Abstract
In the early years of fMRI research, the auditory neuroscience community sought to expand its knowledge of the underlying physiology of hearing, while also seeking to come to grips with the inherent acoustic disadvantages of working in the fMRI environment. Early collaborative efforts between prominent auditory research laboratories and prominent fMRI centers led to development of a number of key technical advances that have subsequently been widely used to elucidate principles of auditory neurophysiology. Perhaps the key imaging advance was the simultaneous and parallel development of strategies to use pulse sequences in which the volume acquisitions were “clustered,” providing gaps in which stimuli could be presented without direct masking. Such sequences have become widespread in fMRI studies using auditory stimuli and also in a range of translational research domains. This review presents the parallel stories of the people and the auditory neurophysiology research that led to these sequences.
Keywords: clustered volume acquisitions, sparse sampling, tonotopy, auditory cortex
Introduction
The following review presents our personal reflections on the massive collaborative efforts undertaken in the early days of fMRI and how they ultimately resulted in one of the key cornerstones for conducting experiments involving sound presentation – the clustered-volume acquisition (CVA) or “sparse sampling” technique. This technique is predominantly used today to conduct fMRI for the purposes of auditory neuroscience, but has a range of other applications in other sensory and cognitive domains. The two authors started to develop this technique independently, unaware that the inherent problems of conducting auditory neuroimaging in an intensely noisy environment had motivated work that was converging on the same solution. We seek to provide an historical perspective on how this process took place in both Boston and Nottingham.
Our Early Goals
Tom
“During late 1993, the second entering class of the MIT-Harvard Division of Health Sciences and Technology Speech and Hearing Sciences Program (of which I was a member) visited the MGH-NMR Center, and received a tour from Bruce Rosen. This tour included a brief presentation by Randy Benson about his research investigating the use of fMRI as an alternative to Wada testing for language lateralization (Benson et al., 1999). Having briefly been exposed to the concepts of neuroimaging in a senior elective while at Purdue, I was hooked.
In March 1994 I began to work at MGH, learning about fMRI and how I might apply it to questions appropriate to my graduate program. I quickly concluded that I would take on the task of elucidating the frequency mapping in the auditory cortex of humans, seeking to demonstrate that the tonotopic maps observed within central auditory fields of cats and monkeys (Merzenich and Brugge, 1973; Merzenich et al., 1973; Morel et al., 1993) were analogous (if not homologous) in man. There was a general consensus in the field that such a finding (e.g., Wessinger et al., 2001) would be of appreciable value to translational research, both from a clinical and basic science perspective, as variations in selectivity for stimulus properties (e.g., amplitude modulation rate; Schreiner and Urbas, 1986, 1988) in experimental animals might then be sought and exploited for the understanding, augmentation or electrical replacement of human hearing. Others at the time were also pursuing similar lines of enquiry (Bilecen et al., 1998; Wessinger et al., 1997).
Working with Randy as my mentor, we conducted our first tonotopy-related experiment in July 1994, modeled after the design of previous works in PET (Lauter et al., 1985) and MEG (Pantev et al., 1988; Romani et al., 1982). This experiment built on pilot data Randy had collected in May 1992, shortly after initial presentation by Tom Brady of Ken Kwong’s work in BOLD-fMRI (Brady, 1991). Our initial experiments were not particularly successful, primarily because our subjects could barely hear our stimuli (750 and 2000 Hz tones), even when presented at full volume.”
Deb
“Under the leadership and vision of Sir Peter Mansfield, the School of Physics and Astronomy, University of Nottingham, had grown from the mid-60s to the mid-90s into one of the most prestigious centres for biomedical imaging in the UK. Sir Peter’s early pioneering work not only transformed NMR into a medical imaging technique, but also foresaw what would be required to make the technique clinically useful. Notably, using the extensive in-house expertise in gradient coil design the group had built the first 3T MR scanner in the UK using an Oxford magnet. By the time a new centre for NMR was built on the campus in 1991 (later renamed The Sir Peter Mansfield Magnetic Resonance Centre, in honour of his Nobel prize), its head, Peter Morris, was welcoming local collaborative opportunities to pursue new NMR applications in the sensory and cognitive neurosciences. Mark Haggard, Director of the MRC Institute of Hearing Research (MRC IHR) in Nottingham saw the huge potential of this emerging methodology and created a new post for a post-doctoral research scientist to lead a fMRI project in the auditory neurosciences. I started working with Mark in December 1996 to explore the spatiotopic organization of auditory cortex with respect to some of the key sound features such as sound level, modulation, pitch etc. I was soon introduced to Richard Bowtell, a reader in experimental physics with expertise in gradient coil design. Working with Richard and one of his bright young PhD students, Stuart Clare, as my MR physics mentors, I started to learn about fMRI and soon realized the many challenges ahead. Stuart’s PhD was focused on developing the technique of fMRI to study visual, motor and auditory brain activation. Under Richard and Peter’s watchful eyes together we worked closely for my first couple of years in Nottingham building up sufficient preliminary data to demonstrate to ourselves that we could reliably detect sound-related activity across multiple auditory cortical fields.”
Imaging-related Acoustic Noise
Despite being separated by over 3000 miles, both of us were being rudely introduced to the vexing issue of the acoustic noise associated with MR imaging. The intensity of the ambient noise is exacerbated in the case of the echo-planar imaging (EPI) pulse sequences used for fMRI.
Tom
“Each time the gradients are switched “on” to force a sweep across k-space for image acquisition (specifically the “readout” portion), current flows through coils that are wound in a spiral manner, in a plane orthogonal to the generated magnetic field. The moving charges comprising this current undergo a Lorentz force that deforms the coil winding ever so slightly. Because these coils are embedded in a rigid tube, the slight motion results in a slight contraction or expansion of the tube, with an associated dull, “knock”-like sound. During EPI, the current polarity is reversed many times per second to effect a trajectory through the entirety of k-space, producing many such sounds in rapid succession. Human hearing has a nominal range of 20 Hz – 20 kHz at birth. As long as these “knock” sounds occur in a train with a period that places their repetition frequency in this nominal range, the subject (and experimenter) will experience a pitch percept associated with image acquisition. In the majority of cases (particularly in the early days of fMRI), the imaging systems produced these sounds at intensities approaching 120 dB SPL, frightfully near the threshold of pain. Needless to say, conducting auditory fMRI was rather a challenging activity.”
Deb
“The spectral characteristics of the EPI noise are mainly a function of the “readout” gradient pulses of the chosen MR pulse sequence and comprise a series of harmonically related peaks together with a background of broadband noise. Most of the energy is at the lower end of the audible range up to about 1 kHz (see Hall et al., 2009). Both groups in Boston and Nottingham made numerous systematic measures of the noise level. The GE/ANMR scanner at the MGH-NMR Center was found to produce a strong harmonic complex with a fundamental frequency at 1 kHz (Ravicz and Melcher, 1998), while the purpose-built 3 T MR scanner in Nottingham had a dominant peak of acoustic energy at 1.9 kHz (Foster et al., 2000).”
How Auditory Cortical Mapping Motivated Solutions to Imaging-related Acoustic Noise
On both sides of the Atlantic, we kept thinking about the problems caused by the hostile acoustic environment and kept searching for creative ways to solve them. Our successes were based upon developing effective multidisciplinary relationships with a group of outstanding collaborators who had the expertise to make the study of the auditory system using fMRI something other than quixotic.
Tom
“By late 1994, I had now established my co-advisors as Bruce Rosen and Jennifer Melcher (Eaton-Peabody Laboratory, EPL), and been connected to Mike Ravicz (an engineer at EPL, specializing in acoustics), Patrick Ledden (then a graduate student at MGH, who dabbled in coil construction), and, thanks to Randy, a number of outstanding people at MGH (including Ken Kwong, Robert Weisskoff and Roger Tootell) who would prove invaluable both in this research and in our efforts to improve auditory fMRI. Intervention by these outstanding colleagues and mentors led to three key advancements that facilitated the investigation of tonotopy, and subsequently enhanced our research into perception of speech and other auditory stimuli.
The first was simply avoiding direct masking of stimuli, by shifting our stimulus frequencies away from the 1 kHz frequency of the readout gradient switching. Coupled with this change, Mike had measured the transfer characteristic of the pneumatic stimulus delivery system and reported significant roll-off above 3 kHz. Therefore, we initially limited ourselves to “high” frequencies in the vicinity of 2.5 kHz. While not optimal, we were at least able to present individual frequencies several octaves apart, lending hope that we might resolve different foci of activation for each stimulus.
The second key advancement was a means to present stimuli effectively while also attenuating scanner noise. In our initial experiments, Randy and I simply placed pneumatic delivery headphones over the subject’s ears, resulting in 15–20 dB attenuation of the scanner noise, but also resulting in loss of much of the acoustic energy of the stimulus into the cavity between the headphones and the ear canal. Therefore, Jennifer and Mike modified a set of highly-rated ear defenders to connect to the pneumatic delivery system by extending tubing from the pneumatic delivery system through the muffs toward the ear canals, and inserted probe tube ear plugs at the end of the tubes. Now, sound delivery was direct to the ear canal, resulting in greater efficiency for stimulation, while also attenuating the scanning noise both by the ear defenders and plugs. This method of sound delivery attenuates the acoustic noise of imaging by nearly 35 dB (Ravicz and Melcher, 1998) and continues to be an extremely effective means of delivering auditory stimuli, whether the source is pneumatic or electrostatic. For our research, masking was greatly reduced and the perceived loudness of our stimuli was notably increased. Having a greater range of super-threshold intensities for stimulation, we introduced an attenuator between our source and the pneumatic transducers, enhancing control of stimulus intensity. This particularly allowed us to (partially) overcome the roll-off of the delivery system by use of stimulus-specific attenuation. We could now work beyond the 3 kHz roll-off of the pneumatic system, and exploit the upper 50% of the normal hearing range for adult humans, making it more likely we would be able to resolve distinct foci of activation across our stimuli.
The third advancement was construction of novel surface coils that could be used with the improved sound delivery system. We had previously observed that use of the birdcage head coil did not yield much sound-related activation, but obtained far better results when using a 5″ surface coil. However, most subjects could not both wear the modified delivery system and have the surface coil placed appropriately. Therefore, we sought out Patrick Ledden – reputed to have a knack for building interesting and effective imaging coils – to help us out. After several revisions, Patrick successfully incorporated the sound delivery and attenuation ear defenders into a set of bilateral auditory surface coils. These coils greatly enhanced the quality of the data we could collect, by improving (by a factor of 4–10) the temporal signal-to-noise ratio in lateral portions of the superior temporal lobes. Surface coils greatly reduced the number of trials/length of time required to obtain usable results on each subject, and likely were the key technical contribution to make my eventual phase-encoding studies at 1.5T feasible.
With the advances noted above, we were now able to conduct probe-based measurements of the frequency-dependent responses in auditory cortex (Talavage et al., 2000), revealing rather consistent constellations of responses to both “high” and “low” categories of frequency stimuli, with these responses being relatively consistent with anticipated boundaries of auditory cortical regions (Galaburda and Sanides, 1980; Rivier and Clarke, 1997). However, while this work identified regions of the cortex that were most responsive to “high” or “low” frequencies, it did not meaningfully address the auditory neuroscience definition of tonotopy, per se, as we had yet to demonstrate continuous gradients of frequency sensitivity no linking our samples.
To address the concern of the continuity of the frequency sensitivity, visual researcher Roger Tootell suggested we collaborate with Marty Sereno and Anders Dale, who had recently published significant retinotopic mapping research revealing boundaries of visual areas (Sereno et al., 1995) through use of phase-encoded techniques (see Engel, current issue). Fortunately, Marty had a strong inherent interest in the auditory system (particularly the frequency organization across cytoarchitectonic fields), and was quick to collaborate with us. Marty generated our first phase-encoded auditory stimuli (using pink noise) and by August 1995, with assistance from Anders, we ran our first subjects.
Introducing a continuous frequency sweep crossing over the fundamental frequency of the 1 kHz scanner noise led to a problem – my subjects could not hear a portion of the sweep, and we were clearly losing the ability to resolve phases associated with the middle of the sweep. By turning up the volume, we could overcome the masking, but cortical tuning becomes broader at higher stimulus intensities, resulting here in a large chunk of cortex exhibiting a phase consistent with the 500 Hz portion of the sweep. This was not unexpected given that the entire cochlea will phase lock to such low-frequency stimuli, particularly at the sound levels necessary to overcome masking – but it was undesirable. Therefore, we began to collect “audiograms” for subjects while they were undergoing an EPI acquisition in the scanner. These audiograms provided session-specific threshold data, from which I could generate a sweep stimulus that followed the contour of the subject’s hearing thresholds under masking conditions. We could now deliver the desired stimulus at a fixed sensation level (i.e., level above that required for threshold perception), making wide-scale synchrony in the cochlea for lower frequencies less likely. This approach provided the added benefit that the consequences of variations in passive attenuation of the scanner noise were greatly reduced.
Subsequently having the help of a number of painfully tolerant subjects (i.e., willing to endure 3-hour sessions, often beginning at 11 PM), I was able to collect data that comprised the bulk of my doctoral thesis and, eventually, our paper presenting the first large-scale, continuous mapping of frequency selectivity gradients in human auditory cortex (Talavage et al., 2004).”
Deb
“The first major step in Nottingham was to create a system that provided the greatest passive noise reduction combined with the high-output and high-fidelity presentation of sound stimuli. Attenuation comparable to that achieved by Jennifer and Mike was obtained using the same approach of circumaural ear defenders. However, we soon realized that tube phone systems were not ideal in terms of frequency response and power output, and corrections for frequency and phase distortions were difficult. As an alternative, we decided to explore the possibility of using electrostatic transducers in the MR scanner. The resulting ingenious solution was the product of several years work by John Chambers and Dave Bullock, engineers at MRC IHR led by Alan Palmer. A series of pilot tests led to the final headset, based on a pair of £1,000 Sennheiser headphones incorporated into a set of ear defenders which Dave pulled apart and re-assembled after removing all ferromagnetic materials. By the end of 1997, we had in place a sound system using the latest digital-signal-processing technology, optic fibres and radio-frequency filtering that was capable of producing a reasonably flat frequency response up to about 20 kHz and outputting sounds over 100 dB SPL. Needless to say, we were all extremely proud of this achievement and in June 1998, Alan presented our work to the growing neuroimaging community at OHBM in Montreal. This meeting was an important milestone because it fostered growing relations with Jennifer, Mike and Tom who were addressing similar technical challenges in Boston.
With these advances, we were now able to conduct our first fMRI studies of modulation (Hall et al., 2000) and sound-level (Hall et al., 2001) coding across auditory cortical fields. The technology also paved the way for the future implementation of active noise cancellation to further reduce the subject’s experience of the scanner acoustic noise (Blackman and Hall, 2011; Hall et al., 2009).”
Development of Clustered Volume Acquisition Sequences for Auditory fMRI
Tom
“While my tonotopy research was ongoing, however, Jennifer and I continued to be concerned about the scanner acoustic noise, as it effectively precluded our ability to compare the fMRI mappings I was deriving to those obtained in quiet from experimental animals using depth electrodes. This concern would ultimately lead to one of the more entertaining and exciting research experiences of my career. In fact, this advancement has almost certainly been one of the most influential developments as far as the use of auditory fMRI is concerned – the clustered volume acquisition (CVA) approach to data collection (Edmister et al., 1999), more commonly known today as “sparse sampling” when combined with relatively long interscan intervals (Hall et al., 1999).
‘We wrote it on a napkin,’ is a common, and typically apocryphal, origin story for many developments, scientific or otherwise, generally taken to have been scribbled upon late at night, over drinks in a bar. In this case, however, the napkin (Figure 1) was scribbled on at a McDonald’s, located in the underground food court across from the Vancouver Trade and Conference Center, during the 1997 ISMRM meeting.
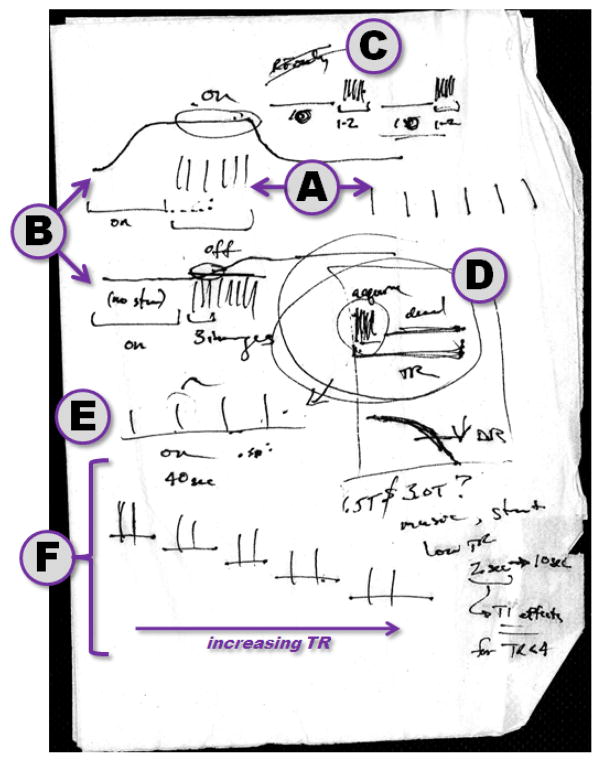
Figure 1.
McDonald’s napkin on which “Hammer” – the initial clustered volume acquisition (CVA) sequence for auditory imaging – was developed by Tom Talavage and Whitney Edmister over a cheap dinner, on Wednesday, April 16, 1997. A: After a description by Tom regarding how the equal spacing between slice acquisitions (right set of vertical lines) led to masking of stimuli, Whitney proposed “clustering” the acquisitions and leaving a gap (left set of vertical lines). B: Given this option, an alternate means to measure the response to any given stimulus, contrasting presentation of the stimulus (top) with no stimulus (bottom), noting that only the first few images of this latter acquisition would be free of the (sketched) hemodynamic response to the volume acquisition. C: Conceptual structure for sequence operation in which a long gap (here, 10s; initially including RF excitation at fixed intervals when not acquiring images) would precede a 1–2s acquisition. D: Initial vision for how the sequence would be constructed within the TR period, including prediction of how the induced change in fMRI response would decrease with increasing TR. E: Given that each acquisition would produce a hemodynamic response, longer TRs would also be expected to minimize interaction across acquisitions. F: Plan for experiment to be conducted using increasing gaps between acquisitions (i.e., increasing TR) to evaluate benefits of CVA for auditory fMRI, ultimately the basis of Edmister et al. (1999).
Whitney Edmister, a fellow graduate student at MGH, and I had just heard Peter Bandettini’s seminal presentation on measurement of the auditory cortical response to the noise generated by EPI during fMRI. Peter had varied the number of gradient readouts (without excitation) prior to the beginning of his experiment, conducted a simple subtraction, and looked at how the approach to steady-state magnetization was affected (Bandettini et al., 1997).
Whitney and I sat in the food court for quite a while, discussing how the measurements could be improved if we could achieve steady-state magnetization prior to the variation in readout train length. Achieving this goal would require some sort of “gap” between volume acquisitions, into which we could somehow insert a variable number of gradient readouts without excitation. Such a “gap” would also be beneficial for my tonotopic work as I could then deliver short bursts of stimuli in these intervals, free of direct masking from the scanner noise. Jennifer, Mike and I had discussed this “gap” concept with Peter Bandettini several years before (when he had been a post-doc in our lab). We had also talked about it several times with some of our auditory neuroimaging colleagues from Nottingham (Deb Hall, Mark Haggard, Alan Palmer) and Magdeburg, Germany (Claus Tempelman). However, the 1.5T and 3.0T ANMR systems at MGH did not provide any control over the rate of a volume acquisition (the slices were automatically distributed over the duration of the repetition time), so this great theoretical concept seemed rather problematic to put into practice.
Pointing to the napkin, I skeptically asked Whitney, “You can do this?” “Yes!” was his emphatic response. And, thus was born a (partial) solution to one of the biggest problems we encounter in auditory fMRI – reducing the direct interference from scanner noise.
Having spoken many times at prior ISMRM meetings with Peter and his former MCW colleague, Eric Wong, about the work that led to the first BOLD-fMRI paper (Bandettini et al., 1992) – and critically, ignorance being bliss – Whitney and I concluded that we, too, could go back to the lab and complete a significant work in a few weeks’ time.
Whitney handled the modifications of the ANMR EPI code and the “Hammer” sequence was born (affectionately named after an offhand comment from Tom Budinger in response to the naming of phMRI – “So, if I hit a cat with a hammer and then image the cat, I should call it ‘HammerMRI’?”). “Hammer” represented the original MGH implementation of an EPI sequence that packed slice acquisitions into the shortest possible time-period, and left a preceding delay. Five days after the end of the 1997 ISMRM meeting, Whitney and I successfully compiled and ran the sequence on a willing volunteer (Dr. Tetsuo Makabe). Taking a couple of days to verify that Hammer was generating useful data, we moved on to introduce sequences that allowed for variable numbers of gradient readouts without RF excitation to be inserted prior to the actual volume acquisition. This led to variations such as “Jackhammer” and “Sledgehammer” that were subsequently used to quantify the effects of the larger number of slice acquisitions in a fixed period of time. By the middle of May, we had acquired the data that would ultimately be published using the “CVA” naming convention (developed with Robert Weisskoff) in both Edmister et al. (1999) and Talavage et al. (1999), and preliminary findings that motivated Talavage and Edmister (2004).”
Deb
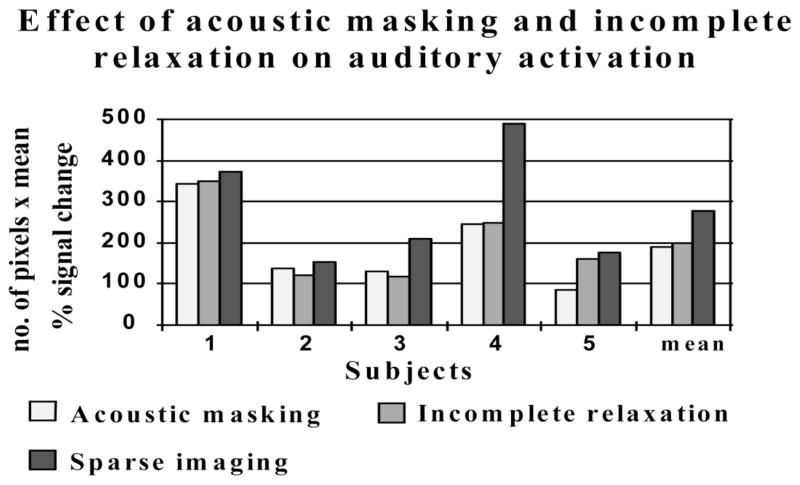
“It is interesting to reflect that independently and in parallel we were developing the exact same solution (though by a different name – “sparse sampling”). Our solution wasn’t quite scribbled on a McDonald’ napkin, nor a pub beer mat for that matter! However, it grew out of an inspired suggestion by Mark Haggard that the easiest way to reduce the scanner acoustic noise would be to modify the temporal sequence of the image acquisition. The great advantage of having a purpose-built MR scanner was that we were able to program new pulse sequences in a flexible manner. By 1997, Mike Elliott (a physics PhD student) had joined our team and was responsible for implementing several new EPI sequences. With Mike’s help, we were able to quantify the various effects of the scanner noise using three different pulse sequences (see Figure 2). The initial “sparse sampling” sequence collected 8 slices as rapidly as possible every 14s, with the scanner inactive during the inter-scan intervals. An “acoustic masking” sequence inserted “continuous” scanner acoustic noise between volume acquisitions by applying readout gradients for six 8-slice volumes, with RF excitation only for the first of these scans. The “incomplete relaxation” sequence examined the effect of short inter-scan intervals but without the intense scanner noise by performing selective excitation (and its associated low intensity, low-frequency “knocking” sound) of the volume 6 times in the 14s period, but with EPI readout for only the first scan. By comparing the results obtained from each of these sequences, we were able to demonstrate that the sparse sampling method was clearly superior in terms of its ability to detect sound-related activity due to a combination of reduced acoustic masking and the more complete recovery of magnetisation between excitations. I took the exciting opportunity to present this work in June 1998 at the OHBM meeting in Montreal, although only the results of the sparse sampling method were ever published (Hall et al., 1999).”
Figure 2.
Using speech as a stimulus, bilateral auditory activity was observed in auditory cortex. Descriptions of the conditions are given in the text. For each condition, the degree of activation was characterized by the product of the number of activated voxels and mean % signal change. For all five subjects, both acoustic masking and incomplete relaxation reduced the overall activation level relative to the activation in the sparse imaging condition (unpublished data)..
It is appropriate to note here, that as users of GE/ANMR and home-built systems, we were both unaware that Siemens had already implemented their EPI sequence in a manner that fundamentally encoded clustered volume acquisitions into their product. We suspect that our first introduction to this came from a brief discussion Tom had with Stefan Posse in late May, at the 1997 OHBM meeting in Copenhagen, after his initial work was already submitted.
Benefits of Clustered Volume Acquisitions (CVA) for Auditory Imaging and Beyond
Tom
“Excitingly for Whitney and I, many researchers at MGH found applications for our “Hammer” sequence – not just those of us interested in auditory phenomena. For example, “Hammer” was tested by Jack Belliveau for acquisition of electrical potentials in the MRI (e.g., see Liebenthal et al., 2003). Further, a refinement of the slice profile that Whitney and I had to effect to reduce crosstalk between adjacent slices was found to be beneficial for multi-slice cardiac gating (e.g., Sigalovsky and Melcher, 2006). Early on, it also became apparent that this type of acquisition had substantial advantages for event-related experimentation – a relatively new approach to fMRI design and analysis that was just taking hold at the time of our development of “Hammer” (e.g., Buckner et al., 1996) – independent of the modality of stimulation. In fact, many people today use “sparse designs” when speaking of event-related paradigms in which stimulus presentations are arranged so as to fall within the gaps between volume acquisitions (e.g., Eden et al., 1999), providing a means to control for modulation of attention, whether to auditory or other stimuli (e.g., Grady et al., 1997; Woodruff et al., 1996).
But did the development of “Hammer” affect my own study of tonotopy? Interestingly, no, it did not. After we had created “Hammer,” I ran a number of subjects using both blocked and phase-encoding techniques and rapidly came to the conclusion that the results were not going to change more than the inherent measurement error of the system, at least not at 1.5 T. In the case of blocked experimental work (e.g., Talavage et al., 2000), this was likely because we had already minimized direct masking of our stimuli. For the phase-encoding paradigm, we likely benefitted from the spread of times over which individual slices were acquired – e.g., if a region of cortex responsive to a particular frequency range was imaged across multiple slices, combining these time-courses during the fitting procedure effected an increase in oversampling of the signal, and thus a more precise measurement of phase. So, while use of CVA and associated sparse designs did not ultimately affect our assessments of tonotopic organization in human auditory cortex, it has been subsequently applied by others for mapping of frequency selectivity using discrete tonal stimuli (e.g., Formisano et al., 2003; Humphries et al., 2010) and it did open the door for many other studies to effectively elucidate key principles of processing in the auditory pathway (e.g., Ahveninen et al., 2006; Jaaskelainen et al., 2004; Langers and van Dijk, 2011).”
Deb
“In the time since those early publications, sparse sampling has proven to be of great importance because most auditory research typically uses broadband stimuli that overlap spectrally with the scanner acoustic noise. Not only does sparse sampling provide a substantial and reliable improvement in detecting sound-evoked activity in auditory cortex, but it also reduces the effort required for frequency discrimination, sound source segregation, and speech perception (Blackman and Hall, 2011). This method has, therefore, become the EPI sequence of choice for all of our subsequent auditory fMRI work in Nottingham enabling spatiotopic mapping of many different sound features, and has been widely adopted elsewhere. For example, sparse sampling has also recently been applied to examine the role of nonauditory networks during basic sound processing using independent components analysis of functional connectivity (Langers and Melcher, 2011).
Sparse sampling can provide considerable benefits in other domains too, since scanner acoustic noise may spoil fMRI experiments, primarily by way of other mechanisms such as distraction (Cho et al., 1998) or increased load on working memory (Tomasi et al., 2005). There is an unrealized potential for sparse sampling to have a much wider impact beyond auditory neuroimaging. Perhaps some researchers are hesitant to apply the method through concern that reducing the amount of acquired data would reduce signal-to-noise ratio and temporal resolution. The former problem is overestimated because autocorrelations in physiological noise limit the added value of contiguous acquisitions; the latter problem is of minor concern at least in block-related designs, which are still highly prevalent. Pleasingly, there is already evidence that sparse temporal sampling has been applied to the study of a range of clinical conditions including schizophrenia (Allen et al., 2007; Fu et al., 2005; Mechelli et al., 2007; Shergill et al., 2000), vegetative state (Owen et al., 2006) and epilepsy (Korsnes et al., 2010). The method has clearly become one of the cornerstones of translational neuroscience leading to insights about how to assess and treat many different medical problems.”
Where are we today?
Sparse sampling has been demonstrated to effectively reduce effects of the acoustic noise generated during fMRI acquisitions on the audibility and processing of any stimulus (including reducing general costs for attention and working memory). More than a decade since our parallel developments of sparse sampling, this technique remains a prominent approach to collecting fMRI data. We are pleased to find that the solution we both developed on the way to addressing questions related to auditory neuroscience has been of greater impact than we originally envisioned, including studies of functional connectivity and translational applications. While mapping studies using subtractive analysis approaches undoubtedly benefits greatly from our methods, we do not necessarily advocate that sparse sampling should be used in all circumstances. For example, effective connectivity explorations of network modes of processing in the brain are probably better suited to continuous acquisitions, although here one can still apply CVA to reduce the reliance on slice timing correction at the post-processing stage.
Although various technological solutions have substantially reduced the perceived level of the scanner acoustic noise, it has not been eliminated and possibly never will completely. The auditory research community continues to push for quieter sequences (e.g., Peelle et al., 2010) and improved understanding of how responses to the scanner acoustic noise and desired stimuli interact (e.g., Langers et al., 2005; Olulade et al., 2011). The quality of delivered stimuli has been greatly advanced by a variety of delivery systems relying on fiber optic signal transmission to electrostatic transducers, providing relatively flat frequency response over the auditory range of humans while minimizing sensitivity to the switched magnetic fields associated with image acquisition. Such systems provide a platform for future commercialization of active noise cancellation (e.g., Hall et al., 2009) which can reduce the sound energy at the listener’s ear by up to 35 dB ear (e.g., from 93 to 58 dB SPL at the peak frequency of the acoustic energy) over and above the attenuation achieved by ear defenders. Other options may come from continued development of parallel imaging, particularly through sparse (k-space) sampling techniques that use compressed sensing approaches. Such sequences enable non-periodic sampling of k-space, and thus can greatly reduce the pitch percept, likely also reducing the induced response in the auditory cortex (see Hall, 2006; Talavage et al., (in press)). These developments can be viewed as an addition to the benefits already provided by sparse sampling, rather than a substitute for them.
While it is probably unrealistic ever to silence EPI, considerable reductions in its intensity should be achievable in the next decade. Until the day that fMRI generates no meaningful acoustic noise, “Hammer” away!
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thomas M. Talavage, Purdue University, School of Electrical and Computer Engineering, Weldon School of Biomedical Engineering, West Lafayette, USA
Deborah A. Hall, NIHR National Biomedical Research Unit in Hearing, University of Nottingham, Nottingham, UK
References
- Ahveninen J, Jaaskelainen IP, Raij T, Bonmassar G, Devore S, Hamalainen M, Levanen S, Lin FH, Sams M, Shinn-Cunningham BG, Witzel T, Belliveau JW. Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci U S A. 2006;103:14608–14613. doi: 10.1073/pnas.0510480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Amaro E, Fu CH, Williams SC, Brammer MJ, Johns LC, McGuire PK. Neural correlates of the misattribution of speech in schizophrenia. Br J Psychiatry. 2007;190:162–169. doi: 10.1192/bjp.bp.106.025700. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Van Kylen J, Birn RM, Hyde JS. fMRI of Scanner Noise Induced Auditory Cortex Activation. Fifth Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine; Vancouver, B.C., Canada. 1997. p. 349. [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, Davis TL, Weisskoff RM, Talavage TM, Logan WJ, Cosgrove GR, Belliveau JW, Rosen BR. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Bilecen D, Scheffler K, Schmid N, Tschopp K, Seelig J. Tonotopic organization of the human auditory cortex as detected by BOLD-FMRI. Hear Res. 1998;126:19–27. doi: 10.1016/s0378-5955(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Blackman GA, Hall DA. Reducing the effects of background noise during auditory functional magnetic resonance imaging of speech processing: qualitative and quantitative comparisons between two image acquisition schemes and noise cancellation. J Speech Lang Hear Res. 2011;54:693–704. doi: 10.1044/1092-4388(2010/10-0143). [DOI] [PubMed] [Google Scholar]
- Brady TJ. Future prospects for MR imaging. Tenth Annual Meeting of the Society for Magnetic Resonance in Medicine; San Francisco, CA. 1991. p. 2. [Google Scholar]
- Buckner RL, Bandettini PA, O’Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ZH, Chung SC, Lim DW, Wong EK. Effects of the acoustic noise of the gradient systems on fMRI: a study on auditory, motor, and visual cortices. Magn Reson Med. 1998;39:331–335. doi: 10.1002/mrm.1910390224. [DOI] [PubMed] [Google Scholar]
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA. Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: the behavior interleaved gradients technique. Magn Reson Med. 1999;41:13–20. doi: 10.1002/(sici)1522-2594(199901)41:1<13::aid-mrm4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7:89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Kim DS, Di Salle F, van de Moortele PF, Ugurbil K, Goebel R. Mirror-symmetric tonotopic maps in human primary auditory cortex. Neuron. 2003;40:859–869. doi: 10.1016/s0896-6273(03)00669-x. [DOI] [PubMed] [Google Scholar]
- Foster JR, Hall DA, Summerfield AQ, Palmer AR, Bowtell RW. Sound-level measurements and calculations of safe noise dosage during EPI at 3 T. J Magn Reson Imaging. 2000;12:157–163. doi: 10.1002/1522-2586(200007)12:1<157::aid-jmri17>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK. Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry. 2005;162:485–494. doi: 10.1176/appi.ajp.162.3.485. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- Grady CL, Van Meter JW, Maisog JM, Pietrini P, Krasuski J, Rauschecker JP. Attention-related modulation of activity in primary and secondary auditory cortex. Neuroreport. 1997;8:2511–2516. doi: 10.1097/00001756-199707280-00019. [DOI] [PubMed] [Google Scholar]
- Hall DA. fMRI of the auditory cortex. In: Faro SH, Feroze MB, editors. Functional MRI: Basic Principles and Clinical Applications. Springer Science and Media Inc; New York: 2006. pp. 364–393. [Google Scholar]
- Hall DA, Chambers J, Akeroyd MA, Foster JR, Coxon R, Palmer AR. Acoustic, psychophysical, and neuroimaging measurements of the effectiveness of active cancellation during auditory functional magnetic resonance imaging. J Acoust Soc Am. 2009;125:347–359. doi: 10.1121/1.3021437. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Summerfield AQ, Palmer AR, Elliott MR, Bowtell RW. Modulation and task effects in auditory processing measured using fMRI. Hum Brain Mapp. 2000;10:107–119. doi: 10.1002/1097-0193(200007)10:3<107::AID-HBM20>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Summerfield AQ, Akeroyd MA, Palmer AR, Bowtell RW. Functional magnetic resonance imaging measurements of sound-level encoding in the absence of background scanner noise. J Acoust Soc Am. 2001;109:1559–1570. doi: 10.1121/1.1345697. [DOI] [PubMed] [Google Scholar]
- Humphries C, Liebenthal E, Binder JR. Tonotopic organization of human auditory cortex. Neuroimage. 2010;50:1202–1211. doi: 10.1016/j.neuroimage.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaskelainen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levanen S, Lin FH, May P, Melcher J, Stufflebeam S, Tiitinen H, Belliveau JW. Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci U S A. 2004;101:6809–6814. doi: 10.1073/pnas.0303760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsnes MS, Hugdahl K, Nygard M, Bjornaes H. An fMRI study of auditory hallucinations in patients with epilepsy. Epilepsia. 2010;51:610–617. doi: 10.1111/j.1528-1167.2009.02338.x. [DOI] [PubMed] [Google Scholar]
- Langers DR, van Dijk P. Robustness of intrinsic connectivity networks in the human brain to the presence of acoustic scanner noise. Neuroimage. 2011;55:1617–1632. doi: 10.1016/j.neuroimage.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Langers DR, Van Dijk P, Backes WH. Interactions between hemodynamic responses to scanner acoustic noise and auditory stimuli in functional magnetic resonance imaging. Magn Reson Med. 2005;53:49–60. doi: 10.1002/mrm.20315. [DOI] [PubMed] [Google Scholar]
- Langers DRM, Melcher JR. Hearing without listening: Functional connectivity reveals the engagement of multiple nonauditory networks during basic sound processing. Brain Connectivity. 2011;1:233–244. doi: 10.1089/brain.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter JL, Herscovitch P, Formby C, Raichle ME. Tonotopic organization in human auditory cortex revealed by positron emission tomography. Hear Res. 1985;20:199–205. doi: 10.1016/0378-5955(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Ellingson ML, Spanaki MV, Prieto TE, Ropella KM, Binder JR. Simultaneous ERP and fMRI of the auditory cortex in a passive oddball paradigm. Neuroimage. 2003;19:1395–1404. doi: 10.1016/s1053-8119(03)00228-3. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Hum Brain Mapp. 2007;28:205–217. doi: 10.1002/hbm.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Brugge JF. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 1973;50:275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Knight PL, Roth GL. Cochleotopic organization of primary auditory cortex in the cat. Brain Res. 1973;63:343–346. doi: 10.1016/0006-8993(73)90101-7. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335:437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- Olulade O, Hu S, Gonzalez-Castillo J, Tamer GG, Jr, Luh WM, Ulmer JL, Talavage TM. Assessment of temporal state-dependent interactions between auditory fMRI responses to desired and undesired acoustic sources. Hear Res. 2011;277:67–77. doi: 10.1016/j.heares.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Pantev C, Hoke M, Lehnertz K, Lutkenhoner B, Anogianakis G, Wittkowski W. Tonotopic organization of the human auditory cortex revealed by transient auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol. 1988;69:160–170. doi: 10.1016/0013-4694(88)90211-8. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Eason RJ, Schmitter S, Schwarzbauer C, Davis MH. Evaluating an acoustically quiet EPI sequence for use in fMRI studies of speech and auditory processing. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz ME, Melcher JR. Reducing imager-generated acoustic noise at the ear during functional magnetic resonance imaging: Passive attenuation. Twenty-First Midwinter Research Meeting of the Association for Research in Otolaryngology; St. Petersburg Beach, FL. 1998. p. 208. [Google Scholar]
- Rivier F, Clarke S. Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: evidence for multiple auditory areas. Neuroimage. 1997;6:288–304. doi: 10.1006/nimg.1997.0304. [DOI] [PubMed] [Google Scholar]
- Romani GL, Williamson SJ, Kaufman L. Tonotopic organization of the human auditory cortex. Science. 1982;216:1339–1340. doi: 10.1126/science.7079770. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Urbas JV. Representation of amplitude modulation in the auditory cortex of the cat. I. The anterior auditory field (AAF) Hear Res. 1986;21:227–241. doi: 10.1016/0378-5955(86)90221-2. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Urbas JV. Representation of amplitude modulation in the auditory cortex of the cat. II. Comparison between cortical fields. Hear Res. 1988;32:49–63. doi: 10.1016/0378-5955(88)90146-3. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Sigalovsky IS, Melcher JR. Effects of sound level on fMRI activation in human brainstem, thalamic and cortical centers. Hear Res. 2006;215:67–76. doi: 10.1016/j.heares.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, Edmister WB. Nonlinearity of FMRI responses in human auditory cortex. Human Brain Mapping. 2004;22:216–228. doi: 10.1002/hbm.20029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, Edmister WB, Ledden PJ, Weisskoff RM. Quantitative assessment of auditory cortex responses induced by imager acoustic noise. Hum Brain Mapp. 1999;7:79–88. doi: 10.1002/(SICI)1097-0193(1999)7:2<79::AID-HBM1>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, Johnsrude IS, Gonzalez Castillo J. Hemodynamic Imaging: fMRI. In: Poeppel D, Overath T, Popper AN, Fay RR, editors. Human Auditory Cortex. Springer Science+Business Media, LLC; New York: in press. [Google Scholar]
- Talavage TM, Ledden PJ, Benson RR, Rosen BR, Melcher JR. Frequency-dependent responses exhibited by multiple regions in human auditory cortex. Hear Res. 2000;150:225–244. doi: 10.1016/s0378-5955(00)00203-3. [DOI] [PubMed] [Google Scholar]
- Talavage TM, Sereno MI, Melcher JR, Ledden PJ, Rosen BR, Dale AM. Tonotopic organization in human auditory cortex revealed by progressions of frequency sensitivity. J Neurophysiol. 2004;91:1282–1296. doi: 10.1152/jn.01125.2002. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27:377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessinger CM, Buonocore MH, Kussmaul CL, Mangun GR. Tonotopy in human auditory cortex examined with functional magnetic resonance imaging. Hum Brain Mapp. 1997;5:18–25. doi: 10.1002/(SICI)1097-0193(1997)5:1<18::AID-HBM3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wessinger CM, VanMeter J, Tian B, Van Lare J, Pekar J, Rauschecker JP. Hierarchical organization of the human auditory cortex revealed by functional magnetic resonance imaging. J Cogn Neurosci. 2001;13:1–7. doi: 10.1162/089892901564108. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, Benson RR, Bandettini PA, Kwong KK, Howard RJ, Talavage T, Belliveau J, Rosen BR. Modulation of auditory and visual cortex by selective attention is modality-dependent. Neuroreport. 1996;7:1909–1913. doi: 10.1097/00001756-199608120-00007. [DOI] [PubMed] [Google Scholar]