Abstract
Glioblastomas are characterized by an aggressive local growth pattern, a marked degree of invasiveness and poor prognosis. Tumor invasiveness is facilitated by the increased activity of proteolytic enzymes which are involved in destruction of the extracellular matrix of the surrounding healthy brain tissue. Elevated levels of matrix metalloproteinases (MMPs) were found in glioblastoma (GBM) cell-lines, as well as in GBM biopsies as compared with low-grade astrocytoma (LGA) and normal brain samples, indicating a role in malignant progression. A careful review of the available literature revealed that both the expression and role of several of the 23 human MMP proteins is controversely discussed and for some there are no data available at all. We therefore screened a panel of 15 LGA and 15 GBM biopsy samples for those MMPs for which there is either no, very limited or even contradictory data available. Hence, this is the first complete compilation of the expression pattern of all 23 human MMPs in astrocytic tumors. This study will support a better understanding of the specific expression patterns and interaction of proteolytic enzymes in malignant human glioma and may provide additional starting points for targeted patient therapy.
Keywords: Astrocytic tumor, Expression pattern, Glioblastoma cell-lines, Glioblastoma multiforme, Matrix metalloproteinase
INTRODUCTION
Glioblastomas (GBM) are the most common malignant brain tumors in adults[1]. High grade glioblastoma (WHO grade IV) may develop from low grade astrocytoma (LGA) (WHO grade II), anaplastic astrocytoma (WHO grade III) or they may manifest de novo without low grade precursor lesions[1,2]. Whereas the prognosis of patients with anaplastic astrocytomas or GBM remains poor, patients with LGA have a better prognosis[3]. Patients with anaplastic astrocytomas or GBM are on average older (median age at diagnosis is 40 years and 53 years, respectively), compared to those with LGA (mean age at diagnosis 35 years)[3]. Despite multidisciplinary treatment which includes surgery, temozolomide chemotherapy and γ-irradiation, the overall median survival time for patients with malignant glioblastoma is as low as 14.6 mo[4] and there are only a few reports of patients who have survived more than 5 years[5].
This limited prognosis of GBM patients is the result of an aggressive local growth pattern and the marked degree of invasiveness displayed by these tumors[6,7]. Glioma cell invasion requires an intricate series of both, host and tumor related steps, involving tumor cell migration and tumor matrix disintegration. Disruption of the extracellular matrix (ECM) is a prerequisite for tumor cell invasion, because it delivers the tracks for the migrating cells[8]. A number of different proteolytic enzymes such as matrix metalloproteinases (MMP) are overexpressed during tumor development[9,10]. Their role is to break down the structural barriers to migration and invasion by dissolving and destroying the matrix proteins of the surrounding normal brain tissue[11,12].
Interference with MMP-9 and one of its upstream regulators by RNA interference led to a reduction in tumor growth and invasion in a mouse model[13]. Understandably, reducing MMP activity has been probed as a new therapeutic measure to stop or at least delay tumor invasion and to ultimately prolong patient survival. However, the inhibition of MMPs with broad-band synthetic and natural inhibitors has, as yet, been of little clinical success due to the development of severe side effects during treatment[14,15]. MMP-9, MMP-2 and its activator MMP-14 are involved in migration and invasion of human GBM cells[16-19] and the first clinical trials using the MMP inhibitor, marimastat, in combination with chemotherapy have recently been performed in GBM patients[20,21]. It stands to reason that a more specific inhibition of individual or combined MMPs may be effective in the treatment of gliomas and have fewer unwanted side-effects. In order to generate these future therapy concepts a thorough knowledge of MMPs expression patterns and their interaction is required. This review summarizes currently available data on the expression of MMPs in human glioblastomas. We also present our own data on those MMPs, not yet published in literature.
MATRIX METALLOPROTEINASES
Matrix metalloproteinases are a family of zinc-dependent endopeptidases. A total of 23 family members have been identified in humans so far. These are numbered in the sequence of their discovery[12] (Table 1). MMPs mediate the degradation of protein components of the ECM and of basement membranes. Both are essential for the interaction of individual cells with their surrounding and for the development and function of multicellular organisms[11]. Thus, MMPs play a central role in a number of physiological processes, including embryonic growth and development, implantation, morphogenesis, bone remodelling, wound healing, angiogenesis, apoptosis, and nerve growth[24], during which they are produced by e.g., trophoblasts, keratinocytes, (pre)osteoclastic cells and fibroblasts[25]. However, increased expression and activation of MMPs also contributes to a number of pathological processes such as rheumatoid arthritis, cardiovascular diseases or cancer progression[26-28].
Table 1.
The human matrix metalloproteinase family
| MMP | Alternative names | Group | Substrates |
| MMP-1 | Collagenase-1, interstitial collagenase, fibroblast collagenase, tissue collagenase | Collagenases | Aggrecan, collagen I,II, III, VII, VIII, X, XI, entactin/nidogen, fibronectin, gelatin I, IGFBPs, laminin, link protein, myelin basic, tenascin, vitronectin, α 1-AC, α 2-M, α1-PI, casein, C1q, fibrin, fibrinogen, IL1β, proTNFα, serpins |
| MMP-2 | Gelatinase A, 72-kDa gelatinase, 72-kDa type IV collagenase, neutrophil gelatinase | Gelatinases | Aggrecan, collagen I, III, IV, V, VII, X, XI, decorin, elastin, entactin/nidogen, fibrillin, fibronectin, fibulins, gelatin I, IGFBPs, laminin, link protein, myelin basic, osteonectin, tenascin, vitronectin, α1-AC, α 1-PI, C1q, fibrin, fibrinogen, IL1β, monocyte chemoattractant protein 3, proTGF β, proTNF α, plasminogen, substance P, T kininogen |
| MMP-3 | Stromelysin-1, transin-1, proteoglycanase, procollagenase activating protein | Stromelysins | Aggrecan, collagen III, IV, V, VII, IX, X, XI, decorin, elastin, entactin/nidogen, fibrillin, fibronectin, gelatin I, IGFBPs, laminin, link protein, myelin basic, osteonectin, tenascin, vitronectin, α1-AC, α 2-M, α 1-PI, casein, C1q, E-cadherin, fibrin, fibrinogen, IL1β, osteopontin, proTNF α, plasminogen, substance P |
| MMP-7 | Matrilysin, matrin, PUMP1, small uterine metalloproteinase | Matrilysins | Aggrecan, collagen I, IV, decorin, elastin, entactin/nidogen, fibronectin, fibulins, gelatin I, laminin, link protein, myelin basic, osteonectin, tenascin, vitronectin, α 1-PI, casein, E-cadherin, fibrinogen, proTNF α, plasminogen, versican |
| MMP-8 | Collagenase-2, neutrophil collagenase, PMN collagenase, granulocyte collagenase | Collagenases | Aggrecan, collagen I,II, III, IX, X, α 2-M, α 1-PI, C1q, E-cadherin, fibrinogen, laminin, serpins, substance P |
| MMP-9 | Gelatinase B, 92-kDa gelatinase, 92-kDa type IV collagenase | Gelatinases | Aggrecan, collagen IV, V, VII, X, XI, XIV, decorin, elastin, fibrillin, gelatin I, laminin, link protein, myelin basic, osteonectin, vitronectin, α 2-M, α 1-PI, casein, C1q, fibrin, fibrinogen, IL1β, proTGF β, proTNF α, plasminogen, substance P |
| MMP-10 | Stromelysin-2, transin-2 | Stromelysins | Aggrecan, collagen III, IV, V, elastin, fibronectin, gelatin I, link protein, casein, fibrinogen, osteopontin |
| MMP-11 | Stromelysin-3 | Other MMPs | IGFBPs, α 2-M, α 1-PI, serpins |
| MMP-12 | Metalloelastase, macrophage elastase, macrophage metalloelastase | Stromelysins | Aggrecan, collagen I, IV, elastin, entactin/nidogen, fibrillin, fibronectin, gelatin I, laminin, myelin basic, vitronectin, apolipoprotein A, α 2-M, α 1-PI, factor XII, fibrinogen, proTNF α, plasminogen |
| MMP-13 | Collagenase-3 | Collagenases | Aggrecan, collagen I,II, III, VI, IX, X, XIV, fibrillin, fibrin, fibronectin, gelatin I, laminin, osteonectin, α 2-M, casein, C1q, factor XII, fibrinogen, perlecan, pro-MMP2, serpins |
| MMP-14 | MT1-MMP, MT-MMP1 | Membrane-type MMPs | Aggrecan, collagen I,II, III, entactin/nidogen, fibrillin, fibronectin, gelatin I, laminin, vitronectin, α 2-M, α 1-PI, factor XII, fibrin, fibrinogen, proMMP2, proTNF α |
| MMP-15 | MT2-MMP, MT-MMP2 | Membrane-type MMPs | Aggrecan, fibronectin, laminin, nidogen, perlecan, tenascin |
| MMP-16 | MT3-MMP, MT-MMP3 | Membrane-type MMPs | Cartilage proteoglycans, casein, collagen III, fibronectin, gelatin, laminin, α 2-M |
| MMP-17 | MT4-MMP, MT-MMP4 | Membrane-type MMPs | Fibrin, fibrinogen, TNF precursor |
| MMP-19 | RASI-1, MMP-18 | Other MMPs | Aggrecan, collagen I, IV, fibronectin, gelatin I, laminin, nidogen, cartilage oligometric matrix protein, casein, tenascin |
| MMP-20 | Enamelysin | Other MMPs | Amelogenin, aggrecan, cartilage oligometric matrix protein |
| MMP-21 | Homologue of Xenopus XMMP | Other MMPs | ND |
| MMP-23 | Cysteine array MMP (CA-MMP), femalysin, MIFR, MMP-21/MMP-22, MMP-23A/MMP-23B1 | Other MMPs | McaPLGLDpaARNH 2 (synthetic MMP substrate) |
| MMP-24 | MT5-MMP, MT-MMP5 | Membrane-type MMPs | Proteoglycans |
| MMP-25 | MT6-MMP, MT-MMP6, leukolysin | Membrane-type MMPs | Collagen IV, gelatin, fibrin, fibronectin |
| MMP-26 | Endometase, matrilysin-2 | Matrilysins | Collagen IV, fibronectin, gelatin I, α 1-PI, fibrinogen, TACE substrates |
| MMP-27 | Other MMPs | ND | |
| MMP-28 | Epilysin | Other MMPs | Casein |
To date, numerous substrates of MMPs have been identified by in vitro and in vivo studies, including collagens, non-collageneous glycoproteins and proteoglycans, which underline their participation in the degradation of ECM proteins. Other ECM components like tenascin, fibronectin and laminin, which often show tumor specific expression are also substrates[29]. So are precursor forms of many growth factors, including tumor growth factor-α (TGF-α). For a more complete overview of specific substrates of MMPs refer to Table 1.
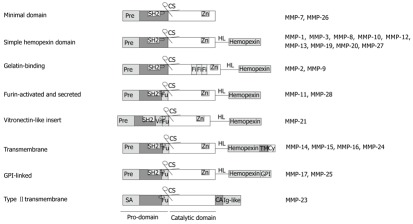
Originally, MMPs were classified according to their respective substrate specificity. However, because of a considerable overlap in substrate preference they are now divided into eight structural subgroups, five of which are secreted and three of which are transmembrane MMPs[12] (Table 1 and Figure 1). Members of each group contain a group-specific prodomain, which is lost during enzyme activation. A zinc-interacting thiol group, and a catalytic domain with a highly conserved zinc-binding site are common to all MMPs, and only MMP-23 lacks an amino-terminal signalling peptide. Connected to the catalytic domain by a hinge linker, a hemopexin-like carboxy-terminal domain is found in all MMPs, except MMP-7, MMP-26 and MMP-23. MMP-2 and MMP-9 contain an additional gelatin-binding domain, which is inserted between the catalytic and the hemopexin domain. All membrane-type (MT)-MMPs have a transmembrane domain added to their C-terminus[30] and contain a furin-like cleavage site, which is important during enzyme activation (Table 1 and Figure 1). This latter site is also found in the furin-activated and secreted MMPs e.g., MMP-11[31].
Figure 1.
Structural groups of matrix metalloproteinases and their domain composition. Pre: Amino-terminal signal sequence, directing matrix metalloproteinases (MMPs) to the endoplasmic reticulum; SA: Signal anchor for cell membrane targeting; Fu: Recognition motif for intracellular furin-like serine proteinases, allowing intracellular activation of MMPs by cutting off the pro-domain at the cleavage site; Vn: Vitronectin-like insert; Fi: Collagen-binding type II repeats of fibronectin, HL: hinge linker, connecting the catalytic domain with the hemopexin domain, which mediates interaction with tissue inhibitors of metalloproteinases (TIMPs), cell-surface molecules and proteolytic substrates; CA: Cysteine array; TM: Single-span transmembrane domain; Cy: Cytoplasmic domain.
Regulation of MMP activity
As MMPs are involved into the breakdown of the ECM in normal tissue, their secretion and activity has to be tightly controlled in order to prevent pathological tissue disruption. Complex regulatory mechanisms of their differential activity involve transcriptional regulation, activation of pro-enzymes and inhibition of active enzymes by specific endogenous inhibitors. Comprehensive reviews are available which specifically focus on this complex process[32-38]. We therefore, concentrate on presenting only a short overview.
Most MMPs are not constitutively expressed in the cell. Their transcription is induced by a variety of growth factors, such as epidermal growth factor (EGF), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF) and various inflammatory cytokines including TNF-α and interleukin-1β (IL-1β)[39]. Physical stress, various chemical agents (e.g., phorbol esters), oncogene products, as well as cell-cell and cell-ECM interactions can also induce or repress the expression of MMPs[24].
MMPs are produced and secreted by cells as inactive zymogens, also referred to as pro-MMPs. These inactive enzymes contain a pro-peptide region with a cysteine-sulphydryl residue near the C-terminal end of the peptide (Figure 1). The zinc ion of the catalytic region, which is essential for MMP activity, is bound to this residue of the pro-peptide, thus blocking the active site. Activation of pro-enzymes begins with the disruption of the cysteine-zinc interaction (cysteine switch) and exposure of the catalytic site[32,40]. Proteinases and non-proteolytic agents such as SH-reactive agents, mercurial compounds, reactive oxygen or denaturants are involved in the activation process[24] with the exception of MMP-11[31], MMP-14[41,42], MMP-16[43] and the mouse homologue of MMP-28 (the human MMP-28 has not yet been investigated)[44], which are activated prior to secretion by furin-like serine proteinases. Moreover, the activation of certain MMPs is dependent on the presence and activity of other MMPs[14], such as activation of MMP-1 by MMP-10[45], or MMP-2 by MMP-14[11,46-48], a fact that may be reflected in the close correlation of their expression patterns. It is also suggested that MMP-15 acts synergistically with MMP-14 in activation of pro-MMP-2[49]. In addition, gelatin-zymography suggested that tumor related overexpression of MMP-24 contributes to generation of the gelatinolytic activity in conjunction with MMP-2[50] and demonstrated the ability of MMP-25 to mediate the membrane activation of pro-MMP-2, thus suggesting that overexpression of this protease by tumor cells facilitates the progression of brain tumors in vivo[51].
The fully active enzyme is generated by proteolytic cleavage of the pro-peptide domain of the partially active intermediate enzyme[11].
Once active, MMPs are regulated by interactions with endogenous inhibitors including α2-macroglobulin, thrombospondin-2, tissue inhibitors of metalloproteinases (TIMPs) and RECK (reversion-inducing cysteine-rich protein with kazal motifs)[12]. Whereas α2-macroglobulins play an important role in the irreversible clearance of MMPs in tissue fluids by forming complexes with them, which are afterwards removed by scavenger receptor-mediated endocytosis[11], the TIMP protein family leads to a locally restricted and reversible inhibition of MMPs. To date, these proteins are the best studied natural inhibitors of MMPs and comprise the four structurally related proteins, TIMP1 to TIMP4. TIMPs are secreted in a soluble form in most tissues and body fluids with the exception of TIMP3, which is closely associated with the ECM[52]. They specifically form non-covalent stoichiometric complexes with the zinc binding sites of active MMPs[53]. Individual TIMPs differ in their ability to modulate the various MMPs. For instance, TIMP1 only inhibits MMP-16 very weakly. TIMP2 and TIMP3 are effective inhibitors of the membrane-type MMPs, e.g., MMP-14, and TIMP3 inhibits MMP-9 with a higher affinity than the other TIMPs[11,54]. TIMP4, in contrast, seems to be a strong inhibitor of all MMPs[55]. However, TIMPs are also involved in the activation of MMPs. Pro-MMP-2 is activated at the plasma membrane through a unique multistep pathway involving both, active and TIMP2-bound MMP-14[46,47], as shown by measuring its gelatinolytic activity using in situ zymography[49,51]. The hemopexin domain of pro-MMP-2 interacts with the C-terminal domain of TIMP2, whereas MMP-14 associates with the N-terminus of TIMP2 and is inhibited in this way. This complex allows an adjacent uninhibited MMP-14 to cleave the N-terminal prodomain of pro-MMP-2, thus generating the intermediate MMP-2, which is then completely activated by removal of the residual portion of the pro-peptide by another MMP-2 molecule[11,48]. Consistently, only the latent form of MMP-2 is found in malignant glioma cells that lack MMP-14 protein[56] and MMP-2 is constitutively produced at low levels during normal tissue maintenance and remodelling[46,57].
MMP EXPRESSION BY GLIOBLASTOMAS
Human glioblastoma cell-lines
Human GBM cell-lines are commonly used for in vitro and in vivo studies of cell migration and invasion[58-62]. Although numerous studies have investigated the expression of selected MMPs in human GBM cell lines[10,13,51,56,63-77], as summerized in Table 2, the only comprehensive study of all 23 MMPs is an analysis of U251 glioma cells by quantitative real time PCR[71]. As it has already been reported that there are differences in the expression patterns of MMPs in different cell-lines, even when they originate from the same type of tissue[67], it is not surprising that MMP expression varies in different glioma cell-lines. It is conspicuous that MMP-13, -17, -19 and -24 were expressed by all analyzed cell-lines, whereas MMP-20 and MMP-21 were not expressed by any of the GBM cells (Table 2). Controversial data on MMP expression have been reported for MMP-2 in U251, MMP-3 in U87, U373 and U138, MMP-7 in U87, U138 and T98G, MMP-8 in U251, MMP-9 in SNB-19, U251, U87, U373, A172 and T98G, MMP-11 in U251, U373 and T98G, MMP-12 in U251 and U138, MMP-14 and -15 in U87 and U373 and MMP-16, -26 and -27 in U251 cells (Table 2). A number of different techniques have been used to detect protease expression patterns including semiquantitative RT-PCR[68,73,75], quantitative real time-PCR[65,71,76], gelatin zymography[13,56,63,65,66,68-70,73], Northern-blotting[56,64,66,69,70], Western-blotting[13,56,66,69,70,73,74], 125I Western-blotting after protein concentration[65] and RNase protection assay[72]. This list implies that the use of different methods may have led to dissimilar conclusions, due to disparate sensitivities and due to comparing mRNA expression with protein expression or protein activity. However, for expression of most MMPs in U251, U87 and U373 cells in most studies reach concordant results, as can be seen in Table 2, suggesting that divergent data may also be caused by other factors. Fluctuations in MMP expression with the number of passages has been reported in some GBM cell-lines[77,78]. It was suggested that these variations in MMP expression may be due to in vitro selection processes or karyotype evolution, where the transcription of either the enzyme and/or its inhibitor may be affected which ultimately leads to an imbalance in the MMP-regulatory network[78]. However, alterations in MMP expression may also depend on the cell environment. MMP-2, -9 and MMP-14 are differentially upregulated by increasing cellular density[79]. MMP-14 expression was enhanced if U87 cells were cultured as neurospheres instead of as monolayers[80]. MMP-12 expression in U251 cells increased during growth in a three-dimensional tenascin-C matrix compared to its expression in a two-dimensional matrix[81]. U87 cells displayed low MMP-7 expression in culture, which increased after the cells were implanted into the brain of RAG 2/γc immune-deficient mice[82], suggesting that the astrocyte environment may also influence MMP expression. Astrocytes in culture produce significant amounts of pro-MMP-2, but no active MMP-2. Co-cultured U251 cells are then able to convert pro-MMP-2 into its active form[83].
Table 2.
Matrix metalloproteinases expression in glioblastoma cell-lines
| SNB-19 | GaMG | U251 | U87 | U373 | U343 | U138 | A172 | T98G | References | |
| MMP-1 | + | + | + | +/- | + | + | + | - | + | [10,63,64,67,71,72,77] |
| MMP-2 | + | + | +/- | + | + | - | + | + | + | [10,56,63-65,67-69,71-73,76] |
| MMP-3 | + | - | + | +/- | +/- | +/- | - | + | [51,64,67,68,71,72] | |
| MMP-7 | + | - | + | + / - | + | +/- | - | +/- | [10,13,64,67,68,71,73,76] | |
| MMP-8 | - | - | + / - | - | - | - | - | - | [67,71,76,77] | |
| MMP-9 | +/- | + | + / - | + / - | +/- | - | - | +/- | +/- | [13,63,64,67,68,72,73,77] |
| MMP-10 | + | + | + | - | + | + | + | - | [67,71,76,77] | |
| MMP-11 | - | + | +/- | - | +/- | + | + | +/- | [10,67,71,77] | |
| MMP-12 | + | - | + / - | + | - | +/- | - | [67,68,71,73] | ||
| MMP-13 | + | + | + | + | + | + | + | + | [67,71,76,77] | |
| MMP-14 | + | + | +/- | +/- | + | + | - | + | [10,56,64,66,67,69,71-74,76] | |
| MMP-15 | + | +/- | +/- | + | + | [10,66,67,71,74] | ||||
| MMP-16 | +/- | + | - | - | + | [10,66,67,71,74,76] | ||||
| MMP-17 | + | + | + | + | + | + | + | [71,77] | ||
| MMP-19 | + | + | + | + | + | + | + | [71,77] | ||
| MMP-20 | - | - | - | - | - | - | - | [71,77] | ||
| MMP-21 | - | - | - | - | - | - | - | [71,77] | ||
| MMP-23 | + | - | + | + | + | + | + | [71,77] | ||
| MMP-24 | + | + | + | + | + | + | + | [71,74,77] | ||
| MMP-25 | - | - | - | - | [71] | |||||
| MMP-26 | - | - | +/- | - | - | - | - | [71,75,77] | ||
| MMP-27 | - | - | +/- | - | - | - | - | [71,77] | ||
| MMP-28 | + | - | + | - | + | + | - | [71,77] |
MMP: Matrix metalloproteinases; +: Expressed; -: Not expressed; +/-: Controversely discussed.
In vivo MMPs are regulated by the surrounding tissue and by growth-factors or cytokines and their downstream signalling pathways[10,16,18,84,85]. In particular, for MMP-9 it has been shown that its production is dependent on a regulation by extracellular signal-regulated kinase (ERK), PKCα/NF-κB and jun amino-terminal kinase (JNK) signaling cascades[84-86]. Glioblastomas are highly hypoxic and hypoxia upregulates MMP-2 mRNA expression in U87, U251, U373 and LN18 glioblastoma cell-lines by activation of the HIF-1α transcription factor, thereby enhancing their invasive potential[87]. Migration and invasion of U87 and T98G GBM cells is also facilitated by NO, which can be found in high concentrations in glioblastoma tissue[88]. NO stimulates MMP-1 expression and activity[88]. EGF raises MMP-14 expression in U251 cells, but does not influence MMP-15, -16 or MMP-24[74]. MMP-2 expression and secretion is induced by IL-6 in U87 cells[89]. However, IL-6 action seems to be cell-line specific, since U343 cells were not affected[89].
The inflammatory cytokine TNF-α and the immunsuppressive cytokine TGF-β have been implicated in migration and invasion of glioma cells in vitro[59,90,91]. In U251 and in U373 cells, TNF-α stimulated the expression of MMP-9 and MMP-19[77]. MMP-1 mRNA expression was significantly increased in U373 cells by TNF-α, whereas its expression in U251 cells remained unaffected. This may be due to the high basal level of MMP-1 expression displayed by U251 cells, where a further increase is not possible, or else it could also be a cell-line specific effect[77]. Such an effect has been observed for MMP-1, -2, -3 and MMP-7 regulation by TNF-α and TGF-β1, which only caused a marked induction of expression in some GBM cell-lines, but not in others[64]. TNF-α enhances the invasivenes of T98G cells through an induction of MMP-3, but has no effect on MMP-1, -2 or MMP-9[92]. However, in U251 cells TNF-α inhibits MMP-2 and decreases invasiveness into the extracellular matrix[93]. In A172 cells, TNF-α induces gene expression and protein secretion of MMP-9[94]. TGF-β1 alone had no effect on MMP-9 production. However, when it was added together with TNF-α a significant dose-dependent inhibition of MMP-9 secretion was observed[94]. TGF-β1 displayed inconsistent effects on adhesion and invasiveness, depending on the cell-line examined. The invasive potential of U138 cells was markedly reduced, whereas U373 cell invasion remained unchanged[95]. TGF-β1 caused a significant induction of MMP-11 and MMP-24 expression in U373 cells, whereas there was no impact on MMP expression in U251 cells[77]. In U87 and LN229 cells, TGF-β upregulates MMP-2[90,91]. Thus, the transcriptional modulation of MMP genes in response to TNF-α or TGF-β is not consistent, but extremely cell-line specific[64].
Together these data indicate that there is a large variety in the MMP expression patterns between different cell-lines, and that these expression patterns can change with duration of cell culture and are highly dependent on specific cell culture conditions and cell-density. Cytokines show divergent effects depending on the cell-line.
MMPs expressed by human malignant gliomas
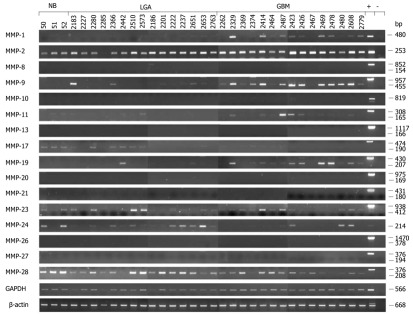
Elevated levels of several MMPs were not only found in cell-lines, but also in malignant glioma tissue samples from patients. Although MMP expression levels are highly variable from one tumor to another (Figure 2)[96,97], their increased expression suggests that they are closely related to malignant progression in vivo[10].
Figure 2.
Expression analysis of matrix metalloproteinases in normal brain and tumor brain samples by semiquantitative real-time reverse transcription PCR. Total RNA from normal brain (NB, lanes 1 to 3), low grade astrocytoma (LGA, lanes 4 to 18) and glioblastoma multiforme tissue samples (GBM, lanes 19 to 33) was used as a template for real-time reverse transcription PCR (RT-PCR) analysis. Primers, specific for each transcript, were designed in flanking exons, resulting in longer amplicons if human genomic DNA was amplified [positive control (+)] and in shorter amplicons representing cDNAs. In several cases (MMP-1, -10 and -24) HBMEC (human brain microvascular endothelial cell) cDNA was used as a positive control. The various cDNA concentrations were normalized to that of the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) which was used as an internal loading control. GAPDH transcripts were amplified in 20 cycles, whereas amplification of MMP transcripts was performed in 33 cycles. All technical procedures, PCR conditions and primer sequences have been published[77].
MMP-1 expression was increased in surgical specimens of GBM compared to LGA and normal brain (NB)[98,99]. In contrast, other groups only found very low gene expression levels in glioma tumor samples[64,100]. We re-analysed the expression of this gene and screened three NB samples, 15 LGA and 15 GBM by semiquantitative RT-PCR (Figure 2)[97]. This analysis confirmed the view of Nakagawa et al[98], since there was a clear increase in MMP-1 mRNA expression in GBM compared to low grade tumors, whereas expression of this gene was not detected in NB tissue (Figure 2)[97]. This increased expression is probably due to a single nucleotide polymorphism in the MMP-1 promoter at position-1607, creating a functional binding site for members of the ETS family of transcription factors[99]. MMP-1 was expressed throughout the tumor section, particularly in the highly cellular areas of the GBM, as determined by immunohistochemistry[97]. There was no specific association with necrosis or the invasive zone. The signal mainly was found in the interstitial matrix, but tumor cells and macrophages also showed strong cytoplasmic staining[97].
RT-PCR, Northern-blotting, Western-blotting and gelatin zymography analysis of surgical tumor samples showed that the gradual expression and activity of MMP-2 is closely related to the malignant progression of human glioblastomas in vivo[49,56,64,69,96,100-107] and may be associated with the invasive behavior of these tumors[108]. Komatsu et al[109] showed that MMP-2 mRNA expression was increased in 62% of glioma samples and protein expression in 38%, however, in contrast to earlier reports, they could not find any correlation between the expression of MMP-2 protein or mRNA and the morphology of the tumors. However, a very similar study analysing 17 LGA, 20 astrocytoma WHO grade III and 12 GBM found a significant elevation of MMP-2 expression with the degree of malignancy of the glioma (53% LGA, 80% astrocytoma WHO grade III and 100% GBM stained positive)[110]. In situ hybridization revealed MMP-2 transcripts to be present in normal neurones and glia, malignant glioma cells and blood vessels[102]. MMP-2 protein expression is restricted to the cytoplasm of tumor cells, as shown by immunohistochemistry[98,100,108,110-112], and the cytoplasm of glial cells around the tumor show strong expression[113]. Additional immunohistochemical analysis of tumor tissue determined intense staining of MMP-2 protein in highly cellular areas and in endothelial cells of tumor blood vessels with a radial spread into the surrounding perivasculature, which suggests an involvement in tissue remodelling during tumor invasion and neoangiogenesis[101-103,110,114,115]. This view is supported by results from a mouse model[116]. GBM tumors derived from cells devoid of MMP-2 exhibited a marked increase in vascular density as well as enhanced vascular branching and sprouting, however, these tumor vessels did not undergo proper maturation and were thus only poorly perfused[116]. The increased but dysfunctional vasculature caused the tumor cells to become more prone to apoptosis, which led to prolonged survival of tumor bearing mice[116]. In humans, MMP-2 expression was correlated with an expression of hepatocyte growth factor (HGF), which has a stimulatory effect on the synthesis of MMP-2[114]. LGA and NB samples showed very weak or no MMP-2 staining[98,100,102] and low levels of HGF[114].
The expression pattern of MMP-7 is very similar to that of MMP-2, although Northern-blot and RT-PCR analysis identified a more diverse expression of its mRNA in native GBM tissues. There was no expression detected in NB, LGA and anaplastic astrocytoma. Therefore, this gene could be closely related to malignant progression of human glioblastomas[64,100,103]. Our data were in line with these findings, showing only a weak MMP-7 immunoreactivity in tumor specimens, around the cytoplasm of tumor cells, macrophages and endothelial cells of small capillaries. Immunostaining of NB samples was restricted to single blood vessel pericytes, supporting a possible role in tumor invasiveness[103]. However, when MMP-7 mRNA expression was analyzed in different primary brain tumors, it showed highly variable levels of expression that were not related to the invasive behavior[82].
Many studies have demonstrated an intimate association between MMP-9 and tumor invasiveness. Data obtained by RT-PCR, Northern-blot, Western-blot and immunohistochemical analyses for MMP-9 were negative in NB tissue, showed weak signals in LGA and strong expression in GBM[64,96,98,100,102-105,107,109,110,117]. Gelatin zymography analyses revealed that MMP-9 activity increases from LGA to malignant tumors[69,96,100,102,105]. MMP-9 is strongly expressed in blood vessels at proliferating margins, as well as tumor cells, as revealed by in situ hybridization[102]. Immunostaining determined MMP-9 localization in the cytoplasm of tumor cells[98,100,108,110-112,117]. In addition, strong staining was seen in the vicinity of necrosis and the tumor vasculature, suggestive of a role in the regulation of tumor neoangiogenesis[102,103,109,110,115]. MMP-9 expression is promoted by epidermal growth factor receptor (EGFR) signalling and the ligand-independent EGFR variant III is frequently overexpressed by primary GBM in contrast to secondary GBM, which are more often characterized by P53 mutations[1]. Expression of active MMP-9 was found in 69% of primary GBM and only in 14% of secondary GBM[118]. In addition, 73% of EGFR-overexpressing GBM, but only 20% of EGFR-negative tumors expressed active MMP-9[118], suggesting a close relation between EGFR-signalling and MMP-9 expression, especially in primary GBM.
Whereas Northern-blotting and RT-PCR analysis identified only a very weak expression of MMP-11[100], other studies have revealed that MMP-11 mRNA expression increased concomitantly with the WHO grading of human gliomas, whereas NB samples remained negative (Figure 2)[97,115]. Immunohistochemistry demonstrates MMP-11 protein expression in the invasive zone of the GBM, predominantly around tumor cells and not in the extracellular matrix itself. MMP-11 staining was also located in the cytoplasm of tumor-associated macrophages and a small number of tumor cells. The highest concentrations of MMP-11, however, were found in the proximity of tumor blood vessels and around their endothelial lining[97]. These data suggest a functional relevance of MMP-11 in GBM development[97,115].
There were no detectable levels of MMP-12 mRNA in both NB and tumor samples by Northern blot analysis[100]. However, MMP-12 mRNA was identified in surgical glioma samples using semiquantitative and quantitative RT-PCR analysis[68,81,119]. Western-blotting confirmed these results[81,96] which is possibly explained by the higher sensitivity of RT-PCR and Western blot detection methods.
Overexpression of MMP-14 in human glioma samples was determined by several studies[9]. Results of Northern blot and real-time PCR expression analysis showed that the level of MMP-14 mRNA was significantly higher in malignant glioblastomas than in low-grade gliomas, whereas it was not detectable in NB tissues[10,49,51,56,69,71,100,104-106]. Consistent with these results, in situ hybridization analysis of MMP-14 mRNA identified its localization in neoplastic astrocytes in glioma specimens[49,120]. Moreover, the latent and active forms of MMP-14 were detected in glioblastoma samples by Western-blot analysis, whereas no MMP-14 protein was found in NB[49]. In malignant glioma sections, the intense heterogeneous immunoreactivity for MMP-14 was seen at the cell membrane and in the cytoplasm of neoplastic astrocytes, the cytoplasm of glial cells around the tumor, endothelial cells and perivascular cells. In low grade gliomas and normal white brain matter it was almost undetectable[49,56,100,105,106,113]. Therefore, the expression of MMP-14 seems to be closely related to the malignant phenotype in vivo.
Analysis of MMP-15 showed that its expression increases gradually with the tumor grade from low-grade glioma to GBM[9]. Strong expression of this gene was determined in malignant tumor samples at both mRNA and protein levels, whereas no expression could be identified in NB and only a weak expression was found in low-grade tumors[71]. In situ hybridization and immunohistochemical analysis of GBM tissues identified signals for MMP-15 in neoplastic astrocytoma cells and some endothelial cells of blood vessels[49]. Moreover, Western-blot analysis identified latent and active forms of MMP-15 in glioma samples, whereas no such species could be detected in NB tissue[49].
MMP-19 expression was not detected in both NB and malignant glioma specimens by Northern-blot analysis[100]. However, our semiquantitative RT-PCR analysis showed a clear increase in the expression of MMP-19 mRNA in high grade tumor tissues compared to low grade tumors, whereas its expression was not detected in NB tissue (Figure 2)[97]. Immunohistochemical staining revealed its expression throughout the tumor section[97].
Llano et al[50,71] examined NB and tumor tissues by Northern-blot analysis and showed that the expression of MMP-24 is related to glioma tumor progression. The MMP-24 transcript is moderately expressed in astrocytoma specimens, strongly expressed in anaplastic astrocytomas and GBM, whereas the few examined samples of NB showed no expression of its mRNA at all. However, since the data on MMP-24 expression in GBM is scarce, we screened NB, LGA and GBM by semiquantitative RT-PCR (Figure 2) and detected a weak expression of the gene in several of the samples, but were not able to confirm any correlation to the tumor grade (Figure 2).
Northern-blot and quantitative RT-PCR analysis of MMP-25 in both, NB and tumor tissues, identified its strong expression in some anaplastic astrocytoma and also expression in GBM, whereas no significant levels were detected in NB tissues[51,71,81].
MMPs without relevance in glioblastoma development
In gliomas a number of MMPs probably have little or even no functional relevance in degradation of the extracellular matrix, as no significant correlation between their expression and the tumor grade could be observed. MMP-3 was only weakly or in some cases not detectable at the mRNA and protein levels in both NB and surgical specimens of patients with malignant gliomas[64,100,103,104,119,121]. Northern-blot and RT-PCR analysis identified very weak expression of the two MT-MMPs, MMP-16 and MMP-17, in different samples, without any correlation to the tumor grade[10,49,51,71,100]. However, one group reported increased expression of MMP-16 in brain tumors compared to normal tissue at the mRNA and protein level[104]. Expression of MMP-8, -10 and -13 could not be detected in both NB and malignant glioma specimens by Northern-blot analysis[100]. Our own analysis confirmed these data by semiquantitative RT-PCR (Figure 2). There was no significant difference in MMP-8 expression between NB and GBM[107], and it has been shown in U251 GBM cells that MMP-8 is epigenetically silenced[76]. Since MMP-8 expression may have tumor-protective functions and has the ability to inhibit melanoma progression and to reduce the metastatic potential of breast and lung cancer cells in both mice and humans[122,123], putatively its repression may contribute to GBM development. MMP-21 expression has been reported to be elevated in mid-grade glioma specimens, but then to decline again in GBM[81]. We could not detect any expression of MMP-21 in the entire tumor panel we analysed (Figure 2).
As yet, the expression of MMP-20, -23, -26, -27 and -28 in glioma has not been covered in the literature. We therefore analysed mRNA levels of these MMPs by semiquantitative RT-PCR (Figure 2). This analysis showed ubiquitous expression of MMP-23 and MMP-28 in all tested samples and detected no expression of MMP-20, -26 and -27, thus suggesting that these MMPs are not involved in astrocytic tumor development (Figure 2).
MMP expression by primary cells derived from GBM specimens
The differences in MMP expression profiles of GBM cell-lines and patient tissue samples led to the question whether primary cells derived from human tumor biopsies will maintain or alter their MMP expression pattern. From four of the GBM analysed (Figure 2), primary cells were isolated, cultured and analysed at passage 1, passage 5 and passage 10[77]. At passage 1, a completely altered MMP expression pattern was seen as compared to the orginal tumor tissue. Again this pattern was not stable, but changed with each further passage[77]. The pattern was similar to the one seen in established GBM cell-lines, although there were some differences. MMP-1, -11, -23 and MMP-24 expression was stronger in the primary cells. MMP-9 expression showed more alterations during passages in primary cells, but was more stably expressed in the cell-lines. MMP-13 and MMP-28 expression was nearly absent in primary cells, whereas it was clearly visible in the cell-lines[77]. In summary, MMP expression is highly variable under cell culture conditions and their expression patterns do not match those seen in the original GBM patient tumor tissue.
OUTLOOK
Expression of MMP-1, -2, -7, -9, -11, -12, -14, -15 and -25 shows correlation with the tumor grade, whereas MMP-3, -8, -10, -13, -16, -17, -20, -21, -23, -26, -27 and -28 do not seem to play a major role during glioblastoma development, since they are either constitutively expressed in NB, LGA and GBM, or they are not expressed at all. The available data for MMP-19 and -24 are contradictory, since some studies including our own suggest their involvement during development of astrocytic tumors, and the results of other groups contradict such a connection. However, the detection of MMP mRNA expression level only offers a first hint, suggesting MMPs might be of functional relevance in glioblastomas. MMP regulation is complex and involves several steps, including signal transduction, transcription factor regulation, inhibitors and interdependency with other MMPs. Studies showing protein concentration, tissue distribution and activity are necessary to gain a more complete picture. So far, comprehensive data are only available for very few MMPs. A correlation of MMPs during activation was shown for MMP-2 in conjunction with MMP-14, -15, -24 and -25[48-51]. A precise understanding of MMP expression and activation will help to identify more specific and effective targets for GBM therapy. The single-agent broad spectrum inhibition of MMPs has been of little clinical benefit[14,15]. However, a simultaneous interference of MMP9 and cathepsin B, the upstream regulator of its activation, by siRNA resulted in decreased glioblastoma cell invasion, tumor growth and angiogenesis in an animal experiment[13]. The inhibition of MMP-2 and MMP-9 in conjunction with temozolomide chemotherapy also showed promising results in cell culture[124,125]. However, COL-3 (6-demethyl-6-deoxy-4-dedimethylaminotetracycline), a compound which targets multiple aspects of MMP regulation such as MMP proenzyme synthesis and activation, did not provide any benefit to GBM patients in a phase I study[126]. On the other hand, phase II clinical trials using the broad-spectrum MMP-inhibitor, marimastat, in conjunction with temozolomide has shown encouraging results[20,21]. The major therapy-related toxicity was joint and tendon pain in 47% of patients and 11% were eliminated from the study because of intolerable joint pain[20]. These multimodal treatment concepts point towards the right therapeutic direction and further tests with a combination of MMP inhibition and chemotherapy seem warranted. A further advancement on these strategies could be the direct local delivery of the inhibitor using novel drug delivery techniques, such as the use of drug-impregnated wafers, convection enhanced delivery, nanoparticles or even the delivery of genes encoding for inhibitory or toxic proteins by virus particles or immune-cells[127-133]. These methods could increase the effectiveness while reducing systemic toxicity. When deciding on appropriate future targets it has to be kept in mind, however, that the MMP expression pattern in established cell lines and primary cells is highly variable and will depend on the individual cell-line, passaging and cell culture conditions.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Stefanie Gerngras and Siglinde Kühnel for their technical assistance.
Footnotes
Supported by Interdisziplinäres Zentrum für Klinische For-schung der Universität Würzburg;, Project B25
Peer reviewer: William Andrew Yeudall, BDS, PhD, Associate Professor, VCU Philips Institute of Oral and Craniofacial Molecular Biology, Virginia Commonwealth University School of Dentistry, 521 N 11th St, Richmond, VA 23298-0566, United States
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC
References
- 1.Reifenberger G, Collins VP. Pathology and molecular genetics of astrocytic gliomas. J Mol Med (Berl) 2004;82:656–670. doi: 10.1007/s00109-004-0564-x. [DOI] [PubMed] [Google Scholar]
- 2.Biernat W, Tohma Y, Yonekawa Y, Kleihues P, Ohgaki H. Alterations of cell cycle regulatory genes in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 1997;94:303–309. doi: 10.1007/s004010050711. [DOI] [PubMed] [Google Scholar]
- 3.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Chandler KL, Prados MD, Malec M, Wilson CB. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993;32:716–20; discussion 720. doi: 10.1227/00006123-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 7.Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM, Maiorka PC, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 8.Friedl P, Hegerfeldt Y, Tusch M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol. 2004;48:441–449. doi: 10.1387/ijdb.041821pf. [DOI] [PubMed] [Google Scholar]
- 9.Fillmore HL, VanMeter TE, Broaddus WC. Membrane-type matrix metalloproteinases (MT-MMPs): expression and function during glioma invasion. J Neurooncol. 2001;53:187–202. doi: 10.1023/a:1012213604731. [DOI] [PubMed] [Google Scholar]
- 10.VanMeter TE, Rooprai HK, Kibble MM, Fillmore HL, Broaddus WC, Pilkington GJ. The role of matrix metalloproteinase genes in glioma invasion: co-dependent and interactive proteolysis. J Neurooncol. 2001;53:213–235. doi: 10.1023/a:1012280925031. [DOI] [PubMed] [Google Scholar]
- 11.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 13.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 14.Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 15.Sela-Passwell N, Rosenblum G, Shoham T, Sagi I. Structural and functional bases for allosteric control of MMP activities: can it pave the path for selective inhibition? Biochim Biophys Acta. 2010;1803:29–38. doi: 10.1016/j.bbamcr.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Levicar N, Nuttall RK, Lah TT. Proteases in brain tumour progression. Acta Neurochir (Wien) 2003;145:825–838. doi: 10.1007/s00701-003-0097-z. [DOI] [PubMed] [Google Scholar]
- 17.Nakada M, Okada Y, Yamashita J. The role of matrix metalloproteinases in glioma invasion. Front Biosci. 2003;8:e261–e269. doi: 10.2741/1016. [DOI] [PubMed] [Google Scholar]
- 18.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005;15:327–341. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kargiotis O, Chetty C, Gondi CS, Tsung AJ, Dinh DH, Gujrati M, Lakka SS, Kyritsis AP, Rao JS. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27:4830–4840. doi: 10.1038/onc.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groves MD, Puduvalli VK, Hess KR, Jaeckle KA, Peterson P, Yung WK, Levin VA. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383–1388. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]
- 21.Levin VA, Phuphanich S, Yung WK, Forsyth PA, Del Maestro R, Perry JR, Fuller GN, Baillet M. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J Neurooncol. 2006;78:295–302. doi: 10.1007/s11060-005-9098-5. [DOI] [PubMed] [Google Scholar]
- 22.Gururajan R, Grenet J, Lahti JM, Kidd VJ. Isolation and characterization of two novel metalloproteinase genes linked to the Cdc2L locus on human chromosome 1p36.3. Genomics. 1998;52:101–106. doi: 10.1006/geno.1998.5401. [DOI] [PubMed] [Google Scholar]
- 23.Velasco G, Pendás AM, Fueyo A, Knäuper V, Murphy G, López-Otín C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274:4570–4576. doi: 10.1074/jbc.274.8.4570. [DOI] [PubMed] [Google Scholar]
- 24.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 25.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 26.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 27.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 31.Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 32.Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 33.Murphy G, Stanton H, Cowell S, Butler G, Knäuper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 34.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 35.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
- 36.Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213:355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- 37.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Borden P, Heller RA. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997;7:159–178. doi: 10.1615/critreveukargeneexpr.v7.i1-2.90. [DOI] [PubMed] [Google Scholar]
- 40.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- 42.Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996;393:101–104. doi: 10.1016/0014-5793(96)00861-7. [DOI] [PubMed] [Google Scholar]
- 43.Kang T, Nagase H, Pei D. Activation of membrane-type matrix metalloproteinase 3 zymogen by the proprotein convertase furin in the trans-Golgi network. Cancer Res. 2002;62:675–681. [PubMed] [Google Scholar]
- 44.Illman SA, Keski-Oja J, Parks WC, Lohi J. The mouse matrix metalloproteinase, epilysin (MMP-28), is alternatively spliced and processed by a furin-like proprotein convertase. Biochem J. 2003;375:191–197. doi: 10.1042/BJ20030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- 46.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 47.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 49.Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol. 1999;154:417–428. doi: 10.1016/S0002-9440(10)65288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llano E, Pendás AM, Freije JP, Nakano A, Knäuper V, Murphy G, López-Otin C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res. 1999;59:2570–2576. [PubMed] [Google Scholar]
- 51.Velasco G, Cal S, Merlos-Suárez A, Ferrando AA, Alvarez S, Nakano A, Arribas J, López-Otín C. Human MT6-matrix metalloproteinase: identification, progelatinase A activation, and expression in brain tumors. Cancer Res. 2000;60:877–882. [PubMed] [Google Scholar]
- 52.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 53.Gomis-Rüth FX, Maskos K, Betz M, Bergner A, Huber R, Suzuki K, Yoshida N, Nagase H, Brew K, Bourenkov GP, et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- 54.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 55.Stratmann B, Farr M, Tschesche H. MMP-TIMP interaction depends on residue 2 in TIMP-4. FEBS Lett. 2001;507:285–287. doi: 10.1016/s0014-5793(01)02987-8. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res. 1996;56:384–392. [PubMed] [Google Scholar]
- 57.Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991;266:14064–14071. [PubMed] [Google Scholar]
- 58.Engebraaten O, Bjerkvig R, Berens ME. Effect of alkyl-lysophospholipid on glioblastoma cell invasion into fetal rat brain tissue in vitro. Cancer Res. 1991;51:1713–1719. [PubMed] [Google Scholar]
- 59.Chicoine MR, Silbergeld DL. Mitogens as motogens. J Neurooncol. 1997;35:249–257. doi: 10.1023/a:1005808315821. [DOI] [PubMed] [Google Scholar]
- 60.Pilkington GJ, Bjerkvig R, De Ridder L, Kaaijk P. In vitro and in vivo models for the study of brain tumour invasion. Anticancer Res. 1997;17:4107–4109. [PubMed] [Google Scholar]
- 61.Hagemann C, Gloger J, Anacker J, Said HM, Gerngras S, Kühnel S, Meyer C, Rapp UR, Kämmerer U, Vordermark D, et al. RAF expression in human astrocytic tumors. Int J Mol Med. 2009;23:17–31. [PubMed] [Google Scholar]
- 62.Hagemann C, Said HM, Flentje M, Roosen K, Vince GH. Proteins involved in cell migration from glioblastoma neurospheres analyzed by overexpression and siRNA-mediated knock-down. Methods Mol Biol. 2010;650:129–143. doi: 10.1007/978-1-60761-769-3_11. [DOI] [PubMed] [Google Scholar]
- 63.Abe T, Mori T, Kohno K, Seiki M, Hayakawa T, Welgus HG, Hori S, Kuwano M. Expression of 72 kDa type IV collagenase and invasion activity of human glioma cells. Clin Exp Metastasis. 1994;12:296–304. doi: 10.1007/BF01753836. [DOI] [PubMed] [Google Scholar]
- 64.Nakano A, Tani E, Miyazaki K, Yamamoto Y, Furuyama J. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gliomas. J Neurosurg. 1995;83:298–307. doi: 10.3171/jns.1995.83.2.0298. [DOI] [PubMed] [Google Scholar]
- 65.Uhm JH, Dooley NP, Villemure JG, Yong VW. Glioma invasion in vitro: regulation by matrix metalloprotease-2 and protein kinase C. Clin Exp Metastasis. 1996;14:421–433. doi: 10.1007/BF00128958. [DOI] [PubMed] [Google Scholar]
- 66.Shofuda K, Moriyama K, Nishihashi A, Higashi S, Mizushima H, Yasumitsu H, Miki K, Sato H, Seiki M, Miyazaki K. Role of tissue inhibitor of metalloproteinases-2 (TIMP-2) in regulation of pro-gelatinase A activation catalyzed by membrane-type matrix metalloproteinase-1 (MT1-MMP) in human cancer cells. J Biochem. 1998;124:462–470. doi: 10.1093/oxfordjournals.jbchem.a022136. [DOI] [PubMed] [Google Scholar]
- 67.Giambernardi TA, Grant GM, Taylor GP, Hay RJ, Maher VM, McCormick JJ, Klebe RJ. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998;16:483–496. doi: 10.1016/s0945-053x(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 68.Wagner S, Stegen C, Bouterfa H, Huettner C, Kerkau S, Roggendorf W, Roosen K, Tonn JC. Expression of matrix metalloproteinases in human glioma cell lines in the presence of IL-10. J Neurooncol. 1998;40:113–122. doi: 10.1023/a:1006146405880. [DOI] [PubMed] [Google Scholar]
- 69.Hur JH, Park MJ, Park IC, Yi DH, Rhee CH, Hong SI, Lee SH. Matrix metalloproteinases in human gliomas: activation of matrix metalloproteinase-2 (MMP-2) may be correlated with membrane-type-1 matrix metalloproteinase (MT1-MMP) expression. J Korean Med Sci. 2000;15:309–314. doi: 10.3346/jkms.2000.15.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kondraganti S, Mohanam S, Chintala SK, Kin Y, Jasti SL, Nirmala C, Lakka SS, Adachi Y, Kyritsis AP, Ali-Osman F, et al. Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res. 2000;60:6851–6855. [PubMed] [Google Scholar]
- 71.Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, Edwards DR. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol Cancer Res. 2003;1:333–345. [PubMed] [Google Scholar]
- 72.Kim SY, Jung SH, Kim HS. Curcumin is a potent broad spectrum inhibitor of matrix metalloproteinase gene expression in human astroglioma cells. Biochem Biophys Res Commun. 2005;337:510–516. doi: 10.1016/j.bbrc.2005.09.079. [DOI] [PubMed] [Google Scholar]
- 73.Wild-Bode C, Weller M, Wick W. Molecular determinants of glioma cell migration and invasion. J Neurosurg. 2001;94:978–984. doi: 10.3171/jns.2001.94.6.0978. [DOI] [PubMed] [Google Scholar]
- 74.Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fillmore HL. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. Neuro Oncol. 2004;6:188–199. doi: 10.1215/S1152851703000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng Y, Li W, Li Y, Yang H, Xu H, Liang S, Zhang L, Li Y. Expression of Matrix Metalloproteinase-26 promotes human glioma U251 cell invasion in vitro and in vivo. Oncol Rep. 2010;23:69–78. [PubMed] [Google Scholar]
- 76.Chernov AV, Baranovskaya S, Golubkov VS, Wakeman DR, Snyder EY, Williams R, Strongin AY. Microarray-based transcriptional and epigenetic profiling of matrix metalloproteinases, collagens, and related genes in cancer. J Biol Chem. 2010;285:19647–19659. doi: 10.1074/jbc.M109.088153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hagemann C, Anacker J, Haas S, Riesner D, Schömig B, Ernestus R-I, Vince GH. Comparative expression pattern of Matrix-Metalloproteinaes in human glioblastoma cell-lines and primary cultures. BMC Res Notes. 2010;3:293. doi: 10.1186/1756-0500-3-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossi M, Rooprai HK, Maidment SL, Rucklidge GJ, Pilkington GJ. The influence of sequential, in vitro passage on secretion of matrix metalloproteinases by human brain tumour cells. Anticancer Res. 1996;16:121–128. [PubMed] [Google Scholar]
- 79.Trog D, Yeghiazaryan K, Fountoulakis M, Friedlein A, Moenkemann H, Haertel N, Schueller H, Breipohl W, Schild H, Leppert D, et al. Pro-invasive gene regulating effect of irradiation and combined temozolomide-radiation treatment on surviving human malignant glioma cells. Eur J Pharmacol. 2006;542:8–15. doi: 10.1016/j.ejphar.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 80.Annabi B, Laflamme C, Sina A, Lachambre MP, Béliveau R. A MT1-MMP/NF-kappaB signaling axis as a checkpoint controller of COX-2 expression in CD133+ U87 glioblastoma cells. J Neuroinflammation. 2009;6:8. doi: 10.1186/1742-2094-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarkar S, Nuttall RK, Liu S, Edwards DR, Yong VW. Tenascin-C stimulates glioma cell invasion through matrix metalloproteinase-12. Cancer Res. 2006;66:11771–11780. doi: 10.1158/0008-5472.CAN-05-0470. [DOI] [PubMed] [Google Scholar]
- 82.Rome C, Arsaut J, Taris C, Couillaud F, Loiseau H. MMP-7 (matrilysin) expression in human brain tumors. Mol Carcinog. 2007;46:446–452. doi: 10.1002/mc.20293. [DOI] [PubMed] [Google Scholar]
- 83.Le DM, Besson A, Fogg DK, Choi KS, Waisman DM, Goodyer CG, Rewcastle B, Yong VW. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci. 2003;23:4034–4043. doi: 10.1523/JNEUROSCI.23-10-04034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lakka SS, Jasti SL, Kyritsis AP, Yung WK, Ali-Osman F, Nicolson GL, Rao JS. Regulation of MMP-9 (type IV collagenase) production and invasiveness in gliomas by the extracellular signal-regulated kinase and jun amino-terminal kinase signaling cascades. Clin Exp Metastasis. 2000;18:245–252. doi: 10.1023/a:1006724826083. [DOI] [PubMed] [Google Scholar]
- 85.Lin CW, Shen SC, Chien CC, Yang LY, Shia LT, Chen YC. 12-O-tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression. J Cell Physiol. 2010;225:472–481. doi: 10.1002/jcp.22226. [DOI] [PubMed] [Google Scholar]
- 86.Gessi S, Sacchetto V, Fogli E, Merighi S, Varani K, Baraldi PG, Tabrizi MA, Leung E, Maclennan S, Borea PA. Modulation of metalloproteinase-9 in U87MG glioblastoma cells by A3 adenosine receptors. Biochem Pharmacol. 2010;79:1483–1495. doi: 10.1016/j.bcp.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Fujiwara S, Nakagawa K, Harada H, Nagato S, Furukawa K, Teraoka M, Seno T, Oka K, Iwata S, Ohnishi T. Silencing hypoxia-inducible factor-1alpha inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol. 2007;30:793–802. [PubMed] [Google Scholar]
- 88.Pullen NA, Fillmore HL. Induction of matrix metalloproteinase-1 and glioma cell motility by nitric oxide. J Neurooncol. 2010;96:201–209. doi: 10.1007/s11060-009-9965-6. [DOI] [PubMed] [Google Scholar]
- 89.Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep. 2010;23:1553–1559. doi: 10.3892/or_00000795. [DOI] [PubMed] [Google Scholar]
- 90.Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech. 2001;52:401–410. doi: 10.1002/1097-0029(20010215)52:4<401::AID-JEMT1025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 91.Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001;53:177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 92.Cheng SM, Xing B, Li JC, Cheung BK, Lau AS. Interferon-gamma regulation of TNFalpha-induced matrix metalloproteinase 3 expression and migration of human glioma T98G cells. Int J Cancer. 2007;121:1190–1196. doi: 10.1002/ijc.22729. [DOI] [PubMed] [Google Scholar]
- 93.Qin H, Moellinger JD, Wells A, Windsor LJ, Sun Y, Benveniste EN. Transcriptional suppression of matrix metalloproteinase-2 gene expression in human astroglioma cells by TNF-alpha and IFN-gamma. J Immunol. 1998;161:6664–6673. [PubMed] [Google Scholar]
- 94.Esteve PO, Tremblay P, Houde M, St-Pierre Y, Mandeville R. In vitro expression of MMP-2 and MMP-9 in glioma cells following exposure to inflammatory mediators. Biochim Biophys Acta. 1998;1403:85–96. doi: 10.1016/s0167-4889(98)00020-2. [DOI] [PubMed] [Google Scholar]
- 95.Paulus W, Baur I, Huettner C, Schmausser B, Roggendorf W, Schlingensiepen KH, Brysch W. Effects of transforming growth factor-beta 1 on collagen synthesis, integrin expression, adhesion and invasion of glioma cells. J Neuropathol Exp Neurol. 1995;54:236–244. doi: 10.1097/00005072-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 96.Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, Del Maestro R, Béliveau R. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis. 1999;17:555–566. doi: 10.1023/a:1006760632766. [DOI] [PubMed] [Google Scholar]
- 97.Stojic J, Hagemann C, Haas S, Herbold C, Kühnel S, Gerngras S, Roggendorf W, Roosen K, Vince GH. Expression of matrix metalloproteinases MMP-1, MMP-11 and MMP-19 is correlated with the WHO-grading of human malignant gliomas. Neurosci Res. 2008;60:40–49. doi: 10.1016/j.neures.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg. 1994;81:69–77. doi: 10.3171/jns.1994.81.1.0069. [DOI] [PubMed] [Google Scholar]
- 99.McCready J, Broaddus WC, Sykes V, Fillmore HL. Association of a single nucleotide polymorphism in the matrix metalloproteinase-1 promoter with glioblastoma. Int J Cancer. 2005;117:781–785. doi: 10.1002/ijc.21207. [DOI] [PubMed] [Google Scholar]
- 100.Lampert K, Machein U, Machein MR, Conca W, Peter HH, Volk B. Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol. 1998;153:429–437. doi: 10.1016/S0002-9440(10)65586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- 102.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vince GH, Wagner S, Pietsch T, Klein R, Goldbrunner RH, Roosen K, Tonn JC. Heterogeneous regional expression patterns of matrix metalloproteinases in human malignant gliomas. Int J Dev Neurosci. 1999;17:437–445. doi: 10.1016/s0736-5748(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 104.Pagenstecher A, Wussler EM, Opdenakker G, Volk B, Campbell IL. Distinct expression patterns and levels of enzymatic activity of matrix metalloproteinases and their inhibitors in primary brain tumors. J Neuropathol Exp Neurol. 2001;60:598–612. doi: 10.1093/jnen/60.6.598. [DOI] [PubMed] [Google Scholar]
- 105.Munaut C, Noël A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer. 2003;106:848–855. doi: 10.1002/ijc.11313. [DOI] [PubMed] [Google Scholar]
- 106.Nakada M, Kita D, Futami K, Yamashita J, Fujimoto N, Sato H, Okada Y. Roles of membrane type 1 matrix metalloproteinase and tissue inhibitor of metalloproteinases 2 in invasion and dissemination of human malignant glioma. J Neurosurg. 2001;94:464–473. doi: 10.3171/jns.2001.94.3.0464. [DOI] [PubMed] [Google Scholar]
- 107.Varga I, Hutóczki G, Petrás M, Scholtz B, Mikó E, Kenyeres A, Tóth J, Zahuczky G, Bognár L, Hanzély Z, et al. Expression of invasion-related extracellular matrix molecules in human glioblastoma versus intracerebral lung adenocarcinoma metastasis. Cent Eur Neurosurg. 2010;71:173–180. doi: 10.1055/s-0030-1249698. [DOI] [PubMed] [Google Scholar]
- 108.Kunishio K, Okada M, Matsumoto Y, Nagao S. Matrix metalloproteinase-2 and -9 expression in astrocytic tumors. Brain Tumor Pathol. 2003;20:39–45. doi: 10.1007/BF02483445. [DOI] [PubMed] [Google Scholar]
- 109.Komatsu K, Nakanishi Y, Nemoto N, Hori T, Sawada T, Kobayashi M. Expression and quantitative analysis of matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor Pathol. 2004;21:105–112. doi: 10.1007/BF02482184. [DOI] [PubMed] [Google Scholar]
- 110.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20:65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 111.Tews DS, Nissen A. Expression of adhesion factors and degrading proteins in primary and secondary glioblastomas and their precursor tumors. Invasion Metastasis. 1999;18:271–284. doi: 10.1159/000024520. [DOI] [PubMed] [Google Scholar]
- 112.Raithatha SA, Muzik H, Muzik H, Rewcastle NB, Johnston RN, Edwards DR, Forsyth PA. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro Oncol. 2000;2:145–150. doi: 10.1093/neuonc/2.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nagashima G, Suzuki R, Asai J, Fujimoto T. Immunohistochemical analysis of reactive astrocytes around glioblastoma: an immunohistochemical study of postmortem glioblastoma cases. Clin Neurol Neurosurg. 2002;104:125–131. doi: 10.1016/s0303-8467(01)00197-4. [DOI] [PubMed] [Google Scholar]
- 114.Yano H, Hara A, Murase S, Hayashi K, Ando H, Shinoda J, Shimokawa K, Sakai N. Expression of hepatocyte growth factor and matrix metalloproteinase-2 in human glioma. Brain Tumor Pathol. 2001;18:7–12. doi: 10.1007/BF02478919. [DOI] [PubMed] [Google Scholar]
- 115.Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res. 2003;23:3937–3944. [PubMed] [Google Scholar]
- 116.Du R, Petritsch C, Lu K, Liu P, Haller A, Ganss R, Song H, Vandenberg S, Bergers G. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008;10:254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rao JS, Yamamoto M, Mohaman S, Gokaslan ZL, Fuller GN, Stetler-Stevenson WG, Rao VH, Liotta LA, Nicolson GL, Sawaya RE. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996;14:12–18. doi: 10.1007/BF00157681. [DOI] [PubMed] [Google Scholar]
- 118.Choe G, Park JK, Jouben-Steele L, Kremen TJ, Liau LM, Vinters HV, Cloughesy TF, Mischel PS. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res. 2002;8:2894–2901. [PubMed] [Google Scholar]
- 119.Tonn JC, Kerkau S, Hanke A, Bouterfa H, Mueller JG, Wagner S, Vince GH, Roosen K. Effect of synthetic matrix-metalloproteinase inhibitors on invasive capacity and proliferation of human malignant gliomas in vitro. Int J Cancer. 1999;80:764–772. doi: 10.1002/(sici)1097-0215(19990301)80:5<764::aid-ijc22>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 120.Forsyth PA, Laing TD, Gibson AW, Rewcastle NB, Brasher P, Sutherland G, Johnston RN, Edwards DR. High levels of gelatinase-B and active gelatinase-A in metastatic glioblastoma. J Neurooncol. 1998;36:21–29. doi: 10.1023/a:1005879027267. [DOI] [PubMed] [Google Scholar]
- 121.Vince GH, Herbold C, Klein R, Kühl J, Pietsch T, Franz S, Roosen K, Tonn JC. Medulloblastoma displays distinct regional matrix metalloprotease expression. J Neurooncol. 2001;53:99–106. doi: 10.1023/a:1012241031138. [DOI] [PubMed] [Google Scholar]
- 122.Gutiérrez-Fernández A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN, et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 123.Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, Davis S, Wang C, Cronin JC, Agrawal NS, et al. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–520. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, Sioka C, Rao JS. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–21892. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 125.Gabelloni P, Da Pozzo E, Bendinelli S, Costa B, Nuti E, Casalini F, Orlandini E, Da Settimo F, Rossello A, Martini C. Inhibition of metalloproteinases derived from tumours: new insights in the treatment of human glioblastoma. Neuroscience. 2010;168:514–522. doi: 10.1016/j.neuroscience.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 126.Rudek MA, New P, Mikkelsen T, Phuphanich S, Alavi JB, Nabors LB, Piantadosi S, Fisher JD, Grossman SA. Phase I and pharmacokinetic study of COL-3 in patients with recurrent high-grade gliomas. J Neurooncol. 2011;105:375–381. doi: 10.1007/s11060-011-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;6:539–546. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Laquintana V, Trapani A, Denora N, Wang F, Gallo JM, Trapani G. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6:1017–1032. doi: 10.1517/17425240903167942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soni V, Jain A, Khare P, Gulbake A, Jain SK. Potential approaches for drug delivery to the brain: past, present, and future. Crit Rev Ther Drug Carrier Syst. 2010;27:187–236. doi: 10.1615/critrevtherdrugcarriersyst.v27.i3.10. [DOI] [PubMed] [Google Scholar]
- 131.Kroeger KM, Muhammad AK, Baker GJ, Assi H, Wibowo MK, Xiong W, Yagiz K, Candolfi M, Lowenstein PR, Castro MG. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discov Med. 2010;10:293–304. [PMC free article] [PubMed] [Google Scholar]
- 132.Invernici G, Cristini S, Alessandri G, Navone SE, Canzi L, Tavian D, Redaelli C, Acerbi F, Parati EA. Nanotechnology advances in brain tumors: the state of the art. Recent Pat Anticancer Drug Discov. 2011;6:58–69. doi: 10.2174/157489211793979990. [DOI] [PubMed] [Google Scholar]
- 133.Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8:415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]