Abstract
Trait-based approaches have long been a feature of physiology and of ecology. While the latter fields drifted apart in the twentieth century, they are converging owing at least partly to growing similarities in their trait-based approaches, which have much to offer conservation biology. The convergence of spatially explicit approaches to understanding trait variation and its ecological implications, such as encapsulated in community assembly and macrophysiology, provides a significant illustration of the similarity of these areas. Both adopt trait-based informatics approaches which are not only providing fundamental biological insights, but are also delivering new information on how environmental change is affecting diversity and how such change may perhaps be mitigated. Such trait-based conservation physiology is illustrated here for each of the major environmental change drivers, specifically: the consequences of overexploitation for body size and physiological variation; the impacts of vegetation change on thermal safety margins; the consequences of changing net primary productivity and human use thereof for physiological variation and ecosystem functioning; the impacts of rising temperatures on water loss in ectotherms; how hemisphere-related variation in traits may affect responses to changing rainfall regimes and pollution; and how trait-based approaches may enable interactions between climate change and biological invasions to be elucidated.
Keywords: climate change, drought, evolutionary physiology, extreme events, latitudinal gradients, pollution
1. Introduction
The investigation of traits lies at the heart of physiology. Although no physiologist imagines that traits can be considered independent of the organisms in which they are manifest, the complexity of physiological investigations and the dictates of experimental biology mean that traits typically form the foundation of investigations [1–4]. This trait-based focus extends to evolutionary physiology and to macrophysiology, which seek to explore the evolution of physiological variation and its ecological implications over a range of spatial and temporal scales [5,6]. It is also entirely characteristic of mechanistic physiology, which includes investigations of the genetic and biochemical foundations of physiological traits [7,8]. In consequence, a trait-based approach is typically neither considered unusual, nor controversial in physiology. Moreover, it is widely appreciated that to understand how an organism functions in a given setting, and how variation in that functioning may affect birth and death rates, so ultimately determining fitness, various trait-based approaches must be integrated [2,9].
Perhaps reflecting both the origin of ecology as a sub-discipline of physiology [3] and their subsequent partial separation, trait-based approaches have, likewise, long been a feature of ecology [10–12], but a more controversial one. Recent calls for a further focus on trait-based approaches [13], and contemporaneous discussions of how such approaches need better integration [14,15], but may still fail to live up to expectations that have been built around them [16], illustrate the point. Nonetheless, considerable attention is now being given to trait-based approaches in ecology. Several areas stand out in this regard. In no particular order these are functional diversity, its measurement and demographic implications [12]; trait-based community ecology [17,18]; community phylogenetics [19,20]; and phylogenetic niche conservatism [21]: all of which are variously concerned with trait variation and its implications.
Although the emphasis of physiology and ecology usually lies either at different levels in the biological hierarchy or on different traits, the latter often simply reflects either interest or data availability [8]. In consequence, it is obvious that much scope exists for integration of the ecological and physiological trait-based approaches, especially to inform conservation biology. At times such integration is almost a matter of routine (e.g. the integration of metabolic rate data into the Pantheria database of life-history traits [22,23]). However, much insight into the mechanisms underlying variation in biodiversity may be gained by taking a more formal approach to such integration. This could be done in several ways, but a useful illustration thereof can be provided by considering the trait-based theoretical framework for understanding community structure and function proposed by Webb et al. [14].
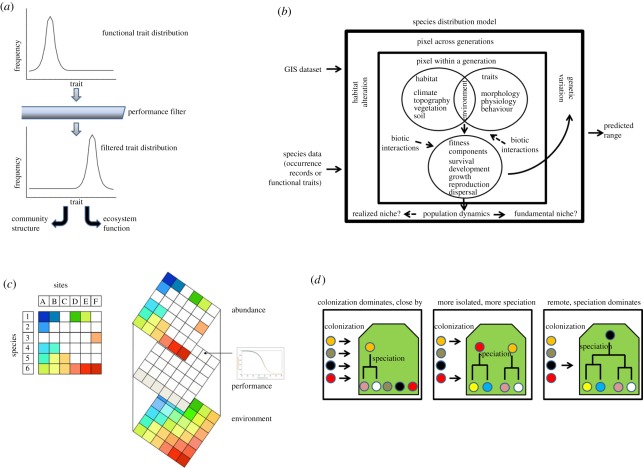
Their framework comprises an underlying trait distribution (from the regional pool of individuals), a performance filter which relates to sorting of individuals and natural selection in a given environmental context, and subsequent community structure and function. Spatial or temporal variation in the environmental context may then lead to variation in community structure and ecosystem functioning. The performance filter is considered as the relationship between the environmental context and performance (e.g. water-efficient organisms perform better in an arid setting). In the context of mechanistic and environmental niche modelling, the effects of the filter are equivalent to those described by the form of species response curves to indirect, direct and resource environmental variables, which result in variation through space in abundance and distribution [24,25]. Likewise, the effects of the performance filter are directly comparable with the entry, exit and transformation rules described for macrophysiology [3]. In this case, the entry rules describe how the performance filter might prevent or allow access to a new assemblage (through differential probabilities of dispersal, establishment, growth and spread, not co-incidentally also representing the stages typical of biological invasion models [26]), or enable access through speciation. The exit rules consider extinction, which might take place because of a change in the environmental context, and the transformation rules describe the responses by organisms, either within generations (usually in the form of phenotypic plasticity [27]) or across generations, to the environmental context or changes therein. How fast such transformations might take place and what that means for community structure and function is well explored in a context that is being recognized as closely allied to trait-based community ecology [28]. The similarity thereof to community phylogenetics [19] can be readily discerned (figure 1).
Figure 1.
Various theoretical representations of the relationship between the environment, some form of trait or species source pool and the final set of traits or abundances. (a) The relationship between trait distributions and performance filters used by Webb et al. [14]. (b) The approach taken by Kearney & Porter [29] to illustrate the relationship between species distribution models (a statistical description of what goes on inside the black square), and mechanistic niche modelling, which is explicit about the internal processes. (c) The macrophysiological approach described by Chown et al. [30]. (d) The relationship between isolation and time and how this may influence community assembly and phylogenetic relationships among species. The different coloured dots represent different species, and the coloured island the filter to colonization of such an isolated area (adapted from Emerson & Gillespie [19]).
These approaches are concerned with environment–assemblage relationships [18] and are thus focussed on what might be considered one of three levels that can be investigated when examining spatial variation in trait diversity—intraspecific, interspecific and assemblage [31]. In a spatially explicit context, the relationship between the three levels can be readily comprehended [30] (table 1). Comparative physiology is most usually undertaken at the intraspecific and interspecific levels, with assemblage-level analyses being much less common [3]. By contrast, trait-based ecological investigations focus on the assemblage level [18] and how variation among individuals and the contributing species pool might result in assemblage level characteristics. Other areas of ecological endeavour also adopt a similar, trait-based approach, but mostly at the intraspecific level to understand environmental variation in abundance and its dynamics. Most recognizable in the former case is mechanistic niche modelling [29], while spatially explicit population viability analyses or individual-based animal population models are characteristic of the latter [33,34]. The spatially explicit approach may also take several other forms [35,36], though all readily familiar as trait-based approaches that seek to understand some composite measure of the assemblage (e.g. abundance or species richness). Indeed, in at least some instances, the relationships between these approaches are simply taken as given, to assess the extent to which particular modelling approaches may provide insights into community composition or changes in species distributions through time [24,37]. These various developments clearly underline a growing recognition of the significance of trait–environment relationships in all aspects of biology, including applied areas such as the origins of wine chemodiversity [38]. Much scope exists for their integration, especially across the physiological and ecological arenas (figure 1).
Table 1.
A matrix of species (i) by sites (j) indicating how physiological variables may be included in such a matrix and can provide insight into intraspecific, interspecific and assemblage-level variation [30]. The variable is critical thermal minimum (CTmin) [32]. The italicized variables indicate intraspecific variation across space. Interspecific variation (entries in bold) is calculated as a mean value for the species at the centre of their latitudinal range, while assemblage characteristics are the mean (bold-italic) and the variance of a trait across all species at a given site.
| species by sites matrix for critical thermal minimum (°C) |
interspecific variation |
|||||
|---|---|---|---|---|---|---|
| i sites/j species | site A (40° S) | site B (38° S) | site C (35° S) | site D (30° S) | mean CTmin | mean latitude (° S) |
| species 1 | 4 | 5 | 6 | 8 | 5.8 | 35.8 |
| species 2 | 3 | 4 | 7 | 4.7 | 37.7 | |
| species 3 | 3 | 4 | 3.5 | 39.0 | ||
| species 4 | 2 | 2.0 | 40.0 | |||
| mean assemblage value | 3 | 4.3 | 6.5 | 8 | ||
| assemblage variance | 0.7 | 0.3 | ||||
At least from the physiological perspective, a key feature of discussions of trait-based, spatially explicit approaches is the claim that they will not only improve understanding of current ecological patterns, but will also facilitate the development of ecological forecasting, especially of the responses of species and ecosystems to environmental change [25,39–41]. While the major drivers of environmental change are now reasonably well understood (habitat alteration, overexploitation, climate change, pollution, species introductions) [42], forecasting the biodiversity outcomes thereof, and especially of interactions among these drivers, remains among biology's greatest challenges [25,43–45]. In consequence, the main focus of this review is to show that this claim is largely being borne out, and a consequence of doing so is the development of science that is of considerable significance in conservation policy. Importantly, this significance does not only flow from evidence that is both robust and widely agreed, to use the IPCC language [46]. Rather, it also emerges from the identification of findings where evidence is more limited and where agreement remains low. The approach taken here will be to examine, from a trait-based perspective, the vulnerability of diversity (sensu [45]) to the major environmental change drivers, with the predominant focus being on sensitivity and to a lesser extent adaptive capacity.
2. Overexploitation
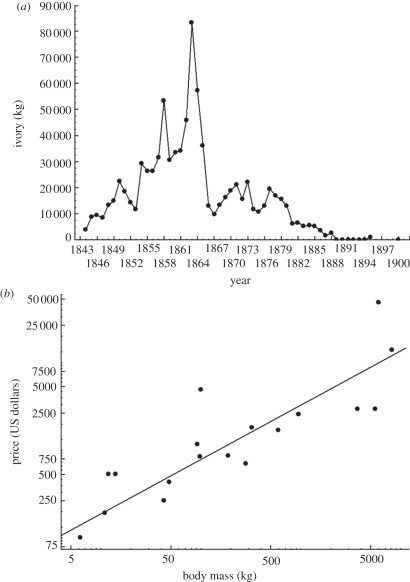
Human overexploitation of biodiversity is now widely appreciated, although the significant consequences of the reduction of populations of once common species perhaps less so [47]. Nonetheless, it is clear that exploitation can be rapid (figure 2), and that larger bodied species and larger individuals of those species are often singled out. One recent survey of the outcomes of human predation revealed that of the 297 cases examined, declines in size attributes of on average 18 per cent had occurred in 95 per cent of them, and at rates typically much faster than under circumstances of natural change [50]. The selection of larger individuals and species is widely recognized both in terrestrial systems, such as in the case of hunting and specimen collection, where large trophies or individuals are preferred or are more valuable [49,51,52] (figure 2), and in aquatic systems, notably fisheries, where larger individuals and species tend to have shown the most substantial declines associated with human interventions [53–55]. More generally, the phenomenon is known as trophic skew [56]. Moreover, although the relationship between extinction probability and size is complicated by several factors, including previous size-based extinction filters [57–59] and human assessments of species' ecology [60], and a positive relationship not always found [61], on average, an association between body size and extinction probability is typical of a wide range of groups [62]. Thus, large-bodied species and individuals not only have been differentially affected by extinction, but are also, in several instances, most prone to on-going threat. Through ecological time, such relationships between size and extinction probability may have been driven by underlying relationships between size and abundance, and size and intrinsic rates of increase [63], but additional pressures are now clearly being brought to bear that are not typical of circumstances prior to the evolution of organized human society [50].
Figure 2.
(a) The trade in ivory through Durban harbour (South Africa) over time in the 1800s. Note how rapidly the supply from across the eastern parts of the sub-continent was diminished (redrawn from data in McCraken [48]). (b) The relationship between body mass and US dollar prices for various African mammal families (redrawn from Johnson et al. [49]).
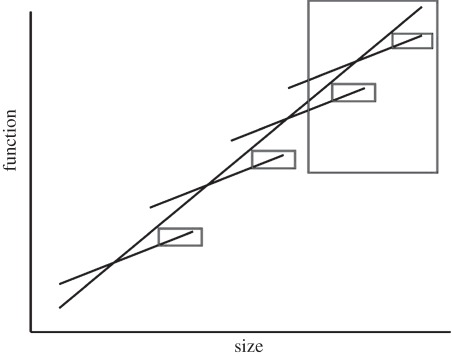
From a conservation physiology perspective, the implications are straightforward, and have been called the ‘erosion of large areas of phenotypic phase space’ [25]. That is, because so many traits are related to size [64], the removal of large individuals and species means the loss of a wide variety of physiological features or portions of particular trait distributions (figure 3). The food web and ecosystem functioning consequences of size and size loss are widely appreciated [25,56,65], but other features may also be affected. These include the loss of particular physiological functions associated with large size, such as regional heterothermy in fish [25], as well as significant characteristics such as stress resistance. The latter case may apply across a wide range of organisms because, typically, storage compounds scale isometrically with body mass (i.e. m1.0) [64,66], while metabolic rates and water loss rates tend to scale allometrically (m0.67–1.0) [67,68]. Large individuals and species are, in consequence, frequently better able to overcome stressful periods than smaller ones, which may explain at least some patterns in size variation through space [69]. Should global climate change-related forecasts of increases in rainfall variability, including, for example, more prolonged drought periods, in many areas be borne out, as seems likely [70], populations may be more prone to extinction than was the case previously, resulting in food web changes that might not have been expected based on assessments of abundances of significant species alone.
Figure 3.
The removal of large-bodied individuals and large-bodied species (indicated by the red rectangles) will eventually lead to the removal of several forms of physiological function owing to the strong relationships between size and function. This may be the case even if relationships are significantly negative, but in the case of no relationship the effect may not be realized.
3. Habitat alteration
Habitat alteration is one of the most significant anthropogenic factors affecting biodiversity [42]. Much attention is rightly focussed on forest loss, but habitat alteration may also be a consequence of biological invasions, such as by trees [71], or trophic cascades as a consequence of species removal [72]. Many of the effects thereof are direct, as a result of changes to food availability and the structure of the environment. A noteworthy example of the latter is the effect of invasive alien vegetation on sex determination in crocodiles [73].
However, substantial impacts as a consequence of changes to climate and microclimate, mediated through specific traits, may also be realized. For example, one of the major challenges facing ectotherms in subtropical and tropical environments is keeping their body temperatures below those that may prove sublethal or lethal [74]. This is largely done through behavioural thermoregulation. Thus, changes in vegetation cover, such as through deforestation, will substantially alter the ability of ectotherms to thermoregulate, especially under circumstances of increasing global temperatures [74]. However, because climate change includes changes in precipitation, and is being accompanied by on-going deforestation, matters may be substantially more complicated, as has been shown in a recent analysis of thermal performance traits in squamate reptiles [75]. Preferred body temperatures (which are often close to optimal temperatures) are most closely (and negatively) related to precipitation rather than to environmental temperature variables. Thus, if tropical climate change includes an increase in cloud cover and precipitation, as has been reported for several areas by the IPCC [76], these animals may suffer much less of an impact than previously thought, especially if their thermal safety margins are not quite as narrow as previously predicted [75]. By contrast, if drying affects forest cover (as detected through productivity, for example) [77,78], and if subtropical regions, where thermal safety margins are already narrow, are most significantly affected by such reductions in cloud cover and precipitation, risks may be elevated [75]. Substantial conversion of subtropical savannah and other subtropical habitats is also taking place [79,80], making vegetation cover less readily available. In combination, these factors could mean considerable future extinction risk for squamate reptiles in these regions. Climate change-related risk to subtropical terrestrial reptiles has been forecast using a different, though related, approach and to some extent borne out through empirical surveys of lizard population extinctions [81]. However, the complexities of variation in available environmental (operative) temperatures might also temper the global extent of the risk [82]. Likewise, substantial differences in drought and net primary productivity (NPP) between the Northern and Southern Hemispheres [83] may further complicate matters. What these trait-based approaches highlight is that interactions between habitat alteration and climate change, including direct effects of vegetation change on precipitation, are likely to make forecasts of extinction probabilities much more complex than they first appear. Thus, while the evidence for extinctions and their on-going likelihood is robust, agreement on how this will play out spatially cannot yet be considered high, emphasizing the significance of mitigation strategies both for climate change and habitat alteration [75,79].
Humans are also markedly affecting a further emergent property of habitats—their primary productivity. Determining just how much of an impact changes in climate are having on NPP or net ecosystem productivity and whether the trends are significant is not straightforward, making the field controversial [77,78,83] (see also responses to some of these works). One contentious study suggests that NPP has declined significantly over the last decade in the Southern Hemisphere, but increased over the Northern Hemisphere [83], while an earlier work recorded an increasing trend between 1982 and 1999 [84]. Moreover, humans appropriate approximately 24 per cent of terrestrial NPP [85]. From a conservation physiology perspective, the implication is that a range of traits may be affected in unpredictable ways, largely through effects on body size. A recent synthesis of the factors underlying spatial and temporal body size variation has suggested that eNPP, or ecologically and evolutionarily relevant NPP, has a significant positive effect on body size, resulting in most of the patterns seen globally [86]. If this turns out to be the case, and humans alter NPP (and accordingly eNPP) profoundly, then responses through variation in body size should be marked. Few studies have sought such an effect, with most investigations of size-related trends being interpreted in the context of changing temperatures [87,88]. Clearly, this is an area where the evidence is limited and agreement less than robust [89], but one that deserves further exploration.
4. Climate change
Trait-based approaches have featured conspicuously in explorations of the biodiversity impacts of climate change, largely through mechanistic niche modelling [29], but also by providing insights into how phenotypic plasticity will affect, and in some instances is affecting, species responses to change [32,44,90]. More recently, they have further come to prominence as a consequence of macrophysiological investigations. These informatics-type approaches have demonstrated limited thermal safety margins for ectotherms in the tropics and subtropics [75,91], a topic recently reviewed in depth [92]. Significantly, they have also shown relatively limited geographical variation in upper thermal limits [93,94], including much more limited phenotypic plasticity, evolutionary lability and heritability than is the case for lower thermal limits and some other physiological traits [32,41,95–97]. The consequences for biodiversity of this conservatism in upper thermal limits are clearly negative given the likelihood that most ecoregions will, by 2070, face monthly temperature averages more than two standard deviations from the 1960–1991 baseline, with tropical regions experiencing these conditions soonest [98]. Indeed, these consequences already seem to be playing themselves out [81]. Threats to tropical organisms are not only most pronounced for ectotherms. A recent, trait-based investigation for mammals suggested that tropical, small-bodied and specialist species are likewise at particular risk from changing climates [23].
Changing thermal regimes have rightly been the focus of much of this trait-based macrophysiology. However, much change in precipitation regimes is not only being forecast, but is also now being realized [76,78], and interactions with changing thermal regimes are affecting several components of terrestrial systems, such as evapotranspiration and NPP [77,83]. Many areas, especially in the Southern Hemisphere, seem to be under increasing water stress [83], at least given current trends, and many of them in any case are water-limited [99]. For ectotherms, this could further spell substantial potential difficulties under climate change. One of the first studies to investigate how such fitness costs might be realized used a standard environmental tolerance evolution model [100], indicating that organisms in tropical and northern high latitude areas would experience the most significant impacts of changes in precipitation. This outcome is, at least for the higher latitude regions, somewhat contrary to recent findings of substantial drought in the Southern Hemisphere [83], but nonetheless highlights the considerable differences between the hemispheres. It also emphasizes the need to consider the potential effects of changing water stress on small ectotherms.
One significant group of ectotherms that may be particularly prone to such effects is the insects, given their small size and susceptibility to desiccation [101]. Just how vulnerable they may be can readily be seen by considering not only the direct effects of changing precipitation regimes, but also the indirect effects of changing temperatures. The main avenues for water loss in insects are the cuticle and respiratory system. The former contributes most significantly [101], but respiratory water loss in insects, especially those exchanging gases discontinuously, may be higher, per unit gas exchanged, than for many other organisms, as a recent trait-based synthetic model has demonstrated [102]. Increasing cuticular rates of water loss with rising temperatures have been widely demonstrated in insects [103], with some studies showing that insects and other small ectotherms may alter their phenotypes to limit such loss [104,105]. High vapour pressure deficit likewise causes increases in water loss rate [103]. Respiratory water loss also rises with increasing temperatures, and some insects may likewise alter metabolic rates to restrict such loss [101,104].
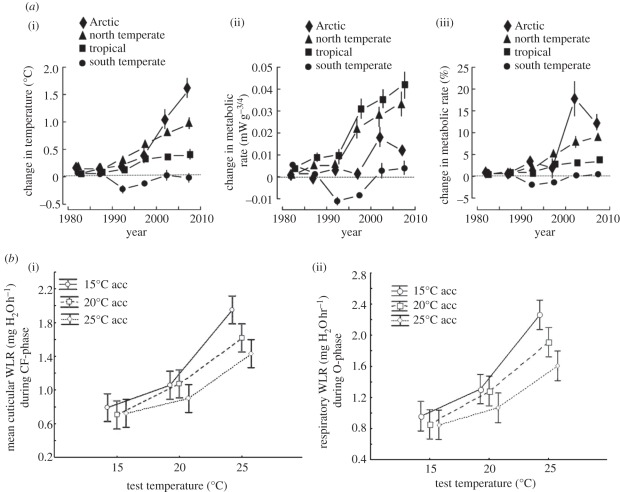
Global increases in temperature therefore have probably not only had the effect of increasing metabolic rates and therefore the overall costs of living [91], but through the associated respiratory water loss [102], and elevated cuticular water loss, have probably also increased water stress in many areas (figure 4). Increases in the frequency and severity of drought or declines in soil moisture are likely to have exacerbated this effect. The trait-based conservation physiology approach clearly indicates the theoretical potential for this effect. However, just as is the case for increased energetic costs [91], whether such an effect has been realized or whether phenotypic plasticity has been sufficient to offset it [106] is not known. What does appear certain, however, is that on average, the outcomes are likely to play out in very different ways across the hemispheres. That southern systems are typically more water-limited than northern ones, and may have experienced greater recent drought conditions is clear [77,78,98,99]. However, insects in these southern areas also tend to have much shallower metabolic rate–temperature relationships than their northern counterparts [107]. Thus, at least from a respiratory water loss perspective, Southern Hemisphere taxa may already be capable of coping with dry conditions to a greater extent than northern groups. In the past, this rate–temperature variation was interpreted as being indicative of some form of metabolic cold adaptation [108], although the latter has proven to be a controversial idea [109]. An interpretation of a shallow slope to effect further water conservation is not only in keeping with the idea of a somewhat profligate respiratory water economy in insects [102], but also is in keeping with empirical information suggesting that acclimation effects on the rate–temperature relationship have more to do with water savings than metabolic responses per se [104]. Moreover, it fits well with findings that mammalian metabolic rates in southern systems may also have evolved in response to variable precipitation regimes [110], making such an interpretation more general. For birds, a different response has been found [111]. However, variation in metabolic rates and the slope of the rate–temperature relationship are probably a consequence of responses both to environmental temperature and water regimes, making a single, simple explanation of such rate variation unlikely. Nonetheless, substantial variation in global change and in forecast species responses among the hemispheres bears out previous arguments for the ecological significance of consistent among-hemisphere variation in traits and at higher levels in the biological hierarchy [112,113]. Such variation deserves further exploration because it is manifest in a wide variety of traits and across many groups [112,114,115].
Figure 4.
(a) Global temperature changes and changes in ectotherm metabolic rates between 1980 and 2010. The panels indicate, in order, changes in temperature, mass-normalized metabolic rate and relative changes in mass-normalized metabolic rate, respectively. Adapted from Dillon et al. [91]. (b) Changes in cuticular (i) and respiratory (ii) water loss rates (WLRs) in the dung beetle Scarabaeus spretus at different temperatures and following different acclimation treatments. Redrawn from Terblanche et al. [104].
5. Pollution
One trait that is known to vary in consistent ways with global geography is clutch size in birds. Typically, clutch sizes are smaller (averaging ca 2.2–3.0) in the tropics and Southern Hemisphere than in the higher latitudes of the Northern Hemisphere (averaging 4.0–4.5), a consequence of greater seasonality in the north [114]. This hemisphere-related variation is found in other features of birds, such as a preponderance of cooperative breeders in the Southern Hemisphere, reflecting greater interannual environmental uncertainty [115], and a tendency for range sizes to be much larger on average in the Northern Hemisphere than elsewhere [116].
The implications of this variation more generally for conservation are only starting to be explored. Perhaps one of the most intriguing, though as yet poorly explored, consequences of clutch size variation is on the susceptibility of avian populations to organochlorine pollutants. Recent assessments of such pollutants in bird eggs in South Africa [117,118] have recognized that low clutch sizes and long lifespans may mean greater pollutant loads in fewer eggs and larger risks of eggshell thinning given similar environmental pollutant levels [117]. Although the data for such effects remain limited, they do suggest that novel, trait-based approaches in conservation physiology may reveal threats not previously recognized. An additional example drawn from the same research group is the threat that pollution by SO2 and from volatile organic compounds (VOCs) emitted by planted forestry trees may have on the blue swallow, a species listed as critically endangered in South Africa, and breeding in a localized area on the eastern escarpment [119,120]. The suggestion is that SO2 pollution from the west and locally emitted VOCs form sulphonates that have a detergent effect on the extremely water-repellent plumage of this swallow species adapted for flying in dense fog. During heavy fog years, the swallows are thought to have a lower flight capability and reduced breeding success. While firm, direct evidence for these effects is lacking [119], this example is a striking one of how conservation physiology focused on particular traits, in this case plumage water repellence, may provide testable hypotheses to account for conservation threats.
A further example of how trait-based studies may inform understanding of conservation threats, and actions to mitigate them, concerns variation in heavy-metal tolerance among indigenous and invasive marine bryozoans in Australia [121]. Comparison of Cu tolerance in laboratory assays revealed marked post-exposure recovery of growth in the invasive species compared with reduced feeding efficiency and reduced growth of the indigenous species, a pattern also seen in field trials. These results bear out a combination of survey and field-trial data from New South Wales showing that increasing heavy metal pollution leads to a decline in indigenous species diversity, and an increase in the richness and dominance of non-indigenous species [122]. Harbours are not only notoriously polluted environments, but also serve as the point of origin of ballast water and hull fouling [123,124]. If pollution serves to favour species that are globally invasive, this may promote the invasion of other areas via shipping traffic. While ballast water agreements to mitigate invasion risks are in place, dealing with hull fouling is a much more complicated matter [125,126].
Pollution not only takes the form of contamination by heavy metals and organic pollutants, but also involves the deposition of elements such as nitrogen [127]. Although the impact of increasing carbon has received much attention from the perspective of ocean acidification, it may also be having a substantial effect on terrestrial systems as a consequence of physiological differences between C3 trees and C4 grasses, changing the balance between shrublands (thickets) and grasslands [128,129]. The idea, based on a carbon-allocation physiological mechanism, and supported by laboratory data [128,130], is that elevated CO2 levels promote C3 tree growth, enabling the trees to escape the fire trap created by flammable C4 grasses. Thus, increasing CO2 concentrations should be leading to an increase in shrublands irrespective of land-management practices. This is indeed what is being seen in parts of southern Africa [131], although the interaction with rainfall still remains an area that needs to be more fully explored (see also [132,133]). Nonetheless, a physiological model of carbon allocation, coupled with laboratory experiments and field data, has demonstrated that rising CO2 levels may have a significant impact on an important, and under-conserved biome in southern Africa, and on similar systems elsewhere. Shrub encroachment effects on other aspects of diversity have been documented, illustrating a growing conservation threat [79,134].
6. Biological invasions
Trait-based approaches have long been a feature of attempts to determine just what makes a species likely to move through the range of steps [26] from being indigenous in one area to an invader with major impacts in another [135]. Although early studies tended to be somewhat equivocal, recent work has drawn attention to show how comparisons should be made to understand the significance of traits during the invasion process and to avoid introducing bias into the analyses [136], and has shown that trait differences do indeed exist between invasive and non-invasive species. For example, a recent meta-analysis of invasive plant species traits demonstrated that size, growth rate, shoot allocation, and aspects of physiology and fitness all contribute to invasiveness, though depending to some extent on the comparisons made [137]. Although it appears, therefore, that trait-based analyses would be helpful for assessing invasion risks (see also [138,139]), it has also been suggested that the traits identified in these analyses are common to species that do well generally in disturbed and/or nutrient-rich environments [140]. Although this view has been contested [141,142], and the argument is likely to continue given that trait-based approaches have always been contentious in the field [135], to some extent it is moot from a conservation physiology perspective. Identifying a suite of traits that might promote an understanding of colonization and of spread in any set of organisms is useful for forecasting impacts of changing environments and for mitigating risks of species introductions, and is similar to the identification of the influence of response and effect traits in understanding the ecological significance of functional diversity [143].
Understanding the consequences of variation in response traits is particularly significant for assessing the extent to which changing climates may alter the consequences for any system of non-indigenous species. Several suggestions have been made that non-indigenous species will benefit from climate change to a greater extent than will their indigenous counterparts [144–146]. Clearly, such a broad brush approach overlooks the fact that climate change will take place in very different ways in different parts of the world, and that interactions with other forms of disturbance will also be significant. Nonetheless, in some temperate environments, differences in the physiology of indigenous and non-indigenous species do indeed seem to be favouring the latter, including in ascidians as a consequence of faster growth rates under warm conditions in the non-indigenous species [147], and in springtails, as a consequence both of greater desiccation resistance under warm conditions in adults and faster egg growth rates in the non-indigenous species [105,148]. How general these effects are is not yet clear, but they certainly point to the value of trait-based approaches to understanding interactions between climate change and biological invasions.
7. Conclusions
Physiology is concerned with how organisms maintain function in the face of a changing environment, and thus, even if indirectly, how trait variation is related to fitness. That a physiological perspective would have much to offer conservation biology should consequently have been self-evident. However, this perspective has been slow to develop, although the pace is now quickening. A major contributor has been the realization that, despite the caveats associated with so doing [149], a macrophysiological approach can provide valuable insights into conservation problems, and indeed identify several that may have been missed. The limited scope for evolution of upper thermal limits in terrestrial ectotherms and the narrow safety margins for tropical and sub-tropical organisms are clear examples. In essence, much of this value has resulted from the application of informatics approaches to trait-based questions in physiology and conservation, in much the same way that the application of informatics approaches has led to the novel insights of macroecology [150]. While the power of informatics is widely recognized in the context of gene and metabolic function, its ability to provide fundamental insights in other areas is growing in appreciation [107,151]. Conservation is in a strong position to benefit significantly in the future from these developments. However, the realization of such benefits will depend fundamentally on the extent to which trait information is compiled, collected and made broadly available in ways similar to that adopted for genetic information. Considerable work on plants has revealed which traits might be most useful, and much progress has been made in compiling trait databases [152]. Similar progress is being made for some vertebrate groups [22], though how useful some of the traits might be more generally (as opposed to simply being available) is not yet clear. For invertebrates, less attention has been given to compiling trait databases, although the significance of doing so is appreciated and in some cases has commenced (e.g. for freshwater taxa [153,154]). For terrestrial taxa, compiling a set of databases does beg the question of what traits might be significant, especially given that few traits are routinely investigated for the same taxa [155]. At least for insects, it would seem that mass, critical thermal minimum and maximum, thermal optimum, preferred temperature and survival of desiccation would be a good place to start given that these variables influence assemblage membership in a host of groups [32,94,156,157]. Irrespective, even traits which are routinely measured, such as mass, are rarely compiled in publicly accessible databases [69]. Conservation physiology [158] provides important reasons and impetus for doing so.
Acknowledgements
I thank Craig Franklin for the invitation to contribute to the broader discussion of conservation physiology, Melodie McGeoch and an anonymous reviewer for useful comments on a previous version of this manuscript, and the South African National Research Foundation (Incentive Fund) and HOPE Project of Stellenbosch University for support.
References
- 1.Prosser C. L. 1986. Adaptational biology. Molecules to organisms. New York, NY: Wiley [Google Scholar]
- 2.McNab B. K. 2002. The physiological ecology of vertebrates. A view from energetics . Ithaca, NY: Cornell University Press [Google Scholar]
- 3.Gaston K. J., et al. 2009. Macrophysiology: a conceptual reunification. Am. Nat. 174, 595–612 10.1086/605982 (doi:10.1086/605982) [DOI] [PubMed] [Google Scholar]
- 4.Terblanche J. S., Hoffmann A. A., Mitchell K. A., Rako L., Le Roux P. C., Chown S. L. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725 10.1242/jeb.061283 (doi:10.1242/jeb.061283) [DOI] [PubMed] [Google Scholar]
- 5.Feder M. E., Bennett A. F., Huey R. B. 2000. Evolutionary physiology. Annu. Rev. Ecol. Syst. 31, 315–341 10.1146/annurev.ecolsys.31.1.315 (doi:10.1146/annurev.ecolsys.31.1.315) [DOI] [Google Scholar]
- 6.Chown S. L., Gaston K. J., Robinson D. 2004. Macrophysiology: large-scale patterns in physiological traits and their ecological implications. Funct. Ecol. 18, 159–167 10.1111/j.0269-8463.2004.00825.x (doi:10.1111/j.0269-8463.2004.00825.x) [DOI] [Google Scholar]
- 7.Mangum C. P., Hochachka P. W. 1998. New directions in comparative physiology and biochemistry: mechanisms, adaptations, and evolution. Physiol. Zool. 71, 471–484 [DOI] [PubMed] [Google Scholar]
- 8.Chown S. L., Storey K. B. 2006. Linking molecular physiology to ecological realities. Physiol. Biochem. Zool. 79, 314–323 10.1086/499989 (doi:10.1086/499989) [DOI] [PubMed] [Google Scholar]
- 9.Chown S. L., Nicolson S. W. 2004. Insect physiological ecology. Mechanisms and patterns. Oxford, UK: Oxford University Press [Google Scholar]
- 10.Weiher E., Keddy P. A. 1995. Assembly rules, null models, and trait dispersion: new questions from old patterns. Oikos 74, 159–164 10.2307/3545686 (doi:10.2307/3545686) [DOI] [Google Scholar]
- 11.Messier J., McGill B. J., Lechowicz M. J. 2010. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 13, 838–848 10.1111/j.1461-0248.2010.01476.x (doi:10.1111/j.1461-0248.2010.01476.x) [DOI] [PubMed] [Google Scholar]
- 12.Weiher E. 2011. A primer of trait and functional diversity. In Biological diversity. Frontiers in measurement and assessment (eds Magurran A. E., McGill B. J.), pp. 175–193 Oxford, UK: Oxford University Press [Google Scholar]
- 13.McGill B. J., Enquist B. J., Weiher E., Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 10.1016/j.tree.2006.02.002 (doi:10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 14.Webb C. T., Hoeting J. A., Ames G. M., Pyne M. I., LeRoy Poff N. 2010. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol. Lett. 13, 267–283 10.1111/j.1461-0248.2010.01444.x (doi:10.1111/j.1461-0248.2010.01444.x) [DOI] [PubMed] [Google Scholar]
- 15.Kearney M., Simpson S. J., Raubenheimer D., Helmuth B. 2010. Modelling the ecological niche from functional traits. Phil. Trans. R. Soc. B 365, 3469–3483 10.1098/rstb.2010.0034 (doi:10.1098/rstb.2010.0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angert A. L., Crozier L. G., Rissler L. J., Gilman S. E., Tewksbury J. J., Chunco A. J. 2011. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689 10.1111/j.1461-0248.2011.01620.x (doi:10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 17.Ackerly D. D., Cornwell W. K. 2007. A trait-based approach to community assembly: partitioning of species trait values into within- and among-community components. Ecol. Lett. 10, 135–145 10.1111/j.1461-0248.2006.01006.x (doi:10.1111/j.1461-0248.2006.01006.x) [DOI] [PubMed] [Google Scholar]
- 18.Weiher E., Freund D., Bunton T., Stefanski A., Lee T., Bentivenga S. 2011. Advances, challenges and developing a synthesis of ecological community assembly theory. Phil. Trans. R. Soc. B 366, 2403–2413 10.1098/rstb.2011.0056 (doi:10.1098/rstb.2011.0056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emerson B. C., Gillespie R. 2008. Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol. Evol. 23, 619–630 10.1016/j.tree.2008.07.005 (doi:10.1016/j.tree.2008.07.005) [DOI] [PubMed] [Google Scholar]
- 20.Kembel S. W. 2009. Disentangling niche and neutral influences on community assembly: assessing the performance of community phylogenetic structure tests. Ecol. Lett. 12, 949–960 10.1111/j.1461-0248.2009.01354.x (doi:10.1111/j.1461-0248.2009.01354.x) [DOI] [PubMed] [Google Scholar]
- 21.Wiens J. J., Graham C. H. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539 10.1146/annurev.ecolsys.36.102803.095431 (doi:10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 22.Jones K. E., et al. 2009. PanTHERIA: a species-level database of life-history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648. 10.1890/08-1494.1 (doi:10.1890/08-1494.1) [DOI] [Google Scholar]
- 23.Cooper N., Freckleton R. P., Jetz W. 2011. Phylogenetic conservatism of environmental niches in mammals. Proc. R. Soc. B 278, 2384–2391 10.1098/rspb.2010.2207 (doi:10.1098/rspb.2010.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin M. 2007. Species distribution models and ecological theory: a critical assessment and some possible new approaches. Ecol. Model. 200, 1–19 10.1016/j.ecolmodel.2006.07.005 (doi:10.1016/j.ecolmodel.2006.07.005) [DOI] [Google Scholar]
- 25.Chown S. L., Gaston K. J. 2008. Macrophysiology for a changing world. Proc. R. Soc. B 275, 1469–1478 10.1098/rspb.2008.0137 (doi:10.1098/rspb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn T. M., Pyšek P., Bacher S., Carlton J. T., Duncan R. P., Jarošík V., Wilson J. R. U., Richardson D. M. 2011. A proposed unified framework for biological invasions. Trends Ecol. Evol. 26, 333–339 10.1016/j.tree.2011.03.023 (doi:10.1016/j.tree.2011.03.023) [DOI] [PubMed] [Google Scholar]
- 27.Scheiner S. M. 1993. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68 10.1146/annurev.es.24.110193.000343 (doi:10.1146/annurev.es.24.110193.000343) [DOI] [Google Scholar]
- 28.Ellner S. P., Geber M. A., Hairston N. G. 2011. Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603–614 10.1111/j.1461-0248.2011.01616.x (doi:10.1111/j.1461-0248.2011.01616.x) [DOI] [PubMed] [Google Scholar]
- 29.Kearney M., Porter M. 2009. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 12, 334–350 10.1111/j.1461-0248.2008.01277.x (doi:10.1111/j.1461-0248.2008.01277.x) [DOI] [PubMed] [Google Scholar]
- 30.Chown S. L., Gaston K. J., van Kleunen M., Clusella-Trullas S. 2010. Population responses within a landscape matrix: a macrophysiological approach to understanding climate change impacts. Evol. Ecol. 24, 601–616 10.1007/s10682-009-9329-x (doi:10.1007/s10682-009-9329-x) [DOI] [Google Scholar]
- 31.Gaston K. J., Chown S. L., Evans K. L. 2008. Ecogeographical rules: elements of a synthesis. J. Biogeogr. 35, 483–500 10.1111/j.1365-2699.2007.01772.x (doi:10.1111/j.1365-2699.2007.01772.x) [DOI] [Google Scholar]
- 32.Chown S. L., Terblanche J. S. 2007. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 33, 50–152 10.1016/S0065-2806(06)33002-0 (doi:10.1016/S0065-2806(06)33002-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll C. 2007. Interacting effects of climate change, landscape conversion, and harvest on carnivore populations at the range margin: Marten and Lynx in the northern Appalachians. Conserv. Biol. 21, 1092–1104 10.1111/j.1523-1739.2007.00719.x (doi:10.1111/j.1523-1739.2007.00719.x) [DOI] [PubMed] [Google Scholar]
- 34.McRae B. H., Schumaker N. H., McKane R. B., Busing R. T., Solomon A. M., Burdick C. A. 2008. A multi-model framework for simulating wildlife population response to land-use and climate change. Ecol. Model. 219, 77–91 10.1016/j.ecolmodel.2008.08.001 (doi:10.1016/j.ecolmodel.2008.08.001) [DOI] [Google Scholar]
- 35.Dray S., Legendre P. 2008. Testing the species traits–environment relationships: the fourth-corner problem revisited. Ecology 89, 3400–3412 10.1890/08-0349.1 (doi:10.1890/08-0349.1) [DOI] [PubMed] [Google Scholar]
- 36.Gotelli N. J., et al. 2009. Patterns and causes of species richness: a general simulation model for macroecology. Ecol. Lett. 12, 873–886 10.1111/j.1461-0248.2009.01353.x (doi:10.1111/j.1461-0248.2009.01353.x) [DOI] [PubMed] [Google Scholar]
- 37.Zurell D., Jeltsch F., Dormann C. F., Schröder B. 2009. Static species distribution models in dynamically changing systems: how good can predictions really be? Ecography 32, 733–744 10.1111/j.1600-0587.2009.05810.x (doi:10.1111/j.1600-0587.2009.05810.x) [DOI] [Google Scholar]
- 38.Gougoen R. D., et al. 2009. The chemodiversity of wines can reveal a metabologeography expression of cooperage oak wood. Proc. Natl Acad. Sci. USA 106, 9174–9179 10.1073/pnas.0901100106 (doi:10.1073/pnas.0901100106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tewksbury J. J., Huey R. B., Deutsch C. A. 2008. Ecology: putting the heat on tropical animals. Science 320, 1296–1297 10.1126/science.1159328 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 40.Pörtner H. O., Farrell A. P. 2008. Physiology and climate change. Science 322, 690–692 10.1126/science.1163156 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann A. A. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880 10.1242/jeb.037630 (doi:10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 42.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute [Google Scholar]
- 43.Brook B. W., Sodhi N. S., Bradshaw C. J. A. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 10.1016/j.tree.2008.03.011 (doi:10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 44.Chevin L. M., Lande R. L., Mace G. M. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson T. P., Jackson S. T., House J. I., Prentice I. C., Mace G. M. 2011. Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58 10.1126/science.1200303 (doi:10.1126/science.1200303) [DOI] [PubMed] [Google Scholar]
- 46.Mastrandrea M. D., et al. 2010. Guidance note for lead authors of the IPCC fifth assessment report on consistent treatment of uncertainties. Intergovernmental Panel on Climate Change (IPCC) See http://www.ipcc.ch [Google Scholar]
- 47.Gaston K. J. 2011. Common ecology. BioScience 61, 354–362 10.1525/bio.2011.61.5.4 (doi:10.1525/bio.2011.61.5.4) [DOI] [Google Scholar]
- 48.McCraken D. P. 2008. Saving the Zululand wilderness. An early struggle for conservation. Johannesburg, South Africa: Jacana [Google Scholar]
- 49.Johnson P. J., Kamsly R., Loveridge A. J., Macdonald D. W. 2010. Size, rarity and charisma: valuing African wildlife trophies. PLoS ONE 5, e12866. 10.1371/journal.pone.0012866 (doi:10.1371/journal.pone.0012866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darimont C. T., Carlson S. M., Kinnison M. T., Paquet P. C., Reimchen T. E., Wilmers C. C. 2009. Human predators outpace other agents of trait change in the wild. Proc. Natl Acad. Sci. USA 106, 952–954 10.1073/pnas.0809235106 (doi:10.1073/pnas.0809235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coltman D. W., O'Donoghue P., Jorgenson J. T., Hogg J. T., Strobeck C., Festa-Bianchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658 10.1038/nature02177 (doi:10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 52.Allendorf F. W., Hard J. J. 2009. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Natl Acad. Sci. USA 106, 9987–9994 10.1073/pnas.0901069106 (doi:10.1073/pnas.0901069106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olden J. D., Hogan Z. S., VanderZanden M. J. 2007. Small fish, big fish, red fish, blue fish; size-biased extinction risk of the world's freshwater and marine fishes. Glob. Ecol. Biogeogr. 16, 694–701 10.1111/j.1466-8238.2007.00337.x (doi:10.1111/j.1466-8238.2007.00337.x) [DOI] [Google Scholar]
- 54.Fisher J. A. D., Frank K. T., Leggett W. C. 2010. Breaking Bergmann's rule: truncation of Northwest Atlantic marine fish body sizes. Ecology 91, 2499–2505 10.1890/09-1914.1 (doi:10.1890/09-1914.1) [DOI] [PubMed] [Google Scholar]
- 55.Genner M. J., et al. 2010. Body size-dependent responses of a marine fish assemblage to climate change and fishing over a century-long scale. Glob. Change Biol. 16, 517–527 10.1111/j.1365-2486.2009.02027.x (doi:10.1111/j.1365-2486.2009.02027.x) [DOI] [Google Scholar]
- 56.Strong D. R., Frank K. T. 2010. Human involvement in food webs. Annu. Rev. Environ. Res. 35, 1–23 10.1146/annurev-environ-031809-133103 (doi:10.1146/annurev-environ-031809-133103) [DOI] [Google Scholar]
- 57.Meiri S. 2008. Evolution and ecology of lizard body sizes. Glob. Ecol. Biogeogr. 17, 724–734 10.1111/j.1466-8238.2008.00414.x (doi:10.1111/j.1466-8238.2008.00414.x) [DOI] [Google Scholar]
- 58.Davidson A. D., Hamilton M. J., Boyer A. G., Brown J. H., Ceballos G. 2009. Multiple ecological pathways to extinction in mammals. Proc. Natl Acad. Sci. USA 106, 10 702–10 705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee T., Jetz W. 2011. Unravelling the structure of species extinction risk for predictive conservation science. Proc. R. Soc. B 278, 1329–1338 10.1098/rspb.2010.1877 (doi:10.1098/rspb.2010.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinsky M. L., Jensen O. P., Ricard D., Palumbi S. R. 2011. Unexpected patterns of fisheries collapse in the world's oceans. Proc. Natl Acad. Sci. USA 108, 8317–8322 10.1073/pnas.1015313108 (doi:10.1073/pnas.1015313108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harnick P. G. 2011. Direct and indirect effects of biological factors on extinction risk in fossil bivalves. Proc. Natl Acad. Sci. USA 108, 13 594–13 599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaston K. J. 2006. Biodiversity and extinction: macroecological patterns and people. Prog. Phys. Geog. 30, 258–269 10.1191/0309133306pp483pr (doi:10.1191/0309133306pp483pr) [DOI] [Google Scholar]
- 63.Polishchuck L. 2010. The three-quarter-power scaling of extinction risk in Late Pleistocene mammals, and a new theory of size selectivity of extinction. Ecol. Evol. Res. 12, 1–22 [Google Scholar]
- 64.Peters R. H. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press [Google Scholar]
- 65.Estes J. A., et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306 10.1126/science.1205106 (doi:10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 66.Lease H. M., Wolf B. O. 2011. Lipid content of terrestrial arthropods in relation to body size, phylogeny, ontogeny and sex. Physiol. Entomol. 36, 29–38 10.1111/j.1365-3032.2010.00767.x (doi:10.1111/j.1365-3032.2010.00767.x) [DOI] [Google Scholar]
- 67.Chown S. L., Marais E., Terblanche J. S., Klok C. J., Lighton J. R. B., Blackburn T. M. 2007. Scaling of insect metabolic rate is inconsistent with the nutrient supply network model. Funct. Ecol. 21, 282–290 10.1111/j.1365-2435.2007.01245.x (doi:10.1111/j.1365-2435.2007.01245.x) [DOI] [Google Scholar]
- 68.White C. R., Cassey P., Blackburn T. M. 2007. Allometric exponents do not support a universal metabolic allometry. Ecology 88, 315–323 10.1890/05-1883 (doi:10.1890/05-1883) [DOI] [PubMed] [Google Scholar]
- 69.Chown S. L., Gaston K. J. 2010. Body size variation in insects: a macroecological perspective. Biol. Rev. 85, 139–169 10.1111/j.1469-185X.2009.00097.x (doi:10.1111/j.1469-185X.2009.00097.x) [DOI] [PubMed] [Google Scholar]
- 70.Knapp A. K., et al. 2008. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience 58, 811–821 10.1641/B580908 (doi:10.1641/B580908) [DOI] [Google Scholar]
- 71.Richardson D. M., Rejmánek M. 2011. Trees and shrubs as invasive alien species: a global review. Divers. Distrib. 17, 788–809 10.1111/j.1472-4642.2011.00782.x (doi:10.1111/j.1472-4642.2011.00782.x) [DOI] [Google Scholar]
- 72.Zavaleta E. S., Hobbs R. J., Mooney H. A. 2001. Viewing invasive species removal in a whole-ecosystem context. Trends Ecol. Evol. 16, 454–459 10.1016/S0169-5347(01)02194-2 (doi:10.1016/S0169-5347(01)02194-2) [DOI] [Google Scholar]
- 73.Leslie A. J., Spotila J. R. 2001. Alien plants threatens Nile crocodile (Crocodylus niloticus) breeding in Lake St. Lucia, South Africa. Biol. Conserv. 98, 347–355 10.1016/S0006-3207(00)00177-4 (doi:10.1016/S0006-3207(00)00177-4) [DOI] [Google Scholar]
- 74.Kearney M., Shine R., Porter W. P. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 10.1073/pnas.0808913106 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clusella-Trullas S., Blackburn T. M., Chown S. L. 2011. Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am. Nat. 177, 738–751 10.1086/660021 (doi:10.1086/660021) [DOI] [PubMed] [Google Scholar]
- 76.Trenberth K. E., et al. 2007. Observations: surface and atmospheric climate change. In Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), Cambridge, UK: Cambridge University Press [Google Scholar]
- 77.Jung M., et al. 2010. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature 467, 951–954 10.1038/nature09396 (doi:10.1038/nature09396) [DOI] [PubMed] [Google Scholar]
- 78.Schwalm C. R., Williams C. A., Schaefer K. 2011. Carbon consequences of global hydrologic change, 1948–2009. J. Geophys. Res. 116, G03042. 10.1029/2011JG001674 (doi:10.1029/2011JG001674) [DOI] [Google Scholar]
- 79.Chown S. L. 2010. Temporal biodiversity change in transformed landscapes: a southern African perspective. Phil. Trans. R. Soc. B 365, 3729–3742 10.1098/rstb.2010.0274 (doi:10.1098/rstb.2010.0274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellis E. C., Ramankutty N. 2008. Putting people in the map: anthropogenic biomes of the world. Front. Ecol. Environ. 6, 439–447 10.1890/070062 (doi:10.1890/070062) [DOI] [Google Scholar]
- 81.Sinervo B., et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 10.1126/science.1184695 (doi:10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 82.Clusella-Trullas S., Chown S. L. 2011. Technical comment on ‘Erosion of lizard diversity by climate change and altered thermal niches’. Science 332, 537. 10.1126/science.1195193 (doi:10.1126/science.1195193) [DOI] [PubMed] [Google Scholar]
- 83.Zhao M., Running S. W. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943 10.1126/science.1192666 (doi:10.1126/science.1192666) [DOI] [PubMed] [Google Scholar]
- 84.Nemani R. R., Keeling C. D., Hashimoto H., Jolly W. M., Piper S. C., Tucker C. J., Myneni R. B., Running S. W. 2003. Climate-driven increase in global terrestrial net primary production from 1982 to 1999. Science 300, 1560–1563 10.1126/science.1082750 (doi:10.1126/science.1082750) [DOI] [PubMed] [Google Scholar]
- 85.Haberl H., Erb K. H., Krausmann F., Gaube V., Bondeau A., Plutzar C., Gingrich S., Lucht W., Fischer-Kowalski M. 2007. Quantifying and mapping the human appropriation of net primary production in earth's terrestrial ecosystems. Proc. Natl Acad. Sci. USA 104, 12 942–12 947 10.1073/pnas.0704243104 (doi:10.1073/pnas.0704243104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huston M. A., Wolverton S. 2011. Regulation of animal size by eNPP, Bergmann's rule, and related phenomena. Ecol. Monogr. 81, 349–405 10.1890/10-1523.1 (doi:10.1890/10-1523.1) [DOI] [Google Scholar]
- 87.Daufresne M., Lengfellner K., Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793 10.1073/pnas.0902080106 (doi:10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gardner J. L., Peters A., Kearney M. R., Joseph L., Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291 10.1016/j.tree.2011.03.005 (doi:10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 89.Yom-Tov Y., Geffen E. 2011. Recent spatial and temporal changes in body size of terrestrial vertebrates: probable causes and pitfalls. Biol. Rev. 86, 531–541 10.1111/j.1469-185X.2010.00168.x (doi:10.1111/j.1469-185X.2010.00168.x) [DOI] [PubMed] [Google Scholar]
- 90.Somero G. N. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 10.1242/jeb.037473 (doi:10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 91.Dillon M. E., Wang G., Huey R. B. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706 10.1038/nature09407 (doi:10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 92.Huey R. B., Kearney M. R., Krockenberger A., Holtum J. A. M., Jess M., Williams S. E. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology, and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679 10.1098/rstb.2012.0005 (doi:10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Addo-Bediako A., Chown S. L., Gaston K. J. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. B 267, 739–745 10.1098/rspb.2000.1065 (doi:10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoffmann A. A., Sørensen J. G., Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Thermal Biol. 28, 175–216 10.1016/S0306-4565(02)00057-8 (doi:10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 95.Chown S. L. 2001. Physiological variation in insects: hierarchical levels and implications. J. Insect Physiol. 47, 649–660 10.1016/S0022-1910(00)00163-3 (doi:10.1016/S0022-1910(00)00163-3) [DOI] [PubMed] [Google Scholar]
- 96.Chown S. L., Jumbam K. R., Sørenson J. G., Terblanche J. S. 2009. Phenotypic variance, plasticity and heritability estimates of critical thermal limits depend on methodological context. Funct. Ecol. 23, 133–140 10.1111/j.1365-2435.2008.01481.x (doi:10.1111/j.1365-2435.2008.01481.x) [DOI] [Google Scholar]
- 97.Mitchell K., Hoffmann A. A. 2010. Thermal ramping rate influences evolutionary potential and species differences for upper thermal limits in Drosophila. Funct. Ecol. 24, 694–700 10.1111/j.1365-2435.2009.01666.x (doi:10.1111/j.1365-2435.2009.01666.x) [DOI] [Google Scholar]
- 98.Beaumont L. J., Pitman A., Perkins S., Zimmermann N. E., Yoccoz N. G., Thuiller W. 2011. Impacts of climate change on the world's most exceptional ecoregions. Proc. Natl Acad. Sci. USA 108, 2306–2311 10.1073/pnas.1007217108 (doi:10.1073/pnas.1007217108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawkins B. A., et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 10.1890/03-8006 (doi:10.1890/03-8006) [DOI] [Google Scholar]
- 100.Bonebrake T. C., Mastrandrea M. D. 2010. Tolerance adaptation and precipitation changes complicate latitudinal patterns of climate change impacts. Proc. Natl Acad. Sci. USA 107, 12 581–12 586 10.1073/pnas.0911841107 (doi:10.1073/pnas.0911841107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chown S. L., Sørensen J. G., Terblanche J. S. 2011. Water loss in insects: an environmental change perspective. J. Insect Physiol. 57, 1070–1084 10.1016/j.jinsphys.2011.05.004 (doi:10.1016/j.jinsphys.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 102.Woods H. A., Smith J. N. 2010. Universal model for water costs of gas exchange by animals and plants. Proc. Natl Acad. Sci. USA 107, 8469–8474 10.1073/pnas.0905185107 (doi:10.1073/pnas.0905185107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hadley N. F. 1994. Water relations of terrestrial arthropods. San Diego, CA: Academic Press [Google Scholar]
- 104.Terblanche J. S., Clusella-Trullas S., Chown S. L. 2010. Phenotypic plasticity of gas exchange pattern and water loss in Scarabaeus spretus (Coleoptera: Scarabaeidae): deconstructing the basis for metabolic rate variation. J. Exp. Biol. 213, 2940–2949 10.1242/jeb.041889 (doi:10.1242/jeb.041889) [DOI] [PubMed] [Google Scholar]
- 105.Chown S. L., Slabber S., McGeoch M. A., Janion C., Leinaas H. P. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537 10.1098/rspb.2007.0772 (doi:10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chown S. L. 2011. Discontinuous gas exchange: new perspectives on evolutionary origins and ecological explanations. Funct. Ecol. 25, 1163–1168 10.1111/j.1365-2435.2011.01879.x (doi:10.1111/j.1365-2435.2011.01879.x) [DOI] [Google Scholar]
- 107.Irlich U., Terblanche J. S., Blackburn T. M., Chown S. L. 2009. Insect rate–temperature relationships: environmental variation and the metabolic theory of ecology. Am. Nat. 174, 819–835 10.1086/647904 (doi:10.1086/647904) [DOI] [PubMed] [Google Scholar]
- 108.Addo-Bediako A., Chown S. L., Gaston K. J. 2002. Metabolic cold adaptation in insects: a large scale perspective. Funct. Ecol. 16, 332–338 10.1046/j.1365-2435.2002.00634.x (doi:10.1046/j.1365-2435.2002.00634.x) [DOI] [Google Scholar]
- 109.Clarke A. 2003. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 18, 573–581 10.1016/j.tree.2003.08.007 (doi:10.1016/j.tree.2003.08.007) [DOI] [Google Scholar]
- 110.Lovegrove B. G. 2000. The zoogeography of mammalian basal metabolic rate. Am. Nat. 156, 201–219 10.1086/303383 (doi:10.1086/303383) [DOI] [PubMed] [Google Scholar]
- 111.White C. R., Blackburn T. M., Martin G. R., Butler P. J. 2007. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B 274, 287–293 10.1098/rspb.2006.3727 (doi:10.1098/rspb.2006.3727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chown S. L., Sinclair B. J., Leinaas H. P., Gaston K. J. 2004. Hemispheric asymmetries in biodiversity: a serious matter for ecology. PLoS Biol. 2, e406. 10.1371/journal.pbio.0020406 (doi:10.1371/journal.pbio.0020406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dunn R. R., et al. 2009. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 12, 324–333 10.1111/j.1461-0248.2009.01291.x (doi:10.1111/j.1461-0248.2009.01291.x) [DOI] [PubMed] [Google Scholar]
- 114.Jetz W., Sekercioglu C. H., Böhning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, 2650–2657 10.1371/journal.pbio.0060303 (doi:10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jetz W., Rubenstein D. R. 2011. Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 1–7 10.1016/j.cub.2010.11.056 (doi:10.1016/j.cub.2010.11.056) [DOI] [PubMed] [Google Scholar]
- 116.Orme C. D. L., et al. 2006. Global patterns of geographic range size in birds. PLoS Biol. 4, 1276–1283 10.1371/journal.pbio.0040208 (doi:10.1371/journal.pbio.0040208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bouwman H., Polder A., Venter B., Skaare J. U. 2008. Organochlorine contaminants in cormorant, darter, egret, and ibis eggs from South Africa. Chemosphere 71, 227–241 10.1016/j.chemosphere.2007.09.057 (doi:10.1016/j.chemosphere.2007.09.057) [DOI] [PubMed] [Google Scholar]
- 118.Polder A., Venter B., Skaare J. U., Bouwman H. 2008. Polybrominated diphenyl esters and HBCD in bird eggs of South Africa. Chemosphere 73, 148–154 10.1016/j.chemosphere.2008.03.021 (doi:10.1016/j.chemosphere.2008.03.021) [DOI] [PubMed] [Google Scholar]
- 119.Evans S. W., Bouwman H. 2000. The influence of mist and rain on the reproductive success of the blue swallow Hirundo atrocaerulea. Ostrich 71, 83–86 10.1080/00306525.2000.9639875 (doi:10.1080/00306525.2000.9639875) [DOI] [Google Scholar]
- 120.Kylin H., Bouwman H., Evans S. W. 2011. Evaluating threats to an endangered species by proxy: air pollution as a threat to the blue swallow (Hirundo atrocaerulea) in South Africa. Environ. Sci. Pollut. Res. 18, 282–290 10.1007/s11356-010-0369-0 (doi:10.1007/s11356-010-0369-0) [DOI] [PubMed] [Google Scholar]
- 121.Piola R. F., Johnston E. L. 2009. Comparing differential tolerance of native and non-indigenous marine species to metal pollution using novel assay techniques. Environ. Pollut. 157, 2853–2864 10.1016/j.envpol.2009.04.007 (doi:10.1016/j.envpol.2009.04.007) [DOI] [PubMed] [Google Scholar]
- 122.Piola R. F., Johnston E. L. 2008. Pollution reduces native diversity and increases invader dominance in marine hard-substrate communities. Divers. Distrib. 14, 329–342 10.1111/j.1472-4642.2007.00430.x (doi:10.1111/j.1472-4642.2007.00430.x) [DOI] [Google Scholar]
- 123.Drake J. M., Lodge D. M. 2004. Global hot spots of biological invasions: evaluating options for ballast-water management. Proc. R. Soc. Lond. B 271, 575–580 10.1098/rspb.2003.2629 (doi:10.1098/rspb.2003.2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Minton M. S., Verling E., Miller A. W., Ruiz G. M. 2005. Reducing propagule supply and coastal invasions via ships: effects of emerging strategies. Frontiers Ecol. Environ. 3, 304–308 10.1890/1540-9295(2005)003[0304:RPSACI]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0304:RPSACI]2.0.CO;2) [DOI] [Google Scholar]
- 125.Davidson I. C., McCann L. D., Fofonoff P. W., Sytsma M. D., Ruiz G. M. 2008. The potential for hull-mediated species transfers by obsolete ships on their final voyages. Divers. Distrib. 14, 518–529 10.1111/j.1472-4642.2008.00465.x (doi:10.1111/j.1472-4642.2008.00465.x) [DOI] [Google Scholar]
- 126.Lee J. E., Chown S. L. 2009. Temporal development of hull fouling assemblages associated with an Antarctic supply vessel. Mar. Ecol. Prog. Ser. 386, 97–105 10.3354/meps08074 (doi:10.3354/meps08074) [DOI] [Google Scholar]
- 127.Phoenix G. K., et al. 2006. Atmospheric nitrogen deposition in world biodiversity hotspots: the need for a greater global perspective in assessing N deposition impacts. Glob. Change Biol. 12, 470–476 10.1111/j.1365-2486.2006.01104.x (doi:10.1111/j.1365-2486.2006.01104.x) [DOI] [Google Scholar]
- 128.Bond W. J., Midgley G. F. 2000. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Change Biol. 6, 865–869 10.1046/j.1365-2486.2000.00365.x (doi:10.1046/j.1365-2486.2000.00365.x) [DOI] [Google Scholar]
- 129.Bond W. J., Midgley G. F., Woodward F. I. 2003. The importance of low atmospheric CO2 and fire in promoting the spread of grasslands and savannas. Glob. Change Biol. 9, 973–982 10.1046/j.1365-2486.2003.00577.x (doi:10.1046/j.1365-2486.2003.00577.x) [DOI] [Google Scholar]
- 130.Kgope B. S., Bond W. J., Midgley G. F. 2010. Growth responses of African savanna trees implicate atmospheric [CO2] as a driver of past and current changes in savanna tree cover. Austral Ecol. 35, 451–463 10.1111/j.1442-9993.2009.02046.x (doi:10.1111/j.1442-9993.2009.02046.x) [DOI] [Google Scholar]
- 131.Wigley B. J., Bond W. J., Hoffman M. T. 2010. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob. Change Biol. 16, 964–976 10.1111/j.1365-2486.2009.02030.x (doi:10.1111/j.1365-2486.2009.02030.x) [DOI] [Google Scholar]
- 132.Staver A. C., Archibald S., Levin S. A. 2011. The global extent and determinants of savannah and forest as alternative biome states. Science 334, 230–232 10.1126/science.1210465 (doi:10.1126/science.1210465) [DOI] [PubMed] [Google Scholar]
- 133.Hirota M., Holmgren M., Van Nes E. H., Scheffer M. 2011. Global resilience of tropical forest and savannah to critical transitions. Science 334, 232–235 10.1126/science.1210657 (doi:10.1126/science.1210657) [DOI] [PubMed] [Google Scholar]
- 134.Bond W. J., Parr C. L. 2010. Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 143, 2395–2404 10.1016/j.biocon.2009.12.012 (doi:10.1016/j.biocon.2009.12.012) [DOI] [Google Scholar]
- 135.Richardson D. M., Pyšek P. 2006. Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geog. 30, 409–431 10.1191/0309133306pp490pr (doi:10.1191/0309133306pp490pr) [DOI] [Google Scholar]
- 136.Van Kleunen M., Dawson W., Schlaepfer D., Jeschke J. M., Fischer M. 2010. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 13, 947–958 10.1111/j.1461-0248.2009.01418.x (doi:10.1111/j.1461-0248.2009.01418.x) [DOI] [PubMed] [Google Scholar]
- 137.Van Kleunen M., Weber E., Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 13, 235–245 10.1111/j.1461-0248.2009.01418.x (doi:10.1111/j.1461-0248.2009.01418.x) [DOI] [PubMed] [Google Scholar]
- 138.Van Kleunen M., Johnson S. D. 2007. South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. J. Ecol. 95, 674–681 10.1111/j.1365-2745.2007.01250.x (doi:10.1111/j.1365-2745.2007.01250.x) [DOI] [Google Scholar]
- 139.Van Kleunen M., Manning J. C., Pasqualetto V., Johnson S. D. 2008. Phylogenetically independent associations between autonomous self-fertilization and plant invasiveness. Am. Nat. 171, 195–201 10.1086/525057 (doi:10.1086/525057) [DOI] [PubMed] [Google Scholar]
- 140.Thompson K., Davis M. A. 2011. Why research on traits of invasive plants tells us very little. Trends Ecol. Evol. 26, 155–156 10.1016/j.tree.2011.01.007 (doi:10.1016/j.tree.2011.01.007) [DOI] [PubMed] [Google Scholar]
- 141.Hulme P. E., Pyšek P., Duncan R. P. 2011. Don't be fooled by a name: a reply to Thompson and Davis. Trends Ecol. Evol. 26, 318. 10.1016/j.tree.2011.03.018 (doi:10.1016/j.tree.2011.03.018) [DOI] [PubMed] [Google Scholar]
- 142.Van Kleunen M., Dawson M., Dostal P. 2011. Research on invasive-plant traits tells us a lot. Trends Ecol. Evol. 26, 317. 10.1016/j.tree.2011.03.019 (doi:10.1016/j.tree.2011.03.019) [DOI] [PubMed] [Google Scholar]
- 143.Naeem S., Wright J. P. 2003. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 6, 567–579 10.1046/j.1461-0248.2003.00471.x (doi:10.1046/j.1461-0248.2003.00471.x) [DOI] [Google Scholar]
- 144.Dukes J. S., Mooney H. A. 1999. Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139 [DOI] [PubMed] [Google Scholar]
- 145.Theoharides K. A., Dukes J. S. 2007. Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol. 176, 256–273 10.1111/j.1469-8137.2007.02207.x (doi:10.1111/j.1469-8137.2007.02207.x) [DOI] [PubMed] [Google Scholar]
- 146.Walther G.-R., et al. 2009. Alien species in a warmer world: risks and opportunities. Trends Ecol. Evol. 24, 686–693 10.1016/j.tree.2009.06.008 (doi:10.1016/j.tree.2009.06.008) [DOI] [PubMed] [Google Scholar]
- 147.Stachowicz J. J., Terwin J. R., Whitlatch R. B., Osman R. W. 2002. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl Acad. Sci. USA 99, 15 497–15 500 10.1073/pnas.242437499 (doi:10.1073/pnas.242437499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Janion C., Leinaas H. P., Terblanche J. S., Chown S. L. 2010. Trait means and reaction norms: the consequences of climate change/invasion interactions at the organism level. Evol. Ecol. 24, 1365–1380 10.1007/s10682-010-9405-2 (doi:10.1007/s10682-010-9405-2) [DOI] [Google Scholar]
- 149.Chown S. L., Addo-Bediako A., Gaston K. J. 2003. Physiological diversity: listening to the large-scale signal. Funct. Ecol. 17, 568–572 10.1046/j.1365-2435.2003.07622.x (doi:10.1046/j.1365-2435.2003.07622.x) [DOI] [Google Scholar]
- 150.Brown J. H., Maurer B. A. 1989. Macroecology: the division of food and space among species on continents. Science 243, 1145–1150 10.1126/science.243.4895.1145 (doi:10.1126/science.243.4895.1145) [DOI] [PubMed] [Google Scholar]
- 151.Dell A. I., Pawar S., Savage V. M. 2011. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. USA 108, 10 591–10 596 10.1073/pnas.1015178108 (doi:10.1073/pnas.1015178108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kattge J., et al. 2011. TRY: a global database of plant traits. Glob. Change Biol. 17, 2905–2935 10.1111/j.1365-2486.2011.02451.x (doi:10.1111/j.1365-2486.2011.02451.x) [DOI] [Google Scholar]
- 153.Wallace J. B., Webster J. R. 1996. The role of macroinvertebrates in stream ecosystem function. Annu Rev. Entomol. 61, 115–139 10.1146/annurev.en.41.010196.000555 (doi:10.1146/annurev.en.41.010196.000555) [DOI] [PubMed] [Google Scholar]
- 154.Bêche L., Statzner B. 2009. Richness gradients of stream invertebrates across the USA: taxonomy- and trait-based approaches. Biodivers. Conserv. 18, 3909–3930 10.1007/s10531-009-9688-1 (doi:10.1007/s10531-009-9688-1) [DOI] [Google Scholar]
- 155.Chown S. L., Addo-Bediako A., Gaston K. J. 2002. Physiological variation in insects: large-scale patterns and their implications. Comp. Biochem. Physiol. B 131, 587–602 10.1016/S1096-4959(02)00017-9 (doi:10.1016/S1096-4959(02)00017-9) [DOI] [PubMed] [Google Scholar]
- 156.Cerdá X., Retana J., Cros S. 1997. Thermal disruption of transitive hierarchies in Mediterranean ant communities. J. Anim. Ecol. 66, 363–374 10.2307/5982 (doi:10.2307/5982) [DOI] [Google Scholar]
- 157.Worthen W. B., Jones M. T., Jetton R. M. 1998. Community structure and environmental stress: desiccation promotes nestedness in mycophagous fly communities. Oikos 81, 45–54 10.2307/3546466 (doi:10.2307/3546466) [DOI] [Google Scholar]
- 158.Seebacher F., Franklin C. E. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614 10.1098/rstb.2012.0036 (doi:10.1098/rstb.2012.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]