Abstract
Of the multiple theories to explain exceptional longevity, the most robust of these has centered on the reduction of three anabolic protein hormones, growth hormone (GH), insulin-like growth factor, and insulin. GH mutant mice live 50% longer and exhibit significant differences in several aspects of energy metabolism as compared with wild-type mice. Mitochondrial metabolism is upregulated in the absence of GH, whereas in GH transgenic mice and dwarf mice treated with GH, multiple aspects of these pathways are suppressed. Core body temperature is markedly lower in dwarf mice, yet whole-body metabolism, as measured by indirect calorimetry, is surprisingly higher in Ames dwarf and Ghr–/– mice compared with normal controls. Elevated adiponectin, a key antiinflammatory cytokine, is also very likely to contribute to longevity in these mice. Thus, several important components related to energy metabolism are altered in GH mutant mice, and these differences are likely critical in aging processes and life-span extension.
Keywords: Hormones, Aging, Mitochondria, Body temperature, Inflammation
MANY theories have been presented over the last several decades to explain exceptional longevity in animals and humans. Perhaps, the most robust of these to date focuses on three interrelated endocrine systems: the growth hormone (GH), insulin and its hormone homologs, and insulin-like growth factor (IGF) pathways. As far down the evolutionary ladder as yeast, there is strong evidence that a carbohydrate regulatory system exists and that if perturbed, life span extension is observed. As one moves up the ladder to nematodes and fruit flies, both insulin and IGF become important in the maintenance of metabolism. Similar to yeast, if ligand or receptor expression or signaling factors in these pathways are disrupted (ie, nematode daf-2; Drosophila chico), an extension of life span is observed. In the mammalian system, the endocrine system becomes more complex with the addition of GH, a hormone that controls circulating IGF1 levels and thus has somatic actions yet also exhibits key metabolic functions that are independent of IGF1. Disturbing the GH pathway either by severely reducing plasma levels or by receptor disruption significantly extends health span and life span in mice.
KEY CHARACTERISTICS OF LONG-LIVED Gh-RELATED MUTANTS
The Ames dwarf, Snell dwarf, and GH receptor knock-out (Ghr–/–) mice are the longest living mouse mutants discovered to date (1–3). The Ames and Snell dwarfs are phenotypically identical with similar hormone deficits caused by loss-of-function mutations that affect proper differentiation of the same pituitary cell types. Mutations in Prop-1 and Pit-1 result in deficient circulating GH, prolactin, and thyrotropin in Ames and Snell mice, respectively (4,5). As a consequence of the lack of plasma GH stimulation, plasma IGF1 levels are barely discernable in these mice (6). On average, Ames mice live 49%–69% longer (males and females, respectively), whereas Snell dwarfs live nearly 50% longer than their normal counterparts (1,2,7). The differences in life span between these dwarf mice result from differences in genetic background rather than the pituitary deficiencies. The other long-living GH mutant, the Ghr–/– mouse, was generated by targeted disruption of the GH receptor and GH-binding protein (8). These mice live up to 46% longer than wild-type siblings and also exhibit undetectable circulating levels of IGF1 owing to dysfunctional GH receptors (no liver IGF1 stimulus; (3)). The current record for the longest-lived Ghr–/– mouse is 1,819 days (Bartke, personal communication 2003). Hence, the major physiological difference between these long-living mice is the presence (Ames and Snell) and absence (Ghr–/–) of an intact GH signaling system. This difference, in turn, results in some heterogeneity in downstream targeting of GH and IGF1 between these mice (comprehensive review in (9)).
Several aspects of GH and IGF status as they relate to energy metabolism have been explored in GH mutant mice and include insulin signaling, adipose tissue metabolism/inflammation, body temperature, and mitochondrial and oxidative pathways. The mitochondria play a central role in energy metabolism through oxidative phosphorylation and ATP synthesis, apoptosis, and in the generation of free radicals (produced as byproducts). These reactive oxygen species induce oxidative stress but have also been shown to regulate cellular signaling and integrate energy state, stress signaling, and survival (10). The oxidative damage resulting from reactive oxygen species and incurred by mitochondria and other cellular components results in disturbed energy budgets at the cellular and tissue levels and likely contributes to the aging phenotype (11–13). Moreover, it has been shown that defects in the electron transport chain (ETC) contribute to the etiology of several disease states (reviewed in (14)). The mitochondria thus drive energy metabolism in the cells and tissues and likely contribute to cellular aging.
MITOCHONDRIAL FUNCTION
Examination of individual oxidative phosphorylation (OXPHOS) components has the potential to uncover areas of altered function that may result from differences in circulating levels of hormones and contribute to longevity differences between mutant and wild-type mice. Complex I of the OXPHOS system is the largest of the multimeric ETC proteins and contributes significantly to the generation of free radicals, respiration rate, and overall control of the ETC in mammalian species (15,16). Inhibition of complex I (as little as 25%) can profoundly impact energy metabolism and contribute to less efficient energy production in aging (17–19). Many reports documented significant declines in complex I activity with aging (20–23). In GH-deficient Ames mice, several alterations in OXPHOS complexes have been observed. Increased complex I activity and protein levels have been demonstrated in liver tissues from healthy old dwarf mice in comparison to age-matched wild-type mice (20 months of age; (24)). In addition, greater declines in activity with age were observed in GH-sufficient wild-type mice versus Ames mice in liver, skeletal muscle, and kidney tissues (24). The liver tissue is a key player in metabolism, as it orchestrates the supply of energy substrates to other tissues. Thus, increased liver complex I activity and protein in old dwarf mice suggest that mitochondrial function is better preserved in these long-living dwarf mice at old ages.
Considering that complex I governs overall ETC function, then the differences observed in complex I in the dwarf mice may underlie the elevated levels of other downstream enzymes in the OXPHOS system (III, IV, and V) of these mice. However, ATP synthase (complex V) is not coupled to ETC processes, so the higher level of complex V in dwarf tissues is not a result of a generalized upregulation of OXPHOS proteins. Instead, it may be indicative of ample energy availability versus energy deficits observed in diseases with decreased ATP synthase protein levels and/or mutations in genes encoding components of the ATP synthase complex (25,26). Ames mouse tissues also exhibited elevated levels of the adenine nucleotide translocator (messenger RNA or protein), a protein involved in both the transport of ATP and the maintenance of the mitochondrial permeability transition pore complex (and thus, apoptosis). If complex I is the major controller of overall ETC function as reported (21) and a decline in activity contributes to decreased energy production and aging processes, then Ames mice exhibit an advantage in many tissues over wild-type mice. This advantage may contribute to the delayed aging phenotype enjoyed by Ames mice (24). A major regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, has also been shown to be upregulated in tissues of Ames mice (and Ghr–/– mice; (24,27–29)). However, an increase in mitochondrial numbers is not evident as indicated by similar mitochondrial DNA:nuclear DNA ratios between dwarf and wild-type mice (24). Therefore, this nuclear hormone activation likely contributes to other aspects of metabolism including antioxidant defense, insulin sensitivity, and β-oxidation.
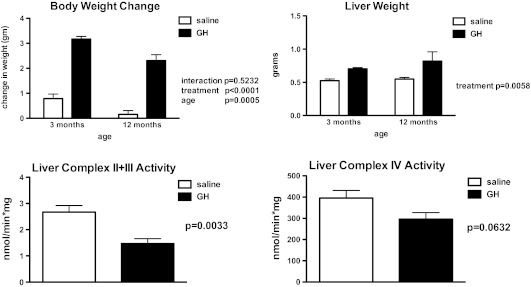
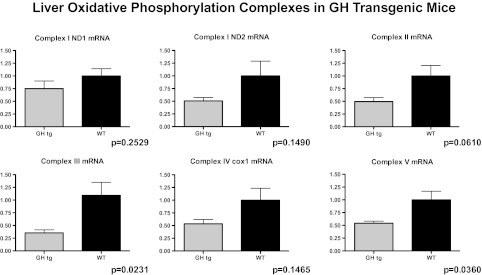
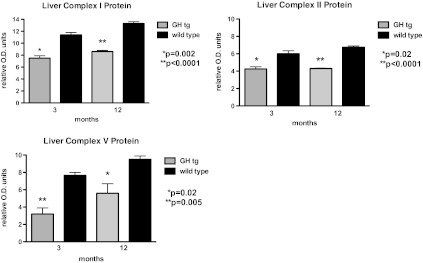
It has been reported that overall mitochondrial protein synthesis decreases by middle age in humans (30), a finding associated with decreasing plasma GH concentrations (31). Factors that regulate the synthesis and activities of the mitochondrial ETC complexes, however, are largely unknown (32). Cellular metabolism is stimulated by anabolic hormones, increasing metabolic activity (oxygen consumption and glucose oxidation), oxidative phosphorylation, and reactive oxygen species production. However, neither GH nor IGF1 have been shown to directly modulate the expression or activities of the OXPHOS proteins. Thus, the prediction that anabolic hormones stimulate mitochondrial function via oxidative phosphorylation activities is tenuous. To examine this assertion directly, we treated Ames mice with GH (25 μg porcine GH/injection, subcutaneous; two times daily; (33)) for 7 days, a treatment regimen has been shown to significantly increase circulating levels of IGF1 (6,33). Body and liver weights increased in response to GH injection (p < .0001; Figure 1A and B), indicative of IGF1 action. We observed a significant suppression in liver complex I protein (12-month-old GH-treated dwarf mice −1.989 ± 0.39 versus saline-treated dwarf mice 4.034 ± 0.79 relative optical density units; n = 6 per treatment; p = .0408) suggesting that GH influences OXPHOS and thus mitochondrial metabolism. Although the electron flow through complexes I and III (I + III activity) was not altered significantly by GH treatment in 12-month-old dwarf mice, electron flow through complexes II + III (II + III activity) was suppressed 45% in comparison to dwarf mice treated with saline (p < .01; Figure 1C). GH treatment also decreased the activity of cytochrome c oxidase (complex IV) by 25% when compared with the saline-treated mice (p = .06; Figure 1D). No differences in complex protein levels were observed in 3-month-old dwarf mice similarly treated with GH. Furthermore, levels of gene expression for complexes II, III, and V as well as protein levels of complexes I, II, and V are markedly decreased in mice with high circulating levels of GH (GH transgenic mice; Figures 2 and 3) and shortened life spans. Taken together, these data are compelling and suggest that the metabolic actions of GH decrease the expression and activities of OXPHOS components. There is also the possibility that GH’s actions are secondary to increased insulin (as insulin promotes mitochondrial activity) in these mice. Nevertheless, Ames mice exhibit negligible levels of plasma GH and IGF1 and reduced levels of insulin, and it is this lack of anabolic activity that may result in the upregulation in OXPHOS and mitochondrial metabolism, potential underlying factors in their long life.
Figure 1.
Body and liver weights and complex enzyme activities in liver tissue from Ames dwarf mice following a short-term 7-day treatment with growth hormone (GH; 25 μg porcine GH per injection × two injections per day for 7 days). Top left: Body weight change (g); top right: Liver weights (g); bottom left: Liver complex II + III activity (ng/min × mg protein) in 12-month-old Ames mice; and bottom right: Liver complex IV activity (nmol/min × mg protein) in 12-month-old Ames mice. Details of enzyme assays described in (24). Values represent means ± SEM, n = 7–8 mice per treatment.
Figure 2.
Gene expression of complex enzymes in 3-month-old male liver tissue from GH transgenic and wild-type control mice. Primer pairs and real-time reverse transcription-PCR conditions described in (24). Values represent means ± SEM, n = 6–7 mice per genotype.
Figure 3.
Liver protein levels of complexes I, II, and V in 3- and 12-month-old male tissue from growth hormone transgenic and wild-type control mice. Protein extraction and immunoblotting assays and antibodies are described in (24). Values represent means ± SEM, n = 7–8 mice per age per genotype.
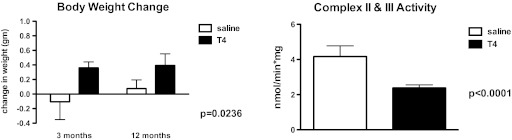
Extremely low thyroid hormone concentrations in Ames dwarf mice also preclude the assertion that enhanced metabolic activity may be responsible for the elevated OXPHOS output in older dwarf mice. We found that treatment of Ames mice with thyroxine (2 μg in 0.9% saline) every other day for 1 week did not affect liver gene expression of the OXPHOS complexes but did reduce the activity of liver complex II + III by 43% (p < .0001; Figure 4). In contrast to Ames mice, hypothyroid rats exhibit reduced complex V activity compared with euthyroid animals and short-term 3,5-L-diiodothyronine injection enhanced this activity (34). As additional information, Panici and coworkers (35) reported that short-term (6-week) T4 administration to Ames mice had no affect on longevity. Although lifelong treatment of Snell dwarf mice with thyroxine reduced their life span (36).
Figure 4.
Body weight change (g) and liver complex II + III activity (nmol/min × mg) in Ames dwarf mice following short-term treatment with thyroxine or saline. Details of complex II + III activity assay described in (24). Values represent means ± SEM, n = 6–8 mice per treatment.
ANTIOXIDATIVE DEFENSES AND OXIDATIVE DAMAGE
Oxidative metabolism is closely linked to mitochondrial metabolism as this system counters the metabolic by-products (free radicals) produced during the oxidative phosphorylation process. Moreover, oxidative stress underlies mitochondrial dysfunction and impaired energetics. Several lines of evidence demonstrate that GH plays a role in antioxidative defense. The high plasma GH and IGF1 concentrations found in short-living GH transgenic mice are strongly associated with increased superoxide radicals, increased oxidative damage, and significantly suppressed tissue antioxidative enzyme levels (MnSOD, CuZnSOD, catalase, and GPX; (37–40)). Both direct and specific effects of GH and IGF1 in vitro (primary hepatocytes) support the in vivo evidence demonstrating that these two hormones downregulate the expression of antioxidative enzymes (41). Therefore, the significant downregulation of oxidative defense capacity and the multiple indices of physiological decline associated with premature aging (early reproductive senescence, glomerulonephritis, glomerulosclerosis, early onset, and increased incidence of tumors, etc) likely lead to the reported 50% reduction in life span in animals with pharmacological levels of plasma GH (42). In comparison, IGF1 transgenic mice do not experience this severe renal pathology suggesting that GH is the main culprit (43).
In striking contrast, GH deficiency in dwarf mice results in significantly enhanced antioxidative defense capacity. Ames mice exhibit elevated catalase, SOD, and GPX in multiple tissues (activities, protein and/or messenger RNA; (38–40,44)). GH administration to dwarf mice suppresses these same enzymes when compared with saline-injected dwarf mice (33). Nonenzymatic antioxidative defense mechanisms such as glutathione and metallothionein are also elevated in dwarf mice. The lack of anabolic stimulation of mitochondrial metabolism and the elevated oxidative defense mechanisms in these mice result in lower liver hydrogen peroxide generation and lower oxidative damage to nuclear and mitochondrial DNA, proteins, and lipids in several tissues (45–48).
Mitochondria play an important role in metabolic rate and energy metabolism as oxidative phosphorylation is responsible for the majority of whole-animal oxygen consumption and in the control of cellular respiration (49). Mitochondrial metabolism is suppressed in many mammals (including mice) during fasting and daily torpor and is mechanistically linked to reductions in reactive oxygen species production and body temperature (50).
BODY TEMPERATURE
Hunter and his colleagues (51) reported in 1999 that core body temperature (Tco) was significantly lower in Ames dwarf mice than in normal animals from the same strain. In this study, Tco was continuously monitored in singly housed animals at an ambient temperature of 26°C using intraperitoneally placed transmitters and telemetric recording. The difference in Tco between normal and dwarf animals was large, approximately 1.6°C. In addition, some dwarf animals in this study exhibited periods of hypothermia with Tco dropping to values near or below 33°C for periods of up to 6 hours (51). Subsequently, we reported that Tco in Ghr–/– animals was also reduced, although the reduction was much smaller than in Ames dwarf mice (approximately 0.4°C) and statistically significant only at some phases of the 24-hour Tco rhythm (52). Major reduction of Tco in Ames dwarfs and a more modest effect in Ghr–/– can be readily related to the reduced action of calorigenic hormones, thyroxine, GH, and insulin in these mutants: Ames dwarfs are severely hypothyroid due to thyroid stimulating hormone deficiency and do not produce GH; Ghr–/– mice are GH resistant and both are hypoinsulinemic.
We propose that reduced Tco contributes to extended longevity of Ames dwarf and Ghr–/– mice. In an elegant study of Conti and colleagues (53), reducing body temperature of mice by hypothalamic overexpression of uncoupling protein 2 led to a significant increase of life span. Association of reduced body temperature with increased longevity was also reported in the human (54). Forced restriction of food intake (calorie restriction, CR) leads to reductions in the levels of thyroid hormones and in body temperature. In mice, CR can induce periods of torpor with associated hypothermia (55,56). Additionally, an enlargement of interscapular brown adipose tissue was observed in Ghr–/– mice over that of wild-type mice as well as elevated levels of uncoupling protein-1 in this tissue (57).
OXYGEN CONSUMPTION AND RESPIRATORY QUOTIENT
Although lower Tco would seem to imply a downward shift in energy and metabolism, studies by indirect calorimetry revealed that oxygen consumption per unit of body mass is significantly increased in both Ames dwarf and Ghr–/– mice in comparison to the corresponding normal controls (58). This finding was not expected. Animals subjected to CR show an initial decrease in metabolic rate but after a period of adaptation and weight loss, consume the same amount of oxygen per unit of lean body mass as controls with unlimited access to food (59). Reporting results of studies of energy metabolism in terms of lean body mass is supported by evidence that metabolic rate scales more closely with this value (generally calculated as body weight0.67 or body weight 0.75) rather than with body weight. However, the differences between findings in long-lived mice with GH-related mutations and in genetically normal animals subjected to CR are not a simple reflection of different ways of presenting the results because the adjustments to “lean body mass” are based on the assumption that smaller animals are leaner, whereas adiposity (body fat as percent of body weight) in long-living dwarf mice is either significantly increased or near normal (60,61). The interpretation of findings from studies of energy metabolism in mice and common laboratory rodents is further complicated by the fact that basal or resting metabolic rates in these animals are difficult to measure, and the average metabolic rate that can be fairly precisely determined over periods of 12 or 24 hours includes energy used for thermogenesis and maintaining body temperature. In mice, thermoneutral environmental temperature is approximately 30°C (86°F) (62), and thus, standard housing conditions (usually about 22°C) represent a thermal stress. For dwarf mice, which are much smaller than normal mice and thus have higher body surface to body mass ratio, the loss of heat by radiation can be assumed to be greater (in spite of increased or normal insulation by subcutaneous fat), and thus, the energy demand for thermogenesis is presumably further increased at “room temperature.”
An increase in oxygen consumption per gram body weight in dwarf mice was associated with a significant reduction in respiratory quotient (RQ; calculated as a ratio of carbon dioxide output to oxygen consumption). RQ provides an estimate of the use of metabolic fuels to generate energy with an RQ of 1.0 representing exclusive reliance on carbohydrates and RQ of 0.7 representing reliance on fats. Increased reliance on “fat burning” or, more precisely, β-oxidation of fatty acids for satisfying energy needs is a normal response to reduced availability of nutrients during fast or long between-meal intervals (such as sleep in the human) and has been associated with improved metabolic homeostasis and extended longevity in a variety of situations (63–65). A shift from carbohydrate to fatty acid utilization in response to CR is believed to represent an important metabolic adaptation of mitochondrial function and one of the key mechanisms of extended longevity (66).
Recent findings in our laboratory indicate that differences in oxygen consumption (VO2) and RQ between normal mice and long-lived mutants can be eliminated or greatly reduced by several days of exposure to thermoneutral temperatures. This raises an intriguing possibility that the metabolic characteristics of GH-related mouse mutants detected by indirect calorimetry at ambient temperatures of 22°C (increased VO2/g and reduced RQ) represent an exaggerated response of these diminutive animals to cold stress and that the resulting phenotype is one of the mechanisms of their extended longevity. In indirect support of this hypothesis, Koizumi and his colleagues (56) reported that maintaining CR mice at a thermoneutral temperature eliminates some of the beneficial effects of CR. This included a significantly shorter survival of calorically restricted C57BL/6 mice at 30°C versus 20–22°C. The authors ascribed these findings to elimination of torpor, which was a daily occurrence under the employed CR protocol (56).
ADIPOSE TISSUE, ADIPOKINES, AND INFLAMMATION
The loss of GH’s somatic effects, the majority of which are due to IGF1, results in animals of small size but extended longevity. However, as mentioned previously, GH has direct metabolic effects that are IGF1-independent, affecting lipids via lipolysis, lipid oxidation and lipid mobilization, and further regulating body composition (67,68). In addition, levels of adipokines, factors produced by adipocytes, are affected by fat mass and composition and have been shown to affect diverse processes such as energy expenditure, carbohydrate and lipid metabolism, and inflammation (69). Circulating levels of adiponectin, an important antiinflammatory adipokine, are elevated (70,71) in long-lived GH-related mouse mutants, whereas the expression of proinflammatory cytokines, interleukin 6 and tumor necrosis factor alpha, are reduced. Increased adiponectin secretion undoubtedly reflects the absence of negative control of its secretion by GH, along with differences in the size and distribution (72) of adipocytes.
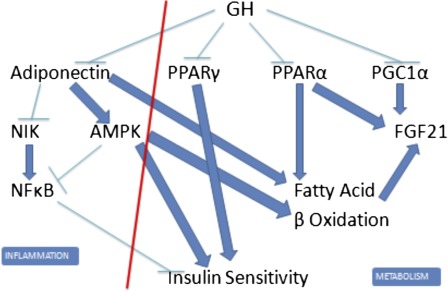
We believe that extended longevity of Ames dwarf and Ghr–/– mice is causally related to phenotypic characteristics induced by elevated adiponectin levels (Figure 5). Adiponectin reduces proinflammatory nuclear factor kappa-light chain-enhancer of activated B cells signaling by reducing expression of NFkB-inducing kinase, an upstream regulator of nuclear factor kappa-light chain-enhancer of activated B cells, and by adenosine monophosphate–activated protein kinase-mediated inhibition of this pathway. Adiponectin activates adenosine monophosphate–activated protein kinase, promotes β-oxidation of fatty acids, and enhances insulin sensitivity. Reduced nuclear factor kappa-light chain-enhancer of activated B cells signaling may contribute to improved responses to insulin, although inflammation and insulin resistance have been dissociated in transgenic mice (73). We have recently shown that removing most of the epididymal and perinephric adipose tissue from adult Ghr–/– mice reduces adiponectin levels, increases RQ, and promotes insulin resistance, thus leading to normalization of the phenotypic characteristic believed to be causally linked to longevity (74). Metabolic characteristics of transgenic mice overexpressing adiponectin are remarkably similar to those of Ames dwarf and Ghr–/– mice, except for the percentage of body fat that is reduced in the transgenics (75,76). Transgenic mice overexpressing human adiponectin in the liver have an increased life span (77).
Figure 5.
Interactions of pathways mediating effects of growth hormone (GH) on metabolism and inflammation. GH inhibits adiponectin, peroxisome proliferator-activated receptor γ, PPARα, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha expression and thus promotes inflammation, insulin resistance, and reduced oxidation of fatty acids. Suppression of GH signaling in long-lived mutants removes these inhibitory effects and thus reduces inflammation and promotes insulin sensitivity and fatty acid oxidation. (This diagram is greatly simplified and is not intended to present all pathways and mediators involved).
In the human, there is considerable evidence for anti-inflammatory and antiatherogenic effects of adiponectin (78–80). Circulating adiponectin levels are increased in centenarians and in offspring of exceptionally long-lived individuals (81–83). Laron dwarfs, which share GH resistance and many of the resulting phenotypic characteristics with Ghr–/– mice, have elevated adiponectin levels (84) and are remarkably protected from age-related disease, including type 2 diabetes and cancer (85,86). It should be pointed out that the average longevity of Laron dwarfs does not differ from that of the normal individuals from the same population, but this may reflect the increased incidence of deaths resulting from accidents and alcohol-related causes in individuals affected by this syndrome (85).
Finally, there is considerable evidence that low-grade inflammation, other inflammatory processes, as well as infections and the resulting “inflammatory load” can profoundly influence longevity and health span in mammals (87,88). GH was reported to exert antiinflammatory effects in experimentally induced sepsis by promoting secretion of interleukin 10 and reducing the levels of tumor necrosis factor alpha (89,90). Moreover, the levels of an important marker of inflammation, C reactive protein, in GH-deficient participants were reduced by GH therapy (91). In contrast to these observations, inflammatory processes and levels of proinflammatory cytokines are increased in transgenic mice overexpressing GH (92,93). Because circulating levels of GH in these animals are extremely high, differences between the results obtained by GH injections and by overexpression of the GH gene may reflect a nonlinear likely biphasic dose–response relationship between GH levels and inflammation. In clinical studies, biphasic inverted U dose response was reported for the effects of GH on cardiac function (94) and for relationship of circulating IGF1 levels to all cause mortality (95).
CONCLUSION
Considering the components of energy metabolism presented (mitochondrial and oxidative metabolism, body temperature, RQ, and adiponectin), the conclusion that in the long-lived mutants, GH deficiency throughout life is beneficial in terms of health span and life span is difficult to debate. In addition, a very recent publication demonstrated that male IGF1 receptor heterozygous mice do not live longer than wild-type mice (nor do they exhibit differences in end-of-life pathology), and the extension of longevity in females was very modest (less than 5%; (96)). These investigators conclude that a reduction in circulating IGF1 levels in CR and dwarf mice plays little if any role in delayed aging and longevity. This evidence supports our own work in dwarf mice that strongly proposes that reduced GH rather than the secondary reduction of IGF1 is the key to longevity in mammalian systems. Further study is warranted regarding the apparent importance of timing of hormonal perturbations (life long vs neonatally or adult only; (35)) and consequent effects on energy metabolism as they relate to aging processes and longevity.
FUNDING
This work was supported by the Ellison Medical Foundation (H.M.B.B.); the National Institutes of Health AG022909 (H.M.B.B.), AG038509 (H.M.B.B.), AG034206 (H.M.B.B.), AG019899 (A.B), and AG038850 (A.B.); and the Glenn Foundation for Medical Research (H.M.B.B.).
References
- 1.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 2.Flurkey K, Papconstantinou J, Miller RA, Harrison DA. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 4.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the prophet of pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Crenshaw BE, III, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 6.Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod. 1993;48:544–551. doi: 10.1095/biolreprod48.3.544. [DOI] [PubMed] [Google Scholar]
- 7.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev. 2002;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 10.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35(9):505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 12.López-Torres M, Gredilla R, Sanz A, Barja G. Influence of aging and long-term caloric restriction on oxygen radical generation and oxidative DNA damage in rat liver mitochondria. Free Radic Biol Med. 2002;32(9):882–889. doi: 10.1016/s0891-5849(02)00773-6. [DOI] [PubMed] [Google Scholar]
- 13.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10(1):12–31. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. GRIM-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2001;276:38345–38348. doi: 10.1074/jbc.C100444200. [DOI] [PubMed] [Google Scholar]
- 16.Chomyn A, Cleeter MW, Ragan CI, Riley M, Doolittle RF, Attardi G. URF6, last unidentified reading frame of human mtDNA, codes for an NADH dehydrogenase subunit. Science. 1986;234:614–618. doi: 10.1126/science.3764430. [DOI] [PubMed] [Google Scholar]
- 17.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292(2):C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 18.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarter RJM. Handbook of Physiology. Oxford, UK: Oxford University Press; 1995. Aging. chapt. 11. [Google Scholar]
- 20.Castelluccio C, Baracca A, Fato R, et al. Mitochondrial activities of rat heart during ageing. Mech Ageing Dev. 1994;76(2–3):73–88. doi: 10.1016/0047-6374(94)91583-0. [DOI] [PubMed] [Google Scholar]
- 21.Ventura B, Genova ML, Bovina C, Formiggini G, Lenaz G. Control of oxidative phosphorylation by Complex I in rat liver mitochondria: implications for aging. Biochim Biophys Acta. 2002;1553(3):249–260. doi: 10.1016/s0005-2728(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113(1):91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama S, Takasawa M, Hayakawa M, Ozawa T. Changes in skeletal muscle, heart and liver mitochondrial electron transport activities in rats and dogs of various ages. Biochem Mol Biol Int. 1993;30(5):937–944. [PubMed] [Google Scholar]
- 24.Brown-Borg HM, Johnson WT, Rakoczy S. Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. Age. 2011;34(1):43–57. doi: 10.1007/s11357-011-9212-x. doi:10.1007/s11357-011-9212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonckheere AI, Huigsloot M, Lammens M, et al. Restoration of complex V deficiency caused by a novel deletion in the human TMEM70 gene normalizes mitochondrial morphology. Mitochondrion. 2011;11(6):954–963. doi: 10.1016/j.mito.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira PC, Santos RH, Fiorelli AI. Selective decrease of components of the creatine kinase system and ATP synthase complex in chronic Chagas disease cardiomyopathy. PLoS Negl Trop Dis. 2011;5(6):e1205. doi: 10.1371/journal.pntd.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corton JC, Brown-Borg HM. Peroxisome proliferator activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60(12):1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 28.Bartke A, Bonkowski M, Masternak MT. How diet interacts with longevity genes. Hormones (Athens) 2008;7(1):17–23. doi: 10.14310/horm.2002.1111033. [DOI] [PubMed] [Google Scholar]
- 29.Gesing A, Masternak MM, Wang F, et al. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011;66A:1062–1076. doi: 10.1093/gerona/glr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 32.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 33.Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. Mech Ageing Dev. 2003;124:1013–1024. doi: 10.1016/j.mad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Cavallo A, Gnoni A, Conte E, et al. 3,5-diiodo-L-thyronine increases FoF1-ATP synthase activity and cardiolipin level in liver mitochondria of hypothyroid rats. J Bioenerg Biomembr. 2011;43(4):349–357. doi: 10.1007/s10863-011-9366-3. [DOI] [PubMed] [Google Scholar]
- 35.Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24(12):5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long-lived and disease resistant. J. Gerontol A Biol Sci Med Sci. 2004;59(12):1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rollo CD. Accelerated aging of giant transgenic mice is associated with elevated free radical processes. Can J Zool. 1996;74:606–620. [Google Scholar]
- 38.Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11(1):41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- 39.Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35(2):199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 40.Hauck SJ, Bartke A. Effects of growth hormone on hypothalamic catalase and Cu/Zn superoxide dismutase. Free Radic Biol Med. 2000;28(6):970–978. doi: 10.1016/s0891-5849(00)00186-6. [DOI] [PubMed] [Google Scholar]
- 41.Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med. 2002;227(2):94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- 42.Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl. 1993;46:61–75. [PubMed] [Google Scholar]
- 43.Doi T, Striker LJ, Quaife C, et al. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulin like growth factor-1. Am J Pathol. 1988;131(3):398–403. [PMC free article] [PubMed] [Google Scholar]
- 44.Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol. 2009;299:64–71. doi: 10.1016/j.mce.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived Ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64(8):819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown-Borg HM, Johnson WT, Rakoczy SG, Romanick MA. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Am Aging Assoc. 2001;24:85–96. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choksi KB, Roberts LJ, 2nd, DeFord JH, Rabek JP, Papaconstantinou J. Lower levels of F2-isoprostanes in serum and livers of long-lived Ames dwarf mice. Biochem Biophys Res Commun. 2007;364(4):761–764. doi: 10.1016/j.bbrc.2007.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanz A. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J Am Aging Assoc. 2002;25:119–122. doi: 10.1007/s11357-002-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 50.Brown JC, Staples JF. Mitochondrial metabolic suppression in fasting and daily torpor: consequences for reactive oxygen species production. Physiol Biochem Zool. 2011;84(5):467–480. doi: 10.1086/661639. [DOI] [PubMed] [Google Scholar]
- 51.Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav. 1999;67:433–437. doi: 10.1016/s0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 52.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med. 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 53.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 54.Roth GS, Lane M, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:81. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 55.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 56.Koizumi A, Wada Y, Tuskada M, et al. A tumor preventive effect of dietary restriction is antagonized by a high housing temperature through deprivation of torpor. Mech Ageing Dev. 1996;92:67–82. doi: 10.1016/s0047-6374(96)01803-9. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Knapp JR, Kopchick JJ. Interscapular brown adipose tissue in growth hormone antagonist and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med. 2003;228:207–215. doi: 10.1177/153537020322800212. [DOI] [PubMed] [Google Scholar]
- 58.Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64A:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCarter R, Masoro EJ, Yu BP. Does food restriction retard aging by reducing the metabolic rate? Am J Physiol. 1985;248:E488–E490. doi: 10.1152/ajpendo.1985.248.4.E488. [DOI] [PubMed] [Google Scholar]
- 60.Heiman M, Tinsley F, Mattison J, Hauck S, Bartke A. Body composition of prolactin-, growth hormone-, and thyrotropin-deficient Ames dwarf mice. Endocrine. 2003;20:149–154. doi: 10.1385/ENDO:20:1-2:149. [DOI] [PubMed] [Google Scholar]
- 61.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 63.Rizzo MR, Mari D, Barbieri M, et al. Resting metabolic rate and respiratory quotient in human longevity. J Clin Endocrinol Metab. 2005;90:409–413. doi: 10.1210/jc.2004-0390. [DOI] [PubMed] [Google Scholar]
- 64.Ukropcova B, Sereda O, de Jonge L, et al. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- 65.Andrews ZB, Erion DM, Beiler R, Choi CS, Shulman GI, Horvath TL. Uncoupling protein-2 decreases the lipogenic actions of ghrelin. Endocrinology. 2010;151:2078–2086. doi: 10.1210/en.2009-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poplawski MM, Mastaitis JW, Yang XJ, Mobbs CV. Hypothalamic responses to fasting indicate metabolic reprogramming away from glycolysis toward lipid oxidation. Endocrinology. 2010;151:5206–5217. doi: 10.1210/en.2010-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fain JN, Cheema P, Tichansky DS, Madan AK. Stimulation of human omental adipose tissue lipolysis by growth hormone plus dexamethasone. Mol Cell Endocrinol. 2008;295:101–105. doi: 10.1016/j.mce.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Yang S, Mulder H, Holm C, Eden S. Effects of growth hormone on the function of beta-adrenoceptor subtypes in rat adipocytes. Obes Res. 2004;12:330–339. doi: 10.1038/oby.2004.41. [DOI] [PubMed] [Google Scholar]
- 69.Harwood HJ., Jr The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.12.010. Dec. 17 Epub. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and caloric restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61A:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 71.Brooks NL, Trent CM, Raetzsch CF, et al. Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J Biol Chem. 2007;282:35069–35077. doi: 10.1074/jbc.M700484200. [DOI] [PubMed] [Google Scholar]
- 72.Wang ZP. Adipocytokines and the Regulation of Lipid Metabolism in Ames Dwarf and Growth Hormone Transgenic Mice Subjected to Calorie Restriction [Ph.D. dissertation] Carbondale, IL: Southern Illinois University; 2006. [Google Scholar]
- 73.Tang T, Zhang J, Yin J, et al. Uncoupling of inflammation and insulin resistance by NF-kB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010;12:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masternak MM, Bartke A, Wang F, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11(1):73–81. doi: 10.1111/j.1474-9726.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauche IB, El Mkadem SA, Pottier AM, et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology. 2007;148:1539–1549. doi: 10.1210/en.2006-0838. [DOI] [PubMed] [Google Scholar]
- 76.Luo N, Wang X, Chung BH, et al. Effects of macrophage-specific adiponectin expression on lipid metabolism in vivo. Am J Physiol Endocrinol Metab. 2011;301:E180–E186. doi: 10.1152/ajpendo.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otabe S, Yuan X, Fukutani T, et al. Overexpression of human adiponectin in transgenic mice results in suppression of fat accumulation and prevention of premature death by high-calorie diet. Am J Physiol Endocrinol Metab. 2007;293:E210–E218. doi: 10.1152/ajpendo.00645.2006. [DOI] [PubMed] [Google Scholar]
- 78.Vaiopoulos AG, Marinou K, Christodoulides C, Koutsilieris M. The role of adiponectin in human vascular physiology. Int J Cardiol. 2012;155(2):188–193. doi: 10.1016/j.ijcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 79.Pischon T, Hu FB, Girman CJ, et al. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis. 2011;219:322–329. doi: 10.1016/j.atherosclerosis.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiu T-Y, Chen C-Y, Chen S-Y, Soon C-C, Chen J-W. Indicators associated with coronary atherosclerosis in metabolic syndrome. Clin Chim Acta. 2012;413:226–231. doi: 10.1016/j.cca.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 81.Kojima T, Kamei H, Aizu T, et al. Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp Gerontol. 2004;39:1595–1598. doi: 10.1016/j.exger.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Baranowska B, Bik W, Baranowska-Bik A, et al. Neuroendocrine control of metabolic homeostasis in Polish centenarians. J Physiol Pharmacol. 2006;57:55–61. [PubMed] [Google Scholar]
- 83.Atzmon G, Rincon M, Schechter CB, et al. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4:e113. doi: 10.1371/journal.pbio.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanety H, Hemi R, Ginsberg S, et al. Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur J Endocrinol. 2009;161:837–844. doi: 10.1530/EJE-09-0419. [DOI] [PubMed] [Google Scholar]
- 85.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 87.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 88.DeMartinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 89.Jaworek J, Leja-Szpak A, Dembinski A, et al. Involvement of sensory nerves in the protective effect of growth hormone on acute pancreatitis. Growth Horm IGF Res. 2009;19:517–522. doi: 10.1016/j.ghir.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Yi C, Cao Y, Mao SH, et al. Recombinant human growth hormone improves survival and protects against acute lung injury in murine Staphylococcus aureus sepsis. Inflamm Res. 2009;58:855–862. doi: 10.1007/s00011-009-0056-0. [DOI] [PubMed] [Google Scholar]
- 91.Deepak D, Daousi C, Javadpour M, et al. The influence of growth hormone replacement on peripheral inflammatory and cardiovascular risk markers in adults with severe growth hormone deficiency. Growth Horm IGF Res. 2010;20:220–225. doi: 10.1016/j.ghir.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148:2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- 93.Coschigano KT, Wetzel AN, Obichere N, et al. Identification of differentially expressed genes in the kidneys of growth hormone transgenic mice. Growth Horm IGF Res. 2010;20:345–355. doi: 10.1016/j.ghir.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sacca L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev. 1994;15:555–573. doi: 10.1210/edrv-15-5-555. [DOI] [PubMed] [Google Scholar]
- 95.Burgers AM, Biermasz NR, Schoones JW, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-1) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- 96.Bokov AF, Garg N, Ikeno Y, et al. Does reduced IGF-1R signaling in Igf1r+/- mice alter aging? PLoS One. 2011;6(11):e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]