Abstract
Oestradiol exerts a profound influence upon multiple brain circuits. For the most part, these effects are mediated by oestrogen receptor (ER)α. We review here the roles of ERβ, the other ER isoform, in mediating rodent oestradiol-regulated anxiety, aggressive and sexual behaviours, the control of gonadotrophin secretion, and adult neurogenesis. Evidence exists for: (i) ERβ located in the paraventricular nucleus underpinning the suppressive influence of oestradiol on the stress axis and anxiety-like behaviour; (ii) ERβ expressed in gonadotrophin-releasing hormone neurones contributing to oestrogen negative-feedback control of gonadotrophin secretion; (iii) ERβ controlling the offset of lordosis behaviour; (iv) ERβ suppressing aggressive behaviour in males; (v) ERβ modulating responses to social stimuli; and (vi) ERβ in controlling adult neurogenesis. This review highlights two major themes; first, ERβ and ERα are usually tightly inter-related in the oestradiol-dependent control of a particular brain function. For example, even though oestradiol feedback to control reproduction occurs principally through ERα-dependent mechanisms, modulatory roles for ERβ also exist. Second, the roles of ERα and ERβ within a particular neural network may be synergistic or antagonistic. Examples of the latter include the role of ERα to enhance, and ERβ to suppress, anxiety-like and aggressive behaviours. Splice variants such as ERβ2, acting as dominant negative receptors, are of further particular interest because their expression levels may reflect preceeding oestradiol exposure of relevance to oestradiol replacement therapy. Together, this review highlights the predominant modulatory, but nonetheless important, roles of ERβ in mediating the many effects of oestradiol upon adult brain function.
Keywords: androgen, oestradiol, gonadotrophin-releasing hormone, fertility, anxiety, stress, hypothalamic-pituitary-adrenal axis, 3β-diol, paraventricular nucleus, aggressive behaviour, sexual behaviour, hormone replacement therapy, neurogenesis
Introduction
In mammals, 17β-oestradiol (E2) has powerful effects on multiple neural networks underpinning reproductive and nonreproductive physiology and behaviour. These effects are not limited by the animal’s genetic sex because the brains of both males and females are exposed to considerable amounts of oestrogens, albeit with higher circulating levels found in females. A role for oestradiol in the aetiology of neurological and neuropsychiatric diseases is also likely, particularly because oestradiol has been shown to modulate inflammatory processes, pain, anxiety, depressive-like behaviours and cognitive function.
The actions of oestradiol in brain are mediated for the most part by two distinct intracellular oestrogen receptors (ERs). The two major types of ERs include ERα (or NR3A1) and ERβ (or NR3A2) and presumably arose as a gene duplication event early in evolution. Of the two receptors, ERα was the first ER to be described and later cloned (1), whereas ERβ is a relative newcomer to the steroid hormone receptor field, having been first described in 1996 (2). Both ERα and ERβ are members of the nuclear receptor super-family of proteins and their classically described function is as ligand-activated transcription factors (Fig. 1). Such receptors are characterised by their ability to alter transcriptional activity by binding to oestrogen response elements in the DNA sequence of gene promoters, thereby providing a direct link between steroid hormone signals and gene transcriptional responses (3,4). The identification of multiple splice variants for ERβ in rodents and humans in particular (Fig. 1) (5–7) has added a further layer of complexity to the genomic regulation of the cell by oestradiol. In addition to their direct transcriptional regulation, it is now apparent that both ER isoforms also participate in nonclassical, often termed ‘rapid’, oestrogen actions in the brain (8). In this case, ERα and ERβ located in the plasma membrane and cytoplasm have been found to be involved in regulating the phosphorylation status of multiple kinases and other proteins to control intracellular signalling (9,10). Because these rapid actions often end up modulating gene expression, and vice versa, the relationship between rapid and genomic actions of ERα and ERβ signalling is complex (11).
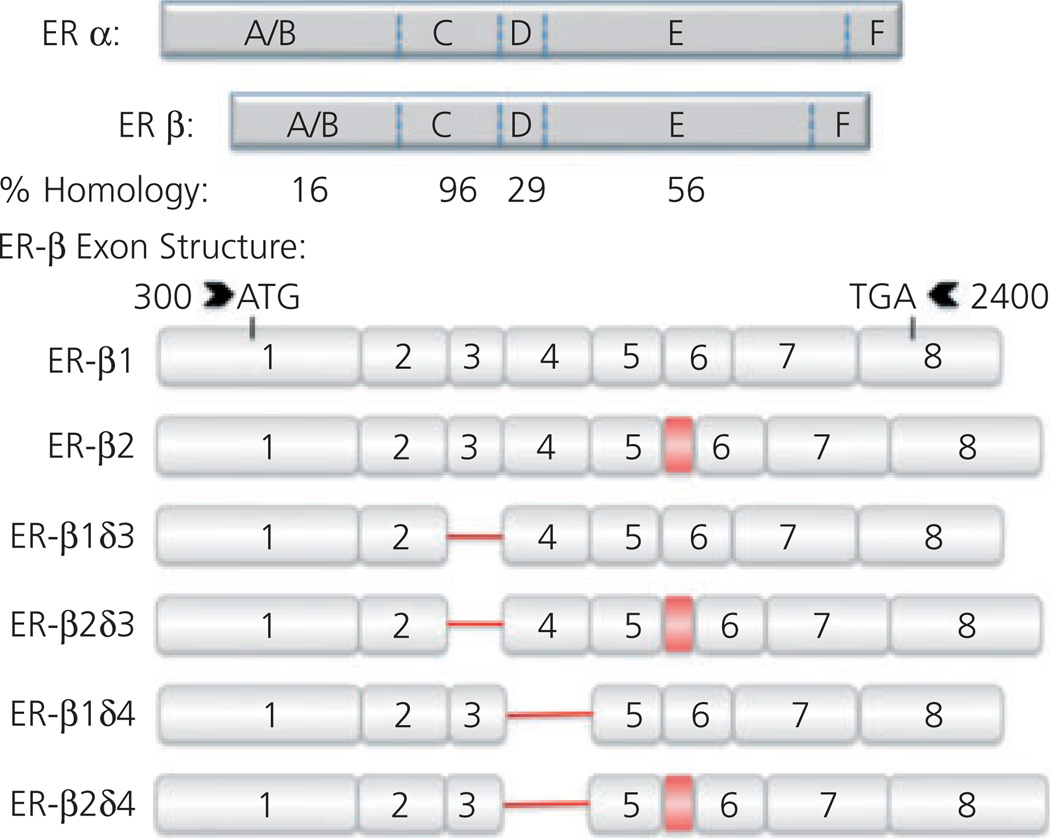
Fig. 1.
Oestrogen receptor (ER)α and ERβ homology and ERβ splice variants. Schematic representations of ERα and ERβ protein structure with relative percentage homology shown below. Letters refer to different domains of receptors; amino terminal domain (A/B), DNA-binding domain (C), a hinge region (D), ligand-binding domain (E) and caudal C-terminal (F). ERβ splice variant exon structure shown below. Exons 1–8 are numbered. Deletions are indicated by red line and insertions are indicated by a red box. The insertion between exons 5 and 6 (ERβ2) results in a modified ligand-binding domain (E). Splice variant data from J. M. Wang (unpublished data) and Price et al. (169).
In the mammalian nervous system, ERα and ERβ are expressed throughout the brain and spinal cord where they have unique but overlapping expression patterns (Fig. 2). Brain regions such as the preoptic area, bed nucleus of the stria terminalis (BNST), medial amygdala, periaqueductal grey and nucleus of the solitary tract have been reported to express both ERs. Moreover, ERα is the predominant receptor found in the ventromedial nucleus of the hypothalamus, whereas ERβ is the predominant form found in the suprachiasmatic, supraoptic, paraventricular hypothalamic nuclei and cerebellum (Fig. 2). ERα has also been reported to be more prevalent in neurones of the arcuate nucleus, whereas ERβ predominates in the dorsal raphe, hippocampus and cortex (12–19). Recent studies have also shown that ERα and ERβ may be expressed by glial cells as well (20–22). Together, these data emphasise the likelihood that ERα and ERβ are not merely functional duplicates but can affect differentially the complex behavioural and homeostatic repertoires of animals.
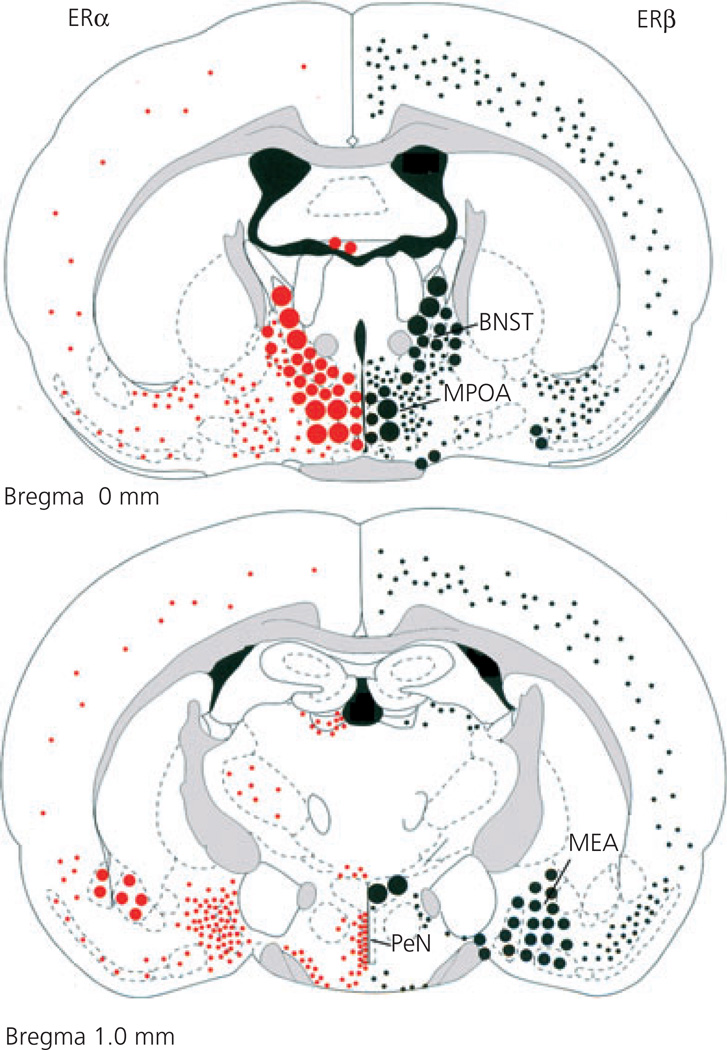
Fig. 2.
Differential distribution of oestrogen receptor (ER) α and ERβ in the rodent brain. Two coronal planes through the brain (one at bregma and one at −1 mm to bregma for mouse) showing the anatomical distribution of ERα (left) and ERβ (right). Note the overlapping as well as differentially distributed expression of the two ERs. BNST, bed nucleus of the stria terminalis; MPOA, medial preoptic area; MEA, medial amygdala; PeN, periventricular nucleus. Grey shading shows white matter tracts. Adapted with permission (17).
Defining the roles of ERα and ERβ in oestradiol-regulated brain function continues to be a major challenge in the field of neuroscience. Initially, the use of ERα and ERβ knockout (KO) mice was useful, although interpretations were limited by the global nature of ER deletion throughout the whole mouse. New generation transgenic approaches are now overcoming this issue with tissue/cell-specific, and inducible, KO paradigms. In addition, the ongoing development of ERα- and ERβ-selective ligands and antagonists has been important in defining the roles of these receptors in specific brain regions. When examined using these tools, it is very often the case that the effects of oestradiol in the brain are found to depend more on upon ERα than ERβ. It is the intention of this review to focus on roles of ERβ in the adult nervous system. For an update on roles of ERβ within the developing brain, a recent review by Fan et al. (23) is recommeded. Here, we examine the roles of ERβ in the neural regulation of fertility, steroid-dependent social, emotional and anxiety behaviours, and conclude with an assessment of potential roles for ERβ in hormone replacement therapy (HRT).
ERβ and the neural regulation of fertility
It is well established that oestradiol secreted from the ovary plays an important homeostatic role in controlling the activity of the neuronal network regulating fertility. This network uses the gonadotrophin-releasing hormone (GnRH) neurones as the final output neurones to drive pituitary secretion of gonadotrophin hormones. The ways in which oestradiol regulates the activity of the GnRH neurones has been investigated intensively and is divided into a negative-feedback component suppressing GnRH release, and a positive-feedback component responsible for stimulating GnRH secretion to evoke the luteinising hormone (LH) surge that triggers ovulation (24,25). Despite receiving much attention, our understanding of the molecular and cellular pathways underlying negative and positive feedback remains rudimentary. Recent studies have highlighted the key role of classical genomic oestradiol signalling through ERα in bringing about positive feedback via a trans-synaptic mechanism involving the rostral hypothalamus (26). Although lacking the same mechanistic detail, it is apparent from global ERαKO that ERα is also critical for normal negative feedback (27). Together, these studies suggest that ERα is the predominant ER involved in the feedback modulation of the neuronal network controling fertility.
The question remains as to whether ERβ has any role in oestrogen feedback. The primary reason for posing this question arises from the observation that adult GnRH neurones express ERβ and not ERα. Although initially controversial (28), it is now accepted that GnRH neurones express ERβ mRNA and protein in all mammalian species examined to date, including mice (29), rats (30–32), sheep (33) and humans (34). Thus, although the effects of oestradiol on GnRH neurones through ERα are indirect, there is the potential for direct actions of oestradiol on GnRH neurones through ERβ.
Reproductive phenotypes of ERβKO mice
One of the principal difficulties for understanding the potential role of ERβ in regulating GnRH neurone behaviour has come from the highly variable reproductive phenotype of ERβKO mouse lines. The initial mouse line produced in 1998 by Krege et al. (35) was reported to have only modestly impaired fertility in that female KO mice had fewer litters and fewer pups. The next ERβKO line produced in Strasbourg exhibited a more variable reproductive phenotype, with female mice displaying normal fertility through to infertility (36). Because some of this variation was considered to result from the presence of ERβ splice variants missing exon 3, an ERβKO null mouse was generated in which ERβ and all of its known splice variants were deleted (37). The male and female mice of this line were infertile, with females also displaying disordered oestrous cyclicity. Thus, the picture from KO mouse lines has evolved from one of variable, modest roles for ERβ to it being a key player in reproductive physiology. Although much of the reproductive phenotype of these mice is attributed to abnormalities in the gonads (27,36), it remains possible that ERβ expressed by GnRH neurones may also be involved.
Potential roles for ERβ expressed by GnRH neurones
Evidence for a role of ERβ in the oestrogen modulation of GnRH neurones has been accruing slowly over recent years. In keeping with its role as a transcriptional regulator, ERβ has been shown to be involved in the suppression of GnRH mRNA expression in GnRH-secreting cell lines such as the immortalised GT1-7 cells (38–40). However, GnRH mRNA expression was found to be equivalent in ERβKO and wild-type mice (41), suggesting that ERβ does not have a critical role in suppressing GnRH transcript levels in vivo. Galanin is another gene known to be regulated by oestradiol in GnRH neurones (42) and ERβ-selective ligands have been show to recapitulate the stimulatory effects of oestradiol on galanin mRNA levels in GnRH neurones in the rat (43). Although these experiments have not been able to determine whether it is ERβ in the GnRH neurones or elsewhere in the neuronal network that is involved, they raise the possibility that ERβ may exert transcriptional actions in GnRH neurones (Fig. 3).
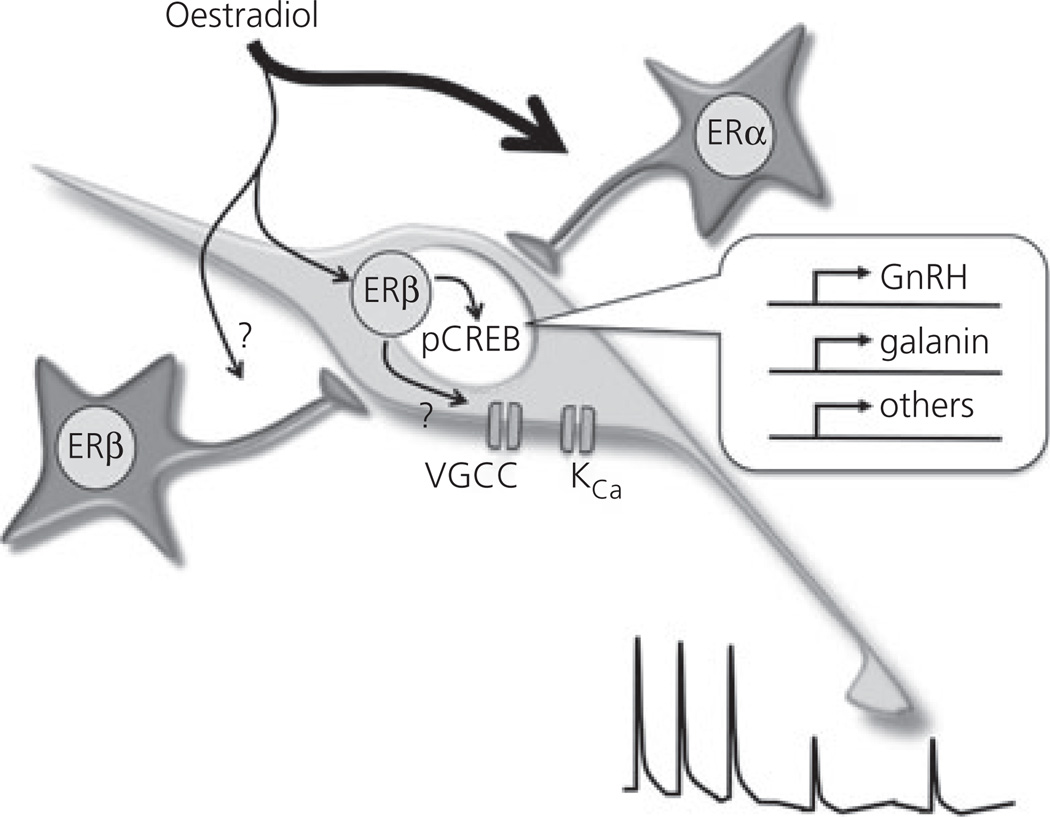
Fig. 3.
Schematic diagram showing the various pathways underpinning oestrogen negative feedback in the rodent. The principal pathway involves indirect modulation of gonadotrophin-releasing hormone (GnRH) neurones through oestrogen receptor (ER)α. A second pathway that involves direct and indirect ERβ-dependent modulation is shown. Genes reported to be regulated by oestradiol through ERβ in GnRH neurones are shown in the inset. Within GnRH neurones, oestradiol is considered to modulate the phosphorylation status of cAMP response element binding (CREB) protein, providing another mechanism for transcriptional regulation. Two ion channels modulated by ERβ-dependent signalling within or outside the GnRH neurone are the voltage-gated calcium channels (VGCC) and calcium-activated potassium channels (KCa). Together, these pathways may help suppress pulsatile luteinising hormone secretion (bottom right).
Oestradiol is also considered to exert rapid effects on gene transcription and the electrical excitability of GnRH neurones (44). Studies by Abraham et al. (45) demonstrated that oestradiol could modulate directly the phosphorylation status of cAMP response element binding protein in mouse GnRH neurones within 15 min, and that this effect was absent in ERβKO mice. More recent studies have begun to highlight the signalling pathways involved in this response by showing a key role of the mitogen-activated protein kinase pathway in mediating the actions of oestradiol on cAMP response element binding protein in GnRH neurones (46). Recent investigations have also detected rapid effects of ERβ-selective ligands on the activity of certain ion channels expressed by GnRH neurones (47,48). Specifically, it was found that the ERβ-selective ligand diarylpropionitrile (DPN) rapidly potentiates L-type voltage-gated calcium channels (47) at the same time as reducing the after hyperpolarising current (48) in mouse GnRH neurones. Although the experiments outlined above have not, for the most part, been able to show that the effects of oestradiol or DPN are direct on GnRH neurones (Fig. 3), they do provide support for the hypothesis that ERβ has a physiological role in mediating the feedback actions of oestradiol in this network.
If ERβ expressed by GnRH neurones is important, what might its role be in the feedback regulation of GnRH secretion? As noted above, there is now compelling evidence that ERα is the key ER in the positive feedback mechanism in rodents (49–52). Positive feedback is absent in global and neurone-specific ERαKO mice and an ERα-selective ligand, 16α-LE2, is able to generate the LH surge in mice (26). There appears to be little room for an important role of ERβ in positive feedback; the mechanism occurs normally in ERβKO mice (26) and an ERβ ligand, 8β-VE2, cannot evoke the LH surge (R. Porteous & A. E. Herbison, unpublished data).
By contrast, despite the predominance of ERα in the negative-feedback mechanism, this receptor is not likely to function alone. One study has shown that basal LH levels are elevated in ERβKO mice (41), although this was not observed in other studies (53). Also, classical ERα genomic mechanisms account for only approximately half of the negative-feedback actions of oestradiol on LH secretion in mice (51). Thus, there is a possibility that ERβ may play a minor but significant role in the oestradiol negative-feedback mechanism (Fig. 3). It has been suggested previously that, unlike the positive feedback mechanism, negative feedback involves many different cellular mechanisms, including direct effects, as well as indirect trans-synaptic and glial cell influences upon GnRH neurones (54). Future studies will need to dissect the importance of each of these pathways and attribute the influence of ERβ, or not, on their functioning. This is a difficult proposition and one that can probably only be undertaken at present through the use of conditional- and cell-specific KO approaches.
ERβ, androgen metabolites and the hypothalamic-pituitary-adrenal (HPA) axis in the regulation of anxiety-like behaviours
A role for ERβ in modulating anxiety-like behaviour
Studies examining the neurobiological actions of ERβ suggested that one of its roles might be in controlling the expression of fear-and anxiety-like behaviours. Investigations into the effects of oestradiol on anxiety and depressive-like behaviours have shown that different doses, treatment regimens or animal models can result in either oestradiol-induced increases or decreases in the expression of these behaviours (55–59). These diverse effects of oestradiol may be the result of its ability to bind both ER subtypes with near equivalent affinity (60). By contrast, activation or inhibition of a particular receptor subtype might allow greater insight into the role of these receptors in controlling particular behaviours. For example, female ERβKO mice have increased anxiety-like behaviours (61), suggesting that ERβ might transmit an anxiolytic signal. Furthermore, the selective ERβ agonist DPN administered to ovariectomised female rats has anxiolytic activity when animals are tested in the elevated plus maze, open field arena and light dark box (62). Similar effects of other ERβ agonists, such as WAY200070 or DPNS, with the latter being the more selective enantiomer of racemic DPN, also show anxiolytic and anti-depressive like effects (63). By contrast, ERα agonists appear to have anxiogenic like properties (62,63). Further support for ERβ mediating an anxiolytic signal also comes from studies showing that the anxiolytic actions of ERβ agonists occur after administration to wild-type but not ERβKO mice (64,65). Moreover, treatment with ERβ agonists can also prevent experimentally-induced anxiogenic states such as those caused by glucocorticoid receptor stimulation of the central nucleus of the amygdala (66). Together, these studies indicate an important role for ERβ within the brain in mediating anxiety-like behaviours (Fig. 4).
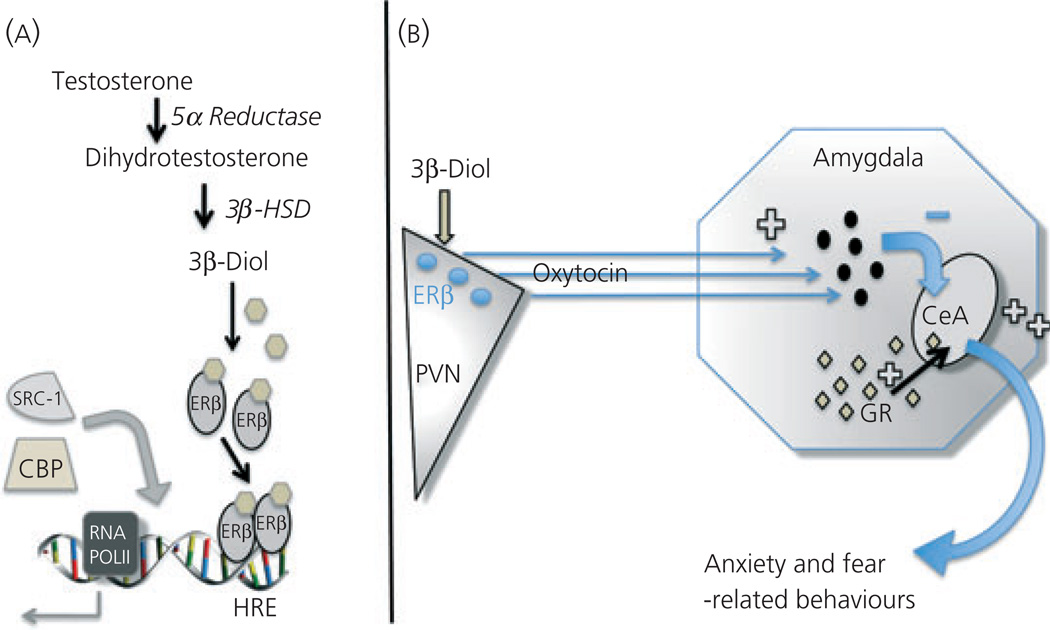
Fig. 4.
Putative mechanisms for 5α-androstane-3β,17β-diol (3β-diol) inhibition of anxiety-like behaviours. (A) 3β-Diol is produced through oxidation of dihydrotestosterone by the enzyme, 3β-hydroxysteroid dehydrogenase (3β-HSD) and others. Within oxytocin (OT) neurones, it binds ERβ, which dimerises and activates OT gene transcription by binding the hormone response element located at −160 in the ot promoter. In doing so, it attracts co-regulatory proteins such as SRC1 and CBP to regulate transcription of the ot gene. (B) 3β-Diol binds and activates ERβ found in OT neurones of the paraventricular nucleus (PVN) to activate inhibitory neurones in the amygdala and correspondingly reduce activity of neurones in the central nucleus of the amygdala (CeA). The activation of CeA neurones is involved in increased anxiety- and fear-related behaviours. By contrast, glucocorticoid receptor (GR) containing neurones of the amygdala and CeA will increase the tone of CeA neurones, thereby potentiating fear- and anxiety-related behaviours. HRE, hormone response element
ERβ modulates activity of the HPA axis
In mammals, adrenal corticosterone (CORT) secretion is tightly controlled by the neuroendocrine HPA axis that involves the hypothalamus, the anterior pituitary and the adrenal gland. This HPA axis represents the integration of a cascade of neural and humoral signals driven by both the circadian pacemaker, as well as the environment. Threats to homeostasis, whether real or perceived, activate the HPA by funnelling information through neurones located within the hypothalamic paraventricular nucleus (PVN), a major integratory node of the hypothalamus. Within the parvocellular part of the PVN are neurones that contain corticotrophin-releasing factors, most notably corticotrophin-releasing hormone (CRH) and vasopressin (AVP). The release of these hormones into the hypophyseal portal system enhances synthesis and release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland. In turn, ACTH acts on the adrenal cortex to cause a rise in plasma CORT. Circulating CORT subsequently acts at the level of the pituitary, hypothalamus and higher brain areas to limit further hormone secretion (67). Glucocorticoid hormones can also act upon select brain areas to modulate behaviour (66).
After the initial report identifying ERβ (2), its mRNA and protein were shown to be highly expressed within neurones of the PVN (12,17,18,68,69), raising the possibility that these ERβ containing neurones might represent an important neuroendocrine regulatory system. A large percentage of ERβ expressing cells in the PVN are oxytocin, vasopressin and prolactin-immunoreactive neurons (69–73) and ERβ is also colocalised in a small number (10–15%) of CRH containing neurones of the PVN (12,73). This distribution suggests that oestradiol could have direct impact upon the function of PVN neurones through ERβ. By contrast, ERα is found sporadically in the periventricular PVN (74) and not in CRH, AVP or OXY neurones (73,75). Indeed, in vivo studies demonstrated that ERβ agonists inhibit the stress-induced increases in ACTH and CORT when given peripherally or when applied to the PVN (63,76).
Androgens are metabolised to important ERβ ligands
The metabolism of steroid hormones in both central and peripheral tissues has been studied for several decades. In both males and females, testosterone serves not only as a ligand for the androgen receptor (AR), but also as a precursor for other steroids. We now know that testosterone can be converted in brain tissue to oestradiol by the aromatase enzyme (77), or to dihydrotestosterone (DHT) by 5α reductase (78). Historically, DHT has been used as a potent and selective agonist for ARs because is not a substrate for aromatisation. However, recent studies have proposed that DHT may be a precursor for other steroids that can act on receptors other than the AR (79,80). DHT is metabolised to 5α-androstane-3α,17β-diol (3α-diol) or to 5α-androstane-3β,17β-diol (3β-diol) by the actions of several enzymes including 3α-hydroxysteroid dehydrogenase (3α-HSD), 3β hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase (79,81–84).
3α-Diol and 3β-diol possess only weak AR binding activity, although it is now apparent that they can initiate cellular responses through other receptor types. 3α-Diol, similar to other 3α tetrahydrosteroids, is a potent allosteric modulator of GABAA receptors, whereas 3β-diol does not posses this activity (83,85). As a result, 3α-diol has been implicated in regulating a number of behaviours (2,86–89) by modulating GABAergic pathways. Alternatively, 3β-diol will preferentially bind and activate transcription through ERβ, whereas 3α-diol does not have this capability (2,87).
The conversion of DHT to 3α-diol is a reversible reaction utilising 3α-HSD and ‘RoDH-like’ 3α-HSD to drive the reaction in the forward and reverse directions (90,91). Therefore, it appears that 3α-diol serves as a sink for further DHT and 3β-diol synthesis. By contrast, synthesis of 3β-diol is unidirectional (83). Ultimately, 3β-diol is converted to inactive 6α- or 7α-triols (5α-androstan-3β,6α,17β-triol; 5α-androstan-3β,7α,17β-triol) by CYP7B1 (92). Consequently, CYP7B1 may be an important pre-receptor regulatory mechanism for this pathway (79). Down-regulation of CYP7B1 activity would allow accumulation of 3β-diol, whereas CYP7B1 up-regulation could limit the action of 3β-diol. CYP7B1 has been found at high levels in brain (93) and, although its distribution has never been carefully examined, studies show that its mRNA is found in PVN (62). Because the brain contains the necessary steroid metabolising enzymes to convert DHT to 3β-diol (93), it was hypothesised that in brain, the actions of testosterone are mediated by its conversion to DHT and then to 3β-diol. The net result is that the AR is bypassed and ERβ pathways are activated (Fig. 4). This endocrine pathway exists in several androgen-dependent tissues, and its functional significance was first suggested for regulating prostate growth (79). Moreover, recent data show that mRNAs for 5α reductase, 3α-HSD and 17α-HSD are also present in the PVN of male rats (76) and this is consistent with enzyme activity assays indicating that PVN homogenate can make 3β-diol from 3H-DHT precursor (L. R. Hinds and R. J. Handa, unpublished data). The active enzyme(s) for brain conversion of DHT to 3β-diol remain unknown.
3β-diol regulates the HPA axis via ERβ
Lund et al. (94) first described the ability of 3β-diol to regulate the HPA axis by testing the ability of peripherally administered 3β-diol-diproprionate to alter stress-responsive CORT and ACTH secretion in castrated adult male mice. These studies demonstrated that peripheral 3β-diol treatment was as effective as peripheral DHT administration to reduce stress-induced CORT and ACTH secretion. The effects of 3β-diol could be blocked by co-administration of the ER antagonist, tamoxifen, but not by the AR antagonist, flutamide, thus implicating ERs. Furthermore, ERβ agonists inhibited HPA reactivity in a fashion similar to DHT and 3β-diol. Subsequently, it was shown that both DPN and 3β-diol are not active in ERβKO mice (64), providing evidence that 3β-diol mediates the effects of DHT on HPA reactivity by activating ERβ. Similarly, evidence for the PVN being a neural target of the HPA inhibiting activity of 3β-diol has been found (76). By using small pellets of beeswax as a carrier for hormone, it was found that the stereotaxic application of 3β-diol to the PVN of castrated male rats mimicked the actions of both central and peripherally administered DHT. Moreover, local application of DPN could also mimic the actions of DHT. These inhibitory actions of 3β-diol and DPN are blocked by co-administration of the ER antagonist, tamoxifen, whereas the AR antagonist, flutamide has little effect. These data suggest that local 3β-diol synthesis by cells in or around the PVN could profoundly impact the function of HPA reactivity to stressors and that compounds that bind ERβ are inhibitors of stressor reactivity (Fig. 4). By contrast, oestradiol appears to act primarily through ERα to augment HPA reactivity because oestradiol and the ERα selective agonist, propylpyrazoletriol, have the opposite action of ERβ agonists and increase HPA reactivity to restraint stress (76).
How the HPA axis can distinguish the enhancing from inhibiting actions of a single compound, such as oestradiol, that binds equivalently to both ERα and ERβ is currently under investigation. One possible explanation lies in the ratio of ERα to ERβ that exists within neurones in and around of the PVN. A greater ERα/ERβ ratio could cause a shift towards enhanced gain and the opposite would be true under conditions where ERβ was elevated compared to ERα. Indeed, it has been demonstrated that levels of ERβ might change in response to circulating glucocorticoid hormone levels, as well as oestradiol levels (68,95,96), thus shifting the gain of the system.
Another intriguing possibility comes from observation that activation of a gene promoter sequence by oestradiol-bound ERβ is not equivalent to that after activation of the same promoter by 3β-diol bound ERβ. This is true for both the vasopressin promoter (97) and the oxytocin promoter (98). Furthermore, using chromatin immunoprecipitation, it has been demonstrated that 3β-diol treatment of N38 hypothalamic cells increased ERβ occupancy of a composite hormone response element in the oxytocin promoter, whereas oestradiol treatment did not (99). Hence, there is the added potential for ligand identity in controlling the inhibitory actions of ERβ, and this may exemplify a unique feature of 3β-diol-mediated transcription that differs from that of oestradiol-mediated transcription through ERβ binding.
ERβ in the regulation of sexual, aggressive, and social behaviours
Role of ERβ in the regulation of female sexual behaviour
It is well established that the gonadal steroid hormone E2 plays a central role in female reproductive behaviour, particularly, lordosis behaviour, a dorsiflexion posture displayed by a sexually receptive female in response to mounting by a male. A series of behavioural analyses using KO mouse models (100,101) and, more recently, brain site-specific gene knockdown methods (102) revealed that binding of oestradiol to intracellular ERα in the ventromedial nucleus of the hypothalamus (VMN), is critical for normal expression of lordosis behaviour. These studies also demonstrated that both ERαKO and VMN-specific ERα knockdown female mice had reduced levels of proceptive posture and vigorous rejection when male mice approached, sexually investigated, and attempted to mount. Lack of functional ERα protein in the VMN in both models also greatly attenuated induction of progesterone receptor (PR), one of the transcriptional gene products of ER activation, by oestrogen in this brain area.
On the other hand, behavioural analysis in ERβKO has revealed that a lack of functional ERβ does not affect lordosis and courtship behaviour (103), despite reduced fertility (35). On the day of oestrus, ERβKO mice are as receptive as wild-type littermate mice, showing approximately 80% of lordosis quotient (% of number of lordosis/number of mounts and intromissions). As expected, PR induction by oestrogen in the VMN in ERβKO mice was comparable to that in wild-type littermate mice. However, detailed behavioural analysis during the entire oestrous cycle revealed that sexual receptivity of ERβKO mice was not restricted on the day of oestrus, but significantly extended beyond the day of behavioural oestrus. Similarly, ovariectomised ERβKO female mice showed high receptivity not only in the tests carried out after 48 h of oestrogen and 6 h of progesterone treatment, but also those carried out 24 h later, when wild-type littermate mice are no longer as receptive. These behavioural observations suggest that activation of ERβ may be necessary to turn ‘off’ receptivity at the appropriate timing and play a critical role in the fine-tuning of mating behaviour. Although the exact neuroendocrine mechanism of this phenomenon remains to be determined, it is possible that ERβ-containing neurones in brain areas other than the VMN (13,17) may be responsible for this type of behavioural control. These include ERβ-expressing neurones in the medial amygdala (MA), which is involved in processing mating-related information, those in the serotonergic midbrain dorsal raphe nuclei (DRN), and/or those in noradrenergic locus coeruleus (LC). Indeed, PR induction by oestrogen in serotoninergic neurones in the DRN is greatly attenuated in ERβKO, but not in ERαKO, female mice (104). In a recent study, DRN site-specific ERβ knockdown also lowered the number of PR and tryptophan hydroxylase double-labelled cells. Similarly, oestrogen increased the number of PR and tyrosine hydroxylase double positive cells in the LC in ERαKO mice, as well as in wild-type mice, but failed to do so in ERβKO mice (105). Thus, although ERα is the key receptor within the VMN enabling lordosis, it is likely that ERβ, possibly located outside of the VMN, has a facilitatory role in limiting the behaviour to the appropriate time.
Role of ERβ in the regulation of male aggressive behaviour
ERβ also plays a role in the modulation of aggressive behaviour in male mice. By contrast to the almost complete abolition of male-typical aggressive behaviour in ERαKO mice (106–108), ERβ disruption does not lower the levels of aggression at all. Instead, detailed analysis revealed that ERβKO male mice were more aggressive than wild-type littermate mice, depending on social experience and age, suggesting that ERβ activation might rather have an inhibitory role in the expression of aggressive behaviour. Adult ERβKO mice are found to be more aggressive than wild-type littermate mice during the first aggression test but, with the repetition of aggression tests, these genotype differences disappeared (103,106). A more striking effect of ERβ gene disruption was observed in male mice during the adolescent period where ERβKO mice were significantly more aggressive than wild-type littermate at 5–6 weeks of age. These genotype differences were not apparent at older ages, when wild-type littermate male mice started to be more aggressive than during pubertal period (109). Therefore, it is possible that ERβ activation may also be necessary to fine-tune the timing of onset of aggression during peri-pubertal period. To date, behavioural data collectively suggest that: (i) activation of ERα and ERβ has opposing effects on male aggression and (ii) ERβ may inhibit aggressive behaviour induced by activation of ERα, either alone or in combination with AR activation.
Because possible brain mechanisms underlie the inhibitory regulation of aggressive behaviour by ERβ, attention has been focused on the brain areas showing clear differences in the distribution of ERα and ERβ (13,17) (Fig. 2). It is known that ERβ mRNA and protein are highly concentrated in a number of brain areas not particularly rich in ERα, such as the PVN and midbrain DRN. ERβ is also localised in limbic areas such as the MA and BNST, which are implicated in the regulation of emotional behaviours, including aggressive behaviours. Among these areas, the total number of ERβ-immunoreactive cells was almost twice that of ERα in the ventral subdivision of the DRN, in contrast to the adjacent periaqueductal grey, in which the number of ERβ positive cells is approximately one-third that of ERα (110). Furthermore, dual-label immunocytochemistry revealed that more than 90% of ERβ immunoreactive cells in the DRN were also positive for TPH, the rate-limiting enzyme for the serotonergic system known as a major neurotransmitter for the control of aggression. These findings raise the possibility that ERβ activation may contribute to the regulation of aggression, in part, by acting directly on serotonergic neurones in the DRN. It is also possible that ERβ activated by oestradiol or 3β-diol from DHT (Fig. 4A) may modulate aggressive behaviour in male mice by regulating the gene expression of neuropeptides, such as oxytocin and AVP, and/or their receptors in a number of hypothalamic and limbic areas, including PVN, MA and BNST (111). With the development of ERβ specific compounds, it may soon be possible to investigate roles of ERβ in behavioural regulation in the therapeutic setting.
Possible role of ERβ in the regulation of behavioural responses in social context
As noted above, studies from different laboratories have demonstrated that ERβ is involved in the oestrogenic regulation of anxiety levels measured in behavioural tests such as elevated plus and light/dark transition (65,112,113). For example, the anxiolytic effects of low-dose oestrogen treatment in ovariectomised female mice, as indicated by a longer time spent in the light compartment in the light/dark transition test, were not observed in ERβKO mice (59). Behavioural analysis also revealed that ERβ might also be involved in the regulation of anxiety in social context and, in turn, behavioural responses to other animals.
Because (i) aggressive behaviours of young ERβKO mice were very impulsive and (ii) adult ERβKO mice are more aggressive than wild-type littermate mice in the very first aggression test, it is possible that social reactivity may be altered in ERβKO male mice compared to wild-type littermate mice. To more precisely control and measure behavioural responses at the first encounter with an opponent mouse, we presented an intruder mouse in a protective shield (a clear perforated plexiglass cylinder) placed in the center of the home cage for 30 min (social instigation procedure) before regular 15 min aggression tests (S. Ogawa unpublished data). Mice in control groups were presented with an empty cylinder. Social instigation potentiated the levels of aggression in ERβKO mice, although it had no effects on wild-type littermate mice. We also found that ERβKO mice in the instigated group showed elevated levels of social investigation (i.e. sniffing toward the holes of the cylinder) than instigated wild-type littermate mice, as well as non-instigated control ERβKO mice. These results suggest that ERβKO male mice may be hyper-reactive to social stimuli. Instigated ERβKO mice also showed higher levels of c-fos induction than instigated wild-type littermate mice, as well as non-instigated control ERβKO mice in a number of brain areas, including the MA and BNST, which are known to be involved in the regulation of aggressive behaviour. These results suggest that ERβ may play a role in the regulation of animal’s reactivity to social stimuli in male mice.
Similar behavioural phenotypes have also been observed in female ERβKO mice. When tested for social recognition using an habituation–dishabituation paradigm, both ERαKO and ERβKO female mice showed impairments, in that their habituation responses (a gradual decrease of social investigation time) to a repeated presentation of the same opponent mouse, and their dishabituation responses (a restoration of social investigation time) to one-time presentation of a new stimulus mouse, were much less than those of wild-type mice (114). However, ERβKO mice showed much less habituation compared to ERαKO mice and almost the same high levels of social investigation throughout all five trials. The lack of habituation response to social stimuli in ERβKO was even more obvious when they were tested in a discrimination test paradigm, in which the same two opponents were presented in the first four trials and one of the opponents was replaced with a new opponent in the fifth trial (115). Again, both KO mice failed to show discrimination in the fifth trail, although there was a clear difference in the changes of the total social investigation time between ERαKO and ERβKO mice. Although ERαKO showed a gradual decrease of total investigation time over five trials, ERβKO mice showed a persistently high total investigation time throughout the five trials. These findings suggest that a lack of ERβ activation induces hyper-reactivity to social stimuli.
ERβ and post-menopausal oestrogen therapy
Menopause and the Womens’ Health Initiative (WHI)
A persistent decline in circulating levels of oestradiol is associated with surgical and natural menopause. This fall in oestradiol levels in menopausal women has been found to affect cognition, such as declarative memory (116–118). The association between the decline of gonadal hormones and cognition is supported by studies demonstrating that cognitive deficits caused by low levels of sex hormones are reversed by exogenous oestradiol administration under pathological conditions such as Turner syndrome (119) or after surgical procedures such as oophorectomy (120).
From the early 1960s onward, HRT became increasingly popular for post-menopausal women. This was based on numerous observational studies reporting that HRT ameliorated menopausal symptoms, including mental and cognitive deficits. The idea that oestradiol would improve menopausal-related memory and mental impairment was further supported by numerous reports on the neuroprotective and neurotrophic actions of oestradiol in laboratory animal studies and basic scientific experiments. However, the concept of beneficial oestradiol actions in menopausal women was challenged by the Women’s Health Initiative and ancillary Memory Study (WHIMS), which was terminated prematurely in 2002 (121,122). The WHIMS study indicated that HRT did not significantly protect cognition and mental disorders and may even cause harm when administered to women over the age of 65 years, with a reduction in brain volume, neuronal size and dendritic spine numbers being detected in HRT participants.
Why were the results from basic science studies and early observational trials so different from those found in WHI/WHIMS? Several factors may be responsible, including differences in the oestrogen compounds used, their route of administration, cyclic versus continuous regimens, and the concomitant use of progestins. However, the most important factor may be the time of initiation of HRT. In the WHIMS study, subjects were recruited between the ages of 65 and 79 years and had been post-menopausal in a hypoestrogenic state for 10–21 years at the time HRT was initiated (121,122). In earlier observational studies, in which beneficial results were reported, HRT treatment was initiated before or around the age of 50 years. Therefore, it has been proposed that a critical period, or therapeutic window, close to the time of menopausal transition may exist during which HRT should be initiated to obtain beneficial effects (123–125). The therapeutic window theory is supported by re-analysis of WHIMS data (126), meta-analyses of early clinical trials (127) and studies of the response to oestrogen treatment after bilateral oophorectomy (125). However, the molecular basis of this therapeutic window is unknown.
ERβ splice variants in the brain
ERα and ERβ share common nuclear receptor super family features such as an amino terminal domain (A/B/domain), a highly conserved DNA-binding domain comprised of two Cys4 zinc fingers (C domain), a hinge region (D domain), a less well conserved C-terminal ligand-binding domain (E domain) and a caudal C-terminal F domain (Fig. 1) (128,129). Importantly the cassette nature of these receptors, coupled with the capability of cells to selectively splice out functional domains of the receptors, predicts that multiple splice variants exist and these have now been identified (Fig. 1) (5,6,130).
Of especial interest is the potential dominant negative role of the ERβ2 isoform (Fig. 1). In the rodent, the additional 54bp nucleotides of ERβ2 code for an extra 18 amino acids within the ligand-binding domain and these reduce the binding affinity to oestradiol by up to 30-fold (6,131). In addition, ERβ2 also exhibits weaker interactions with TIF2 and RAP250, which are two transcription coactivators (6,131). The changes in binding affinity and the ability for interaction with other coactivators make ERβ2, in part, a dominant negative receptor (129,132).
ER-mediated neurogenesis and a therapeutic window
Neurogenesis is observed well into adulthood in two brain regions, the dentate gyrus of the hippocampus and the lateral walls of the lateral ventricles, in many mammals, including humans. This observation raises the possibility that neurodegenerative conditions, such as Alzheimer’s disease (AD), could be ameliorated by the generation of new neurones. Indeed, the expression of AD pathology markers is accompanied by a decline of neurogenesis in the brains of transgenic rodents (133–136) and the cognitive deficits induced by human familiar AD mutations can be reversed by neurogenic agents (135,136). In addition, conditions associated with major depression, such as social stress, suppress hippocampal neurogenesis in rodents and primates (137–139). Furthermore, selective serotonin reuptake inhibitors, the most common antidepressant drugs, increase the extracellular level of serotonin and reverse depression-induced deficits of neurogenesis within the dentate gyrus in the adult rodent and primate brain (140–144). These studies suggest that this neurogenic rescue may underlie the behavioural effects of these antidepressant drugs (144–149) and might possibly provide clues regarding the cellular mechanisms involved in the antidepressive effects of oestradiol observed in rodents.
Oestradiol promotes neural progenitor cell proliferation in the rat hippocampus in vivo under both physiological (150,151) and pathological (152) conditions. For example, cell proliferation in the dentate gyrus increases during pro-oestrus, when ovarian hormone levels are highest, compared to oestrus and di-oestrus (151) and the augmentation of neural progenitor proliferation results in a transient increase in the number of new granular neurons (153,154). Further support for oestradiol increasing neurogenesis comes from the observation that more new cells are found in the dentate gyrus of male pups during the breeding season (when they receive oestradiol from milk) than during the nonbreeding season (155,156).
Oestradiol-promoted neurogenesis is mediated by its receptors, of which, ERβ is the most important for hippocampus-dependent cognition. Accumulated data suggest that E2 promotes hippocampal neural progenitor cell proliferation in vitro, in vivo and after brain injury (157). The E2-promoted neural progenitor cell proliferation in rodents is mediated by both ERα and β. Although, in cultured human neural progenitor cells, oestradiol-induced proliferation is mediated by ERβ (158), in human neural progenitor cells, ERβ expression was predominant relative to ERα, which was barely detectable in human neural progenitor cells. Activation of ERβ by the ERβ-specific ligand, DPN, led to an increase in phosphorylated extracellular signal-regulated kinase. Furthermore, subsequent centrosome amplification and human neural progenitor cell proliferation were blocked by the mitogen-activated protein kinase/extracellular-signal-regulated kinase kinase kinase antagonist, UO126, but not its inactive analogue, UO124.
Studies in animals and humans suggest that the brain responds differently to oestrogen therapy depending on age and proximity to menopause (159). Oestradiol appears to reduce the risk of dementia and depression in younger, recently post-menopausal women, but not in older post-menopausal women (160,161). In rats, oestradiol increases the proliferation of neural progenitor cells in the dentate gyrus of recently (within 6 days) ovariectomised (OVX) rats after either a single injection (151) or chronic E2 treatment (150). However, this effect was attenuated when given to rats OVX for 14 days and had no effect in mice OVX for > 21 days (150). The data derived from different species (rats and mice) suggest a common phenomenon: time since ovariectomy is inversely proportional to the beneficial effects of E2 on hippocampal neurogenesis.
The menopausal transition and alterations in ERβ isoform expression
Several ERβ splice variants are known to exist (Fig. 1) and many of these have been identified in the rat hippocampus (162,163). Although the functions of these splice variants are yet unclear, oestradiol binds with low affinity to ERβ2 (6,131,164) and has been proposed to be a dominant negative receptor when forming a homodimer with ERβ or heterodimer with ERα. Interestingly, a variant named ERβ2 (also named hERβcx) has also been identified in humans and nonhuman primates, where it results in an additional 26 unique amino acid residues in the C-terminal part of the ligand-binding domain. This variant is unable to bind ligands or coactivators and has no transcriptional activity in reporter assays (165,166). Therefore, although the ERβ2 variants in rodents and humans are structurally different, the variations in both result in diminished ERβ ligand-binding and also preferential dimerisation with ERα. Variations in RNA splicing result from alternative splicing mechanisms in which the exons of the primary gene transcript, the pre-mRNA, are separated and reconnected so as to produce alternative ribonucleotide arrangements for translation. Therefore, it is important to note that alternative splicing is regulated by factors independent of the genomic DNA.
Recent studies suggest that the duration of a period of low gonadal hormone exposure determines the expression profile of ERβ2 (62,162–164,167–169). Ishunina and Swaab (167) have shown that ERα expression increases with age in post-mortem human brains of both men and women and that, although ERβ levels did not change in aged men, they decreased in aged women (167). Studies in the rodent, however, show an increase of ERβ levels after OVX (62,164) and there is evidence that this involves an increase in the expression of ERβ2 in the hippocampus of OVX rats (162,163). This finding is similar to the observation that ERβ2 increases in the pituitary of female Wistar rats OVX for > 2 weeks (168). Together, these data suggest that ERβ2 expression increases with the time period of gonadal hormone deprivation. As such, determining ERβ2 expression and identifying the factors that regulate its gonadal steroid-dependent expression may provide biomarkers for the determination of the most efficient ET window. The determination of the specific function of each receptor isoform will also help in the design and development of more receptor-isoform-specific drugs for menopause- and age-related diseases.
Conclusions
We have highlighted the recent advances in our understanding of how ERβ may contribute to the oestrogenic-regulation of adult brain function. The major theme to emerge is that, despite the differential distribution of ERα and ERβ within the brain, both receptors appear to be active in mediating the effects of oestrogen on specific brain functions. As such, the role of one receptor isoform cannot be considered in isolation and the search for brain functions controlled exclusively by ERα or ERβ may be forlorn. For example, in terms of the neural networks underpinning fertility, female sexual behaviour and male aggressive behaviour, it is clear that ERα is the dominant receptor. The effective output of these networks collapses in the absence of ERα in the ERαKO mice. However, investigators are now finding that ERβ is not without a role in mediating some effects of oestradiol in these ERα-dominated neural circuits. Evidence is accruing for a role of ERβ in: (i) the regulation of GnRH neurones at the time of negative feedback; (ii) the turning off of lordosis behaviour; and (iii) the suppression of aggressive behaviour in male mice. In some cases, such as aggressive and anxiety-like behaviours, ERα and ERβ appear to play counter-balancing roles. This may be the case particularly for splice variants such as ERβ2 that can act as a dominant negative receptor. In other cases, exemplified by the reproductive networks, ERβ appears to exert a modulatory role upon critical ERα-mediated effects. By contrast to these behaviours, the effects of oestradiol on neurogenesis in the adult brain may be dependent solely on ERβ; a unique phenomenon that may represent an avenue for ERβ-selective compounds in the future modulation of neural stem cell therapies. Although it is appreciated that much remains to be carried out, the recent development of tools enabling the selective modulation of ERβ in specific brain regions has been of great use in going beyond the phenotype of the global ERKO mice to define the typically subtle neuromodulatory roles of ERβ. We expect that the future development of more powerful investigative tools will allow further clarification of the roles of ERβ in modulating brain function.
Acknowledgements
The authors’ research programmes are supported by NIH R01-039951 (R.J.H.); Grants-in-Aid for Scientific Research from the MEXT Japan, University of Tsukuba Research Grant (S.O.), Alzheimer’s Association NIIG grant, Public Health Service Grant P20 RR17701, University of Mississippi Medical Center Intramural Research Support grant (J.M.W.); New Zealand Health Research Council and Marsden grants (A.E.H.).
References
- 1.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 4.Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585–586. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- 5.Poola I, Abraham J, Baldwin K. Identification of ten exon deleted ERbeta mRNAs in human ovary, breast, uterus and bone tissues: alternate splicing pattern of estrogen receptor beta mRNA is distinct from that of estrogen receptor alpha. FEBS Lett. 2002;516:133–138. doi: 10.1016/s0014-5793(02)02521-8. [DOI] [PubMed] [Google Scholar]
- 6.Lu B, Leygue E, Dotzlaw H, Murphy LJ, Murphy LC, Watson PH. Estrogen receptor-beta mRNA variants in human and murine tissues. Mol Cell Endocrinol. 1998;138:199–203. doi: 10.1016/s0303-7207(98)00050-1. [DOI] [PubMed] [Google Scholar]
- 7.Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998;139:1082–1092. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Revs. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 9.Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Mol Cell Endocrinol. 2009;308:17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 14.Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 15.Price RH, Jr, Handa RJ. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci Lett. 2000;288:115–118. doi: 10.1016/s0304-3940(00)01221-0. [DOI] [PubMed] [Google Scholar]
- 16.Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- 17.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 19.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 20.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor alpha and beta in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathology. 2009;29:55–62. doi: 10.1111/j.1440-1789.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Ovejero D, Veiga S, Garcia-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Comp Neurol. 2002;450:256–271. doi: 10.1002/cne.10325. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Xu H, Warner M, Gustafsson JA. ERbeta in CNS: new roles in development and function. Prog Brain Res. 2010;181:233–250. doi: 10.1016/S0079-6123(08)81013-8. [DOI] [PubMed] [Google Scholar]
- 24.Herbison AE. Physiology of the GnRH neuronal network. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd edn. San Diego, CA: Academic Press; 2006. pp. 1415–1482. [Google Scholar]
- 25.Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2009;21:327–333. doi: 10.1111/j.1365-2826.2009.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal (HPG) axis in estrogen receptor null mice reveals hypergonadism and endocrine sex-reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 28.Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- 29.Skynner MJ, Sim JS, Herbison AE. Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- 30.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- 31.Legan SJ, Tsai HW. Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J Neuroendocrinol. 2003;15:1164–1170. doi: 10.1111/j.1365-2826.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- 32.Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol. 2001;13:741–748. doi: 10.1046/j.1365-2826.2001.00708.x. [DOI] [PubMed] [Google Scholar]
- 33.Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroendocrinol. 2005;17:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- 34.Hrabovszky E, Kallo I, Szlavik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J Clin Endocrinol Metab. 2007;92:2827–2830. doi: 10.1210/jc.2006-2819. [DOI] [PubMed] [Google Scholar]
- 35.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 37.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy D, Angelini NL, Belsham DD. Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1-7 GnRH neurons. Endocrinology. 1999;140:5045–5053. doi: 10.1210/endo.140.11.7117. [DOI] [PubMed] [Google Scholar]
- 39.Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin-releasing hormone promoter activity. Endocrinology. 2006;147:1924–1931. doi: 10.1210/en.2005-1297. [DOI] [PubMed] [Google Scholar]
- 40.Ng Y, Wolfe A, Novaira HJ, Radovick S. Estrogen regulation of gene expression in GnRH neurons. Mol Cell Endocrinol. 2009;303:25–33. doi: 10.1016/j.mce.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 42.Marks DL, Smith MS, Vrontakis M, Clifton DK, Steiner RA. Regulation of galanin gene expression in gonadotropin-releasing hormone neurons during the estrous cycle of the rat. Endocrinology. 1993;132:1836–1844. doi: 10.1210/endo.132.4.7681766. [DOI] [PubMed] [Google Scholar]
- 43.Merchenthaler I, Hoffman GE, Lane MV. Estrogen and estrogen receptor-beta-selective ligands induce galanin expression within gonadotropin hormone-releasing hormone-immunoreactive (GnRH-i) neurons in the female rat brain. Endocrinology. 2005;146:2760–2765. doi: 10.1210/en.2004-1562. [DOI] [PubMed] [Google Scholar]
- 44.Herbison AE. Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology? J Physiol. 2009;587:5025–5030. doi: 10.1113/jphysiol.2009.179838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham IM, Han K, Todman MG, Korach KS, Herbison AE. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheong RY, Kwakowsky A, Herbison AE, Abraham IA. Rapid Estrogen Actions on Multiple Signaling Pathways in Gonadotropin-Releasing Hormone Neurons. Rouen: 7th International Congress of Neuroendocrinology; 2010. [Google Scholar]
- 47.Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. Opposite roles of estrogen receptor (ER)-alpha and ERbeta in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology. 2008;149:1627–1637. doi: 10.1210/en.2007-1540. [DOI] [PubMed] [Google Scholar]
- 53.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Magler JF, Sar M, Korach KS, Gustafsson J-A, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 55.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 56.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 57.Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 58.Nomikos GG, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27:691–696. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- 59.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuiper GGM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci USA. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 63.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oyola MG, Portilla W, Reyna R, Foradori C, Kudwa A, Hinds L, Handa R, Mani S. Neurobiology of estrogen receptor beta. Annual Meeting of the Society for Behavioral Neuroendocrinology. 2009 [Google Scholar]
- 65.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 69.Simonian SX, Herbison AE. Differential expression of estrogen receptor alpha and beta immunoreactivity by oxytocin neurons of rat paraventricular nucleus. J Neuroendocrinol. 1997;9:803–806. doi: 10.1046/j.1365-2826.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- 70.Alves SE, Weiland NG, Hayashi S, McEwen BS. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol. 1998;391:322–334. [PubMed] [Google Scholar]
- 71.Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 72.Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-beta in rat vasopressin and oxytocin neurons. J Neurosci. 2003;23:4261–4269. doi: 10.1523/JNEUROSCI.23-10-04261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 74.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 75.Estacio MA, Yamada S, Tsukamura H, Hirunagi K, Maeda K. Effect of fasting and immobilization stress on estrogen receptor immunoreactivity in the brain in ovariectomized female rats. Brain Res. 1996;717:55–61. doi: 10.1016/0006-8993(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 76.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 78.Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Brain Res Rev. 2001;37:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 79.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17beta-hydroxysteroid dehydrogenase. FASEB J. 2003;17:274–276. doi: 10.1096/fj.02-0397fje. [DOI] [PubMed] [Google Scholar]
- 82.Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- 83.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 84.Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305:37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]
- 85.Steckelbroeck S, Lutjohann D, Bauman DR, Ludwig M, Friedl A, Hans VH, Penning TM, Klingmuller D. Non-stereo-selective cytosolic human brain tissue 3-ketosteroid reductase is refractory to inhibition by AKR1C inhibitors. Biochim Biophys Acta. 2010;1801:1221–1231. doi: 10.1016/j.bbalip.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 87.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 88.Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res Brain Res Rev. 2001;37:162–171. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 89.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 90.Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha,17-beta-diol to 5alpha-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol. 2006;20:444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- 91.Penning TM, Bauman DR, Jin Y, Rizner TL. Identification of the molecular switch that regulates access of 5alpha-DHT to the androgen receptor. Mol Cell Endocrinol. 2007;266:77–82. doi: 10.1016/j.mce.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sundin M, Warner M, Haaparanta T, Gustafsson JA. Isolation and catalytic activity of cytochrome P-450 from ventral prostate of control rats. J Biol Chem. 1987;262:12293–12297. [PubMed] [Google Scholar]
- 93.Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- 94.Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 95.Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- 96.Somponpun SJ, Holmes MC, Seckl JR, Russell JA. Modulation of oestrogen receptor-beta mRNA expression in rat paraventricular and supraoptic nucleus neurones following adrenal steroid manipulation and hyperosmotic stimulation. J Neuroendocrinol. 2004;16:472–482. doi: 10.1111/j.1365-2826.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- 97.Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148:3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 98.Hiroi R, Handa RJ. Regulation of the human oxytocin promoter by 3beta diol. Endocrine Rev. 2010;31:S9000. [Google Scholar]
- 99.Sharma D, Handa RJ, Uht R. The testosterone metabolite 5alpha-androstane-3beta, 17beta-diol (3beta diol) leads to differential ERalpha and beta occupancy of the oxytocin (OT) promoter, a stress-related gene. Nuclear Receptors and Disease, Cold Springs Harbor laboratory meeting. 2010 [Google Scholar]
- 100.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 101.Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 102.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alves SE, McEwen BS, Hayashi S, Korach KS, Pfaff DW, Ogawa S. Estrogen-regulated progestin receptors are found in the midbrain raphe but not hippocampus of estrogen receptor alpha (ER alpha) gene-disrupted mice. J Comp Neurol. 2000;427:185–195. doi: 10.1002/1096-9861(20001113)427:2<185::aid-cne2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 105.Helena C, Gustafsson J-Å, Korach KS, Pfaff DW, Anselmo-Franci J, Ogawa S. Effects of estrogen receptor and gene deletion on estrogenic induction of progesterone receptors in the locus coeruleus in female mice. Endocrine. 2009;36:169–177. doi: 10.1007/s12020-009-9207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogawa S, Washburn TF, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Modifications of testosterone-dependent behaviors by estrogen receptor-alpha gene disruption in male mice. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 109.Nomura M, Durbak L, Chan J, Smithies O, Gustafsson JA, Korach KS, Pfaff DW, Ogawa S. Genotype/age interactions on aggressive behavior in gonadally intact estrogen receptor beta knockout (betaERKO) male mice. Horm Behav. 2002;41:288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- 110.Nomura M, Akama KT, Alves SE, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: predominant role of ER-beta in midbrain serotonergic systems. Neuroscience. 2005;130:445–456. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 111.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;109:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 112.Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–163. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 116.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 117.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 118.Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 119.Ross J, Roeltgen D, Zinn A. Cognition and the sex chromosomes: studies in Turner syndrome. Horm Res. 2006;65:47–56. doi: 10.1159/000090698. [DOI] [PubMed] [Google Scholar]
- 120.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–579. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- 121.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women’s health initiative memory study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 122.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the women’s health initiative memory study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 123.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 127.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 128.Heldring N, Pawson T, McDonnell D, Treuter E, Gustafsson JA, Pike AC. Structural insights into corepressor recognition by antagonist-bound estrogen receptors. J Biol Chem. 2007;282:10449–10455. doi: 10.1074/jbc.M611424200. [DOI] [PubMed] [Google Scholar]
- 129.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 130.Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142:2039–2049. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- 131.Chu S, Fuller PJ. Identification of a splice variant of the rat estrogen receptor beta gene. Mol Cell Endocrinol. 1997;132:195–199. doi: 10.1016/s0303-7207(97)00133-0. [DOI] [PubMed] [Google Scholar]
- 132.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS ONE. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang JM, Irwin RW, Liu L, Chen S, Brinton RD. Regeneration in a degenerating brain: potential of allopregnanolone as a neuroregenerative agent. Curr Alzheimer Res. 2007;4:510–517. doi: 10.2174/156720507783018262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- 138.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 140.Marcussen AB, Flagstad P, Kristjansen PE, Johansen FF, Englund U. Increase in neurogenesis and behavioural benefit after chronic fluoxetine treatment in Wistar rats. Acta Neurol Scand. 2008;117:94–100. doi: 10.1111/j.1600-0404.2007.00910.x. [DOI] [PubMed] [Google Scholar]
- 141.Keith JR, Wu Y, Epp JR, Sutherland RJ. Fluoxetine and the dentate gyrus: memory, recovery of function, and electrophysiology. Behav Pharmacol. 2007;18:521–531. doi: 10.1097/FBP.0b013e3282d28f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antide-pressant drugs reverse the loss of adult neural stem cells following chronic stress. J Neurosci Res. 2007;85:3574–3585. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- 143.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 145.Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007;6:311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- 146.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 147.Newton SS, Duman RS. Regulation of neurogenesis and angiogenesis in depression. Curr Neurovasc Res. 2004;1:261–267. doi: 10.2174/1567202043362388. [DOI] [PubMed] [Google Scholar]
- 148.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 149.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA. 2007;104:4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 151.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 153.Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 154.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 155.Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- 156.Ormerod BK, Galea LA. Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett. 2003;346:25–28. doi: 10.1016/s0304-3940(03)00546-9. [DOI] [PubMed] [Google Scholar]
- 157.Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- 158.Wang JM, Liu L, Brinton RD. Estradiol-17beta-induced human neural progenitor cell proliferation is mediated by an estrogen receptor beta-phosphorylated extracellularly regulated kinase pathway. Endocrinology. 2008;149:208–218. doi: 10.1210/en.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stahl SM. Effects of estrogen on the central nervous system. J Clin Psychiatry. 2001;62:317–318. doi: 10.4088/jcp.v62n0501. [DOI] [PubMed] [Google Scholar]
- 160.Almeida OP, Lautenschlager NT, Vasikaran S, Leedman P, Gelavis A, Flicker L. A 20-week randomized controlled trial of estradiol replacement therapy for women aged 70 years and older: effect on mood, cognition and quality of life. Neurobiol Aging. 2006;27:141–149. doi: 10.1016/j.neurobiolaging.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 161.Pefanco MA, Kenny AM, Kaplan RF, Kuchel G, Walsh S, Kleppinger A, Prestwood K. The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. J Am Geriatr Soc. 2007;55:426–431. doi: 10.1111/j.1532-5415.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 162.Brinton RD, Wang JM. Estrogen Receptor Splice Variants as Candidate Estrogen Membrane Receptor in Hippocampus and Cortex. San Diego, CA: Society For Neuroscience Annual Meeting; 2004. [Google Scholar]
- 163.Wang JM, Brinton RD. Estrogen Receptor Splice Variants in Hippocampus and Cortex. San Diego, CA: Endocrine Society’s 87 Annual Meeting; 2005. [Google Scholar]
- 164.Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol. 2007;505:249–267. doi: 10.1002/cne.21490. [DOI] [PubMed] [Google Scholar]
- 165.Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 166.Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor of estrogen action in human. Nucleic Acids Res. 1998;26:3505–3512. doi: 10.1093/nar/26.15.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ishunina TA, Swaab DF. Alterations in the human brain in menopause. Maturitas. 2007;57:20–22. doi: 10.1016/j.maturitas.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 168.Tena-Sempere M, Navarro VM, Mayen A, Bellido C, Sanchez-Criado JE. Regulation of estrogen receptor (ER) isoform messenger RNA expression by different ER ligands in female rat pituitary. Biol Reprod. 2004;70:671–678. doi: 10.1095/biolreprod.103.021378. [DOI] [PubMed] [Google Scholar]
- 169.Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Res Mol Brain Res. 2000;80:260–268. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]