Abstract
Objective
To determine if plasma levels of follistatin-like protein 1 (FSTL-1), a pro-inflammatory protein produced by mesenchymal tissue, including cardiac myocytes, correlate with the development of Kawasaki disease (KD) and coronary artery aneurysms (CAA).
Study design
FSTL-1 plasma levels were measured serially by ELISA in 48 patients with KD at time of diagnosis and, when available, 2 weeks, 6 weeks and 6 months following onset of disease. These were compared with 23 controls. Data were analyzed using Generalized Estimating Equations.
Results
Plasma FSTL-1 levels were elevated in patients with acute KD as compared with controls (p=0.0086). FSTL-1 levels remained significantly elevated at 2 weeks after disease onset but returned to control levels by 6 months. Seven patients with CAA had significantly higher FSTL-1 levels at the time of diagnosis than those who did not develop aneurysms (p=0.0018). Sensitivity and specificity for CAA at a specific FSTL-1 cutpoint (178ng/ml) was 85% and 71%.
Conclusions
Plasma levels of FSTL-1 are elevated in acute KD and may predict cardiac morbidity in this disease. These results suggest a possible role for FSTL-1 in the formation of coronary artery aneurysms.
Kawasaki disease (KD) is the major cause of acquired coronary artery aneurysms (CAA) in childhood [2]. The etiology of KD remains unknown. It is believed that a possible undefined infectious agent triggers systemic inflammation and vasculitis in predisposed individuals[3, 4]. The incidence of CAA has decreased due to treatment with IVIG, however up to 5% of treated patients still develop aneurysms, as compared with up to 25% of untreated patients[6–9]. Follistatin-like protein 1 (FSTL-1) is an extracellular glycoprotein which was originally cloned from an osteoblast cell line as a TGF-β inducible gene[12]. FSTL-1 is highly conserved across mammalian species. FSTL-1 is secreted by cells of the mesenchymal lineage, including cardiac myocytes, and suppression of FSTL-1 expression by siRNA treatment leads to increased cardiomyocyte apoptosis[13]. FSTL-1 has also been shown to have a role in promoting revascularization of skeletal muscle after ischemic injury[14]. FSTL-1 expression has been found to be elevated in patients with heart failure but returns to normal levels upon recovery[15].
We have previously shown that FSTL-1 can have proinflammatory effects. Overexpression of FSTL-1 leads to exacerbation of arthritis in mice while its neutralization ameliorates arthritis[16]. Furthermore, overexpression of FSTL-1 in monocytes leads to upregulation of IL-1β, IL-6, and TNF-α[17]. The current study was designed to determine if plasma levels of FSTL-1 correlate with the development of KD and development of CAA.
METHODS
Banked frozen plasma samples were obtained from patients with typical KD whose diagnosis was made through established clinical criteria[19]. These samples were obtained through the pediatric cardiology clinics and inpatient services at Cincinnati Children’s Hospital Medical Center and from Children’s Memorial Hospital in Chicago. Serial samples from 48 individual patients were obtained, when available, at acute presentation (prior to IVIG), at 2 weeks, 6 weeks, and 6 months following presentation. One of these patients developed CAA. Six patients in this group did not have an acute sample but had samples collected at later time points. Six additional acute samples from patients with KD who were known to have subsequently developed CAA were obtained from Children’s Memorial Hospital. In total, plasma samples from 54 patients with KD were analyzed. Coronary artery aneurysms were identified by echocardiogram according to the American Heart Association guidelines of Dajani et al. There was a greater proportion of Hispanic and Asian patients in the aneurysm group as compared with the other groups. However, there was no significant inter-ethnic difference in FSTL-1 concentration within the aneurysm group itself. Control plasma samples were obtained from 23 children of similar age and sex with no history of inflammatory disease who underwent surgical procedures including hernia repair, tonsillectomy and adenoidectomy, and simple ophthalmologic procedures. One additional control sample was excluded from the analysis because its FSTL-1 value measured above the limit of detection. Assigning a random very high value to this sample did not alter the statistical significance of the results and, because a value could not accurately be assigned to it, this sample was excluded from the analysis. Use of samples was approved by the Institutional Review Board at the University of Pittsburgh. FSTL-1 ELISA assays were performed at the Children’s Hospital of Pittsburgh.
For detection of human FSTL-1 in plasma, Nunc Immunomodule MaxiSorp F8 Framed ELISA plates were coated with 5ug/ml polyclonal anti-FSTL1 (AF1694; R&D Systems, Minneapolis, MN) in phosphate buffered saline (PBS) and incubated at 4° C overnight. Plates were then washed with PBS/0.05%Tween 20 and blocked for one hour with bovine serum albumin (BSA) buffer (1% BSA and 5% sucrose in PBS). Plates were washed again, and human plasma samples diluted 1:10 in Serum Dilution Buffer (20mM Tris, 150mM NaCL 0.1 % BSA, 0.05% Tween 20: pH 7.3) were added and incubated for one hour. After washing, 2.5µg/ml biotinylated monoclonal anti-FSTL1 (MAB1694; R&D systems) was added for 1 hour. Plates were washed again and incubated with Streptavidin-HRP conjugate at 0.25µg/ml for 20 minutes. BD OptEIA TMB Substrate Reagent was added, and plates were incubated for an additional hour, following which development was stopped with addition of 1M H2SO4. Plate absorbance was read on a microplate reader with dual measurement of 450nm and 570nm reference level. A titration of purified FSTL-1 was used to generate a standard curve from which plasma concentration of samples was calculated.
Statistical Analysis
Statistical analysis was performed using STATA version 11. A series of comparisons between study groups were made using t-tests and Generalized Estimating Equations (GEE) to account for the repeated measures for comparisons of FSTL-1 levels over time. Among KD patients, n=48, excluding the 6 patients specifically selected for aneurysm (to avoid statistical oversampling), mean FSTL-1 levels were compared over acute, 2 weeks, 6 weeks and 6 month time points, using GEE with an independent correlation structure and robust variance estimates. Mean baseline FSTL-1 levels were then compared between controls, KD patients without aneurysms who had samples available at the acute time point (n=41) and KD patients with aneurysms (n=7). All reported p-values are 2-sided and considered significant at p < 0.05, and are corrected for multiple comparisons using the Bonferroni method. The two KD patient groups were combined in order to calculate sensitivity and specificity for aneurysm development by FSTL-1 concentration cutpoint, and a Receiver Operating Curve (ROC) was constructed.
RESULTS
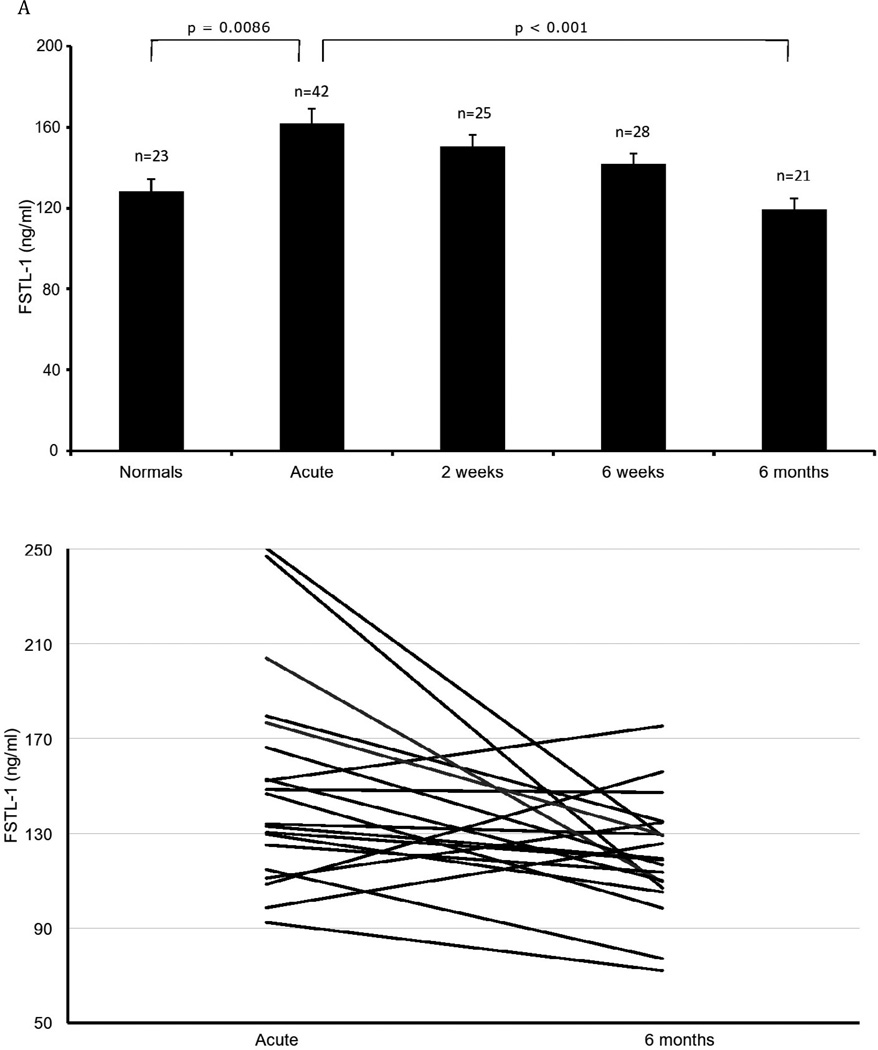
Patient demographics are summarized in the Table. As shown in Figure 1, the mean FSTL-1 level in acute KD was significantly elevated compared to controls (161.7 ± SEM 7.0 ng/ml vs. 128.3 ± SEM 6.1 ng/ml, p = 0.0086). Mean FSTL-1 level was still significantly elevated at 2 weeks (150.3 ± SEM 5.6 ng/ml, p=0.0424) but by 6 weeks was no longer significantly different from controls (141.9 ± SEM 5.2 ng/ml). Mean FSTL-1 levels declined further to 119.6 ± 5.2 ng/ml at 6 months, which was not significantly different from normal controls, but was significantly different from the acute time point (p <0.0001). Using a paired t-test, comparison of patients with both an initial (acute) time point and a recovery (6 month post presentation) time point was statistically significant, p=0.012. In patients with KD without aneurysms, there was a positive correlation between FSTL-1 level and height of fever (p = 0.02).
TABLE 1.
Baseline Patient Characteristics
| Patient Characteristic | KD without aneurysm (n=47) |
KD with aneurysm (n=7) |
Control (n=23) |
|---|---|---|---|
| Age, months* (SD) | 42.95 (32.7) | 41.7 (51.3) | 43.22 (25.2) |
| Sex† | |||
| Male | 29 (64%) | 5 | 17 (74%) |
| Female | 17 (36%) | 2 | 6 (26%) |
| Unknown | 1 (2%) | - | - |
| Race‡ | |||
| White | 26 | 2 | 17 |
| Black | 17 | 1 | 6 |
| Other/unknown | 4 (1 Asian, 1 Hispanic, 2 unknown) |
4 (1 Asian, 3 Hispanic) |
- |
p value age: 0.9943 (Test of heterogeneity), age missing for 3 patients in KD without aneurysm.
p value sex: 0.7065 (Exact test)
p value race: 0.0035 (Exact test)
FIGURE 1.
FSTL-1 plasma levels in KD. Each bar represents the mean ± SEM. n=number of patients.
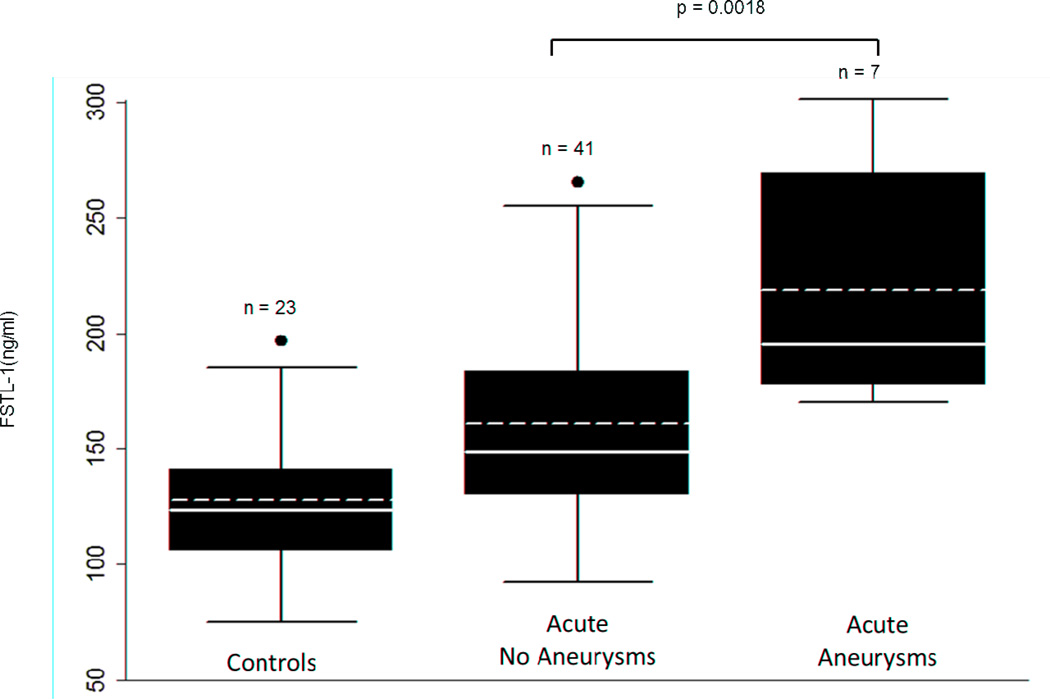
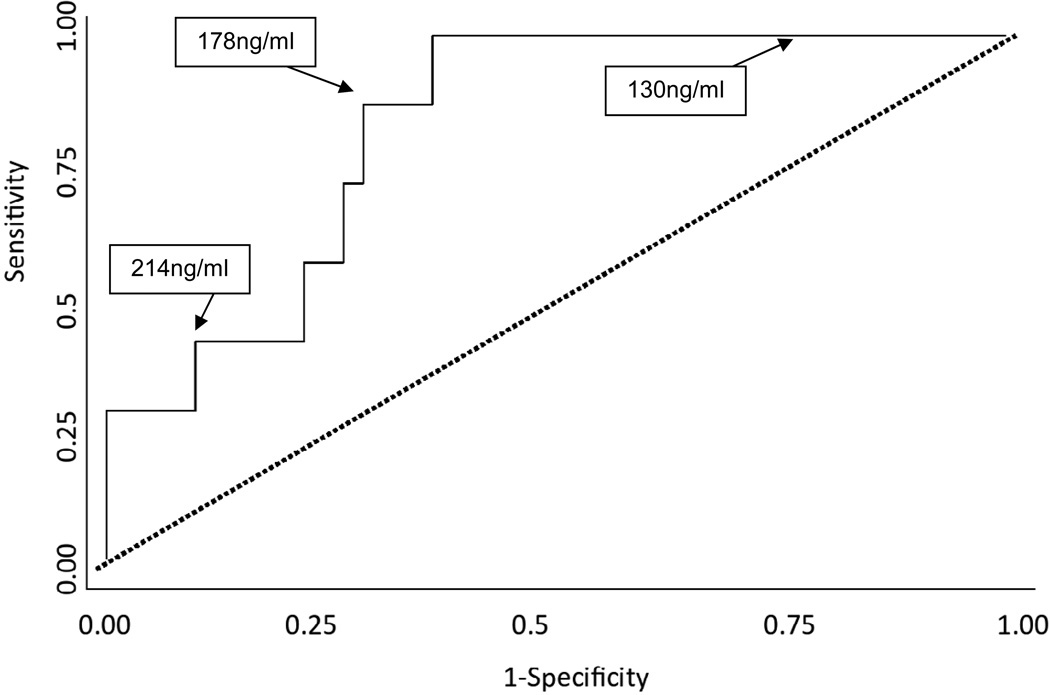
As shown in Figure 2, the mean FSTL-1 level in patients who developed aneurysms was significantly higher than in patients who did not (219.2 ± SEM 19.4 ng/ml versus 161.2 ± SEM 7.2 ng/ml; p = 0.0018). Patients who developed CAA had significantly higher levels of FSTL-1 at presentation than did patients without CAA at all stages of disease. FSTL-1 level had a high sensitivity and specificity as a biomarker for aneurysm development. The ROC analysis for FSTL-1 levels at non-aneurysm and aneurysm status had an area under the curve of 0.8223, (95% CI 0.6863, 0.9583) (Figure 3). Using the ROC, a threshold of 178 ng/ml yielded a sensitivity of 85%, with specificity of 71%.
FIGURE 2.
Acute FSTL-1 plasma levels in patients with and without CA and controls. Solid line = median FSTL-1 level, dashed line = mean FSTL-1 level. n=number of patients.
FIGURE 3.
Receiver Operator Curve analysis shows an area under the curve of 0.8223, (95% CI 0.6863, 0.9583). Sensitivity of 85% and Specificity of 71% corresponded to 178 ng/ml.
DISCUSSION
Development of CAA is the major cause of morbidity and mortality associated with KD. Efforts to define clinical or serological risk factors for the development of CAA have been described over the last 30 years. These risk factors have included non-coronary cardiac abnormalities[20],[21], incomplete clinical presentation at very young age, higher body temperature during certain phases of disease[22], and resistance to IVIG therapy at older ages[23, 24]. Clinical scoring mechanisms to predict development of CAA have been developed that utilize neutrophil and band counts, hemoglobin concentrations, platelet counts, and temperature on the day after infusion of IVIG[25]. Lab markers such as high white blood cell count, thrombocytopenia, anemia, C-reactive protein levels, age of presentation and male sex have been identified as risk factors for development of aneurysm formation[25, 26].
Lin et al investigated other serologic markers for development of CAA including IL-6, TNF-α and soluble IL-2 receptor[27]. This evaluation included multiple monthly blood samples and showed that elevated levels of IL-6 and IL-8 in the first week of illness could predict development of CAA. The findings reported here indicate that elevated FSTL-1 levels correlate with increased risk of development of CAA in KD, suggesting that a simple blood test at the time of diagnosis might be highly sensitive and specific in identifying patients at high risk.
Limitations to our study include the relatively small numbers of patients in the CAA group, as well as the overrepresentation of Hispanic patients in this group. There were no Asians or Hispanics in the control population. Additionally, all of our patients met the criteria for complete KD, and we cannot generalize the findings to patients with atypical or incomplete KD. Additional prospective studies with a larger numbers of patients with CAA would provide further validation of our findings.
Despite these limitations, other studies on FSTL-1 and cardiac disease suggest a rationale for our conclusions. FSTL-1 expression has been shown to be increased in the heart in the setting of cardiac injury and may play a protective role against hypoxic injury to myocytes[13]. FSTL-1 was also found to be elevated in patients with heart failure, and FSTL-1 staining was seen in myocytes and vascular endothelial cells of capillaries, small vessels, and smooth muscle cells of larger vessels in these patients[15].
Although the mechanism by which FSTL-1 might promote CAA is speculative, possible effects on endothelial nitric oxide synthase (eNOS) expression are worth consideration. Alterations in eNOS expression have been found to be associated with aneurysm formation both in mouse and human models of aneurysmal disease. Aged eNOS knockout mice have decreased aneurysm formation in comparison to wild type mice[28] suggesting that eNOS activity contributes to aneurysm formation. Polymorphisms in the eNOS gene have been shown to predispose humans to develop abdominal aortic aneurysms[29], and alterations in nitric oxide production has been shown to cause development of cerebral aneurysms in rats[30]. Finally, histopathologic analysis of coronary artery aneurysms in KD demonstrates decreased eNOS staining, among other features of coronary artery senescence[31]. FSTL-1 overexpression was recently shown to improve the revascularization of ischemic limbs in wild type mice, to enhance endothelial cell differentiation and migration, and to lead to phosphorylation and activation of eNOS[14]. Furthermore, FSTL-1 overexpression did not lead to revascularization in mice deficient in eNOS. These actions of FSTL-1 on vascular endothelium through the Akt-eNOS signaling pathway thus suggests a mechanism by which elevated expression of FSTL-1 might contribute to aneurysm formation in KD.
Our findings suggest that high levels of FSTL-1, which is produced by cardiac myocytes, might be a biomarker for development of CAA in KD.
Acknowledgments
Supported by National Institutes of Health (grants RO1 AI073556, R01 AR056959, T32 AR052282 (Hirsch) and the Children’s Hospital of Pittsburgh of UPMC. A patent on FSTL-1 as a therapeutic target in inflammatory conditions listing R.H. and D. W. as inventors has been filed by the University of Pittsburgh.
List of abbreviations
- FSTL-1
Follistatin like protein 1
- KD
Kawasaki Disease
- CAA
Coronary artery aneurysms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991;119:279–282. doi: 10.1016/s0022-3476(05)80742-5. [DOI] [PubMed] [Google Scholar]
- 3.Galeotti C, Bayry J, Kone-Paut I, Kaveri SV. Kawasaki disease: aetiopathogenesis and therapeutic utility of intravenous immunoglobulin. Autoimmun Rev. 9:441–448. doi: 10.1016/j.autrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowley AH, Shulman ST. New developments in the search for the etiologic agent of Kawasaki disease. Curr Opin Pediatr. 2007;19:71–74. doi: 10.1097/MOP.0b013e328012720f. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2007–2008 nationwide survey. J Epidemiol. 20:302–307. doi: 10.2188/jea.JE20090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 7.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 8.Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888–893. doi: 10.1016/s0022-3476(97)70038-6. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 10.Gordon JB, Kahn AM, Burns JC. When children with kawasaki disease grow up myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–1920. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negoro N, Nariyama J, Nakagawa A, Katayama H, Okabe T, Hazui H, et al. Successful catheter interventional therapy for acute coronary syndrome secondary to kawasaki disease in young adults. Circ J. 2003;67:362–365. doi: 10.1253/circj.67.362. [DOI] [PubMed] [Google Scholar]
- 12.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem. 1993;217:13–19. doi: 10.1111/j.1432-1033.1993.tb18212.x. [DOI] [PubMed] [Google Scholar]
- 13.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, et al. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–5827. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 16.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, et al. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–4762. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 17.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234–239. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 62:2510–2516. doi: 10.1002/art.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335–336. doi: 10.1161/01.cir.103.2.335. [DOI] [PubMed] [Google Scholar]
- 20.Gidding SS, Duffy CE, Pajcic S, Berdusis K, Shulman ST. Usefulness of echocardiographic evidence of pericardial effusion and mitral regurgitation during the acute stage in predicting development of coronary arterial aneurysms in the late stage of Kawasaki disease. The American journal of cardiology. 1987;60:76–79. doi: 10.1016/0002-9149(87)90988-x. [DOI] [PubMed] [Google Scholar]
- 21.Printz BF, Sleeper LA, Newburger JW, Minich LL, Bradley T, Cohen MS, et al. Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory inflammatory markers in acute Kawasaki disease. J Am Coll Cardiol. 57:86–92. doi: 10.1016/j.jacc.2010.08.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr. 1986;108:388–392. doi: 10.1016/s0022-3476(86)80878-2. [DOI] [PubMed] [Google Scholar]
- 23.Song D, Yeo Y, Ha K, Jang G, Lee J, Lee K, et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr. 2009;168:1315–1321. doi: 10.1007/s00431-009-0925-0. [DOI] [PubMed] [Google Scholar]
- 24.Yeo Y, Kim T, Ha K, Jang G, Lee J, Lee K, et al. Incomplete Kawasaki disease in patients younger than 1 year of age: a possible inherent risk factor. Eur J Pediatr. 2009;168:157–162. doi: 10.1007/s00431-008-0722-1. [DOI] [PubMed] [Google Scholar]
- 25.Beiser AS, Takahashi M, Baker AL, Sundel RP, Newburger JW. A predictive instrument for coronary artery aneurysms in Kawasaki disease. US Multicenter Kawasaki Disease Study Group. Am J Cardiol. 1998;81:1116–1120. doi: 10.1016/s0002-9149(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 26.Nakano H, Ueda K, Saito A, Tsuchitani Y, Kawamori J, Miyake T, et al. Scoring method for identifying patients with Kawasaki disease at high risk of coronary artery aneurysms. Am J Cardiol. 1986;58:739–742. doi: 10.1016/0002-9149(86)90348-6. [DOI] [PubMed] [Google Scholar]
- 27.Lin CY, Lin CC, Hwang B, Chiang BN. Cytokines predict coronary aneurysm formation in Kawasaki disease patients. Eur J Pediatr. 1993;152:309–312. doi: 10.1007/BF01956740. [DOI] [PubMed] [Google Scholar]
- 28.Pimiento JM, Maloney SP, Tang PC, Muto A, Westvik TS, Fitzgerald TN, et al. Endothelial nitric oxide synthase stimulates aneurysm growth in aged mice. J Vasc Res. 2008;45:251–258. doi: 10.1159/000112940. [DOI] [PubMed] [Google Scholar]
- 29.Atli FH, Manduz S, Katrancioglu N, Ozum U, Disli OM, Atahan E, et al. eNOS G894T polymorphism and abdominal aortic aneurysms. Angiology. 61:125–130. doi: 10.1177/0003319709339589. [DOI] [PubMed] [Google Scholar]
- 30.Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M, et al. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens. 2009;27:1284–1292. doi: 10.1097/HJH.0b013e328329d1a7. [DOI] [PubMed] [Google Scholar]
- 31.Fukazawa R, Ikegam E, Watanabe M, Hajikano M, Kamisago M, Katsube Y, et al. Coronary artery aneurysm induced by Kawasaki disease in children show features typical senescence. Circ J. 2007;71:709–715. doi: 10.1253/circj.71.709. [DOI] [PubMed] [Google Scholar]