Abstract
How mechanical signals are transmitted in the cardiac myocyte is poorly understood. In this study, we produced a tamoxifen-inducible mouse model in which β1 integrin could be reduced specifically in the adult cardiomyocyte, so that the function of this integrin could be assessed in the postnatal and mechanically stressed heart. The expression of β1 integrin was reduced to 35% of control levels, but function remained normal at baseline. With aortic constriction, the knockout mice survived but had a blunted hypertrophic response. Integrin knockout myocytes, in contrast to controls, showed reduced integrin-linked kinase expression both at baseline and after hemodynamic stress; focal adhesion kinase expression was reduced after stress. Alterations in multiple signaling pathways were detected in the integrin knockout group after acute and chronic hemodynamic stress. Most remarkably, when we challenged the knockout mice with short-term loading, the robust responses of several kinases (extracellular signal–regulated kinase 1/2, p38, and Akt) evident in control mice were essentially abolished in the knockout mice. We also found that reduction of myocyte β1 integrin expression modified adrenergic-mediated signaling through extracellular signal–regulated kinase, p38, and Akt. Reduction of β1 integrin expression in the mature cardiac myocyte leads to a varied response compared with when this protein is reduced during either the embryonic or perinatal period. These results show that β1 integrin expression is required for proper mechanotransductive and adrenergic responses of the adult heart.
Hypertrophic growth of the adult heart is caused by signals beginning at the cell surface through receptors or channels that, in turn, activate intracellular signaling cascades and effect nuclear cues that alter gene expression.1,2 The molecular machinery that directs mechanical sensing in the cardiac myocyte is incompletely understood. Important detectors of mechanical load are the cell surface adhesion receptors, termed integrins. These receptors transmit information through a complex of proteins at the Z–disk, where the cytoskeleton, sarcomere, sarcoplasmic reticulum, and sarcolemma are interfaced.3–7
Mammals express more than 18 α and 8 β integrin subunits, which heterodimerize to produce more than 24 different receptors. A dominant β integrin subunit in the cardiac myocyte is β1 integrin. β1D is a β1 splice variant isoform that associates with α subunits expressed in myocytes and is the primary β1 integrin isoform expressed in the postnatal heart.5 The cytoplasmic tail of β1 integrins connects to actin filaments via bridging proteins, such as α-actinin, talin, paxillin, and vinculin-metavinculin.8 Thus, integrins connect the extracellular matrix to the cytoskeleton and to cytoplasmic proteins. In addition to their structural and mechanical role, integrins also transduce signals.9 Because integrins do not possess enzymatic activity, they signal by triggering downstream molecules. Examples include activation of tyrosine kinases, such as focal adhesion kinase (FAK), proline-rich tyrosine kinase 2, or small GTPases, such as Rho or Rac.10 They also regulate assembly of cytoskeletal adapter molecules that also propagate intracellular signals, such as paxillin, integrin-linked kinase (ILK), Src, Grb2, and Sos. In addition to FAK, integrin cytoplasmic domains bind numerous other molecules, including melusin and muscle integrin binding protein, both of which are preferentially expressed in muscle.5 Integrins may synergize with other types of receptors to cooperatively signal, as exemplified by the collaborative interactions between integrins and growth factors.7,11
Global knockout of the murine β1 integrin gene caused early embryonic lethality.12,13 When β1 integrin expression was specifically reduced in cardiac myocytes early in cardiogenesis with Nkx2.5-Cre, ventricular compaction was perturbed and myocardial proliferation was reduced.14 In addition, when the β1 integrin gene was excised only in ventricular myocytes using a constitutively active Cre-recombinase driven by the endogenous myosin light chain-2 ventricular promoter, we found the mice developed myocyte reduction of β1 integrin protein soon after birth, showed significant and progressive myocardial fibrosis, developed dilated cardiomyopathy by the age of 6 months, and were intolerant of hemodynamic loading.15
In the current study, we hypothesized that reduced expression of β1 integrin in the mature myocyte would cause defective mechanical signaling but would have unique effects compared with our prior work when this integrin gene was deleted from cardiomyocytes early in cardiogenesis or perinatally. We found that induced cardiac myocyte–specific excision of the β1 integrin gene in the adult heart reduced integrin protein expression effectively and lead to a blunted hypertrophic response and defective hypertrophic-stress signaling in the intact heart, most dramatically with short-term mechanical loading. In addition, we found that adrenergic-mediated signaling was abnormal in the isolated β1 integrin–deficient myocyte. These results indicate the importance of β1 integrin as a cardiomyocyte mechanotransducer and signaling protein in the mature cardiac myocyte.
Materials and Methods
Generation of Mice with Inducible Cardiac Myocyte–Specific Excision of the β1Integrin Gene
Floxed β1 integrin mice were constructed as previously reported15 and diagrammed in Figure 1A. β1 Integrin floxed mice were bred to the transgenic strain, α-myosin heavy chain (MHC) Mer-Cre-Mer,16 which contains a tamoxifen-inducible, cardiac myocyte–specific Cre-recombinase driven by the α-MHC promoter. The background strain was mixed C57 Bl6/Swiss-black.
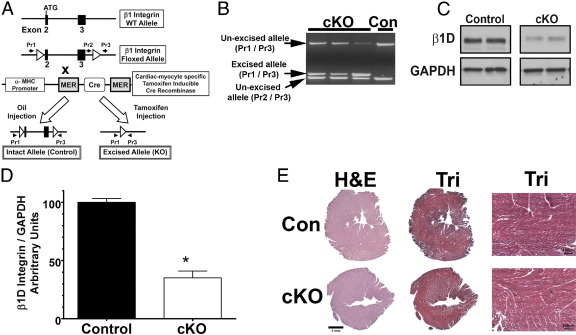
Figure 1.
Generation and basal analysis of the cardiac myocyte–specific β1 integrin knockout mice. A: Diagrams of β1 integrin wild-type, floxed, and excised alleles. Primers used for identification of the unexcised and excised allele are designated as Pr1, Pr2, and Pr3. WT, wild type. B: Genotypes of the β1 cardiac-myocyte specific knockout (cKO) mice were identified by PCR from heart genomic DNA. PCR fragments from floxed bands that were not excised were 3460 and 329 bp in control (Con) mice. These were identified with PCR primer pairs Pr1-Pr3 and Pr2-Pr3, respectively. A 469-bp excised band was detected by PCR of the β1 integrin KO mouse DNA, generated after tamoxifen injection. PCR primers Pr1-Pr3 were used for this reaction. C and D: β1 Integrin protein expression was analyzed by using Western blot analysis (C) and then quantified by densitometry (D) to show cKO mice with 35% ± 5% of control values 4 to 8 weeks after tamoxifen injection (n = 6 in each group). *P < 0.0001 versus oil injection. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. E: Histological analysis of myocardial tissue after reduction of myocyte β1D integrin. No significant differences were detected on histological analysis of the myocardial tissue. Representative LV apical samples from the various mice are shown. Tri, Mallory's trichrome stain.
Detection of Cre-Mediated Excision of the Floxed Integrin Allele via PCR
PCR was used to determine that Cre-mediated excision of the β1 integrin floxed gene had occurred. For this, we used primers as shown in Figure 1A: primer (Pr) 1, 5′-AGGAGACTGTGTAGATGGACATT-3′; Pr2, 5′-TATGAGGCTCCTTGATTGGTCA-3′; and Pr3, 5′-GACCAGGATGAAAGGTCAAA-3′. The PCR conditions were as follows: 2 minutes at 94°C; 12 cycles of 10 seconds at 94°C, 30 seconds at 57°C, and 3 minutes at 68°C; 18 cycles of 15 seconds at 94°C, 30 seconds at 57°C, and 3 minutes 30 seconds at 68°C; and 7 minutes at 68°C. PCR was performed with the Expand long Template PCR System (Roche Diagnostics, Indianapolis, IN) containing 1.75 mmol/L MgCl2, 3.75 U Expand long Template enzyme, 0.35 mmol/L deoxy-NTPs, 0.3 μmol/L of each primer, and 1 μg of genomic DNA from the heart. Resultant bands were 3460 and 329 bp in control mice and a 469-bp excised band in the β1 integrin cardiac-myocyte specific knockout (cKO) mice.
Tamoxifen Induction
Age-matched, 2- to 3-month-old β1 flox/flox/α-MHC Cre mice were injected i.p. with either 4-OH tamoxifen (0.5 mg/100 μL) in peanut oil once daily for 10 days or tamoxifen base (40 μg/g body weight) in corn oil for 10 days, followed by an additional 5 days of injections at 30 days after completion of the first injection sequence. The identical injection of oil alone was used to generate control mice in littermates. For all studies, except as noted, mice were analyzed at 60 days after the conclusion of the injection period or were subjected to surgical procedures as described.
Protein Preparation, Western Blot Analysis, and Immunomicroscopy
Whole hearts were excised from mice, and tissue sections were frozen in liquid nitrogen. Protein preparation and Western blot analysis procedures were performed as previously described.17 Immunostaining was performed by standard methods.15 Deconvolution microscopy was performed using Deltavision software (Applied Precision, Inc., Seattle, WA) or a Zeiss AxioObserver (Carl Zeisss MicroImaging GmbH, Göttingen, Germany) microscope and analyzed with Axiovision software. Cross-sectional measurements were performed, and calculations were made using ImageJ software (NIH, Bethesa, MD).
Cardiac Myocyte Isolation, Culture, Stimulation, Kinase, and TUNEL Assays
Single cardiac myocytes were isolated from adult mice via enzymatic digestion of Langendorff perfused hearts.18 For analysis of protein expression after surgery, cells were prepared for analysis without culturing. For isoproterenol stimulation studies, cells were studied at 30 days after the conclusion of tamoxifen injection, cultured overnight on laminin-coated plates, and then, as indicated, stimulation was performed with isoproterenol at 1 μmol/L for 30 minutes. After isoproterenol stimulation, Western blot analysis was performed (as previously described) or in vitro kinase activities for p38, p42/44 mitogen-activated protein kinase (MAPK), and Akt–protein kinase B were detected with nonradioactive assay kits using appropriate substrates for the immunoprecipitated proteins (Cell Signaling Technology, Beverly, MA). For TUNEL assays, cryotome sections of heart samples were obtained from the control and cKO mice subjected to sham or transverse aortic constriction (TAC) surgical procedures. Sections were processed per manufacturer's instructions (ApopTaq Red In Situ Apoptosis Detection Kit, catalogue number S7165; Millipore Inc., Hayward, CA). Monoclonal anti-α-sarcomeric actinin (number A7811; Sigma, St Louis, MO) was used to counterstain cardiomyocytes. Greater than 1000 myocytes for at least three different mice in each of the four groups were evaluated for the presence of signal, and results were expressed as percentage myocytes detected as TUNEL positive.
Echocardiography, Hemodynamic Analysis, and TAC
Methods for these procedures were previously detailed.15 TAC was performed using anesthesia with ketamine (100 mg/kg) and xylazine (2.5 mg/kg) injected i.p. Transthoracic echocardiography was performed using a Vevo 770 (Visualsonics, Toronto, ON, Canada) machine, with mice anesthetized with isoflurane anesthesia by use of a vaporizer (model 100-F; Ohio Medical Instruments, Cincinnati, OH). Isoflurane was maintained during spontaneous breathing of 1.25% isoflurane in 100% O2 at a flow rate of 1 L per minute via a small nose cone.
Antibodies
The primary antibodies used in these experiments were to detect the following: β1D-integrin, produced in our own laboratory19; glyceraldehyde-3-phosphate dehydrogenase [RDI-TRK5G4-6C5 (Research Diagnostics, Flanders, NJ) or SC32233 (Santa Cruz Biotechnology, Santa Cruz, CA)]; phosphorylated FAK(PY397) (number 44624G; Invitrogen, Carlsbad, CA); total FAK (number 06-543) and β3 integrin (number 04-1060; Millipore, Billerica, MA); vinculin (number V9131; Sigma); paxillin (number 2541; BD Transduction Labs, San Jose, CA); and ILK (number 3862), phosphorylated paxillin (number 2541), phosphorylated p44/42 MAPK (number 9101), total p44/42 MAPK (number 9102), phosphorylated Elk-1 (number 9181), Elk-1 (number 9182), phosphorylated p38 MAPK (numbers 9211 and 9216), total p38 (number 9212), phosphorylated Akt (number 9271), total Akt (numbers 9272 and 4691), phosphorylated glycogen synthetase kinase (GSK) 3α/β (number 9331), total GSK3β (number 9315), phosphorylated activating transcription factor 2 (ATF-2) (number 9221), ATF-2 (number 9226), cleaved caspase 3 (number 9664), and β5 integrin (number 4708) (all from Cell Signaling Technology).
RNA Isolation, cDNA Synthesis, and RT-qPCR Analysis
Total RNA was isolated from adult mouse ventricular myocytes from control and β1 cKO mice by using RNeasy (Qiagen, Valencia, CA), according to the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA in 20 μL with Superscript III, containing reverse transcriptase with random hexamer primers (Invitrogen) by incubation for 1 hour at 50°C. RT-qPCR analysis was performed (7300 Real-Time PCR System; Applied Biosystems, Carlsbad, CA) by using sets of primer pairs, as listed in Table 1. Data were analyzed with 7300 software version 1.3.1 (Applied Biosystems). mRNA levels were measured in three replicates. Fold changes in integrin subunit mRNA were normalized to ribosomal protein large P0 levels.
Table 1.
Primer Sets Used for Analysis of Integrin Transcript Expression
| Integrin | Forward primer | Reverse primer |
|---|---|---|
| β1D | 5′-CAAGTGGGACACGCAAGAAA-3′ | 5′-CGTCCATAGTTTGGATTCTTGAAAT-3′ |
| β3 | 5′-TATGTGGTGGAAGAGCCTGA-3′ | 5′-CAGAGTAGCAAGGCCAATGA-3′ |
| β5 | 5′-TGGGTAGACACCATCGTCAA-3′ | 5′-TGGGACGTTCTGTGTAGCTG-3′ |
| β6 | 5′-CAGGCTGCTGTGTGTAAGGA-3′ | 5′-TGCTGTCCATTCCAAAATGA-3′ |
| β7 | 5′-GAGTCAACCAGACGGTGGAT-3′ | 5′-TCCTCTGAGAAGCCAAGAGC-3′ |
| β8 | 5′-GGAGTGTGAAGGTGGCAGAT-3′ | 5′-GGCCTTGGAGTTGACACAGT-3′ |
Statistical Analyses
All results were expressed as the mean ± SEM. Statistical analysis was performed using the two-tailed Student's t-test or an analysis of variance, with P < 0.05 considered statistically significant.
Results
Generation of Inducible, Cardiac Myocyte–Specific β1 Integrin Knockout Mice
Floxed β1 integrin mice were constructed such that the exon 2 (containing the translational start site) and exon 3, of the β1 integrin gene, were flanked by loxP recognition sites15 (Figure 1A). Homozygous β1 integrin flox/flox mice were bred to α-MHC Mer-Cre-Mer transgenic mice, which contain a tamoxifen-inducible Cre-recombinase driven by the cardiac myocyte–specific α-MHC promoter.16 The interbred mice (β1 integrin Lox/Lox × Mer-Cre-Mer, termed cKO) were injected with tamoxifen. β1 Integrin Lox/Lox × Mer-Cre-Mer mice injected with oil (diluent) were used as controls for the study. No effect of tamoxifen injection into the α-MHC Mer-Cre-Mer was seen on either expression of β1 integrin or physiological function (as assessed by echocardiography) when evaluated at up to 12 weeks after injection (data not shown). Therefore, diluent-injected mice were used as controls. At 60 days after completion of the injections, we assessed total heart DNA for gene excision and protein for expression of the muscle-specific isoform β1D integrin (Figure 1, B–D). Gene excision occurred, and a reduction in β1D integrin protein to 35% ± 5% of control values was detected by using Western blot analysis in the tamoxifen-injected versus control mice. To assess if the expression of other β integrin subunits might be increased to compensate for loss of β1 from the myocyte, we performed quantitative RT-PCR (RT-qPCR) on RNA from control and cKO myocyte samples (see Supplemental Figure S1A at http://ajp.amjpathol.org). In agreement with the Western blot analysis data, β1D integrin transcript levels (the dominant β1 integrin in myocytes) was reduced. Of the other β subunits tested, only β7 integrin was statistically increased. Therefore, we performed Western blot analysis for this subunit on myocyte protein samples. Also, because the β3 subunit has received attention as being important for cardiac myocyte hypertrophy,20 we evaluated if protein expression of this integrin in the cKO group was increased compared with control cardiac myocytes. No differences were detected between control and cKO protein lysates for either subunit (see Supplemental Figure S1, B and C, at http://ajp.amjpathol.org).
Cardiac Form and Function in Basal and Hemodynamically Loaded Hearts after Adult β1 Integrin Gene Excision
Next, we characterized mice in the basal state. Histologically, there were no abnormalities and, in particular, no significant fibrosis noted in the gene-excised mice (Figure 1E). The functional importance of β1 integrin in the mature, fully developed heart was evaluated. Baseline echocardiographic data, analyzed up to 8 weeks after gene excision, showed that hearts of the integrin cKO mice had no significant differences from control-injected littermates (Table 2).
Table 2.
Baseline Echocardiographic Data Show No Statistically Significant Differences between Control and β1 cKO Mice
| Variable | Control group (n = 10) | β1 cKO group (n = 10) |
|---|---|---|
| BW (g) | 23.5 ± 0.91 | 24.9 ± 0.6 |
| HR (bpm) | 545 ± 6 | 525 ± 14 |
| LV EDD (mm) | 3.67 ± 0.04 | 3.66 ± 0.9 |
| LV ESD (mm) | 2.15 ± 0.09 | 2.26 ± 0.04 |
| LV SDd (mm) | 0.54 ± 0.01 | 0.55 ± 0.01 |
| LV PWd (mm) | 0.65 ± 0.02 | 0.66 ± 0.02 |
| %FS | 41 ± 2 | 39.6 ± 1.1 |
| LV mass (mg) | 69.2 ± 3 | 69.9 ± 2 |
| Mean Vcf (cir/second) | 8.3 ± 0.3 | 7.7 ± 0.4 |
BW, body weight; cir, circumferences; EDD, end-diastolic diameter; ESD, end-systolic diameter; %FS, percentage fractional shortening; HR, heart rate; PWd, posterior wall thickness in diastole; SDd, septal thickness in diastole; Vcf, velocity of circumferential fiber shortening.
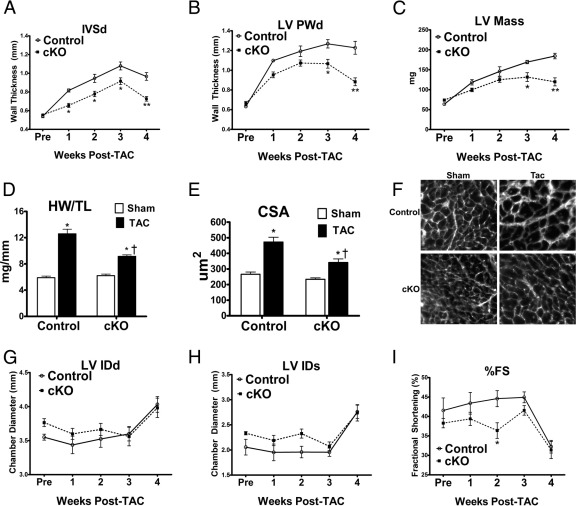
Previously, when we had produced mice with β1 integrin reduction in cardiac myocytes beginning soon after birth, we found that the mice spontaneously developed cardiomyopathy and were intolerant of hemodynamic loading for even a short period.15 We hypothesized that reduced expression of this mechanotransduction protein in the adult might have a varied effect given that protein reduction would occur after the myocyte had fully matured, with normal amounts of this β integrin subunit. Tamoxifen-injected and control mice were subjected to TAC beginning 60 days after gene excision. Transaortic pressure gradients, determined via catheterization of left and right carotid arteries, were comparable between the cKO and control mice (110 ± 55 versus 144 ± 21 mmHg). Serial echocardiographic analyses of these mice showed that they had a blunted hypertrophic response after hemodynamic loading [reduced increases in wall thicknesses and left ventricular (LV) mass] compared with controls (Figure 2). The echocardiographic parameters were in agreement with morphometric and myocyte cross-sectional area measurements that also were reduced in the cKO mice versus controls. Despite this response, with long-term hemodynamic loading, both groups of mice developed ventricular dilation and decreased cardiac function. No histological differences were noted in heart specimens obtained from the various groups after TAC, with only mild fibrosis noted in both (see Supplemental Figure S2 at http://ajp.amjpathol.org). These results indicated that if β1 integrin expression is reduced after the cardiac myocyte is fully mature, normal mechanical responses to hemodynamic load are altered.
Figure 2.
Decreased expression of cardiac myocyte β1 integrin in the cKO mice alters response of the adult heart to hemodynamic loading. A–C: The hypertrophic response of the cKO mice was altered. Serial echocardiography revealed a blunted hypertrophic response in the integrin-deficient cKO mice. Serial echocardiographic analyses demonstrated that LV intraventricular septal thickness in diastole (IVSd), LV posterior wall thickness in diastole (PWd), and LV mass had varied responses in the cKO versus control mice. *P < 0.01, **P < 0.001 versus control at the same time point (n = 6 each). D: Heart weight/tibia length (HW/TL) was reduced in cKO versus control, commensurate with echocardiographic data. *P < 0.05 versus sham of each group, †P < 0.05 versus control TAC (n = 6 each). E: Myocyte cross-sectional area (CSA) was reduced in cKO versus control mice, in agreement with echocardiographic data. *P < 0.05 versus sham of each group, †P < 0.05 versus control TAC (n > 60 for each group). F: Representative microscopy of myocardial tissue from various groups illustrating cell sizes when staining tissue with wheat germ agglutinin. G and H: Chamber dimensions were similar between groups [G, LV internal dimension in diastole (IDd); and H, LV internal dimension in systole (IDs)] (n = 6). I: The percentage fractional shortening (%FS) was reduced at 2 weeks after TAC in the cKO group but is otherwise similar between groups. *P < 0.05 versus control at the same time point (n = 6).
Integrin and Adapter Protein Responses Following Long-Term Hemodynamic Loading Are Altered by β1 Integrin Deficiency
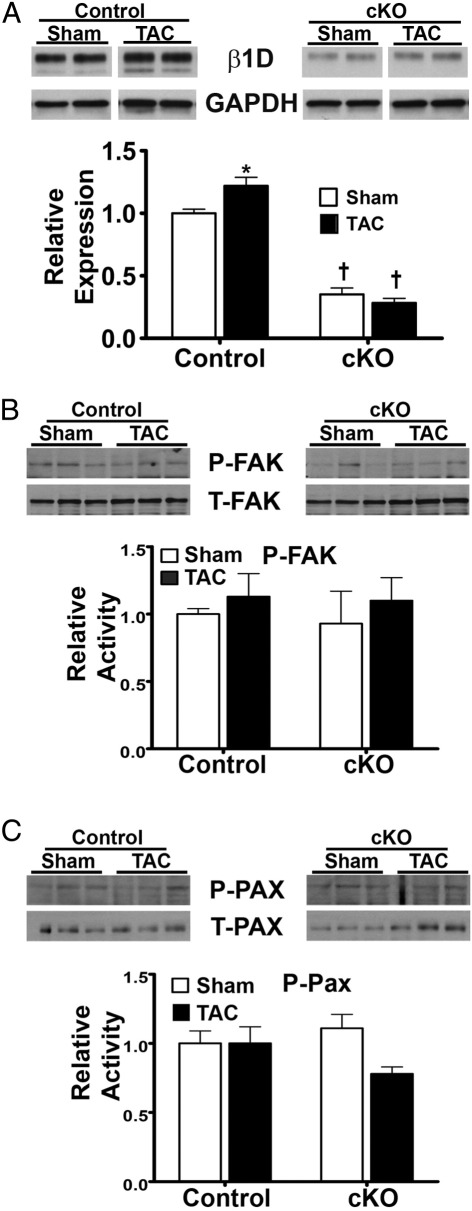
After surgical manipulations, we first assessed the expression of β1 integrin in control and cKO samples (Figure 3A). This was important because the tamoxifen-induced β1 integrin gene excision allowed for residual expression of the protein. We found that, with TAC, the control mice increased integrin expression significantly to 122% ± 7% versus sham control, whereas the cKO expression levels remained lower than that noted in sham cKO (28% ± 4% of control sham). Therefore, with TAC, β1 integrin expression in cKO hearts was 23% of control values. Given the blunted hypertrophic response of these mice, we next assessed the expression and activity levels of proteins that are membrane proximal to integrins. We first examined expression of activated FAK (p-Y397) or paxillin proteins in whole heart tissue at 4 weeks after sham or TAC surgery (Figure 3, B and C). FAK or paxillin activity was not increased by TAC in either the control or the cKO group, nor was there any difference noted between the control and integrin-deficient mice in either the sham or TAC state.
Figure 3.
Expression of β1 integrin and activity of proximal integrin effectors in sham and TAC heart samples after 4 weeks of aortic constriction. A: β1 Integrin expression was increased by TAC in control mice, whereas it remains significantly reduced in cKO mice. *P < 0.05 versus control sham, †P < 0.05 versus control sham or TAC. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Data for FAK (B) and paxillin (C) are shown. No differences in activated FAK or paxillin were noted in either group after TAC, nor were there differences between groups. The expression of phosphorylated (P) protein was normalized to total (T) protein in all cases (n = 5 to 7).
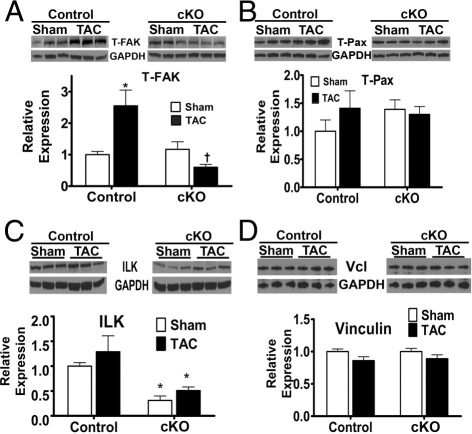
Next, we examined expression of various integrin-linked adapter proteins specifically in myocytes isolated 4 weeks after the sham or TAC surgery (Figure 4). Unlike the whole heart, we found that, with TAC, myocytes from control mice had an approximate 2.5-fold increase in total FAK protein, whereas the cKO myocytes did not have an increase. Total paxillin expression neither changed with hemodynamic loading nor produced a difference between control or cKO expression between sham or TAC groups. ILK, a critical adapter that has been linked to myocardial growth,21,22 showed reduced expression in both the sham and TAC cKO mice, relative to controls. No difference was detected in vinculin expression between groups.
Figure 4.
The expression of proteins that are membrane proximal to integrins was altered in the cKO myocytes. Myocytes were isolated from sham and TAC hearts 4 weeks after surgery. A: Total FAK (T-FAK) was similar in control and cKO basal myocytes but increased in the control, although not cKO, myocytes after TAC. *P < 0.05 versus sham control, †P < 0.02 versus control TAC (n = 3 each). B: Total paxillin (T-Pax) expression was similar in control and cKO myocyte samples (n = 6 each). C: ILK expression was reduced in the basal (sham) and hemodynamically loaded cKO myocytes versus control. *P < 0.05 versus control of each respective group, sham and TAC (n = 6 each). D: Vinculin levels were similar in control and cKO sham and TAC myocytes (n = 6). The expression of noted proteins was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression in all cases.
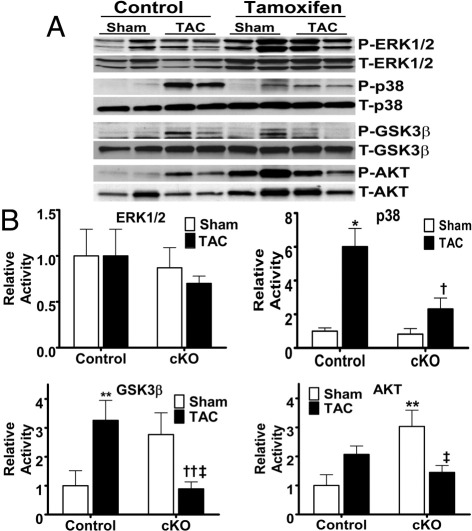
We then examined the effects of the long-term loading (4 weeks after TAC) on kinases modulated by integrins and important for the hypertrophic response (Figure 5). Although no significant changes were noted in extracellular signal–regulated kinase (ERK) 1 or 2, p38 activity was significantly increased in the control samples but had a blunted response in the cKO samples. Sham-treated cKO mice also showed increased Akt activity versus control. After TAC, the cKO mice showed reduced Akt activity compared with sham cKO mice, whereas control mice showed no significant change. GSK3β showed a robust response with loading of control mouse hearts, but this response was reduced after TAC in cKO versus both sham cKO and control TAC. Finally, we evaluated for differences in programmed cell death between the control and cKO mice in both the sham state and after TAC. Although the number of TUNEL-positive cells increased in TAC compared with sham, no differences were seen between the control and cKO groups (see Supplemental Figure S3 at http://ajp.amjpathol.org). In addition, no difference in caspase-3 cleavage was detected between groups (data not shown).
Figure 5.
Long-term mechanotransductive signals were altered in the cKO mice; 4 weeks of TAC produced robust activation of several kinases in the myocardium of control mice, with altered responses in the cKO group. A: Representative Western blot analyses of respective proteins. B: Graphical analysis of normalized activities of noted proteins. No significant change in ERK1 or ERK2 activation was noted in either the control or cKO group; no differences between these groups were found. p38 activity was increased by TAC in the control, but not in the cKO, group. Akt activity was increased in sham cKO relative to control but reduced in cKO with TAC in contrast to no significant change in control. GSK3β was increased by TAC in control, but reduced in cKO, relative to both sham cKO and TAC control groups. In all cases, activity was determined by normalizing phosphorylated kinase to total kinase protein expression. *P < 0.002, **P < 0.05 versus control sham; †P < 0.02, ††P < 0.005 versus control TAC; and ‡P < 0.05 versus cKO sham (n = 5 to 7 for all).
Short-Term Mechanotransductive Signaling Is Significantly Blunted When Cardiac Myocyte β1 Integrin Is Reduced
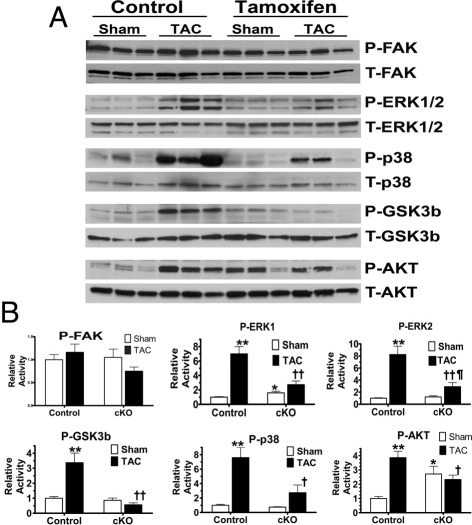
Mechanotransductive reactions of the myocardium have an initial response on short-term stimulation; thus, we next examined if a range of kinases might be activated in the heart with acute hemodynamic stress. Thus, sham or TAC surgical procedures were performed in the control and cKO mice, with analyses performed on tissues harvested after only 10 minutes of stress (Figure 6). This time period was based on preliminary studies in control mice, which showed maximal short-term activation of this group of proteins at this time point (data not shown). With this short-term event, no differences in the amount of FAK activation were noted in either sham group, nor was there any significant change with TAC. Next, we evaluated the activities of ERK1, ERK2, Gsk3β, and p38. With sham surgery, no differences were noted between control and cKO group samples. TAC produced robust responses in the control mice, ranging from a greater than threefold activity increase (over sham) in GSK3β to a greater than eightfold increase in ERK2. In contrast, the response of all of these kinases was essentially abolished in the cKO group. Furthermore, in the sham state, the cKO group had an approximate threefold up-regulation of Akt activity relative to control, although the response to TAC was also blunted, like the other kinases.
Figure 6.
Short-term mechanotransductive signals were blunted in the cKO mice; 10 minutes of TAC produces robust activation of numerous kinases in the myocardium of control mice and blunted responses in cKO. A: Representative Western blot analyses of respective proteins. B: Graphical analysis of normalized activities of noted proteins. No significant changes in FAK activation were noted, but in contrast, activation of ERK1, ERK2, Gsk3β, and p38 was robust in control mice, but significantly blunted in cKO animals. In addition, ERK1 and Akt activity was increased in sham cKO versus sham control, although the activation of Akt after TAC was also blunted. In all cases, activity was determined by normalizing phosphorylated kinase to total kinase protein expression. *P < 0.05, **P < 0.005 versus control sham; †P < 0.05, ††P < 0.01 versus control TAC; and ¶P < 0.05 versus cKO sham (n = 6 for all).
Signaling Events Are Altered in the β1 Integrin KO Adult Cardiomyocyte Stimulated with Isoproterenol
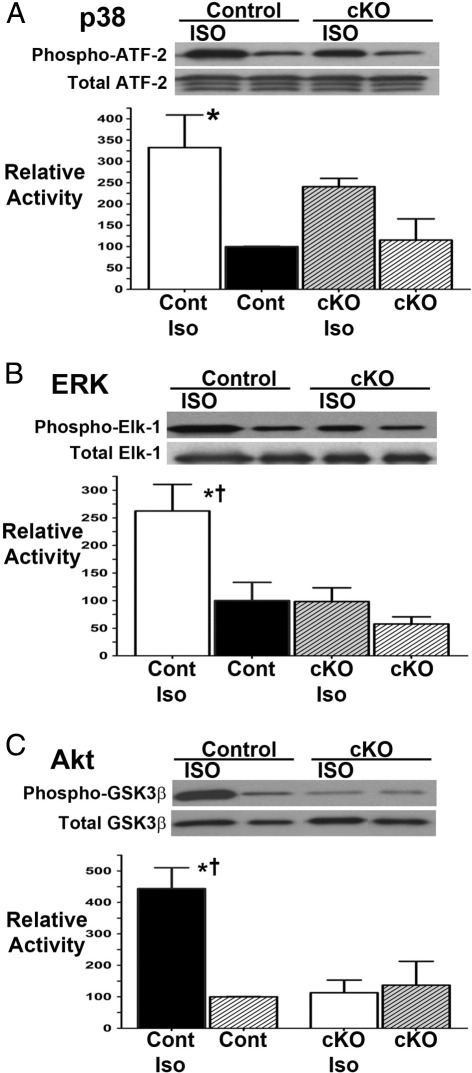
Because, in addition to mechanotransduction, integrin and G-protein–coupled signaling events have been linked in various cell types, we next assessed isolated adult cardiac myocytes from the cKO and control animals. We evaluated cells in the basal state and investigated if the cardiac myocytes deficient in β1 integrin would have altered adrenergically mediated signaling properties. Cells were isolated from the mice, cultured overnight, and then stimulated with the β-adrenergic receptor agonist isoproterenol for 30 minutes. For increased sensitivity, we studied the activity of p38 MAPK, ERK1/2, and Akt using substrate activity assays as opposed to using Western blot analysis techniques with phosphorylated specific antibodies (Figure 7). In all cases, basal activities of the kinases in cKO myocytes were comparable to those in control cells. However, with isoproterenol stimulation, the control cells displayed a robust activation of all three enzymatic activities, whereas the cKO cells showed no significant change from the baseline value.
Figure 7.
Adrenergically stimulated signaling events were altered in the isolated cKO adult cardiomyocytes. Short-term isoproterenol (ISO or Iso) stimulation caused increased activity levels of p38 (A), ERK (B), and Akt (C) in control (Cont) adult myocytes but not in myocytes from β1 integrin knockout mice. *P < 0.05 for basal versus Iso-stimulated control cells, †P < 0.05 versus Iso-stimulated KO cells. Representative Western blot analyses are shown. Cells were studied at 30 days after conclusion of tamoxifen injection (n = 3 in each group).
Discussion
There are numerous diseases that result in hypertrophy of the myocardium, many of which may progress to heart failure. Given this, there has been much interest in understanding how mechanical and humoral responses are transmitted to growth responses in the heart. Indeed, mechanical stresses may activate adrenergic signals. Because integrins are important mechanotransducers, we hypothesized that reduced expression of β1 integrin in the mature myocyte would lead to altered responses of the heart and myocyte to stress, and that this response would be varied in the mature heart as opposed to when the cardiomyocyte was made deficient in this integrin from early in development. Adult mice were made deficient in β1 integrin to levels approximately 35% of control values. Basally, the integrin-deficient adult heart showed no alterations in cardiac structure or function. When subjected to hemodynamic loading of the LV by TAC, the development of hypertrophy was blunted, yet with continued loading, ventricular function declined in cKO, as in controls. More important, TAC significantly increased β1D integrin expression in the control mouse heart, a response that was not mounted in the cKO group despite maintenance of some residual β1D protein. Isolated myocytes showed reduced ILK expression in both the basal and hemodynamically stressed cKO mice versus controls, with reduced FAK expression after TAC. Paxillin and vinculin expression levels were unchanged in the cKO group. In addition, alterations in the response of p38, Akt, and GSK3β were detected in cKO relative to control. Most remarkably, when we challenged the mice with short-term hemodynamic loading, the control mice had robust increases in activation of ERK1/2, GSK3β, p38, and Akt, whereas the cKO group did not. In addition, the activity of ERK1 and Akt was significantly increased in the cKO sham group versus controls. At this point, FAK was not activated by TAC. In addition to alterations in mechanotransduction, reduction of myocyte β1 integrin also modified adrenergic-mediated signaling through p38, ERK, and Akt.
These data indicate that if the heart has fully matured before myocyte β1 integrin expression is significantly reduced, basal function can be maintained, but when challenged with increased mechanical loading, the hypertrophic response is blunted. Prior work14,15 showed that reduction of β1 integrin in the early embryonic myocyte or in the perinatal period produced developmental abnormalities or rapid alterations in the formed myocardium. In contrast to the mice examined herein, when we reduced β1 integrin expression in the myocyte near birth, TAC caused rapid mortality.15 Therefore, we conclude that reduction of β1 integrin protein expression is better tolerated by the adult myocyte than when protein is reduced during the important period of cardiac maturation. This is likely related to the varied roles of β1 integrin protein during cardiogenesis (eg, myocyte proliferation and ventricular maturation14) versus that necessary after the myocyte and heart have fully formed.
Integrins do not themselves possess intrinsic kinase activity. Rather, their signaling functions require assembly of scaffolding proteins and activation of downstream cytoplasmic kinases, such as ILK and FAK. Our data showed that, with reduction of β1 integrin, ILK expression was also reduced both in the basal mice and after TAC. As a protein that binds to the β integrin cytoplasmic domain, it is highly likely that with loss of integrin expression in the myocyte, ILK cannot be positioned appropriately in the costamere and, therefore, may have accelerated degradation. ILK deletion specifically in murine cardiac myocytes also leads to dilated cardiomyopathy.22 FAK is also an important kinase necessary for integrin function. We found that, after hemodynamic loading, the cKO did not increase FAK expression in cardiac myocytes, like controls. Differences in FAK expression were only detected in isolated myocytes, but not in whole heart tissue, because the amount of FAK in nonmyocytes is considerably more than that in myocytes themselves (A.M.M. and R.S.R., unpublished data). These data regarding FAK are interesting in that cardiac myocyte–specific excision of the FAK gene in mice has resulted in relevant, but varied, phenotypes.23,24 In one study,23 reduction of myocyte FAK had no basal effect on the heart but had a modest effect on the TAC-induced hypertrophic response of the myocyte/heart, predisposed to long-term TAC-induced cardiac dysfunction, and resulted in a blunted activation of ERK, at 1 and 4 days after TAC. Deletion of a variant FAK-floxed allele by another group24 also showed no basal phenotype but resulted in LV dilation, without changes in wall thickness by 10 days after TAC. Given these results and that our integrin-deficient mice could not increase the expression of ILK or FAK in response to hemodynamic load, it is likely that these proteins downstream of β1 integrin played substantial roles in the abnormal hypertrophic response detected in our mice.
In addition to evaluation of these proximal integrin linkers, we also tested how loss of β1 integrin altered myocyte mechanical and adrenergic-mediated signaling, because both types of signaling events can stimulate hypertrophic growth. With acute mechanical stress, the normal robust activation of ERK1/2, p38, Akt, and GSK3β was all strongly blunted in β1 cKO mice, indicating an essential role of β1 integrins in the early response to mechanical stimulation. Similarly, the activities of ERK, p38, and Akt were also inhibited in adrenergically stimulated, isolated adult myocytes from cKO mice. Therefore, cardiac myocyte integrins are mediators of both mechanical and humoral events. The roles of the kinase pathways analyzed have been extensively studied in cardiac hypertrophy, although without a full consensus about the details of their function.25,26 Studies,27,28 including some of our own, have shown that ERK1/2 is rapidly activated after short-term mechanical loading of the heart and that integrin activation may specifically lead to ERK1/2 activation. Remarkably, cardiac myocyte– specific Cre-mediated ablation of ERK2 in an ERK1-null background allowed for survival of the mice, although with eventual development of a dilated cardiomyopathic phenotype.29 With TAC, the ERK1−/−/ERK2 cKO group died rapidly, whereas normal responses were detected to TAC in both the ERK1−/− and the ERK2 cKO group alone. We found that integrin deficiency caused reduced ERK activity after short-term hemodynamic and adrenergic stimulation; however, in the long-term, the activity was normal. Thus, it is possible that contributions to the blunted hypertrophic response we detected in our cKO mice might come from these short-term perturbations in ERK activity, which is not dissimilar from the reduction in ERK activity and the reduced hypertrophic responses, detected in FAK cKO mice challenged with TAC.23
Our data also showed reduced activation of p38 in cKO hearts and cells after both short- and long-term mechanical loading, as well as adrenergic stimulation. The function of p38 in cardiac growth, stress responses, and progression to heart failure has also been complex,30–34 with some studies suggesting p38 is prohypertrophic and others indicating that p38 was not required for pressure overload–induced hypertrophy. Similarly, the Akt–protein kinase B pathway has also been linked to cardiac growth and its manipulation has provoked a spectrum of phenotypes, ranging from profound cardiac failure to sudden death and to supranormal contractility.35–39 The variance of expression level and the type of Akt construct used for these studies influenced the phenotypic response. Furthermore, GSK3β can be inhibited (phosphorylated) by hypertrophic stimuli. With cardiac myocyte–specific transgenic increases in GSK3β activity, the mouse heart was basally normal but had a reduced ability to hypertrophy in response to adrenergic stimulation or hemodynamic loading.40 The changes we noted in our mice speak to the complexities of these response pathways in that, with integrin deficiency, the basal myocytes had increased Akt activity, relative to control. It is possible that the basal activation of Akt in the cKO mice occurred as a protective response of the integrin-deficient cardiac myocytes to cellular stress, despite the absence of functional abnormalities at this time point. Still, both acute and chronic TAC lead to a reduced stimulation of Akt and GSK3β in the cKO mice. Likewise, adrenergic stimulation of the integrin-deficient cells had a reduced Akt activation. These blunted responses may have also contributed to the blunted hypertrophic response, and this hypothesis is supported by a prior study focused on the integrin-binding protein, melusin. Like our mice, these melusin KO mice also showed a blunted hypertrophic response after TAC and then were predisposed to heart failure.41 In the short-term, these mice also showed reduced phosphorylation of Akt and GSK3β. These data are directly in line with our findings herein.
A limitation of our study is lack of a full understanding of why the integrin cKO mice had only a blunted hypertrophic response but did not dilate or fail significantly faster than controls. We hypothesize that this can be attributed to the residual integrin expression that remained in our Cre-lox–deleted mice and also to the extremely high degree of aortic constriction (>100-mmHg gradient) that ultimately leads to decreased cardiac function, even in the control mice that retained normal integrin expression. Having mice in a mixed genetic background may have influenced some variability of our data, but littermate controls mitigated any concern that the mixed background may have altered our results.
What relevance might these data have for human disease? To our knowledge, there are no genetic mutations in β1 integrin that have been detected in humans. Because β1 integrin is ubiquitously expressed, it is likely that genetic mutations in this integrin subunit would be embryonically lethal. Still, α7 integrin, which combines with β1 as a dominant muscle heterodimer, has identified mutations in patients with congenital muscular dystrophies, although the mutant effects on cardiac function are unknown.42 Immunolocalization of α7β1 was also disturbed in patients with muscular dystrophy who had a merosin deficiency.43 In addition, α7β1 expression was increased in patients with Duchenne's muscular dystrophy and may be a potential protective therapy for patients with this disease.44,45 Also germane is that β1D integrin protein has been either reduced or cleaved in patients with ischemic and dilated cardiomyopathy,46,47 and that integrin expression levels return to normal as the reverse remodeling occurs with mechanical unloading of the failing heart through use of an LV assist device. Finally, in addition to integrins themselves, mutations in integrin-linked protein ILK, which we found decreased in expression in our cKO mice, have also been identified in patients with cardiomyopathy,48 and ILK expression has been detected as being up-regulated >10-fold in the failing human myocardium.49 Given this, we would suggest that mutation of α7β1 integrins could potentially lead to cardiac dysfunction, particularly in the face of a second hit, such as hypertension or valvular disease, and that augmented expression of these integrins could likewise be tested as a therapy for cardiomyopathies of many types. Further work is necessary to explore this tenet.
In conclusion, our results clearly show novel information that β1 integrins are critically involved in short- and long-term cardiac myocyte mechanotransduction and in the response of cardiac myocytes to adrenergic stimulation. Our data speak to the complexities in understanding how short- and long-term mechanical and adrenergic events summate in the hypertrophic and potentially failing myocardial phenotype. How modulation of integrin expression or function might predispose to cardiac dysfunction, and whether augmented integrin function could be used therapeutically to prevent myocardial disease, clearly requires further study.
Acknowledgments
We thank Tomo Yajima for excellent experimental and technical assistance and Dr. Jeff Molkentin for original supply of the tamoxifen-inducible α-MHC–Cre mice.
Footnotes
Supported by grants from the NIH and The Veterans Administration (Merit Award) (R.S.R.).
R.L., Y.W., and A.M.M. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.12.007.
Supplementary data
Evaluation of β integrin transcripts and proteins in cardiac myocytes from control and cKO mice. A: RT-qPCR analysis of myocyte transcripts shows a statistically significant reduction in the β1D integrin transcript and an increase in the β7 transcript in cKO versus control samples (n = 3 each). *P < 0.05 versus control. B and C: Western blot analyses of myocyte lysates from control and cKO mice show no differences in either β3 or β7 integrin protein expression. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
No differences are noted in histological analyses of control versus cKO hearts after hemodynamic loading provoked by TAC. Specimens from sham– and TAC–operated mice are shown for each group.
TUNEL-positive cells are increased by hemodynamic loading of the left ventricle, but no significant differences are noted between control and cKO hearts either in the basal state or after TAC. A: Representative specimens from control and cKO ventricles stained for a myocyte-specific marker (α-sarcomeric actinin, green) and for TUNEL-positive cells (red) in both the sham– and TAC–operated states. B: Graphic depiction of TUNEL-positive cells in the sham and TAC specimens shows no significant differences between the control and cKO samples in either the sham or TAC state (n = 3 mice in each group, with >1000 cells assayed from each group).
References
- 1.Olson E.N., Schneider M.D. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin J.D., Dorn G.W., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 3.Pyle W.G., Solaro R.J. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- 4.Sadoshima J., Izumo S. Mechanotransduction in stretch-induced hypertrophy of cardiac myocytes. J Recept Res. 1993;13:777–794. doi: 10.3109/10799899309073692. [DOI] [PubMed] [Google Scholar]
- 5.Ross R.S., Borg T.K. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 6.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meder B., Huttner I.G., Sedaghat-Hamedani F., Just S., Dahme T., Frese K.S., Vogel B., Köhler D., Kloos W., Rudloff J., Marquart S., Katus H.A., Rottbauer W. PINCH proteins regulate cardiac contractility by modulating integrin-linked kinase-protein kinase B signaling. Mol Cell Biol. 2011;31:3424–3435. doi: 10.1128/MCB.05269-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaff M., Liu S., Erle D.J., Ginsberg M.H. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J Biol Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- 9.Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 10.Hynes R.O. Cell adhesion: old and new questions. Trends Cell Biol. 1999;9:M33–M37. [PubMed] [Google Scholar]
- 11.Ross R.S. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381–390. doi: 10.1016/j.cardiores.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Fassler R., Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 13.Stephens L.E., Sutherland A.E., Klimanskaya I.V., Andrieux A., Meneses J., Pedersen R.A., Damsky C.H. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 14.Ieda M., Tsuchihashi T., Ivey K.N., Ross R.S., Hong T.T., Shaw R.M., Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shai S.Y., Harpf A.E., Babbitt C.J., Jordan M.C., Fishbein M.C., Chen J., Omura M., Leil T.A., Becker K.D., Jiang M., Smith D.J., Cherry S.R., Loftus J.C., Ross R.S. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 16.Sohal D.S., Nghiem M., Crackower M.A., Witt S.A., Kimball T.R., Tymitz K.M., Penninger J.M., Molkentin J.D. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 17.Zemljic-Harpf A.E., Miller J.C., Henderson S.A., Wright A.T., Manso A.M., Elsherif L., Dalton N.D., Thor A.K., Perkins G.A., McCulloch A.D., Ross R.S. Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol Cell Biol. 2007;27:7522–7537. doi: 10.1128/MCB.00728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yajima T., Yasukawa H., Jeon E.S., Xiong D., Dorner A., Iwatate M., Nara M., Zhou H., Summers-Torres D., Hoshijima M., Chien K.R., Yoshimura A., Knowlton K.U. Innate defense mechanism against virus infection within the cardiac myocyte requiring gp130-STAT3 signaling. Circulation. 2006;114:2364–2373. doi: 10.1161/CIRCULATIONAHA.106.642454. [DOI] [PubMed] [Google Scholar]
- 19.Pham C.G., Harpf A.E., Keller R.S., Vu H.T., Shai S.Y., Loftus J.C., Ross R.S. Striated muscle-specific beta(1D)-integrin and FAK are involved in cardiac myocyte hypertrophic response pathway. Am J Physiol Heart Circ Physiol. 2000;279:H2916–H2926. doi: 10.1152/ajpheart.2000.279.6.H2916. [DOI] [PubMed] [Google Scholar]
- 20.Harston R.K., Kuppuswamy D. Integrins are the necessary links to hypertrophic growth in cardiomyocytes. J Signal Transduct. 2011;2011:521742. doi: 10.1155/2011/521742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H., Fedak P.W., Dai X., Du C., Zhou Y.Q., Henkelman M., Mongroo P.S., Lau A., Yamabi H., Hinek A., Husain M., Hannigan G., Coles J.G. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006;114:2271–2279. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 22.White D.E., Coutu P., Shi Y.F., Tardif J.C., Nattel S., St A.R., Dedhar S., Muller W.J. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiMichele L.A., Doherty J.T., Rojas M., Beggs H.E., Reichardt L.F., Mack C.P., Taylor J.M. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99:636–645. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng X., Kraus M.S., Wei H., Shen T.L., Pariaut R., Alcaraz A., Ji G., Cheng L., Yang Q., Kotlikoff M.I., Chen J., Chien K., Gu H., Guan J.L. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose B.A., Force T., Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehat I., Molkentin J.D. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation. 2010;122:2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babbitt C.J., Shai S.Y., Harpf A.E., Pham C.G., Ross R.S. Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem Cell Biol. 2002;118:431–439. doi: 10.1007/s00418-002-0476-1. [DOI] [PubMed] [Google Scholar]
- 28.Shyy J.Y., Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9:707–713. doi: 10.1016/s0955-0674(97)80125-1. [DOI] [PubMed] [Google Scholar]
- 29.Kehat I., Davis J., Tiburcy M., Accornero F., Saba-El-Leil M.K., Maillet M., York A.J., Lorenz J.N., Zimmermann W.H., Meloche S., Molkentin J.D. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Q., Molkentin J.D. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003;35:1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S., Weinheimer C., Courtois M., Kovacs A., Zhang C.E., Cheng A.M., Wang Y., Muslin A.J. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R.P., Kass D.A., Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci U S A. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishida K., Yamaguchi O., Hirotani S., Hikoso S., Higuchi Y., Watanabe T., Takeda T., Osuka S., Morita T., Kondoh G., Uno Y., Kashiwase K., Taniike M., Nakai A., Matsumura Y., Miyazaki J., Sudo T., Hongo K., Kusakari Y., Kurihara S., Chien K.R., Takeda J., Hori M., Otsu K. p38α Mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braz J.C., Bueno O.F., Liang Q., Wilkins B.J., Dai Y.S., Parsons S., Braunwart J., Glascock B.J., Klevitsky R., Kimball T.F., Hewett T.E., Molkentin J.D. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latronico M.V., Costinean S., Lavitrano M.L., Peschle C., Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann N Y Acad Sci. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 36.Matsui T., Li L., Wu J.C., Cook S.A., Nagoshi T., Picard M.H., Liao R., Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 37.DeBosch B., Sambandam N., Weinheimer C., Courtois M., Muslin A.J. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBosch B., Treskov I., Lupu T.S., Weinheimer C., Kovacs A., Courtois M., Muslin A.J. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 39.Shiojima I., Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 40.Antos C.L., McKinsey T.A., Frey N., Kutschke W., McAnally J., Shelton J.M., Richardson J.A., Hill J.A., Olson E.N. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo: proceedings of the National Academy of Sciences of the United States of America. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brancaccio M., Fratta L., Notte A., Hirsch E., Poulet R., Guazzone S., De Acetis M., Vecchione C., Marino G., Altruda F., Silengo L., Tarone G., Lembo G. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi Y.K., Chou F.L., Engvall E., Ogawa M., Matsuda C., Hirabayashi S., Yokochi K., Ziober B.L., Kramer R.H., Kaufman S.J., Ozawa E., Goto Y., Nonaka I., Tsukahara T., Wang J.Z., Hoffman E.P., Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 43.Vachon P.H., Xu H., Liu L., Loechel F., Hayashi Y., Arahata K., Reed J.C., Wewer U.M., Engvall E. Integrins (alpha7beta1) in muscle function and survival: disrupted expression in merosin-deficient congenital muscular dystrophy. J Clin Invest. 1997;100:1870–1881. doi: 10.1172/JCI119716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodges B.L., Hayashi Y.K., Nonaka I., Wang W., Arahata K., Kaufman S.J. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110(Pt 22):2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- 45.Engvall E., Wewer U.M. The new frontier in muscular dystrophy research: booster genes. FASEB J. 2003;17:1579–1584. doi: 10.1096/fj.02-1215rev. [DOI] [PubMed] [Google Scholar]
- 46.Fedak P.W., Moravec C.S., McCarthy P.M., Altamentova S.M., Wong A.P., Skrtic M., Verma S., Weisel R.D., Li R.K. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation. 2006;113:238–245. doi: 10.1161/CIRCULATIONAHA.105.571414. [DOI] [PubMed] [Google Scholar]
- 47.Pfister R., Acksteiner C., Baumgarth J., Burst V., Geissler H.J., Margulies K.B., Houser S., Bloch W., Flesch M. Loss of β1D-integrin function in human ischemic cardiomyopathy. Basic Res Cardiol. 2007;102:257–264. doi: 10.1007/s00395-006-0640-1. [DOI] [PubMed] [Google Scholar]
- 48.Knoll R., Postel R., Wang J., Kratzner R., Hennecke G., Vacaru A.M., Vakeel P., Schubert C., Murthy K., Rana B.K., Kube D., Knoll G., Schafer K., Hayashi T., Holm T., Kimura A., Schork N., Toliat M.R., Nurnberg P., Schultheiss H.P., Schaper W., Schaper J., Bos E., Den Hertog J., van Eeden F.J., Peters P.J., Hasenfuss G., Chien K.R., Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116:515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- 49.Sopko N., Qin Y., Finan A., Dadabayev A., Chigurupati S., Qin J., Penn M.S., Gupta S. Significance of thymosin beta4 and implication of PINCH-1-ILK-alpha-parvin (PIP) complex in human dilated cardiomyopathy. PLoS One. 2011;6:e20184. doi: 10.1371/journal.pone.0020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of β integrin transcripts and proteins in cardiac myocytes from control and cKO mice. A: RT-qPCR analysis of myocyte transcripts shows a statistically significant reduction in the β1D integrin transcript and an increase in the β7 transcript in cKO versus control samples (n = 3 each). *P < 0.05 versus control. B and C: Western blot analyses of myocyte lysates from control and cKO mice show no differences in either β3 or β7 integrin protein expression. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
No differences are noted in histological analyses of control versus cKO hearts after hemodynamic loading provoked by TAC. Specimens from sham– and TAC–operated mice are shown for each group.
TUNEL-positive cells are increased by hemodynamic loading of the left ventricle, but no significant differences are noted between control and cKO hearts either in the basal state or after TAC. A: Representative specimens from control and cKO ventricles stained for a myocyte-specific marker (α-sarcomeric actinin, green) and for TUNEL-positive cells (red) in both the sham– and TAC–operated states. B: Graphic depiction of TUNEL-positive cells in the sham and TAC specimens shows no significant differences between the control and cKO samples in either the sham or TAC state (n = 3 mice in each group, with >1000 cells assayed from each group).