ABSTRACT
Cryptococcosis is caused by the opportunistic pathogen Cryptococcus neoformans or by the primary pathogen Cryptococcus gattii. Epidemiological studies suggest that patients infected with C. gattii mainly present with pulmonary disease, while those infected with C. neoformans commonly manifest meningoencephalitis. We compared the pathogenesis of the two species using the C. neoformans H99 and C. gattii R265 strains in a murine inhalation model. C. neoformans grew faster in the brain and caused death by meningoencephalitis, while C. gattii grew faster in the lungs and caused death without producing fulminating meningoencephalitis. Despite the consistent failure to recover R265 cells from blood, a fraction of the R265 population was detected in the extrapulmonary organs, including the brain. Upon intravenous (i.v. ) inoculation of 104 cells via the tail vein, however, C. gattii produced severe meningoencephalitis, demonstrating that C. gattii cells can efficiently cross the blood-brain barrier. Interestingly, i.v. inoculation with five cells caused brain infection in only 10% of C. gattii-infected mice, compared to 60% of mice infected with C. neoformans. In mice that had been initially inoculated via the pulmonary route and subsequently challenged intravenously, a protective effect was observed only in mice infected with C. gattii. C. neoformans cells grew 10 to 100 times faster than C. gattii cells in blood or serum collected from naive mice. The paucity of meningoencephalitis upon inhalation of C. gattii, therefore, may be partly due to an unknown factor(s) in the host’s blood coupled with immune protection that reduces dissemination to the brain and fosters lung infection.
IMPORTANCE
While Cryptococcus neoformans is the most common cause of fatal meningoencephalitis, especially in HIV patients, Cryptococcus gattii causes disease mainly in non-HIV patients. Clinical studies revealed that most patients infected with C. gattii VGII strains have lung infections with minimal brain involvement. Despite extensive clinicopathological studies on cryptococcosis in animal models, only a few have included C. gattii. We compared the pathogenesis of the two species in mice using an inhalation model. Similar to infection in humans, even though C. gattii can cross the blood-brain barrier, it failed to cause fatal meningoencephalitis but caused fatal lung infection. We show that growth of C. gattii in mouse blood is significantly slower than that of C. neoformans and that a secondary protective phenomenon, though weak, manifests itself only in C. gattii infection. Our study provides a model for understanding the clinicopathological differences between these two closely genetically related pathogens.
Introduction
The members of the Cryptococcus neoformans/Cryptococcus gattii species complex comprise the most common fungal pathogens that cause fatal central nervous system infection in humans and also cause infections in a wide range of mammals (1, 2). C. neoformans has been categorized as an AIDS-defining opportunistic pathogen, while C. gattii is classified as a primary pathogen (3). Clinical perspectives on the diseases caused by the two species have been well documented (4). The two species have been categorized into eight molecular types: C. neoformans includes molecular types VNI and VNII in serotype A, molecular type VNIV in serotype D, and molecular type VNIII in the AD hybrid, and C. gattii includes molecular types VGI to VGIV in serotype B or C (5–8).
Among the 4 molecular types within C. neoformans, VNI is the most common cause of infection, occurring in approximately 80% of cases in HIV-infected patients and also in animal infections worldwide (9). Moreover, VNII, VNIII, and VNIV, though significantly less prevalent than VNI, have also been found to cause disease, mainly in immunocompromised patients, with the highest prevalence of VNIV being found in Europe (10, 11). Recently, the status of C. neoformans as an opportunistic pathogen was challenged by studies in Far East Asian countries reporting that C. neoformans, especially VNI, infects mostly non-HIV patients with or without underlying conditions (12, 13). Whether susceptibility to cryptococcosis is affected by the patients’ genetic background and whether there are unique strain-dependent virulence factors warrant investigation.
Although cryptococcosis due to C. gattii is endemic in Australia (14), the species did not receive much attention until the recent cryptococcosis outbreak emerged in Vancouver, Canada, in 1999 (15). Since then, C. gattii has attracted considerable attention as a public health issue, and the number of research articles on C. gattii has been increasing. C. gattii molecular type VGI is endemic in Australia, with the highest incidence being found in aboriginal populations of the Northern part of Australia, and was the first molecular type to be rigorously studied (14). Detailed studies on the pathogenesis of C. gattii VGI were performed using a rat model and found that in contrast to the cases of C. neoformans infection which resulted in spontaneous recovery (16), all rats succumbed to infection. These results suggested that C. gattii is a primary pathogen (17). Recently, VGII has drawn more attention than any other molecular type of C. gattii, since it was the cause of the Canadian outbreak (15). As a result, literature on the epidemiology and pathogenesis of C. gattii molecular type VGII has accumulated rapidly (4, 10, 11, 17–20). The C. gattii genome project initially selected WM276, the Australian environmental VGI strain, and R265, the Canadian clinical VGII strain (21), for sequencing. C. gattii VGIII and VGIV, two less frequently isolated molecular types, were also initially believed to be primary pathogens (8, 9). However, recent studies in Africa (22) and North America (23) revealed that in contrast to VGI and VGII, the prevalence of VGIII and VGIV was relatively high among isolates from HIV/AIDS patients with cryptococcosis.
While C. neoformans primarily causes central nervous system (CNS) infection and only 35% of C. neoformans-infected patients suffer from pulmonary infection (14, 24), approximately 70% of the cases caused by C. gattii include evidence of lung infection (14, 24). The small number of CNS infections caused by C. gattii compared to those caused by C. neoformans is even more striking when the disease caused by C. gattii VGII type is considered. Galanis and Macdougall reported that only 7.8% of cryptococcosis patients infected with VGII had CNS involvement (24). Recently, host responses to C. neoformans VNI (H99) and C. gattii VGII (R265) were compared using the mouse model (18). That report demonstrated that R265 suppressed host immune responses more effectively than H99 and suggested that this finding may partially explain why C. gattii infects immunocompetent patients more readily than C. neoformans (18). The fate of cells of the two species inoculated into mice, however, has not been compared, and the difference in their pathogenesis is poorly understood. Thus, we conducted a comparative study on pathogenesis of the two species using a mouse model.
RESULTS
Strains.
The genome-sequenced strains C. neoformans H99 (VNI) and C. gattii R265 (VGII) were selected as representative strains for the following reasons: (i) both strains were isolated from clinical cases; (ii) H99 belongs to molecular type VNI, representing the most common opportunistic pathogenic clade, while R265 belongs to VGII, representing a primary pathogen and the major strain of the Vancouver outbreak; (iii) both strains have been most widely used as reference strains of the two species in studies of genetics and pathogenesis; and (iv) the completed whole genome sequences of both strains are invaluable for subsequent genetic studies (21).
Fulminant meningoencephalitis occurs in mice infected with H99 but not in those infected with R265.
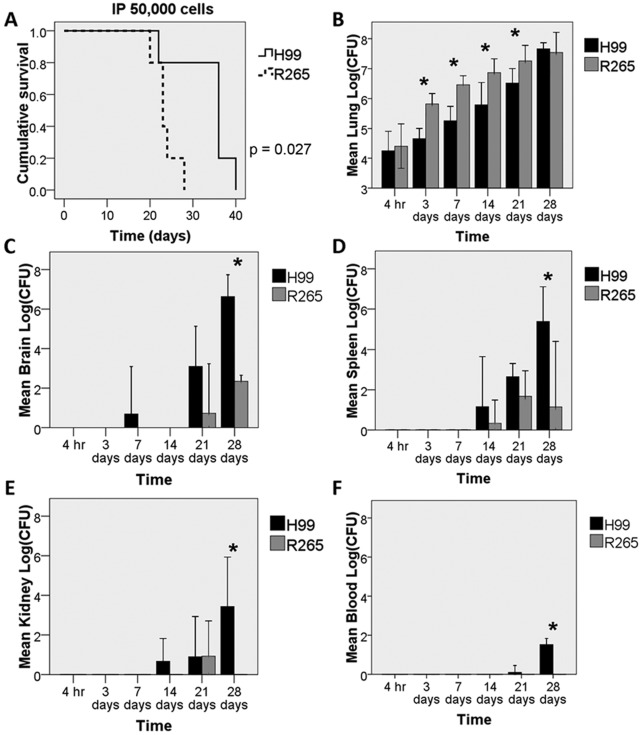
We first compared the differences in pathogenesis between H99 and R265 using an inhalation model by inoculating yeast cells via intrapharyngeal (i.p.) aspiration. A high inoculum (50,000 cells/mice) was used, since both species consistently killed C57BL/6 mice after infection with this inoculum (10, 20, 25, 26). Mice infected with either species began to die 3 weeks after inoculation. However, the mice infected with R265 died faster than those infected with H99 (Fig. 1A) (P = 0.027). Both species produced severe pulmonary fungal burdens during the infection period (Fig. 1B). However, the pulmonary fungal burden was consistently higher in mice infected with R265 than in those infected with H99 from day 3 until just before the time of death (Fig. 1B). By 2 weeks after inoculation, lungs of the mice infected with R265 were visibly larger than those inoculated with H99 (see Fig. S1 in the supplemental material). In the brain, H99 cells were detected as early as the first week after inoculation and reached 106 to 108 CFU/brain by the time of death (Fig. 1C) with severe meningoencephalitis (see Fig. S2 in the supplemental material). In contrast, R265 cells were not detected in the brain until the third week after inoculation, and only 0 to 102 CFU were recovered from tissue without any sign of meningoencephalitis at the time of death (Fig. 1C; also, see Fig. S2 in the supplemental material). Similar to the results for fungal burdens obtained from the brains, higher numbers of H99 CFU than R265 CFU were recovered from spleens and kidneys (Fig. 1D and E). Surprisingly, although yeast cells could be recovered from every extrapulmonary organ examined, R265 was never recovered from blood, even at the time of death. Blood cultured from mice infected with H99, on the other hand, yielded CFU occasionally at 3 weeks after inoculation and consistently near the time of death (Fig. 1F). Since no R265 cells could be recovered from blood of the infected mice and yet R265 cells disseminated to the extrapulmonary organs, we speculate that fungemia due to R265 occurred transiently and/or at an undetectable level. These data suggest that both R265 and H99 can exit from the lung and disseminate to other organs but R265 fails to establish infection in the extrapulmonary organs as readily as the H99 strain.
FIG 1 .
Heavy brain fungal burden is found only in mice infected with H99, not in those infected with R265. C57BL/6 mice were inoculated by intrapharyngeal (i.p.) aspiration of 50,000 yeast cells/mouse. At least 3 mice were sacrificed at each time point for fungal load determination. (A) Survival curves were obtained from a study of 10 mice per group, which was independent of the time course study. (B to F) CFU in each organ at the indicated time points. Error bars show 2 standard deviations (SD). *, P < 0.05.
Host immune response in the lungs of mice infected with R265 is minimal compared to that in mice infected with H99.
A previous study showed that the host immune response of the lung to cryptococcal infection included increased numbers of activated macrophages, neutrophils, and lymphocytes (18). Similar results were found in this study. Leukocyte infiltrations in the lungs of mice infected with R265 were mostly minimal relative to those in mice infected with H99 in both early (see Fig. S3A and B in the supplemental material) and late (see Fig. S3C and D in the supplemental material) time points. Moreover, despite being smaller, R265 produced as many giant cells as H99, which was consistent with previous reports (27, 28).
Results with BALB/c mice are congruent with findings in C57BL/6 mice.
To confirm that the difference in pathogenesis between the two species is not mouse strain specific, we repeated the experiment with BALB/c mice, which were used in previous studies for both C. neoformans and C. gattii infections (20, 26). In the BALB/c strain, no difference was found in the virulence between the two strains (see Fig. S4A in the supplemental material). As in C57BL/6 mice, both strains caused a progressive increase in the fungal burden in the lungs after i.p. inoculation, although the numbers were higher from day 3 to day 14 with R265 than with H99 (see Fig. S4B in the supplemental material). Only 0 to 10 CFU were recovered from the brains of mice infected with R265 without any visible lesions at the time of death (see Fig. S3C in the supplemental material), while fulminant meningoencephalitis with 106 to 108 CFU was found in the brains of mice infected with H99 at the time of death (see Fig. S3C in the supplemental material; also data not shown).
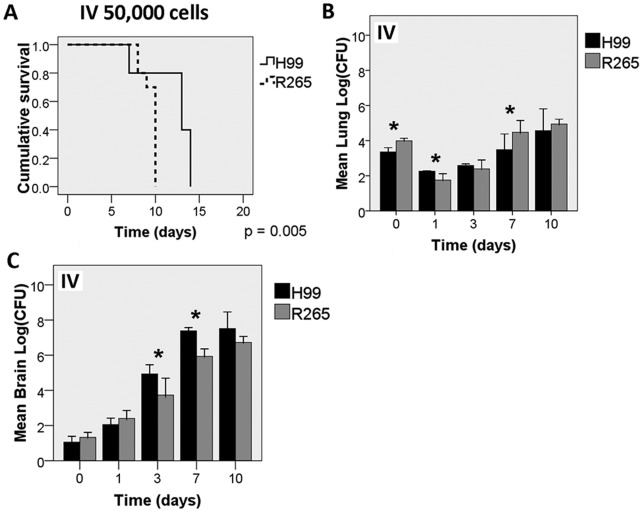
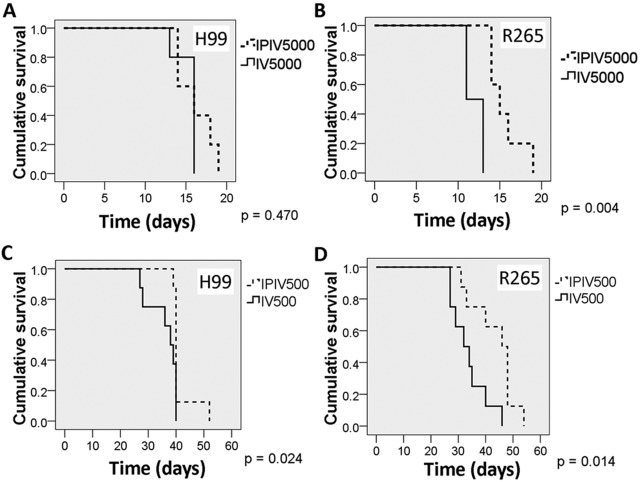
Since the fungal burden in the extrapulmonary organs was much lower for R265-infected mice, we investigated whether such a difference would be present in mice infected by a different route. BALB/c mice were injected intravenously (i.v.) with 50,000 cells of H99 or R265 via the tail vein. The mice infected with R265 died faster than those infected with H99 (Fig. 2A) (P = 0.005). Both strains established infection equally well in the lungs (Fig. 2B). All mice infected i.v. with H99 showed signs of severe brain infection with a high load of cryptococcal cells (Fig. 2C). Cystic lesions were found throughout brain sections. In contrast to mice inoculated via the i.p. route, mice inoculated i.v. with R265 did manifest signs of severe brain infection with a high load of cryptococcal cells, although the rate of increase of the fungal burdens was slightly lower than that in H99-infected mice (Fig. 2C). Interestingly, brain lesions showing prominent cryptococcomas with hemorrhage were common with R265 but rare with H99 (see Fig. S5 in the supplemental material). These data clearly indicate that R265 can invade the brain and establish infection if large numbers of cells are inoculated into the bloodstream. Therefore, the reason for the lower fungal burden in the extrapulmonary organs in mice infected with R265 i.p. remains to be determined.
FIG 2 .
R265 causes severe brain infection in an intravenous (i.v.) model. BALB/c mice were inoculated with 50,000 cells/mouse of H99 or R265 via the i.v. route. At least 3 mice each were sacrificed at each time point for the determination of the fungal tissue load. Survival curves (A) were generated from the results obtained with 10 mice per group, which are independent of the time course study (B and C). Error bars show 2 SD. *, P < 0.05.
Intravenous inoculation of mice with low inocula causes infection more consistently with H99 than R265.
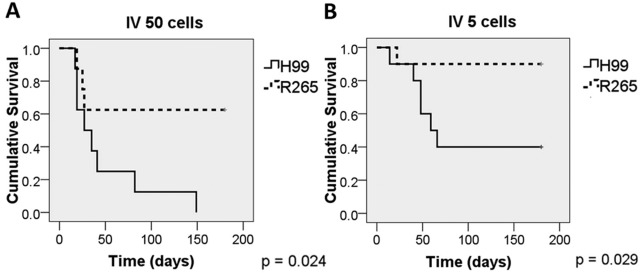
In our i.p. model, numbers of R265 CFU in the extrapulmonary organs were significantly lower than those for H99 and were undetectable in circulating blood (Fig. 1). We hypothesize that the lower fungal burdens of R265 in extrapulmonary organs was due to the very low number of circulating cells in the blood. Since R265 fungemia was below the level of detection (Fig. 1F), we assumed that the number of cryptococcal cells in bloodstream was below 3 per ml. To mimic fungemia in i.p. infection, we inoculated very low numbers of cells (5 and 50 cells) of either H99 or R265 into BALB/c mice intravenously. In contrast to the result with the high inoculum (Fig. 2), H99 was far more virulent than R265 with 5 or 50 cells (Fig. 3). With the inoculum of 5 cells, as many as 9 of 10 mice infected with R265 cleared the infection, while only 4 of 10 mice infected with H99 cleared the infection (Table 1). Similarly, with an inoculum of 50 cells, H99 caused infection more consistently than R265 (Table 1). These results suggest that when very low numbers of the cells are introduced into the bloodstream, R265 cells are less able to establish infection in host tissues (brain, lung, and spleen).
FIG 3 .
H99 is more virulent than R265 when mice are inoculated with very low numbers of cells i.v. Eight to ten BALB/c mice were inoculated i.v. with approximately 5 or 50 cells of H99 or R265, and the survival of mice was monitored.
TABLE 1 .
Numbers of mice that succumbed to or cleared infection after i.v. inoculation with a very small inoculum
| Inoculum size (no. of cells) | Strain | No. (%) of mice that: |
|
|---|---|---|---|
| Succumbed to infection | Cleared infection and surviveda | ||
| 50 | H99 | 8 (100) | 0 (0) |
| R265 | 3 (38) | 5 (62) | |
| 5 | H99 | 6 (60) | 4 (40) |
| R265 | 1 (10) | 9 (90) | |
“Cleared” is defined as no CFU detected in the brain, lungs, or spleen.
Growth of R265 is inferior to that of H99 in whole blood or serum of mice.
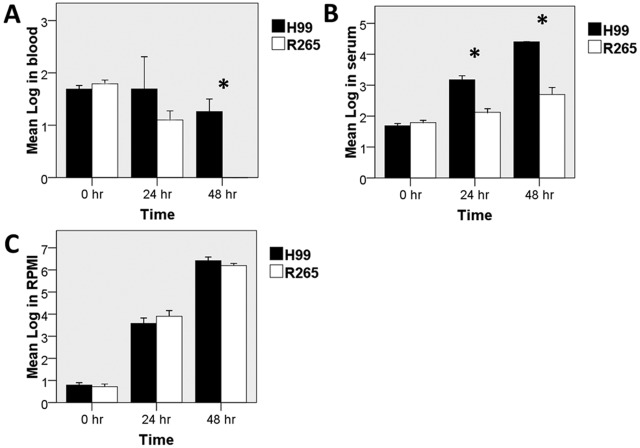
The low recovery of R265 from blood and reduced ability to cause infection in experiments using very low i.v. inocula suggested that R265 might not survive or grow as well as H99 in the blood. Thus, we compared the growth rates of H99 and R265 in whole blood as well as serum collected from naive mice in vitro. To mimic mammalian body conditions, both strains were incubated in blood, serum, or RPMI at 37°C with 5% CO2. Interestingly, H99 grew significantly better than R265 in both whole blood and serum (Fig. 4A and B), while both strains grew equally well in RPMI (Fig. 4C). In order to determine if the disparity in growth rates between H99 and R265 in mouse serum was related to nutrient availability, cells were incubated in phosphate-buffered saline (PBS) containing 10% (vol/vol) RPMI or in serum supplemented with 10% (vol/vol) RPMI. The numbers of CFU of the two strains were similar in PBS containing 10% RPMI after 48 h of incubation, but the growth difference between H99 and R265 persisted in the serum supplemented with 10% RPMI (see Fig. S6 in the supplemental material). We also used heated versus nonheated serum, and the growth difference between two strains remained in those two conditions (data not shown). These results indicate that the difference is not due to either nutrient limitation or complement differentially suppressing the growth of C. gattii.
FIG 4 .
Lower growth of R265 than H99 in murine blood and serum. Blood or serum was collected from naive C57BL/6 mice. Yeast cells were diluted and incubated in 100 µl of whole blood, serum, or RPMI with 5% CO2 at 37°C for the indicated period, and numbers of CFU were recorded. Error bars show 1 SD. *, P < 0.05.
Intrapulmonary infection with R265 slightly enhances tolerance of the mice to subsequent infection via i.v. inoculation.
We hypothesized that the establishment of infection first in the lungs (by i.p. inoculations) might activate the host immune system and affect the ability of yeast cells to disseminate to extrapulmonary organs in R265-infected mice. As a result, significantly lower fungal burden in extrapulmonary organs might be found in these mice than in H99-infected mice. Thus, we first inoculated mice via i.p. with 50 yeast cells of H99 or R265 and challenged them 2 weeks later with 500 or 5,000 cells of the same strains i.v. (dual inoculation). The survival of the mice was monitored. The 2-week interval before the second challenge was chosen because it reflects the time when yeast cells start to appear in the extrapulmonary organs (Fig. 1; also, see Fig. S4 in the supplemental material). Compared to the control mice (i.e., mice that were not preinfected), the dual inoculation of either 500 or 5,000 cells prolonged the survival of R265 preinfected mice for several days but not the survival of H99 preinfected mice (Fig. 5; Table 2). In a control group of mice inoculated i.p. with approximately 50 CFU of H99 or R265 with no subsequent i.v. challenge, all survived throughout the experiment (data not shown). These results suggest that preintrapulmonary infection with R265 may afford the mice better immune protection than infection with H99 during subsequent exposure to cryptococci introduced via the intravenous route.
FIG 5 .
Preintrapulmonary infection slightly prolongs the survival of mice subsequently infected with R265 via i.v. inoculation. Five to eight BALB/c mice per group were used. Mice were first inoculated i.p. with approximately 50 cells of H99 or R265. After 2 weeks, the preinfected and naive mice were injected i.v. with approximately 500 or 5,000 cells of H99 or R265. The survival of the mice was recorded.
TABLE 2 .
Comparison of median survival times of mice infected by i.v. inoculation and dually infected mice
| Inoculum size (CFU) | Infection route | Median survival (days) |
|
|---|---|---|---|
| H99 | R265 | ||
| 5,000 | i.v. | 16 | 11 |
| Dual | 16 | 14 | |
| 500 | i.v. | 38 | 32 |
| Dual | 40 | 46 | |
DISCUSSION
Relative to C. neoformans, C. gattii more frequently causes lung infections, and C. gattii VGII strains especially are less frequently reported to be neurotropic in the human host (4, 14, 24, 29). This clinical observation was confirmed by our animal experiments. Mice infected via the pulmonary route with H99 appeared to have been killed by brain infection, while those infected with R265 were killed by lung infection, in both BALB/c and C57BL/6 strains. Interestingly, while inferior to H99 in extrapulmonary dissemination, R265 was superior to H99 in intrapulmonary growth. This may be explained by stronger pulmonary immunosuppression with R265, as fewer inflammatory cells were present in lungs infected with R265 than in lungs infected with H99. Cheng et al. (18) reported similar findings with C. gattii strains, including the R265 strain, which was isolated from Vancouver outbreak cases. All the C. gattii strains induced a less protective inflammatory response in C57BL/6 mice by suppressing neutrophil migration to the infection sites. Cheng et al. also found that C. gattii strains failed to elicit the production of protective cytokines, such as tumor necrosis factor, in comparison with H99 (18). However, the difference between C. gattii and C. neoformans in terms of their fate during infection has not been elucidated. We expanded on the pathological differences between the two species by monitoring the fate of the yeast cells inoculated via two different routes. Our results support the previous report (18), but details of the immunosuppressive mechanisms that C. gattii employs have yet to be deciphered.
The adaptive immune response, both antibody mediated and cell meditated, has been proven to be essential against cryptococcal infection (30). C. gattii cannot cause fulminant meningoencephalitis upon pulmonary inoculation, although the yeast cells must have been released into the bloodstream, since they can cross the blood-brain barrier (BBB). We speculate, therefore, that the host may manifest stronger protective immunity against R265. This speculation is supported by the fact that mice infected intravenously with R265 survived longer than those infected with H99 when they had been preinfected via the pulmonary route 2 weeks prior to the intravenous challenge. On the other hand, when mice were preinfected with R265 via the pulmonary route and challenged by intravenous inoculation after 5 days, their survival was not prolonged (data not shown). These findings suggest that an adaptive systemic immune response likely affects the dissemination to the brain and delays the establishment of severe meningoencephalitis in mice infected with R265 but not those infected with H99.
Since the protective effect of dual infections was not robust enough to prolong survival in R265 infected mice, additional mechanisms may prevent R265 from causing fulminating brain infection. In fact, even with a single high-dose i.v. inoculum, the fungal load of R265 in the brains was lower than that of H99 (Fig. 2). Furthermore, while most mice infected with a very low i.v. inoculum of R265 cleared the infection, the majority of mice infected with the same number of H99 cells did not (Table 1). In light of the consistent failure to recover R265 from the blood of mice inoculated intrapharyngeally (i.p.) and the poor yield of R265 in blood or serum cultures in vitro, it was not surprising to find limited numbers of CFU in extrapulmonary organs. Although the detailed immunological mechanism has yet to be investigated, our results suggest differences in the host innate immune responses to the two species. Therefore, comparative studies on the detailed host immunological response to C. neoformans versus C. gattii are warranted.
The mechanism by which C. neoformans cells cross the blood-brain barrier (BBB) has been studied extensively, and both direct transcytosis (31) and a Trojan horse mechanism (32) have been proposed. A recent study using intravital microscopy reported that crossing the BBB by C. neoformans is urease dependent, requires viability, and involves cellular deformation, which suggest direct invasion (33). To date, however, neither mechanism has been confirmed directly within the host. It is possible that C. neoformans may use both mechanisms to cross the BBB (34). Whether and how C. gattii crosses the BBB has never been studied. When mice were inoculated via the i.p. route, C. gattii disseminated to the brain far less effectively than C. neoformans and failed to cause meningoencephalitis despite its higher growth rate in the lungs. At the time of death, only 0 to 200 and 0 to 20 CFU were found in the brains of R265-infected C57BL/6 and BALB/c mice, respectively, indicating that death was not caused by a brain disease. This was also true even with i.v. inoculation when the inoculum size was 50 cells or less. Since growth of C. gattii in blood or serum in vitro was significantly slower than that of C. neoformans, there may be a factor(s) in mouse blood or serum that inhibits C. gattii cells from multiplying and eventually reaching the brain. However, R265 caused severe meningoencephalitis when the i.v. inoculum was large (50,000 cells/mouse). Even with the large inoculum, the brain fungal load of R265 trailed that of H99. These data suggest that R265 can cross the BBB and invade the brain but less efficiently than H99. The pattern of CNS infection is also different between the two species, since prominent cryptococcomas with hemorrhage were commonly observed in mice infected intravenously with R265 but were rarely seen in those infected with H99 (see Fig. S5 in the supplemental material; also data not shown).
In conclusion, our study validates previous reports on clinical manifestations of the two agents of cryptococcosis (4): mice infected with C. gattii R265 strain via the pulmonary route died due to overwhelming intrapulmonary growth, while those infected with H99 died due to fulminating brain disease. Despite the differences in their clinicopathology, both H99 and R265 crossed the blood-brain barrier effectively and established fatal brain infection upon intravenous inoculation. Although the growth rate of R265 in the blood was lower than that of H99 in vitro, this does not explain why mice infected with R265 via the pulmonary route failed to exhibit fatal meningoencephalitis. For instance, mice infected via inhalation with 50 R265 cells survived for more than 90 days without noticeable brain lesions, while an i.v. inoculum of five R265 cells established fatal brain infection in 10% of mice in less than 30 days. It is possible that some immunological protection resulting during lung infection provided an additional mechanism(s) for reducing R265 dissemination to the extrapulmonary organs. Thus, it can be assumed that both innate and adaptive immune responses are responsible for such containment of C. gattii. However, it is paradoxical, since R265 induces a relatively poor inflammatory response compared to H99 and yet can produce more adaptive immunity. The observation that growth of C. gattii in the blood or serum is clearly slower than that of C. neoformans raises the question of whether there are factors in mouse blood that actively suppress R265 but not H99 and might be further induced during infection. This would offer some protection against blood exposure to R265 but not H99. It is important to find anti-C. gattii compounds in serum, since the agent in question does not appear to be complement. We believe our results have laid the foundation for further in-depth studies of the host immune response to the two species.
MATERIALS AND METHODS
Strains and media.
The strains H99 and R265 were chosen as representative strains of C. neoformans and C. gattii, respectively. Strains were maintained on YPD agar (2% glucose, 1% yeast extract, 2% peptone and 2% Bacto agar) throughout the experiment.
Animal study.
The animal experiments were carried out with the approval and oversight of the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Six- to eight-week-old C57BL/6 and BALB/c mice were purchased from DCT (National Cancer Institute, NCI-Frederick, MD). Mice were inoculated with the designated number of yeast cells via intrapharyngeal (i.p.) aspiration (inhalation method) (35, 36) or tail vein (i.v.) injection. Briefly, yeast cells were grown in YPD broth overnight and diluted in 1× phosphate-buffered saline (PBS) to the desired concentrations. In the case of i.p. inoculation, 20 µl of the yeast suspension was administered intrapharyngeally after mice were anesthetized with isoflurane. In the case of i.v. injection, 200 µl of the yeast suspension was injected into the tail vein. Mice were provided with food and water ad libitum, and survival was monitored up to 6 months.
Organ fungal burdens.
Mice were euthanized by inhalation of CO2 or an overdose of pentobarbitone (200 mg/kg intraperitoneally). The lungs, spleens, kidneys, and brain were dissected for the analysis of CFU. Briefly, each organ was homogenized in PBS, diluted, and plated on YPD agar for colony counts. While mice were under the effect of overdose pentobarbitone, 300 to 800 µl of blood was withdrawn from heart using a needle and syringe containing 0.01% EDTA and then plated directly on YPD agar.
Histology.
Each organ was fixed in 4% neutral buffered formalin, processed, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) or Grocott’s methenamine silver (GMS) stain. The presence or absence of yeast cells, their morphology and location (intracellular or extracellular), and the host inflammatory response and the distribution of immune cells within the tissues were recorded.
Growth in whole blood or serum.
Whole blood was collected from naive mice via heart puncture as mentioned above. EDTA (0.01%) or heparin (30 IU/ml) was used as an anticoagulant for blood collection. Serum was collected by drawing off the supernatant after centrifugation of clotted whole blood at 1,300 × g for 15 min, and 10 to 100 yeast cells of each strain were inoculated into 100 µl of whole blood, serum, or RPMI in round-bottom 96-well plates and incubated for 24 and 48 h at 37°C with 5% CO2. CFU were counted by plating the incubation mixtures on YPD agar.
Statistical analysis.
The t test and Kaplan-Meier survival test were performed using an SPSS statistics 17.0 program.
SUPPLEMENTAL MATERIAL
Lungs of mice infected with H99 and R265. The lungs of mice (C57BL/6) inoculated with 50,000 yeast cells were removed on day 7 and day 14 and fixed in formalin. By day 14, the lungs infected with R265 (B) were significantly larger than those from H99 infected mice (A). Bar, 5 mm. Download Figure S1, TIF file, 5.4 MB.
Only H99 produces severe meningoencephalitis in the murine inhalation model. C57BL/6 mice were infected i.p. with H99 or R265 cells (50,000 cells/mouse). Brains were removed at the time of death and fixed in 4% neutral buffered formalin before processing for histopathological sections. Tissue sections were stained with hematoxylin and eosin (H&E). (a) H99; (b) R265. Download Figure S2, TIF file, 1.5 MB.
Fewer inflammatory cells are found in the lungs of mice infected with R265. C57BL/6 mice were infected i.p. with 50,000 yeast cells. At the time of death, lungs were fixed in 4% neutral buffered formalin. Tissue sections were stained with hematoxylin and eosin (H&E). Download Figure S3, TIF file, 4.2 MB.
The pattern of infection caused by H99 and R265 is similar in BALB/c and C57BL/6 mice. BALB/c mice were each inoculated with 50,000 cells of H99 or R265 via the i.p. route. At least 3 mice each were sacrificed at each time point for the determination of the fungal tissue load. Survival curves (A) were generated from the results obtained with 10 mice per group, which are independent of the time course study (B and C). Error bars show 2 SD. *, P < 0.05. Download Figure S4, TIF file, 0.2 MB.
Hemorrhagic cryptococcoma in mice infected with R265. Mice were inoculated i.v. with 5,000 cells of either H99 or R265. Mice were euthanized by carbon dioxide when signs of severe hydrocephalus were observed. Brains were removed and photographed. Download Figure S5, TIF file, 0.4 MB.
Growth of H99 is better than that of R265 in serum. Serum was collected by drawing supernatant after centrifugation of clotted whole blood of BALB/c mice at 1,300 × g for 15 min, and 100 to 200 yeast cells of H99 and R265 were inoculated in PBS, RPMI, or serum supplemented with 10% (vol/vol) RPMI in a round-bottom 96-well plate and incubated for 48 h at 37°C with 5% CO2. Numbers of CFU were determined by plating the incubation mixtures on YPD agar. The labels on the x axis are as follows: 1, PBS at 0 h; 2, PBS at 48 h; 3, 100% RPMI at 48 h; 4, PBS plus 10% (vol/vol) RPMI at 48 h; 5, serum at 48 h; 6, serum plus 10% (vol/vol) RPMI at 48 h. Download Figure S6, TIF file, 5.4 MB.
ACKNOWLEDGMENTS
This study was supported by funds from the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Daniel Barber and Katrin Mayer Barber and Ashok Varma for their suggestions and critical readings of the manuscript.
Footnotes
Citation Ngamskulrungroj P, Chang Y, Sionov E, and Kwon-Chung KJ. 2012. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3(3):e00103-12. doi:10.1128/mBio.00103-12.
REFERENCES
- 1. Kwon-Chung KJ. 1976. A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 68:943–946 [PubMed] [Google Scholar]
- 2. Sorrell TC. 2001. Cryptococcus neoformans variety gattii. Med. Mycol. 39:155–168 [PubMed] [Google Scholar]
- 3. Kwon-Chung KJ, Boekhout T, Fell JW, Diaz M. 2002. (1557) Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. basillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon 51:804–806 [Google Scholar]
- 4. Sorrell TC, Chen SCA, Phillips P, Marr KA. 2010. Clinical perspectives on Cryptococcus neoformans and Cryptococcus gattii: implications for diagnosis and management, p 595–606 In Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC [Google Scholar]
- 5. Boekhout T, et al. 2001. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 147:891–907 [DOI] [PubMed] [Google Scholar]
- 6. Kwon-Chung KJ, Varma A. 2006. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 6:574–587 [DOI] [PubMed] [Google Scholar]
- 7. Meyer W, et al. 2009. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 47:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyer W, et al. 2003. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect Dis. 9:189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer W, et al. 2011. Molecular typing of the Cryptococcus neoformans/C. gattii species complex, p 620 In Heitman J, Kozel TR, Kwon-Chung J, Perfect JR, Casadevall A, Cryptococcus: from human pathogen to model yeast. ASM Press, Washington, DC [Google Scholar]
- 10. Fraser JA, et al. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360–1364 [DOI] [PubMed] [Google Scholar]
- 11. Narasipura SD, Chaturvedi V, Chaturvedi S. 2005. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol. Microbiol. 55:1782–1800 [DOI] [PubMed] [Google Scholar]
- 12. Chen J, et al. 2008. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg. Infect. Dis. 14:755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi YH, et al. 2010. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the republic of Korea. FEMS Yeast Res. 10:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen S, et al. 2000. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Clin. Infect. Dis. 31:499–508 [DOI] [PubMed] [Google Scholar]
- 15. Kidd SE, et al. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. U. S. A. 101:17258–17263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldman D, Lee SC, Casadevall A. 1994. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect. Immun. 62:4755–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krockenberger MB, et al. 2010. Pathogenesis of pulmonary Cryptococcus gattii infection: a rat model. Mycopathologia 170:315–330 [DOI] [PubMed] [Google Scholar]
- 18. Cheng PY, Sham A, Kronstad JW. 2009. Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect. Immun. 77:4284–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kidd SE, Guo H, Bartlett KH, Xu J, Kronstad JW. 2005. Comparative gene genealogies indicate that two clonal lineages of Cryptococcus gattii in British Columbia resemble strains from other geographical areas. Eukaryot. Cell 4:1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ngamskulrungroj P, Serena C, Gilgado F, Malik R, Meyer W. 2011. Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver island outbreak genotype. Clin. Microbiol. Infect. 17:251–258 [DOI] [PubMed] [Google Scholar]
- 21. D’Souza CA, et al. 2011. Genome variation in Cryptococcus gattii, an emerging pathogen of immunocompetent hosts. mBio 2(1):e00342-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Litvintseva AP, Thakur R, Reller LB, Mitchell TG. 2005. Prevalence of clinical isolates of Cryptococcus gattii serotype C among patients with AIDS in sub-Saharan Africa. J. Infect. Dis. 192:888–892 [DOI] [PubMed] [Google Scholar]
- 23. Byrnes EJ, III, et al. 2011. A diverse population of Cryptococcus gattii molecular type VGIII in southern Californian HIV/AIDS patients. PLoS Pathog. 7:e1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galanis E, Macdougall L. 2010. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis. 16:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma H, et al. 2009. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc. Natl. Acad. Sci. U. S. A. 106:12980–12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ngamskulrungroj P, et al. 2009. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect. Immun. 77:4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okagaki LH, et al. 2010. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 6:e1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaragoza O, et al. 2010. Fungal cell gigantism during mammalian infection. PLoS Pathog. 6:e1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwon-Chung KJ, Sorrell TC, Dromer F, Fung E, Levitz SM. 2000. Cryptococcosis: clinical and biological aspects. Med. Mycol. 38(Suppl. 1):205–213 [PubMed] [Google Scholar]
- 30. Voelz K, May RC. 2010. Cryptococcal interactions with the host immune system. Eukaryot. Cell 9:835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang YC, et al. 2004. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 72:4985–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charlier C, et al. 2009. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 77:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi M, et al. 2010. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 120:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Casadevall A. 2010. Cryptococci at the brain gate: break and enter or use a Trojan horse? J. Clin. Invest. 120:1389–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao GV, et al. 2003. Efficacy of a technique for exposing the mouse lung to particles aspirated from the pharynx. J. Toxicol. Environ. Health A 66:1441–1452 [DOI] [PubMed] [Google Scholar]
- 36. Sugui JA, et al. 2010. Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J. Clin. Microbiol. 48:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lungs of mice infected with H99 and R265. The lungs of mice (C57BL/6) inoculated with 50,000 yeast cells were removed on day 7 and day 14 and fixed in formalin. By day 14, the lungs infected with R265 (B) were significantly larger than those from H99 infected mice (A). Bar, 5 mm. Download Figure S1, TIF file, 5.4 MB.
Only H99 produces severe meningoencephalitis in the murine inhalation model. C57BL/6 mice were infected i.p. with H99 or R265 cells (50,000 cells/mouse). Brains were removed at the time of death and fixed in 4% neutral buffered formalin before processing for histopathological sections. Tissue sections were stained with hematoxylin and eosin (H&E). (a) H99; (b) R265. Download Figure S2, TIF file, 1.5 MB.
Fewer inflammatory cells are found in the lungs of mice infected with R265. C57BL/6 mice were infected i.p. with 50,000 yeast cells. At the time of death, lungs were fixed in 4% neutral buffered formalin. Tissue sections were stained with hematoxylin and eosin (H&E). Download Figure S3, TIF file, 4.2 MB.
The pattern of infection caused by H99 and R265 is similar in BALB/c and C57BL/6 mice. BALB/c mice were each inoculated with 50,000 cells of H99 or R265 via the i.p. route. At least 3 mice each were sacrificed at each time point for the determination of the fungal tissue load. Survival curves (A) were generated from the results obtained with 10 mice per group, which are independent of the time course study (B and C). Error bars show 2 SD. *, P < 0.05. Download Figure S4, TIF file, 0.2 MB.
Hemorrhagic cryptococcoma in mice infected with R265. Mice were inoculated i.v. with 5,000 cells of either H99 or R265. Mice were euthanized by carbon dioxide when signs of severe hydrocephalus were observed. Brains were removed and photographed. Download Figure S5, TIF file, 0.4 MB.
Growth of H99 is better than that of R265 in serum. Serum was collected by drawing supernatant after centrifugation of clotted whole blood of BALB/c mice at 1,300 × g for 15 min, and 100 to 200 yeast cells of H99 and R265 were inoculated in PBS, RPMI, or serum supplemented with 10% (vol/vol) RPMI in a round-bottom 96-well plate and incubated for 48 h at 37°C with 5% CO2. Numbers of CFU were determined by plating the incubation mixtures on YPD agar. The labels on the x axis are as follows: 1, PBS at 0 h; 2, PBS at 48 h; 3, 100% RPMI at 48 h; 4, PBS plus 10% (vol/vol) RPMI at 48 h; 5, serum at 48 h; 6, serum plus 10% (vol/vol) RPMI at 48 h. Download Figure S6, TIF file, 5.4 MB.