Mechanical stimulation induces zyxin-dependent actin cytoskeletal reinforcement. Stretch induces MAPK activation, zyxin phosphorylation, and recruitment to actin stress fibers, independent of p130Cas. Zyxin's C-terminal LIM domains are required for stretch-induced targeting to stress fibers, and zyxin's N-terminus is necessary for actin remodeling.
Abstract
Reinforcement of actin stress fibers in response to mechanical stimulation depends on a posttranslational mechanism that requires the LIM protein zyxin. The C-terminal LIM region of zyxin directs the force-sensitive accumulation of zyxin on actin stress fibers. The N-terminal region of zyxin promotes actin reinforcement even when Rho kinase is inhibited. The mechanosensitive integrin effector p130Cas binds zyxin but is not required for mitogen-activated protein kinase–dependent zyxin phosphorylation or stress fiber remodeling in cells exposed to uniaxial cyclic stretch. α-Actinin and Ena/VASP proteins bind to the stress fiber reinforcement domain of zyxin. Mutation of their docking sites reveals that zyxin is required for recruitment of both groups of proteins to regions of stress fiber remodeling. Zyxin-null cells reconstituted with zyxin variants that lack either α-actinin or Ena/VASP-binding capacity display compromised response to mechanical stimulation. Our findings define a bipartite mechanism for stretch-induced actin remodeling that involves mechanosensitive targeting of zyxin to actin stress fibers and localized recruitment of actin regulatory machinery.
INTRODUCTION
The ability of cells to respond to mechanical stimulation is key for normal physiology and function. Cells of the cardiovascular, respiratory, urogenital, and locomotory systems are exposed to mechanical stress under both normal and pathophysiological conditions (Jaalouk and Lammerding, 2009). Given the clear impact of mechanical signals on cell physiology, much research has focused on understanding how physical force is sensed by cells and how physical signals are converted into chemical information that can directly influence cell behavior.
Recent efforts have been made to simulate physiological conditions of mechanical stimulation in the laboratory in order to learn more about how cells sense and respond to physical cues (Eyckmans et al., 2011). For example, to model the mechanical stress experienced by vascular endothelial cells in vivo, flow chambers that deliver calibrated levels of shear stress have been devised (Davies, 1995). To model the cyclic stretch that occurs with inspiration or rhythmic cardiac beating, chambers have been developed in which cells are grown on elastic membranes that can be deformed according to precise experimental parameters (Banes et al., 1985; Yoshigi et al., 2003). By employing these devices for controlled mechanical stimulation of cells, it has been possible to demonstrate activation of signaling cascades (Orr et al., 2006; Cohen et al., 2010), actin remodeling and reinforcement (Wille et al., 2004; Yoshigi et al., 2005), and even changes in gene expression (Kessler et al., 2001; Wojtowicz et al., 2010) in response to physical stress.
The cell membrane defines the interface between the cell interior and the extracellular environment. It plays a central role in sensing and transducing mechanical signals. Focal adhesions (FAs)—regions of the cell surface that are specialized for cell–substratum attachment—provide sites for transmission of mechanical signals to the cell interior. Integrins—transmembrane receptors for extracellular matrix—are concentrated at FAs and play central roles influencing cell behavior downstream of physical cues (Geiger et al., 2009; Puklin-Faucher and Sheetz, 2009; Wang et al., 2009; Parsons et al., 2010; Schwartz, 2010). Actin stress fibers (SFs) are cytoskeletal structures that terminate at FAs, where they are linked to integrins bound to extracellular matrix. Prominent in cultured cells, SFs also assemble in vivo in locations where cells experience mechanical stress (Wong et al., 1983; Byers et al., 1984). Mechanical force can also influence the conductivity of stretch-gated ion channels to directly influence signaling cascades via alterations in membrane potential or intracellular calcium levels (Sukharev and Corey, 2004).
Stretch-induced signaling is also promoted in demembranated cells, illustrating the potential for non–membrane-bound effectors to contribute to the force response (Sawada and Sheetz, 2002). For example, tyrosine phosphorylation of the integrin effector p130Cas is stimulated upon direct mechanical extension of isolated cytoskeletons (Tamada et al., 2004). Tyrosine phosphorylation of p130Cas creates a docking site for the SH2-adaptor CRK, which in turn recruits a Rap1-guanine nucleotide exchange factor, C3G, to activate the Rap1 GTPase and mitogen-activated protein kinase (MAPK) signaling (Sawada et al., 2001, 2006; Chodniewicz and Klemke, 2004; Defilippi et al., 2006). Similarly, stretching of the integrin-binding protein talin activates its capacity to bind vinculin (del Rio et al., 2009). Examples such as these provide evidence for one mechanism by which application of physical force might be converted into novel chemical information by inducing exposure of a phosphorylation site or new protein interaction site.
Several recent studies have highlighted the mechanosensitive features of the cytoskeletal protein zyxin. Zyxin is a FA constituent that mobilizes to actin SFs in cells exposed to mechanical stress (Yoshigi et al., 2005). A retrograde flux of zyxin along FA-anchored actin filaments is promoted by high substratum rigidity (Guo and Wang, 2007), and live-cell imaging revealed a positive correlation between SF tension and zyxin accumulation (Colombelli et al., 2009). Zyxin's retention at FAs is also sensitive to mechanical signals. Conditions that reduce the forces impinging on a FA, such as chemical inhibition of cellular contractility or laser severing of a FA-associated SF, increases the kOFF of zyxin at the FA, leading to a reduced concentration of zyxin at cell–substratum adhesion sites (Lele et al., 2006).
The influence of physical stress on zyxin's subcellular distribution has functional consequences for local actin remodeling. Zyxin facilitates force-induced actin polymerization at FAs (Hirata et al., 2008), and zyxin contributes to actin polymerization at force-bearing cell–cell junctions (Nguyen et al., 2010). Exposure of cells to either uniaxial cyclic stretch or shear stress results in dramatic reorientation and reinforcement of the actin cytoskeleton. Zyxin-null cells reorient their actin SFs in response to uniaxial cyclic stretch but fail to thicken or reinforce them normally, illustrating zyxin-independent and zyxin-dependent facets of the stretch response (Yoshigi et al., 2005). The SF reinforcement response is postulated to facilitate the cell's ability to withstand the physical stress associated with a cell-stretching regimen (Yoshigi et al., 2005), and, indeed, cells that lack zyxin display higher-frequency breakage of their actin SFs (Smith et al., 2010).
Here we investigate the mechanism by which cells reinforce their actin SFs when exposed to mechanical stimulation. We demonstrate that stretch-induced SF reinforcement occurs independent of new transcription or translation, leading us to focus on posttranslational response mechanisms. We show that zyxin is phosphorylated in response to uniaxial cyclic stretch and that this response is independent of p130Cas, an integrin effector and zyxin-binding partner that is also posttranslationally modified in response to mechanical stress. We provide evidence that stretch-stimulated zyxin phosphorylation depends on activation of MAPK pathways and identify nonoverlapping domains in zyxin that are responsible for its force-sensitive targeting to actin SFs and recruitment of the actin-remodeling machinery. Binding of both α-actinin and Ena/VASP proteins to zyxin is essential for robust actin reinforcement downstream of physical stimulation. Zyxin's ability to influence SF maintenance and reinforcement is retained in the presence of Rho kinase inhibitors, further highlighting the novelty of this actin-remodeling pathway.
RESULTS
Uniaxial cyclic stretch triggers mobilization of a subset of FA constituents to SFs and induces actin reinforcement independent of transcription and translation
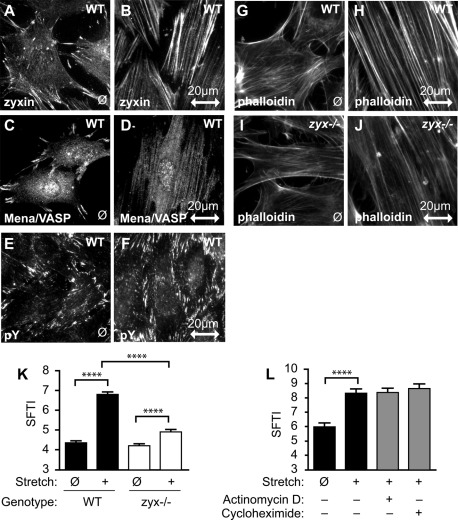
Integrin-rich FAs provide a transmembrane molecular link between the extracellular matrix and the actin cytoskeleton. Thus they serve as conduits for bidirectional communication of mechanical stress between the interior and exterior of cells. In vivo, normal tidal breathing induces 10% distension in the lung, and in vitro a similar magnitude stretch of lung cells is sufficient to induce cytoskeletal reorganization (Geiger et al., 2006; Eldib and Dean, 2011). Similarly, in vivo vasculature changes of 2–15% have been reported (Dobrin, 1978). By culturing fibroblasts on a pliable silicone membrane, it is possible to subject cells to uniaxial cyclic stretch of defined frequency and amplitude and induce cellular responses (Yoshigi et al., 2003, 2005; Jungbauer et al., 2008; Faust et al., 2011). On exposure to cyclic stretch (15%, 0.5 Hz), the FA proteins zyxin (Figure 1, A and B) and Mena/VASP (Figure 1, C and D) show diminished localization at sites of substratum adhesion and accumulate on actin SFs. Many other FA constituents, including vinculin, talin, and FAK, are retained at FAs for the duration of the mechanical stimulation (unpublished data). Staining of cells with anti-phosphotyrosine antibody confirms the integrity of FAs after cyclic stretch (Figure 1, E and F) and also illustrates that integrin-dependent signaling to tyrosine kinases resident at FAs is retained, and possibly even enhanced, in stretched cells. The anti-phosphotyrosine labeling of stretched cells also reveals that whereas a subset of proteins, including zyxin and Mena/VASP, accumulate on SFs in mechanically stimulated cells, other FA constituents, including those that are tyrosine phosphorylated, remain concentrated at FAs (Figure 1F). The elongated FA distribution of tyrosine-phosphorylated proteins in stretched cells is reminiscent of the retrograde flux of focal adhesion kinase that occurs when cells are grown on a stiff substrate (Guo and Wang, 2007).
FIGURE 1:
Uniaxial cyclic stretch induces changes in focal adhesion constituents and actin cytoskeletal reinforcement. Wild-type (WT) fibroblasts on unstretched membranes (ø) or after uniaxial cyclic stretch (2 h, 15%, 0.5 Hz), were fixed and immunostained for zyxin (A, B), Mena/VASP (C, D), and phosphotyrosine (E, F). The direction of stretch is shown in the horizontal plane, designated by a double-headed arrow scaled to 20 μm. Uniaxial cyclic stretch of WT (G, H) and zyxin-null (I, J) fibroblasts followed by phalloidin staining indicated abrogated actin thickening response in the absence of zyxin. (K) SFTI analysis of phalloidin-stained WT (black bars) and zyxin−/− (white bars) fibroblasts, unstretched (ø) and after 1 h of uniaxial cyclic stretch (+). (L) SFTI analysis of WT fibroblasts stretched in the presence of actinomycin D or cycloheximide (gray bars). ****p < 0.0001, n >100 SFTI measurements.
Coincident with the accumulation of zyxin and Mena/VASP proteins on the actin SFs, the SFs are reoriented perpendicular to the stretch vector and show measurable thickening, as evidenced by visual inspection (Figure 1, G and H). Stretch-induced SF reinforcement is abrogated in cells isolated from mice that harbor a targeted disruption of the gene encoding zyxin (Figure 1, I and J; Hoffman et al., 2003; Yoshigi et al., 2005). Calculation of the stress fiber thickness index (SFTI; Yoshigi et al., 2005) provides quantitative evidence that, although detectable SF thickening occurs in stretched cells that lack zyxin, zyxin is clearly required for robust SF reinforcement downstream of uniaxial cyclic stretch (Figure 1K).
The striking labeling of SFs with anti-zyxin antibodies that is observed in cells exposed to uniaxial cyclic stretch raised the possibility that mechanical stimulation might promote expression of zyxin; however, we did not detect an increase in zyxin by Western immunoblot analysis. It should also be noted that green fluorescent protein (GFP)–zyxin can be directly observed to accumulate on SFs of cells exposed to cyclic stretch. Thus we are observing bulk movement of zyxin onto the SF compartment and not simply enhanced epitope availability and antibody accessibility. Moreover, the ability of cells to reinforce the actin cytoskeleton in response to mechanical stimulation is not abrogated by gene transcription or protein translation inhibitors (actinomycin D and cycloheximide, respectively; Figure 1L). These results illustrate that posttranslational mechanisms are sufficient to promote the cytoskeletal changes that occur in response to mechanical stress.
Posttranslational signaling in response to uniaxial cyclic stretch
Phosphorylation is a common posttranslational modification downstream of transmembrane signaling. Because zyxin is required for the mechanical stress response and is phosphorylated in vivo (Beckerle, 1986; Crawford and Beckerle, 1991; Hervy et al., 2010), we explored whether zyxin is phosphorylated in response to mechanical stress. Phosphorylation of zyxin affects its electrophoretic mobility (Hervy et al., 2010). Therefore we used Western immunoblot analysis to explore whether uniaxial cyclic stretch is associated with altered mobility of zyxin by SDS–PAGE. As can be seen in Figure 2A (compare lanes 1 and 2), slower-mobility zyxin isoforms displayed enhanced prominence in lysates derived from stretched cells, suggesting that stretch induced a posttranslational modification such as phosphorylation that might account for the altered electrophoretic mobility of zyxin. Incubation of lysates with phosphatase before electrophoresis results in reduced prominence of the slower-mobility isoform (Figure 2A, lanes 3 and 4), consistent with the view that stretch induces phosphorylation of zyxin. Western immunoblot analysis with antibodies specific for a phospho-zyxin isoform reveals a baseline of zyxin phosphorylation in unstretched cells with enhancement of phospho-zyxin, and the appearance of a slower-mobility phospho isoform, upon stretch (Figure 2B). Quantitative analysis reveals a 2.6-fold enhancement of phosphorylated zyxin detected by Western immunoblot (Figure 2B), whereas the total amount of zyxin remains constant during the stretch response. Immunocytochemistry illustrates the presence of phospho-zyxin at both FAs of unstretched cells (Figure 2C) and enhanced at SFs of cells exposed to uniaxial cyclic stretch (Figure 2D).
FIGURE 2:
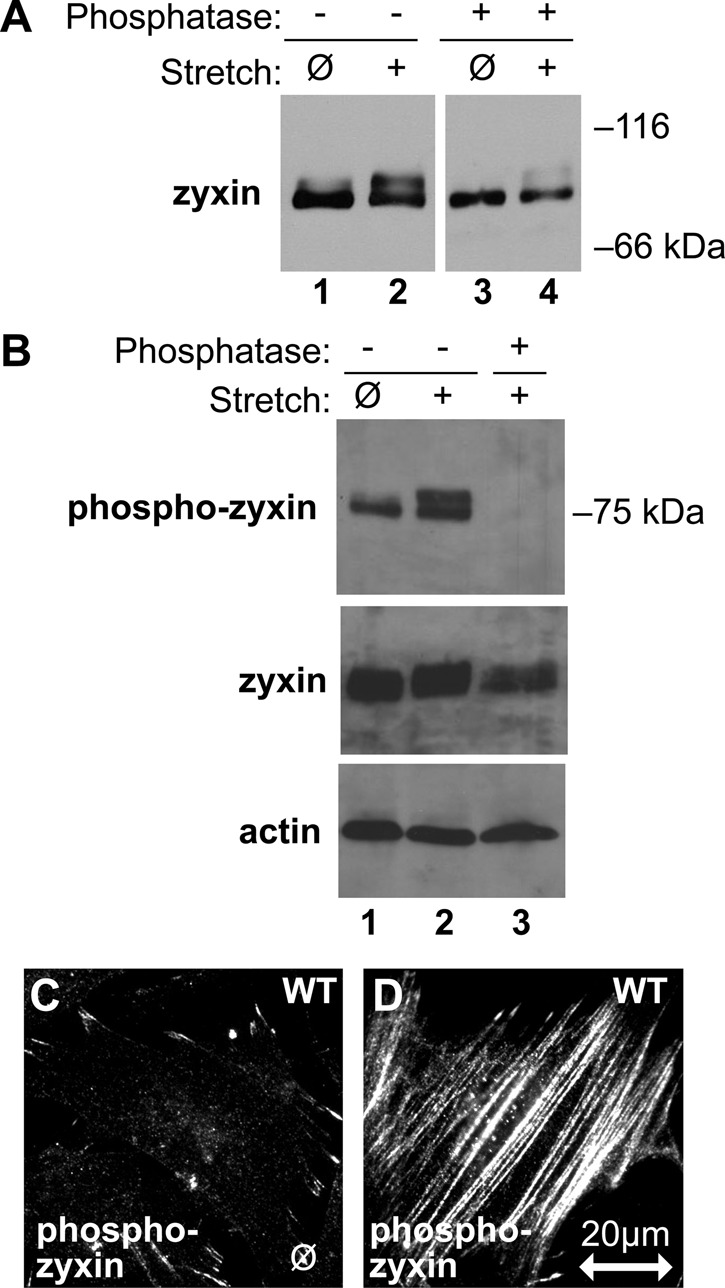
Posttranslational modification of zyxin in response to stretch. (A) Immunoblot analysis of zyxin from unstretched (ø) and stretched (+) WT fibroblasts (lanes 1 and 2) identified a stretch-induced shift of zyxin, which was reversed after incubation with phosphatase (lanes 3 and 4). (B) Immunoblot analysis with a phosphospecific zyxin antibody on unstretched (ø) and stretched (+) cell lysates (lanes 1and 2) and elimination of the phospho-zyxin signal by incubation with phosphatase (lane 3). Immunoblots for total zyxin and β-actin show that their signals are retained following phosphatase treatment. (C, D) Indirect immunofluorescence microscopy on unstretched (ø) and stretched (+) fibroblasts with phospho-zyxin antibody. Phospho-zyxin signal is low at focal adhesions of unstretched cells, but it increases and appears along stress fibers in stretched cells. Stretch direction is in the horizontal plane (double-headed arrow; scale, 20 μm).
The integrin effector p130Cas is not essential for stretch-stimulated actin SF reinforcement or zyxin phosphorylation
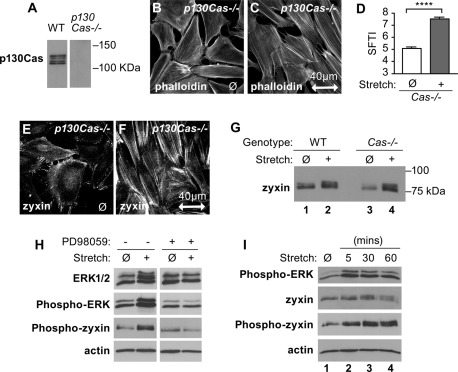
The ability of cells to realign and reinforce actin SFs in response to uniaxial cyclic stretch was previously shown to depend on integrin-mediated adhesion (Yoshigi et al., 2005). The integrin effector p130Cas is conformationally modulated by mechanical stress and is recovered in complex with zyxin via native immunoprecipitation (Yi et al., 2002). These observations raised the possibility that p130Cas might be upstream of zyxin in the mechanical stress response pathway. If that were the case, stretch-induced actin reinforcement would be blunted in cells that lack p130Cas. To test this hypothesis, we used cells that harbor a targeted deletion of the gene encoding p130Cas (Honda et al., 1998) and lack detectable p130Cas by Western immunoblot analysis (Figure 3A). We evaluated the capacity of these cells to mount a response to uniaxial cyclic stretch. Cells that lack p130Cas display a somewhat compromised resting SF organization, with accumulations of filamentous actin at cell borders (Figure 3B; Honda et al., 1998). Nevertheless, when stimulated by exposure to uniaxial cyclic stretch, the p130Cas−/− cells realign their actin cytoskeletal arrays perpendicular to the stretch vector (Figure 3C) and reinforce their SFs (Figure 3D), illustrating that they retain the capacity to sense and respond to mechanical signals. Consistent with our demonstration that stretch-induced SF reinforcement is dependent on zyxin and independent of p130Cas, zyxin retains the ability to localize at FAs (Figure 3E) and mobilizes to SFs in response to mechanical stress (Figure 3F) in cells devoid of p130Cas. Moreover, the electrophoretic mobility shift between unstretched and stretched p130Cas−/− cell lysates (Figure 3G), indicates that the stretch-activated signaling cascades required for phosphorylation of zyxin do not depend on p130Cas.
FIGURE 3:
Zyxin responds independent of signaling molecule p130Cas. (A) Western immunoblot for p130Cas in WT and Cas-null fibroblasts. (B, C) Fibroblasts from p130Cas−/− mice were stained for F-actin (phalloidin) on unstretched (ø) membranes or after uniaxial cyclic stretch (1 h, 15%, 0.5 Hz). Stretch direction is in the horizontal plane (double-headed arrow; scale, 40 μm). (D) SFTI analysis of the stretch-induced actin thickening in Cas−/− cells, ****p < 0.0001. (E, F) Immunofluorescence microscopy of zyxin in fibroblasts from p130Cas−/− mice on unstretched (ø) and stretched membranes. (G) Immunoblot analysis of zyxin in cell lysates from unstretched (ø) and stretched (+) membranes (WT, lanes 1 and 2; Cas−/−, lanes 3 and 4) showed the stretch-induced zyxin shift seen in WT cells persists in p130Cas−/− cells. (H) Fibroblasts on unstretched (ø) membranes or after uniaxial cyclic stretch (5 min, 15%, 0.5 Hz) with dimethyl sulfoxide control or with ERK activation inhibitor PD98059 (100 μM) were immunoblotted for total ERK1/2 (p44/p42), P-ERK, P-zyxin, and β-actin. (I) WT fibroblasts unstretched (ø) and stretched for 5, 30, and 60 min, electrophoresed, and immunoblotted for P-ERK, total zyxin, P-zyxin, and β-actin indicate maximal ERK phosphorylation at 5 min, followed by zyxin phosphorylation.
Stretch activation of p130Cas stimulates the GTPase Rap1, promoting the activation of MAPK signaling (Sawada et al., 2001). Although p130Cas clearly contributes to Rap1 activation downstream of mechanical stress, p130Cas-independent stretch activation of Rap1 has also been observed (Sawada et al., 2006). Therefore, even though p130Cas is not required for stretch-induced zyxin phosphorylation or SF realignment and reinforcement, it remained possible that zyxin phosphorylation depends on MAPK activation. Consistent with this possibility, zyxin displays multiple phosphorylation sites with sequences characteristic of MAPK consensus sites (Hervy et al., 2010). Cyclic stretch activates MAPK pathways in endothelial (Kito et al., 2000) and epithelial cells (Cohen et al., 2010), and we find that extracellular signal-regulated kinase (ERK) activation is evident in fibroblasts exposed to uniaxial cyclic stretch (Figure 3H). We next examined the impact of ERK activation inhibitor PD98059 on stretch-activated zyxin phosphorylation. As can be seen in Figure 3H, inhibition of ERK signaling abrogates the appearance of the activated phospho-ERK and also inhibits the zyxin phosphorylation detected by phospho-zyxin antibody. Temporal analysis of ERK and zyxin phosphorylation during the stretch response reveals that peak zyxin phosphorylation lags ERK activation (Figure 3I). Both the pharmacological inhibition studies and the phosphorylation kinetics are consistent with the conclusion that zyxin phosphorylation is directly or indirectly dependent on ERK activation.
Mapping the SF reinforcement response by molecular dissection of zyxin
Zyxin comprises 564 amino acids and is characterized by the presence of three C-terminal LIM domains, double zinc finger modules that support protein–protein interaction (Perez-Alvarado et al., 1994; Schmeichel and Beckerle, 1994). Murine zyxin exhibits two nuclear export signals at residues 144–156 and 343–359 (Nix and Beckerle, 1997; Renfranz et al., 2003). To define how zyxin contributes to reinforcement of SFs downstream of uniaxial cyclic stretch, we mapped the regions of zyxin that are necessary and sufficient for targeting of the protein to FAs, for recruitment to SFs in mechanically stimulated cells, and for reinforcement of the actin SFs in response to uniaxial cyclic cell stretch. We reconstituted zyxin−/− mouse embryo fibroblasts with enhanced GFP (eGFP)–tagged wild-type zyxin (zyxin1-564) or deletion variants (Table 1), creating a condition in which the only zyxin protein within the cells is the engineered construct, thus reducing competition or synergy that might occur if native protein were present. Because MEFs display a low DNA transfection efficiency, we used a lentiviral infection/expression system and selected cells with comparable eGFP expression by fluorescence-activated cell sorting (FACS). Western immunoblot analysis was used to verify that the proteins migrated as expected on SDS–PAGE (Figure 4A) and retained both zyxin and GFP epitopes.
TABLE 1:
Zyxin domain analysis.
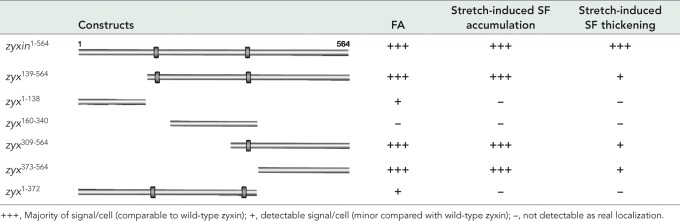 |
FIGURE 4:
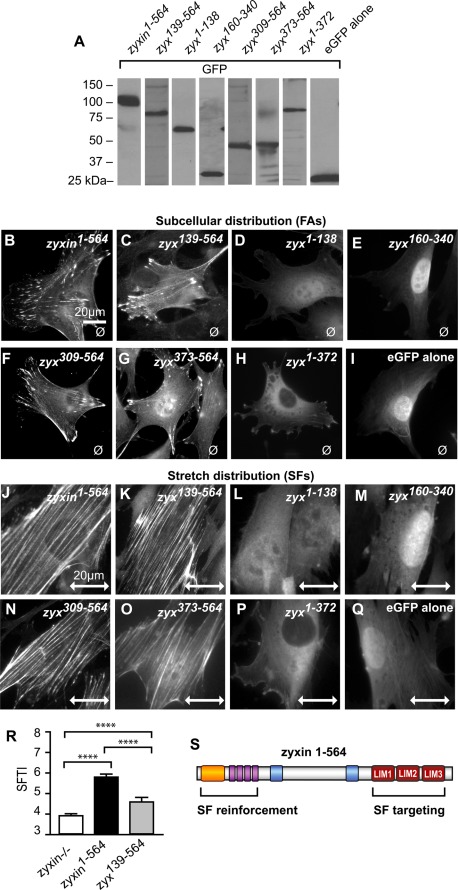
Zyxin mechanosensitivity-domain resides in the C-terminal LIM domains. (A) Western immunoblot analysis of zyxin domain–GFP fusion proteins to confirm expression and relative mobility of proteins. (B–I) Subcellular distribution of GFP by fluorescence microscopy of zyxin1-564 (B), zyx139-564 (C), zyx1-138 (D), zyx160-340 (E), zyx309-564 (F), zyx373-564 (G), zyx1-372 (H), and eGFP alone (I). (J–Q) After stretch stimulation (uniaxial, 15%, 0.5 Hz, 1 h), the GFP signals of zyxin1-564 (J), zyx139-564 (K), zyx309-564 (N), and zyx373-564 (O) were detected along actin stress fibers, whereas the other constructs—zyx1-138 (L), zyx160-340(M), zyx1-372(P), and eGFP alone (Q)—were not detected on stress fibers. Stretch direction is in the horizontal plane (double-headed arrow; scale, 20 μm). (R) Stretch-stimulated zyxin-null cells and cells expressing the zyxin1-564 and zyx139-564 variants were stained by phalloidin, and the SF thickening was measured. Zyx139-564 lacking the N-terminus is insufficient for normal stress fiber thickening, although it is able to target to stress fibers; **** p <0.0001. (S) Diagram of the stress fiber–targeting C-terminal LIM domains (red boxes) and the N-terminal SF reinforcement region of zyxin with α-actinin binding (yellow), Ena/VASP binding (purple), and the nuclear export sequences (blue).
We first mapped the regions of zyxin that are necessary and sufficient for FA targeting by analyzing unstretched cells. Direct fluorescence imaging of the subcellular distribution of the eGFP-tagged zyxin variants revealed that only variants containing residues 373–564 retained the capacity to target to focal adhesions (Figure 4, B, C, F, and G). The zyxin variants zyx1-138 and zyx1-372 (lacking LIM domains) did not exhibit significant focal adhesion accumulation (Figure 4, D and H). Although focal adhesions were clearly detectable by vinculin staining, zyxin variants zyx1-138 and zyx1-372 failed to accumulate at those focal adhesions (Supplemental Figure S1). As reported by others (Seibel et al., 2007), eGFP alone accumulates in the nucleus (Figure 4I). Consistent with previous studies that identified two potent nuclear export sequences (NESs) in zyxin (Nix and Beckerle, 1997; Renfranz et al., 2003), exclusion of the NESs results in nuclear accumulation of the zyxin variant (Figure 4, D and E). Inclusion of the zyx373-564 FA targeting sequence is sufficient to partially overcome the eGFP-driven nuclear localization and enable FA localization (compare Figure 4, G and I). Retention of a single NES, as in zyx309-564, eliminates the nuclear accumulation and reveals even more robust FA targeting (Figure 4F).
To identify the region(s) of zyxin that are critical for targeting of the protein to SFs in response to mechanical stimulation, we cultured the cells on elastic membranes and subjected them to uniaxial cyclic stretch before fixation and imaging (Figure 4, J–Q). Similar to what was observed for FA targeting, zyx373-564 harbors the sequence determinants that are necessary and sufficient for SF targeting in response to uniaxial cyclic stretch (Figure 4O). SF accumulation was not observed for constructs lacking the LIM region, such as the zyx1-138 and zyx1-372 variants (Figure 4, L and P). To ensure that the defect in SF targeting was not due to a complete lack of SFs in those cells, counterstaining with phalloidin was performed (Supplemental Figure S2). Although SFs were detectable in cells with the zyxin variants zyx1-138 and zyx1-372, these constructs lacking the LIM domains did not accumulate on the SFs. This domain analysis provides evidence that the C-terminal LIM domains localize zyxin to FAs in unstretched conditions and target zyxin to actin SFs after uniaxial cyclic stretch.
To map the region(s) of zyxin responsible for the stretch-induced SF reinforcement response, we introduced full-length zyxin or deletion variants (Table 1) into zyxin−/− cells, imaged filamentous actin in cells exposed to uniaxial cyclic stretch, and used our SF thickness algorithm to provide a quantitative assessment of SF reinforcement. In comparison with cells expressing full-length zyxin1-564, cells expressing a zyxin deletion variant lacking the N-terminal 138 amino acids, zyx139-564, display a statistically significant reduction in the SF reinforcement response (Figure 4R), suggesting that zyx1-138 contains critical information necessary for actin remodeling downstream of mechanical stimulation. We tested directly the ability of zyx1-138 to rescue the actin reinforcement response in zyxin−/− cells and found that it was incapable of doing so (Table 1), displaying an SFTI after stretch that was statistically indistinguishable from that observed in zyxin−/− cells (unpublished data). The failure of zyx1-138 to localize effectively at FAs or to SFs in stretched cells (Figure 4, D and L) is likely responsible for its lack of SF reinforcement activity. In comparison with cells lacking zyxin or reconstituted with zyx1-138, zyxin constructs harboring the C-terminal LIM domains retain some residual SF reinforcement function (Table 1), although clearly reduced relative to full-length protein.
This functional analysis of zyxin deletion variants provides evidence that the primary SF targeting and SF reinforcement activities of zyxin are contained in nonoverlapping areas of zyxin's primary sequence, identifying physically separable SF targeting and reinforcement domains that display unique structural features (Figure 4S). The C-terminal 192 amino acids, residues 373–564, a region comprising three tandemly arrayed LIM domains, is sufficient to target zyxin to SFs in stretched cells. The major sequences associated with zyxin's ability to contribute to stretch-induced SF reinforcement are found in the N-terminal 138 amino acids; however, since these sequences lack the subcellular targeting information, they are ineffective in promoting SF reinforcement. Two protein groups implicated in actin organization and dynamics—α-actinin and members of the Ena/VASP family—have been reported to associate with zyx1-138 (Golsteyn et al., 1997; Niebuhr et al., 1997; Reinhard et al., 1999; Drees et al., 2000; Li and Trueb, 2001). Therefore we investigated the importance of zyxin's ability to dock each of these proteins for an effective SF reinforcement response.
Deletion of zyxin's α-actinin–binding site compromises, but does not eliminate, the stress fiber reinforcement response
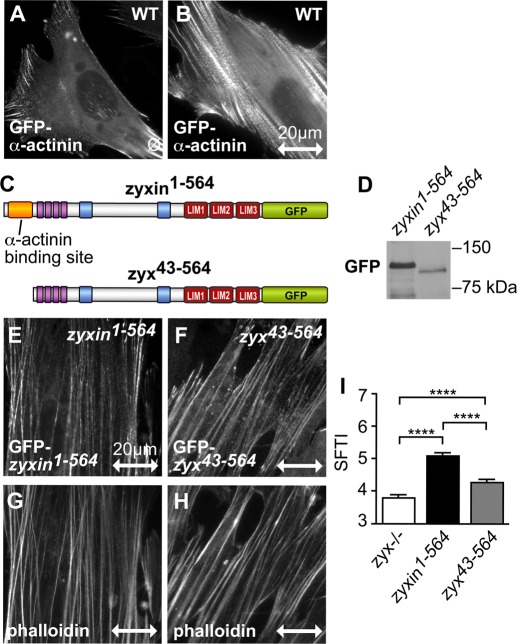
To define the mechanism by which zyxin residues 1–138 contribute to SF reinforcement, we first examined the importance of zyxin's α-actinin–binding capacity. The actin cross-linking protein α-actinin is prominently associated with SFs and has many binding partners (Otey and Carpen, 2004; Naumanen et al., 2008). Like zyxin, α-actinin is present at FAs of unstretched cells (Figure 5A; Pavalko et al., 1995) and accumulates on central SFs when cells are exposed to uniaxial cyclic stretch (Figure 5B). Zyxin's ability to dock α-actinin has been mapped to amino acids 1–42 (Drees et al., 1999; Reinhard et al., 1999; Li and Trueb, 2001). To probe the importance of this aspect of zyxin function for the SF reinforcement response, we expressed zyx43-564 in zyxin−/− cells and compared this to null cells reconstituted with full-length zyxin (Figure 5, C and D). Like full-length zyxin, zyx43-564 accumulates on SFs in cells challenged by exposure to uniaxial cyclic stretch (Figure 5, E and F), consistent with our finding that the C-terminal LIM region of zyxin harbors the primary SF targeting information. Analysis of the SF reinforcement response revealed that deletion of zyxin's α-actinin–binding domain compromises, but does not completely abrogate, the stretch response (Figure 5, G–I).
FIGURE 5:
Zyxin and α-actinin respond to stretch independently, but together they contribute to actin thickening. (A, B) Fluorescence microscopy of GFP–α-actinin in WT fibroblasts on unstretched (ø) and stretched membranes (uniaxial cyclic stretch, 15%, 0.5 Hz, 2 h; double-headed arrow; 20-μm scale). (C) GFP-tagged full-length mouse zyxin (1–564) with the α-actinin interaction site (yellow box) and the N-terminal deletion mutant zyxin (43–564) missing the α-actinin interaction site. (D) Western immunoblot analysis of both zyxin constructs expressed in zyxin-null fibroblasts. (E, F) Subcellular distribution of zyxin1-564 and the N-terminal mutant zyx43-564 along stretch-induced actin SF, coincident with phalloidin signal (G, H). (I) SFTI analysis of stretch-stimulated actin in zyxin-null cells (white bar) compared with cells expressing comparable levels of zyxin1-564 (black bar) and zyx43-564 (gray bar). Stretch direction is in the horizontal plane (double-headed arrow; scale, 20 μm); ****p <0.0001.
The ability of zyxin to recruit Ena/VASP family members is crucial for cytoskeletal stability
Members of the Ena/VASP family include Mena, VASP, and EVL, all three of which are expressed in mammalian fibroblasts (Krause et al., 2003). Ena/VASP proteins are characterized by the presence of conserved EVH1domains (Renfranz and Beckerle, 2002) that bind to ActA repeats—proline-rich sequences present in the Listeria cell surface protein ActA (Purich and Southwick, 1997). Zyx71-121 contains four ActA repeats, each of which has the capacity to dock Ena/VASP (Golsteyn et al., 1997; Niebuhr et al., 1997; Drees et al., 2000).
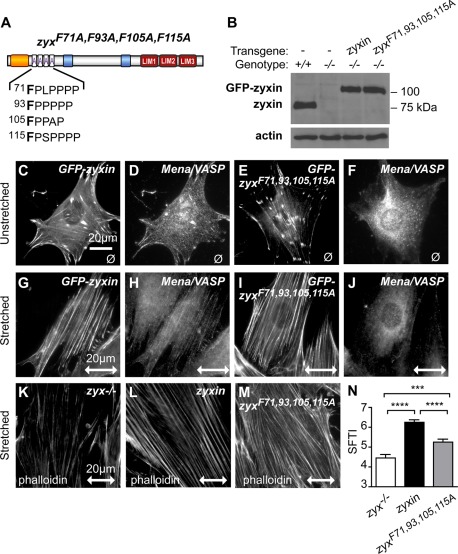
An absolutely conserved phenylalanine residue present in all ActA repeats is critical to support docking of Ena/VASP proteins (Drees et al., 2000; Machner et al., 2001). Therefore, to abolish the ability of zyxin to interact with members of the Ena/VASP family while minimizing nonspecific consequences that might arise with larger deletions, we mutated the individual phenylalanines (F) in each of zyxin's ActA repeats to alanine (A) to generate what we refer to as the zyxF71,93,105,115A mutant (Figure 6A). GFP-tagged wild-type zyxin or mutant zyxF71,93,105,115A was expressed in zyxin−/− fibroblasts and FACS sorted for equivalent GFP expression. Western immunoblot analysis confirmed the lack of endogenous zyxin protein and the effective expression of the transgenic variants (Figure 6B). Both wild-type zyxin and zyxF71,93,105,115A localize to FAs (Figure 6, C and E); however, zyxF71,93,105,115A fails to support docking of VASP at FAs (compare Figure 6, F with D). After stretch stimulation, wild-type zyxin accumulates on SFs (Figure 6G) and recruits VASP to SFs (Figure 6H). Mutant zyxF71,93,105,115A also accumulates on SFs in stretch-challenged cells (Figure 6I) but fails to recruit VASP (Figure 6J), illustrating that VASP depends on zyxin for its appropriate targeting to both FAs and stretch-stimulated SFs.
FIGURE 6:
Stretch-induced actin cytoskeletal response is perturbed by disruption of zyxin–VASP interaction. (A) Diagram of mouse zyxin protein demarcates four ActA repeats for Mena/VASP interaction, targeted for site-directed mutagenesis of F71A, F93A, F105A, and F115A as zyxF71,93,105,115A (purple letters). (B) Western immunoblot of zyxin from wild-type fibroblasts (+/+), zyxin-null fibroblasts (−/−), and zyxin-null fibroblasts programmed to express GFP-tagged wild-type zyxin and mutant zyxF71,93,105,115A. Immunoblot for β-actin confirmed equivalent protein loading of the cell lysates. (C, D) GFP-zyxin and Mena/VASP proteins colocalized at focal adhesions. (E, F) GFP-zyxF71,93,105,115A accumulated normally at focal adhesions but failed to recruit Mena/VASP. After stretch stimulation, zyxin (G) and Mena/VASP (H) accumulated along actin SF, as did zyxF71,93,105,115A (I), but Mena/VASP remained mislocalized (J). Phalloidin-stained, stretch-stimulated stress fibers in zyxin-null cells (K) and cells expressing GFP-zyxin (L) and GFP- zyxF71,93,105,115A (M) were analyzed for thickening. (N) SFTI analysis of zyx−/− cells (white bar) and cells expressing comparable levels of zyxin (black bar) and zyxF71,93,105,115A (gray bar). Stretch direction is in the horizontal plane (double-headed arrow; scale, 20 μm); ****p <0.0001.
Quantitative analysis of cells exposed to uniaxial cyclic stretch revealed that the ability of zyxin to dock Ena/VASP proteins contributes significantly to the SF reinforcement response (Figure 6, K–N). In particular, zyxin−/− cells reconstituted with zyxF71,93,105,115A and subjected to cyclic stretch fail to reinforce actin SFs to the level achieved when wild-type zyxin is present (compare Figure 6, L and M, and Figure 6N). However, cells reconstituted with zyxF71,93,105,115A achieved a level of actin SF reinforcement that was superior to that observed in zyxin−/− cells (Figure 6N), showing that factors in addition to zyxin's capacity to recruit Mena/VASP proteins contribute to the SF reinforcement response.
Failure of zyxin to bind Ena/VASP proteins enhances the rate of SF demise and accumulation of disorganized actin aggregates in response to jasplakinolide
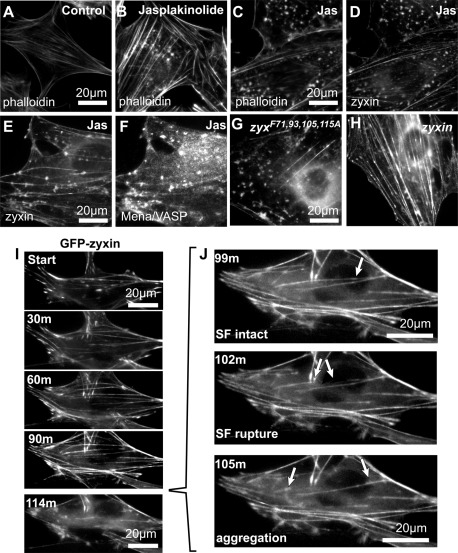
These results illustrate a role for zyxin-dependent recruitment of Ena/VASP proteins for maintenance and reinforcement of the actin cytoskeleton in the face of mechanical stress. Chemical probes provide an alternative mechanism for stressing the actin cytoskeleton in living cells and probing the functional significance of zyxin's ability to bind Ena/VASP proteins. The marine toxin jasplakinolide reduces the critical concentration of monomeric actin required for actin assembly and increases the rate of actin nucleation in vivo (Bubb et al., 2000). Jasplakinolide is also used to stabilize actin filaments in vivo (Lee et al., 1998; Cramer, 1999), and it may function as an inhibitor of actin depolymerization (Miyoshi et al., 2006). When applied to living cells, jasplakinolide induces an initial thickening of SFs (Figure 7, A and B) that is associated with recruitment of zyxin to the SF compartment (Hoffman et al., 2006). Over time, there is an accumulation of disorganized filamentous actin aggregates throughout the cytoplasm (Figure 7C). The actin aggregates contain both zyxin (Figure 7, D and E) and VASP (Figure 7 F), an observation that led us to investigate the importance of the zyxin–VASP interaction for the cellular response to jasplakinolide.
FIGURE 7:
Actin stress fibers formed without VASP recruitment exhibit enhanced sensitivity to actin inhibitor jasplakinolide (Jas). (A, B) In comparison to controls, WT cells treated with jasplakinolide (100 nM, 2 h) accumulated stress fibers and aggregates of F-actin (phalloidin) visible by fluorescence microscopy. F-Actin (phalloidin; C) and zyxin (D) colocalized within the jasplakinolide-induced aggregates. Zyxin (E) and binding partner Mena/VASP (F) also colocalized at the jasplakinolide-induced aggregates. Jasplakinolide treatment (100 nM, 2 h) of zyxin-null cells expressing either GFP- zyxF71,93,105,115A mutant (G) or GFP-zyxin (H) induced a more pronounced actin phenotype, with disruption of zyxin-Mena/VASP interaction (zyxF71,93,105,115A). (I) Time-lapse microscopy of GFP-zyxin in cells (0, 30, 60, 90, and 114 min after 200 nM jasplakinolide addition) showed mobilization from focal adhesions to actin stress fibers, followed by rupture of fiber and accumulation of aggregates (J, arrows; 99, 102, 105 min). See Supplemental Video S1.
The accumulation of actin aggregates in response to jasplakinolide treatment is both concentration and time dependent (Bubb et al., 2000). Therefore we explored the kinetics of the jasplakinolide response in zyxin−/− cells reconstituted with wild-type zyxin or zyxF71,93,105,115A.. Zyxin−/− cells reconstituted with zyxF71,93,105,115A display significant cytoskeletal breakdown and actin aggregate formation in response to a 2-h exposure to jasplakinolide (Figure 7G) compared with cells reconstituted with wild-type zyxin, which retain many SFs under these conditions (Figure 7H).
The accumulation of actin aggregates in response to jasplakinolide has been suggested to result from the de novo polymerization of actin into amorphous masses, with the subsequent loss of SFs due to insufficient actin monomer for SF maintenance during normal remodeling and turnover (Bubb et al., 2000). However, if this were the explanation, why would an inability of zyxin to bind Ena/VASP accelerate this process? We reasoned that an alternative explanation might be a failure of SF maintenance and/or repair processes when zyxin is unable to recruit Ena/VASP proteins. In that scenario, the actin aggregates might reflect the remnants of broken SFs that arise when SF maintenance is compromised. To explore this possibility directly, we imaged cells expressing GFP-tagged zyxin during exposure to jasplakinolide to determine the genesis of the actin and zyxin-rich aggregates (Figure 7I and Supplemental Video S1). From this analysis, it is evident that the amorphous aggregates of filamentous actin in the jasplakinolide-treated cells represent remnants of ruptured SFs, not newly assembled structures (Figure 7J). These results support the view that zyxin's ability to recruit Ena/VASP is important for the maintenance of SF architecture in response to both mechanical and pharmacological stress.
The zyxin-dependent actin remodeling response is independent of Rho kinase
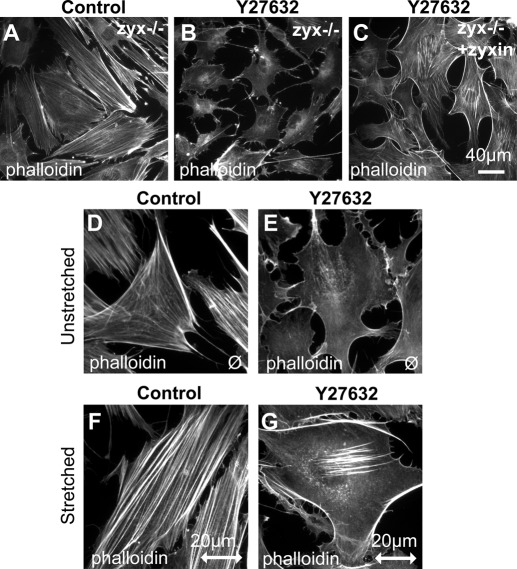
SF assembly is triggered by activation of Rho kinase, which promotes phosphorylation and activation of myosin light chain to stimulate myosin-dependent contractility (Jaffe and Hall, 2005; Guilluy et al., 2011a). We defined a zyxin, VASP, and α-actinin–dependent pathway of SF remodeling that results in SF thickening in response to uniaxial cyclic stretch. To test whether this pathway is regulated by Rho kinase, we first compared the effect of the Rho kinase inhibitor Y27632 on both zyxin−/− cells and zyxin−/− cells reconstituted with zyx1-564. The eGFP-zyx1-564 cells were selected to express at levels similar to that of endogenous zyxin in wild-type cells, as seen in Figure 6B. SFs are present in untreated cells (Figure 8A), but exposure to 3 μM Y27632 for 2 h results in loss of SFs from zyxin−/− cells (Figure 8B). In contrast, although diminished compared with pretreatment, residual SFs are still present in Y27632-treated cells that express zyxin (Figure 8C). These results provide a preliminary indication that zyxin promotes SF assembly or maintenance by a mechanism that does not require Rho kinase activity.
FIGURE 8:
Stretch-induced actin remodeling persists in the presence of Rho kinase inhibition. Zyx−/− cells untreated (A) or after 2 h of treatment with Y27632 (3 μM; B) and cells expressing GFP-zyxin treated with 3 μM Y27632 (C) were fixed and then visualized by phalloidin staining and fluorescence microscopy. Scale, 40 μm. (D, F) WT control cells were unstretched (D) and stretch stimulated (F) for 1 h, followed by fixation, phalloidin staining, and fluorescence microscopy, and then compared with WT cells in the presence of Y27632 that were unstretched (E) and stretch stimulated (G) for 1 h. Stretch direction is in the horizontal plane (double-headed arrow; scale, 20 μm).
To explore this possibility further, we tested whether inhibition of Rho kinase would influence the response of cells to uniaxial cyclic stretch (Figure 8, D–G). Unstretched cells exposed to Y27632 displayed diminished SFs relative to unstretched control cells (compare unstretched cells in Figure 8, D and E). However, cells exposed to Rho kinase inhibitor retained the capacity to launch a SF reinforcement response upon exposure to uniaxial cyclic stretch (Figure 8G). Cells treated with the Rho kinase inhibitor failed to realign their SFs perpendicular to the stretch vector (compare stretched cells in Figure 8, F and G), providing independent evidence that the Rho kinase inhibitor is interfering with the SF reorientation response while not fully eradicating the SF reinforcement response.
DISCUSSION
Cells reorient and reinforce their actin cytoskeletons when exposed to mechanical force, establishing a robust interior framework composed of actin stress fibers. The LIM protein zyxin is mobilized from focal adhesions to actin stress fibers in response to mechanical stimulation, and it is required for the ensuing reinforcement of intracellular actin arrays. The results presented here refine our understanding of the pathways that lead from application of uniaxial cyclic stretch in fibroblasts and address the mechanism by which zyxin promotes SF remodeling and reinforcement.
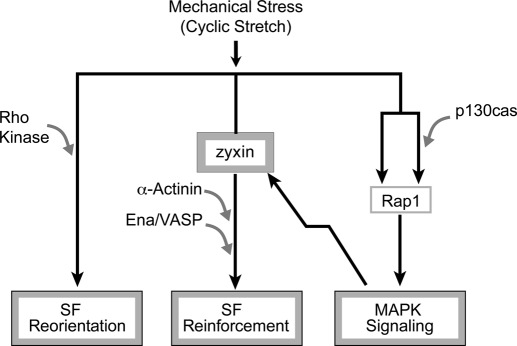
Three mechanistically separable cellular response pathways downstream of mechanical stress can now be described (Figure 9):
FIGURE 9:
Model of mechanotransduction. Mechanical stress induces three separable responses: stress fiber reorientation, stress fiber reinforcement, and MAPK signaling. Our data support the notion that Rho kinase signaling contributes to SF reorientation, that zyxin, α-actinin, and Ena/VASP proteins contribute to SF reinforcement, and that p130Cas, Rap1, and MAPK signaling form the third response to mechanical stress applied by uniaxial cyclic stretch.
1. SF reorientation. Cells align their SFs perpendicular to the stretch vector. SF reorientation is independent of zyxin and is inhibited by agents that block Rho kinase.
2. SF reinforcement. Thickening of the SFs requires zyxin. It depends on targeting of zyxin to SFs via the LIM domains and actin remodeling that involves the zyxin-binding partners α-actinin and VASP. Zyxin is phosphorylated in response to uniaxial cyclic stretch. Zyxin-dependent SF reinforcement can occur independent of Rho kinase activation.
3. p130Cas-dependent activation of MAPK signaling. p130Cas is tyrosine phosphorylated in response to mechanical stress, a modification that stimulates MAPK activation (Tamada et al., 2004; Sawada et al., 2006). Uniaxial cyclic stretch promotes both SF alignment and SF reinforcement in p130Cas−/− cells, and thus there is not an obligatory link from p130Cas activation to these processes. p130Cas-independent activation of MAPK is likely responsible for stretch-induced phosphorylation of zyxin since MAPK inhibitors block this posttranslational modification and zyxin phosphorylation occurs normally in p130Cas−/− cells. Zyxin displays several MAPK consensus phosphorylation sites (Hervy et al., 2010), consistent with the view that it may be a direct substrate of a MAPK.
Molecular dissection of the stretch-induced actin reinforcement response
By reconstitution of zyxin-null cells with a series of zyxin deletion variants, we defined critical functional domains of zyxin that are necessary and sufficient to support key steps in the stretch response. First, we discovered that the essential determinants of zyxin's stretch-induced actin reinforcement capacity map within the N-terminal 138 amino acids of the protein. Zyxin's N-terminus has been reported to bind a number of partners, including the actin filament cross-linker α-actinin (Drees et al., 1999; Reinhard et al., 1999; Li and Trueb, 2001), the actin assembly modulator Ena/VASP (Golsteyn et al., 1997; Niebuhr et al., 1997; Drees et al., 2000), and the cytoskeletal proteins LIM-nebulette and LASP-1 (Li et al., 2004; Grunewald et al., 2007). Because of their well-established roles in actin assembly and organization, we focused our analysis on the roles of α-actinin and Ena/VASP in the SF reinforcement response. Reconstitution of zyxin−/− cells with constructs harboring the minimal deletion or amino acid substitution necessary to compromise zyxin's ability to bind either α-actinin or Ena/VASP family members revealed that zyxin can be recruited to SFs in mechanically stimulated cells independent of binding to either of these partners. However, the loss of capacity to bind either α-actinin or Ena/VASP compromises the SF reinforcement response that occurs downstream of uniaxial cyclic stretch. These results illustrate the contributions of both of these binding partners to the zyxin-dependent actin reinforcement response. α-Actinin binding by zyxin could facilitate SF reinforcement by recruitment and cross-bridging of short actin filaments at sites of zyxin accumulation. Ena/VASP could promote actin polymerization at barbed filament ends. Alternatively, we showed previously that binding of Ena/VASP proteins to zyxin enhances the rate at which zyxin is recruited to sites of local SF damage (Smith et al., 2010). Phosphorylation promotes Ena/VASP binding by zyxin by inhibiting an intramolecular (head–tail) interaction within the zyxin protein (Moody et al., 2009; Call et al., 2011). Thus it is intriguing to consider the possibility that the MAPK-dependent phosphorylation of zyxin that occurs in response to uniaxial cyclic stretch might relieve an intramolecular zyxin interaction, simultaneously enhancing Ena/VASP binding and stabilizing a protein conformation in which the LIM region is accessible.
Distinct domains for SF targeting and remodeling
Although the core capacity of zyxin to promote SF reinforcement resides in the N-terminal 138 amino acids, this region of the protein on its own fails to localize to SFs in stretched cells and fails to support SF remodeling. This observation suggested that a physically separable region of zyxin might be responsible for targeting the protein to the SFs in mechanically stimulated cells. Here we determined that the LIM region of zyxin is both necessary and sufficient to promote SF localization in cells exposed to uniaxial cyclic stretch. The LIM region is also sufficient for accumulation of zyxin at FAs of unstretched cells (Nix et al., 2001) and migrating cells (Uemura et al., 2011). Although the LIM region of zyxin is capable of associating with FAs and SFs, it is not sufficient to restore the actin reinforcement response in zyxin-null cells exposed to mechanical stress. This observation is consistent with a report that zyxin LIM domains displace endogenous zyxin and disrupt actin polymerization at FAs (Hirata et al., 2008). Collectively, these findings support the view that zyxin harbors two structurally separable, discrete functional domains that contribute obligatorily to the stretch-induced actin reinforcement response: a localization or targeting domain and an actin-remodeling domain.
p130Cas is not required for stretch-induced actin reinforcement
The LIM region of zyxin that controls the protein's subcellular localization interacts directly with the integrin effector p130Cas (Yi et al., 2002), which has itself been shown to undergo conformational change in response to cyclic stretch (Sawada et al., 2006). Zyxin interacts with the same region of p130Cas that both is modified by tyrosine phosphorylation and provides a docking site for the SH2-SH3 adaptor CRK under conditions of mechanical stimulation (Yi et al., 2002; Tamada et al., 2004). We were intrigued by the possibility that uniaxial cyclic stretch might simultaneously cause release of zyxin from p130Cas and enable the binding of CRK and activation of a Rap1-dependent signaling cascade to MAPK. However, in our experiments we found that both zyxin's recruitment to SFs and the actin reorientation/reinforcement that occur in response to uniaxial cyclic stretch are retained in p130Cas-null cells. Thus both zyxin and p130Cas display mechanosensitivity but appear to influence independent responses to mechanical stimulation (Figure 9).
Although p130Cas has been implicated in the stretch-induced activation of MAPK signaling, elimination of p130Cas by targeted gene disruption does not disturb zyxin's stretch-dependent phosphorylation. This is perhaps not surprising, given work showing that although knockdown of p130Cas leads to a 50% reduction in stretch-induced Rap1 activation, Rap1 activity is not completely eliminated in Cas-deficient cells exposed to cyclic stretch (Sawada et al., 2006). Thus it appears that both p130Cas-dependent and -independent activation of Rap1 occurs in response to mechanical stimulation. The mechanisms by which various MAPKs influence the mechanotransduction response remain to be deciphered and will likely be complex. One recent hint regarding how MAPK signaling contributes to the response of cells to mechanical tension is the work of Guilluy et al. (2011b) that suggests a role for ERK activation of a particular Rho-directed guanine nucleotide exchange factor (Rho GEF-H1).
Cellular response to global or local mechanical stress
Here we show that exposure of cells to uniaxial cyclic stretch causes zyxin to accumulate on SFs, illustrating a pancellular, global mechanical stress response. Of interest, localized application of mechanical stress triggers local zyxin accumulation on individual SFs as well. For example, zyxin recruitment to a localized SF domain can be induced by targeted application of mechanical stress via direct prodding with a microprobe (Smith et al., 2010) or by micromanipulation using atomic force microscopy (Colombelli et al., 2009). In a complementary manner, if strain is relieved by release of an internal SF segment from its FA anchors by a pair of internal nanosurgical cuts, zyxin localization along the released SF fragment declines (Colombelli et al., 2009). Thus, under a variety of conditions that exert either a positive or negative effect on zyxin accumulation on SFs, the application of force is a common factor that promotes zyxin localization. Consistent with this view, application of the nonmuscle myosin II inhibitor blebbistatin to reduce SF contractility causes release of zyxin from SFs in cultured cells (Colombelli et al., 2009). It remains to be determined whether zyxin is accumulating at sites of high SF strain because it is directly detecting mechanical stress by recognizing a novel strain-induced protein conformation, for example, or whether the mechanical stress is inducing local SF damage, such as actin filament breaks, that are recruitment targets for zyxin and its partners.
SFs in cultured cells undergo strain-induced, local thinning and elongation (Smith et al., 2010). Zyxin is rapidly recruited to these areas where SF integrity is compromised and is required for the restoration of SF structure via a mechanism that involves both α-actinin and VASP (Smith et al., 2010). The response of cells to uniaxial cyclic stretch might reflect the accumulation of a large number of strain-induced local thinning and elongation events, which, in aggregate, would result in zyxin-coated SFs and actin remodeling and reinforcement, as we observed.
Zyxin contributes to Rho kinase–independent SF reinforcement
Activation of the small GTPase Rho stimulates SF assembly (Ridley and Hall, 1992). Rho-GTP interacts with Rho kinase, stimulating its activity and ultimately enhancing myosin-dependent contractility (Jaffe and Hall, 2005; Guilluy et al., 2011a). Because zyxin promotes SF reinforcement, it was of interest to assess whether Rho kinase was an upstream modulator of the zyxin-dependent actin-remodeling response to cyclic stretch. Eliminating zyxin in concert with inhibiting Rho kinase activity results in a nearly complete loss of actin stress fibers in both stretched and unstretched cells. However, reconstitution of the cells with zyxin is sufficient to partially restore actin SFs, revealing a zyxin-dependent, Rho kinase–independent machinery that influences SF architecture. Treatment of cells with Rho kinase inhibitor abrogates the SF reorientation response downstream of uniaxial cyclic stretch, illustrating a critical role for Rho kinase–dependent actin-remodeling processes in the alignment of SFs within mechanically stimulated cells. Some new SFs are produced within stretched cells even under conditions of Rho kinase inhibition. Although the SFs are generally less abundant in cells in which Rho kinase is inhibited compared with controls, it is clear that some Rho kinase–independent mechanism contributes to SF reinforcement downstream of mechanical stress, and this likely involves zyxin. Consistent with our findings, previous studies revealed that Rho activation is required for SF reorientation in aortic endothelial cells exposed to cyclic stretch (Kaunas et al., 2005; Lee et al., 2010). Our work extends these studies and reveals that the zyxin/α-actinin/VASP actin-remodeling machinery is a key contributor in a Rho kinase–independent pathway of SF reinforcement downstream of mechanical stress.
MATERIALS AND METHODS
Reagents
Mouse antibodies for vinculin hVIN-1, β-actin AC-74, and α-actinin BM75.2 (all Sigma-Aldrich, St. Louis, MO), rabbit anti-ERK1/2 and anti–phospho-ERK1/2 (4695 and 4370; Cell Signaling Technology, Beverly, MA), mouse anti-p130cas (BD Transduction Laboratories, Lexington, KY), and anti–pY 4G10 (Upstate, Millipore, Billerica, MA) were used as recommended by manufacturers. To generate the anti–Mena/VASP polyclonal rabbit serum B119, rabbits were immunized against KLH-coupled peptides GLAAAIAGAKLRKVSKQE and EKPPKDESASQEESEARL (Harlan Bioproducts for Science, Indianapolis, IN). Anti-zyxin rabbit polyclonal serum B71 was previously described (Hoffman et al., 2003). Phospho-zyxin (human Ser-142/143) rabbit antibody (4863; Cell Signaling Technology) detected mouse zyxin p-Ser144 (Western blots 1:2000, indirect immunofluorescence microscopy 1:200). Secondary antibodies and phalloidin were Alexa Fluor conjugates used for cell staining (Molecular Probes/Invitrogen, Carlsbad, CA) and horseradish peroxidase (HRP) conjugates used for Western blots (GE Healthcare, Piscataway, NJ). Actinomycin D and cycloheximide were from Sigma-Aldrich, jasplakinolide and Y27632 from Calbiochem (La Jolla, CA), and PD98059 from Cell Signaling Technology.
Cells and constructs
Wild type and zyxin-null fibroblasts (with or without expression constructs) were maintained in high-glucose DMEM supplemented with pyruvate, glutamine, penicillin/streptomycin (Invitrogen), and 10% fetal bovine serum (Hyclone Labs, Logan, UT) as described (Hoffman et al., 2006). Wild-type and p130cas-null fibroblasts (Honda et al., 1998) were cultured as recommended.
Mouse zyxin cDNA was used as template to PCR amplify domains with homologous ends for recombination cloning (Gateway Technology, Invitrogen). DNA sequencing of each entry clone confirmed the correct zyxin sequence before subcloning into a lentivirus expression system following manufacturer's recommendations for a three-part recombination with human cytomegalovirus promoter and C-terminal eGFP tag (ViraPower HiPerform Lentiviral Expression Systems, Invitrogen). Lentivirus was produced in 293FT cells, and the cell supernatant was used to infect zyxin-null fibroblasts. After at least 1 wk in culture, cells were FACS sorted for GFP expressors. Zyxin and GFP epitopes were confirmed for constructs containing 1–564, 139–564, 1–138, 309–564, or 1–372, but no antibodies were available for zyxin sequences in 160–340 and 373–564.
Zyxin mutant 43–564, previously described (Smith et al., 2010), was subcloned into Gateway pLentivirus expression (Invitrogen) and cells sorted for GFP expression. Site-directed mutagenesis of four phenylalanines in the Ena/VASP–binding region of zyxin (zyx4F>A; F71A, F93A, F105A, F115A) was performed with the QuikChange II Mutagenesis Kit (Invitrogen). Mutant zyxF71,93,105,115A was subcloned with eGFP tag into the retroviral vector pLINX using methylation-sensitive ClaI restriction sites (Hoshimaru et al., 1996). Retrovirus was made in Phoenix-Eco producer cells (American Type Culture Collection, Manassas, VA) and used to infect zyxin-null fibroblasts. Stable cell lines expressing GFP-zyxF71,93,105,115A were selected for GFP expression by FACS. GFP–α-actinin nucleofection followed manufacturer recommendations (Amaxa Biosystems, Lonza, Cologne, Germany).
Cell stretching, protein detection, and SFTI analysis
Cells were seeded onto precoated silicone membranes (25 μg/ml collagen I, 2 μg/ml fibronectin) as previously described (Yoshigi et al., 2005). Briefly, three 26 × 33 mm membranes in a 100-mm dish were seeded with 1.2–1.5 million cells. After 24 h growth in DMEMc and 10% serum, cells were subjected to uniaxial cyclic stretch (15%, 0.5 Hz, up to 2 h) using a custom-made stretch device driven by a step motor (Yoshigi et al., 2003). At specified times, cells were fixed (15 min, 3.7% formaldehyde) and permeabilized (5 min, 0.5% Triton X-100) directly on membranes, followed by staining and imaging, or cells were lysed on membranes for protein analysis (150–300 μl/membrane; 50 mM Tris-HCl, pH 8, 150 mM NaCl, 0.5% NP-40, 0.1% SDS, 0.1 mM NaF, 0.2 mM sodium orthovanadate with protease inhibitors [Boehringer-Mannheim, Mannheim, Germany]). Cell images are always shown with the stretch vector maintained in the horizontal direction (double-headed arrow). SFs within 30° of perpendicular to the horizon were considered aligned (Yoshigi et al., 2005). For inhibition of transcription and translation, cells on membranes were preincubated for 30 min before stretch and for the duration of a 1-h stretch with actinomycin D (1 μg/ml) or cycloheximide (10 μg/ml; Tamura et al., 2000; Sun et al., 2001; Momberger et al., 2005), concentrations that are sufficient to inhibit >90% transcription (Sawicki and Godman, 1971) and >90% protein translation in this time frame (Sundell and Singer, 1990). For Table 1 showing the zyxin domain analysis, cells were scored for GFP-zyxin signal localization at FAs and along SFs. SF thickening was phalloidin signal/cell increased by stretch, comparison (relative to zyx-null and wild-type cells) between independent stretch experiments.
For SFTI measurements, images of phalloidin-stained F-actin (using concomitant GFP signal to confirm expression) were analyzed with a custom erosion/brightness decay software (Yoshigi et al., 2005) written in LabVIEW (National Instruments, Austin, TX). Exposure of GFP signal was held constant for image capture, and cells with comparable expression levels were selected for SFTI analysis of phalloidin signal. For each cell type, multiple stress fibers/cell in >10 fields (Zeiss 40×; Carl Zeiss, Jena, Germany) were evaluated (n > 100 measurements); statistical analysis (means ± SEMs, unpaired t tests, analysis of variance) and graphing were performed with Prism software (GraphPad, La Jolla, CA). At least three independent stretch experiments were performed for each construct and cell type, and the relative SFTI values within each experiment were compared.
Cell microscopy
Cells were seeded onto glass coverslips and grown for 18 h in complete DMEM and 10% serum, followed by fixation (15 min, 3.7% formaldehyde) and permeabilization (5 min, 5% Triton X-100). Proteins were localized by antibody immunostaining and phalloidin. Cell images were captured with a CoolSnap HQ camera and Zeiss Axiophot fluorescence microscope (Plan-Apochromat 63×, 1.40 numerical aperture [NA], oil objective; 40×, 0.75 NA, dry objective) and OpenLab software (Improvision, PerkinElmer, Waltham, MA) or ImageQuant software (Andor Technology, South Windsor, CT). A Zeiss Axioskop2 mot plus microscope (40×, 0.75 NA, dry objective) with automatic shutter and a Zeiss AxioCamMRm camera with Zeiss AxioVision 4.8.1 software were also used to capture images. Time-lapse imaging used a stage heater (Bioptechs, Butler, PA) on an Olympus microscope (60×, 1.45 NA, objective; Olympus, Center Valley, PA), a digital camera (Orca; Hamamatsu, Hamamatsu, Japan), and MetaMorph software (Molecular Devices, Sunnyvale, CA). Photoshop, version 8 (Adobe, San Jose, CA), was used for image processing and Adobe Illustrator, version 11.0, for figure preparation.
Electrophoresis and Western immunoblots
Cell lysates (10–25 μg/lane) were electrophoresed through denaturing 10% polyacrylamide gels (Bio-Rad, Hercules, CA) with prestained Precision Plus molecular weight markers (Bio-Rad) and then electroblotted onto nitrocellulose filters, probed with HRP-conjugated antibodies, and detected by enhanced chemiluminescence (GE Healthcare). For phosphatase experiments, cell lysates were incubated with calf intestinal phosphatase or lambda phosphatase (New England BioLabs, Ipswich, MA) for 30 min before electrophoresis.
Supplementary Material
Acknowledgments
We are grateful to Hiroaki Honda, Hisamura Hirai, and Carol Otey for cell lines and constructs. We thank Martial Hervy and Mark Toda, who participated in early stages of this work, and Mark Smith and Diana Lim for help with manuscript preparation. A National Cancer Institute Cancer Center Support Grant (2 P30 CA042014) provided essential shared resources. This work was supported by National Institutes of Health Grant GM50877 (to M.C.B.) and the Huntsman Cancer Institute.
Abbreviations used:
- FA
focal adhesion
- MAPK
mitogen-activated protein kinase
- NES
nuclear export sequence
- SF
stress fiber
- SFTI
stress fiber thickness index
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-12-1057) on March 28, 2012.
REFERENCES
- Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci. 1985;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- Beckerle MC. Identification of a new protein localized at sites of cell-substrate adhesion. J Cell Biol. 1986;103:1679–1687. doi: 10.1083/jcb.103.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- Byers HR, White GE, Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. A review. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Call GS, Chung JY, Davis JA, Price BD, Primavera TS, Thomson NC, Wagner MV, Hansen MD. Zyxin phosphorylation at serine 142 modulates the zyxin head-tail interaction to alter cell-cell adhesion. Biochem Biophys Res Commun. 2011;404:780–784. doi: 10.1016/j.bbrc.2010.12.058. [DOI] [PubMed] [Google Scholar]
- Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cohen TS, Gray Lawrence G, Khasgiwala A, Margulies SS. MAPK activation modulates permeability of isolated rat alveolar epithelial cell monolayers following cyclic stretch. PLoS One. 2010;5:e10385. doi: 10.1371/journal.pone.0010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, Stelzer EH. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci. 2009;122:1665–1679. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- Cramer LP. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr Biol. 1999;9:1095–1105. doi: 10.1016/s0960-9822(99)80478-3. [DOI] [PubMed] [Google Scholar]
- Crawford AW, Beckerle MC. Purification and characterization of zyxin, an 82,000-dalton component of adherens junctions. J Biol Chem. 1991;266:5847–5853. [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin PB. Mechanical properties of arteries. Physiol Rev. 1978;58:397–460. doi: 10.1152/physrev.1978.58.2.397. [DOI] [PubMed] [Google Scholar]
- Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000;275:22503–22511. doi: 10.1074/jbc.M001698200. [DOI] [PubMed] [Google Scholar]
- Drees BE, Andrews KM, Beckerle MC. Molecular dissection of zyxin function reveals its involvement in cell motility. J Cell Biol. 1999;147:1549–1560. doi: 10.1083/jcb.147.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldib M, Dean DA. Cyclic stretch of alveolar epithelial cells alters cytoskeletal micromechanics. Biotechnol Bioeng. 2011;108:446–453. doi: 10.1002/bit.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker's guide to mechanobiology. Dev Cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust U, Hampe N, Rubner W, Kirchgessner N, Safran S, Hoffmann B, Merkel R. Cyclic stress at mHz frequencies aligns fibroblasts in direction of zero strain. PLoS One. 2011;6:e28963. doi: 10.1371/journal.pone.0028963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Geiger RC, Taylor W, Glucksberg MR, Dean DA. Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther. 2006;13:725–731. doi: 10.1038/sj.gt.3302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn RM, Beckerle MC, Koay T, Friederich E. Structural and functional similarities between the human cytoskeletal protein zyxin and the ActA protein of Listeria monocytogenes. J Cell Sci. 1997;110(Pt 16):1893–1906. doi: 10.1242/jcs.110.16.1893. [DOI] [PubMed] [Google Scholar]
- Grunewald TG, Kammerer U, Winkler C, Schindler D, Sickmann A, Honig A, Butt E. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer. 2007;96:296–305. doi: 10.1038/sj.bjc.6603545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011a;21:718–726. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011b;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WH, Wang YL. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol Biol Cell. 2007;18:4519–4527. doi: 10.1091/mbc.E07-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervy M, Hoffman LM, Jensen CC, Smith MA, Beckerle MC. The LIM protein Zyxin binds CARP-1 and promotes apoptosis. Genes Cancer. 2010;1:506–515. doi: 10.1177/1947601910376192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Jensen CC, Kloeker S, Wang CL, Yoshigi M, Beckerle MC. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J Cell Biol. 2006;172:771–782. doi: 10.1083/jcb.200512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LM, et al. Targeted disruption of the murine zyxin gene. Mol Cell Biol. 2003;23:70–79. doi: 10.1128/MCB.23.1.70-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- Hoshimaru M, Ray J, Sah DW, Gage FH. Differentiation of the immortalized adult neuronal progenitor cell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. Proc Natl Acad Sci USA. 1996;93:1518–1523. doi: 10.1073/pnas.93.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jungbauer S, Gao H, Spatz JP, Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J. 2008;95:3470–3478. doi: 10.1529/biophysj.107.128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA. 2005;102:15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem. 2001;276:36575–36585. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- Kito H, Chen EL, Wang X, Ikeda M, Azuma N, Nakajima N, Gahtan V, Sumpio BE. Role of mitogen-activated protein kinases in pulmonary endothelial cells exposed to cyclic strain. J Appl Physiol. 2000;89:2391–2400. doi: 10.1152/jappl.2000.89.6.2391. [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- Lee CF, Haase C, Deguchi S, Kaunas R. Cyclic stretch-induced stress fiber dynamics—dependence on strain rate, Rho-kinase and MLCK. Biochem Biophys Res Commun. 2010;401:344–349. doi: 10.1016/j.bbrc.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Lee E, Shelden EA, Knecht DA. Formation of F-actin aggregates in cells treated with actin stabilizing drugs. Cell Motil Cytoskeleton. 1998;39:122–133. doi: 10.1002/(SICI)1097-0169(1998)39:2<122::AID-CM3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- Li B, Trueb B. Analysis of the alpha-actinin/zyxin interaction. J Biol Chem. 2001;276:33328–33335. doi: 10.1074/jbc.M100789200. [DOI] [PubMed] [Google Scholar]
- Li B, Zhuang L, Trueb B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J Biol Chem. 2004;279:20401–20410. doi: 10.1074/jbc.M310304200. [DOI] [PubMed] [Google Scholar]
- Machner MP, Urbanke C, Barzik M, Otten S, Sechi AS, Wehland J, Heinz DW. ActA from Listeria monocytogenes can interact with up to four Ena/VASP homology 1 domains simultaneously. J Biol Chem. 2001;276:40096–40103. doi: 10.1074/jbc.M104279200. [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Tsuji T, Higashida C, Hertzog M, Fujita A, Narumiya S, Scita G, Watanabe N. Actin turnover-dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing. J Cell Biol. 2006;175:947–955. doi: 10.1083/jcb.200604176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momberger TS, Levick JR, Mason RM. Hyaluronan secretion by synoviocytes is mechanosensitive. Matrix Biol. 2005;24:510–519. doi: 10.1016/j.matbio.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody JD, Grange J, Ascione MP, Boothe D, Bushnell E, Hansen MD. A zyxin head-tail interaction regulates zyxin-VASP complex formation. Biochem Biophys Res Commun. 2009;378:625–628. doi: 10.1016/j.bbrc.2008.11.100. [DOI] [PubMed] [Google Scholar]
- Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231:446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TN, Uemura A, Shih W, Yamada S. Zyxin-mediated actin assembly is required for efficient wound closure. J Biol Chem. 2010;285:35439–35445. doi: 10.1074/jbc.M110.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl UD, Walter U, Gertler FB, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix DA, Beckerle MC. Nuclear-cytoplasmic shuttling of the focal contact protein, zyxin: a potential mechanism for communication between sites of cell adhesion and the nucleus. J Cell Biol. 1997;138:1139–1147. doi: 10.1083/jcb.138.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix DA, Fradelizi J, Bockholt S, Menichi B, Louvard D, Friederich E, Beckerle MC. Targeting of zyxin to sites of actin membrane interaction and to the nucleus. J Biol Chem. 2001;276:34759–34767. doi: 10.1074/jbc.M102820200. [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton. 2004;58:104–111. doi: 10.1002/cm.20007. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Schneider G, Burridge K, Lim SS. Immunodetection of alpha-actinin in focal adhesions is limited by antibody inaccessibility. Exp Cell Res. 1995;217:534–540. doi: 10.1006/excr.1995.1119. [DOI] [PubMed] [Google Scholar]
- Perez-Alvarado GC, Miles C, Michelsen JW, Louis HA, Winge DR, Beckerle MC, Summers MF. Structure of the carboxy-terminal LIM domain from the cysteine rich protein CRP. Nat Struct Biol. 1994;1:388–398. doi: 10.1038/nsb0694-388. [DOI] [PubMed] [Google Scholar]
- Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purich DL, Southwick FS. ABM-1 and ABM-2 homology sequences: consensus docking sites for actin-based motility defined by oligoproline regions in Listeria ActA surface protein and human VASP. Biochem Biophys Res Commun. 1997;231:686–691. doi: 10.1006/bbrc.1997.6158. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Zumbrunn J, Jaquemar D, Kuhn M, Walter U, Trueb B. An alpha-actinin binding site of zyxin is essential for subcellular zyxin localization and alpha-actinin recruitment. J Biol Chem. 1999;274:13410–13418. doi: 10.1074/jbc.274.19.13410. [DOI] [PubMed] [Google Scholar]
- Renfranz PJ, Beckerle MC. Doing (F/L)PPPPs: EVH1 domains and their proline-rich partners in cell polarity and migration. Curr Opin Cell Biol. 2002;14:88–103. doi: 10.1016/s0955-0674(01)00299-x. [DOI] [PubMed] [Google Scholar]
- Renfranz PJ, Siegrist SE, Stronach BE, Macalma T, Beckerle MC. Molecular and phylogenetic characterization of Zyx102, a Drosophila orthologue of the zyxin family that interacts with Drosophila Enabled. Gene. 2003;305:13–26. doi: 10.1016/s0378-1119(02)01173-3. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Sawada Y, et al. Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MAP kinase. J Cell Sci. 2001;114:1221–1227. doi: 10.1242/jcs.114.6.1221. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Sheetz MP. Force transduction by Triton cytoskeletons. J Cell Biol. 2002;156:609–615. doi: 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki SG, Godman GC. On the differential cytotoxicity of actinomycin D. J Cell Biol. 1971;50:746–761. doi: 10.1083/jcb.50.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel KL, Beckerle MC. The LIM domain is a modular protein-binding interface. Cell. 1994;79:211–219. doi: 10.1016/0092-8674(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel NM, Eljouni J, Nalaskowski MM, Hampe W. Nuclear localization of enhanced green fluorescent protein homomultimers. Anal Biochem. 2007;368:95–99. doi: 10.1016/j.ab.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Smith MA, Blankman E, Gardel ML, Luettjohann L, Waterman CM, Beckerle MC. A zyxin-mediated mechanism for actin stress fiber maintenance and repair. Dev Cell. 2010;19:365–376. doi: 10.1016/j.devcel.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE. 2004;2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- Sun R, Chen X, Yang VW. Intestinal-enriched Kruppel-like factor (Kruppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–6900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell CL, Singer RH. Actin mRNA localizes in the absence of protein synthesis. J Cell Biol. 1990;111:2397–2403. doi: 10.1083/jcb.111.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Tamura K, et al. Molecular mechanism of fibronectin gene activation by cyclic stretch in vascular smooth muscle cells. J Biol Chem. 2000;275:34619–34627. doi: 10.1074/jbc.M004421200. [DOI] [PubMed] [Google Scholar]
- Uemura A, Nguyen TN, Steele AN, Yamada S. The LIM domain of zyxin is sufficient for force-induced accumulation of zyxin during cell migration. Biophys J. 2011;101:1069–1075. doi: 10.1016/j.bpj.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Wille JJ, Ambrosi CM, Yin FC. Comparison of the effects of cyclic stretching and compression on endothelial cell morphological responses. J Biomech Eng. 2004;126:545–551. doi: 10.1115/1.1798053. [DOI] [PubMed] [Google Scholar]
- Wojtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res. 2010;107:898–902. doi: 10.1161/CIRCRESAHA.110.227850. [DOI] [PubMed] [Google Scholar]
- Wong AJ, Pollard TD, Herman IM. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983;219:867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]
- Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- Yoshigi M, Clark EB, Yost HJ. Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytometry A. 2003;55:109–118. doi: 10.1002/cyto.a.10076. [DOI] [PubMed] [Google Scholar]
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.