Abstract
Hypertriglyceridemia and associated high circulating free fatty acids are important risk factors of atherosclerosis. In contrast to omega-3 fatty acids, linoleic acid, the major omega-6 unsaturated fatty acid in the American diet, may be atherogenic by amplifying an endothelial inflammatory response. We hypothesize that omega-6 and omega-3 fatty acids can differentially modulate TNF-α-induced endothelial cell activation and that functional plasma membrane microdomains called caveolae are required for endothelial cell activation. Caveolae are particularly abundant in endothelial cells and play a major role in endothelial trafficking and the regulation of signaling pathways associated with the pathology of vascular diseases. To test our hypothesis, endothelial cells were pre-enriched with either linoleic acid or α-linolenic acid prior to TNF-α-induced endothelial activation. Measurements included oxidative stress and NF-κB-dependent induction of COX-2 and PGE2 under experimental conditions with intact caveolae and with cells in which caveolin-1 was silenced by siRNA. Exposure to TNF-α induced oxidative stress and inflammatory mediators, such as p38 MAPK, NF-κB, COX-2 and PGE2, which were all amplified by pre-enrichment with linoleic acid but blocked or reduced by α-linolenic acid. The p38 MAPK inhibitor SB203580 blocked TNF-α-mediated induction of COX-2 protein expression, suggesting a regulatory mechanism through p38 MAPK signaling. Image overlay demonstrated TNF-α-induced co-localization of TNF receptor type 1 (TNFR-1) with caveolin-1. Caveolin-1 was significantly induced by TNF-α, which was further amplified by linoleic acid and blocked by α-linolenic acid. Furthermore, silencing of the caveolin-1 gene completely blocked TNF-α-induced production of COX-2 and PGE2 and significantly reduced the amplified response of linoleic acid plus TNF-α. These data suggest that omega-6 and omega-3 fatty acids can differentially modulate TNF-α-induced inflammatory stimuli and that caveolae and its fatty acid composition play a regulatory role during TNF-α-induced endothelial cell activation and inflammation.
Keywords: atherosclerosis, inflammation, endothelial activation, omega-6 and omega-3 polyunsaturated fatty acids, tumor necrosis factor-α, oxidative stress, caveolae
1. Introduction
Severe endothelial cell activation and injury can lead to necrotic and apoptotic cytotoxicity, and ultimately to disruption of endothelial integrity. Dysfunction of endothelial cells and associated inflammatory events are critical underlying causes of the initiation of cardiovascular diseases such as atherosclerosis [1, 2]. The mechanisms by which selected fatty acids induce endothelial cell activation, oxidative stress and inflammation are not fully understood. Oxidative stress-induced transcription factors, which regulate inflammatory cytokine and adhesion molecule production, are important regulatory elements in the induction of inflammatory responses. One of these transcription factors, nuclear factor κB (NF-κB), plays a significant role in these regulatory processes [3]. Binding sites for NF-κB and related transcription factors were identified in the promoter regions of a variety of inflammatory genes [4, 5] such as interleukin 6 (IL-6), vascular cell adhesion molecule-1 (VCAM-1) or cyclooxygenase-2 (COX-2), all of which are up-regulated by TNF-α [6, 7].
Numerous risk factors for the development of atherosclerosis have been identified, including obesity [8] and hypertriglyceridemia [9]. Increased circulating free fatty acid levels are associated with hypertriglyceridemia and obesity [10], and high plasma free fatty acids may contribute to an environment of increased oxidative stress and inflammation in the vasculature and especially in vascular endothelial cells [11, 12]. Dietary balance of long-chain fatty acids may influence processes involving leukocyte-endothelium interactions, such as atherogenesis and inflammation [13]. Even though diets high in omega-6 fatty acids may lead to a decrease in serum cholesterol [14], replacing saturated with unsaturated omega-6 rich lipids may not be desirable because of their ability to easily oxidize. High intake of linoleic acid-rich oils or fats will lead to an increase in cellular oxidative stress and can elicit an inflammatory response [15], events which have been implicated in most chronic diseases. Omega-6 fatty acids, and especially linoleic acid can cause endothelial cell dysfunction as well as potentiate tumor necrosis factor-α (TNF-α)-mediated endothelial injury [16]. We have recently demonstrated that both the extracellular signal regulated kinase (ERK1/2) and phosphoinositide-3 kinase/amino kinase terminal (PI3K/Akt) signaling pathways can contribute to the effect of linoleic acid on nuclear factor-kappa B (NF-κB)-dependent transcription and endothelial cell activation [17].
In contrast to omega-6 fatty acids, omega-3 fatty acids can influence cardiovascular disease pathology by beneficially modulating inflammation. Epidemiological and interventional studies have shown a dose-dependent decrease in risk of cardiovascular disease endpoints with increased dietary consumption of moderate amounts of omega-3 fatty acids, either plant or marine derived [14]. Current estimates indicate that over 90% of the omega-3 consumed by U.S. citizens is in the form of α-linolenic acid, not the longer chain omega-3 fatty acids found in fish oils [18]. Independent of their dietary source, omega-3 fatty acids contribute to cardio-protective properties, which include down-regulation of proinflammatory and proatherogenic genes, including adhesion molecules and cytokines, during early atherogenesis and possibly also during later stages of plaque development and plaque rupture [19]. For example, an α-linolenic acid-rich oil decreased oxidative stress and CD40 ligand in patients with mild hypercholesterolemia [20], reduced levels of soluble cell adhesion molecules in plasma [21] and recurrence of coronary heart disease [22]. Also, by partially replacing omega-6 analogues in membrane phospholipids with omega-3 fatty acids, it is possible to decrease the transcriptional activation of inflammatory and pro-atherogenic genes involved in endothelial cell activation and atherosclerosis [23].
There is increasing evidence that lipid raft proteins and lipids play an important role in health and disease [24]. A major subclass of lipid rafts is caveolae, i.e., membrane domains which have been implicated in the pathology of atherosclerosis [25]. The lack of the caveolin-1 gene may provide protection against the development of atherosclerosis [26]. This may be important in understanding mechanisms of atherosclerosis, because caveolae are particularly abundant in endothelial cells, where they are believed to play a major role in the regulation of endothelial vesicular trafficking as well as the uptake of lipids and related lipophilic compounds [27]. There is evidence that fatty acids can alter localization and function of caveolae-associated signaling proteins in mouse colonic mucosa [28]. Besides their role in cellular uptake of lipophilic substances, including fatty acids [29], caveolae house an array of cell signaling molecules, and numerous genes involved in endothelial cell dysfunction and inflammation are associated with caveolae [25]. Examples of caveolae facilitated targeting of proteins involved in pro-inflammatory signal transduction include activators of p44/p42 (ERK) MAPK pathway, H-Ras [30, 31], non-receptor tyrosine kinase c-Src [31], and the upstream regulator of NF-κB, IkappaB kinase (IKK) [32]. Furthermore, caveolins have been reported to co-localize with cyclooxygenase, suggesting that caveolins play a role in regulating the function of this enzyme [33, 34].
A major objective of the current study was to explore specific mechanisms involved in fatty acid-mediated activation of endothelial cells. Our current data support our hypothesis that omega-6 and omega-3 fatty acids can differentially modulate TNF-α-induced endothelial cell activation and that regulatory mechanisms are associated within caveolae and linked to caveolae function and associated gene inductions.
2. Materials and Methods
2.1 Materials
Linoleic acid and α-linolenic acid (> 99% pure by gas-liquid chromatography) were obtained from Nu-Chek Prep (Elysian, MN). Human TNF-α was purchased from Sigma (St. Louis, MO). P38 MAPK inhibitor SB203580 was purchased from Calbiochem (EMD Biosciences, San Diego, CA).
2.2 Cell culture and experimental media
Endothelial cells were isolated from porcine pulmonary arteries and cultured as previously described [35]. Arteries obtained during routine slaughter were donated from the College of Agriculture, University of Kentucky. The basic culture medium consisted of medium 199 (M-199) (GIBCO Laboratories, NY) containing 10% (v/v) fetal bovine serum (FBS, HyClone Laboratories, UT). The experimental media were composed of M-199 enriched with 5% (v/v) FBS and enriched with 20 μmol/L linoleic acid or α-linolenic acid. Preparation of experimental media with fatty acids was performed as described earlier [36].
2.3 Measurement of oxidative stress
Cellular oxidation was determined by 2',7'-dichlorofluorescein (DCF) fluorescence as described earlier [37]. This method is based on the conversion of 2’,7’-dichlorofluorescin into fluorescent 2’,7’-dichlorofluorescein by oxygen reactive species, primarily peroxyl radicals and peroxides. Cells were first pretreated with different fatty acids followed by TNF-α treatment. Then cells were incubated with 100 μmol/L 2,7-dichlorofluorescin diacetate (Molecular Probes, Inc., OR) for 30 minutes. A multi-well fluorescent plate reader (Molecular Devices, CA) was utilized for the imaging study. Excitation and emission wavelengths were 490 and 520 nm, respectively.
2.4 Transcription factor NF-κB activation studies: electrophoretic mobility shift assay (EMSA)
Nuclear extracts containing active proteins were prepared from cells according to the method of Beg et al. [38], with minor modifications. Binding reactions were performed in a 20 μL volume containing 7 μg of nuclear protein extracts. Nuclear extracts were incubated for 25 minutes with 32P-end-labeled oligonucleotide probes containing enhancer DNA element NF-κB (5’ AGTTGAGGGGACTTTCCCAGGC 3’) (Santa Cruz Biotech, CA). Following binding, the protein-DNA complex and uncomplexed DNA in the mixture were resolved on native 5% polyacryamide gels using 0.5× TBE buffer (50 mmol/L Tris-Cl, 45 mmol/L boric acid, 0.5 mmol/L EDTA, pH 8.4) and visualized by autoradiography. Control reactions using supershift assay were performed to demonstrate the specificity of the shifted DNA-protein complexes for NF-κB.
2.5 Measurement of caveolae-1 protein expression, p38 MAPK phosphorylation, and COX-2 protein levels by Western blotting
Cells were harvested using cell lysis buffer as previously described [17]. Protein samples were resolved by SDS-PAGE using 12% gradient gels for caveolin-1 and p38 MAPK, and 10% gradient gels for COX-2, and then transferred to nitrocellulose membranes using a Bio-Rad immunoblot transfer apparatus (Bio-Rad Laboratories, CA) according to the manufacturer's instructions. The nonspecific sites on the membrane were blocked for 1 h at room temperature with 5% nonfat dry milk in TBST followed by incubation with caveolin-1 (Affinity BioReagents, CO), p38 MAPK (total and phosphorylation forms, respectively), or COX-2 (Santa Cruz Biotech, CA) primary antibodies overnight at 4 °C. β-actin (Sigma, St. Louis, MO) was used as a housekeeping gene in caveolin-1 and COX-2 protein measurements. Bands were visualized using the appropriate horseradish peroxidase-conjugated secondary antibody followed by ECL immunoblotting detection reagents (Amersham Biosci, UK). Detection and quantitative analysis were performed using a digitizing system (UN-SCAN-IT, Silk Scientific Corporation, UT).
2.6 Immunofluorescence microscopy
This technique was adapted from previous protocols [39]. Cells were plated on LAB-TEKII chamber slides (Nalge Nunc International, Naperville, IL) and grown to confluence. After experimental treatments, cells were fixed in 4% (vol/vol) paraformaldehyde (in PBS) for 1 hour at room temperature. After permeabilization with 0.1% (vol/vol) Triton ×-100 in PBS for 5 min, cells were washed three times with PBS. Nonspecific binding sites were blocked with 5% (vol/vol) donkey serum in PBS. Cells were then incubated with anti-caveolin-1 antibody (Affinity BioReagents, Golden, CO) and anti-TNFR-1 antibody (R&D Systems, Minneapolis, MN), followed by incubation with Alexa Fluor 488-labeled donkey anti-mouse IgG antibody and Alexa Fluor 546-labeled donkey anti-goat IgG antibody, respectively (Invitrogen-Molecular Probes, Carlsbad, CA). Cell nuclei were stained using Hoechst (AnaSpec Inc., San Jose, CA). Slides were mounted in Aqueous Mounting Medium with Anti-fading Agents (Biomeda Corp., Foster City, CA) and covered with coverslips. Images were captured digitally by Fluoview 300 Confocal Microscopy (Olympus America Inc., PA).
2.7 PGE2 determination
Cells were seeded in 60 mm culture dishes (Becton Dickinson Labware, NJ) and grown to confluence. After pre-treatment with fatty acids, cells were exposed to PCB77. Subsequently, supernatants of cell cultures were collected into microcentrifuge tubes (Isc BioExpress, UT), centrifuged at 4 °C to remove cellular debris and stored at -80 °C. PGE2 levels were assessed using a PGE2-specific enzyme immunoassay (EIA) (Cayman Chemicals, MI) following the manufacturer's protocol. Absorbance at 405 nm was detected using a microplate spectrophotometer SpectraMaxPro M2 (Molecular Devices Corporation, CA).
2.8 Caveolin-1 siRNA and transfection
Caveolin-1 gene silencer was designed as previously described [40]. Cells were transfected with control siRNA or caveolin-1 siRNA at a final concentration of 40 nmol/L using GeneSilencer (Genlantis, San Diego, CA) in Optimem I medium (Invitrogen, Carlsbad, California). Cells were incubated with transfection mixtures for 4 hours and then replaced with regular medium. 48 hours after transfection, cells were treated with TNF-α in the presence or absence of linoleic acid.
2.9 Statistical analysis
Values are reported as mean ± standard error of the mean (SEM) of at least three independent experiments. Data were analyzed using Sigma Stat software (Jandel Corp., Wan Rafael, CA). One way ANOVA was followed by post hoc comparisons using the least significant difference (LSD) method. A statistical probability of p< 0.05 was considered significant.
3. Results
3.1 Linoleic acid and α-linolenic acid modulate cellular oxidative stress, NF-κB activity and COX-2 expression induced by TNF-α
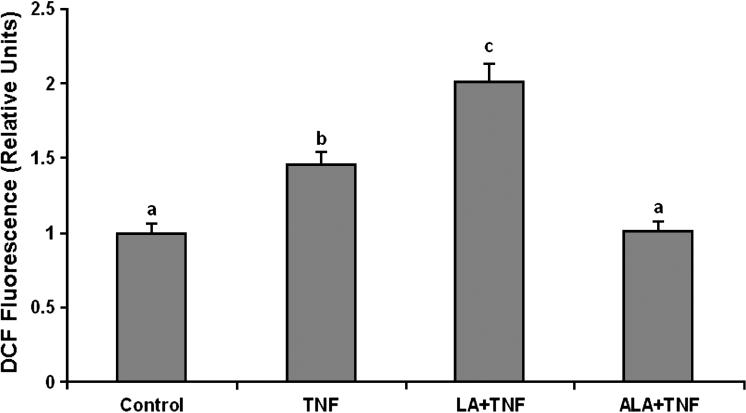
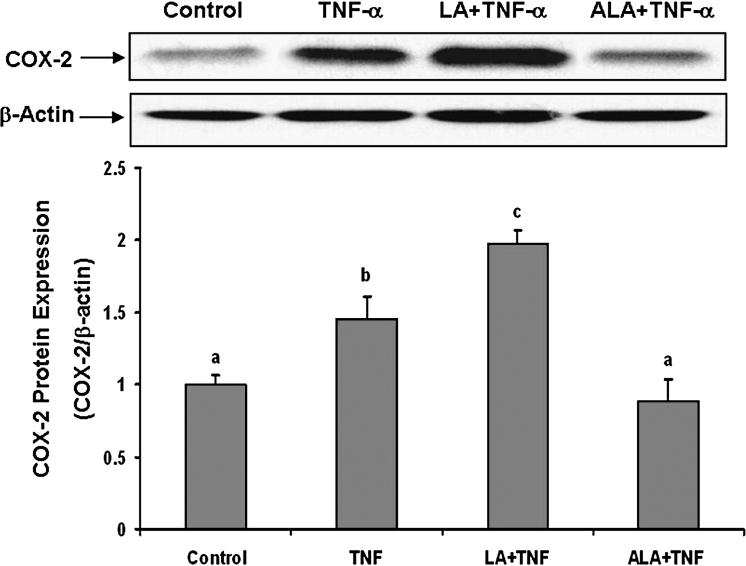
To examine the effects of omega-6 and omega-3 fatty acids on TNF-α-induced endothelial activation, cells were pretreated with either linoleic acid or α-linolenic acid, followed by exposure to TNF-α. As indicated in Figures 1-3, exposure to TNF-α alone significantly increased cellular oxidative stress (ROS), NF-κB DNA binding activity, and COX-2 protein expression, respectively. Pretreatment with linoleic acid following by exposure to TNF-α further induced these effects compared to cultures treated only with TNF-α (Figures 1-3). In contrast, pretreatment with α-linolenic acid blocked the TNF-α-induced oxidative stress and subsequent induction of NF-κB and COX-2.
Figure 1.
Effect of linoleic acid (LA) and α-linolenic acid (ALA) on TNF-α-induced oxidative stress. Cultures were pretreated in media supplemented with 20 μmol/L LA or ALA for 24 hours followed by exposure to 0.5 ng/mL TNF-α for additional 3 hours. Values are means ± SEM (n = 3). Different letters represent significant differences among the treatment groups.
Figure 3.
Effect of linoleic acid (LA) and α-linolenic acid (ALA) on TNF-α-induced expression of COX-2. Cells were pretreated with 20 μmol/L of LA or ALA for 24 hours and then exposed to 0.5 ng/mL TNF-α for an additional 8 hours. COX-2 protein levels were measured by Western blotting and normalized according to β-actin expression. Experiments were repeated three times, and the blots shown are representative of one of the experiments. The bar graph shows the corresponding densitometric analysis of the blots. Values are means ± SEM. Different letters represent significant difference among treatment groups.
3.2 Linoleic acid and α-linolenic acid modulate TNF-α-induced activation of p38 MAPK
In mammalian cells, MAPKs are strongly activated by growth factors, environmental stresses, and inflammatory cytokines [41]. As shown in Figure 4A, p38 MAPK was significantly activated by TNF-α, and pre-treatment with linoleic acid followed by exposure to TNF-α further increased p38 MAPK phosphorylation. In contrast, p38 MAPK activation was blocked by preenrichment with α-linolenic acid.
Figure 4.
Effect of linoleic acid (LA) and α-linolenic acid (ALA) on TNF-α-induced p38-MAPK activation and the effect of p38-MAPK inhibitor on TNF-α-induced COX-2 expression. (A) Endothelial cells were pretreated with 20 μmol/L of LA or ALA for 24 hours and then exposed to 0.5 ng/mL TNF-α for 10 minutes. Phosphorylated and total p38 MAPK were detected by Western blotting using specific dually phosphorylated p38 MAPK antibody (Thr 180/Tyr 182) or anti-total p38 MAPK antibody, respectively. (B) Cells were exposed to 0.5 ng/mL TNF-α for 8 hours, or first pre-enriched with 10 μmol/L SB203508 for 30 minutes followed by co-exposure to TNF-α for additional 8 hours. COX-2 protein levels were measured by Western blotting and normalized according to β-actin expression. Experiments were repeated three times and the blots shown are a representative of one of the experiments. The bar graph shows the corresponding densitometric analysis of the blots. Values are means ± SEM. Different letters represent significant difference among treatment groups.
In order to further evaluate the role of p38 MAPK in TNF-α-induced inflammatory gene expression, endothelial cells were pretreated with the specific p38 MAPK inhibitor SB203580 prior to exposure to TNF-α. As shown in Figure 4B, SB203580 significantly decreased TNF-α-induced COX-2 expression.
3.3 Linoleic acid and α-linolenic acid modulate TNF-α-induced PGE2 production
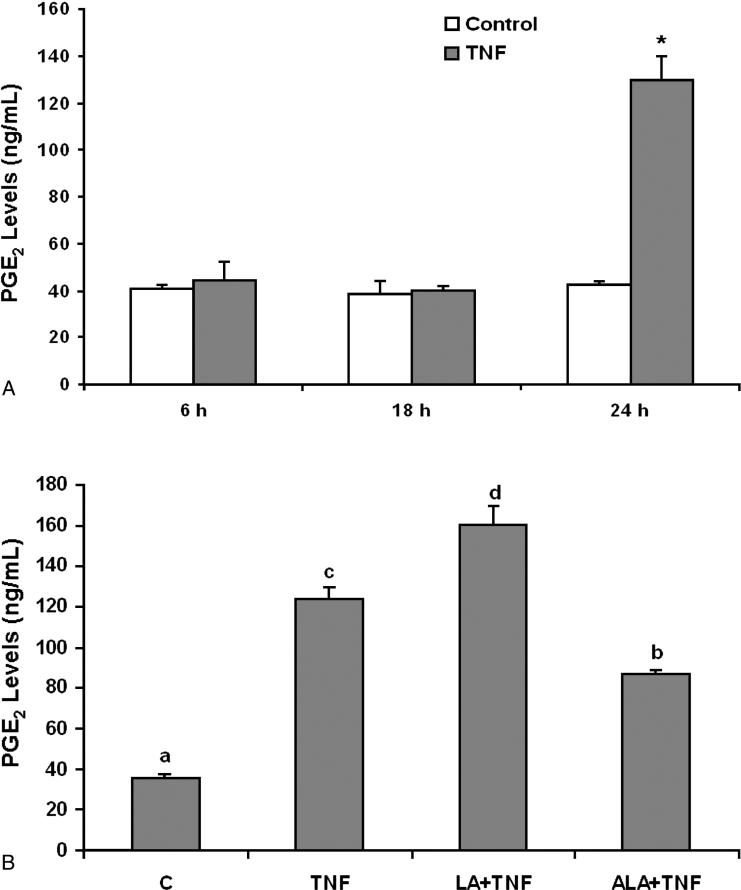
COX-2 plays a key role in inflammation, and PGE2 is a major inflammatory mediator. As shown in Figure 5A, a 24 hours exposure to TNF-α significantly induced PGE2 production. Compared to the TNF-α treatment group, enriching endothelial cells with linoleic acid further enhanced TNF-α-induced PGE2 levels (Figure 5B). In contrast, cellular exposure to α-linolenic acid markedly reduced the proinflammatory effect of TNF-α (Figure 5B).
Figure 5.
Effect of linoleic acid (LA) and α-linolenic acid (ALA) on TNF-α-stimulated release of PGE2 from endothelial cells. (A) Cells were exposed to 0.5 ng/mL TNF-α for 6, 18 and 24 hours. Supernatants of cell cultures were collected and PGE2 levels were measured by enzyme immunoassay (EIA). Bars represent means ± SEM from three independent experiments. * represents significant difference compared with the control treatment. (B) Cells were pretreated with 20 μmol/L of LA or ALA for 24 hours and then exposed to 0.5 ng/mL TNF-α for additional 24 hours. Supernatants of cell cultures were collected and PGE2 levels were measured by EIA. Values are means ± SEM (n=3). Different letters represent significant difference among treatment groups.
3.4 Linoleic acid and α-linolenic acid modulate TNF-α-induced caveolin-1 up-regulation and the co-localization of TNFR-1and caveolin-1
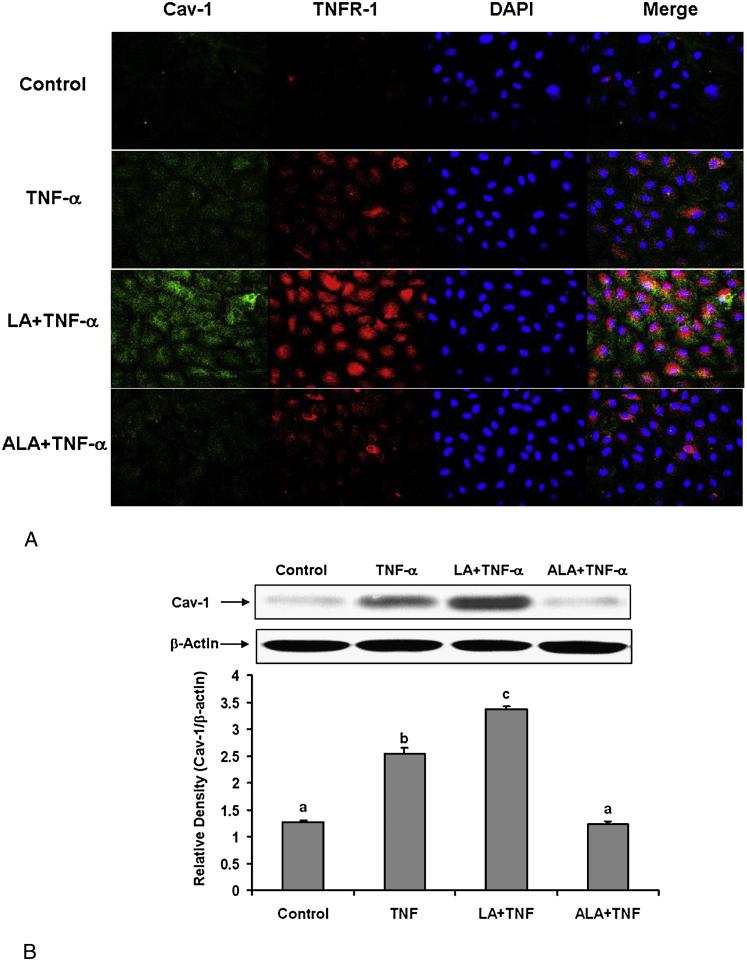
The first step in the TNF-α-induced inflammatory response is the binding of the cytokine to its cognate receptor. TNF receptor type 1 (TNFR-1) has been reported to be enriched in caveolae [42]. Caveolin-1 has also been shown to form a complex with the TNF receptor [43]. To assess the effects of linoleic acid and α-linolenic acid on caveolin-1 expression and its co-localization with TNFR-1, endothelial cells were first pre-incubated with either fatty acid, followed by treatment with TNF-α. The immunofluorescence images in Figure 6A demonstrate that TNF-α can increase caveolin-1 and TNFR-1 expression compared with the control group, and that pretreated cells with linoleic acid followed by TNF-α can further increase these effects compared with TNF-α treatment alone. The overlapped images also indicate that the co-localization of caveolin-1 and TNFR-1 is markedly increased in cells pretreated with linoleic acid followed by TNF-α compared with TNF-α treatment alone. In contrast, cells pretreated with α-linolenic acid followed by exposure to TNF-α diminished both TNF-α-induced caveolin-1 up-regulation and the co-localization of TNFR-1 and caveolin-1. The effects of linoleic acid and α-linolenic acid on TNF-α–induced caveolin-1 expression were confirmed by measuring caveolin-1 protein levels by Western blotting (Figure 6B).
Figure 6.
Effect of linoleic acid (LA) and α-linolenic acid (ALA) on TNF-α-induced up-regulation of caveolin-1 and colocalization of TNFR-1 and caveolin-1. Cells were treated with 0.5 ng/mL TNF-α alone for 4 hours, or pretreated with 20 μmol/L LA or ALA for 24 hours followed by exposure to TNF-α for 4 hours. (A) Immunofluorescence staining for caveolin-1 (FITC green fluorescence), TNF Receptor-1 (TNFR-1) (Texas red fluorescence), and nuclei (DAPI blue fluorescence). Regions of co-localization of caveolin-1 and TNFR-1 are depicted in yellow in the merged images. Experiments were repeated three times and the epifluorescence micrographs shown are representative fields of one of the experiments (original magnification, × 400). (B) Caveolin-1 protein levels were detected by Western blotting and normalized according to β-actin expression. Experiments were repeated three times, and the blots shown are a representative of one of the experiments. The bar graph shows the corresponding densitometric analysis of the blots. Values are means ± SEM. Different letters represent significant differences among treatment groups.
3.5 Caveolin-1 silencing decreases TNF-a-induced COX-2 expression
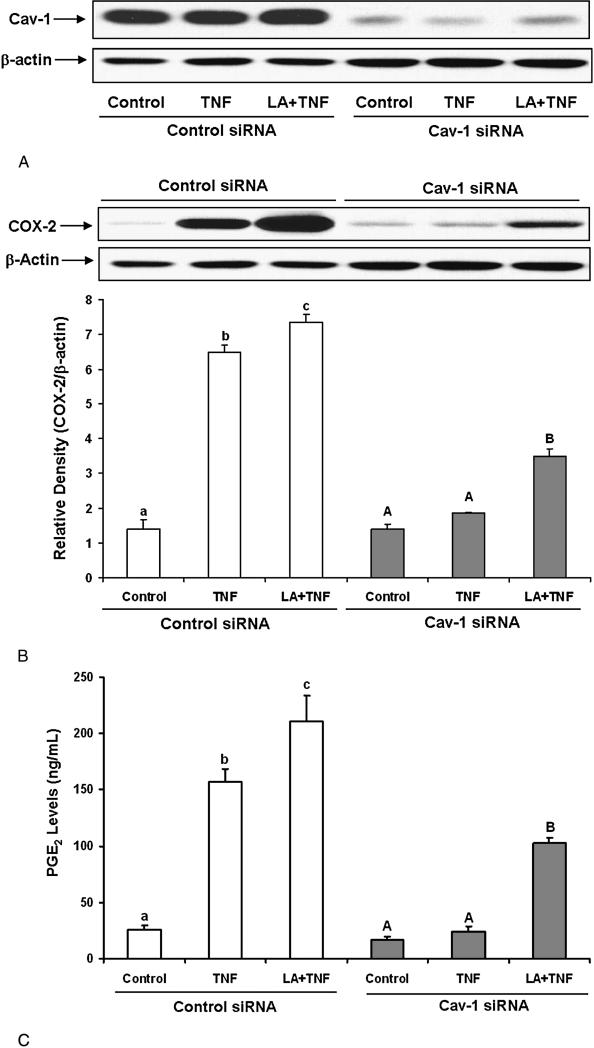
In order to determine the role of caveolin-1 in TNF-α-induced endothelial cell activation, we utilized small interfering RNA to specifically down-regulate caveolin-1. As indicated in Figure 7A, caveolin-1 siRNA reduced caveolin-1 expression by approximately 80% as compared with control siRNA. TNF-α-induced COX-2 expression was totally blocked by caveolin-1 silencing (Figure 7B). Caveolin-1 silencing also diminished the combined COX-2 induction induced by both linoleic acid and TNF-α. Similar to the COX-2 data, caveolin-1 silencing blocked TNF-α-induced PGE2 production and also diminished TNF-α-induced PGE2 production in the presence of linoleic acid (Figure 7C).
Figure 7.
Effect of caveolin-1 silencing on TNF–α- and linoleic acid (LA)-induced COX-2 expression and PGE2 synthesis. Cells were transfected with siRNA for caveolin-1 (Cav-1 siRNA) or with scrambled control siRNA. Then, cells were treated with 0.5 ng/mL TNF-α alone for 24 hours, or pretreated with 20 μmol/L LA for 24 hours followed by exposure to TNF-α for additional 24 hours. Caveolin-1 protein levels were measured by Western blotting and normalized according to β-actin expression (Figure 7A). COX-2 protein levels were measured by Western blotting under the same experimental conditions as in Figure 7A (Figure 7B). Supernatants of cell cultures were collected and PGE2 levels were measured by EIA (Figure 7C). Experiments were repeated three times and the blots shown are a representative of one of the experiments. Values are means ± SEM. Different letters represent significant difference among treatment groups.
4. Discussion
Atherosclerosis is considered an inflammatory disease, which involves the interplay of prooxidative activities, induction of inflammatory cytokines and adhesion molecules, and activation of vascular endothelial cells, all events which promote vascular leukocyte infiltration and plaque development [1]. Inflammatory events also include the cyclooxygenase and subsequent eicosanoid pathways [44, 45]. For example, inflammatory cytokines like interleukin-1beta (IL-1-β) can induce COX-2 expression and the release of PGE2, events which may be mediated through activation of p42/44 and p38 MAPKs, and the NF-κB pathway [46]. Our present data demonstrate that TNF-α can markedly induce oxidative stress, p38 MAPK, NF-κB, COX-2 and PGE2 in vascular endothelial cells.
Hypertriglyceridemia has been identified as an independent risk factor for atherosclerosis, and increased circulating free fatty acid levels are associated with hypertriglyceridemia and obesity [10]. High plasma free fatty acids may contribute to an environment of increased oxidative stress and inflammation in the vasculature [11, 12], and we have previously shown that linoleic acid can markedly amplify a TNF-α-mediated endothelial inflammatory response [47]. We also have recently demonstrated that both the extracellular signal regulated kinase (ERK1/2) and phosphoinositide-3 kinase/amino kinase terminal (PI3K/Akt) signaling pathways can contribute to the stimulatory effect of linoleic acid on NF-κB-dependent transcription and endothelial cell activation [17]. In the current study, exposure to TNF-α induced oxidative stress, p38 MAPK, NF-κB, COX-2 and PGE2, which was amplified by pre-enrichment with linoleic acid. This clearly supports our hypothesis that diets high in omega-6 fatty acids, and especially in linoleic acid, are proinflammatory and thus may contribute to the early pathology of atherosclerosis.
In contrast to omega-6 fatty acids, epidemiological and interventional studies have shown a dose-dependent decrease in risk of cardiovascular disease endpoints with increased dietary consumption of moderate amounts of omega-3 fatty acids, either plant or marine derived [14]. Our data clearly demonstrate the anti-inflammatory properties of omega-3 fatty acids. In fact, TNF-α induced oxidative stress, p38 MAPK, NF-κB, COX-2 and PGE2 in endothelial cells was blocked or reduced when cells were enriched with α-linolenic acid. Mechanisms of vascular inflammation are largely regulated through p38 MAPK signaling [48-51], and our data suggest that p38 MAPK is an important player in fatty acid modification of the TNF-α-induced inflammatory signaling cascade. The p38 MAPK inhibitor SB203580 blocked TNF-α-mediated induction of COX-2 protein expression, suggesting a regulatory mechanism through p38 MAPK signaling. This was supported by evidence that p38 MAPK increases nuclear recruitment of NF-κB in cells exposed to inflammatory stimuli [52].
Mechanisms of linoleic acid-mediated induction of proinflammatory events, including enhanced expression and production of COX-2 and PGE2, as well as the observed protective properties by linolenic acid, are not clear. One possible reason for the linoleic acid-mediated endothelial activation and dysfunction is the observation that the conversion of linoleic acid to more unsaturated metabolites is diminished due to very low delta6 desaturase activity in endothelial cells [2]. We have reported previously that, when enriching endothelial cells with linoleic acid or linolenic acid, both fatty acids can incorporate into cellular phospholipids, but only linoleic acid accumulated into cell triglycerides [53]. Furthermore, eicosapentaenic acid (the bioactive elongation and desaturation metabolite of linolenic acid) increased in cellular phospholipids following enrichment with linolenic acid, whereas arachidonic acid levels decreased following enrichment with linoleic acid [53]. It is very likely, that linoleic acid can displace arachidonic acid from cell membrane phospholipids and thus make arachidonic acid more available for prostaglandin synthesis.
Even though our data suggest that exposure to TNF-α can cause endothelial cell dysfunction and that omega-6 fatty acids (and in particular linoleic acid) can amplify TNF-α-induced endothelial activation [47], mechanisms of this interaction may be multi-layered and are not known. We hypothesize that caveolae play a critical role in facilitating the cellular uptake and trafficking of fatty acids and trigger the initial cell signaling induced by TNF-α and lipids. Our present data suggest that functional caveolae are necessary for TNF-α and/or linoleic acid-mediated activation of endothelial cells. TNF-α markedly induced both caveolin-1 and TNFR-1, the events which were further induced when cells were first enriched with linoleic acid. TNFR-1 contains a death domain and this region is required for TNF-α-induced pro-inflammatory cellular responses, such as activation of NF-κB [54]. Furthermore, silencing caveolin-1 totally blocked both TNF-α-induced COX-2 expression and cellular release of PCE2. We recently investigated caveolin-1-regulated mechanisms associated with PCB-induced markers of peroxynitrite formation and DNA binding of NF-κB [55] and demonstrated that cellular exposure to PCB77 (a persistent organic pollutant) increased eNOS phosphorylation and NO production, as well as peroxynitrite levels. We also found that caveolin-1 silencing abolished the PCB-stimulated eNOS phosphorylation, suggesting a regulatory role of caveolae in PCB-induced eNOS signaling. In another study, perturbation of membrane raft structural integrity with cholesterol-sequestering compounds caused delocalization of eNOS from caveolae and inhibited TNF-α-induced ROS production and protein tyrosine nitration [56].
It is very likely that selected fatty acids either stabilize or perturb caveolae functions, thus leading to modifications of caveolae-dependent signaling. There is evidence that fatty acids can alter localization and function of caveolae-associated signaling proteins in mouse colonic mucosa [28]. Our data provide evidence that TNF-α-induced upregulation of caveolin-1 is further enhanced when endothelial cells are first enriched with linoleic acid. In contrast, pre-enrichment with α-linolenic acid totally blocked TNF-α-mediated induction of caveolin-1. These data suggests that omega-3 fatty acids may in part be anti-inflammatory by decreasing caveolin-1 expression or by causing dysfunctional caveolae, leading to down-regulation of caveolin-dependent down-stream signaling. Using human breast cancer cells, others have shown recently that omega-3 fatty acids can alter lipid raft composition and a decrease in epidermal growth factors [57], suggesting that selected fatty acids can modify caveolae-associated cell functions. There is evidence that caveolae play a role in lipid trafficking and uptake and transport of fatty acids [24]. Furthermore, preferential uptake of fatty acids via the FAT/CD36, a receptor exclusively located in lipid rafts such as caveolae, may explain the fatty acid effects we observed. In fact, working with mouse embryonic fibroblasts, it was recently demonstrated that mistargeting of FAT/CD36 in cells lacking the caveolin-1 gene resulted in reduced fatty acid uptake compared with the wild-type cells [58].
Our observations that linoleic acid can amplify the TNF-α-mediated induction of both caveolin-1 and COX-2 is significant because of the link between caveolins (caveolae) and the pathology of atherosclerosis [25]. Caveolin-1 has been reported to co-localize with interleukin-1beta-induced COX-2 [59], suggesting the dependence of COX-2 induction on functional caveolae. Recent evidence suggests that high-fat diets can up-regulate caveolin-1 expression in aorta of diet-induced obese rats [60], suggesting that our fatty acid data may mimic an in vivo response by activating COX-2. High-fat diets contribute to hypertriglyceridemia, and the vascular endothelium can be exposed to significant levels of free fatty acids derived from lipoprotein lipase-mediated hydrolysis of triglyceride-rich lipoproteins [16].
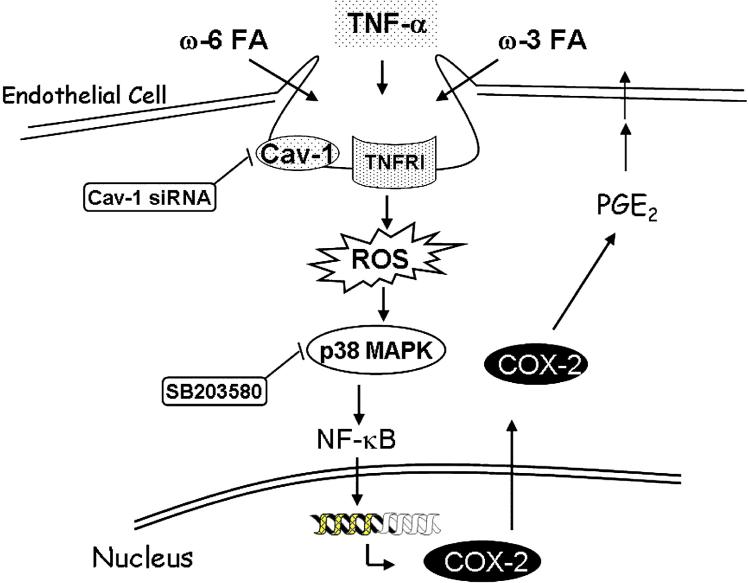
In summary, we provide novel data demonstrating that omega-6 and omega-3 fatty acids can differentially modulate TNF-α-induced inflammatory stimuli and that these events require functional caveolae (Figure 8). Furthermore, functional changes of caveolae associated with modifications by dietary fatty acids appear to affect critical phases of induction of oxidative stress-sensitive transcription factors and inducible inflammatory parameters during endothelial cell activation. Because caveolae and caveolins have been implicated in several human diseases and in particular vascular diseases, our data may have implications in understanding novel mechanisms of inflammatory diseases modulated by dietary lipids.
Figure 8.
Proposed mechanism for fatty acid-mediated modulation of endothelial cell activation induced by TNFα. Omega-6 or omega-3 fatty acids can differentially modulate TNFα-induced up-regulation of caveolin-1 and the activation of TNFR-1 mediated signaling pathway, which includes induction of oxidative stress (ROS), p38 MAPK, NF-κB and COX-2. TNF-α induced cell signaling and PGE2 production are further enhanced by linoleic acid but blocked by α-linolenic acid. Finally, targeted knockdown of caveolin-1 completely abrogates TNF-α-induced PGE2 production, indicating that caveolin-1 plays a mechanistic role in TNF-α-induced endothelial cell activation and modification by dietary fatty acids.
Figure 2.
Effect of linoleic acid (LA) and α-linolenic acid (ALA) on TNF-α-induced activation of NF-κB. Cells were treated with 20 μmol/L of LA or ALA for 24 hours prior to exposure to 0.5 ng/mL TNF-α for an additional 6 hours. Experiments were repeated three times, and the blots shown are a representative of one of the experiments. The bar graph shows the corresponding densitometric analysis of the blots. Values are means ± SEM. Different letters represent significant differences among treatment groups.
Acknowledgment
This study was supported in part by grants from NIH/NIEHS (P42 ES 07380), and the University of Kentucky Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Spector AA, et al. Utilization of arachidonic and linoleic acids by cultured human endothelial cells. J Clin Invest. 1981;68(4):1003–11. doi: 10.1172/JCI110322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Winther MP, et al. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25(5):904–14. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 4.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85(8):753–66. doi: 10.1161/01.res.85.8.753. [DOI] [PubMed] [Google Scholar]
- 5.Muller JM, Rupec RA, Baeuerle PA. Study of gene regulation by NF-kappa B and AP-1 in response to reactive oxygen intermediates. Methods. 1997;11(3):301–12. doi: 10.1006/meth.1996.0424. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, et al. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S9–12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- 7.Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10(1):27–39. doi: 10.1016/s1359-6101(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Corral A, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 9.Cullen P. Evidence that triglycerides are an independent coronary heart disease risk factor. Am J Cardiol. 2000;86(9):943–9. doi: 10.1016/s0002-9149(00)01127-9. [DOI] [PubMed] [Google Scholar]
- 10.Mostaza JM, et al. Abnormal metabolism of free fatty acids in hypertriglyceridaemic men: apparent insulin resistance of adipose tissue. J Intern Med. 1998;243(4):265–74. doi: 10.1046/j.1365-2796.1998.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.Austin MA, et al. Cardiovascular disease mortality in familial forms of hypertriglyceridemia: A 20-year prospective study. Circulation. 2000;101(24):2777–82. doi: 10.1161/01.cir.101.24.2777. [DOI] [PubMed] [Google Scholar]
- 12.Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med. 2001;47:111–36. [PubMed] [Google Scholar]
- 13.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71(1 Suppl):213S–23S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 14.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;98(4A):3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Grimble RF. Dietary lipids and the inflammatory response. Proc Nutr Soc. 1998;57(4):535–42. doi: 10.1079/pns19980078. [DOI] [PubMed] [Google Scholar]
- 16.Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J Am Coll Nutr. 2001;20(2 Suppl):97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]
- 17.Hennig B, et al. Linoleic acid induces proinflammatory events in vascular endothelial cells via activation of PI3K/Akt and ERK1/2 signaling. J Nutr Biochem. 2006;17(11):766–72. doi: 10.1016/j.jnutbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Trumbo P, et al. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 19.De Caterina R, et al. Nutritional mechanisms that influence cardiovascular disease. Am J Clin Nutr. 2006;83(2):421S–426S. doi: 10.1093/ajcn/83.2.421S. [DOI] [PubMed] [Google Scholar]
- 20.Alessandri C, et al. Alpha-linolenic acid-rich wheat germ oil decreases oxidative stress and CD40 ligand in patients with mild hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26(11):2577–8. doi: 10.1161/01.ATV.0000242795.08322.fb. [DOI] [PubMed] [Google Scholar]
- 21.Thies F, et al. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36(11):1183–93. doi: 10.1007/s11745-001-0831-4. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med. 2005;11(3):24–30. quiz 31, 79. [PubMed] [Google Scholar]
- 23.De Caterina R, Massaro M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J Membr Biol. 2005;206(2):103–16. doi: 10.1007/s00232-005-0783-2. [DOI] [PubMed] [Google Scholar]
- 24.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99(3):129–40. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 25.Frank PG, et al. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23(7):1161–8. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 26.Frank PG, et al. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(1):98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 27.Matveev S, et al. The role of caveolae and caveolin in vesicle-dependent and vesicle-independent trafficking. Adv Drug Deliv Rev. 2001;49(3):237–50. doi: 10.1016/s0169-409x(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 28.Ma DW, et al. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. Faseb J. 2004;18(9):1040–2. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 29.Pohl J, et al. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16(1):24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy S, et al. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1(2):98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- 31.Stan RV, et al. Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae). Mol Biol Cell. 1997;8(4):595–605. doi: 10.1091/mbc.8.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter I, Nixon GF. Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways. J Biol Chem. 2006;281(45):34705–15. doi: 10.1074/jbc.M605738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha SH, et al. Evidence for cyclooxygenase-1 association with caveolin-1 and -2 in cultured human embryonic kidney (HEK 293) cells. IUBMB Life. 2004;56(4):221–7. doi: 10.1080/15216540410001699312. [DOI] [PubMed] [Google Scholar]
- 34.Kwak JO, et al. Evidence for cyclooxygenase-2 association with caveolin-3 in primary cultured rat chondrocytes. J Korean Med Sci. 2006;21(1):100–6. doi: 10.3346/jkms.2006.21.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennig B, et al. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis. 1984;4(5):489–97. doi: 10.1161/01.atv.4.5.489. [DOI] [PubMed] [Google Scholar]
- 36.Toborek M, et al. Measurement of inflammatory properties of fatty acids in human endothelial cells. Methods Enzymol. 2002;352:198–219. doi: 10.1016/s0076-6879(02)52020-6. [DOI] [PubMed] [Google Scholar]
- 37.Mattson MP, et al. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- 38.Beg AA, et al. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13(6):3301–10. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosswig N, et al. Class A scavenger receptor-mediated adhesion and internalization require distinct cytoplasmic domains. J Biol Chem. 2003;278(36):34219–25. doi: 10.1074/jbc.M303465200. [DOI] [PubMed] [Google Scholar]
- 40.Repetto S, et al. Insulin and IGF-I phosphorylate eNOS in HUVECs by a caveolin-1 dependent mechanism. Biochem Biophys Res Commun. 2005;337(3):849–52. doi: 10.1016/j.bbrc.2005.09.125. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, et al. MAP kinases. Chem Rev. 2001;101(8):2449–76. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 42.D'Alessio A, et al. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol. 2005;166(4):1273–82. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng X, et al. Caveolin-1 associates with TRAF2 to form a complex that is recruited to tumor necrosis factor receptors. J Biol Chem. 2001;276(11):8341–9. doi: 10.1074/jbc.M007116200. [DOI] [PubMed] [Google Scholar]
- 44.Vila L. Cyclooxygenase and 5-lipoxygenase pathways in the vessel wall: role in atherosclerosis. Med Res Rev. 2004;24(4):399–424. doi: 10.1002/med.10065. [DOI] [PubMed] [Google Scholar]
- 45.Bishop-Bailey D, Mitchell JA, Warner TD. COX-2 in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26(5):956–8. doi: 10.1161/01.ATV.0000219672.68024.bc. [DOI] [PubMed] [Google Scholar]
- 46.Yang CM, et al. Interleukin-1beta-induced cyclooxygenase-2 expression is mediated through activation of p42/44 and p38 MAPKS, and NF-kappaB pathways in canine tracheal smooth muscle cells. Cell Signal. 2002;14(11):899–911. doi: 10.1016/s0898-6568(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 47.Toborek M, et al. Linoleic acid and TNF-alpha cross-amplify oxidative injury and dysfunction of endothelial cells. J Lipid Res. 1996;37(1):123–35. [PubMed] [Google Scholar]
- 48.Li YP, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. Faseb J. 2005;19(3):362–70. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark JE, Sarafraz N, Marber MS. Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacol Ther. 2007;116(2):192–206. doi: 10.1016/j.pharmthera.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 50.Gustin JA, et al. Tumor necrosis factor activates CRE-binding protein through a p38 MAPK/MSK1 signaling pathway in endothelial cells. Am J Physiol Cell Physiol. 2004;286(3):C547–55. doi: 10.1152/ajpcell.00332.2002. [DOI] [PubMed] [Google Scholar]
- 51.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 52.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 53.Hennig B, Watkins BA. Linoleic acid and linolenic acid: effect on permeability properties of cultured endothelial cell monolayers. Am J Clin Nutr. 1989;49(2):301–5. doi: 10.1093/ajcn/49.2.301. [DOI] [PubMed] [Google Scholar]
- 54.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis. 2000;59(Suppl 1):i6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim EJ, et al. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- 56.Yang B, Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292(2):H954–62. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 57.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137(3):548–53. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 58.Ring A, et al. Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim Biophys Acta. 2006;1761(4):416–23. doi: 10.1016/j.bbalip.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 60.Yang N, et al. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc Res. 2007 doi: 10.1016/j.cardiores.2007.05.028. [DOI] [PubMed] [Google Scholar]