Type II and NE-II parasite alleles are present in persons with congenital toxoplasmosis in North America. NE-II serotype was more prevalent in certain demographics and associated with prematurity and severity at birth. Both II and NE-II infections improved with treatment.
Abstract
Background. Congenital toxoplasmosis is a severe, life-altering disease in the United States. A recently developed enzyme-linked immunosorbent assay (ELISA) distinguishes Toxoplasma gondii parasite types (II and not exclusively II [NE-II]) by detecting antibodies in human sera that recognize allelic peptide motifs of distinct parasite types.
Methods. ELISA determined parasite serotype for 193 congenitally infected infants and their mothers in the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS), 1981–2009. Associations of parasite serotype with demographics, manifestations at birth, and effects of treatment were determined.
Results. Serotypes II and NE-II occurred in the United States with similar proportions during 3 decades. For persons diagnosed before or at birth and treated in infancy, and persons diagnosed after 1 year of age who missed treatment in infancy, proportions were similar (P = .91). NE-II serotype was more common in hot, humid regions (P = .02) but was also present in other regions. NE-II serotype was associated with rural residence (P < .01), lower socioeconomic status (P < .001), and Hispanic ethnicity (P < .001). Prematurity (P = .03) and severe disease at birth (P < .01) were associated with NE-II serotype. Treatment with lower and higher doses of pyrimethamine with sulfadizine improved outcomes relative to those outcomes of persons in the literature who did not receive such treatment.
Conclusions. Type II and NE-II parasites cause congenital toxoplasmosis in North America. NE-II serotype was more prevalent in certain demographics and associated with prematurity and severe disease at birth. Both type II and NE-II infections improved with treatment.
Clinical Trials Registration. NCT00004317.
Congenital toxoplasmosis is usually diagnosed in the United States when there is fetal loss, prematurity, or severe disease at birth with damage to eyes and brain with lifelong consequences, especially if untreated. This disease also presents as recurrent active retinochoroiditis, loss of cognitive and motor function, and seizures later in life in persons who appeared normal at birth and whose initial disease went unrecognized. Of those who are congenitally infected with Toxoplasma gondii and did not receive treatment, 80%–90% are reported to have eye disease by adolescence [1]. Obstetrical serologic screening is mandated by law in France and Austria, virtually eliminating severe disease in these countries through early treatment of the fetus. In the United States, screening for acute acquired T. gondii infection during gestation is performed only occasionally.
The National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS), which has been ongoing since 1981, has allowed careful evaluation of 2 cohorts of persons with congenital toxoplasmosis. There is one cohort of persons, most often diagnosed with substantial disease in the newborn period and treated in the first year of life, and another in which congenital toxoplasmosis was diagnosed after the first year of life. Sera have been collected from all the congenitally infected persons and almost all of their mothers.
Genetically disparate parasites behave differently in animal models and tissue culture [2–4]. Type I parasites are more virulent, measured as causing death in mice. Type II parasites are less lethal in mice, produce more cysts in brains of mice, and grow more slowly in tissue culture. Type III parasites are intermediate for these phenotypes. Parasites may also be nonarchetypal, containing mixtures of II or I/III specific alleles, or altogether new alleles. In a small series of persons with substantial ophthalmologic disease, there was an unusual abundance of non-II or atypical parasites, suggesting that disease outcomes in humans infected with different parasite types may differ [3]. Immune responses, including production of interferon γ, dendritic cell responses, and numbers of activated T cells, also differ [3]. Effects of type I, II, and III parasites on transcriptomes of a human neuroepithelial cell line have been shown to differ [4]. In the United States there has been only limited study of distribution of parasite types and diseases they cause, with no analyses of substantial cohorts of congenitally infected persons as described herein.
An enzyme-linked immunosorbent assay (ELISA) allows discrimination of infections caused by type II and non-II parasites using a serologic test identifying strain-specific antibodies induced by allelic peptide motifs in dense granule proteins GRA6 and GRA7 [5]. This assay allows us to distinguish strain type (II or not exclusively II [NE-II]) causing congenital toxoplasmosis in our cohorts and to correlate this with demographics of families, manifestations in infants at birth and later in life, and effects of treatment.
METHODS
National Collaborative Chicago-Based Congenital Toxoplasmosis Study
Sera were obtained from 183 mothers who transmitted T. gondii to their fetuses and 151 infants, most diagnosed with substantial disease as newborns, between 1981 and 2009 [6–20]. Forty-two persons who were referred to us after their first year also were studied [15, 16, 19, 20]. All these persons were referred to the NCCCTS by their physicians. Mothers and/or fathers were present at their children’s prespecified evaluations in Chicago (near birth, 1, 3.5, 5, 7.5, 10, and ≥15 years). Serum samples were obtained from mother and child at these times [6–20], and samples obtained closest to the time assays were performed were selected preferentially, depending on availability of sample. Our studies are conducted with ethical standards for human experimentation established in the Declaration of Helsinki, with prior institutional review board approval, and in accordance with Health Insurance Portability and Accountability Act regulations. Informed consent was obtained from all adult participants and from parents or legal guardians of minors.
Demographics
Place of residence, race/ethnicity, and variables to calculate the Four Factor Index of Social Status [21] were determined.
Risk Factors and Maternal Illness
Mothers were questioned about possible exposure to common means by which T. gondii is transmitted and about known symptoms of illness during pregnancy that could indicate infection (eg, flulike symptoms, fever, night sweats, headache, lymphadenopathy).
Host Susceptibility Alleles and Genotyping
Previously we found polymorphisms of COL2A1 and ABCA4 to be associated with congenital toxoplasmosis: COL2A1 with ophthalmologic disease, and ABCA4 with ophthalmologic and brain disease [12].
Evaluation of Congenitally Infected Persons
Evaluations were conducted at the predetermined ages specified above [6–20]. Patients were assigned an eye severity score for each eye, which characterized impact of infection on the patient’s vision. Scores were as follows: 0, normal vision, no lesion; 1, normal vision, nonmacular lesions; 2, normal vision, macular lesions; 3, impaired vision, nonmacular lesions; 4, impaired vision, macular lesions; 4.5, impaired vision, inability to view posterior pole because of cataracts or another etiology; and 5, no observable light perception (retinal detachment and grossly abnormal electroretinogram). Other preestablished outcome endpoints have been described elsewhere [6–20].
T. gondii Serologic Testing
Diagnosis of T. gondii Infection in Mother and Child
Studies performed at the Palo Alto Medical Research Foundation included the Sabin-Feldman Dye test, immunoglobulin G (IgG) [18]; immunoglobulin M (IgM) ELISA [18] (mother); IgM immunosorbent agglutination assay (newborn) [18]; immunoglobulin A (IgA) ELISA (mother and child) [18]; differential agglutination tests (mother) [18], and avidity assays (mother) [18].
Determination of Presence of Antibody to Type II or Non-II Parasites
Presence of strain-specific antibodies in people infected by parasites with type II or non-II alleles was determined using polymorphic peptides derived from 2 T. gondii dense granule proteins (GRA6 and GRA7) and 2 control peptides coupled to keyhole limpet hemocyanin (Biosource, Camarillo, California) in an ELISA, as described elsewhere [5]. Sera from strain type I, II, or III T. gondii–infected individuals (for whom genotype of infecting parasite was known) were included in each experiment as positive controls. The cutoff value for a positive reaction in the ELISA was 1.4, as established previously using a US cohort [5]. For patients who possessed antibodies to both II and non-II peptides, but one or the other was clearly more abundant as determined by comparing serum dilutions, these serotypes were designated IIa (reactivity ≥1.4, II > I/III) or I/IIIa (reactivity ≥1.4, I/III > II), respectively. When antibodies to II and non-II peptides demonstrated near-equivalent reactivities to one or the other type by testing of serum dilutions, these were designated II = I/III. For mothers and congenitally infected persons whose serum samples lacked reactivity using the ELISA, parasite serotype could not be determined. Only persons whose serum antibody response was typable were included in analyses associating parasite serotype with demographics and clinical findings.
Statistical Methods
Associations between presence of serum antibody to peptides of II or NE-II parasites and demographics and clinical findings were examined using the χ2 test. In cases where there were cell counts of ≤5, Fisher exact test was used. Stratified analyses were performed using the Cochran-Mantel-Haenszel test. Logistic regression was employed to assess relevant treatment and parasite serotype interactions. All statistical analyses were performed using Stata software, version 11 [22]. A P value <.05 was considered statistically significant. No adjustment for multiple comparisons was made.
RESULTS
Parasites Causing Congenital Toxoplasmosis in the NCCCTS Have Type I, II, III, and II = I/III Alleles
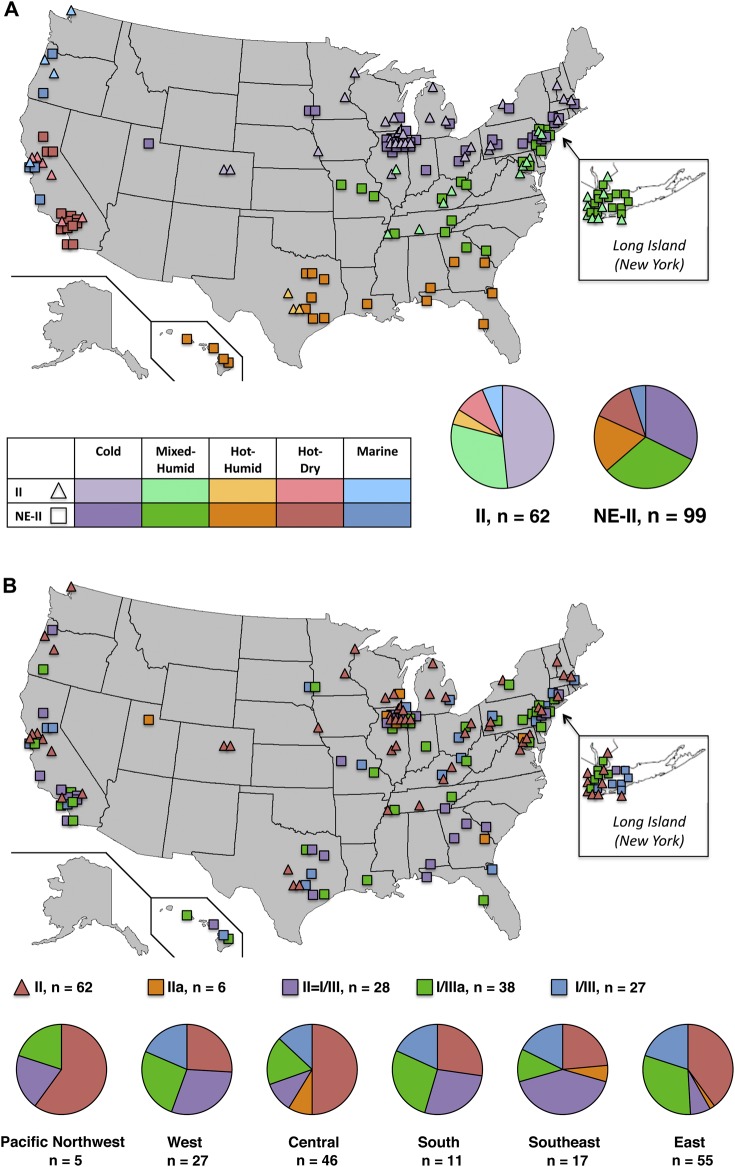
Congenital toxoplasmosis in the NCCCTS was caused by parasites with differing alleles in the same proportions during the past 3 decades (Figure 1). Parasite serotype could not be determined in 10 mothers and their congenitally infected children. They were not included in analyses of the 183 serotyped persons. To remain consistent with designations in previous studies, herein, associations are reported for persons having antibody responses to type II or NE-II (all non–type II serotypes) GRA6/7 peptides (Figure 1A). We noted different patterns of reactivity among serotyped NE-II persons, and the proportions of these persons also remained consistent during the past 3 decades (Figure 1B).
Figure 1.
National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) patients with antibodies to GRA6 and GRA7 peptides from type II or type I/III parasites or both based on decade of birth. A, Distribution of NCCCTS patients with serologic responses designated as type II and not exclusively II (NE-II). The cutoff value for a positive reaction in the enzyme-linked immunosorbent assay was 1.4 [5]. II indicates those persons who have reactivity only to type II GRA6/7 alleles. NE-II indicates all other persons with antibodies to GRA6/7 alleles. B, Distribution of NCCCTS patients with serologic responses designated as II, IIa, II = I/III, I/IIIa, and I/III. I/III indicates those persons who have reactivity only to type I/III GRA6/7 alleles. For persons who possessed antibodies to both II and non-II peptides, but in whom one or the other was clearly more abundant, these serotypes were designated as IIa (reactivity ≥1.4; II > I/III) or I/IIIa (reactivity ≥1.4; I/III > II), respectively. For persons who possessed antibodies to both II and non-II peptides with near-equivalent reactivities to one or the other type, these were designated II = I/III. Rx indicates persons in the NCCCTS cohort who were diagnosed in the perinatal period and treated during the first year of life [6–18]; No Rx, persons in the NCCCTS cohort who missed being treated in the first year of life and were referred to the NCCCTS after that time [16, 19, 20]; All, persons in the Rx plus No Rx cohorts; All decades, 1980–2009 and earlier for a few persons in the No Rx cohort. Specifically, there were 2 untreated persons born between 1940 and 1949, 2 born between 1960 and 1969, and 2 born between 1970 and 1979. There was only 1 untreated person born between 2000 and 2009 with a II = I/III parasite serotype; no histogram is presented for this person. P values are for comparisons of serotype distribution across decades for the All, Rx, or No Rx cohorts and are from χ2 tests or Fisher exact tests. aThe numbers outside parentheses represent the number of persons with the parasite serotype born in each time period for each treatment category (all, treated, or untreated persons). Numbers within parentheses represent the percentage of persons with parasite serotype among those born in each time period and the treatment category. No significant variation for distribution of parasite serotypes across decades was found.
Distribution of Parasite Serotypes in Persons Diagnosed at Birth and Later in Life Is Similar
There was little difference in distribution of parasite serotypes in the 2 cohorts that always were considered separately in other publications (P = .91), that is, those who were diagnosed in the perinatal period and treated [6–18] and those diagnosed after the first year of life and therefore missed being treated [16, 19, 20] (Table 1).
Table 1.
Associations of Parasite Serotype With Country, Demographics, Host Susceptibility Alleles, and Gestational Illness
| Demographics | II | NE-II | P Value |
|---|---|---|---|
| A. Serotypes in US-born, NCCCTS contrasted with literature data for France [23, 24] and Brazil [25] | |||
| NCCCTS | 62/161 (39)a | 99/161 (61) | <.001 |
| France [23, 24] | 72/86 (84) | 14/86 (16) | |
| Brazil [25] | 0/20 (0) | 20/20 (100) | |
| B. Serotypes in NCCCTS by demographics, host susceptibility alleles, and gestational illness | |||
| Cohorts | |||
| Treated | 52/66 (79)b | 93/117 (79) | .91 |
| Untreated | 14/66 (21) | 24/117 (21) | |
| Demographics | |||
| Rural hometownc | 8/66 (12) | 34/117 (29) | <.01 |
| SES ≥3 | 17/62 (27) | 64/116 (55) | <.001 |
| Hispanic | 3/66 (5) | 31/117 (27) | <.001 |
| Host susceptibility alleles | |||
| ≥1 SNP COL2A1, ABC4A | 33/59 (56) | 42/106 (40) | .04 |
| Gestational illness | |||
| ≥1 symptomd | 15/52 (29) | 45/92 (49) | .02 |
Socioeconomic status of 4 persons with the II serotype and 1 person with the NE-II serotype was not calculated because data were not available. SNPs genotyped with significant associations with congenital toxoplasmosis and brain and/or eye diseases were COL2A1: rs2276455, rs1635544 and ABCA4: rs952499. Genotyping was not performed for 18 patients because of technical difficulties. II and NE-II serotypes are defined in the Methods section and the Figure 1 legend. P values are from χ2 tests or Fisher exact tests.
Abbreviations: NCCCTS, National Collaborative Chicago-based Toxoplasmosis Study; SES, socioeconomic status calculated by the Hollinghead Four Factor Index of Social Status; SNP, single-nucleotide polymorphism.
The numerators represent no. of persons with this parasite serotype residing in country; denominators represent total no. of persons residing in country. Numbers within parentheses represent the percentage of persons with this parasite serotype residing in country.
Numerators represent no. of persons who had the characteristic with this parasite serotype; denominators represent total no. of persons with this parasite serotype. Numbers within parentheses represent the percentage of persons with this parasite serotype who had the given characteristic.
Rural: hometown population size <1900 at the time of first visit to NCCCTS.
Symptoms were flulike symptoms, fever, night sweats, headache, and/or lymphadenopathy. Only mothers of infants treated during the first year of life were included. Data were unavailable for 1 mother (NE-II serotype).
Incidence of Parasite Serotypes in the US, NCCCTS Differs From That in France and Brazil
Each parasite serotype was detected in congenitally infected US, NCCCTS persons, with NE-II predominant (61%). This incidence differed from that in France [23, 24] (almost exclusively type II [84%]) and Brazil [25] (predominantly NE-II) (P < .001; Table 1).
Serotypes II and NE-II Are Present Throughout the United States, but NE-II Is Predominant Along the Gulf Coast, Pacific Region, and Hawaii and in Warmer Climates
Serotypes II and NE-II were detected throughout the United States (Figure 2A), with predominance of NE-II serotype in hot, humid climates (P = .02). There were predominant II and NE-II subgroup serotypes in different US regions (Figure 2B; P < .01): East, types II, I/III, and I/IIIa; Central, II with a smaller proportion of I/III; Southeast, II = I/III most frequent; West, II = I/III most frequent with almost the same proportion of II, I/III, and I/IIIa. All 4 persons infected in the Greater Victoria outbreak in March 1995 had NE-II serotypes [26].
Figure 2.
Distribution of parasite serotypes in the United States with locations mapped according to birthplace. A, Climate regions, following designations of the Pacific Northwest National Laboratory and Oak Ridge National Library (2010). Guide to Determining Climate Regions by County. Building America Best Practices Series (7.1). Retrieved from http://apps1.eere.energy.gov/buildings/publications/ pdfs/building_america/ba_climateguide_7_1.pdf. B, Serotypes and US regions. US regions were derived from the 10 standard federal regions as established by the Office of Management and Budget: East (regions I, II, and III); Southeast (region IV); South (region VI); Central (regions V, VII, and VIII); West (region IX); and Pacific Northwest (region X). The serotypes II, IIa, II = I/III, I/IIIa, I/III, and NE-II serotypes are defined in the Methods section and the Figure 1 legend. Pie graphs: Percentage = [number of persons born in climate or geographic region with parasite serotype/total number of persons with parasite serotype] × 100.
Rural Locales, Lower Socioeconomic Status, and Hispanic Ethnicity Are Associated With NE-II Serotype
Persons in rural locales more often had NE-II serotype (P < .01) (Table 1). The II and NE-II serotypes were found in persons in urban and suburban locales. Persons of lower socioeconomic status or of Hispanic ethnicity more often had NE-II serotype (P < .001 and P < .001, respectively; Table 1).
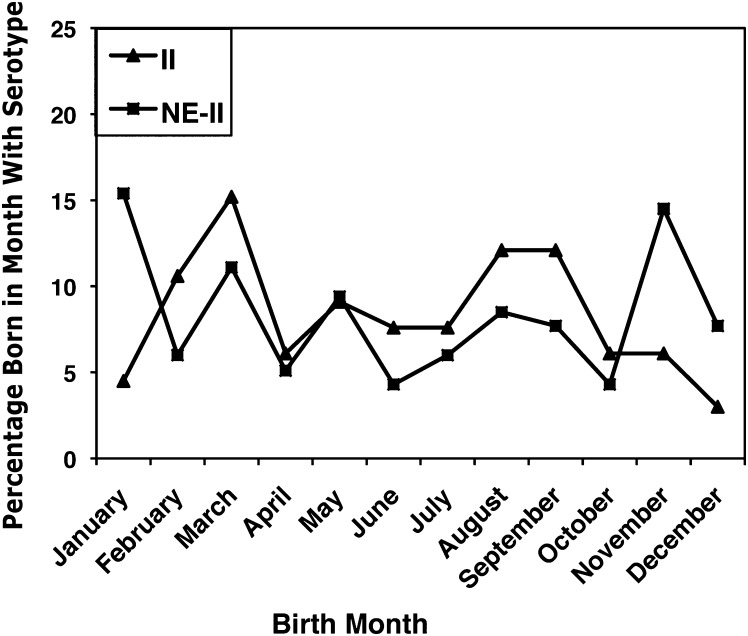
Parasite Serotype and Birth Month
Parasite serotype and birth month were not significantly associated (P = .25). However, 44 persons (38%) with NE-II serotype were born from November to January, versus 9 (14%) with serotype II (Figure 3).
Figure 3.
Distribution of parasite serotypes by birth month. In total, 38% of persons with not exclusively II (NE-II) serotype were born from November to January. Percentage = [number of persons born in month with parasite serotype/total number of persons with parasite serotype]. The serotypes II and NE-II are defined in the Methods section and the Figure 1 legend.
Susceptibility Alleles Are Associated With Serotype II
A larger proportion of persons with II compared with NE-II serotype had susceptibility alleles of COL2A and/or ABCA4 (56% vs 40%; P = .04) (Table 1).
Maternal Illness During Pregnancy Is More Common With NE-II Serotype
Maternal illness during pregnancy was significantly associated with parasite serotype (P = .02) (Table 1). A greater proportion of mothers with NE-II than II serotype had at least 1 symptom of illness during pregnancy (49% vs 29%, respectively). No risk factors for T. gondii infection in mothers were significantly associated with parasite serotype (Table 2).
Table 2.
Maternal Risk Factors for Toxoplasma gondii Infection
| Risk Factor | II | NE-II | P Value |
|---|---|---|---|
| Exposure to cats | 15/65 (23)a | 38/115 (33) | .16 |
| Clean litter pan | 7/65 (11) | 12/115 (10) | .94 |
| Gardening | 17/65 (26) | 31/115 (27) | .91 |
| Sand box | 4/65 (6) | 8/114 (7) | 1.00 |
| Exposure to raw meat | 23/65 (35) | 39/114 (34) | .87 |
| Drink raw milk | 1/65 (2) | 9/114 (8) | .10 |
| Eat raw eggs | 5/64 (8) | 9/113 (8) | 1.00 |
The serotypes II and NE-II are defined in the Methods section and the Figure 1 legend. P values reported are from χ2 tests or Fisher exact tests. No recognized risks for T. gondii transmission were significantly associated with parasite serotype.
Numerators represent no. of mothers with parasite serotype who recognized exposure to risk factor; denominators represent total no. of mothers with parasite serotype. Numbers within parentheses represent the percentage of mothers with parasite serotype who reported exposure to this risk factor.
Prematurity Is More Common in Persons With NE-II Serotype
Prematurity was more common in persons with NE-II serotype (47% vs 30%; P = .03; Table 3). This was the case in each of the two cohorts, ie, infants who were treated or not during their first year of life (Table 3).
Table 3.
Associations of Parasite Serotype With Prematurity, Disease Severity, and Other Manifestations
| Association | II | NE-II | P Value |
|---|---|---|---|
| A. Associations with prematurity (gestational age <38 weeks) | |||
| All | 20/66 (30)a | 54/114 (47) | .03 |
| Rx | 18/52 (35) | 43/92 (47) | .16 |
| No Rx | 2/14 (14) | 11/22 (50) | .04 |
| B. Associations with disease severity at birth and other manifestations for those who were treated during the first year of life | |||
| Severe, S/MN8 | 33/52 (63)b | 77/93 (83) | <.01 |
| Eye severity score ≥2 at birth | 18/46 (39) | 59/88 (67) | <.01 |
| Systemic | |||
| Splenomegaly | 9/51 (18) | 37/91 (41) | <.01 |
| Hepatomegaly | 14/51 (27) | 42/91 (46) | .03 |
| Thrombocytopenia | 18/50 (36) | 39/89 (44) | .37 |
| Anemia | 7/52 (13) | 18/87 (21) | .28 |
| Skin rash | 8/51 (16) | 29/91 (32) | .04 |
| Jaundice | 28/48 (58) | 59/86 (69) | .23 |
| R/O sepsis | 16/52 (31) | 38/93 (41) | .23 |
| Neurologic/ophthalmologic | |||
| Hydrocephalus | 19/52 (37) | 35/93 (38) | .90 |
| Microcephalus | 5/52 (10) | 13/93 (14) | .60 |
| CNS calcifications | 32/52 (62) | 69/93 (74) | .11 |
| Seizures | 8/52 (15) | 12/93 (13) | .68 |
| Chorioretinal scars | 32/52 (62) | 72/93 (77) | .04 |
| Microphthalmia | 10/52 (19) | 13/92 (14) | .42 |
P values reported are from χ2 tests or Fisher exact tests.
Abbreviations: CNS, central nervous system; R/O, rule out sepsis; Rx, persons in the NCCCTS cohort who were diagnosed in the perinatal period and treated during the first year of life [6–18]; No Rx, persons in the NCCCTS cohort who missed being treated in the first year of life and were referred to the NCCCTS after that time [16, 19, 20]; All, persons in the Rx plus No Rx cohorts; S, severe; S/MN, severe/moderate neurologic disease [8]; II and NE-II serotypes are defined in the Methods section and the Figure 1 legend.
Numerators represent no. of persons born at <38 weeks’ gestation with this parasite serotype in the NCCCTS cohort; denominators represent total no. of persons with this parasite serotype in the NCCCTS cohort. Numbers within parentheses represent the percentage of persons with this parasite serotype in the NCCCTS cohort who were born at <38 weeks’ gestation.
Numerators represent no. of persons in the NCCCTS cohort who were treated during the first year of life who had this manifestation at birth and this parasite serotype; denominators represent total no. of persons in the NCCCTS cohort who were treated during the first year of life with this parasite serotype. Numbers within parentheses represent the percentage of persons in the NCCCTS cohort who were treated during the first year of life who had this manifestation and this parasite serotype.
To determine association between prematurity and parasite serotype (II vs NE-II) controlling for socioeconomic status, a Cochran-Mantel-Haenszel stratified test was performed, and the association remained significant (P = .04).
Severe Disease at Birth Is More Common in Persons With NE-II Serotype
Severe disease at birth [8] was more common in infants with NE-II serotype (P < .01; Table 3) as was eye severity at birth (P < .01; Table 3). Systemic manifestations at birth were present more often in infants with NE-II serotype, for example, splenomegaly (P < .01), hepatomegaly (P = .03), and skin rash (P = .04) (Table 3).
To look at association between disease severity and parasite serotype (II vs NE-II) controlling for socioeconomic status in the treated group, a Cochran-Mantel-Haenszel stratified test was performed, and the association trended toward significance (P = .08).
Neurologic and/or ophthalmologic manifestations at birth, other than chorioretinal scars, were not significantly associated with parasite serotype (P > .05; Table 3). Hydrocephalus had no association with parasite serotype (P = .90). Association of chorioretinal scars with NE-II serotype was significant (P = .04).
Parasite Serotype and Treatment During Gestation
Additional analyses examined whether these differences in disease manifestations between parasite serotypes varied based on whether in utero treatment was received (ie, tested for a parasite serotype by treatment interaction) (Table 4). Each manifestation occurred with similar or lower frequency among those with NE-II than among those with II serotypes who had received in utero treatment (although never reaching statistical significance owing to the small number of persons who had received treatment). In contrast, manifestations occurred with greater frequency among those with NE-II compared with II serotypes who had not received in utero treatment and reached statistical significance for all but prematurity and skin rash. However, this differential effect of parasite serotype based on receipt of in utero treatment was significant only for eye severity (P = .04 for interaction), with trends for chorioretinal scars (P = .07) and disease severity (P = .08), owing to limited power for detecting such interactions.
Table 4.
Manifestations at Birth, Treatments, and Outcomes Later in Life
| In Utero [14] Rx | No In Utero Rx | ||||
|---|---|---|---|---|---|
| Manifestation | II | NE-II | II | NE-II | P Value |
| A. Manifestations at birth for groups based on gestational treatment and parasite serotype | |||||
| Gestational age <38 weeks | 3/15 (20)a | 3/13 (23) | 15/37 (41) | 40/79 (51) | .82 |
| Severe, S/MN8 | 8/15 (53) | 6/13 (46) | 25/37 (68) | 71/80 (89) | .08 |
| Eye severity score ≥2 at birth | 6/15 (40) | 4/13 (31) | 12/31 (39) | 55/75 (73) | .04 |
| Splenomegaly | 1/15 (7) | 1/13 (8) | 8/36 (22) | 36/78 (46) | .54 |
| Hepatomegaly | 4/15 (27) | 2/13 (15) | 0/36 (28) | 40/78 (51) | .11 |
| Skin rash | 1/15 (7) | 0/13 (0) | 7/36 (19) | 29/78 (37) | Not estimableb |
| Chorioretinal scars | 8/15 (53) | 5/13 (38) | 24/37 (65) | 67/80 (84) | .07 |
| B. Outcomes later in life based on parasite serotype and treatment group | |||||
| II | NE-II | P Value | |||
| A | C | A | C | ||
| Vision <20/30 | 10/19 (53)c | 5/10 (50) | 19/27 (70) | 28/31 (90) | .17 |
| New eye lesions | 5/17 (29) | 4/7 (57) | 9/24 (38) | 19/27 (70) | .85 |
| New eye lesions–central only | 2/17 (12) | 2/7 (29) | 6/24 (25) | 7/27 (26) | .42 |
| Motor/tone abnormality | 3/19 (16) | 2/10 (20) | 6/27 (22) | 3/31 (10) | .32 |
| IQ <70 | 2/19 (11) | 4/13 (31) | 10/30 (33) | 8/37 (22) | .08 |
| Decrease in IQ ≥15 | 3/19 (16) | 2/10 (20) | 6/27 (22) | 6/31 (19) | .70 |
| Hearing loss >30 db | There was no hearing loss in any group. | ||||
A. P values reported are based on a test of the in utero treatment group by parasite serotype interaction from a logistic regression model. A statistically significant interaction would indicate that the effect of parasite serotype on disease manifestations depends on whether in utero treatment was received. B. Treatment group A, treatment of daily pyrimethamine and sulfadiazine for 2 months, followed by pyrimethamine on Monday, Wednesday, and Friday, and continued daily sulfadiazine for the remainder of the year of therapy. Treatment group C, treatment with daily pyrimethamine and sulfadiazine for 6 months, followed by pyrimethamine on Monday, Wednesday, and Friday, and continued daily sulfadiazine for the remainder of the year. P values reported are based on a test of the treatment group by parasite serotype interaction from a logistic regression model. A statistically significant interaction would indicate that the effect of treatment on outcome depends on parasite serotype. These data are for both randomized and feasibility phases combined and include severely and mildly involved persons. The endpoints were determined at the following ages: vision <20/30, motor/tone abnormality, decrease in IQ ≥15, hearing loss > 30 db, ≥5 years; new eye lesions: central + peripheral, new eye lesions: central only, ≥7.5 years; IQ <70, ≥3.5 years. Manifestations at birth that differed significantly based on parasite serotype without in utero treatment did not differ for those who were treated in utero. Neither parasite serotype or treatment group was associated with different outcomes later in life.
Abbreviations: S, severe; S/MN, severe/moderate neurologic disease [8].The II and NE-II serotypes are defined in the Methods section and the Figure 1 legend.
Numerators represent no. of persons in the NCCCTS cohort with this parasite serotype and in utero [14] treatment status with manifestation at birth; denominators represent total no. of persons in the NCCCTS cohort with this parasite serotype and in utero [14] treatment status. Numbers within parentheses represent the percentage of persons in the NCCCTS cohort with manifestation at birth among those with this parasite serotype and in utero [14] treatment status.
Not estimable due to zero cells.
Numerators represent no. of persons in the NCCCTS treatment cohort with endpoint who have this parasite serotype; denominators represent total no. of persons in the NCCCTS treatment cohort who have this parasite serotype. Numbers within parentheses represent the percentage of persons in the NCCCTS cohort of treated persons who had this parasite serotype with this outcome.
Incidence of Outcome Endpoints Later in Life Is Similar Across Parasite Serotypes
Table 4 provides results of tests showing whether parasite serotype led to a differential effect of treatment during the first year of life (ie, a test of parasite serotype by treatment interaction) regardless of disease severity. There was no strong evidence to suggest such an effect. As has been described elsewhere [11], there were no significant differences between higher (C) and lower (A) dose groups except for new eye lesions (central plus peripheral) where there was a higher rate in C compared with A. Thus, the 2 treatment groups were combined and vision <20/30 (P = .36), new eye lesions (P = .43), new central eye lesions (P = .52), motor/tone abnormality (P = .49), IQ <70 (P = .99), decrease in IQ >15 (P = 1.00), and hearing loss >30 db were not significantly associated with parasite serotype among those with severe disease at birth. Importantly, endpoints later in life were less prevalent in NCCCTS patients with severe illness and treatment during the first year of life, regardless of parasite serotype, compared with those in the literature [27] with severe disease except for vision <20/30, which was more prevalent in NCCCTS patients (Figure 4 and Table 5). This greater severity of eye disease was noted at birth [11, 16], reflecting magnitude of initial disease burden in NCCCTS children.
Figure 4.

Parasite serotype and outcomes later in life in our cohort and in literature data. Rx indicates persons in the National Collaborative Chicago-based Congenital Toxoplasmosis Study cohort who were diagnosed in the perinatal period and treated during the first year of life [6–18]; No Rx, literature [27]. The II and NE-II serotypes are defined in the Methods and the Figure 1 legend.
Table 5.
Associations of Parasite Serotype With Outcomes Later in Life Compared to Literature Data
| II | NE-II | Literature [27] | P Value | |||
|---|---|---|---|---|---|---|
| Rx - Severea | Rx - Severe | No Rxb | II vs NE-II | II vs Literature | NE-II vs Literature | |
| Vision <20/30 | 14/17 (82) | 45/49 (92) | 60/101 (59) | .36 | .10 | <.01 |
| New eye lesions | 8/16 (50) | 27/44 (61) | 90/101 (89) | .56 | <.01 | <.01 |
| New eye lesions–central only | 3/16 (19) | 13/44 (30) | NAc | .52 | Not estimabled | Not estimabled |
| Motor/tone abnormality | 5/17 (29) | 9/49 (18) | 70/101 (69) | .49 | <.01 | <.01 |
| IQ <70 | 6/20 (30) | 17/57 (30) | 86/101 (85) | 1.00 | <.01 | <.01 |
| Decrease in IQ ≥15 | 4/17 (24) | 12/49 (24) | NAc | 1.00 | Not estimabled | Not estimabled |
| Hearing loss >30 db | 0/17 (0) | 0/49 (0) | 14/101 (14) | Not estimable | .22 | <.01 |
Rx indicates persons in the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) cohort who were diagnosed in the perinatal period and treated during the first year of life [6–18]; No Rx, persons in the NCCCTS cohort who missed being treated in the first year of life and were referred to the NCCCTS after that time; All, persons in the Rx plus No Rx cohorts. The II and NE-II serotypes are defined in the Methods section and the Figure 1 legend. P values were computed using Fisher exact test.
Numerators represent no. of persons with this parasite serotype who had all of the following: (1) severe disease at birth [8]; (2) treatment (Rx) during the first year of life in the NCCCTS cohort; and (3) this endpoint later in life; denominators represent no. of persons in the NCCCTS cohort who have this parasite serotype with (1) severe disease at birth and (2) treatment during the first year of life. Nos. within parentheses represent percentage of persons with this parasite serotype, severe disease at birth, and treatment during the first year of life in the NCCCTS cohort who had this endpoint later in life.
Numerators represent no. of persons with endpoint later in life in the Eichenwald study [27]; denominators represent total no. of persons in the Eichenwald study. Nos. within parentheses represent percentage of persons in the Eichenwald study with endpoint later in life.
Data not available.
Not estimable due to 0 cells or data not available.
NE-II Subgroup Analyses
Because it has recently become apparent that there is more global diversity than II and NE-II parasites, and we had noted different relative magnitudes of reactivity to II and I/III GRA6/7 alleles among persons with NE-II serotype, we also performed NE-II subgroup analyses. This was to address whether any subgroups of the NE-II category were responsible for differences noted between II and NE-II serotype associations or whether any new associations became apparent. This analysis assessed whether there were significant differences in manifestations between I/III, I/IIIa, and II = I/III serotypes. Because of the very small number of IIa serotype persons, this subgroup was excluded. Differences found between II and NE-II serotypes were not associated with any particular NE-II subgroup (P > .05). Of note, seizures were most frequent in the II = I/III subgroup (P < .01), and thrombocytopenia was most frequent in the I/IIIa subgroup (P = .01).
Parasite Genotypes and Associated Serologic Phenotypes
To begin to correlate parasite genotypes with serologic phenotypes, we compared our currently available multilocus genotyping of 2 isolates from persons in the NCCCTS cohort with their serotype patterns determined by our ELISA. “JGM,” from a person infected in the Victoria outbreak, had I/III alleles at GRA6/7 and elicited a I/IIIa pattern in mother and child sera. “Ray,” a type X (HG12) strain isolated from an infant with severe congenital infection in Texas, produced a II = I/III serotype in mother and infant. “Ray” and another type X isolate, “Ari” from an immunocompromised patient, also had induced a II = I/III serotype in mice.
DISCUSSION
There are polymorphic allelic variants of T. gondii in North American participants in the NCCCTS, and this has not changed during the last 3 decades. Associations of parasite alleles with prematurity and severity of congenital toxoplasmosis affect lifelong outcomes, including severe brain and eye disease. As in France, where type II parasites clearly have caused severe as well as milder symptoms [23], we also find infants with mild, moderate, and severe disease with all parasite serotypes, in all decades. Serotype is neither necessary nor sufficient to predict outcomes at birth. Outcome endpoints in our study [11] do not differ for treated persons with differing parasite serotypes. The percentage of persons who have antibody to each of these type specific alleles is the same in the untreated and treated cohorts.
Because correlations we have found are not absolute, other factors must be influencing associations besides solely parasite GRA6 and GRA7 alleles. Other secreted protein alleles almost certainly are important. For instance, the strain-specific influence of secreted proteins on host cells varies; for example, GRA 15 (II, NF-κB) [28], ROP 5s (I, II, III, virulence) [29], ROP 16 (STAT3 and 6) [30] and ROP 18 (I, GTPases) [31] all indicate that other Toxoplasma alleles might influence outcomes of congenital toxoplasmosis and suggest mechanisms whereby this may occur. Sixteen gene regions, distinct between recombinant parasites, influence virulence in mice [32]. Future characterization of associations with other parasite alleles will be of interest.
Our finding of an association of parasite serotype with COL2A and ABCA4 alleles and our other work defining a number of host susceptibility and resistance genes both in humans [12, 15, 33–36] and in animal models [37] indicate that host genetics provide additional and more complex interactions. Earlier we found that susceptibility alleles for COL2A and ABCA4 were modified in an imprinted manner. Herein we note that NE-II serotype is less frequent in those with susceptibility alleles, raising a question of whether NE-II serotype, which is associated with increased prematurity and severity, might cause fetal loss if susceptibility alleles are present. This association emphasizes importance of interactions of host genetics and parasite genetics specifying interacting proteins.
In contrast to homogeneous distribution of type II parasites in France [23] versus polymorphic parasites in Brazil [25] and hypervirulent parasites in French Guiana [37], there is considerable heterogeneity of T. gondii in North America. The NE-II serotype was common among persons residing in hot, humid climates or rural locales, and those of lower socioeconomic status and Hispanic ethnicity. Warm, moist soil could allow survival and longer persistence of oocysts, perhaps providing opportunity for genetic crosses. Humidity was reported to increase viability of T. gondii oocysts in water and soil [38]. NE-II serotype is predominant in birth months of November to January, perhaps due to exposure to soil and oocysts in warmer climates during latter months of gestation, when transmission to the fetus is greatest.
Current serotyping methods, although giving robust results herein, do have limitations. Recent population genetic analyses have identified 15 parasite lineages, including type X lineage (haplogroup 12, which possesses unique GRA6/7 alleles) common in North American wildlife [39] (additional references in Supplementary Data). It is unclear what percentage of human toxoplasmosis is caused by type X. Two human US type X isolates (“Ari” and “Ray”) elicited II = I/III serotypes in mice (reactive to 6-I/III and 6-II but not 7-II peptides) [39]. Persons infected with “Ray” in our cohort had II = I/III serotypes, suggesting that our assay may correctly identify type X. There were 33 serotype II = I/III persons of 183 total (18%) in the NCCCTS. Future studies with additional markers using parasite isolates linked to serotyping as performed herein for “Ray” may help deconvolute complex global diversity of infections in humans. Sousa et al [40] identified variation in GRA6/7 alleles in multiple global isolates. This diversity and currently available reagents limit our detection of substantial global variations in GRA6/7 alleles [40]; hence, peptides may need to be developed that are locale-specific. Associations also could be due to factors that influence acquisition earlier in gestation when fetal infection is most severe. For example, NE-II parasites might be preferentially transmitted earlier. Nonetheless, associations of serotypes identified herein and phenotypes are remarkable. This well-characterized NCCCTS cohort will be valuable for future studies as additional typing reagents and approaches become available.
Parasite genetics (alleles) are associated with manifestations of congenital toxoplasmosis in persons in North America. NE-II serotypes are more often present than serotype II in infants born prematurely or with severe disease, and in certain demographics. Outcomes following treatment were independent of parasite allelic type and emphasize the need to identify all patients, as they will benefit from treatment.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We gratefully acknowledge the families who generously participated in this study; Joseph McCammon for his assistance with this manuscript; and J. S. Remington and the Palo Alto Medical Research Foundation for performing the serologic testing to diagnose NCCCTS patients, for advice and guidance with the NCCCTS work throughout the past 3 decades, and for performing preliminary studies together with Rima McLeod, which provided a conceptual foundation for the present work.
Other members of the Toxoplasmosis Study Group include Dianna Bardo, Delilah Burrowes, Audrey Cameron, Ellen Holfels, Paul Latkany, Douglas Mack, John Marcinak, James McAuley, Marilyn Mets, Sanford Meyers, William Mieler, Dushyant Patel, Jeanne Perkins, James Rago, Nancy Roizen, Lazlo Stein, Andrew Suth, Marie Weissbourd, Teri Hull, Kathy Zebracki, and Caitlin Roache.
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant number R01AI027530 to R. M.), the Research to Prevent Blindness Foundation, The Stanley Foundation and Medical Research Institute (grant number 07R-1890 to R. M.), the Intramural Research Program of the National Institutes of Health and NIAID (to M. E. G.); and gifts from the Blackmon, Brennan, Cornwell, Cussen, Dougiello, Jackson, Kapnick, Kiewiet, Koshland, Langel, Lipskar, Mann, Morel, Rooney-Alden, Rosenstein, Samuel, and Taub families.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254–6. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- 2.Denkers EY, Butcher BA, Del Rio L, Bennouna S. Neutrophils, dendritic cells, and Toxoplasma. Int J Parasitol. 2004;34:411–21. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis. 2001;184:633–9. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- 4.Xiao J, Jones-Bando L, Talbot CC, Jr, Yolken RH. Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infect Immun. 2011;79:1363–73. doi: 10.1128/IAI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong JT, Grigg ME, Uyetake L, Parmley S, Boothroyd JC. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis. 2003;187:1484–95. doi: 10.1086/374647. [DOI] [PubMed] [Google Scholar]
- 6.Boyer KM, Holfels E, Roizen N, et al. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. Am J Obstet Gynecol. 2005;192:564–71. doi: 10.1016/j.ajog.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 7.McLeod R, Mack D, Foss R, et al. Levels of pyrimethamine in sera and cerebrospinal and ventricular fluids from infants treated for congenital toxoplasmosis. Antimicrob Agents Chemother. 1992;36:1040–8. doi: 10.1128/aac.36.5.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McAuley J, Boyer KM, Patel D, et al. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 9.Swisher CN, Boyer KM, McLeod R. Congenital toxoplasmosis. The Toxoplasmosis Study Group. Sem Ped Neurol. 1994;1:4–25. [PubMed] [Google Scholar]
- 10.Patel DV, Holfels EM, Vogel NP, et al. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology. 1996;199:433–40. doi: 10.1148/radiology.199.2.8668790. [DOI] [PubMed] [Google Scholar]
- 11.McLeod R, Boyer KM, Karrison T, et al. Outcomes of treatment of congenital toxoplasmosis, 1981–2004, the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) Clin Infect Dis. 2006;42:1383–94. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson SE, de Roubaix LA, Cortina-Borja M, et al. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS One. 2008;3:e2285. doi: 10.1371/journal.pone.0002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in treated congenital toxoplasmosis. Ophthalmology. 2008;115:553–9. doi: 10.1016/j.ophtha.2007.06.022. e8.84. [DOI] [PubMed] [Google Scholar]
- 14.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem Inst Oswaldo Cruz. 2009;104:320–44. doi: 10.1590/s0074-02762009000200029. ISSN 0074–0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson SE, Cordell H, Petersen E, McLeod R, Gilbert RE, Blackwell JM. Host genetic and epigenetic factors in toxoplasmosis. Mem Inst Oswaldo Cruz. 2009;104:162–9. doi: 10.1590/s0074-02762009000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mets M, Holfels E, Boyer KM, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1996;122:309–24. doi: 10.1016/s0002-9394(14)72057-4. [DOI] [PubMed] [Google Scholar]
- 17.Roizen N, Kasza K, Karrison T, et al. Impact of visual impairment on measures of cognitive function for children with congenital toxoplasmosis: implications for compensatory intervention strategies. Pediatrics. 2006;118:e379–90. doi: 10.1542/peds.2005-1530. [DOI] [PubMed] [Google Scholar]
- 18.Remington JS, McLeod R, Thulliez P, Wilson C, Desmonts G. Congenital toxoplasmosis. In: Remington JS, Klein J, Wilson C, Maldonado B, editors. Infectious diseases of the fetus and newborn infant. 7th ed. Philadelphia: Elsevier; 2010. [Google Scholar]
- 19.Benevento J, Jager R, Noble AG, et al. Toxoplasmosis associated neovascular lesions treated successfully with ranibizumab and antiparasitic therapy. Arch Ophthalmol. 2008;126:1152–5. doi: 10.1001/archopht.126.8.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan L, Kasza K, Jalbrzikowski J, et al. Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol. 2008;146:375–84. doi: 10.1016/j.ajo.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. . Unpublished manuscript. [Google Scholar]
- 22.StataCorp. Stata statistical software: release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 23.Ajzenberg D, Cogné N, Paris L, et al. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186:684–9. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- 24.Peyron F, Lobry JR, Musset K, et al. Serotyping of Toxoplasma gondii in chronically infected pregnant women: predominance of type II in Europe and types I and III in Colombia (South America) Microbes Infect. 2006;8:2333–40. doi: 10.1016/j.micinf.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira Ade M, Vitor RW, Gazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol. 2006;6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Bowie WR, King AS, Werker DH, et al. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet. 1997;350:173–7. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- 27.Eichenwald HF. A study of congenital toxoplasmosis, with particular emphasis on clinical manifestations, sequelae, and therapy. In: Siim JC, editor. Human toxoplasmosis. Copenhagen: Munksgaard; 1960. pp. 41–9. [Google Scholar]
- 28.Rosowki EE, Lu D, Julien L, et al. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J Exp Med. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci U S A. 2011;108:9625–30. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeij JP, Coller S, Boyle JP, et al. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–7. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fentress SJ, Behnke MS, Dunay IR, et al. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe. 2010;8:484–95. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle JP, JSaeij JP, Harada SY, Ajioka JW, Boothroyd JC. Expression quantitative trait locus mapping of Toxoplasma genes reveals multiple mechanisms for strain-specific differences in gene expression. Eukaryot Cell. 2008;7:1403–14. doi: 10.1128/EC.00073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mack D, Johnson J, Roberts F, et al. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol. 1999;29:1351–8. doi: 10.1016/s0020-7519(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 34.Witola WH, Mui E, Hargrave A, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79:756–66. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, et al. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–83. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lees MP, Fuller SJ, McLeod R, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040–6. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carme B, Demar M, Ajzenberg D, Dardé ML. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg Infect Dis. 2009;15:656–8. doi: 10.3201/eid1504.081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rifaat MA, Arafa MS, Sadek MS, et al. Toxoplasma infection of stray cats in Egypt. J Trop Med Hyg. 1976;79:67–70. [PubMed] [Google Scholar]
- 39.Grigg ME. Population genetics, sex, and the emergence of clonal lines of Toxoplasma gondii. In: Ajioka JW, Soldati D, editors. Toxoplasma—molecular and cellular biology. Philadelphia: Taylor & Francis; 2007. pp. 227–40. [Google Scholar]
- 40.Sousa S, Ajzenberg D, Marle M, et al. Selection of polymorphic peptides from GRA6 and GRA7 sequences of Toxoplasma gondii strains to be used in serotyping. Clin Vaccine Immunol. 2009;16:1158–69. doi: 10.1128/CVI.00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.