Abstract
Adrenomedullin is a highly conserved peptide implicated in a variety of physiological processes ranging from pregnancy and embryonic development to tumor progression. This review highlights past and present studies that have contributed to our current appreciation of the important roles adrenomedullin plays in both normal and disease conditions. We provide a particular emphasis on the functions of adrenomedullin in vascular endothelial cells and how experimental approaches in genetic mouse models have helped to drive the field forward.
Keywords: Adrenomedullin, angiogenesis, endothelial, lymphangiogenesis, mouse model, permeability, CLR, RAMPs.
INTRODUCTION
The Multifunctional Adrenomedullin Peptide
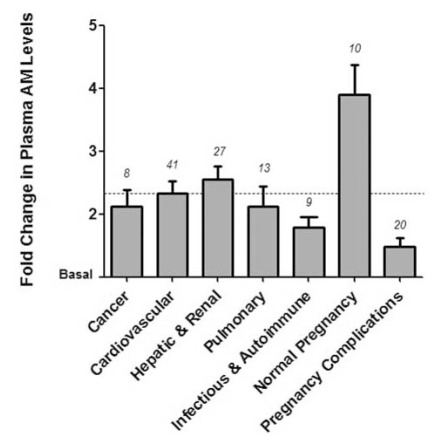
Adrenomedullin (gene=Adm; protein=AM) is a highly conserved multifunctional peptide that is implicated in a wide variety of physiological processes including angiogenesis and cardiovascular homeostasis [1]. For over a decade, the association of ~2-fold elevations in plasma levels of AM peptide with a wide variety of cardiovascular disease conditions has prompted intense inquiry into understanding the functions and roles of AM in human disease (Fig. 1). Moreover, the recent development of highly precise methods for the quantitation of midregional proadrenomedullin (MR-proADM) as a reliable surrogate of mature AM plasma levels [2], has paved the way for the introduction of AM as a clinically useful biomarker for the staging of adverse cardiovascular events, including myocardial infarction, sepsis and community acquired pneumonia [3-6]. While it is clear that AM can elicit powerful effects on vascular smooth muscle cells and thus acutely modulate vascular tone, numerous studies in the past 5 years have elucidated essential functions of AM on vascular endothelial cells. In the following sections we review the multi-faceted role of AM in endothelial cells during development, consider how perturbations in AM signaling may lead to vascular pathologies, and highlight recent discoveries regarding AM that have contributed in substantial ways to the broader field of vascular biology. Many of these discoveries have been unraveled through the use of sophisticated genetic animal models (Tables 1 and 2), and so we have placed a special emphasis on describing the merits and shortcomings of these approaches and also highlighting current questions that are of predominant interest to the field today.
Fig. (1).
Fold Change in Plasma Adrenomedullin Levels in a Variety of Human Conditions. Bars indicate average fold change in circulating AM levels in various disease categories or conditions based on published human clinical data. The dashed horizontal line at 2.33 represents the average fold increase in plasma AM levels across all conditions depicted. Number above each bar indicates the number of published observations assessing plasma AM levels in each category. The clinical papers that were used for our analysis are listed according to the following broad categories: cancer [117- 122], cardiovascular [4, 117, 119, 123-150], hepatic and renal [130, 131, 133, 134, 151-162], pulmonary [6, 131, 163-168], infectious & autoimmune [169-175], normal pregnancy [63, 64, 66-67, 176-180], and pregnancy complications [63, 67, 180-192].
Table 1.
Gene Targeted Mouse Models for Studying Adrenomedullin Signaling
| Mouse Model | Development or Adulthood | Result | Reference |
|---|---|---|---|
| Adm-/- | Development | -Embryonic lethal (e14.5), edema, smaller hearts, reduced myocyte proliferation and increased apoptosis, increased left ventricle trabecularization, thinner aorta and carotid artery walls, increased vascular permeability, hypoplastic jugular lymph sac | [22,30-32] |
| Adm+/- | Adulthood |
-Pregnancy: Disrupted fertility, placentation, and fetal growth -Cardiovascular: Increased damage including hypertrophy, reactive oxygen species (ROS), and fibrosis -Liver cold injury: increased apoptosis of the sinusoidal endothelial cells |
[32,70,81,90] |
| Admfl/fl/Tubulin Tα-1-Cre+ | Adulthood | -High anxiety, hyeractive, impaired motor coordination | [116] |
| Calcrl-/- | Development | -Embryonic lethal (e13.5), similar phenotype as Adm-/- mice | [22,33] |
| CalcrlLoxP/-/Tie2Cre+ | Development | -Embryonic lethal (e16.5) and recapitulation of Adm-/-, Calcrl-/-, and Ramp2-/-phenotype | [22] |
| Ramp2-/- and Ramp2fl/fl/CAG-Cre+ | Development | -Embryonic lethal (e15.5), similar phenotype as Adm-/- mice | [18,22,34] |
| Ramp2+/- | Adulthood |
-Increased vascular permeability and decreased neovascularization -Liver cold injury: increased apoptosis of the sinusoidal endothelial cells |
[18,90] |
| PAM-/- | Development | -Embryonic lethal (e14.5) and phenocopy of Adm-/-, Calcrl-/-, and Ramp2-/-mice due to loss of amidation of AM peptide | [35] |
Adrenomedullin (Adm); Calcitonin receptor-like receptor (Calcrl); Receptor activity modifying protein (RAMP); Peptidylglycine alpha-amidating monooxygenase (PAM)
Table 2.
Vascular Assays for Studying Adrenomedullin Function
| Assay | Result | Reference |
|---|---|---|
| Atherogenic Model | -Atherogenic diet and AM treatment in ApoE-/-mice resulted in reduced formation of atherosclerotic lesions | [87] |
| Tail microlymphography | -AM injected mice showed reduced permeability of the dermal lymphatic capillaries | [25] |
| Matrigel plug | -AM increased vascular regeneration -Ramp2+/- mice exhibited reduced neovascularization | [12,13,18] |
| Aortic ring | -Ramp2+/-mice exhibited reduced neovascularization in response to growth factor stimulation | [18] |
| AngII/high-salt | AM+/- mice exhibited increased reactive oxygen species (ROS), vascular fibrosis, and intimal thickening | [39] |
| Prolonged mechanical ventilation | -AM treatment reduced lung vascular permeability resulting from ventilator use | [21] |
| Chronic cerebral hypofusion | -AM promoted arteriogenesis and angiogenesis | [98] |
| Hind-limb ischemia | -AM promotes endothelial cell proliferation and capillary formation -Adm+/- mice showed reduced blood flow and capillary development | [58] |
| Wound healing (Pressure Ulcer -Ischemia reperfusion model) | -AM reduced wound area and increased angiogenesis and lymphangiogenesis | [99] |
| Tail lymphedema | -AM improved lymphedema and increased number of lymph and blood vessels | [11] |
| Tumor xenografts | -Blocking AM signaling results in reduced vascular development | [58, 114, 115] |
Adrenomedullin GPCR-Mediated Signaling in Endothelial Cells
G-protein coupled receptors (GPCRs) are widely expressed proteins that span the cell membrane 7 times and respond to a variety of stimuli including peptides, proteins, small organic compounds, lipids, amino acids, and cations. AM binds and signals through the GPCR calcitonin receptor-like receptor (gene=Calcrl; protein=CLR). The discovery of a novel class of GPCR associated proteins called receptor activity-modifying proteins (gene=Ramp; protein=RAMP) [7] provided insight into how GPCRs signal. The RAMPs are single-pass transmembrane accessory proteins that regulate the translocation of GPCRs to the plasma membrane as well as provide ligand specificity to these receptors. The tissue specific and temporal expression pattern of RAMPs determines the responsiveness of GPCRs to particular ligands. For example, AM binds to the CLR receptor when CLR is associated with either RAMP2 or RAMP3. However, co-expression of CLR with RAMP1 changes the ligand specificity to another potent vasodilator called calcitonin gene-related peptide (CGRP), a related family member of the AM peptide. The ability of CLR to bind multiple ligands provides a unique mechanism by which the receptor can initiate a variety of signaling pathways. Since the AM receptor CLR and the 3 mammalian RAMPs are highly expressed in the vasculature, this cell signaling paradigm is being intensely investigated to determine how it can be exploited for the potential treatment of conditions such as pulmonary hypertension [8], cardiovascular disorders [9], and the inhibition of cancer metastasis [10].
The binding of AM to its receptor CLR results in a myriad of downstream effects including modulation of endothelial cell survival, proliferation, and vessel permeability. For example, AM-induced proliferation and migration of lymphatic endothelial cells is mediated in part by cAMP and downstream MEK/ERK pathways [11]. Similar results were shown using cultured human umbilical vein endothelial cells (HUVECs). AM-mediated induction of HUVEC proliferation and migration through activation of PKA, PI3K, and focal adhesion kinase were observed and then further substantiated in whole animal studies [12, 13]. AM induced the proliferation and migration of cultured HUVECs [12] and numerous studies have shown a direct role for AM in endothelial growth and survival [14-16].
Using in vitro experiments, AM was found to regulate the permeability and migration of HUVECs [17]. Previous studies indicated that adult Ramp2+/- mice had increased vascular permeability and overexpression of Ramp2 in BECs resulted in reduced permeability [18]. AM also reduces the permeability of HUVECs and pulmonary artery endothelial cells treated with permeabilizing agents including hydrogen peroxide and thrombin [19]. AM has been shown to regulate the transport of molecules across the blood brain barrier in cerebral endothelial cells by modulating permeability [20]. In cerebral endothelial cells, AM regulated various functions of the blood brain barrier including increasing transendothelial electrical resistance, reducing fluid-phase endocytosis, and reducing permeability for sodium fluorescein which indicate that the cerebral endothelial cell junctions are tightened by AM [20]. Also in an in vivo model, AM treatment reduced lung vascular permeability resulting from ventilator use in a mouse model where prolonged mechanical ventilation was administered [21]. Overall, these data provide evidence for the role of AM as a junctional tightening factor to help regulate endothelial cell permeability.
Although AM functions to promote endothelial cell growth and proliferation in both the blood and lymphatic vasculatures, Fritz-Six et al. have shown that there is an enhanced effect of AM on lymphatic endothelial cells (LECs) as compared to blood endothelial cells (BECs) [22]. This biological distinction in AM function is based upon the finding that LECs are enriched in the expression of AM and its receptor components, Calcrl and Ramp2 [22-24]. This increase in Calcrl expression is mediated in part by induction of the transcriptional regulator of lymphatic specification, Prox1 [22]. It is therefore not surprising that loss of any component of the AM signaling axis (Adm, Calcrl, or Ramp2) results in embryonic lethality associated with profound lymphatic vascular defects [22]. Furthermore, several in vitro and in vivo experiments reveal that AM controls lymphatic permeability and flow through reorganization of junctional proteins ZO-1 and an adherens protein VE-Cadherin, independent of changes in junctional protein gene expression [25]. Administration of AM to a monolayer of LECs resulted in tightening of the lymphatic endothelial barrier by reorganization of a tight junction protein at the plasma membrane to form continuous cell-cell contacts. Through the use of in vivo tail microlymphography, local administration of AM in a SvEv129/6 mouse tail resulted in decreased velocity of lymph uptake from the interstitial space and movement through the lymphatic dermal capillaries in the tail [25]. Thus, it becomes critically important to consider the pleiotropic effects of AM not just on blood endothelial cells, but also on neighboring lymphatic vessels—a dynamic that may ultimately help resolve the complex functions of AM peptide in cardiovascular disease, tumor progression and inflammation.
While activation of GPCRs typically leads to induction of classical second messenger signaling systems, it is now appreciated that more complex levels of regulation exist [26, 27]. Therefore, it is not surprising that pathway cross-talk is one mechanism through which AM modulates certain endothelial cell functions. For example, Yurugi-Kobayashi et al. describe a novel embryonic stem cell differentiation system to study mechanisms of arterial-venous specification. They demonstrated that coordinated signaling of AM/cAMP, VEGF, and Notch induces arterial endothelial cell differentiation from vascular progenitors [28]. Furthermore, GPCR-induced transactivation of receptor tyrosine kinases is another mechanism that allows interaction between signaling molecules. Evidence exists that AM and VEGF pathways are likely to interact in endothelial cells. Although an earlier study claimed that AM-induced capillary tube formation in HUVECs was independent of VEGF activation [14], a more recent study by Guidolin et al. demonstrated that VEGFR2 inactivation inhibited AM-mediated angiogenesis in HUVECs [29]. This latter finding suggests that the pro-angiogenic effects of AM require transactivation of the receptor tyrosine kinase VEGFR2. Although controversy still exists regarding the degree of cooperation between pathways, it is certainly intriguing to consider that regulation of endothelial cell biology may very likely involve coordination of multiple signaling molecules. We now must begin to unravel these complexities and elucidate whether these interactions occur differentially in blood and lymphatic endothelial cells and identify the intermediate molecular players involved in pathway cross-talk in the vasculature.
DEVELOPMENT
Endothelial Adrenomedullin Signaling is Essential for Embryonic Development
Work by multiple independent groups has established the importance of AM signaling during development. The use of gene targeted mouse models clearly indicates that functional AM signaling is essential for embryonic survival. The genetic ablation of Adm [30-32], Calcrl [33], Ramp2 [18, 22, 34] or the enzyme responsible for functional AM amidation, peptidylglycine alpha-amidating monooxygenase (PAM) [35] all result in midgestational lethality associated with severe interstitial edema and cardiovascular defects. The conserved phenotypes between these knockout (KO) mice is compelling genetic evidence that the CLR/RAMP2 complex is the main receptor of AM during development, and also is the first in vivo confirmation that RAMP2 functionally interacts with CLR [22].
Although the overt phenotypes of these KO mice are conserved, the physiological cause of edema and lethality is still debated. One possible hypothesis is that loss of AM signaling causes developmental cardiac abnormalities that lead to heart failure, thus resulting in edema and death that is similar to previously characterized KO mice with developmental heart failure [36-38]. Supporting this line of thought, our lab showed that Adm-/-, Calcrl-/-, and Ramp2-/- mice have smaller hearts due to decreased myocyte proliferation and increased apoptosis. Additionally, they have increased left ventricle trabecularization, which leads to decreased ventricular volume [22, 30, 33]. However, an alternative hypothesis is that vascular defects are responsible for the phenotypes, since Adm [30], Calcrl [33], and Ramp2 [18] are abundantly expressed in the developing endothelium and vascular smooth muscle cells (vSMC). To help resolve between the two hypotheses, we generated an endothelial-specific Calcrl-/- mouse using a Tie2 promoter to drive Cre recombinase expression which recapitulated the phenotype observed in global KO mice [22], indicating that AM signaling in endothelial cells is essential for embryonic development. A remaining caveat to this conclusion is the fact that Tie2-Cre mediated excision also occurs in developing endocardial cells. Therefore, to definitively determine if cardiac abnormalities contribute to this phenotype the reverse experiment using Cre lines specific to cardiac myocytes would be beneficial.
Although vascular defects are responsible for the edema in these KO mice, it remained unclear whether defects in the blood or lymphatic endothelium were the principle cause of the phenotypes. Given the role of AM in regulating vascular permeability, it seems reasonable that loss of AM signaling could lead to increased vascular permeability and a resulting build up of interstitial fluid. In support of this idea, the KO mice have thinner aorta and carotid artery walls due to a decrease in vSMC proliferation [18, 30, 33], although the endothelium lining the aorta appeared to be normal [33]. There are reported abnormalities in endothelial basement membranes and a down-regulation of junctional proteins in Adm-/- and Ramp2-/- embryos that may lead to increased vascular permeability and hemorrhage [18, 31], but these phenotypes were observed in a small proportion of animals and not conserved in all studies. In addition, the severity of the edema and their survival beyond e10.5 does not resonate with other knockout mouse models with established vascular permeability defects [39-41]. In contrast, the onset (Calcrl=E13.5, Adm=E14.5, Ramp2=E15.5) and severity of the phenotype closely resembles other genetic mouse models that delete genes essential for lymphatic development, including Prox1 [42], Sox18 [43], and Vegfc [44].
To determine whether lymphatic vasculature defects may contribute to the edema observed in AM signaling KO animals, we performed a comprehensive study of AM signaling expression and function during lymphatic vascular development [22]. Adm is temporally and spatially expressed on the endothelium of the jugular vein in a polarized fashion towards the budding primary lymph sac in vivo, which is identical to the master lymphatic regulator, Prox1 [42, 45, 46]. Moreover, Calcrl and Ramp2 are preferentially up-regulated in LECs, partially under the control of the lymphatic-specific transcriptional regulator, Prox1. While loss of AM signaling did not affect the differentiation and migration of LECs to form the primary lymph sac or dermal lymphatics, it did lead to a hypoplastic jugular lymph sac due to decreased LEC proliferation. This result indicates that AM signaling is essential for normal LEC proliferation in vivo and the KO mice develop edema due to smaller jugular lymphatic vessels that are unable to maintain homeostatic fluid balance. It is also interesting to note that the jugular lymphatic trunk is affected by loss of AM signaling, while retroperitoneal and dermal lymphatic vessels appear normal. This indicates that there are different cellular mechanisms regulating different lymphatic beds during development. However, AM does appear to be an essential growth factor for developing LECs in vivo [22]. Thus, it is most likely that a combination of both blood and lymphatic defects leads to the edema and lethality in the KO mice given the integrated physiology between the two vasculatures. However, more specialized genetic assays are required to resolve the relative contributions of each vasculature within these KO mice [47].
An alternative approach to assess the role of AM signaling in development would be to use transgenic mouse models that overexpress Adm, Calcrl, or Ramp2. Interestingly, no developmental phenotypes have been reported in gain-of-function mouse models of AM signaling, either by vascular Adm overexpression [48] or vSMC-specific Ramp2 overexpression [49], though these models displayed adult cardiovascular phenotypes. Given the essential nature of AM signaling within the endothelium, it would be interesting to over express Calcrl or Ramp2 specifically in the endothelium, which to our knowledge, has not yet been reported.
Adrenomedullin vs. Proadrenomedullin
One potential caveat with the majority of Adm-/- studies is that the gene targeting strategies delete the entire Adm coding sequence [30, 31], which results in the genetic KO of two functionally active peptides, AM and proadrenomedullin N-terminal 20 amino acid peptide (PAMP) [50]. PAMP is a small peptide that is produced during post-transcriptional splicing of preproadrenomedullin and has numerous actions to complement or antagonize AM signaling [50-53]. For two of the reported Adm deficient mouse lines, the design of the targeted allele could not rule out whether the observed phenotypes in the KO animals were due to loss of AM, PAMP, or both [30, 31]. This controversy was partially resolved using a third independent Adm-/- mouse, which left PAMP intact, and illustrated that loss of AM alone was enough to recapitulate embryonic lethality [32]. However, these mice lacking only AM had a milder phenotype (less edema and no cardiovascular abnormalities) when compared to KO mice lacking both peptides. This inconsistency in phenotypes could be attributed to differences in mouse strain and/or gene targeting approach [32]. However, a more intriguing hypothesis, which remains to be vigorously experimentally addressed, is that AM and PAMP may have non-redundant functions during cardiovascular development [54].
Developmental Role of RAMP2 vs. RAMP3
While Ramp2-/- mice recapitulated the Adm-/- and Calcrl-/- phenotypes, it appears that RAMP3, another RAMP that associates with CLR and binds AM, is not essential for embryonic survival since Ramp3-/- mice develop normally to adulthood. There also appears to be no functional redundancy between RAMP2 and RAMP3 in development, since there is no transcriptional compensatory mechanism of either RAMP in response to loss of the other [18, 34]. Although RAMP3 has been implicated in receptor trafficking [7, 55, 56], the functional role of the AM/CLR/RAMP3 signaling complex is not well understood in vivo.
New Developmental Insights of Adrenomedullin Pathway
A recent study by Nicoli et al. expanded our knowledge regarding the role of CLR during embryonic vascular development using a zebrafish model. By knocking down crlr they showed drastic vascular defects due to decreased expression of vegf. While vegf appears to be the critical mediator in the vascular development since overexpression of vegf is able to rescue the crlr knockdown phenotype, it still appears that crlr is essential for appropriate levels of vegf. This study provides in vivo evidence that crlr is downstream of sonic hedgehog, but upstream of vegf and notch signaling in arterial differentiation and development [57]. Modulation of vegf levels by AM signaling were previously reported in mice [58] but a complete characterization of AM and VEGF interactions is not well understood. It is novel that sonic hedgehog appears to regulate crlr expression and further dissection of this pathway in animal models would improve our understanding of how CLR is regulated during development. The zebrafish model system has recently been used to study lymphatic development [59-61] and it would be interesting if phenotypes seen in Adm-/-, Calcrl-/-, and Ramp2-/- mice could be recapitulated in zebrafish.
PHYSIOLOGY AND PATHOLOGY
Adrenomedullin Signaling in Pregnancy
AM signaling is known to be a critical component for initiation and progression of normal pregnancy. By the third trimester of a normal pregnancy, plasma levels of AM increase 4- to 5-fold [62-67]. AM is highly expressed in all vascular tissues which include the placenta and uterus [68-69]. Our previous studies in Adm+/- female mice expressing 50% less adrenomedullin revealed that there is disrupted fertility, placentation, uterine receptivity, and fetal growth resulting from reduced AM expression [70]. AM signaling components are also expressed in the trophoblast cells [71-76], which take on an endothelial-like function during the process of decidual maternal spiral artery remodeling during pregnancy. The trophoblast giant cells deriving from the trophectoderm invade and replace the vascular wall by inducing a loss of endothelial cells and smooth muscle cell coverage to allow for higher blood flow to the fetus through the spiral arteries. Failure of this remodeling process to occur is a hallmark feature of pre-eclampsia. Further research needs to be performed to determine the extent to which AM signaling affects trophoblast cells in the process of maternal spiral artery remodeling during pregnancy.
Adrenomedullin Signaling and Cardiovascular Biology
AM has been reported to be upregulated in various cardiovascular conditions [1, 77, 78] and is a potent angiogenic factor as well as a cardioprotective factor [1]. Plasma AM increases 2-fold in conditions such as essential hypertension, renal failure and congestive heart failure [79, 80] (Fig. 1). Previous studies with gene-targeted KO mice for Adm and Calcrl indicated that AM signaling is important for cardiovascular development [22, 30, 33]. Genetic reduction of Adm results in enhanced cardiovascular damage including increased cardiac hypertrophy in male Adm+/- mice [81] and marked perivascular fibrosis, coronary artery intimal hyperplasia and oxidative stress with AngII/high-salt treatment [32]. AM protects the heart from hypertrophy and fibrosis during cardiovascular stress such as hypertension and cardiac hypertrophy, myocardial infarction, heart failure and atherosclerosis [82, 83], but the exact mechanisms of AM-mediated cardioprotection have not been fully elucidated. A comprehensive review of the cardioprotective function of AM during hypertension and heart failure has recently been provided by several groups [9, 84].
Endothelial dysfunction is characterized by reduced endothelium-dependent vascular relaxation which is associated with most forms of cardiovascular disease. It is partially impacted by reduced nitric oxide and upregulation of adhesion molecules to result in a proinflammatory and prothrombotic state [85]. Research also suggests that endothelial dysfunction may act as an early marker of atherosclerosis [86]. One study indicated that Adm and its receptor components, Calcrl and Ramps were upregulated in the aorta of apolipoprotein E-deficient (ApoE-/-) mice [87]. Loss of apoE ultimately results in a mouse model of spontaneous atherosclerosis because apoE is important in the removal of circulating lipoproteins [88]. When these mice were fed an atherogenic diet and treated with AM, the appearance of atherosclerotic lesions was reduced [87]. This study further indicates that AM may help to protect against the progression of atherosclerosis, but the exact mechanism for this action remains to be understood. Expression of adhesion molecules in LECs [89] as well as liver sinusoidal endothelial cells [90] were reduced in response to AM treatment. Similar results were seen with VEGF-treated HUVECs [91]. Thus, AM may impact endothelial dysfunction partially by modulating adhesion molecule expression. With respect to endothelium-dependent vascular relaxation, AM is known to induce vasodilation which is mediated partially by endothelium-derived nitric oxide [92-96]. Also, in a rat model of sepsis induced by cecal ligation and puncture, administration of AM and AM-binding protein (AMBP-1 also known as complement factor H) were shown to prevent against endothelial cell dysfunction and decreased endothelium-dependent vascular relaxation in thoracic aorta [97]. These studies implicate AM as having a protective role in cardiovascular disease and endothelial dysfunction, but further research needs to be performed to investigate how AM directly impacts cardiac endothelial cells to regulate their function.
The Role of Adrenomedullin Signaling in Response to Injury, Vascular Dysfunction and Wound Healing
Endothelial proliferation and angiogenesis are known to be impacted by AM signaling. In a hind-limb ischemia model, AM promotes endothelial cell proliferation and capillary formation and conversely, Adm+/- mice showed reduced blood flow and capillary development [58]. Other whole animal studies using matrigel plugs demonstrated the role of AM in vascular regeneration because AM increased blood flow and capillary densities through PKA- and PI3K-dependent pathways [12, 13]. AM also induced tube-formation of HUVECs cultured on matrigel [14]. Another study pertaining to RAMP2 expression also revealed similar findings. An aortic ring assay and matrigel plug assay with adult Ramp2+/- mice revealed that with decreased Ramp2 expression there was reduced neovascularization in response to growth factor stimulation [18]. Collectively, these studies indicate the importance of AM in endothelial cell proliferation and angiogenesis in adult mice.
AM signaling is known to impact the blood and lymphatic vasculature in other physiological processes and pathological conditions. In a pathological mouse model of subcortical vascular dementia (chronic cerebral hypofusion), AM was shown to promote arteriogenesis and angiogenesis as well as inhibit oxidative stress and preserve white matter in the brain [98]. AM signaling can also induce anti-apoptotic and anti-inflammatory effects in response to injury. In the sinusoidal endothelial cells of the liver, AM helps to protect these cells from cold injury during the process of cold preservation for a liver transplant by decreasing endothelial cell apoptosis and inflammation [90]. Conversely, in Adm+/- and Ramp2+/- mice there is increased apoptosis of the sinusoidal endothelial cells in the liver after cold injury [90] further indicating that AM signaling helps to regulate apoptosis. Wound healing is an essential physiological process that requires angiogenesis and lymphangiogenesis for proper healing. Since AM is a known angiogenic factor and lymphangiogenic factor [22], it is not surprising that AM signaling is necessary in the wound healing process. In an ischemia/reperfusion mouse model of a pressure ulcer, AM administration reduced the wound area and accelerated angiogenesis as well as lymphangiogenesis [99]. Also in a wounded HUVEC monolayer, AM promoted vascular regeneration via activation of endothelial Akt in a PKA- PI3K- dependent manner [12]. Lymphedema is a hallmark condition of lymphatic dysfunction resulting in the swelling of one or more limbs due to accumulation of interstitial fluid. In Balb/C mice with tail lymphedema, AM treatment improved lymphedema and increased the number of lymphatic and blood vessels near the injury site [11]. Taken together, these data indicate that AM is an essential component for proper endothelial cell function in both physiological and pathological states to regulate apoptosis, inflammation, and lymphangiogenesis as well as angiogenesis.
An important issue to still address is to determine the exact role of AM signaling during adulthood by using temporal and spatial KO mice for components of the AM signaling system to evaluate physiology and function of the vascular beds in these mice. Previous studies with genetic KO mice for the AM signaling system reveal an enhanced impact of AM on lymphatic vascular development relative to blood vascular development [22]. It has also been shown that the gene expression of AM receptor components, Calcrl and Ramp2, are enhanced in LECs compared to BECs [23, 24]. Due to these known differences of AM signaling between BECs and LECs, it would be interesting to determine whether there is also an enhanced effect of AM on the lymphatic vasculature in adult physiology and pathology. The underlying mechanisms through which AM impacts the lymphatic vasculature, blood vasculature as well as the more specialized cardiac tissue during adulthood also needs to be identified.
Adrenomedullin Expression in Tumor Progression
The AM peptide was initially isolated from a human adrenal tumor (pheochromocytoma) due to its platelet cAMP elevating activity [78]. Since this discovery almost 20 years ago, investigation into the role of AM in tumors has greatly expanded. Early studies noticed elevated levels of AM in lung and brain tumors [100, 101] and a comprehensive survey of human tumor cell lines from lung, breast, brain, ovary, colon, and prostate substantiated those reports [102]. AM has been implicated in a variety of pro-tumor functions including acting as an autocrine growth factor [102-104], apoptosis survival factor [15], promoter of tumor cell motility and invasion [104-106], and molecular intermediate to enhance communication between tumor cells and immune cell infiltrates [107]. Furthermore, it has been suggested that the presence of AM in tumors may signify a more aggressive tumor phenotype due to correlation between Adm gene expression and histological tumor grade [104, 108].
The mechanism(s) by which Adm gene expression is transcriptionally regulated in tumors remains unclear. It is likely that AM can be both an autocrine and paracrine factor [109] by providing tumor cells a growth advantage in addition to acting on surrounding endothelial cells to promote proliferation and changes in vessel permeability to perhaps facilitate metastasis. Moreover, it has been suggested that hypoxia may play a role in AM production [8, 110]. Tumors often develop hypoxic zones in areas where blood flow is inadequate to supply the necessary oxygen required for the growing tumor cells. As a result of this unfavorable state, hypoxia inducible factor-1 (HIF-1) is activated which in turn upregulates a number of genes to compensate for the reduced oxygen microenvironment. Interestingly, a HIF-1 dependent mechanism was found to increase the expression of Adm in hypoxic human tumor cell lines [111]. Furthermore, Adm and Calcrl were found to be upregulated in microvascular endothelial cells cultured under low oxygen conditions [112]. Together, these results show that both tumor cells and surrounding endothelial cells are responsive to hypoxic conditions and may provide a mechanism for elevated AM levels in a tumor setting.
Although the precise role of AM in tumor development and progression is still unresolved, significant progress has been made to better understand how AM affects not only a tumor cell, but also the endothelial cells in the surrounding microenvironment. Analysis of immunohistochemical staining of human ovarian cancer found that in addition to tumor cells, AM was also localized to the endothelial cells of the surrounding stroma [108]. Furthermore, an in vitro co-culture system found that HUVECs became activated upon exposure to tumor cells and consequently increased transcriptional activity of Adm, among other factors [113]. Since AM directly impacts endothelial cell proliferation and permeability, AM induced modulation of vessels may affect the spread of cancer cells to distant sites via blood or lymphatic vasculature. Research groups have been performing the in vivo studies necessary to confirm that AM promotes tumor progression through its known angiogenic properties. Several reports have shown that inhibition of AM action by neutralizing antibodies or AM antagonist AM22-52 have reduced the growth of tumor xenografts in vivo by suppressing vascular development [58, 114, 115].
While much of the focus in understanding the process of tumor (lymph)angiogenesis has been upon the VEGF protein family, the contribution of AM to this process should not be underappreciated. Clearly, the studies described above point to AM as a valid target for potential cancer therapies although more research is necessary. Generation and validation of preclinical mouse models that are able to rigorously test AM as a target are greatly needed. Since the embryonic lethal phenotype of Adm-/- mice makes studying this signaling pathway more complicated, novel genetic mouse models (Table 1) using conditional alleles [18, 22, 116] and vascular endothelium specific Cre animals are a starting point for such tumor studies. Furthermore, these mouse models will be needed to refine our understanding of the metastatic process. Given the knowledge that AM can act on both the blood and lymphatic endothelium, a key question that remains to be answered is by what mechanisms do tumor cells disseminate into the blood and/or lymphatic vessels.
SUMMARY AND FUTURE DIRECTIONS
The use of genetic animal models in the field of AM research has produced significant contributions toward understanding the biology of this pleiotropic molecule, with a renewed appreciation for it's critical regulation of endothelial cell function during development and vascular diseases. To date, AM has been implicated in lymphatic vascular development, in proper functioning of blood and lymphatic endothelial cells and in a variety of conditions such as pregnancy, cardiovascular disease, and tumor progression (Fig. 2). Despite the strides that have been made, there is much more to learn regarding the mechanisms mediating AM function and regulation. With the generation of additional sophisticated molecular biology tools such as genetic mouse models, we are poised to refine our current knowledge as well as discover other novel roles for this peptide and signaling partners in normal and disease physiology.
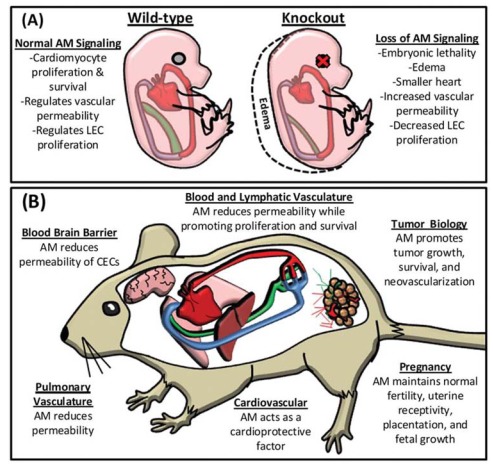
Fig. (2).
Adrenomedullin Signaling in Development and Vascular Biology. (A.) Loss of AM signaling causes embryonic lethality due to severe edema associated with impaired lymphatic vascular development. (B.) In the adult, AM is an angiogenic, lymphangiogenic, and a cardioprotective factor that also regulates vascular permeability and inflammation. Expression of AM is also implicated in pregnancy and tumor progression.
ACKNOWLEDGEMENTS
We respectfully acknowledge the international community of investigators studying adrenomedullin signaling and regret that space limitations precluded us from referencing all relevant studies in this review. We thank members of the Caron lab for stimulating and insightful discussions. This work was supported in part by grants to KMC from NIH HL091973, HD060860 and The Burroughs Wellcome Fund.
CONFLICT OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Gibbons C, Dackor R, Dunworth W, Fritz-Six K, Caron KM. Receptor Activity-Modifying Proteins: RAMPing up Adrenomedullin Signaling. Mol Endocrinol. 2007;21(4):783–96. doi: 10.1210/me.2006-0156. [DOI] [PubMed] [Google Scholar]
- 2.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823–9. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- 3.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302(1):49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SQ, O'Brien RJ, Struck J, et al. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2007;49(14):1525–32. doi: 10.1016/j.jacc.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Muller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9(6):R816–24. doi: 10.1186/cc3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christ-Crain M, Morgenthaler NG, Stolz D, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397] Crit Care. 2006;10(3):R96. doi: 10.1186/cc4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N, Mori H, Murakami S, Kangawa K, Kitamura S. Adrenomedullin: angiogenesis and gene therapy. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1432–7. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ishimitsu T, Ono H, Minami J, Matsuoka H. Pathophysiologic and therapeutic implications of adrenomedullin in cardiovascular disorders. Pharmacol Ther. 2006;111(3):909–27. doi: 10.1016/j.pharmthera.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Julian M, Cacho M, Garcia MA, et al. Adrenomedullin: a new target for the design of small molecule modulators with promising pharmacological activities. Eur J Med Chem. 2005;40(8):737–50. doi: 10.1016/j.ejmech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Jin D, Harada K, Ohnishi S, Yamahara K, Kangawa K, Nagaya N. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovasc Res. 2008;80(3):339–45. doi: 10.1093/cvr/cvn228. [DOI] [PubMed] [Google Scholar]
- 12.Miyashita K, Itoh H, Sawada N, et al. Adrenomedullin provokes endothelial Akt activation and promotes vascular regeneration both in vitro and in vivo. FEBS Lett. 2003;544(1-3):86–92. doi: 10.1016/s0014-5793(03)00484-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Moon SO, Sung MJ, et al. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J. 2003;17(13):1937–9. doi: 10.1096/fj.02-1209fje. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Sauze S, Delfino C, Mabrouk K, et al. Effects of adrenomedullin on endothelial cells in the multistep process of angiogenesis: involvement of CRLR/RAMP2 and CRLR/RAMP3 receptors. Int J Cancer. 2004;108(6):797–804. doi: 10.1002/ijc.11663. [DOI] [PubMed] [Google Scholar]
- 15.Kato H, Shichiri M, Marumo F, Hirata Y. Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology. 1997;138(6):2615–20. doi: 10.1210/endo.138.6.5197. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg. 2004;240(2):321–30. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyashita K, Itoh H, Sawada N, et al. Adrenomedullin promotes proliferation and migration of cultured endothelial cells. Hypertens Res. 2003;(26 Suppl):S93–8. doi: 10.1291/hypres.26.s93. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J Clin Invest. 2008;118(1):29–39. doi: 10.1172/JCI33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippenstiel S, Witzenrath M, Schmeck B, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91(7): 618–25. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- 20.Kis B, Deli MA, Kobayashi H, et al. Adrenomedullin regulates blood-brain barrier functions in vitro. Neuroreport. 2001;12(18):4139–42. doi: 10.1097/00001756-200112210-00055. [DOI] [PubMed] [Google Scholar]
- 21.Muller HC, Witzenrath M, Tschernig T, et al. Adrenomedullin attenuates ventilator-induced lung injury in mice. Thorax. 2010;65(12):1077–84. doi: 10.1136/thx.2010.135996. [DOI] [PubMed] [Google Scholar]
- 22.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118(1):40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrova TV, Makinen T, Makela TP, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo J. 2002;21(17):4593–9. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirakawa S, Hong YK, Harvey N, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162(2):575–86. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunworth WP, Fritz-Six KL, Caron KM. Adrenomedullin stabilizes the lymphatic endothelial barrier in vitro and in vivo. Peptides. 2008;29(12):2243–9. doi: 10.1016/j.peptides.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22(7):368–76. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 27.Lowes VL, Ip NY, Wong YH. Integration of signals from receptor tyrosine kinases and g protein-coupled receptors. Neurosignals. 2002;11(1):5–19. doi: 10.1159/000057317. [DOI] [PubMed] [Google Scholar]
- 28.Yurugi-Kobayashi T, Itoh H, Schroeder T, et al. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol. 2006;26(9):1977–84. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- 29.Guidolin D, Albertin G, Spinazzi R, et al. Adrenomedullin stimulates angiogenic response in cultured human vascular endothelial cells: Involvement of the vascular endothelial growth factor receptor 2. Peptides. 2008;29(11):2013–23. doi: 10.1016/j.peptides.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98(2):615–9. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shindo T, Kurihara Y, Nishimatsu H, et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104(16):1964–71. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- 32.Shimosawa T, Shibagaki Y, Ishibashi K, et al. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation. 2002;105(1):106–11. doi: 10.1161/hc0102.101399. [DOI] [PubMed] [Google Scholar]
- 33.Dackor RT, Fritz-Six K, Dunworth WP, Gibbons CL, Smithies O, Caron KM. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol Cell Biol. 2006;26(7):2511–8. doi: 10.1128/MCB.26.7.2511-2518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dackor R, Fritz-Six K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem. 2007;282(25):18094–9. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- 35.Czyzyk TA, Ning Y, Hsu MS, et al. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev Biol. 2005;287(2):301–13. doi: 10.1016/j.ydbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121(2):549–60. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 37.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18(3):271–5. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 38.Jaber M, Koch WJ, Rockman H, et al. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA. 1996;93(23):12974–9. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. Embo J. 1995;14(23):5884–91. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 41.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA. 2000;97(4):1731–6. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98(6):769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 43.Francois M, Caprini A, Hosking B, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456(7222):643–7. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 44.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 45.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002;21(7):1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong YK, Detmar M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003;314(1):85–92. doi: 10.1007/s00441-003-0747-8. [DOI] [PubMed] [Google Scholar]
- 47.Kahn ML. Blood is thicker than lymph. J Clin Invest. 2008;118(1):23–6. doi: 10.1172/JCI34485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shindo T, Kurihara H, Maemura K, et al. Hypotension and resistance to lipopolysaccharide-induced shock in transgenic mice overexpressing adrenomedullin in their vasculature. Circulation. 2000;101(19):2309–16. doi: 10.1161/01.cir.101.19.2309. [DOI] [PubMed] [Google Scholar]
- 49.Tam CW, Husmann K, Clark NC, et al. Enhanced vascular responses to adrenomedullin in mice overexpressing receptor-activity-modifying protein 2. Circ Res. 2006;98(2):262–70. doi: 10.1161/01.RES.0000200737.63865.58. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura K, Ichiki Y, Tanaka M, et al. Immunoreactive adrenomedullin in human plasma. FEBS Lett. 1994;341(2-3):288–90. doi: 10.1016/0014-5793(94)80474-5. [DOI] [PubMed] [Google Scholar]
- 51.Martinez A, Hodge DL, Garayoa M, Young HA, Cuttitta F. Alternative splicing of the proadrenomedullin gene results in differential expression of gene products. J Mol Endocrinol. 2001;27(1):31–41. doi: 10.1677/jme.0.0270031. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura M, Yoshida H, Hiramori K. Comparison of vasodilator potency of adrenomedulling and proadrenomedullin N-terminal 20 peptide in human. Life Sci. 1999;65(20):2151–6. doi: 10.1016/s0024-3205(99)00480-4. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Ren Y, Dong X, Zhong G, Wu S, Tang C. Roles of different peptide fragments derived from proadrenomedullin in the regulation of vascular tone in isolated rat aorta. Peptides. 2003;24(4):563–8. doi: 10.1016/s0196-9781(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 54.Hay DL, Smith DM. Knockouts and transgenics confirm the importance of adrenomedullin in the vasculature. Trends Pharmacol Sci. 2001;22(2):57–9. doi: 10.1016/s0165-6147(00)01617-5. [DOI] [PubMed] [Google Scholar]
- 55.Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS. Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem. 2005;280(10):9297–307. doi: 10.1074/jbc.M413786200. [DOI] [PubMed] [Google Scholar]
- 56.Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N. Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem. 2005;280(25):23926–35. doi: 10.1074/jbc.M501751200. [DOI] [PubMed] [Google Scholar]
- 57.Nicoli S, Tobia C, Gualandi L, De Sena G, Presta M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood. 2008;111(10):4965–72. doi: 10.1182/blood-2007-10-118166. [DOI] [PubMed] [Google Scholar]
- 58.Iimuro S, Shindo T, Moriyama N, et al. Angiogenic effects of adrenomedullin in ischemia and tumor growth. Circ Res. 2004;95(4):415–23. doi: 10.1161/01.RES.0000138018.61065.d1. [DOI] [PubMed] [Google Scholar]
- 59.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12(6):711–6. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 60.Isogai S, Hitomi J, Yaniv K, Weinstein BM. Zebrafish as a new animal model to study lymphangiogenesis. Anat Sci Int. 2009;84(3):102–11. doi: 10.1007/s12565-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 61.Geudens I, Herpers R, Hermans K, et al. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol. 2010;30(9):1695–702. doi: 10.1161/ATVBAHA.110.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Iorio R, Marinoni E, Scavo D, Letizia C, Cosmi EV. Adrenomedullin in pregnancy. Lancet. 1997;349(9048):328. doi: 10.1016/s0140-6736(05)62827-9. [DOI] [PubMed] [Google Scholar]
- 63.Minegishi T, Nakamura M, Abe K, et al. Adrenomedullin and atrial natriuretic peptide concentrations in normal pregnancy and pre-eclampsia. Mol Hum Reprod. 1999;5(8):767–70. doi: 10.1093/molehr/5.8.767. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi K, Kubota T, Aso T, Hirata Y, Imai T, Marumo F. Immunoreactive adrenomedullin (AM) concentration in maternal plasma during human pregnancy and AM expression in placenta. Eur J Endocrinol. 2000;142(6):683–7. doi: 10.1530/eje.0.1420683. [DOI] [PubMed] [Google Scholar]
- 65.Hoshimoto K, Hayashi M, Ohkura T. Mature adrenomedullin concentrations in plasma during pregnancy. J Matern Fetal Neonatal Med. 2002;11(2):126–9. doi: 10.1080/jmf.11.2.126.129. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi Y, Ueyama H, Mashimo T, Kangawa K, Minamino N. Circulating mature adrenomedullin is related to blood volume in full-term pregnancy. Anesth Analg. 2005;101(6):1816–20. doi: 10.1213/01.ANE.0000182329.02880.83. [DOI] [PubMed] [Google Scholar]
- 67.Senna AA, Zedan M, el-Salam GE, el-Mashad AI. Study of plasma adrenomedullin level in normal pregnancy and preclampsia. Medscape J Med. 2008;10(2):29. [PMC free article] [PubMed] [Google Scholar]
- 68.Marinoni E, Casciani V, Marianetti V, Di Rocco A, Moscarini M, Di Iorio R. Localization and distribution of adrenomedullin receptor in the human placenta: changes with gestational age. J Reprod Med. 2007;52(9):831–8. [PubMed] [Google Scholar]
- 69.Cameron VA, Autelitano DJ, Evans JJ, et al. Adrenomedullin expression in rat uterus is correlated with plasma estradiol. Am J Physiol Endocrinol Metab. 2002;282(1):E139–46. doi: 10.1152/ajpendo.2002.282.1.E139. [DOI] [PubMed] [Google Scholar]
- 70.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116(10):2653–62. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nikitenko LL, Brown NS, Smith DM, MacKenzie IZ, Bicknell R, Rees MC. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol Hum Reprod. 2001;7(7):655–64. doi: 10.1093/molehr/7.7.655. [DOI] [PubMed] [Google Scholar]
- 72.Gratton RJ, Gluszynski M, Mazzuca DM, Nygard K, Han VK. Adrenomedullin messenger ribonucleic acid expression in the placentae of normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2003;88(12):6048–55. doi: 10.1210/jc.2003-030323. [DOI] [PubMed] [Google Scholar]
- 73.Marinoni E, Di Iorio R, Letizia C, Villaccio B, Scucchi L, Cosmi EV. Immunoreactive adrenomedullin in human fetoplacental tissues. Am J Obstet Gynecol. 1998;179(3 Pt 1):784–7. doi: 10.1016/s0002-9378(98)70083-3. [DOI] [PubMed] [Google Scholar]
- 74.Montuenga LM, Martinez A, Miller MJ, Unsworth EJ, Cuttitta F. Expression of adrenomedullin and its receptor during embryogenesis suggests autocrine or paracrine modes of action. Endocrinology. 1997;138(1):440–51. doi: 10.1210/endo.138.1.4881. [DOI] [PubMed] [Google Scholar]
- 75.Yotsumoto S, Shimada T, Cui CY, Nakashima H, Fujiwara H, Ko MS. Expression of adrenomedullin, a hypotensive peptide, in the trophoblast giant cells at the embryo implantation site in mouse. Dev Biol. 1998;203(2):264–75. doi: 10.1006/dbio.1998.9073. [DOI] [PubMed] [Google Scholar]
- 76.Tsatsaris V, Tarrade A, Merviel P, et al. Calcitonin gene-related peptide (CGRP) and CGRP receptor expression at the human implantation site. J Clin Endocrinol Metab. 2002;87(9):4383–90. doi: 10.1210/jc.2002-020138. [DOI] [PubMed] [Google Scholar]
- 77.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21(2):138–67. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 78.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192(2):553–60. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 79.Eto T, Kitamura K, Kato J. Biological and clinical roles of adrenomedullin in circulation control and cardiovascular diseases. Clin Exp Pharmacol Physiol. 1999;26(5-6):371–80. doi: 10.1046/j.1440-1681.1999.03047.x. [DOI] [PubMed] [Google Scholar]
- 80.Jougasaki M, Burnett JC., Jr Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66(10):855–72. doi: 10.1016/s0024-3205(99)00358-6. [DOI] [PubMed] [Google Scholar]
- 81.Caron K, Hagaman J, Nishikimi T, Kim HS, Smithies O. Adrenomedullin gene expression differences in mice do not affect blood pressure but modulate hypertension-induced pathology in males. Proc Natl Acad Sci USA. 2007;104(9):3420–5. doi: 10.1073/pnas.0611365104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuwasako K, Cao YN, Nagoshi Y, Kitamura K, Eto T. Adrenomedullin receptors: pharmacological features and possible pathophysiological roles. Peptides. 2004;25(11):2003–12. doi: 10.1016/j.peptides.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Nishikimi T, Yoshihara F, Mori Y, Kangawa K, Matsuoka H. Cardioprotective effect of adrenomedullin in heart failure. Hypertens Res. 2003;(26 Suppl):S121–7. doi: 10.1291/hypres.26.s121. [DOI] [PubMed] [Google Scholar]
- 84.Yanagawa B, Nagaya N. Adrenomedullin: molecular mechanisms and its role in cardiac disease. Amino Acids. 2006 doi: 10.1007/s00726-005-0279-5. [DOI] [PubMed] [Google Scholar]
- 85.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15(8):1983–92. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 86.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 87.Pan CS, Zhang J, Yu F, et al. Adrenomedullin ameliorates the development of atherosclerosis in apoE-/- mice. Peptides. 2010;31(6):1150–8. doi: 10.1016/j.peptides.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258(5081):468–71. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 89.Jin D, Otani K, Yamahara K, Ikeda T, Nagaya N, Kangawa K. Adrenomedullin reduces expression of adhesion molecules on lymphatic endothelial cells. Regul Pept. 2011;166(1-3):21–7. doi: 10.1016/j.regpep.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Iinuma N, Sakurai T, Kamiyoshi A, et al. Adrenomedullin in sinusoidal endothelial cells play protective roles against cold injury of liver. Peptides. 2010;31(5):865–71. doi: 10.1016/j.peptides.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Kim W, Moon SO, Lee S, Sung MJ, Kim SH, Park SK. Adrenomedullin reduces VEGF-induced endothelial adhesion molecules and adhesiveness through a phosphatidylinositol 3'-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23(8):1377–83. doi: 10.1161/01.ATV.0000081740.65173.D1. [DOI] [PubMed] [Google Scholar]
- 92.Nossaman BD, Feng CJ, Kaye AD, et al. Pulmonary vasodilator responses to adrenomedullin are reduced by NOS inhibitors in rats but not in cats. Am J Physiol. 1996;270(5 Pt 1):L782–9. doi: 10.1152/ajplung.1996.270.5.L782. [DOI] [PubMed] [Google Scholar]
- 93.Nakamura M, Yoshida H, Makita S, Arakawa N, Niinuma H, Hiramori K. Potent and long-lasting vasodilatory effects of adrenomedullin in humans. Comparisons between normal subjects and patients with chronic heart failure. Circulation. 1997;95(5):1214–21. doi: 10.1161/01.cir.95.5.1214. [DOI] [PubMed] [Google Scholar]
- 94.Nishimatsu H, Suzuki E, Nagata D, et al. Adrenomedullin induces endothelium-dependent vasorelaxation via the phosphatidylinositol 3-kinase/Akt-dependent pathway in rat aorta. Circ Res. 2001;89(1):63–70. doi: 10.1161/hh1301.092498. [DOI] [PubMed] [Google Scholar]
- 95.Takahashi Y, de Vroomen M, Gournay V, Roman C, Rudolph AM, Heymann MA. Mechanisms of adrenomedullin-induced increase of pulmonary blood flow in fetal sheep. Pediatr Res. 1999;45(2):276–81. doi: 10.1203/00006450-199902000-00020. [DOI] [PubMed] [Google Scholar]
- 96.De Matteo R, May CN. Direct coronary vasodilator action of adrenomedullin is mediated by nitric oxide. Br J Pharmacol. 2003;140(8):1414–20. doi: 10.1038/sj.bjp.0705572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou M, Maitra SR, Wang P. Adrenomedullin and adrenomedullin binding protein-1 protect endothelium-dependent vascular relaxation in sepsis. Mol Med. 2007;13(9-10):488–94. doi: 10.2119/2007-00113.Zhou. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maki T, Ihara M, Fujita Y, et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42(4):1122–8. doi: 10.1161/STROKEAHA.110.603399. [DOI] [PubMed] [Google Scholar]
- 99.Harada K, Yamahara K, Ohnishi S, et al. Sustained-release adrenomedullin ointment accelerates wound healing of pressure ulcers. Regul Pept. 2011;168(1-3):21–6. doi: 10.1016/j.regpep.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 100.Martinez A, Miller MJ, Unsworth EJ, Siegfried JM, Cuttitta F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology. 1995;136(9):4099–105. doi: 10.1210/endo.136.9.7649118. [DOI] [PubMed] [Google Scholar]
- 101.Satoh F, Takahashi K, Murakami O, et al. Adrenomedullin in human brain, adrenal glands and tumor tissues of pheochromocytoma, ganglioneuroblastoma and neuroblastoma. J Clin Endocrinol Metab. 1995;80(5):1750–2. doi: 10.1210/jcem.80.5.7745031. [DOI] [PubMed] [Google Scholar]
- 102.Miller MJ, Martinez A, Unsworth EJ, et al. Adrenomedullin expression in human tumor cell lines. Its potential role as an autocrine growth factor. J Biol Chem. 1996;271(38):23345–51. doi: 10.1074/jbc.271.38.23345. [DOI] [PubMed] [Google Scholar]
- 103.Metellus P, Voutsinos-Porche B, Nanni-Metellus I, et al. Adrenomedullin expression and regulation in human glioblastoma, cultured human glioblastoma cell lines and pilocytic astrocytoma. Eur J Cancer. 2011;47(11):1727–35. doi: 10.1016/j.ejca.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 104.Ramachandran V, Arumugam T, Hwang RF, Greenson JK, Simeone DM, Logsdon CD. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67(6):2666–75. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 105.Martinez A, Vos M, Guedez L, et al. The effects of adrenomedullin overexpression in breast tumor cells. J Natl Cancer Inst. 2002;94(16):1226–37. doi: 10.1093/jnci/94.16.1226. [DOI] [PubMed] [Google Scholar]
- 106.Keleg S, Kayed H, Jiang X, et al. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int J Cancer. 2007;121(1):21–32. doi: 10.1002/ijc.22596. [DOI] [PubMed] [Google Scholar]
- 107.Zudaire E, Martinez A, Garayoa M, et al. Adrenomedullin is a cross-talk molecule that regulates tumor and mast cell function during human carcinogenesis. Am J Pathol. 2006;168(1):280–91. doi: 10.2353/ajpath.2006.050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hata K, Takebayashi Y, Akiba S, et al. Expression of the adrenomedullin gene in epithelial ovarian cancer. Mol Hum Reprod. 2000;6(10):867–72. doi: 10.1093/molehr/6.10.867. [DOI] [PubMed] [Google Scholar]
- 109.Cuttitta F, Pio R, Garayoa M, et al. Adrenomedullin functions as an important tumor survival factor in human carcinogenesis. Microsc Res Tech. 2002;57(2):110–9. doi: 10.1002/jemt.10059. [DOI] [PubMed] [Google Scholar]
- 110.Ribatti D, Nico B, Spinazzi R, Vacca A, Nussdorfer GG. The role of adrenomedullin in angiogenesis. Peptides. 2005;26(9):1670–5. doi: 10.1016/j.peptides.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 111.Garayoa M, Martinez A, Lee S, et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol. 2000;14(6):848–62. doi: 10.1210/mend.14.6.0473. [DOI] [PubMed] [Google Scholar]
- 112.Nikitenko LL, Smith DM, Bicknell R, Rees MC. Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. FASEB J. 2003;17(11):1499–501. doi: 10.1096/fj.02-0993fje. [DOI] [PubMed] [Google Scholar]
- 113.Khodarev NN, Yu J, Labay E, et al. Tumour-endothelium interactions in co-culture: coordinated changes of gene expression profiles and phenotypic properties of endothelial cells. J Cell Sci. 2003;116(Pt 6):1013–22. doi: 10.1242/jcs.00281. [DOI] [PubMed] [Google Scholar]
- 114.Ouafik L, Sauze S, Boudouresque F, et al. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol. 2002;160(4):1279–92. doi: 10.1016/S0002-9440(10)62555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaafarani I, Fernandez-Sauze S, Berenguer C, et al. Targeting adrenomedullin receptors with systemic delivery of neutralizing antibodies inhibits tumor angiogenesis and suppresses growth of human tumor xenografts in mice. FASEB J. 2009;23(10):3424–35. doi: 10.1096/fj.08-127852. [DOI] [PubMed] [Google Scholar]
- 116.Fernandez AP, Serrano J, Tessarollo L, Cuttitta F, Martinez A. Lack of adrenomedullin in the mouse brain results in behavioral changes, anxiety, and lower survival under stress conditions. Proc Natl Acad Sci USA. 2008;105(34):12581–6. doi: 10.1073/pnas.0803174105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ehlenz K, Koch B, Preuss P, Simon B, Koop I, Lang RE. High levels of circulating adrenomedullin in severe illness: correlation with C-reactive protein and evidence against the adrenal medulla as site of origin. Exp Clin Endocrinol Diabetes. 1997;105(3):156–62. doi: 10.1055/s-0029-1211745. [DOI] [PubMed] [Google Scholar]
- 118.Letizia C, Tamburrano G, Alo P, et al. Adrenomedullin, a new peptide, in patients with insulinoma. Eur J Endocrinol. 2001;144(5):517–20. doi: 10.1530/eje.0.1440517. [DOI] [PubMed] [Google Scholar]
- 119.Letizia C, De Toma G, Caliumi C, et al. Plasma adrenomedullin concentrations in patients with adrenal pheochromocytoma. Horm Metab Res. 2001;33(5):290–4. doi: 10.1055/s-2001-15281. [DOI] [PubMed] [Google Scholar]
- 120.Oehler MK, Fischer DC, Orlowska-Volk M, et al. Tissue and plasma expression of the angiogenic peptide adrenomedullin in breast cancer. Br J Cancer. 2003;89(10):1927–33. doi: 10.1038/sj.bjc.6601397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cotesta D, Caliumi C, Alo P, et al. High plasma levels of human chromogranin A and adrenomedullin in patients with pheochromocytoma. Tumori. 2005;91(1):53–8. doi: 10.1177/030089160509100110. [DOI] [PubMed] [Google Scholar]
- 122.Letizia C, Di Iorio R, De Toma G, et al. Circulating adrenomedullin is increased in patients with corticotropin-dependent Cushing's syndrome due to pituitary adenoma. Metabolism. 2000;49(6):760–3. doi: 10.1053/meta.2000.6241. [DOI] [PubMed] [Google Scholar]
- 123.Miyao Y, Nishikimi T, Goto Y, et al. Increased plasma adrenomedullin levels in patients with acute myocardial infarction in proportion to the clinical severity. Heart. 1998;79(1):39–44. doi: 10.1136/hrt.79.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagaya N, Nishikimi T, Uematsu M, et al. Plasma adrenomedullin as an indicator of prognosis after acute myocardial infarction. Heart. 1999;81(5):483–7. doi: 10.1136/hrt.81.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoshitomi Y, Nishikimi T, Kojima S, et al. Plasma levels of adrenomedullin in patients with acute myocardial infarction. Clin Sci (Lond) 1998;94(2):135–9. doi: 10.1042/cs0940135. [DOI] [PubMed] [Google Scholar]
- 126.Balat A, Cekmen M, Yurekli M, et al. Adrenomedullin and nitrite levels in children with Bartter syndrome. Pediatr Nephrol. 2000;15(3-4):266–70. doi: 10.1007/s004670000474. [DOI] [PubMed] [Google Scholar]
- 127.Evereklioglu C, Yurekli M, Er H, et al. Increased plasma adrenomedullin levels in patients with Behcet's disease. Dermatology. 2000;201(4):312–5. doi: 10.1159/000051544. [DOI] [PubMed] [Google Scholar]
- 128.Ueda S, Nishio K, Minamino N, et al. Increased plasma levels of adrenomedullin in patients with systemic inflammatory response syndrome. Am J Respir Crit Care Med. 1999;160(1):132–6. doi: 10.1164/ajrccm.160.1.9810006. [DOI] [PubMed] [Google Scholar]
- 129.Yoshibayashi M, Kamiya T, Nishikimi T, Saito Y, Matsuo H, Kangawa K. Elevated plasma levels of adrenomedullin in congenital cyanotic heart disease. Clin Sci (Lond) 1999;96(6):543–7. [PubMed] [Google Scholar]
- 130.Tanaka M, Kitamura K, Ishizaka Y, et al. Plasma adrenomedullin in various diseases and exercise-induced change in adrenomedullin in healthy subjects. Intern Med. 1995;34(8):728–33. doi: 10.2169/internalmedicine.34.728. [DOI] [PubMed] [Google Scholar]
- 131.Cheung B, Leung R. Elevated plasma levels of human adrenomedullin in cardiovascular, respiratory, hepatic and renal disorders. Clin Sci (Lond) 1997;92(1):59–62. doi: 10.1042/cs0920059. [DOI] [PubMed] [Google Scholar]
- 132.Jougasaki M, Wei CM, McKinley LJ, Burnett JC., Jr Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92(3):286–9. doi: 10.1161/01.cir.92.3.286. [DOI] [PubMed] [Google Scholar]
- 133.Ishimitsu T, Nishikimi T, Saito Y, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994;94(5):2158–61. doi: 10.1172/JCI117573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caliumi C, Cianci R, Celi M, et al. Plasma adrenomedullin concentrations in patients with renovascular or malignant hypertension. Minerva Cardioangiol. 2004;52(4):313–22. [PubMed] [Google Scholar]
- 135.Yamamoto K, Ikeda U, Sekiguchi H, Shimada K. Plasma levels of adrenomedullin in patients with mitral stenosis. Am Heart J. 1998;135(3):542–9. doi: 10.1016/s0002-8703(98)70333-3. [DOI] [PubMed] [Google Scholar]
- 136.Wolk R, Svatikova A, Otto ME, Hoffmann MS, Duenwald CJ, Somers VK. Plasma adrenomedullin and obstructive sleep apnea. Am J Hypertens. 2004;17(1):74–6. doi: 10.1016/j.amjhyper.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 137.Suzuki Y, Horio T, Hayashi T, et al. Plasma adrenomedullin concentration is increased in patients with peripheral arterial occlusive disease associated with vascular inflammation. Regul Pept. 2004;118(1-2):99–104. doi: 10.1016/j.regpep.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 138.Gouya G, Sturm G, Lamina C, et al. The association of mid-regional pro-adrenomedullin and mid-regional pro-atrial natriuretic peptide with mortality in an incident dialysis cohort. PLoS One. 2011;6(3):e17803. doi: 10.1371/journal.pone.0017803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maisel A, Mueller C, Nowak R, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55(19):2062–76. doi: 10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 140.Dhillon OS, Khan SQ, Narayan HK, et al. Prognostic value of mid-regional pro-adrenomedullin levels taken on admission and discharge in non-ST-elevation myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) II study. J Am Coll Cardiol. 2010;56(2):125–33. doi: 10.1016/j.jacc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 141.von Haehling S, Filippatos GS, Papassotiriou J, et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail. 2010;12(5):484–91. doi: 10.1093/eurjhf/hfq031. [DOI] [PubMed] [Google Scholar]
- 142.Iacobellis G, di Gioia CR, Di Vito M, et al. Epicardial adipose tissue and intracoronary adrenomedullin levels in coronary artery disease. Horm Metab Res. 2009;41(12):855–60. doi: 10.1055/s-0029-1231081. [DOI] [PubMed] [Google Scholar]
- 143.Gombos T, Forhecz Z, Pozsonyi Z, et al. Adrenomedullin and endothelin-1 are related to inflammation in chronic heart failure. Inflamm Res. 2009;58(6):298–305. doi: 10.1007/s00011-008-8184-5. [DOI] [PubMed] [Google Scholar]
- 144.Nishida H, Horio T, Suzuki Y, et al. Plasma adrenomedullin as an independent predictor of future cardiovascular events in high-risk patients: comparison with C-reactive protein and adiponectin. Peptides. 2008;29(4):599–605. doi: 10.1016/j.peptides.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 145.Teramoto S, Yamaguchi Y, Yamamoto H, et al. Effects of age and sex on plasma adrenomedullin levels in patients with obstructive sleep apnea syndrome. J Am Geriatr Soc. 2007;55(11):1891–2. doi: 10.1111/j.1532-5415.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 146.Schulz R, Flototto C, Jahn A, et al. Circulating adrenomedullin in obstructive sleep apnoea. J Sleep Res. 2006;15(1):89–95. doi: 10.1111/j.1365-2869.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 147.Katayama T, Nakashima H, Takagi C, Honda Y, Suzuki S, Yano K. Predictors of mortality in patients with acute myocardial infarction and cardiogenic shock. Circ J. 2005;69(1):83–8. doi: 10.1253/circj.69.83. [DOI] [PubMed] [Google Scholar]
- 148.Katayama T, Nakashima H, Furudono S, Honda Y, Suzuki S, Yano K. Evaluation of neurohumoral activation (adrenomedullin, BNP, catecholamines, etc.) in patients with acute myocardial infarction. Intern Med. 2004;43(11):1015–22. doi: 10.2169/internalmedicine.43.1015. [DOI] [PubMed] [Google Scholar]
- 149.Zhang D, Sun XF, Ma ZF, Zhu HY, Wang YD, Chen XM. Effects of high-flux hemodialysis on plasma adrenomedullin and sustained hypotension in elderly hemodialysis patients. Chin Med J (Engl) 2011;124(6):907–10. [PubMed] [Google Scholar]
- 150.Shinomiya K, Ohmori K, Ohyama H, et al. Association of plasma adrenomedullin with carotid atherosclerosis in chronic ischemic stroke. Peptides. 2001;22(11):1873–80. doi: 10.1016/s0196-9781(01)00507-1. [DOI] [PubMed] [Google Scholar]
- 151.Guevara M, Gines P, Jimenez W, et al. Increased adrenomedullin levels in cirrhosis: relationship with hemodynamic abnormalities and vasoconstrictor systems. Gastroenterology. 1998;114(2):336–43. doi: 10.1016/s0016-5085(98)70486-x. [DOI] [PubMed] [Google Scholar]
- 152.Fernandez-Rodriguez CM, Prada IR, Prieto J, et al. Circulating adrenomedullin in cirrhosis: relationship to hyperdynamic circulation. J Hepatol. 1998;29(2):250–6. doi: 10.1016/s0168-8278(98)80010-x. [DOI] [PubMed] [Google Scholar]
- 153.Tahan V, Avsar E, Karaca C, et al. Adrenomedullin in cirrhotic and non-cirrhotic portal hypertension. World J Gastroenterol. 2003;9(10):2325–7. doi: 10.3748/wjg.v9.i10.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mallamaci F, Zoccali C, Parlongo S, Cutrupi S, Tripepi G, Postorino M. Plasma adrenomedullin during acute changes in intravascular volume in hemodialysis patients. Kidney Int. 1998;54(5):1697–703. doi: 10.1046/j.1523-1755.1998.00136.x. [DOI] [PubMed] [Google Scholar]
- 155.Tokura T, Kinoshita H, Fujimoto S, Hisanaga S, Kitamura K, Eto T. Plasma levels of mature form of adrenomedullin in patients with haemodialysis. Nephrol Dial Transplant. 2001;16(4):783–6. doi: 10.1093/ndt/16.4.783. [DOI] [PubMed] [Google Scholar]
- 156.Cases A, Esforzado N, Lario S, et al. Increased plasma adrenomedullin levels in hemodialysis patients with sustained hypotension. Kidney Int. 2000;57(2):664–70. doi: 10.1046/j.1523-1755.2000.00888.x. [DOI] [PubMed] [Google Scholar]
- 157.Yamasaki H, Nagake Y, Akagi S, Sugimoto T, Ichikawa H, Makino H. Plasma adrenomedullin levels in patients on hemodialysis. Nephron. 2001;89(1):20–5. doi: 10.1159/000046038. [DOI] [PubMed] [Google Scholar]
- 158.Eto T, Washimine H, Kato J, Kitamura K, Yamamoto Y. Adrenomedullin and proadrenomedullin N-terminal 20 peptide in impaired renal function. Kidney Int Suppl. 1996;55:S148–9. [PubMed] [Google Scholar]
- 159.Ishihara T, Yokota N, Hisanaga S, et al. Increased plasma levels of mature form of adrenomedullin in patients with chronic renal failure. Clin Nephrol. 1999;52(2):119–23. [PubMed] [Google Scholar]
- 160.Kojima H, Tsujimoto T, Uemura M, et al. Significance of increased plasma adrenomedullin concentration in patients with cirrhosis. J Hepatol. 1998;28(5):840–6. doi: 10.1016/s0168-8278(98)80235-3. [DOI] [PubMed] [Google Scholar]
- 161.Kalman S, Buyan N, Yurekli M, Ozkaya O, Bakkaloglu S, Soylemezoglu O. Plasma and urinary adrenomedullin levels in children with acute pyelonephritis. Nephrology (Carlton) 2005;10(5):487–90. doi: 10.1111/j.1440-1797.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 162.Dieplinger B, Mueller T, Kollerits B, et al. Pro-A-type natriuretic peptide and pro-adrenomedullin predict progression of chronic kidney disease: the MMKD Study. Kidney Int. 2009;75(4):408–14. doi: 10.1038/ki.2008.560. [DOI] [PubMed] [Google Scholar]
- 163.Ceyhan BB, Karakurt S, Hekim N. Plasma adrenomedullin levels in asthmatic patients. J Asthma. 2001;38(3):221–7. doi: 10.1081/jas-100000109. [DOI] [PubMed] [Google Scholar]
- 164.Kohno M, Hanehira T, Hirata K, et al. An accelerated increase of plasma adrenomedullin in acute asthma. Metabolism. 1996;45(11):1323–5. doi: 10.1016/s0026-0495(96)90109-2. [DOI] [PubMed] [Google Scholar]
- 165.Vizza CD, Letizia C, Sciomer S, et al. Increased plasma levels of adrenomedullin, a vasoactive peptide, in patients with end-stage pulmonary disease. Regul Pept. 2005;124(1-3):187–93. doi: 10.1016/j.regpep.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 166.Kakishita M, Nishikimi T, Okano Y, et al. Increased plasma levels of adrenomedullin in patients with pulmonary hypertension. Clin Sci (Lond) 1999;96(1):33–9. [PubMed] [Google Scholar]
- 167.Potocki M, Breidthardt T, Reichlin T, et al. Midregional pro-adrenomedullin in addition to b-type natriuretic peptides in the risk stratification of patients with acute dyspnea: an observational study. Crit Care. 2009;13(4):R122. doi: 10.1186/cc7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest. 2008;134(2):263–72. doi: 10.1378/chest.08-0047. [DOI] [PubMed] [Google Scholar]
- 169.Hamada H, Saisyo K, Sekimoto T, Chosa E. Plasma adrenomedullin and proadrenomedullin N-terminal 20 peptide in patients diagnosed as having early rheumatoid arthritis. Mod Rheumatol. 2010;20(4):389–95. doi: 10.1007/s10165-010-0295-5. [DOI] [PubMed] [Google Scholar]
- 170.Mok MY, Cheung BM, Lo Y, Leung RY, Wong WS, Lau CS. Elevated plasma adrenomedullin and vascular manifestations in patients with systemic sclerosis. J Rheumatol. 2007;34(11):2224–9. [PubMed] [Google Scholar]
- 171.Garcia-Unzueta MT, Martinez-Taboada VM, Amado-Senaris JA, Rodriguez-Valverde V. Plasma adrenomedullin levels in patients with polymyalgia rheumatica and giant cell arteritis. Clin Exp Rheumatol. 2006;24(2 Suppl 41):S6–9. [PubMed] [Google Scholar]
- 172.Balat A, Islek I, Cekmen M, et al. Adrenomedullin and total nitrite levels in children with familial Mediterranean fever. J Paediatr Child Health. 2006;42(5):240–3. doi: 10.1111/j.1440-1754.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 173.Balat A, Kilinc M, Cekmen MB, et al. Adrenomedullin and total nitrite levels in children with acute rheumatic fever. Clin Biochem. 2005;38(6):526–30. doi: 10.1016/j.clinbiochem.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 174.Michels M, Djamiatun K, Faradz SM, Koenders MM, de Mast Q, van der Ven AJ. High plasma mid-regional pro-adrenomedullin levels in children with severe dengue virus infections. J Clin Virol. 2011;50(1):8–12. doi: 10.1016/j.jcv.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 175.Mak A, Cheung BM, Mok CC, Leung R, Lau CS. Adrenomedullin--a potential disease activity marker and suppressor of nephritis activity in systemic lupus erythematosus. Rheumatology (Oxford) 2006;45(10):1266–72. doi: 10.1093/rheumatology/kel105. [DOI] [PubMed] [Google Scholar]
- 176.Di Iorio R, Marinoni E, Letizia C, Villaccio B, Alberini A, Cosmi EV. Adrenomedullin production is increased in normal human pregnancy. Eur J Endocrinol. 1999;140(3):201–6. doi: 10.1530/eje.0.1400201. [DOI] [PubMed] [Google Scholar]
- 177.Nagata N, Kato J, Kitamura K, et al. Dissociation of adrenomedullin concentrations in plasma and cerebrospinal fluid in pregnant and non-pregnant women. Eur J Endocrinol. 1998;139(6):611–4. doi: 10.1530/eje.0.1390611. [DOI] [PubMed] [Google Scholar]
- 178.Poyhonen-Alho M, Viitasalo M, Nicholls MG, Lindstrom BM, Vaananen H, Kaaja R. Imbalance of the autonomic nervous system at night in women with gestational diabetes. Diabet Med. 2010;27(9):988–94. doi: 10.1111/j.1464-5491.2010.03062.x. [DOI] [PubMed] [Google Scholar]
- 179.Shinozaki H, Aoki H, Kasahara Y, Kangawa K, Minegishi T. Plasma adrenomedullin levels during multiple pregnancy. Gynecol Obstet Invest. 2010;69(3):169–73. doi: 10.1159/000265526. [DOI] [PubMed] [Google Scholar]
- 180.Hata T, Miyazaki K, Matsui K. Decreased circulating adrenomedullin in pre-eclampsia. Lancet. 1997;350(9091):1600. doi: 10.1016/S0140-6736(05)64016-0. [DOI] [PubMed] [Google Scholar]
- 181.Martinez A, Elsasser TH, Bhathena SJ, et al. Is adrenomedullin a causal agent in some cases of type 2 diabetes? Peptides. 1999;20(12):1471–8. doi: 10.1016/s0196-9781(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 182.Di Iorio R, Marinoni E, Urban G, Costantini A, Cosmi EV, Letizia C. Fetomaternal adrenomedullin levels in diabetic pregnancy. Horm Metab Res. 2001;33(8):486–90. doi: 10.1055/s-2001-16942. [DOI] [PubMed] [Google Scholar]
- 183.Di Iorio R, Marinoni E, Letizia C, Gazzolo D, Lucchini C, Cosmi EV. Adrenomedullin is increased in the fetoplacental circulation in intrauterine growth restriction with abnormal umbilical artery waveforms. Am J Obstet Gynecol. 2000;182(3):650–4. doi: 10.1067/mob.2000.103944. [DOI] [PubMed] [Google Scholar]
- 184.Makino I, Makino Y, Yoshihara F, et al. Decreased mature adrenomedullin levels in feto-maternal tissues of pregnant women with histologic chorioamnionitis. Biochem Biophys Res Commun. 2003;301(2):437–42. doi: 10.1016/s0006-291x(02)03059-0. [DOI] [PubMed] [Google Scholar]
- 185.Jerat S, Morrish DW, Davidge ST, Kaufman S. Effect of adrenomedullin on placental arteries in normal and preeclamptic pregnancies. Hypertension. 2001;37(2):227–31. doi: 10.1161/01.hyp.37.2.227. [DOI] [PubMed] [Google Scholar]
- 186.Makino Y, Shibata K, Makino I, Ono Y, Kangawa K, Kawarabayashi T. Expression of adrenomedullin in feto-placental circulation of human normotensive pregnant women and pregnancy-induced hypertensive women. Endocrinology. 1999;140(11):5439–42. doi: 10.1210/endo.140.11.7243. [DOI] [PubMed] [Google Scholar]
- 187.Di Iorio R, Marinoni E, Letizia C, Alo P, Villaccio B, Cosmi EV. Adrenomedullin, a new vasoactive peptide, is increased in preeclampsia. Hypertension. 1998;32(4):758–63. doi: 10.1161/01.hyp.32.4.758. [DOI] [PubMed] [Google Scholar]
- 188.Lu B, Wang A, Fei Y. [Study of the plasma adrenomedullin value in pregnancy induced hypertension patients] Zhonghua Fu Chan Ke Za Zhi. 1999;34(1):17–9. [PubMed] [Google Scholar]
- 189.Lauria MR, Standley CA, Sorokin Y, Yelian FD, Cotton DB. Adrenomedullin levels in normal and preeclamptic pregnancy at term. J Soc Gynecol Investig. 1999;6(6):318–21. doi: 10.1016/s1071-5576(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 190.Di Iorio R, Marinoni E, Letizia C, et al. Influence of labor on fetoplacental adrenomedullin concentrations. Am J Obstet Gynecol. 2001;185(3):697–702. doi: 10.1067/mob.2001.117189. [DOI] [PubMed] [Google Scholar]
- 191.El-mashad AI, Mohamed MA, Farag MA, Ahmad MK, Ismail Y. Role of uterine artery Doppler velocimetry indices and plasma adrenomedullin level in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2011;37(1):51–7. doi: 10.1111/j.1447-0756.2010.01318.x. [DOI] [PubMed] [Google Scholar]
- 192.Dikensoy E, Balat O, Pence S, Balat A, Cekmen M, Yurekli M. The changes of plasma malondialdehyde, nitric oxide, and adrenomedullin levels in patients with preeclampsia. Hypertens Pregnancy. 2009;28(4):383–9. doi: 10.3109/10641950802629634. [DOI] [PubMed] [Google Scholar]