Richdale K, Sinnott LT, Grillott LE, Bailey MD. Semiautomatic extraction algorithm for images of the ciliary muscle. Optom Vis Sci. 2011;88:275–289. doi: 10.1097/OPX.0b013e3182044b94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candy TR, Gray KH, Hohenbary CC, Lyon DW. The accommodative lag of the young hyperopic patient. Invest Ophthalmol Vis Sci. 2012;53:143–149. doi: 10.1167/iovs.11-8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulp MT, Schmidt PP. Visual predictors of reading performance in kindergarten and first grade children. Optom Vis Sci. 1996;73:255–262. doi: 10.1097/00006324-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Shankar S, Evans MA, Bobier WR. Hyperopia and emergent literacy of young children: pilot study. Optom Vis Sci. 2007;84:1031–1038. doi: 10.1097/OPX.0b013e318157a67a. [DOI] [PubMed] [Google Scholar]
- 19.Egashira SM, Kish LL, Twelker JD, Mutti DO, Zadnik K, Adams AJ. Comparison of cyclopentolate versus tropicamide cycloplegia in children. Optom Vis Sci. 1993;70:1019–1026. doi: 10.1097/00006324-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel GM, Mutti DO. Evaluation of infant accommodation using retinoscopy and photoretinoscopy. Optom Vis Sci. 2009;86:208–215. doi: 10.1097/OPX.0b013e3181960652. [DOI] [PubMed] [Google Scholar]
- 21.Dirckx JJ, Kuypers LC, Decraemer WF. Refractive index of tissue measured with confocal microscopy. J Biomed Opt. 2005;10:44014. doi: 10.1117/1.1993487. [DOI] [PubMed] [Google Scholar]
- 22.Tearney GJ, Brezinski ME, Southern JF, Bouma BE, Hee MR, Fujimoto JG. Determination of the refractive index of highly scattering human tissue by optical coherence tomography. Opt Lett. 1995;20:2258. doi: 10.1364/ol.20.002258. [DOI] [PubMed] [Google Scholar]
- 23.Lossing LA, Sinnott LT, Kao CY, Richdale K, Bailey MD. Measuring changes in ciliary muscle thickness with accommodation in young adults. Optom Vis Sci. 2012;89 doi: 10.1097/OPX.0b013e318252cadc. XXX-XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snijders TA, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 25.Goss DA, Cox VD, Herrin-Lawson GA, Nielsen ED, Dolton WA. Refractive error, axial length, and height as a function of age in young myopes. Optom Vis Sci. 1990;67:332–338. doi: 10.1097/00006324-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Helmholtz HF. Treatise on Physiological Optics (translated by Southall JPC) New York: Dover Publications; 1969. [Google Scholar]
- 27.Fincham EF. The mechanism of accommodation. Br J Ophthalmol. 1937;8:7–80. [Google Scholar]
- 28.Duane A. Studies in monocular and binocular accommodation, with their clinical application. Trans Am Ophthalmol Soc. 1922;20:132–157. [PMC free article] [PubMed] [Google Scholar]
- 29.Graves B. The response of the lens capsules in the act of accommodation. Trans Am Ophthalmol Soc. 1925;23:184–198. [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira C, Tello C, Liebmann JM, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005;140:324–325. doi: 10.1016/j.ajo.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Bailey MD. How should we measure the ciliary muscle? Invest Ophthalmol Vis Sci. 2011;52:1817–1818. doi: 10.1167/iovs.11-7313. [DOI] [PubMed] [Google Scholar]
- 32.Pierscionek BK. Age-related response of human lenses to stretching forces. Exp Eye Res. 1995;60:325–332. doi: 10.1016/s0014-4835(05)80114-9. [DOI] [PubMed] [Google Scholar]
- 33.Richdale K, Bullimore MA, Zadnik K. Lens thickness with age and accommodation by optical coherence tomography. Ophthalmic Physiol Opt. 2008;28:441–447. doi: 10.1111/j.1475-1313.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolz M, Prinz A, Drexler W, Findl O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br J Ophthalmol. 2007;91:360–365. doi: 10.1136/bjo.2006.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aiello AL, Tran VT, Rao NA. Postnatal development of the ciliary body and pars plana. A morphometric study in childhood. Arch Ophthalmol. 1992;110:802–805. doi: 10.1001/archopht.1992.01080180074031. [DOI] [PubMed] [Google Scholar]
- 36.Lehman BM, Berntsen DA, Bailey MD, Zadnik K. Validation of optical coherence tomography-based crystalline lens thickness measurements in children. Optom Vis Sci. 2009;86:181–187. doi: 10.1097/OPX.0b013e318198198d. [DOI] [PMC free article] [PubMed] [Google Scholar]