Abstract
Resolution of acute inflammation is an active process that involves the biosynthesis of specialized proresolving lipid mediators. Among them, resolvin D1 (RvD1) actions are mediated by two G protein–coupled receptors (GPCRs), ALX/FPR2 and GPR32, that also regulate specific microRNAs (miRNAs) and their target genes in novel resolution circuits. We report the ligand selectivity of RvD1 activation of ALX/FPR2 and GPR32. In addition to RvD1, its aspirin-triggered epimer and RvD1 analogs each dose dependently and effectively activated ALX/FPR2 and GPR32 in GPCR-overexpressing β-arrestin systems using luminescence and electric cell–substrate impedance sensing. To corroborate these findings in vivo, neutrophil infiltration in self-limited peritonitis was reduced in human ALX/FPR2-overexpressing transgenic mice that was further limited to 50% by RvD1 treatment with as little as 10 ng of RvD1 per mouse. Analysis of miRNA expression revealed that RvD1 administration significantly up-regulated miR-208a and miR-219 in exudates isolated from ALX/FPR2 transgenic mice compared with littermates. Overexpression of miR-208a in human macrophages up-regulated IL-10. In comparison, in ALX/FPR2 knockout mice, RvD1 neither significantly reduced leukocyte infiltration in zymosan-induced peritonitis nor regulated miR-208a and IL-10 in these mice. Together, these results demonstrate the selectivity of RvD1 interactions with receptors ALX/FPR2 and GPR32. Moreover, they establish a new molecular circuit that is operative in the resolution of acute inflammation activated by the proresolving mediator RvD1 involving specific GPCRs and miRNAs.
Chemical mediators regulate acute inflammation and resolution, exerting their actions via cell surface G protein–coupled receptors (GPCRs).1,2,3 Results from this laboratory demonstrated that resolution of self-limited inflammation is an active process that involves local and temporal biosynthesis of a new genus of specialized proresolving lipid mediators. Specialized proresolving lipid mediators include several structurally distinct families, including lipoxins from arachidonic acid, resolvins of the E-series, resolvins of the D-series, protectins, and, most recently, maresins, which are enzymatically biosynthesized from the ω-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid (DHA) (for a recent review, see the article by Serhan3). Specialized proresolving lipid mediators are bioactive chemical autacoids, carry potent and protective actions in disease models, and act on specific cellular targets in a stereoselective manner.
Specialized proresolving lipid mediators exert their actions via cell surface GPCRs.3 Lipoxin A4 (LXA4) interacts with ALX/FPR2,4–6 signals to stop further polymorphonuclear neutrophil (PMN) infiltration, and stimulates nonphlogistic monocyte recruitment7 and macrophage phagocytosis of apoptotic PMNs.8,9 In humans, the aspirin-triggered 15-epimer of LXA4 and the expression level of ALX/FPR2 in skin blisters delineated between resolving versus delayed resolution.10 A member of the D-series resolvins, namely, resolvin D1 (RvD1), biosynthesized from the n-3 essential fatty acid DHA, was first identified in resolving inflammatory exudates from acute self-limited murine peritonitis.11 RvD1 biosynthesis and structure were elucidated,11 and its complete stereochemistry was established as 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid.12
Low-dose aspirin treatment triggers epimers in lipoxins and resolvins, initiating novel aspirin-triggered biosynthetic pathways. For example, these aspirin-triggered routes lead to the biosynthesis of 15R-LXA413 and 17R-RvD111 and to the inhibition of classic cyclooxygenase pathway products, eg, prostaglandins and thromboxanes.3,14 These aspirin-triggered endogenous epimers of natural anti-inflammatory and proresolving mediators are of particular interest because they resist the rapid local enzymatic inactivation of these autacoids.12,15 Stable analogs, including 17(R/S)-methyl RvD1, were designed to mimic the aspirin-triggered form of RvD1 and were prepared by total organic synthesis to give potent and protective actions in ischemia-reperfusion injury; RvD1 and 17(R/S)-methyl RvD1 protected lungs from PMN infiltration and second organ injury (see Kasuga et al16 and the recent review by Serhan and Petasis17).
RvD1 and aspirin-triggered RvD1 (AT-RvD1) display similar actions, each reducing PMN infiltration by ∼50% in the murine dorsal air pouch at equal doses of 100 ng per mouse. In murine peritonitis, RvD1 and AT-RvD1 also proved equipotent (at nanogram dosages), limiting PMN infiltration in a dose-dependent manner. They were as potent as indomethacin, a well-characterized nonsteroidal anti-inflammatory drug, in reducing PMN infiltration.11,12,18 In mouse kidneys, RvD1 is generated in response to bilateral ischemia-reperfusion injury.19 Administration of RvDs before or after ischemia results in a reduction in functional and morphologic kidney injury. RvD1 also displays potent ocular actions, reducing retinopathy20 and suture-induced corneal neovascularization.21 As a potent resolution agonist, RvD1 also attenuates inflammatory pain.22 AT-RvD1 is antihyperalgesic in adjuvant-induced arthritis23, and RvD1 and AT-RvD1 each block activities of specific transient receptor potential channels, attenuating acute pain behaviors and reversing mechanical and thermal hypersensitivity associated with inflammation.24 Recently, AT-RvD1 proved to prevent experimental colitis.25 In these studies, systemic treatment with AT-RvD1 (in nanogram ranges) greatly improved disease severity, including colonic damage, PMN infiltration, and body weight loss in dextran-sulfate-sodium– and 2,4,6-trinitrobenzene-sulfonic-acid–induced colitis. In obese diabetic mice, RvD1 improves insulin sensitivity and reduces macrophage accumulations in adipose tissues.26 Hence, these results suggest that RvD1, AT-RvD1, and their stable analogs could provide a novel approach to stimulate resolution, treating a range of inflammatory disorders.
Two specific human GPCRs, ALX/FPR2 and GPR32, mediate RvD1's proresolving actions on human phagocytes.27 Actions of RvD1 in vivo are mediated, in part, by molecular circuits involving microRNAs (miRNAs) miR-146b, miR-219, and miR-208a and their target genes, which are regulated by these receptors.28 miRNAs are emerging as key regulators of the immune response and inflammation-related diseases such as cancer.29 Earlier, we identified the first miRNA signature of resolution as part of the molecular circuit that actively drives the return to homeostasis.28 Herein, we determined the ligand selectivity of RvD1, its aspirin-triggered epimer AT-RvD1, and their stable analogs using the recently identified cognate receptors human GPR32 (hGPR32: DRV1) and human ALX/FPR2 (hALX/FPR2) and their ability to regulate PMN infiltration and specific miRNAs in vivo.
Materials and Methods
GPCR–β-Arrestin System and Ligand-Receptor Interactions
Ligand-receptor interactions were monitored using the β-arrestin PathHunter system (DiscoveRx, Fremont, CA)30 and were performed essentially as in the study by Krishnamoorthy et al,27 with CHO cells stably overexpressing recombinant hGPR32 (CHO-hGPR32) or HEK cells stably expressing hALX/FPR2 receptors (HEK-hALX/FPR2) tagged with the Pro-Link label of β-galactosidase and β-arrestin linked to the enzyme acceptor fragment of β-galactosidase. Briefly, specific ligand-receptor interactions on incubation with tested compounds (1 hour at 37°C) were determined by measuring chemiluminescence using the PathHunter detection kit (DiscoveRx). RvD1 and AT-RvD1 were prepared by total organic synthesis, and their physical and spectroscopic properties were matched with biogenic products using published criteria as reported in several studies.11,12,18 17(R/S)-methyl RvD1 was also synthesized by total organic synthesis.16 Ligand-receptor interactions were monitored using HEK-hALX/FPR2 or CHO-hGPR32. Each compound was tested from 10-12 to 10-7 mol/L. EC50 values were calculated using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). For methyl ester preparation, RvD1 was treated with excess ethereal diazomethane, taken to dryness with nitrogen gas, suspended in methanol, and isolated using high-performance liquid chromatography. The physical integrity of RvD1 and each related compound was confirmed just before the bioassays.
Ligand Selectivity Using an ECIS System
Ligand-receptor interactions were monitored by measuring impedance31 across cultured CHO-hGPR32 cell monolayers using an electric cell–substrate impedance sensing (ECIS) system (Applied Biophysics, Troy, NY). Briefly, cells were plated at 0.1 × 106 per well of an 8-well ECIS array (8W10E+). Cells were examined 24 hours after plating. RvD1 and related compounds were added to the chambers in serum-free medium, and real-time impedance changes were monitored (0 to 30 minutes, 37°C).
Genetically Engineered Mice, Peritonitis, and Fluorescence-Activated Cell Sorter Staining
Genetically engineered mice were produced and genotyped as reported earlier.32,33 All the animal experiments were performed in accordance with the Harvard Medical School Standing Committee on Animals guidelines for animal care (Protocol 02570). Briefly, mice were anesthetized with isoflurane (Aerrane; Baxter, Deerfield, IL) and were treated with either RvD1–methyl ester (RvD1-ME; 10 ng) or vehicle, i.v. in 100 μL of sterile saline followed by i.p. with 1 mg of zymosan A in 1 mL of sterile saline. Twenty-four hours after injection, mice were euthanized with an overdose of isoflurane, and peritoneal exudates were collected to determine exudate leukocyte numbers and flow cytometry of PMN (with anti-Ly-6G antibody; eBioscience Inc., San Diego, CA) and monocytes/macrophages (with anti-F4/80 antibody; eBioscience).
miRNA Isolation, Reverse Transcription, and Real-Time PCR
miRNA isolation and real-time PCR were performed as in the study by Recchiuti et al.28 Briefly, miRNA fractions from exudate cells collected from mice or human macrophages were isolated using an miRNA isolation kit (Roche Applied Science, Indianapolis, IN) and were quantitated using a spectrophotometer (NanoDrop, Thermo Fisher Scientific Inc., Wilmington, DE) at the Biopolymers Facility at Harvard Medical School (Boston, MA). miRNA samples were then reverse transcribed using the miScript reverse transcription kit (Qiagen Inc., Valencia, CA), followed by real-time PCR with specific primers for each miRNA in a SYBR green–based detection system. PCR was performed using an ABI7900HT thermal cycler (Applied Biosystems, Foster City, CA), and the results were analyzed using SDS software version 2.4 (Applied Biosystems). miRNA expression levels were calculated using the 2–ΔCT relative quantitation34 after normalization to housekeeping genes using RNAU1A and small Cajal body-specific RNA 17 (SCARNA17, NCBI accession no. NR_003003) for mouse miRNA and the small nuclear RNA U1 (RNAU1A; NCBI accession no. NR_004421) for human macrophages.
Human Macrophages, Transfection, and IL-10 Production
Human monocytes were isolated by adherence from peripheral blood mononuclear cells27 from deidentified healthy human volunteers from Children's Hospital Boston blood bank. The cells were cultured in RPMI supplemented with recombinant granulocyte-monocyte colony-stimulating factor (10 ng/mL) for macrophage differentiation. On day 7, macrophages were transfected with miR-208a expression vector or mock vector (OriGene Technologies Inc., Rockville, MD) using the jetPEI macrophage transfection reagent (Polyplus, Ilkrich, France) with 5 μg of expression vector per 2.5 × 106 cells. Seventy-two hours after transfection, miRNA was isolated and subjected to real-time PCR to verify overexpression of miR-208a. Supernatants were collected, and IL-10 levels were determined using an enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) and were normalized to total supernatant proteins measured using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA).
Statistical Analysis
Statistical significance between two groups was evaluated using the 2-tailed Student's t-test. A P < 0.05 was considered significant. For determining significance among groups in the peritonitis experiment, one-way analysis of variance was used, and P < 0.05 across the groups was considered significant.
Results
RvD1, Its Aspirin-Triggered Epimer AT-RvD1, and Their Stable Analogs Directly Activate hALX/FPR2 and hGPR32
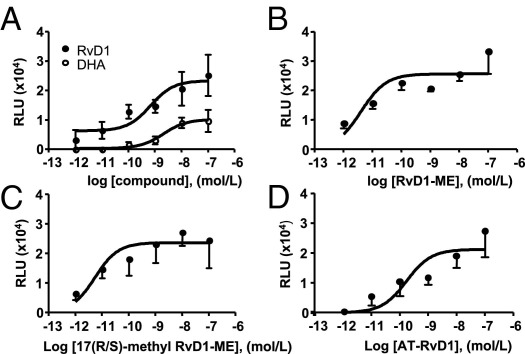
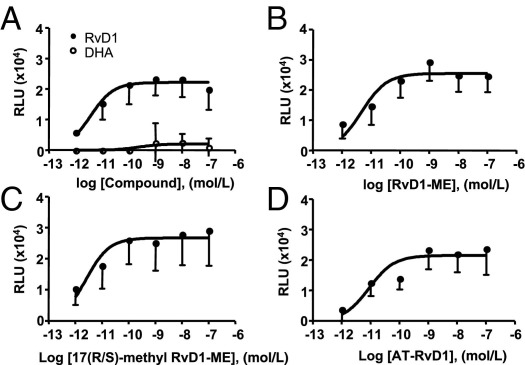
We first evaluated the activation of hGPR32 and hALX/FPR2 by AT-RvD1, RvD1-ME, 17(R/S)-methyl RvD1-ME and its precursor n-3 fatty acid DHA using the β-arrestin–based ligand-receptor interaction system.27 Each of the related RvD1 products are potent bioactive analogs.12,16 At equimolar concentrations, RvD1, AT-RvD1, RvD1-ME, and 17(R/S)-methyl RvD1-ME directly activated hALX/FPR2 (Figure 1) and hGPR32 (Figure 2), with EC50 values in the low picomolar range (Table 1). In comparison, the RvD1 precursor DHA required log orders of magnitude higher concentrations to activate the tagged β-arrestin recruitment in these cells (Figures 1 and 2). At 10−9 mol/L, within the bioactive range of RvD1,11,12 DHA was far less effective (Figure 1A), displaying ∼10% maximal activity of RvD1 with GPR32 and ∼50% maximal activity with the hALX/FPR2 recombinant system.
Figure 1.
RvD1, AT-RvD1, and their stable analogs directly act on hALX/FPR2. Ligand-receptor interaction was monitored using the HEK-ALX/FPR2 β-arrestin system (see Materials and Methods). Dose-response activation curves were obtained for RvD1 and DHA (A), RvD1-ME (B), 17(R/S)-methyl RvD1-ME (C), and AT-RvD1 (D). Results are expressed as mean ± SEM (n = 3 to 4). RLU, relative luminescence units.
Figure 2.
RvD1, AT-RvD1, and their stable analogs directly act on hGPR32. Ligand-receptor interactions were determined using the GPR32 β-arrestin system as in Figure 1. Dose-response activation curves are shown for RvD1 and DHA (A), RvD1-ME (B), 17(R/S)-methyl RvD1-ME (C), and AT-RvD1 (D). Results are expressed as mean ± SEM (n = 4 to 6). RLU, relative luminescence units.
Table 1.
EC50 Values and Maximal Activity for RvD1 and Related Structures with Recombinant hALX/FPR2 and hGPR32 in a β-Arrestin Receptor System
| Compound | EC50, mean (95% CI) (mol/L) | RLUmax (×104) | % of max |
|---|---|---|---|
| hALX/FPR2 | |||
| RvD1 | 4.5 × 10−11 (4.7 × 10−12 to 4.4 × 10−10) | 2.0 | 100 |
| RvD1-ME | 3.7 × 10−12 (3.3 × 10−13 to 4.0 × 10−11) | 2.4 | 125 |
| 17(R/S)-methyl RvD1 | 5.4 × 10−12 (1.2 × 10−12 to 2.5 × 10−11) | 2.3 | 104 |
| AT-RvD1 | 1.8 × 10−10 (9.9 × 10−12 to 3.1 × 10−9) | 2.1 | 115 |
| DHA | 1.9 × 10−9 (8.3 × 10−10 to 4.2 × 10−9) | 1.0 | 50 |
| hGPR32 | |||
| RvD1 | 3.6 × 10−12 (1.6 × 10−12 to 8.2 × 10−12) | 1.1 | 100 |
| RvD1-ME | 4.6 × 10−12 (1.0 × 10−12 to 2.1 × 10−11) | 1.3 | 116 |
| 17(R/S)-methyl RvD1 | 2.5 × 10−12 (7.8 × 10−13 to 8.1 × 10−12) | 1.3 | 98 |
| AT-RvD1 | 8.8 × 10−12 (1.5 × 10−12 to 5.0 × 10−11) | 1.0 | 124 |
| DHA | 2.2 × 10−10 (2.0 × 10−12 to 2.5 × 10−12) | 0.1 | 10 |
Ligand-receptor interactions were monitored using HEK-hALX/FPR2 or CHO-hGPR32, a β-arrestin cell system (see Materials and Methods). Percentage of maximal activity was calculated relative to the maximal RLU values of RvD1. Results are expressed as mean (95% CI, n = 4 to 6).
CI, confidence interval; RLU, relative luminescence units.
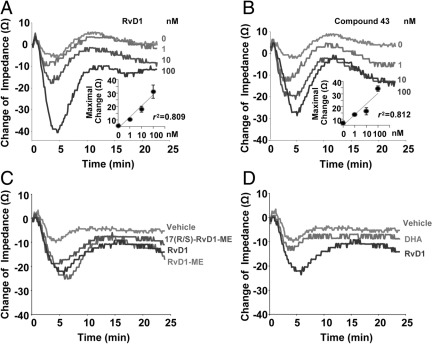
RvD1 and Compound 43 Evoke Rapid Receptor-Dependent Impedance Changes
To further examine ligand-receptor relationships, we used an ECIS system, which monitors changes in impedance on ligand binding to receptors.31 In this system, RvD1 dose dependently (ie, 1, 10, and 100 nmol/L) elicited rapid changes in impedance with CHO-hGPR32 β-arrestin cells (Figure 3A). The anti-inflammatory hALX/FPR2 agonist compound 4335 also evoked dose-dependent changes in impedance with hGPR32-ovexpressing cells similar to those elicited by RvD1 (Figure 3B). Equimolar concentrations of RvD1-ME, 17(R/S)-methyl RvD1-ME, and AT-RvD1 (Figure 3C) elicited similar changes in impedance with CHO-hGPR32 β-arrestin cells. Again, DHA was less effective (Figure 3D).
Figure 3.
RvD1, Rv analogs, and compound 43 produce hGPR32-dependent changes in impedance. Ligand-receptor–dependent changes in impedance were continuously recorded with real-time monitoring across cell monolayers using an ECIS system with hGPR32-expressing β-arrestin cells. Results are tracings obtained from incubations with CHO-GPR32 β-arrestin cells plus RvD1 (1, 10, and 100 nmol/L) (A); compound 43 (1, 10, and 100 nmol/L) (B); 100 nmol/L RvD1, RvD1-ME, and 17(R/S)-methyl RvD1-ME (C); and 100 nmol/L RvD1 directly compared with native DHA (D). Each tracing is representative of n = 3. Insets in A and B show dose-dependent changes in cell impedance determined at 5 minutes of incubation of GPR32-overexpressing cells with RvD1 or compound 43 or vehicle alone. Results are expressed as mean ± SEM (n = 3).
RvD1 Controls PMN Infiltration and Specific miRNAs in Genetically Engineered Mice
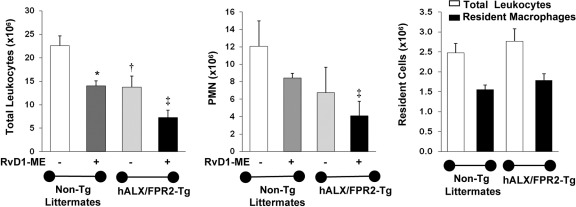
To assess in vivo and pinpoint RvD1 receptor-dependent actions, we tested RvD1-ME in zymosan-induced peritonitis with transgenic mice overexpressing hALX/FPR2. hALX/FPR2 transgene was placed under the control of a human CD11b promoter that drives high levels of transgene expression in mature murine myeloid cells.32 As shown in Figure 4, RvD1 (10 ng per mouse, i.v.) significantly reduced (∼38%) the total peritoneal leukocyte count at 24 hours in nontransgenic littermates (mean ± SD: 14.0 ± 1.5 × 106 versus 22.6 ± 3.0 × 106 in zymosan-treated mice; P < 0.05, one-way analysis of variance). In hALX/FPR2 transgenic mice, RvD1 administration resulted in a further decrease (∼53%) in mean ± SD total leukocyte numbers from 15.6 ± 3.2 × 106 in zymosan alone to 7.3 ± 2.1 × 106 (P < 0.05, one-way analysis of variance) (Figure 4). RvD1 reduced PMN numbers in hALX/FPR2 transgenic mice and nontransgenic littermates by 30% and 40%, respectively (Figure 4). With overexpression of hALX/FPR2, PMN numbers were ∼50% lower than in nontransgenic littermates at 10 ng of RvD1 per mouse. In the absence of zymosan and RvD1, resident peritoneal cell numbers were not significantly different in ALX/FPR2 transgenic mice and their nontransgenic littermates (Figure 4).
Figure 4.
RvD1 reduction in PMN infiltration is enhanced in hALX/FPR2-overexpressing transgenic (Tg) mice peritonitis. hALX/FPR2-Tg mice and WT littermates were injected with zymosan (1 mg per mouse i.p.), with or without RvD1-ME at 10 ng per mouse i.v. Inflammatory exudates were collected at 24 hours and total exudate leukocytes were determined (see Materials and Methods): total leukocytes (left panel) and PMNs (middle panel). The right panel shows numbers of total leukocytes and resident macrophages in control mice. Results are expressed as mean ± SEM (n = 3 mice). *P < 0.05, WT, zymosan versus zymosan + RvD1; †P = 0.05, zymosan, WT versus ALX/FPR2-Tg; ‡P < 0.01, zymosan + RvD1 WT versus ALX/FPR2-Tg.
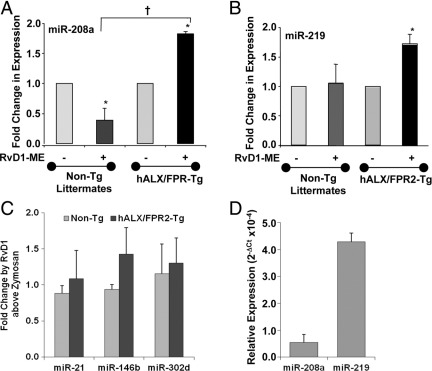
RvD1 regulates miRNAs and their target genes associated with resolution of acute inflammation.28 Since RvD1 activates hALX/FPR2, we next tested whether RvD1 can regulate some of these miRNAs during self-limited acute peritonitis in hALX/FPR2 transgenic mice. Small RNA fractions were isolated from peritoneal lavages collected 24 hours after injection of zymosan. RvD1 significantly up-regulated miR-208a by 1.7-fold (P = 0.01) (Figure 5A) and miR-219 by 1.8-fold (P < 0.01) (Figure 5B) compared with zymosan alone in hALX/FPR2 transgenic mice. RvD1 significantly down-regulated miR-208a levels by 0.4 fold (P < 0.05) in wild-type (WT) mice (Figure 5A) at 24 hours peritonitis, whereas it did not regulate miR-21, miR-146b, and miR-302d levels (Figure 5C). These results indicate that RvD1 regulates specific miRNAs endogenously expressed in resident peritoneal cells (Figure 5D), ie, miR-219 and miR-208a, in hALX/FPR2 transgenic mice, which also confirms those miRNA identified28 as proresolving in vivo.
Figure 5.
RvD1 stimulates specific proresolving miRNAs in hALX/FPR2 transgenic (Tg) mice. miRNA fractions were isolated from peritonitis exudate samples of WT littermates and ALX/FPR2-Tg mice challenged with zymosan (1 mg per mouse) compared with mice given RvD1 (10 ng per mouse with zymosan). Real-time PCR analyses of indicated miRNAs were performed and results were analyzed by the 2−ΔCT method. Fold change in expression levels in RvD1-treated mice compared with zymosan alone were calculated from 2−ΔCT values. Results are expressed as mean ± SEM (n = 3 in each group) fold change in levels of miR-208a (A), miR-219 (B), and miR-21, miR-146b, and miR-302d (C). *P < 0.05 for RvD1-treated versus zymosan-treated WT or Tg mice; †P < 0.05 for zymosan + RvD1–treated Tg mice versus zymosan + RvD1–treated WT mice. D: miR-208a and miR-219 expression levels determined by real-time PCR in peritoneal cells from untreated mice.
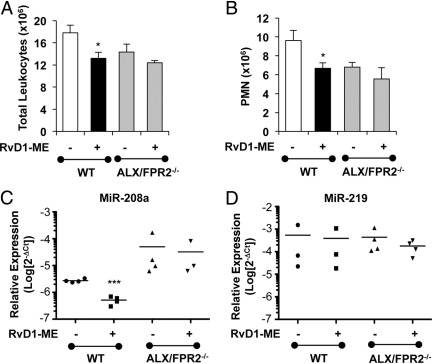
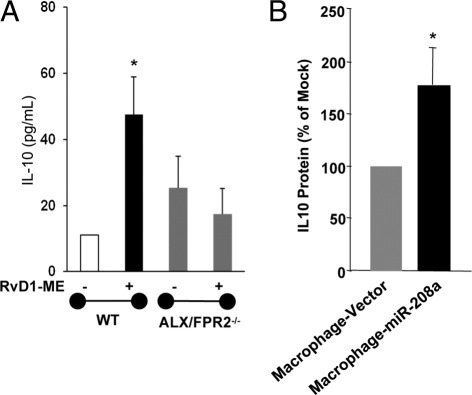
To further investigate ALX/FPR2-dependent actions of RvD1 in vivo, we next tested whether RvD1 could control leukocyte infiltration and regulate proresolving miRNAs in ALX/FPR2 receptor knockout mice, where LXA4 did not regulate leukocyte trafficking.33 As shown in Figure 6, A and B, RvD1 (10 ng per mouse, i.v.) significantly reduced zymosan-initiated leukocyte (by ∼30%) and PMN (by ∼27%) infiltration 24 hours after zymosan injection in WT mice. In ALX/FPR2−/− mice, RvD1 did not regulate PMN infiltration (Figure 6). This finding is consistent with recent results with RvD1 in ALX/FPR2−/− mice and leukocyte trafficking.36 With zymosan alone, total leukocyte and PMN values were not significantly different between ALX/FPR2−/− and WT mice. We also investigated the regulation of miR-219 and miR-208a by RvD1 in ALX/FPR2−/− mice. As shown in Figure 6, C and D, in receptor-deficient mice, RvD1 did not significantly alter miR-208a or miR-219 expression at 24 hours. In addition, RvD1 significantly enhanced IL-10 levels in WT mice that was lost in ALX/FPR2−/− mice (Figure 7A). Taken together, these results indicate that RvD1 regulation of leukocyte trafficking, miR-208a, and IL-10 in mice depends on the endogenous ALX/FPR2 receptor.
Figure 6.
Regulation of acute inflammation and miRNA by RvD1 in ALX/FPR2 knockout mice. Number of total leukocytes (A) and PMNs (B) in peritoneal lavage fluid from WT or ALX/FPR2−/− mice injected with zymosan (1 mg per mouse, i.p.) with or without RvD1-ME (10 ng per mouse, i.v.). Lavage fluid samples were collected 24 hours after initiation of peritonitis, and cells were stained with anti-Ly-6G and F4/80 antibodies. Results are expressed as mean ± SEM (n = 6 mice per group). *P < 0.05 versus zymosan-treated group. Expression of miR-208a (C) and miR-219 (D) in exudate cells from WT or ALX/FPR2−/− mice 24 hours after injection of zymosan plus RvD1-ME (10 ng per mouse, i.v.) or zymosan plus saline. Relative expression of miRNAs was determined using real-time PCR. Results are expressed as mean ± SEM of fold changes in expression (n = 6 mice per group). **P < 0.001 versus zymosan-treated group.
Figure 7.
Regulation of IL-10 levels by RvD1 in peritonitis and by miR-208a in human macrophages. A: IL-10 levels were determined in mALX/FPR2−/− mice (n = 3 to 6). *P < 0.05 versus mice receiving zymosan alone. B: IL-10 levels were determined in human macrophages transiently overexpressing miR-208a or control vector (n = 3). *P < 0.05 versus control vector–transfected macrophages.
Overexpression of miR-208a Up-Regulates IL-10 Secretion in Human Macrophages
miR-208a down-regulates mRNA levels of the proinflammatory PDCD4, a suppressor of IL-10 production37 in human macrophages. Since RvD1 significantly up-regulated miR-208a in human macrophages overexpressing hALX/FPR228 and in hALX/FPR2 transgenic mice (vide supra), we sought to determine whether miR-208a directly regulates the anti-inflammatory cytokine IL-10. As shown in Figure 7B, overexpression of miR-208a in human macrophages resulted in a significant increase in released IL-10 levels compared with mock transfected macrophages.
Discussion
In the present study, we determined the ligand selectivity of the novel proresolving chemical mediator RvD1 and related structures with two recently identified GPCRs, hGPR32 and hALX/FPR2, that are activated by RvD1. Results from these analyses demonstrate that RvD1-hALX/FPR2 interactions regulate acute inflammatory responses in vivo and selected miRNA expression in hALX/FPR2 transgenic mice to reduce inflammation. Along these lines, an RvD1-regulated miR-208a identified in hALX/FPR2 transgenic mice also controlled release of the anti-inflammatory cytokine IL-10 in human macrophages.
We examined RvD1, AT-RvD1, and their analogs in hALX/FPR2 and hGPR32 receptor–overexpressing cells using β-arrestin reporter systems to access ligand potencies since each is known to display potent actions in vivo in a range of animal models (vide supra).3 In these experiments, the RvD1 precursor DHA proved to be less active with these receptors (Figures 1–3). Ligand-receptor–stimulated changes were also monitored with electrode-based impedance measurements, which reflect changes in the actin cytoskeletal framework.38 Because RvD1 regulates actin cytoskeletal changes,27 we sought to use this system where these changes reflect GPCR activation. As shown in Figure 3, A and B, RvD1 and the anti-inflammatory compound 4335 each elicited dose-dependent changes in impedance in hGPR32-overexpressing CHO cells. Similarly, RvD1, AT-RvD1, and their analogs evoked rapid changes in impedance at equimolar concentrations (Figure 3, C and D) with these cells, indicating that activation of this receptor is consistent with the bioactivity of RvD1 and related structures. These results also indicate that in the 17S-containing RvD1 and the 17R- as in the aspirin-triggered epimer AT-RvD1, each configuration at carbon position C17, ie, C17 alcohol groups, is efficiently recognized by receptors hGPR32 and hALX/FPR2. RvD1 showed a lower EC50 for the hALX/FPR2 receptor in the β-arrestin system, suggesting that in these conditions, the S configuration of the C17 hydroxyl group is preferred for interactions with hALX/FPR2 receptor. Also, the addition of a carboxyl methyl ester group to RvD1 (denoted RvD1-ME) retained its ability to activate with these receptors. In contrast, the native DHA structure that lacks functional alcohol groups was far less effective than was RvD1 at activating these receptors within the active concentration/dose range of RvD1 and closely related structures. Although DHA at 10−9 mol/L can partially activate ALX/FPR2, this seems to be relatively weak compared with RvD1 (Table 1). RvD1 and its stable analogs were 2 log orders of magnitude more potent (EC50 ∼10−12 to 10−11 mol/L). In this regard, RvD1 is present in human plasma at ∼5 × 10−11 mol/L,39 consistent with its agonist actions.
Earlier, we demonstrated, using a single cell–monitoring microfluidic chamber, the specific PMN actions of RvD1 at nanomolar levels and its direct actions with human PMN that clearly require conversion of DHA, the precursor of RvD1, to stimulate actions on human PMN.16 Thus, the present results also confirm that specific actions of RvD1 and its analogs are mediated via interactions with surface receptors. Because RvD1 interacts with hALX/FPR2,27 we also tested whether RvD1 can dampen inflammation in hALX/FPR2 transgenic mice. As shown in Figure 4, RvD1 as low as 10 ng i.v. decreased total leukocyte infiltration in zymosan-induced murine peritonitis in WT mice. In parallel, 10 ng of RvD1 significantly reduced total leukocyte and PMN numbers in hALX/FPR2 mice compared with zymosan alone. These results indicate that RvD1 signals via ALX/FPR2 in vivo for its anti-inflammatory actions. Of interest, RvD1 did not change the expression of ALX/FPR2 in peritoneal exudate cells from WT mice, which was determined by quantitative real-time PCR analyses (see Supplemental Figure S1 at http://ajp.amjpathol.org). hALX/FPR2 was initially identified as the receptor for LXA44 and was later shown to be activated by aspirin-triggered lipoxin (15-epimer of LXA4),40 the glucocorticoid-derived annexin peptide,41 and RvD127 and by many proinflammatory peptides (see reviews1,42). Each of these proresolving mediators interacting with hALX/FPR2 signals for anti-inflammatory and proresolving actions. By comparison, as low as 10 ng of aspirin-triggered lipoxin per mouse blocked zymosan-stimulated PMN infiltration by 50% in hALX/FPR2 transgenic mice compared with in WT littermates.32
In the present study, we also assessed the anti-inflammatory actions of an ALX/FPR2 agonist, compound 43 (see Supplemental Figure S2 at http://ajp.amjpathol.org), that was identified by a medical chemical screening approach.35 In Supplemental Figure S2 (available at http://ajp.amjpathol.org), this receptor agonist, compound C43, blocked total leukocyte and PMN infiltration in vivo in murine zymosan peritonitis, confirming its anti-inflammatory actions. Recently, it was reported that targeted knockdown of the murine ALX/FPR2 receptor diminished the anti-PMN activities of its ligands, namely, LXA4, annexin peptide, and compound 43, and gave an exacerbated inflammatory phenotype in mice.33 In these ALX/FPR2-null mice, RvD1's protective action (ie, reducing peritoneal PMN infiltration at 4 hours with 1 and 10 ng per mouse) was also diminished, indicating the crucial role of ALX/FPR2 expression in RvD1 biology in the mouse.36 Along these lines, low-dose aspirin in humans is protective and anti-inflammatory in a cantharidin-induced acute skin inflammation model. These actions of aspirin depended on the endogenous biosynthesis of 15-epimer of LXA4 in skin blisters and on the up-regulation of hALX/FPR2 expression in peripheral blood leukocytes.43 Also, the amplitude and time course of 15-epimer of LXA4 and hALX/FPR2 appearance were key determinants in the severity of inflammation and the onset of resolution in these individuals,10 thus unveiling in humans the important role of hALX/FPR2 and its agonists in governing host responses. Recently, it was shown that ALX/FPR2 expression is up-regulated during the menstrual phase of the cycle and in decidua tissue from the first trimester of pregnancy. LXA4 reduces the inflammatory cytokines in human endometrium and decidua tissue, indicating that the LXA4-ALX/FPR2 axis regulates inflammatory responses in human endometrium and decidua of early pregnancy.44 Hence, it would not be surprising if the ligand-receptor interactions documented in the present study can also be of interest in other organ systems in addition to the immune response and its endogenous resolution mechanisms.
In the effector immune system, certain GPCRs can be activated by bias ligands that couple different cellular responses, as in the case of ALX/FPR2, which interacts with peptides and lipid mediators that function as proinflammatory or anti-inflammatory and proresolving.8,42 This also seems relevant for the resolvin E1 receptor ChemR23, which binds both peptides and is stereoselective for RvE1 and its proresolving actions.45 In view of these findings, it is possible that proresolving ligands use receptors that play a role in initiation and in resolution and termination of the inflammatory response to evoke novel tissue signals required for resolution and homeostasis. RvD1 acts via specific miRNAs that control the inflammatory response, defining the first RvD1-GPCR miRNA signature of resolution in inflammatory exudates.28 To further address these novel proresolving mechanisms in the present experiments, we also assessed regulation of these key miRNAs by RvD1 in vivo using transgenic mice that overexpress the human receptor for LXA4, hALX/FPR2. Among the analyzed miRNAs, RvD1 given at 10 ng per mouse i.v. selectively up-regulated miR-208a and miR-219 in hALX/FPR2 transgenic mice at 24 hours of peritonitis compared with those challenged with zymosan alone or with their nontransgenic littermates. In comparison, RvD1 did not up-regulate either miR-208a or one of its target gene products, IL-10, in genetically modified ALX/FPR2 knockout mice. This finding in receptor-deficient mice further corroborates the evidence that in vivo actions of RvD1 are mediated, at least in part, by the ALX/FPR2 receptor, which, in turn, regulates proresolving miRNAs and target genes. In human macrophages, miR-208a and miR-219 were identified as RvD1-GPCR–regulated miRNAs along with miR-146b and miR-21. These miRNAs were also regulated by RvD1 in murine peritoneal exudate leukocytes. These miRNAs and their target genes seem to belong to an integral network of regulatory molecules that promote resolution of acute inflammation.28 In the present experiments, miR-208a is down-regulated by RvD1 in WT littermates and is up-regulated in hALX/FPR2-overexpressing receptor transgenic mice. These findings indicate the existence of a specific RvD1 receptor-dependent circuit that is required for the regulation of miR-208a. These results also suggest that human and mouse ALX/FPR2 regulate miR-208a in vivo differently.
Recently, we found that RvD1 up-regulates miR-208a and reduces miR-219 expression in human macrophages overexpressing hGPR32, which was not the case for human ALX. RvD1 with human ALX overexpression did not increase miR-208a in human macrophages,28 whereas overexpression of hALX/FPR in transgenic mice (Figure 5A) gave an increase in miR-208a when RvD1 was administered. Hence, the direct actions of RvD1 with isolated human macrophage overexpression of ALX28 versus in vivo administration of RvD1, as in the present results (Figure 5A), give different levels of miR-208a. This difference with miR-208a might reflect the in vivo inflammatory exudate environment or possibly species differences with RvD1 postreceptor signal transduction. Also, it is possible that miR-208a regulation in vivo in mice could reflect its regulation via macrophages and other inflammatory exudate cells.
As for their target genes, miR-219 targets 5-lipoxygenase and regulates leukotriene B4 production.28 miR-208a overexpression in human macrophages increased IL-10 (Figure 7). miR-208a specifically targets and down-regulates PDCD4, a proinflammatory regulatory protein that reduces IL-10 and promotes activation of the NF-κB pathway.28,37 The present experiments with RvD1 are consistent with this and demonstrate that miR-208a overexpression in human macrophages up-regulates IL-10. This is likely important because IL-10 is a pivotal cytokine in inflammation and its resolution. Hence, IL-10 regulation by a local mediator, namely, RvD1, that is biosynthesized from an essential dietary ω-3 fatty acid may have wide-ranging implications in human health and in diseases such as cardiovascular disease (recently reviewed by De Caterina46). Unlike human macrophages, the down-regulation of miR-208a by RvD1 was not associated with concurrent increases in PDCD4 levels in mice.28 This finding suggests that PDCD4 might not be a direct target of miR-208a in murine peritoneal exudate cells. Indeed, it is also known that expression patterns and functions of miRNAs are not strictly conserved between species.47,48 This may also explain, in part, the increases in IL-10 levels in WT mice in response to RvD1 (Figure 7), albeit decreases in miR-208a levels in mice. Along these lines, IL-10 is expressed in several cell types and, hence, may have cell-specific transcriptional and posttranscriptional regulatory mechanisms.49 Since RvD1 up-regulated IL-10 levels in peritoneal exudate cells, it is possible that RvD1 may use additional mechanisms in these cells that are independent of miR-208a to regulate IL-10 production and secretion.
Along these lines, the original complete structural elucidation of the first bioactive coined RvE1 relied on its ability to reduce PMN infiltration and proinflammatory mediators and to evoke the nonphlogistic activation of monocytes/macrophages.45,50 In more aggressive disease models associated with inflammation, infection, and tissue destruction, such as TNBS-induced mouse colitis,51 ocular diseases,20,21 or even an infection-initiated chronic inflammation, as in rabbit periodontal disease,52 low doses of RvE1 proved tissue protective and in certain settings stimulated regeneration of the local tissues injured during PMN-mediated inflammatory responses. In support of this proresolving mechanism being tissue protective, RvD2 and AT-RvD1 were recently demonstrated by Bento et al25 to be potent protective mediators in TNBS and DDS colitis in murine systems with AT-RvD1 relying on ALX/FPR2 for their protective actions in murine tissues. Hence, by activating different proresolving receptors and tissue circuits, specific proresolving mediators, such as LXA4, RvE1, and RvD1 as ligands, each biosynthesized locally from different substrates, regulate leukocyte functional responses and stimulate tissue resolution.
In summation, the potent proresolving mediator RvD1, its aspirin-triggered epimer AT-RvD1, and the synthetic analogs RvD1-ME and 17(R/S)-methyl RvD1 each selectively activates recombinant hALX/FPR2 and hGPR32 receptors. RvD1 dampens acute inflammation in vivo in part via the hALX/FPR2 receptor, as demonstrated in transgenic mice in situ. In peritoneal exudate leukocytes from these mice, RvD1 up-regulates miR-208a, which targets PDCD4, a proinflammatory signaling molecule that gives up-regulation of IL-10 in human macrophages. Together, the findings of the present study establish the ligand selectivity for proresolving agonists of hALX/FPR2 and hGPR32 and indicate that RvD1 via its interactions with these specific GPCRs regulates miRNAs that target specific resolvers. They also shed light on new GPCR-dependent mechanisms for RvD1 and related structures and their actions in controlling the magnitude of the inflammatory response and stimulating its resolution.
Acknowledgments
We thank Mary Halm Small for expert assistance in manuscript preparation, Prof. Mauro Perretti (William Harvey Research Institute, Barts and The London School of Medicine, Queen Mary University of London, London, UK) for providing inflammatory exudates from ALX/FPR2−/− mice, Drs. Lucy V. Norling and Jesmond Dalli for help with peritonitis experiments, the Institute for Cell and Chemical Biology–Longwood, Harvard Medical School, for use of the multiwell plate reader and real-time PCR equipment, Dr. Jim Lederer (Brigham and Women's Hospital) for cytokine measurements, and Prof. Nicos Petasis for the original preparation of 17(R/S)-methyl RvD1-ME.
Footnotes
Supported in part by NIH grant R01-GM038765 (C.N.S.).
S.K. and A.R. contributed equally to this manuscript.
CME Disclosures: C.N.S. is an inventor on patents (resolvins) assigned to Brigham and Women's Hospital and licensed to Resolvyx Pharmaceuticals, is a scientific founder of Resolvyx Pharmaceuticals, and owns equity in the company. C.N.S.' interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. None of the other authors disclosed any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2012.01.028.
Current address of S.K., Biogen Idec Hemophilia Preclinical Pharmacology, Waltham, Massachusetts; of A.R., “G.d'Annunzio” University Foundation, Chieti, Italy; and of G.F., Columbia University, New York, New York.
Supplementary data
Expression of ALX/FPR2 in murine peritoneal cells. Murine peritoneal cells were collected 24 hours after initiation of peritonitis from zymosan- and zymosan + RvD1–treated mice. Real-time PCR analyses were performed as described in Materials and Methods. Results are expressed as mean ± SEM (n = 6 mice per group).
Compound 43 regulates leukocyte trafficking in murine peritonitis. Mice were injected i.v. with compound 43, followed by i.p. injection of 1 mg of zymosan (Zym). Exudates were collected 4 hours after injection, and total cells were enumerated using a hemocytometer. PMN numbers were determined by Ly-6G staining using flow cytometry. Results are expressed as mean ± SEM (n = 4). *P < 0.05 versus Zym alone.
References
- 1.Brink C., Dahlén S.-E., Drazen J., Evans J.F., Hay D.W.P., Nicosia S., Serhan C.N., Shimizu T., Yokomizo T. International Union of Pharmacology XXXVII: nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 3.Serhan C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiore S., Maddox J.F., Perez H.D., Serhan C.N. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maderna P., Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maderna P., Cottell D.C., Toivonen T., Dufton N., Dalli J., Perretti M., Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddox J.F., Hachicha M., Takano T., Petasis N.A., Fokin V.V., Serhan C.N. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 8.Chiang N., Serhan C.N., Dahlen S.E., Drazen J.M., Hay D.W., Rovati G.E., Shimizu T., Yokomizo T., Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 9.Godson C., Mitchell S., Harvey K., Petasis N.A., Hogg N., Brady H.R. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 10.Morris T., Stables M., Colville-Nash P., Newson J., Bellingan G., de Souza P.M., Gilroy D.W. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y.P., Oh S.F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S.P., Petasis N.A., Serhan C.N. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 13.Clària J., Serhan C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 15.Maddox J.F., Serhan C.N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasuga K., Yang R., Porter T.F., Agrawal N., Petasis N.A., Irimia D., Toner M., Serhan C.N. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serhan C.N., Petasis N.A. Resolvins and protectins in inflammation-resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong S., Gronert K., Devchand P., Moussignac R.-L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 19.Duffield J.S., Hong S., Vaidya V., Lu Y., Fredman G., Serhan C.N., Bonventre J.V. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 20.Connor K.M., SanGiovanni J.P., Lofqvist C., Aderman C.M., Chen J., Higuchi A., Hong S., Pravda E.A., Majchrzak S., Carper D., Hellstrom A., Kang J.X., Chew E.Y., Salem N.N., Jr, Serhan C.N., Smith L.E.H. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y., Arita M., Zhang Q., Saban D.R., Chauhan S.K., Chiang N., Serhan C.N., Dana M.R. Novel anti-inflammatory and pro-resolving lipid mediators block inflammatory angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z.Z., Zhang L., Liu T., Park J.Y., Berta T., Yang R., Serhan C.N., Ji R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima-Garcia J., Dutra R., da Silva K., Motta E., Campos M., Calixto J. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;164:278–293. doi: 10.1111/j.1476-5381.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang S., Yoo S., Yang T.J., Cho H., Kim Y.G., Hwang S.W. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bento A.F., Claudino R.F., Dutra R.C., Marcon R., Calixto J.B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- 26.Hellmann J., Tang Y., Kosuri M., Bhatnagar A., Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.H., Yang R., Petasis N.A., Serhan C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C.N. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iliopoulos D., Hirsch H.A., Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson K.R., Eglen R.M. Beta galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev Technol. 2007;5:137–144. doi: 10.1089/adt.2006.052. [DOI] [PubMed] [Google Scholar]
- 31.Peters M.F., Scott C.W. Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J Biomol Screen. 2009;14:246–255. doi: 10.1177/1087057108330115. [DOI] [PubMed] [Google Scholar]
- 32.Devchand P.R., Arita M., Hong S., Bannenberg G., Moussignac R.L., Gronert K., Serhan C.N. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 2003;17:652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- 33.Dufton N.P., Hannon R., Brancaleone V., Dalli J., Patel H.B., Gray M., D'Acquisto F., Buckingham J.C., Perretti M., Flower R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Burli R.W., Xu H., Zou X., Muller K., Golden J., Frohn M., Adlam M., Plant M.H., Wong M., McElvain M., Regal K., Viswanadhan V.N., Tagari P., Hungate R. Potent hFPRL1 (ALXR) agonists as potential anti-inflammatory agents. Bioorg Med Chem Lett. 2006;16:3713–3718. doi: 10.1016/j.bmcl.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 36.Norling L.V., Dalli J., Serhan C.N., Perretti M. Resolvin D1 limits PMN recruitment to inflammatory loci: receptor dependent bioactions (Abstract OC-073) Inflamm Res. 2011;60(suppl 1):S60. [Google Scholar]
- 37.Sheedy F.J., Palsson-McDermott E., Hennessy E.J., Martin C., O'Leary J.J., Ruan Q., Johnson D.S., Chen Y., O'Neill L.A. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 38.Yu N., Atienza J.M., Bernard J., Blanc S., Zhu J., Wang X., Xu X., Abassi Y.A. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Anal Chem. 2006;78:35–43. doi: 10.1021/ac051695v. [DOI] [PubMed] [Google Scholar]
- 39.Psychogios N., Hau D.D., Peng J., Guo A.C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B., Young N., Xia J., Knox C., Dong E., Huang P., Hollander Z., Pedersen T.L., Smith S.R., Bamforth F., Greiner R., McManus B., Newman J.W., Goodfriend T., Wishart D.S. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiang N., Fierro I.M., Gronert K., Serhan C.N. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perretti M., Chiang N., La M., Fierro I.M., Marullo S., Getting S.J., Solito E., Serhan C.N. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perretti M., D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 43.Morris T., Stables M., Hobbs A., de Souza P., Colville-Nash P., Warner T., Newson J., Bellingan G., Gilroy D.W. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 44.Macdonald L.J., Boddy S.C., Denison F.C., Sales K.J., Jabbour H.N. A role for lipoxin A4 as an anti-inflammatory mediator in the human endometrium. Reproduction. 2011;142:345–352. doi: 10.1530/REP-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N.A., Serhan C.N. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 47.Ha M., Pang M., Agarwal V., Chen Z.J. Interspecies regulation of microRNAs and their targets. Biochim Biophys Acta. 2008;1779:735–742. doi: 10.1016/j.bbagrm.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore K.W., De Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 50.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J.N., Petasis N.A., Blumberg R.S., Serhan C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N.A., Levy B.D., Serhan C.N., Van Dyke T.E. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of ALX/FPR2 in murine peritoneal cells. Murine peritoneal cells were collected 24 hours after initiation of peritonitis from zymosan- and zymosan + RvD1–treated mice. Real-time PCR analyses were performed as described in Materials and Methods. Results are expressed as mean ± SEM (n = 6 mice per group).
Compound 43 regulates leukocyte trafficking in murine peritonitis. Mice were injected i.v. with compound 43, followed by i.p. injection of 1 mg of zymosan (Zym). Exudates were collected 4 hours after injection, and total cells were enumerated using a hemocytometer. PMN numbers were determined by Ly-6G staining using flow cytometry. Results are expressed as mean ± SEM (n = 4). *P < 0.05 versus Zym alone.