Abstract
Neglect is often perceived as a “heterogeneous collection of symptoms” with controversial anatomical correlates. However, a clear framework for core and satellite symptoms exists. Here we review the literature when viewed from the perspective of these different syndromes, and find clear pattern of anatomical injury. Specifically, the combined symptoms of biased gaze direction and search – with no awareness of these symptoms – is seen following structural damage to (particularly right hemisphere) perisylvian regions. Object centered deficits such as biased line bisection are due to more posterior (and possibly inferior) injury. Finally, extinction is associated with damage to the temporo-parietal junction. Further, we describe key choices that must be made to parse the spatial and attentional syndromes that result from right hemisphere injury, including the investigation of both acute and chronic injury as well as the use of functional and structural modalities.
Keywords: Spatial neglect, extinction, attention, parietal, temporal, right hemisphere function, stroke, human
Despite the symmetrical anatomical appearance, human brain function is dramatically lateralized. This fact is demonstrated by observing behavioral deficits following unilateral brain injury: left hemisphere strokes often result in profound language impairments whereas injury to the right hemisphere typically leads to spatial and attentional biases. Understanding these syndromes can provide insights into healthy brain function, help predict long term prognosis and guide brain stimulation based therapies. Here we describe how recent advances regarding the anatomy of spatial neglect can enhance our models of spatial orienting and attention.
A large number of brain regions have become associated with spatial neglect, and therefore there appears to be little consensus regarding the core anatomy. While this variability can be seen as frustrating, we suggest that this heterogeneity may actually reveal important features of the human networks involved in spatial orienting and attention. Specifically, differences in clinical definition of spatial neglect (symptomatology), chronicity (acute versus chronic), and method (structural versus functional) across studies may help explain these apparent discrepancies.
Defining the disorder: “spatial neglect”
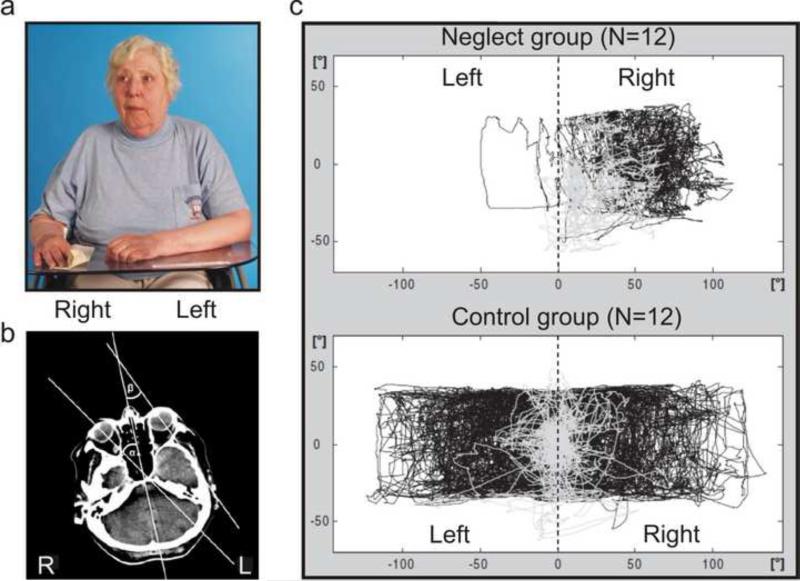
While “spatial neglect” is often operationally defined by a specific cognitive task, most neurologists instinctively diagnose the disorder when a patient is admitted in the absence of any explicit task. Such individuals with acute (particularly right hemisphere) injury show spontaneous and sustained deviation of their eyes and head toward the ipsilesional side (Fig. 1a; Fruhmann-Berger and Karnath, 2005; Fruhmann-Berger et al., 2006). Indeed, this biased gaze direction is even evident on the clinical scans taken at admission where the participant is simply asked to remain still (Fig. 1b; Becker & Karnath, 2010). Crucially, these individuals are physically able to disengage attention and shift their head and eyes into any direction (Fig. 1c; Niemeier & Karnath, 2000), but they exhibit a biased “default position” for eye and head orientation. This rightward bias is in the same direction as their perceived spatial egocenter, i.e. their perception of “straight ahead” orientation of body midline (Karnath, 1994a; Kapoor et al., 2001). Indeed, while extensive work has demonstrated that some right-hemisphere patients have difficulty “disengaging” from visual stimuli (e.g. Posner et al., 1984; Morrow & Ratcliff, 1988), the biased orientation in neglect patients is seen even when they are seated in a completely dark room and asked to look for a non-existent visual target (Hornak, 1992; Karnath & Fetter, 1995); i.e. when being in a situation where nothing is present that could attract and engage the subject's attention. We assert that the second hallmark feature of this disorder is that the individuals are unaware of their shifted spatial egocenter and (eye and head) default position. Despite variations in performance of cognitive tasks, this spontaneous behavior appears remarkably consistent and suggests that there is a unitary, homogenous core deficit which we define as “spatial neglect” (illustrated in Fig. 1).
Figure 1.
(A) Example of the spontaneous eye and head orientation of patients with spatial neglect following a right hemispheric stroke while “doing nothing”, i.e. just sitting and waiting. The patients typically orient eyes and head towards the ipsilesional, right side. One could have the impression that the patient was fixating a certain target situated on the right side. However, the room was empty with only the photographer standing right in front of her. (Modified from Fruhmann-Berger & Karnath, 2005). (B) The eye-in-head deviation is even evident on the clinical brain scans taken at admission where the participant is simply asked to remain still. The egocentric bias is specifically associated with spatial neglect rather than with brain damage per se. (Modified from Becker & Karnath, 2010). (C) Scan paths (=gaze [combined eye and head orientation]) of a group of 12 patients with spatial neglect (upper panel) during active visual search (black lines) as well as at rest (gray lines) as compared with a group of 12 control patients without neglect (lower panel). The neglect patients show a marked bias of their active and their passive behavior toward the ipsilesional, right side. (Modified from Fruhmann-Berger et al., 2008.)
A variety of clinical observations are a consequence of this core deficit: patients orient towards the right whenever addressed (even from the front or the left), and ignore contralesionally located people or objects. Moreover, when they actively search for targets they concentrate their exploratory eye and hand movements toward the right side of space (Heilman et al., 1983; Karnath, 1994b; Behrmann et al., 1997). These behaviors are precisely documented by recordings of these movements (Fig. 1c; Karnath et al., 1998; Karnath & Perenin, 1998) and are reliably measured by popular clinical tasks such as copying or cancellation tasks (where individiuals are asked to identify numerous targets in a cluttered array) (Rorden & Karnath, 2010).
A perisylvian network for spatial neglect
For this core deficit, studies based on structural brain imaging suggest three major cortical areas straddling the sylvian fissure: the temporo-parietal junction (TPJ) and inferior parietal lobule (IPL) (Heilman et al., 1983; Vallar & Perani, 1986; Mort et al., 2003; Chechlacz et al., 2010; Karnath et al., 2011; Rengachary et al., 2011), the superior/middle temporal cortex and underlying insula (Karnath et al., 2001, 2004, 2011; Buxbaum et al., 2004; Corbetta et al., 2005; Committeri et al., 2007; Sarri et al., 2009; Chechlacz et al., 2010; Rengachary et al., 2011), as well as the ventrolateral prefrontal cortex (Husain & Kennard, 1996; Committeri et al., 2007; Rengachary et al., 2011). These cortical areas also are involved in the human left hemisphere when patients show spatial neglect after a left hemisphere stroke (Suchan & Karnath, 2011).
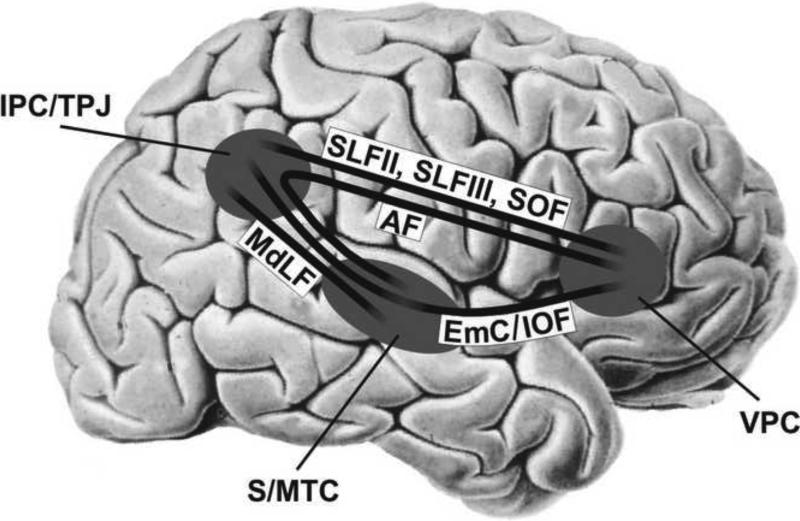
Findings from tract tracing, myelin staining, and diffusion-tensor imaging techniques suggest a dense perisylvian network interconnecting these three cortical sites (Fig. 2; Karnath, 2009): the inferior parietal lobule with the lateral prefrontal cortex (via subcomponents II/III of the superior longitudinal fasciculus [SLF II/III] and the superior occipitofrontal fascicle [SOF]), lateral prefrontal cortex with superior/middle temporal cortex (via arcuate fasciculus [AF] and extreme capsule/inferior occipitofrontal fasciculus [EmC/IOF]), and superior temporal cortex with the inferior parietal lobule (via posterior parts of the middle longitudinal fasciculus [MdLF] and EmC/IOF). Involvement of these connections – in particular the SLF, IOF, and SOF – in the brain damage of patients with spatial neglect has been reported from single or small number case studies (He et al., 2007; Thiebaut de Schotten et al., 2005; Urbanski et al., 2008, 2011; Shinoura et al., 2010) as well as from a recent group study by using a statistical voxelwise lesion-behavior mapping (VLBM) approach in a large cohort of 140 patients with an acute right hemispheric stroke (Karnath et al., 2009).
Figure 2.
Sketch of the perisylvian neural network linking the inferior parietal lobule with the ventrolateral frontal cortex (via SLF II, SLF III, SOF), ventrolateral frontal cortex with superior/middle temporal cortex and insula (via AF, EmC/IOF), and superior temporal cortex with the inferior parietal lobule (via MdLF, EmC/IOF). SLF II/III, subcomponents II/III of the superior longitudinal fasciculus; SOF, superior occipitofrontal fasciculus; AF, arcuate fasciculus; EmC, extreme capsule; IOF, inferior occipitofrontal fasciculus; MdLF, middle longitudinal fasciculus; IPC, inferior parietal cortex; TPJ, temporo-parietal junction; S/MTC, superior/middle temporal cortex; VPC, ventrolateral prefrontal cortex. (Modified from Karnath, 2009).
Karnath (2009) has suggested that this densily interconnected perisylvian neural network consisting of superior/middle temporal, inferior parietal, and ventrolateral frontal cortices in the human right hemisphere represents the anatomical basis for processes involved in spatial orienting (Fig. 2). Neurons of these regions provide us with redundant information about the position and motion of our body in space. They seem to play an essential role in adjusting body position relative to external space (Karnath & Dieterich, 2006). Evidence for this relationship comes from functional imaging studies (e.g., Bottini et al., 2001; Bense et al., 2001; Dieterich et al., 2003; Stephan et al., 2005) as well as electrical stimulation carried out directly on the human cortex (Kahane et al., 2003) showing that these areas are important for the processing of head and body orientation in space. It is also supported by the observation that stimulation of one vestibular organ (Rubens, 1985; Karnath et al., 1996; Rode et al., 1998) or the asymmetrical manipulation of the head-on-trunk signal by posterior neck muscle vibration (Karnath et al., 1993; Karnath, 1995) has compensatory effects on the clinical signs of patients with spatial neglect. By analogy, the opposite behavior is induced in healthy subjects by these types of stimulation, resulting in a bias of the scan path that resembles the spontaneous, asymmetrical egocentric bias of patients with spatial neglect (Karnath et al., 1996). Thus it appears that (part of) the perisylvian neural network illustrated in Figure 2 is important for the neural transformation of converging vestibular, auditory, neck propriopceptive, and visual input into higher order (egocentric) spatial representations (Karnath, 1994c). Other additional functions have been suggested to be associated with lesion of these brain regions, such as a bias in spatial attention as well as deficits of arousal, reorienting, and detection (Corbetta & Shulman, 2011). The tight perisylvian anatomical connectivity between superior/middle temporal, inferior parietal and ventrolateral frontal cortices might explain why lesions at these distant cortical sites around the sylvian fissure in the human right hemisphere can lead to the same egocentric bias of orienting behavior, namely to spatial neglect. In the human left hemisphere, a similar perisylvian network exists but is serving different functions, namely language and praxis (Karnath, 2009).
Spatial neglect can also be observed following injury that appears restricted to subcortical regions as assessed by structural scans. Specifically, lesions of the caudate nucleus (Caplan et al., 1990; Kumral et al., 1999), the putamen (Karnath et al., 2002), and the thalamic pulvinar (Karnath et al., 2002) are associated with spatial neglect. It has been a long-standing debate whether damage to these subcortical structures directly causes the disturbance, or whether these injuries disrupt the subcortical-cortical projections, or whether the relationship is indirect with subcortical damage leading to knock-on functional or metabolic abnormalities in cortical areas. Using perfusion-weighted imaging (PWI) recent studies revealed that cognitive disorders following such subcortical lesions is induced by the dysfunction of structurally intact but abnormally perfused cortical tissue (Hillis et al., 2002; Karnath et al., 2005). PWI revealed that strokes centering on the right basal ganglia which provoke spatial neglect induce abnormal perfusion in circumscribed areas of intact cortex that typically involves those regions that are known to provoke spatial neglect when damaged directly by cortical infarction (see above): the superior/middle temporal cortex, the inferior parietal lobule, and the ventrolateral prefrontal cortex (Karnath et al., 2005). The data thus suggest that spatial neglect following a right basal ganglia lesion typically is caused by dysfunction of (part of) the cortical perisylvian network illustrated in Figure 2.
Additional lateralized attentional and spatial deficits
In addition to this core disorder, it should be noted that patients with right hemisphere injury often exhibit a variety of additional attentional and spatial deficits. We suggest that those who attempt to unify all of these symptoms under the umbrella term “spatial neglect” will conclude that the resulting syndrome is “heterogenous in nature” and is even a “meaningless entity” (Halligan & Marshall, 1992). Also, lesion analysis studies that pool across these symptoms may have low statistical power (not be able to detect crucial regions) and could yield a biased estimate regarding the crucial brain regions. On the other hand, we contend that if one can accurately segregate the underlying distinct syndromes, one should be able to identify the unique anatomical signature for each. Successfully dissociating these symptoms based on anatomy has clear theoretical and clinical implications.
For example, many patients show pathological spatial biases on line bisection tasks – where participants are asked to identify the midpoint for a horizontal line. Conceptually, this deficit seems similar to the classic measures of spatial neglect (gaze direction, cancellation, and drawing biases), as individuals act as if they are ignoring the left side of the object. Curiously, the line bisection task behaviorally can dissociate from the core neglect disorder. For example, Ferber and Karnath (2001) have observed that 40% of patients with core symptoms of spatial neglect were unimpaired in the line bisection task. Moreover, lesion mapping demonstrates that patients with line bisection deficits (with and without the additional core symptoms of spatial neglect) have more posterior injury than those who only have spatial neglect (Binder et al., 1992; Rorden et al., 2006; Verdon et al., 2010; Vossel et al., 2011). Indeed, this anatomical dissociation can even be observed by carefully reviewing literature that did not explicitly attempt to differentiate these symptoms (Mort et al., 2003). Therefore, while the line bisection task does appear to indentify a profound perceptual disorder, it appears to be anatomically and behaviorally independent from the core symptoms of neglect.
One possible explanation for the dissociation between line bisection deficits and measures of the core neglect deficits (gaze direction, exploration, and cancellation biases) is that the line bisection task draws on allocentric (object-based) representation whereas the core deficit in spatial neglect is egocentric (relative to the body of the individual) (Rorden et al., 2006; Chechlacz et al., 2010). This account suggests that studies which explicitly measure allocentric and egocentric biases should show a similar anatomical dichotomy as comparisons between line bisection and the measures of the core deficit. Indeed, studies with patients who have both acute (Medina et al., 2009) and chronic (Chechlacz et al., 2010) cortical injury showed this separation, with egocentric measures near the superior temporal cortex whereas object-centered deficits appear more posterior and inferior (Grimsen et al., 2008; Medina et al., 2009; Chechlacz et al., 2010; Verdon, 2010). Taken together with the anatomical studies of line bisection, one gets a sense that a consensus is building regarding the anatomical dissociation of these functions. However, there is also one (partly) discrepant finding: Hillis and colleagues (2005) examined cortical hypoperfusion in acute patients with subcortical injury. In line with the other studies, they found a dissociation of the anatomical representation between object-centered and egocentric deficits. However, the authors reported the reverse anatomical pattern, namely allocentric neglect was associated with injury to the superior temporal gyrus, whereas egocentric neglect was associated with injury to the inferior parietal cortex. Chechlacz and colleagues (2010) argued that this apparent discrepancy may be an artifact of the large regions of interest used by Hillis and colleagues, whereas the other studies used voxelwise analysis to provide improved spatial resolution (see Medina et al., 2009 for the authors’ own explanation for this discrepancy).
Verdon and colleagues (2010) conducted an analysis of 80 acute and subacute right hemisphere stroke patients who completed a battery of different behavioral tests. A novel component of this study was that a factorial analysis was conducted that detected three significant factors that could explain more than 80% of the behavioral variance. Classic cancellation tasks (which we have suggested track closely with the core deficit of spatial neglect) were correlated with frontal and temporal injury. Whereas the best predictors of deficits in the line bisection and reading tasks were more posterior, in agreement with the previously described dichotomy (Binder et al., 1992; Rorden et al., 2006). Likewise, object-based (allocentric) deficits appeared to correlate with cortical injury to the middle and inferior temporal gyrus and underlying structures (including parahippocampal gyrus). This latter observation is consistent with the suggestions by Medina et al. (2009) that object-based deficits may result from more ventral injury, and supports findings that the injury near the parahippocampal area was associated with spatial neglect (Mort et al., 2003). Therefore, the results from Verdon et al. (2010) largely are in line with the previously described lesion patterns.
Visual extinction is another perceptual deficit commonly seen following right hemisphere injury. Individuals with extinction are able to report a single target presented at any location but fail to report the more contralesional item when confronted with two simultaneous items. While spatial neglect and extinction occur with similar incidence, they appear behaviorally dissociable (Becker & Karnath, 2007; Vossel et al., 2011). Based on structural scans, Karnath et al. (2003) argued that injury to the temporo-parietal junction (TPJ) is the most reliable predictor for extinction. Support to this observation has been given by recent transcranial magnetic stimulation (TMS) findings in healthy volunteers. For example, Meister et al. (2006) observed that single-pulse TMS over right TPJ caused extinction-like performance in a detection task of unilaterally versus bilaterally presented visual stimuli, whereas more rostral application of TMS over superior temporal gyrus (STG) had no such extinction effects. Further, a recent study by Grandjean et al. (2008) has revealed that damage to the TPJ is correlated with the extinction of auditory stimuli. Moreover, a recent study compared areas of abnormal perfusion despite intact structure between extinction patients and control patients suffering from a basal ganglia lesion and has demonstrated that extinction is strongly associated with hypoperfusion-induced impaired functioning of the TPJ (Ticini et al., 2010). Taken together, these studies strongly suggest that extinction is linked to the TPJ (of particularly the right hemisphere).
In sum, there is growing evidence that the behaviorally dissociating symptoms seen following right hemisphere injury correlate with different underlying anatomy, providing unique insights into the functions of the human brain. However, this domain is still in its infancy. There has been considerable speculation regarding further functionally dissociable components that are observed in addition to the core deficit of spatial neglect. For example, it has been noted that neglect patients often exhibit non-spatial deficits (e.g. Husain et al., 1997; Husain & Rorden, 2003), and it remains unclear whether these deficits dissociate anatomically from the core deficit.
Acute versus chronic injury
A classic dilemma when trying to associate the location of stroke injury with a resulting symptom is whether to focus on acute or chronic brain injury. There are several compelling reasons to focus on acute injury. First, it is challenging to detect the accurate location of an injury based on chronic images, as the shape, location, and quantity of brain tissue changes. Second, at the acute stage the brain has not had time to reorganize. Finally, as a related issue, there is a dramatic evolution of symptoms, with many patients showing spontaneous recovery. We will discuss each of these concerns in turn.
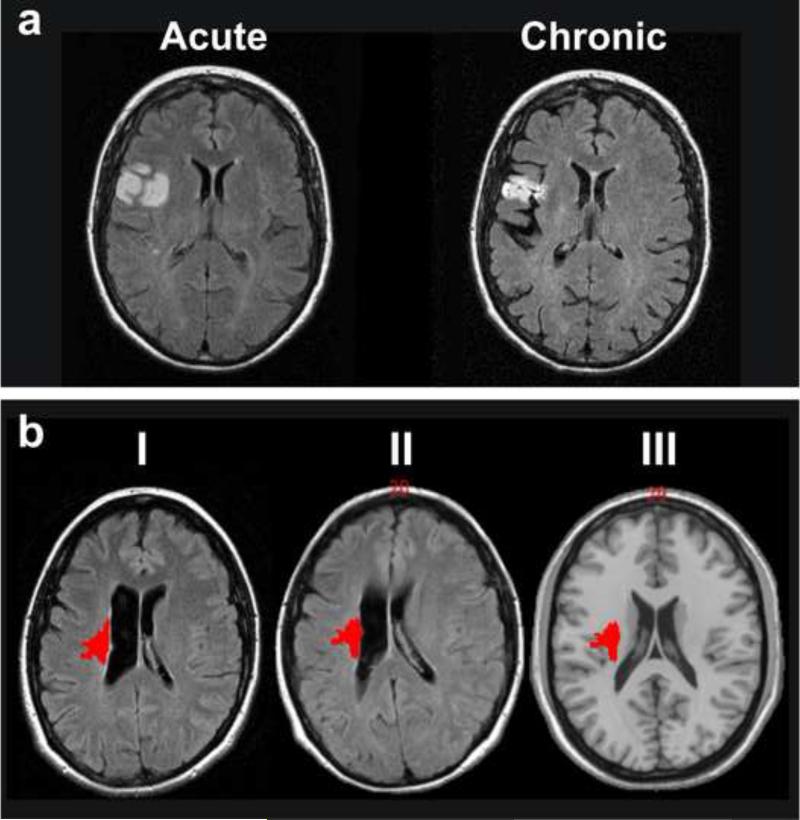
During the acute phase, all brain structures are typically still at their original locations (if one excludes individuals with mass shifts due to extensive hemorrhage or edema). However, chronic images show the effects of tissue resorption leading to structural distortions, sulcal widening, and widening of the ventricle (Fig. 3a). During normalization, these features can lead to unrealistic estimations of the location and extent of injury. For example, anatomical structures that are located close to the ventricles are frequently misclassified as being “intact/undamaged” when superimposed on a template image, although the original, non-normalized scan of the individual clearly indicates that it was affected (Fig. 3b). Such errors will impact the resulting statistical analysis of lesion anatomy. Until improved normalization routines are developed, great care must be taken when conducting analyses of chronic images, and often manual tracing of lesion extent on a standard template image by an expert who can identify key landmarks may be the only available solution in the chronic phase.
Figure 3.
(A) FLAIR images of the same patient in the acute and the chronic phase of a stroke lesion. Secondary lesion shrinkage is accompanied by sulcal widening and leads to diminished size of the chronic lesion. (B) Lesion ROI of a chronic periventricular lesion demarcated on a clinical FLAIR image before (I) and after SPM normalization (II). The same normalized ROI projected onto the standard template of a healthy brain (Ch2 single subject brain distributed with MRIcron) (III) results in a more lateral localization and erroneously indicates that the corpus of the caudate nucleus was “intact”, although it was actually lesioned in this individual (cf. I).
Acute studies also benefit from the fact that the brain still has not had time to functionally reorganize. Therefore, these studies may provide better insight into how the healthy brain functions, without being influenced by compensatory changes. For example, spatial neglect often resolves within weeks to months, with incidence and severity influenced by the time since injury. Indeed, about two thirds of patients who exhibited neglect during the acute phase of the stroke recover when tested 1.3 years post-injury (Karnath et al., 2011). Thus, a study that classifies individuals as having spatial neglect or being a control based on chronic behavior will actually classify a certain percentage of individuals as “controls” although these subjects had shown spatial neglect in the acute phase of the stroke (see Karnath et al., 2011 for an example). Therefore, while such an analysis may demonstrate the regions involved with persistent neglect, it does not give a complete picture of the syndrome. In other words, a study that only examines chronic behavior will not accurately identify all of the regions related to spatial neglect.
Despite these advantages, there are also concerns regarding studies that attempt to assess acute lesions. Specifically, while T1 images have proved popular for normalization because they provide high spatial resolution and good gray-white matter contrast, this modality often does not show the full extent of structural damage, especially in the acute phase. Therefore, acute studies in particular should include other modalities for lesion demarcation. T2-weighted sequences provide high sensitivity for acute cerebral infarcts (including fluid-attenuated inversion-recovery sequences [T2-FLAIR]), while diffusion-weighted imaging (DWI) has proved to be particularly sensitive for the detection of hyperacute infarcts and shows high accuracy in predicting final infarct size (Brant-Zawadzki et al., 1996; Noguchi et al., 1997; Ricci et al., 1999; Schaefer et al., 2002). When acute T1-images are available, these can be used for precise normalization, with the lesion demarcated on a pathological modality that is coregistered to the T1. A pragmatic behavior for lesion analyses thus is to use DWI within the first 48 hours post stroke and T2 or T2-FLAIR sequences when imaging is conducted 48 hours or later after the stroke.
However, a general issue to be noted is that structural scans may not show the full extent of dysfunctional tissue, in particular during the acute phase. One potential solution is to acquire perfusion-weighted imaging (PWI), which detects structurally intact but abnormally perfused brain tissue. In the acute stage of a stroke, regions with normal diffusion but abnormal perfusion, i.e. regions showing a PWI/DWI mismatch, often surround the irreversibly damaged ischemic core region and are thought to represent the “ischemic penumbra” (Schlaug et al., 1999). They represent zones that are receiving enough blood supply to remain structurally intact, but not enough to function normally. New techniques, namely spatial normalisation of PWI maps as well as symmetric voxel-wise interhemispheric comparisons (Karnath et al., 2005; Zopf et al., in prep.), allow to compare the structurally intact but abnormally perfused areas of different individuals in the same stereotaxic space, and at the same time avoid problems due to regional perfusion differences and to possible observer-dependent biases. A limitation of PWI is that the threshold between spared and disrupted function is difficult to assess but some studies have provided reasonable criteria (e.g., Neumann-Haefelin et al., 1999; Hillis et al., 2001; Zopf et al., in prep.).
Regardless of the technical challenges with respect to both acute and chronic lesion imaging, there are clear advantages for examining behavior of stroke survivors at both timepoints. Longitudinal studies provide valuable information regarding recovery, prognosis and treatment. For example, one could imagine different rehabilitation strategies for patients who have injury to regions associated with spontaneous recovery versus patients with injury associated with persistent spatial neglect.
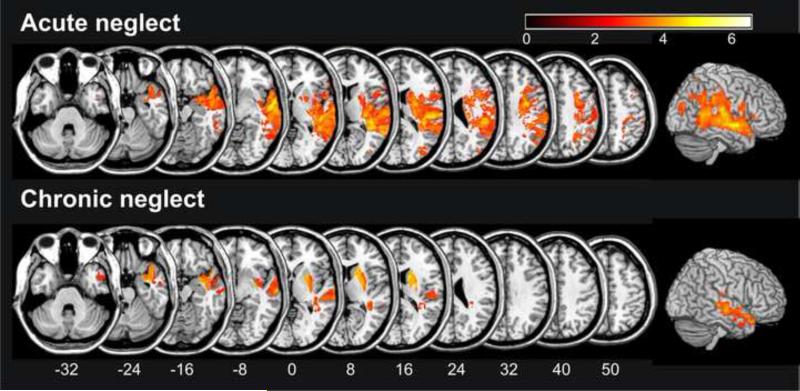
Pioneering work by Sammuelson et al. (1997) identified the white matter in the temporal lobe as the best predictor of persistent spatial neglect. Further, several studies have examined post-acute stroke, reporting the superior and middle temporal cortex (Commiteri et al., 2007; Golay et al., 2008), insula (Commiteri et al., 2007; Golay et al., 2008), inferior and middle frontal cortex (Commiteri et al., 2007), as well as the inferior parietal cortex (Mort et al., 2003; Golay et al., 2008) as strong anatomical predictors. Recently, Karnath and colleagues (2011) conducted a voxelwise analysis of acute scans based on longitudinal behavior that identified the superior and middle temporal cortex as well as the subcortical basal ganglia as the best predictors of persistent neglect (Fig. 4). The authors inferred that individuals who experience spatial neglect in the acute phase of the stroke yet do not have extensive injury to these structures are likely to recover, and thus have a favorable prognosis. New methods – such as machine learning (Krishnapuram et al., 2005) – will help to further develop research in this as well as other fields of anatomo-behavioral analysis. For example, machine learning will allow us to investigate whether the best predictor for (persistent) spatial neglect is damage to several of these identified key anatomical regions, complete injury to any one of these modules, or more likely a combination of these factors (Smith et al., 2011).
Figure 4.
Statistical voxelwise lesion-behavior mapping (VLBM) analyses of 54 right hemisphere damaged patients based on their neglect severity scores measured in the acute and in the chronic phase of the stroke. Injury to highlighted regions predicts acute (upper panel) and chronic (lower panel) spatial neglect. Lesion of the superior and middle temporal cortex predicted both acute as well as chronic neglect. At the subcortical level the basal ganglia as well as the inferior occipitofrontal fasciculus/extreme capsule appeared to play a significant role for both acute as well as chronic neglect. MNI coordinates of each transverse section are given. (Modified from Karnath et al., 2011)
Structural versus functional imaging
The majority of the work to date examining the anatomical correlates of neglect has focused on structural imaging techniques such as CT and structural MRI sequences (T1, T2, FLAIR, DWI). However, while regions that appear damaged using these modalities are clearly dysfunctional, regions that appear structurally intact may in fact be dysfunctional due to a wide range of issues including disconnection, diaschisis and misery perfusion. As outlined above, these concerns are partially ameliorated by perfusion imaging (which reveals the amount and latency of regional perfusion). On the other hand, techniques that can infer task-modulated brain activation hold great promise in contributing to reveal the full consequences of brain injury.
Both task-related (Corbetta et al., 2005) and resting state (He et al., 2007; Carter et al., 2010) magnetic resonance brain imaging (fMRI) studies report imbalanced dorsal parietal and frontal cortex signals between the injured and spared hemispheres, with the level of bias correlating with behavioral measures of spatial deficits. Further, these imbalances reduced along with behavioral biases during the chronic stage (Corbetta et al., 2005; He et al., 2007; Thimm et al., 2008). Corbetta and colleagues thus argued that the core structural damage causes (often transient) functional disruption of dorsal frontal and parietal regions, with the effective disruption of all of these regions leading to neglect (Corbetta et al., 2005; Corbetta & Shulman, 2011). One attractive feature of this hypothesis is that it helps explain why many patients show robust recovery despite permanent brain injury, reflecting reactivation of the structurally intact dorsal network. A second important point is that this work nicely links to functional imaging in healthy adults, which routinely shows robust activation of these dorsal regions associated with spatio-attentional orienting (Corbetta & Shulman, 2002). Despite the theoretical elegance, the clinical implications remain unknown. Specifically, it is still not clear whether the location of the stuctural damage is not highly predictive of the functional disruption. Particularly in acute stroke it might be that structural damage per se causes functional disruptions. It is possible that the functional disruptions turn out to be an epiphenomenon of the structural damage. In this case, functional imaging may not provide additional prognostic utility versus structural scans alone, i.e. it would not provide additional areas of the brain that critically subserve a behavioural deficit beyond structural imaging alone.
It should be noted that functional magnetic resonance imaging does not directly measure brain activity; rather it measures an overcompensation of regional oxygen that peaks about five seconds after brain activity. It is theoretically possible that a functionally viable region might not show a detectable fMRI signal due to either misery or luxury perfusion (Rorden & Karnath, 2004). Likewise, there is evidence that the hemodynamic response can be altered in acute and chronic stroke (Fridriksson et al., 2006; Bonakdarpour et al., 2007; Altamura et al., 2009). Therefore, while both the structural and functional imaging findings appear to reveal clinically useful biomarkers for neglect symptoms and its recovery, future work will be required to fully understand these effects. There are opportunities for complementary techniques such as TMS but also other techniques that can provide the spatial resolution of MRI with a more direct measure of brain activity (for example, event related potentials using MRI for source localization).
Conclusions
There is a tradition to regard spatial neglect as a “heterogeneous mosaic of symptoms” with a tremendous amount of variability across individuals. However, we do not consider this variability as reason to despair. Rather, we worry that in academic journals the term “spatial neglect” has become a catch-all phrase for a wide variety of attentional and spatial deficits observed following brain injury. Yet, visiting an acute stroke unit provides an immediate sense for a common core set of symptoms observed following (predominantly) right hemisphere injury including biased gaze orientation and search combined with a lack of insight regarding these symptoms. We suggest that by recognizing this core deficit of spatial neglect as distinct from the other spatial and attentional deficits often observed following right hemisphere injury one can recognize consistent patterns in the literature. Specifically, a perisylvian network consisting of superior/middle temporal, inferior parietal, and ventrolateral frontal cortices is compromised in individuals exhibiting the core egocentric symptoms termed “spatial neglect”. More posterior (and potentially inferior) injury seems to be involved with allocentric deficits (including line bisection), whereas extinction symptoms correlate with damage near the temporo-parietal junction.
In the human left hemisphere, a homologous perisylvian neural network exists (cf. Karnath, 2009). However, injury to this network only rarely causes full-blown spatial neglect; it typically evokes disturbances of language processing and praxis. The dominant role of the left hemisphere perisylvian network thus is related to these latter functions, although a representation of spatial orienting in such areas also seems to exist (Suchan & Karnath, 2011). Suchan and Karnath (2011) speculated that a representation of spatial orienting in left hemisphere language areas might be a phylogenetic relict in humans, though this representation stays subdominant in the vast majority of individuals.
A clear understanding of human right hemisphere function will require us not only to accurately segregate the underlying syndromes, but to also understand additional sources of variability across studies. One fundamental aspect is the selection of acute versus chronic injury, as there are clear patterns of recovery and compensation. Likewise, we need to recognize the different contributions of structural and functional modalities. Furthermore, we need to harness the complementary strengths of the different modalities available (e.g. CT, T2, DWI, PWI, fMRI) to image the brain.
Our clear understanding of the behaviorally and anatomically dissociable right hemisphere syndromes promises to provide dramatic clinical and theoretical insights. Specifically, longitudinal studies of these syndromes should have clear prognostic value – helping to predict both symptoms and recovery. Further, these anatomical findings also make clear theoretical predictions that should be directly tested with independent methods also in neurologically healthy adults. This additional work is required as no current methods reveal the full impact of brain injury, and statistical power with lesion techniques is constrained by vasculature.
Highlights.
Neglect is often perceived as a “heterogeneous collection of symptoms” with controversial anatomical correlates. However, a clear framework for core and satellite symptoms exists. Here we review the literature when viewed from the perspective of these different syndromes, and find clear pattern of anatomical injury. Specifically, the combined symptoms of biased gaze direction and search – with no awareness of these symptoms – is seen following structural damage to (particularly right hemisphere) perisylvian regions. Object centered deficits such as biased line bisection are due to more posterior (and possibly inferior) injury. Finally, extinction is associated with damage to the temporo-parietal junction. Further, we describe key choices that must be made to parse the spatial and attentional syndromes that result from right hemisphere injury, including the investigation of both acute and chronic injury as well as the use of functional and structural modalities.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (KA 1258/10-1), and the National Institutes of Health (R01 NS054266). We thank Henrike Planert, Graduate School of Neural and Behavioral Sciences Tuebingen, for providing Figure 3 and Bianca de Haan, Center of Neurology Tuebingen, for discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altamura C, Reinhard M, Vry MS, Kaller CP, Hamzei F, Vernieri F, Rossini PM, Hetzel A, Weiller C, Saur D. The longitudinal changes of BOLD response and cerebral hemodynamics from acute to subacute stroke. A fMRI and TCD study. BMC Neuroscience. 2009;10:151. doi: 10.1186/1471-2202-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E, Karnath H-O. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38:3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Becker E, Karnath H-O. Neuroimaging of eye position reveals spatial neglect. Brain. 2010;133:909–914. doi: 10.1093/brain/awq011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Watt S, Black SE, Barton JJ. Impaired visual search in patients with unilateral neglect: an oculographic analysis. Neuropsychologia. 1997;35:1445–1458. doi: 10.1016/s0028-3932(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). Journal of Neurophysiology. 2001;85:886–899. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP. Distinct syndromes of hemineglect. Archives of Neurology. 1992;49:1187–1194. doi: 10.1001/archneur.1992.00530350109026. [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B, Parrish TB, Thompson CK. Hemodynamic response function in patients with stroke-induced aphasia: implications for fMRI data analysis. Neuroimage. 2007;36:322–331. doi: 10.1016/j.neuroimage.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Karnath H-O, Vallar G, Sterzi R, Frith CD, Frackowiak RS, Paulescu E. Cerebral representations for egocentric space : functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain. 2001;124:1182–1196. doi: 10.1093/brain/124.6.1182. [DOI] [PubMed] [Google Scholar]
- Brant-Zawadzki M, Atkinson D, Detrick M, Bradley WG, Scidmore G. Fluid-attenuated inversion-recovery (FLAIR) for assessment of cerebral infarction: initial clinical experience in 50 patients. Stroke. 1996;27:1187–1191. doi: 10.1161/01.str.27.7.1187. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, Frassinetti F, Coslett HB. Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology. 2004;62:749–756. doi: 10.1212/01.wnl.0000113730.73031.f4. [DOI] [PubMed] [Google Scholar]
- Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, Gorelik PB, Helgason C, Hier DB. Caudate infarcts. Archives of Neurology. 1990;47:133–143. doi: 10.1001/archneur.1990.00530020029011. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of Neurology. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Bickerton WL, Hansen PC, Deb S, Humphreys GW. Separating neural correlates of allocentric and egocentric neglect: distinct cortical sites and common white matter disconnections. Cognitive Neuropsychology. 2010;27:277–303. doi: 10.1080/02643294.2010.519699. [DOI] [PubMed] [Google Scholar]
- Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota-Scancerbeg A, Piccardi L, Guariglia C, Pizzamiglio L. Neural bases of personal and extrapersonal neglect in humans. Brain. 2007;130:431–441. doi: 10.1093/brain/awl265. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annual Review of Neuroscience. 2011;34 doi: 10.1146/annurev-neuro-061010-113731. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nature Neuroscience. 2005;8:1424–1425. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Brandt T, Bartenstein P. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003;13:994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Ferber S, Karnath H-O. How to assess spatial neglect-line bisection or cancellation tasks. Journal of Clinical and Experimental Neuropsychology. 2001;23:599–607. doi: 10.1076/jcen.23.5.599.1243. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Rorden C, Morgan PS, Morrow KL, Baylis GC. Measuring the hemodynamic response in chronic hypoperfusion. Neurocase. 2006;12:146–50. doi: 10.1080/13554790600598816. [DOI] [PubMed] [Google Scholar]
- Fruhmann-Berger M, Karnath H-O. Spontaneous eye and head position in patients with spatial neglect. Journal of Neurology. 2005;252:1194–1200. doi: 10.1007/s00415-005-0831-y. [DOI] [PubMed] [Google Scholar]
- Fruhmann-Berger M, Proß RD, Ilg UJ, Karnath H-O. Deviation of eyes and head in acute cerebral stroke. BMC Neurology. 2006;6:23. doi: 10.1186/1471-2377-6-23. Corrigendum, 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhmann-Berger M, Johannsen L, Karnath H-O. Time course of eye and head deviation in spatial neglect. Neuropsychology. 2008;22:697–702. doi: 10.1037/a0013351. [DOI] [PubMed] [Google Scholar]
- Golay L, Schnider A, Ptak R. Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behavioral and Brain Functions. 2008;4:43. doi: 10.1186/1744-9081-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean D, Sander D, Lucas N, Scherer KR, Vuilleumier P. Effects of emotional prosody on auditory extinction for voices in patients with spatial neglect. Neuropsychologia. 2008;46:487–496. doi: 10.1016/j.neuropsychologia.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Grimsen C, Hildebrandt H, Fahle M. Dissociation of egocentric and allocentric coding of space in visual search after right middle cerebral artery stroke. Neuropsychologia. 2008;46:902–914. doi: 10.1016/j.neuropsychologia.2007.11.028. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC. Left visuo-spatial neglect: a meaningless entity? Cortex. 1992;28:525–535. doi: 10.1016/s0010-9452(13)80225-0. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Damasio AR. Localization of lesions in neglect. In: Kertesz A, editor. Localization in Neuropsychology. Academic Press; New York: 1983. pp. 471–492. [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Annals of Neurology. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: Insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J. Ocular exploration in the dark by patients with visual neglect. Neuropsychologia. 1992;30:547–552. doi: 10.1016/0028-3932(92)90057-s. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C. Visual neglect associated with frontal lobe infarction. Journal of Neurology. 1996;243:652–657. doi: 10.1007/BF00878662. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385:154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nature Reviews Neuroscience. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Kahane P, Hoffmann D, Minotti L, Berthoz A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Annals of Neurology. 2003;54:615–624. doi: 10.1002/ana.10726. [DOI] [PubMed] [Google Scholar]
- Kapoor N, Ciuffreda KJ, Suchoff IB. Egocentric localization in patients with visual neglect. In: Suchoff IB, Ciuffreda KJ, Kapoor N, editors. Visual & Vestibular Consequences of Acquired Brain Injury. Optometric Extension Program Foundation; Santa Ana, CA: 2001. pp. 131–144. [Google Scholar]
- Karnath H-O. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain. 1994a;117:1001–1012. doi: 10.1093/brain/117.5.1001. [DOI] [PubMed] [Google Scholar]
- Karnath H-O. Spatial limitation of eye movements during ocular exploration of simple line drawings in neglect syndrome. Cortex. 1994b;30:319–330. doi: 10.1016/s0010-9452(13)80202-x. [DOI] [PubMed] [Google Scholar]
- Karnath H-O. Disturbed coordinate transformation in the neural representation of space as the crucial mechanism leading to neglect. Neuropsychological Rehabilitation. 1994c;4:147–150. [Google Scholar]
- Karnath H-O. Transcutaneous electrical stimulation and vibration of neck muscles in neglect. Experimental Brain Research. 1995;105:321–324. doi: 10.1007/BF00240969. [DOI] [PubMed] [Google Scholar]
- Karnath H-O. A right perisylvian neural network for human spatial orienting. In: Gazzaniga MS, editor. The Cognitive Neurosciences IV. MIT Press; Cambridge, Mass.: 2009. pp. 259–268. [Google Scholar]
- Karnath H-O, Fetter M. Ocular space exploration in the dark and its relation to subjective and objective body orientation in neglect patients with parietal lesions. Neuropsychologia. 1995;33:371–377. doi: 10.1016/0028-3932(94)00115-6. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Perenin M-T. Tactile exploration of peripersonal space in patients with neglect. Neuroreport. 1998;9:2273–2277. doi: 10.1097/00001756-199807130-00024. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Dieterich M. Spatial neglect – a vestibular disorder? Brain. 2006;129:293–305. doi: 10.1093/brain/awh698. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Christ K, Hartje W. Decrease of contralateral neglect by neck muscle vibration and spatial orientation of trunk midline. Brain. 1993;116:383–396. doi: 10.1093/brain/116.2.383. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Fetter M, Dichgans J. Ocular exploration of space as a function of neck proprioceptive and vestibular input-observations in normal subjects and patients with spatial neglect after parietal lesions. Experimental Brain Research. 1996;109:333–342. doi: 10.1007/BF00231791. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Niemeier M, Dichgans J. Space exploration in neglect. Brain. 1998;121:2357–2367. doi: 10.1093/brain/121.12.2357. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus, and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Himmelbach M, Küker W. The cortical substrate of visual extinction. Neuroreport. 2003;14:437–442. doi: 10.1097/01.wnr.0000059778.23521.88. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Fruhmann-Berger M, Küker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cerebral Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Zopf R, Johannsen L, Fruhmann-Berger M, Nägele T, Klose U. Normalised perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain. 2005;128:2462–2469. doi: 10.1093/brain/awh629. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Rorden C, Ticini LF. Damage to white matter fiber tracts in acute spatial neglect. Cerebral Cortex. 2009;19:2331–2337. doi: 10.1093/cercor/bhn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H-O, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain. 2011;134:903–912. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnapuram B, Carin L, Figueiredo MA, Hartemink AJ. Sparse multinomial logistic regression: fast algorithms and generalization bounds. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2005;27:957–968. doi: 10.1109/TPAMI.2005.127. [DOI] [PubMed] [Google Scholar]
- Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30:100–108. doi: 10.1161/01.str.30.1.100. [DOI] [PubMed] [Google Scholar]
- Medina J, Kannan V, Pawlak M, Kleinman JT, Newhart M, Davis C, Heidler-Gary JE, Herskovits EH, Hillis AE. Neural substrates of visuospatial processing in distinct reference frames: Evidence from unilateral spatial neglect. Journal of Cognitive Neuroscience. 2009;21:2073–2084. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister IG, Wienemann M, Buelte D, Grünewald C, Sparing R, Dambeck N, Boroojerdi B. Hemiextinction induced by transcranial magnetic stimulation over the right temporo-parietal junction. Neuroscience. 2006;142:119–123. doi: 10.1016/j.neuroscience.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Morrow L, Ratcliff G. The disengagement of covert attention and the neglect syndrome. Psychobiology. 1988;16:261–269. [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack H-J, Wenserski F, Siebler M, Seitz RJ, Mödder U, Freund H-J. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- Niemeier M, Karnath H-O. Exploratory saccades show no direction-specific deficit in neglect. Neurology. 2000;54:515–518. doi: 10.1212/wnl.54.2.515. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ogawa T, Inugami A, Fujita H, Hatazawa J, Shimosegawa E, Okudera T, Uemura K, Seto H. MRI of acute cerebral infarction: a comparison of FLAIR and T2-weighted fast spin-echo imaging. Neuroradiology. 1997;39:406–410. doi: 10.1007/s002340050433. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman G, Corbetta M. A behavioral analysis of spatial neglect and its recovery after stroke. Frontiers in Human Neuroscience. 2011;5:29. doi: 10.3389/fnhum.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci PE, Burdette JH, Elster AD, Reboussin DM. A comparison of fast spin-echo, fluid-attenuated inversion-recovery, and diffusion-weighted MR imaging in the first 10 days after cerebral infarction. American Journal of Neuroradiology. 1999;20:1535–1542. [PMC free article] [PubMed] [Google Scholar]
- Rode G, Perenin M-T, Honoré J, Boisson D. Improvement of the motor deficit of neglect patients through vestibular stimulation: evidence for a motor neglect component. Cortex. 1998;34:253–261. doi: 10.1016/s0010-9452(08)70752-4. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nature Reviews Neuroscience. 2004;5:813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O. A simple measure of neglect severity. Neuropsychologia. 2010;48:2758–2763. doi: 10.1016/j.neuropsychologia.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Fruhmann-Berger M, Karnath H-O. Disturbed line bisection is associated with posterior brain lesions. Brain Research. 2006;1080:17–25. doi: 10.1016/j.brainres.2004.10.071. [DOI] [PubMed] [Google Scholar]
- Rubens AB. Caloric stimulation and unilateral visual neglect. Neurology. 1985;35:1019–1024. doi: 10.1212/wnl.35.7.1019. [DOI] [PubMed] [Google Scholar]
- Samuelsson H, Jensen C, Ekholm S, Naver H, Blomstrand C. Anatomical and neurological correlates of acute and chronic visuospatial neglect following right hemisphere stroke. Cortex. 1997;33:271–285. doi: 10.1016/s0010-9452(08)70004-2. [DOI] [PubMed] [Google Scholar]
- Sarri M, Greenwood R, Kalra L, Driver J. Task-related modulation of visual neglect in cancellation tasks. Neuropsychologia. 2009;47:91–103. doi: 10.1016/j.neuropsychologia.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer PW, Hunter GJ, He J, Hamberg LM, Sorensen AG, Schwamm LH, Koroshetz WJ, Gonzalez RG. Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. American Journal of Neuroradiology. 2002;23:1785–1794. [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad K-O, Parker RA, Edelmann RR, Warach S. The ischemic penumbra. Operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K. Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia. 2009;47:2600–2603. doi: 10.1016/j.neuropsychologia.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Smith DV, Clithero JA, Rorden C, Karnath H-O. Investigating the neuroanatomy of spatial neglect with multi-voxel pattern analysis. Journal of Cognitive Neuroscience. 2011;23(Suppl):101. [Google Scholar]
- Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, Dieterich M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005;26:721–732. doi: 10.1016/j.neuroimage.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Suchan J, Karnath H-O. Spatial orienting by left hemisphere language areas: a relict from the past? Brain. 2011 doi: 10.1093/brain/awr120. in press. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Thimm M, Fink GR, Sturm W. Neural correlates of recovery from acute hemispatial neglect. Restorative Neurology and Neuroscience. 2008;26:481–492. [PubMed] [Google Scholar]
- Ticini LF, de Haan B, Klose U, Nagele T, Karnath H-O. The role of temporo-parietal cortex in subcortical visual extinction. Journal of Cognitive Neuroscience. 2010;22:2141–2150. doi: 10.1162/jocn.2009.21315. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touzé E, Chokron S, Méder J-F, Lévy R, Dubois B, Bartolomeo P. Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79:598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Oppenheim C, Touzé E, Méder JF, Moreau K, Loeper-Jeny C, Dubois B, Bartolomeo P. DTI-MR tractography of white matter damage in stroke patients with neglect. Experimental Brain Research. 2011;208:491–505. doi: 10.1007/s00221-010-2496-8. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of unilateral neglect after right hemisphere stroke lesions. A clinical/CT-scan correlation study in man. Neuropsychologia. 1986;24:609–622. doi: 10.1016/0028-3932(86)90001-1. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133:880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Vossel S, Eschenbeck P, Weiss PH, Weidner R, Saliger J, Karbe H, Fink GR. Visual extinction in relation to visuospatial neglect after right-hemispheric stroke: quantitative assessment and statistical lesion-symptom mapping. Journal of Neurology, Neurosurgery & Psychiatry. 2011 doi: 10.1136/jnnp.2010.224261. in press. [DOI] [PubMed] [Google Scholar]
- Zopf R, Klose U, Karnath H-O. Improvement in detecting perfusion abnormalities after stroke in dysfunctional brain regions. In prep. [DOI] [PubMed]