Abstract
A challenge to ecologists and evolutionary biologists is predicting organismal responses to the anticipated changes to global ecosystems through climate change. Most evidence suggests that short-term global change may involve increasing occurrences of extreme events, therefore the immediate response of individuals will be determined by physiological capacities and life-history adaptations to cope with extreme environmental conditions. Here, we consider the role of hormones and maternal effects in determining the persistence of species in altered environments. Hormones, specifically steroids, are critical for patterning the behaviour and morphology of parents and their offspring. Hence, steroids have a pervasive influence on multiple aspects of the offspring phenotype over its lifespan. Stress hormones, e.g. glucocorticoids, modulate and perturb phenotypes both early in development and later into adulthood. Females exposed to abiotic stressors during reproduction may alter the phenotypes by manipulation of hormones to the embryos. Thus, hormone-mediated maternal effects, which generate phenotypic plasticity, may be one avenue for coping with global change. Variation in exposure to hormones during development influences both the propensity to disperse, which alters metapopulation dynamics, and population dynamics, by affecting either recruitment to the population or subsequent life-history characteristics of the offspring. We suggest that hormones may be an informative index to the potential for populations to adapt to changing environments.
Keywords: climate change, dispersal, glucocorticoids, maternal effects, phenotypic plasticity, stress axis
1. Introduction
Abundant evidence suggests the world's climate is changing at an unprecedented rate [1]. Extended warm spells, prolonged drought and shifts in precipitation are among the anticipated changes to the climate, beyond the predicted rise in average temperatures. Moreover, as a consequence of disruption of temperature patterns, large-scale climatic cycles, e.g. El Niño–Southern Oscillation events, are expected to increase in frequency and duration, further exacerbating environmental change and disrupting patterns of primary productivity, perturbing the timing of reproduction, altering life-history traits and ultimately affecting population dynamics [2].
The deleterious effects on population persistence of extreme and unpredictable climatic events are likely to be enhanced by other anthropogenic changes impinging on global ecosystems, including habitat fragmentation, degradation and pollutants, e.g. polychlorinated biphenyls and other chemicals [3]. Species occupying altered environments are likely to experience multiple stressors of varying severity, frequency and duration. Of particular concern is how organisms can cope with the anticipated changes in their environment given the associated challenges imposed by other anthropogenic factors (multiple stressors), particularly because temperature and precipitation influence key life-history traits and population dynamics as well as act as selective agents. Although much attention has been focused on endothermic vertebrates, these factors are especially likely to affect ectothermic organisms, because habitat degradation either lowers the available resources or raises the energetic costs of maintenance so as to lower realized recruitment [3].
In the face of rapid environmental change, populations may persist, given sufficient time, by dispersal and shifting distributions that track their original environment [4,5]. A second compensatory response is adaptation by microevolution to new ecological conditions, e.g. thermal adaptation [6–8]. Third, individuals within a population may persist in rapidly changing environments by behavioural or physiological compensation, i.e. phenotypic plasticity [5,9–11]. Phenotypic plasticity may entail different phenomena including within-individual plasticity (i.e. acclimatization within or across years), genotype × environment interactions (i.e. the reaction norm of a trait to the current environment) or developmental plasticity [3]. Predicting the responses of organisms to climate change is complicated by two characteristics of the expected changes to global ecosystems. Sustained warming should impose directional selection favouring adaptation to higher temperatures [9]. However, climate change also is expected to yield circumstances involving unpredictable and episodic extreme climatic events. Consequently, even temporal and spatial variation in selection is unlikely to allow species to adapt to such environmental conditions. Rather, theory suggests that populations inhabiting environments with unpredictable change should evolve a plastic strategy [12–15]. It is likely that species characterized by phenotypic flexibility (i.e. plasticity) should be more resilient to climate change [9,11]. Key questions are how can environmental cues modulate changes in the phenotype [16] and what constraints may limit the potential for plasticity to facilitate coping with changing environments [10,17].
An accumulation of studies shows that species are currently responding to a changing climate [18,19]. There is evidence of shifts in distribution, both latitudinal and elevational, for invertebrates and vertebrates [20–24] as well as changes in phenology [25]. Finally, local extinctions in lizards have been attributed to climate change [8]. Limited data are available that show rapid evolutionary adaptation to climate change [26,27]. However, based on current rates of temperature increases, the estimated selection differential necessary for species to adapt exceeds most published estimates of selection [28]. Moreover, in some taxa, selection for higher body temperature may have genetic correlations with other behaviours, e.g. basking, which may limit the response to selection [29]. Immediate strategies for coping with climate change must entail behavioural and physiological plasticity [7].
The emerging field of conservation physiology attempts to determine the physiological responses of organisms to anthropogenic changes to the environment [30]. In particular, conservation physiology integrates ecology, evolution and physiology to elucidate constraints imposed by the environment on the ability of an organism to cope with the multiple shifts in the abiotic and biotic environment. Ultimately, information on physiological capacity can be used to predict fitness and population dynamic consequences associated with climate change and the persistence of species in an altered environment [31]. Yet, the role of physiological plasticity in coping with changing environments is largely unexplored, particularly within-individual variation and developmental plasticity. In this review, we focus on the role of hormonally mediated maternal effects in inducing phenotypic plasticity as a response to rising temperatures and extreme climatic events [32]. The maternal environment can affect offspring phenotypes and fitness, for example, through variation in investment in offspring size or number [33]. However, the discovery of maternal androgens and glucocorticoids in egg yolks and intra-clutch variation of these hormones [34] indicates that the offspring phenotype can be manipulated in response to environmental conditions experienced by the female [35]. Consequently, maternal effects can be considered intergenerational phenotypic (developmental) plasticity and may be critical in coping with unpredictable environments. In this paper, we describe the organizational effects of maternal hormones, specifically the hormonal cascades of the hypothalamus–pituitary–adrenal (HPA) and hypothalamus–pituitary–gonadal (HPG) axes (figure 1), in shaping offspring phenotypes and the resulting variation in individual strategies and effects on survival and reproduction. Hormones are a critical link between the environment and the genome. Thus, hormones may mediate the expression of phenotypic variation, generate trait integration, shape multi-variate trade-offs [36] and either directly or indirectly shape phenotypic plasticity during ontogeny and later into adulthood [37]. Moreover, the hormonal cascades involved in organizational effects during development may be modulated by environmental stressors and the maternal response as given by the duration and magnitude of elevated glucocorticoids. We discuss the phenotypic and population dynamic consequences of prenatal exposure to steroid hormones resulting in context-dependent expression of traits by the offspring. Finally, we propose how hormone-mediated maternal effects may enhance rapid adaptation to changing environmental conditions.
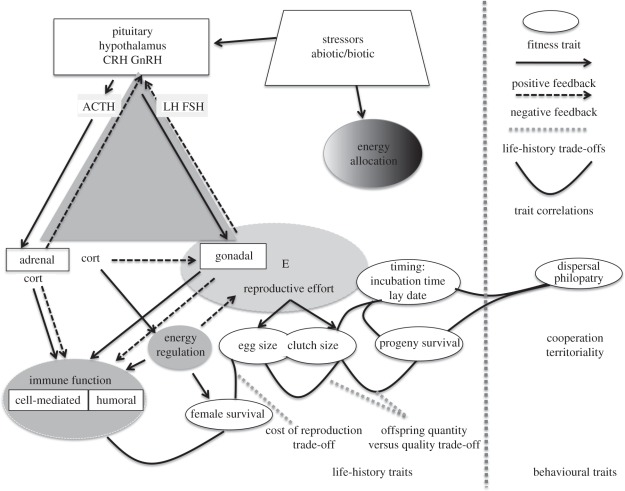
Figure 1.
Integration of endocrine control of maternal life history traits and behavior, given that resources are allocated to multiple competing functions: growth regulation, immunocompetence and immune system regulation, reproduction (egg size, egg number), behaviours including parental care, and dispersal. The figure highlights the synergism between the endocrine system and functional modules tied to fitness. The triangle includes the two main endocrine cascades, hypothalamic–pituitary–adrenal (HPA) axis, and hypothalamic–pituitary–gonadal (HPG) axis. Endocrine systems at each vertex interact in positive and negative feedback loops. The principal regulators of life history traits and behaviour are found within the hypothalamus and pituitary, which synthesize releasing hormones, e.g. GnRH, CRF. The HPG axis regulates clutch size and progeny size and is involved with selection and organization of life history trade-offs, via physiological epistasis. Environmental stressors, e.g. extreme climatic events, trigger the HPA axis, which results in activation of the stress response. Release of glucocorticoids mediates the reproductive hormones that affect life history traits. Other trade-offs include immune function modulating reproductive costs that are a result of constraints on resource allocation. Within the ovary, the release of progesterone (via corpora lutea) regulates egg retention, incubation time/parturition. FSH, follicle stimulating hormone; LH, luteinizing hormone; CRF, corticotropin releasing factor; ACTH, adrenocorticotropic hormone; GnRH, gonadotropin releasing hormone; E, estrogen, cort, glucocorticoid.
2. Endocrine influence on the phenotype
(a). Organizational and activational effects of hormones
The earliest influence of hormones on the phenotype involves the critical organizational effect of steroids for the neurodevelopment and sexual differentiation of male and female behaviours [38]. Organizational effects structure the principal sex-specific behavioural repertoire, but may also influence physiological and morphological attributes into adulthood [39,40]. For example, sex steroid hormones have organizational effects during development in zebra finches (Taeniopygia guttata). Indeed, oestradiol production just after hatching masculinizes the brain in young males so that as adults they can respond to the activational effect of testosterone and sing [41]. In the turtle Trachemys scripta, sex is determined by the temperature of the egg's incubation; administration of oestradiol to eggs at a temperature that normally produces only males can overcome the effects of temperature and produce females [42].
Steroids also influence the expression of secondary sexual characteristics, i.e. activational effects, at the onset of sexual maturity when the sexes begin reproduction [38]. Activational effects also initiate changes in behaviour and physiology in adults in response to proximate factors. The role of testosterone in controlling sexual behaviour in reptiles has been demonstrated by experimental manipulations involving castration followed by exogenous testosterone, or pharmacologically lowering testosterone levels by blocking androgen receptors or inhibiting androgen synthesis [43]. Such manipulations revealed the effects of androgens in courtship and copulation behaviours [44]. Changes in behaviour or energy allocation to reproduction stimulated by steroids can be under direct selection; survival or reproductive success of males or females is associated with the temporal changes in behaviour during maturation. However, direct selection acting on organizational events is unlikely, given that differentiation is incomplete [44]. If the same genes influence both organizational and activational events, it is possible for indirect selection to occur on organizational effects during maturation. Whereas ontogenetic processes dictate organizational effects, activational events are usually initiated in response to an environmental cue, e.g. typically photoperiod, or in some species rainfall. For example, marine toads (Bufo marinus) initiate reproductive activity during heavy rainfall [45]. Because breeding was associated with elevated corticosterone levels without a change in androgen levels, they concluded androgens played a permissive role, whereas corticosterone played an activating role in the initiation of reproductive behaviour. Activational effects result in changes in behaviour, e.g. aggression and territoriality, [46,47], nesting, morphology and physiology [48]. However, the magnitude of post-maturational response to rising steroids may be diminished or enhanced as a consequence of early exposure during pre-maturational development.
(b). Integration of environmental variation and endocrine cascades coordinates the expression of traits
Determining the response of an organism to stressors induced by climate change entails understanding the interaction between the stress response (HPA axis) and hormonal control of reproduction (HPG axis). The HPG axis regulates reproductive activities through positive (stimulation by luteinizing hormone (LH) and follicle stimulating hormone (FSH)) and negative feedback (rising testosterone or oestrogen). This regulation is further mediated by the HPA axis (figure 1). Physiological mechanisms for coping with stressors are coordinated by endocrine cascades of the HPA axis, which modulates activities associated with reproduction. The regulation of the gonads and reproductive traits is accomplished through glucocorticoid (stress) hormones [49].
The response to abiotic stressors involves an endocrine cascade that ultimately leads to the release of corticosterone by the adrenal glands. Corticosterone has diverse targets (see below) including glucocorticoid receptors in the hypothalamus, which regulate the secretion of FSH. Consequently, corticosterone can inhibit the endocrine cascades that regulate the gonads or suppress sexual behaviours and ultimately affect an organism's fitness [50]. Stressors are likely to alter timing and magnitude of hormonal cascades. Because hormones affect multiple traits and generate trait integration, the ability of organisms to respond to selection may be limited owing to trait correlations. Trait integration arises as a consequence of multiple traits influencing fitness [51], and correlational selection should build genetic correlations among the traits resulting in constraints in response to selection [52,53]. Alternatively, selection acting on hormonal control of traits upstream may induce shifts in hormonal cascades and lead to rapid reorganization of trade-offs [44].
(c). Hormones and the immune system
The neuroendocrine and immune systems are now recognized as linked and involved in feedback loops [54,55]. The crosstalk between immune and endocrine systems is important to homeostasis, since the interactions can produce various appropriate adaptive responses when homeostasis is threatened by stressors [56–58]. Substantial evidence shows that mothers can transmit antibodies to their offspring [59]. Therefore, a cost of phenotypic plasticity may be a reallocation of energy from immune function to growth or maintenance functions that reduce the risk of mortality [60]. Furthermore, to fully understand the impacts of global change on long-term population persistence, potential immunosuppressive effects of chronic and unpredictable stressors must be taken into account.
Corticosterone is known to induce immunosuppression [61,62] by inhibiting the differentiation of circulating monocytes into tissue-dwelling macrophages, and inhibiting macrophage production as well as depressing T-cell proliferation [63]. Recent studies have documented that females increase the levels of steroid hormones in the yolks of their eggs in response to stressors. An obvious question is how does raising glucocorticoids alter the immune response of their offspring. Increasing corticosterone in the eggs of yellow-legged gulls (Larus michahellis) depressed T-cell but not humoral immunity in hatchlings [64]. This reduction in chick T-cell-mediated immunity following egg corticosterone treatment is consistent with a large body of literature showing similar effects in adult vertebrates [65].
The stress-induced suppression of immune function may also be evolutionarily adaptive because immunosuppression may conserve energy that is required to deal with immediate demands imposed by the stressors. Indeed, immunological defences against pathogens may compete for a host's resources that are required for other energetically demanding processes, including maintenance, growth and reproduction [66–68]. Moreover, there is no doubt that glucocorticoids are powerful regulators of immune function, but their immunosuppressive effects are known from in vitro studies on immune cells or using pharmacological doses [69]; these in vitro effects do not represent the complexity of glucocorticoid actions observed in vivo [57,70]. Corticosterone administration has been reported to either increase or have no effect on antibody production depending on specific conditions [71–73]. Past studies have shown that corticosterone exhibits a bi-directional effect on immune function, such that acute elevated corticosterone levels may be immune enhancing, whereas chronically elevated levels may be immunosuppressive [70].
Corticosterone and testosterone can also exert an interactive effect on immunity [73]. Manipulation of both hormones in zebra finches resulted in no effect of corticosterone on cell-mediated immunity. In contrast, there was a significant, positive relationship between the plasma levels of testosterone and corticosterone and the birds' secondary antibody response to diphtheria [73]. In bank voles, Myodes glareolus, there is evidence of a genetic tradeoff between immune response and testosterone level [74]. Selection for high immune response resulted in indirect selection for low testosterone levels both in the laboratory and under semi-natural field conditions.
3. Hormones and potential responses to global change
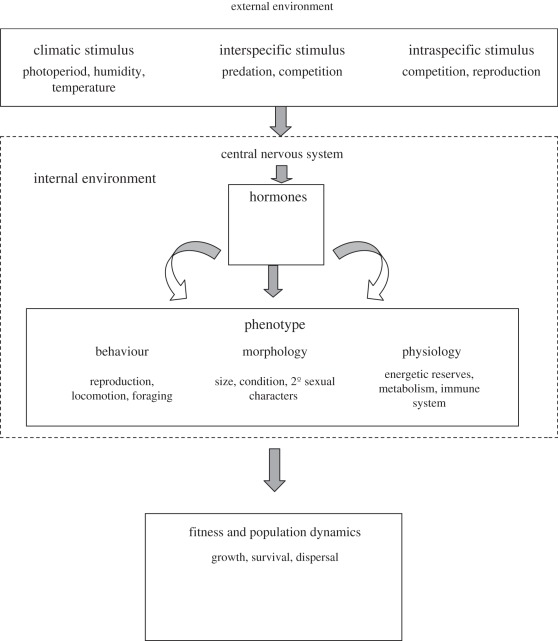
Global changes are expected to induce a myriad of profound environmental perturbations. These include variation in abiotic factors, such as increased extremes of weather, as well as biotic factors, including increased competition with new species contacted through changed geographical distribution, exposure to invasive species introduced by humans and changes in predator density. Furthermore, species are likely to become exposed to new pathogens either from contact with invasive species or by expanding their distributional ranges [75]. As a consequence, faced with a myriad of novel environmental changes arising from human activities, individuals and populations are confronted with novel selective pressures and stressors that require responses on a facultative basis and are disruptive to the predictable life cycle [76]. The effects of the global change may also range across scales from cells to populations. Indeed, the endocrine system is the major link with the nervous system, between the environment and the morphological, physiological and behavioural responses (figure 2) [37,77].
Figure 2.
Life history traits are the consequences of reciprocal interaction and coordination of external and internal environments. The environment can be divided in broad outline into two components: (1) abiotic such as climatic parameters and physical structure of the habitat and (2) biotic that include inter- and intraspecific relationships. Animals have to collect the information to take adequate decision. In pluricellular organisms, these are the nervous and endocrine systems, which integrate the information. Phenotype is in large part regulated by hormones of the hypothalamus–pituitary–gonadal axis.
Hormonal cascades that affect major components of fitness among individuals and consequently population dynamics may facilitate population persistence in the face of climate change.
(a). Hormone correlates with climate
In the song sparrow (Melospiza melodia), Wingfield [78] presented an example of an environmental perturbation that was directly linked with increased plasma corticosterone levels. He observed that a late spring snowstorm led to markedly increased plasma corticosterone levels along with decreased oestradiol levels in females [78]. More importantly, while corticosterone levels returned to pre-storm levels within a week and a half, plasma oestradiol remained depressed for considerably longer. In ensuing years, several studies confirmed that corticosterone levels often increase in birds in response to inclement weather [79,80]. However, the stress response may be limited in species that routinely encounter extreme climates, such as species breeding in high-latitude environments [81]. Birds breeding in high-arctic environments have a reduced stress response compared with the same species breeding in the lower arctic, thus affording some measure of protection from environmental extremes. Similarly, willow warblers (Phylloscopus trochilus) breeding in northern Sweden, where environmental conditions are more extreme, had a reduced response to stress compared with individuals breeding in southern Sweden, where conditions are milder and the breeding season longer [82,83].
Episodes of drought also trigger an endocrine response. The El Niño of 1998 resulted in a collapse of food supplies and a period of famine for Galápagos marine iguanas. Corticosterone levels increased in individuals during the El Niño, and correlated with a diminished probability of survival. However, this pattern depended on body condition [84,85]. Baseline corticosterone levels were elevated once body condition dropped below a critical threshold. The rise in corticosterone was hypothesized to be an adaptive response to starvation [84]. Their study shows the selective consequences of variation in physiology affecting survival through El Niño events via food availability. Climatic events need not be of large magnitude to induce an endocrine response. Minor changes in temperature may affect hormone levels. Wild-caught European starlings (Sturnus vulgaris) exposed to a small decrease in air temperature in the laboratory, e.g. 3°C, increased heart rate and corticosterone and induced behavioural changes, an increase in perch hopping and adjustment of feather position, consistent with a classical stress response [86].
(b). Human disturbance, invasive species, pollution and hormones
Interactions with humans can also affect the endocrine systems of species living in the wild. For example, individuals of yellow-eyed penguins (Megadyptes antipodes) appear to be sensitized to tourists. Yet, birds found in high-impact tourist areas have higher stress-induced corticosterone levels and lower reproductive success than birds at sites with limited tourist impact [87]. Chicks did not habituate as well as adults to tourist activity [88]. Expected deleterious consequences are likely to include reduced juvenile survival and recruitment at the tourist site, while the changed hormonal stress responses may ultimately have an effect on adult fitness and survival. Nevertheless, if the presence of humans is damaging for many avian species, there are a number of species that seemingly thrive in cities (e.g. house sparrows, Passer domesticus, and European starlings, S. vulgaris). Adaptation to human-dominated ecosystems may be sex-specific as shown in white-crowned sparrows (Zonotrichia leucophrys). Males inhabiting urban habitats had higher baseline corticosterone than conspecifics in rural habitats; in contrast, females showed no difference across habitat [89]. In contrast, European blackbirds (Turdus merula) from cities exhibited a dampened corticosterone response to capture and handling [90], which could be interpreted as microevolutionary change allowing urban birds to cope with stressful environments.
Invasive species may also alter a species, response to stressors. A recent study in eastern fence lizards (Sceloporus undulatus) suggests that invasive species modulated the relationship between physiological and behavioural stress response of native species [91]. They found that the effect of elevated corticosterone on behaviour differed for lizards living from sites with fire ants versus those sites without the ants. Marine iguanas (Amblyrhynchus cristatus) on islands in Galápagos without predators compared with individuals with novel predators displayed different behavioural and corticosterone (stress) responses to novel predators [92]. Experiments involving novel predators resulted in longer flight initiation distances and higher corticosterone response only in iguanas inhabiting islands with feral cats and dogs.
Moreover, anthropogenic chemicals, e.g. pollutants, are increasingly released into the environment. In 2001, the tanker Jessica ran aground and released fuel oil and bunker oil. Marine Iguanas exposed to low-level oil contamination of their food showed a strong stress response [93]. After the spill, contaminated iguanas had elevated corticosterone levels. Critically, these elevated corticosterone levels in the oiled iguanas predicted mortality.
Another group of chemical pollutants, known as endocrine disrupters, mimic the action of hormones. Recently, one endocrine disruptor that has received a large amount of attention is 17α-ethinyloestradiol (EE2). EE2 is a synthetic oestrogen that is one of the major components of oral contraceptives, which presents a high resistance to degradation in the human body and consequently can pass into the environment through domestic wastewater [94]. High concentrations of EE2 have been found in wastewater effluent and rivers [95,96]. It has been determined in gulf pipefish (Syngnathus scovelli) [97] and three-spined stickleback (Gasterosteus aculeatus) [98] that even low levels of exposure to the endocrine disruptor EE2 are sufficient to disrupt mating dynamics. More precisely, in gulf pipefish, males exposed to EE2 become more female-like with development of secondary sexual traits that normally appear only in females and consequently decrease male-mating opportunities, potentially impacting their reproductive fitness. Similarly, exposure to environmental oestrogens in reptiles induces sex reversal and a number of abnormalities in characteristics that are usually sexually dimorphic [99]. For example, red-eared slider turtles exposed experimentally to pesticides or their metabolites display altered sex ratios at hatching, and the males are demasculinized as demonstrated by an increased oestrogen/androgen ratio of testicular hormone synthesis [100]. American alligators (Alligator mississippiensis) naturally or experimentally exposed to various oestrogens or pesticides (or their metabolites) exhibit altered gonadal steroidogenesis and abnormally developed phalli [101–103].
4. Hormonal effects on components of fitness
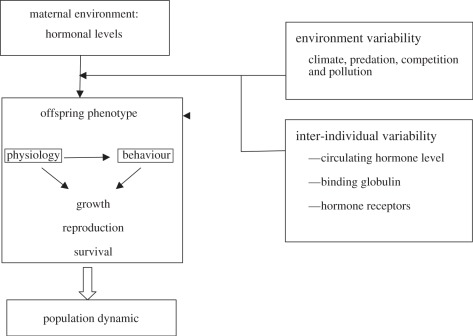
In an interesting review, Knapp [104] presents steroid hormones as ‘key molecules influencing polymorphism’. Indeed, between the secretion of steroid hormones and their effects, there are different components that interact to determine the strength of the hormonal influence. Once the hormones are synthesized and released into the blood, binding globulins can regulate hormone availability to target tissues [105]. Finally, the presence of specific receptors in the target tissue is required for a hormone to have its effects. Variability at each physiological level can explain phenotypic differences among individuals (figure 3).
Figure 3.
Mothers can control different aspects of offspring phenotype such as physiology and behaviour through hormonal levels. These modifications can have profound impacts on different fitness components that also affect the population dynamic. But it is important to conduct integrative studies to reveal the maternal hormonal effect by including environmental and inter-individual variability. Indeed, hormonal levels are known to be dependent on the environment such as the climate, the predation pressure and the pollution but the hormonal response will also depend on the individual characteristics. Binding globulins can regulate hormone availability to target tissues and the presence and the affinity of specific receptor for a hormone in the target tissue will modulate its effects.
It is important to understand the value of particular traits in terms of the selection pressures that act on them either directly or owing to trade-offs due to resource allocation and other factors such as environmental changes [16,37,106] and how endocrine mechanisms act as selective agents operating on phenotypic plasticity.
(a). Hormone-mediated life-history traits and trade-offs
Organizational effects of hormones (testosterone and oestrogen) have been repeatedly described as orchestrating alternative physiological and behavioural reaction patterns [107]. Hormones may mediate important trade-offs either between physiological functions, for example between the immune and reproductive systems [69,108], or between physiological and behavioural profiles [109,110]. Ketterson and co-workers demonstrated in dark-eyed juncos (Junco hyemalis) that experimentally enhanced testosterone increased male mating effort, as measured by song, courtship behaviour and success at obtaining extra-pair fertilizations [111,112], but also generated negative effects on parental behaviour, as measured by nestling feeding rate and nest defence, and to self-maintenance, as measured by body condition, immune function and survival [113,114]. Hence, variation in hormones can influence how energy is allocated to competing functions and constrain or limit life-history traits and trade-offs.
(b). Testosterone and fitness
Several studies have demonstrated the influence of testosterone on life-history traits. Ketterson et al. [115] studied the influence of testosterone in the life history of male dark-eyed juncos (Junco hyemalis). By manipulating testosterone, they found that high-testosterone males have higher song rates, larger territory size, are more attractive to females, and gain more extra-pair fertilizations relative to control males, although these advantages are offset by lower survival, perhaps related to lower body fat and higher plasma corticosterone. Nevertheless, the fitness of testosterone-implanted males exceeded control males, because the benefits of enhanced mating success outweighed the costs of reduced survival and parental care [116]. However, a cost of enhanced testosterone involved the progeny of testosterone-treated males, which were smaller and had lower post-fledging survival [116].
Endocrine cascades initiated by manipulating hormones other than testosterone can also modify life-history traits. A recent experiment involving the manipulation of LH and FSH revealed a suite of changes in the reproductive phenotype in male side-blotched lizards (Uta stansburiana). Treatment males displayed enhanced nuptial coloration, elevated levels of testosterone and increased locomotor performance [117]. In contrast, manipulation of FSH and follicle number in female side-blotched lizards illuminated the endocrine control of the trade-off between egg size and egg number [118]. FSH-manipulated females treated during vitellogenesis resulted in an increased clutch size with a concomitant decrease in egg mass [119].
(c). Glucocorticoids, performance and fitness: the corticosterone–fitness hypothesis
Studying whole-organismal performance of individuals allows the investigation of integration between multiple traits and simultaneous analysis of potential selective agents [120]. Most behaviours entail locomotion, (e.g. foraging activities, predator avoidance and courtship displays), therefore sprint speed or endurance is likely to have important fitness consequences [121–124]. Several studies also highlight the dependence of locomotor abilities on underlying, lower-level traits, such as morphology and physiology [125,126]. Therefore, sprint speed and endurance are ecologically relevant indices of overall performance capacity [127] and are strongly environment dependent at the species level [128]. Individual variation in sprint and endurance can be quite pronounced and may have consequences for the ability to avoid predators, or for dominance interactions among males [129].
Circumstances where an individual must escape from a predator or engage in dominance interactions are likely to be stress inducing. We may expect stress-related hormones to have an important role in shaping multiple whole-organism physiological traits. Several aspects of the action of corticosterone suggest a link between stress and physiological performance. Corticosterone is intimately involved in energy balance and homeostasis [105,108]. In particular, corticosterone facilitates the transfer of energy from storage to the bloodstream by stimulating gluconeogenesis and the generation of glucose substrates from non-carbohydrate sources, e.g. release of amino acids and mobilization of free fatty acids from muscle, fat tissue and liver [130]. However, prolonged periods of elevated corticosterone also induce the catabolism of muscle tissue, negative nitrogen balance, reproductive suppression and immunocompetence [49,105]. Thus, the benefits of elevated corticosterone may entail a cost in terms of diminished reproduction and elevated mortality [131]. This has been demonstrated in males of the dasyurid marsupial species, Phascogale calura, where high level of corticosterone suppresses the immune system and leads to higher mortality during the breeding season [132]. Indeed implantation of exogenous corticosterone stimulates activity or increases endurance in mammals [133,134], birds [135–137], lizards [131,138,139] and turtles [140]. However, corticosterone may also have contrasting effects depending on the species and on the environmental context [141–143].
Estimating levels of circulating glucocorticoid levels has become a standard method for characterizing physiological indices of individual and population condition [30]. Nevertheless, the relationship between corticosterone and fitness remains unclear; few studies have tested this relationship in the wild and results from birds and reptiles yield contradictory results [144]. Some studies have shown that elevated corticosterone may reduce fitness and survival, i.e. the corticosterone-fitness hypothesis [145], through infertility, impaired resistance to disease and inhibition of growth [49,84]. In contrast, other studies suggest that elevated corticosterone promotes adaptive advantages to the individual forming the corticosterone-adaptation hypothesis, [131,146]. Experimental manipulations of corticosterone reveal the complexity of the relationship between corticosterone and fitness. In some years, exogenous corticosterone implants may have a positive effect on total clutch mass and survivorship in females and a negative effect in others [147]. These differences could be explained by a quadratic relationship between corticosterone and fitness [146]. A reduced corticosterone level may indicate acclimatization allowing individuals to maintain good body condition and will not be sufficient to produce a stress-mediated phenotype to cope with a novel environment and consequently suffer lower survival. In contrast, high corticosterone levels induce deleterious changes such as muscle catabolism, which reduces survival. Intermediate levels of corticosterone promote an adequate response to environmental perturbations and therefore enhances survival [146]. Such a pattern was detected in the cliff swallow (Pterochelidon pyrrhonota); individuals with low or high values for corticosterone experienced the lowest survival, whereas intermediate values corresponded with higher survival [148]. The relationship between corticosterone and fitness may also vary within a study, depending on the sex of the individual [149,150] and the measure of fitness [146,149]. The inconsistency of these results with the corticosterone-fitness hypothesis may result in part from variation in the association between corticosterone and components of fitness across life-history stages and environmental contexts [145].
(d). Constraints, adaptation and plasticity
Environmental stressors are likely to alter timing and magnitude of hormonal cascades. Because hormones affect multiple traits and generate trait integration, the ability of organisms to respond to selection may be limited owing to traits being linked. Alternatively, selection acting on hormonal control of traits upstream may induce shifts in hormonal cascades and lead to rapid reorganization of trade-offs among physiological and life-history traits. There is some debate regarding the roles of phenotypic plasticity and maternal effects in adapting to novel environments. Some authors consider phenotypic plasticity unlikely to enable organisms to adapt to altered environments, at least in the short term [3]. Other authors argue that the pace of environmental change exceeds the potential evolutionary response to selection, hence plasticity is likely to be a major avenue for viability of species in novel environments [9,151].
Hormonal control mechanisms regulate the expression of numerous phenotypic traits associated with fitness, including growth, body size, survival, as well as a suite of life-history traits, [38,152]. Because of the link between environmental cues and the resulting phenotype, hormones have been suggested to be critical in shaping evolution to changing environments. However, a single hormone can affect the expression of multiple traits (physiological epistasis, [44,51] or hormonal pleiotropy, [52], hence selection acting on one trait may be constrained by the underlying correlational structure with other traits. In particular, when the covariation between a trait and fitness is conditional on several traits, selection acting on a trait is correlational; the magnitude and direction of selection coefficients depend on the product of two traits. A consequence of correlational selection is the functional integration of traits [51,153]. Under correlational selection, we expect to see physiological epistasis in which several physiological pathways interact to affect the expression of endocrine cascades and the traits controlled by hormones.
Phenotypic integration that arises from hormone-mediated expression of correlated traits has been implied to present limited opportunity for rapid evolution in response to climate-change scenarios [52]. However, phenotypic integration does not necessarily suggest phenotypic rigidity [151]. Despite endocrine cascades displaying tight coordination, the feedback loops and interaction among hormones suggests the potential for rapid response to fluctuating environmental conditions.
Adaptive responses of environmental stressors can occur over the complete life stage of an individual or across generations via maternal effects. Depending on the age of the organism, the integration of environmental stimuli signalling challenging conditions may initiate endocrine cascades that alter the expression of phenotypic characteristics. However, the range and permanence of the induced phenotypes will depend on whether stressors occur early in ontogeny (alteration of organizational events) or after attainment of sexual maturity [107]. Both the external and maternal environment can initiate endocrine cascades that result in a shift in the phenotype.
Hormone-mediated maternal effects are likely to be critical for manipulating the phenotype of individuals early in ontogeny so as to enhance growth or survival in challenging environments. Both viviparous and oviparous species may have maternal transfer of androgens and glucocorticoids during pre-natal or peri-natal periods of development [34], whose effects may be critical in shaping the phenotype of the offspring or have trans-generational effects.
5. Hormones and maternal effects
(a). Hormone-mediated maternal effects: testosterone
Maternal effects can be manifested through developmental responses induced by hormones. Indeed, variation in endocrine signals can also affect physiological and behavioural responses in offspring [154–156] in ways that are thought to influence their survival and reproductive success in a given environment [157]. In particular, vertebrate embryos are exposed to substantial amounts of steroid hormones from their mother. Embryos and foetuses can be exposed to stress hormones during development via the placenta in mammals and viviparous reptiles or their presence in eggs of oviparous species. Indeed, testosterone can also act via maternal effects on juvenile morphology. In the dragon lizard Ctenophorus fordi, yolk testosterone concentration was not correlated with hatchling morphology or survival under natural conditions, but higher concentrations correlated with greater hatchling growth rates [158]. A positive relationship between prenatal androgen exposure and postnatal growth rate has been documented previously in birds and lizards [159,160]. The similarity of response among species despite variation in life history suggests that testosterone is a powerful hormonal tool for adaptive maternal programming. In birds, the levels of steroid hormones in an egg vary both within and between clutches in relation to a range of factors such as position in the laying order, season, food availability, and have short- and long-lasting effects on offspring phenotype [161]. Zebra finches (Taeniopygia guttata) are a species that shows a naturally decreasing pattern of within-clutch testosterone allocation. Boncoraglio [162] experimentally elevated yolk testosterone levels in eggs 2 to 6 to the level of egg 1, and assessed fitness measures for junior offspring (eggs 2 to 6) and senior offspring (egg 1). They found that junior, but not senior, chicks in testosterone-treated broods attained poorer phenotypic quality compared with control broods. Thus, naturally decreasing within-clutch yolk testosterone allocation appears to benefit all siblings [162] (table 1).
Table 1.
Indirect and direct effects of maternal steroid hormones on offspring fitness.
| hormone | animal model | direct and indirect fitness effect | reference |
|---|---|---|---|
| increased cortisol concentrations within eggs are correlated with: | |||
| Atlantic salmon (Salmo salar) | reduced embryo fork length, mass and yolk sac volume and increased offspring mortality | Eriksen et al. [163] | |
| Coral Reef damsel fish (Pomacentrus amboinsensis) | increased egg mortality; increased asymmetry of hatchlings; hatchlings had higher survival | Gagliano & McKormick [164] | |
| increased corticosteroid concentrations in eggs are correlated with: | |||
| barn swallow (Hirundo rustica) | reduced offspring growth | Saino et al. [165] | |
| yellow-legged gull (Larus michahellis) | reduced begging display, lower T-cell-mediated immunity | Rubolini et al. [64] | |
| chicken (Galus domesticus) | reduced growth | Mashaly [166] | |
| European starling (Sturnus vulgaris) | increased weight at hatch and male embryonic mortality | Love et al. [167] | |
| common gecko (Hoplodactylus maculatus) | decreased offspring number | Cree et al. [168] | |
| Japanese quail (Coturnix coturnix japonica) | reduced growth | Hayward & Wingfield [169] | |
| pied flycatcher (Ficedula hypoleuca) | decreased number of young fledged | Silverin [170] | |
| common lizard (Lacerta vivipara) | reduced juvenile body size, condition and growth but increased male juvenile's survival | Meylan & Clobert [150] | |
| side-blotched lizard (Uta stansburiana) | increased total clutch mass | Sinervo & Denardo [147] | |
| western garter snake (Thamnophis elegans) | more stillborn offspring, higher reproductive failure; change in antipredator behaviour | Robert et al. [171] | |
| increased testosterone concentrations in eggs are correlated with: | |||
| American kestrels (Falco sparverius) | delayed hatching and reduced nestling growth and survival rates | Sockman & Schwabl [172] | |
| black-headed gull (Larus ridibundus) | enhanced postnatal growth | Eising et al. [159] | |
| Japanese quail (Coturnix japonica) | suppressed early skeletal growth and mass gain | Hegyi & Schwabl [173] | |
| yellow-legged gull (L. michahellis) | depressed post-hatching body mass and reduced female embryonic survival | Rubolini et al. [174] | |
(b). Hormone-mediated maternal effects: glucocorticoids
Maternal corticosterone levels are also a good candidate in shaping an offspring's phenotype during development [175]. Corticosterone has been demonstrated to mediate long-term maternal effects between rodent mothers living under stressful conditions and their independent offspring. Pollard [154] demonstrated that stress effects were persistent in second-generation rats bred from females whose own mothers had been stressed during pregnancy. It is noteworthy that these effects persisted into adulthood [154]. Daughters of physically stressed mothers are less fertile and less fecund than daughters of unstressed mothers [157,176,177].
Similar variation in yolk hormonal composition has been found in other oviparous species [178,179]. Significant variation in yolk corticosterone was detected at three biological levels: intra-clutch, inter-clutch and among species. Exposure to elevated yolk glucocorticoids consistently reduced the ‘quality’ of offspring, but the effects differed between first and second broods [178]. Hatchling males in manipulated treatment from the first brood begged less and suffered higher mortality than daughters. Nestlings from sham-treated nests in the second brood were heavier. Sons from the corticosterone treatment were characterized by higher cell-mediated immune response, but fledged at a lighter mass. Intra-clutch variation of yolk corticosterone may play an adaptive role in mediating competition within broods [165,179]. By producing eggs that differ in levels of maternally derived glucocorticoids, mothers seem to produce a variety of phenotypes, perhaps as a diversified bet-hedging strategy to cope with unpredictable environmental conditions [180]. However, an increase in trait variation under stressful conditions may also be the result of increased non-adaptive developmental instability [181], or inability of mothers to precisely allocate resources equally among offspring [182].
Maternal glucocorticoid hormone transfer to offspring has traditionally been considered to be maladaptive. However, evolutionary biologists have recently suggested that prenatal stress has an adaptive function. Hormonally induced maternal effects may favour the mother or the offspring, but the outcome is context-dependent [183]. In birds, experimentally elevated plasma corticosterone in the mother significantly affected the growth rates of her offspring [169]. Chicks from eggs laid by corticosterone-implanted females tended to hatch lighter and grew significantly more slowly than control chicks. The authors suggested that high corticosterone levels in adult quails issued from eggs laid by mothers with experimentally elevated corticosterone levels may prove adaptive under conditions where maternal corticosterone levels are elevated owing to high predation risk. In this case, positive anti-predator behaviour mediated by high corticosterone levels would compensate for the negative effects on growth. In contrast, maternal stress induced lower hatching success and reduced growth rate of barn swallow chicks, leading to smaller size at fledging. Because body size at independence is associated with survival, Saino et al. [165] suggest that lower offspring quality may have a large influence on population dynamics. The pattern of reduced embryo body mass and higher mortality with an increase in maternal corticosterone levels was also observed in domestic species [166].
In reptiles, the effects of prenatal exposure to corticosterone may affect size, body condition, growth and sex determination [150,184–186]. In most cases, these corticosterone-mediated morphological changes seem deleterious; the decrease of body condition observed can be caused by impairing immune system function and mobilization of energy stores [187]. However, in the common lizard (Zootoca (=Lacerta) vivipara), Meylan & Clobert [150] experimentally demonstrated that elevated maternal corticosterone during gestation increased the survival of juvenile males.
6. Hormonally mediated maternal effects on dispersal and population dynamics
Hormonally mediated maternal effects particularly due to glucocorticoids result in a diversity of impacts on the offspring phenotype and its fitness. Ultimately, the pleiotropic effects of corticosterone on growth rate, body size, life history and behavioural traits has consequences for the propensity to disperse as well as recruitment to the population, which in turn influences population dynamics. Because maternal effects can generate alternative phenotypes in response to environmental fluctuations, this phenotypic change, i.e. plasticity, may be critical for responding to rapidly changing environments [188].
(a). Dispersal
Natal dispersal is a key trait influencing the genetic and social structuring within populations, and gene flow between populations can be influenced by multiple factors [189]. Maternal hormones can be major proximate factors affecting the propensity for dispersal. In different experimental manipulations of yolk testosterone in great tits, Tschirren and co-workers [190,191] proposed that the transfer of low concentrations of yolk androgens into the eggs might play an important role in modulating the offspring's dispersal behaviour. Birds hatched from eggs with experimentally elevated yolk testosterone concentrations dispersed over significantly longer distances. Moreover, in a previous study, these authors showed that female great tits transferred lower concentrations of yolk androgens into the eggs when their nest was infested with ectoparasites compared with unexposed control females [192]. These two results suggest that the deposition of low concentrations of yolk androgens into the eggs of nests with parasites might thus be a female strategy to promote philopatry of parasitized offspring and thus to increase her offspring's and thereby her own fitness. Similarly, exposure to prenatal corticosterone decreases juvenile dispersal in the common lizard, but this result depended on interactions with maternal-age or physical condition [141,185]. Juveniles from older females treated with corticosterone showed a lower propensity for dispersal; in contrast young females treated with corticosterone produced offspring with higher dispersal behaviours [141].
In spatially structured populations, the viability and dynamics of the population depend critically upon dispersal behaviour. High levels of dispersal in the copepod Calanus finmarchicus appeared to maintain a stable effective population size throughout previous episodic fluctuations in the Earth's climate [193]. Moreover, at the population level, the phenotypic composition of recently colonized populations differs from that of older populations for traits linked with dispersal [194]. By modifying the dispersal behaviour, a mother can have a profound impact on population dynamics. An understanding of the different causes of departure might therefore shed new light on the understanding of spatially structured populations and species invasiveness in the context of global climate changes [189].
(b). Population dynamics
Hormone-mediated maternal effects are known to induce pleiotropic effects on offspring phenotypes, which may persist into adulthood as well as subsequent generations. Maternal effects on the offspring phenotype are likely to alter population dynamics as a result of variation in offspring survivorship, growth rate, body size and other life-history traits. Indeed, theory suggests that maternal effects should induce population cycles as a consequence of delayed density dependence [195]. Furthermore, maternal-age effects, which are manifested in terms of allocation of energy to reproduction and provisioning of antibodies and steroids to the progeny, generate drastic changes in cohort structure and consequently affect population dynamics [196]. This is especially likely if climate change differentially impacts on old versus young cohorts [197]. Key demographic traits, including growth rate and survival exhibited higher variability in younger cohorts, specifically juveniles and subadults, than adults, which was attributable to climatic variation (temperature and rainfall). Furthermore, population projection models found that population growth rates were most sensitive to variation in survival in younger cohorts [30].
Cyclical behaviour in population dynamics also bears the imprint of hormonal effects. At least two species of lizards (Uta stansburiana and Zootoca vivipara) have been described with cyclical changes in the frequency of adult male phenotypes. Interestingly, these cycles are ascribed to be the result of changes in male phenotypes that have behavioural traits organized by the action of testosterone [198,199]. Because organizational effects are, in part, responsible for the expression of male behavioural traits, the disruption of hormonal cascades by environmental change can potential result in chaotic population dynamics, given the link between testosterone and fitness, and the elimination of one or more morphs.
Cyclical behaviour in population density is also germane for understanding the ability of species to cope with climate change. Explanations for the population dynamics of high-latitude rodents have been largely dominated by extrinsic factors, e.g. predator–prey dynamics. However, several studies have shown a role for maternal effects and intrinsic attributes of individuals for the generation of complex population dynamics [200,201]. Maternal effects are expected to result in delayed density dependence, leading to cyclical population behaviour. Maternal life-history attributes and offspring quality varies between population peaks and troughs; peaks have females with a reduction of energy apportioned to reproduction and lightweight offspring. In contrast, at population troughs, the adults are heavier and invest more in reproduction. In addition, changes in the HPA axis with variation in density may exacerbate the trajectory of the population excursions [202].
Population cycles can be driven by differential investment in offspring by females that have morphs with different reproductive strategies [203]. In the lizard U. stansburiana females adopting an r- versus K- strategy have a fitness advantage contingent on density cycles. At low population densities, orange-throated females, that have low levels of corticosterone, produce many eggs and are favoured by selection. In contrast, at high densities, yellow-throated females, that have high levels of corticosterone, and that produce fewer, larger eggs, have a fitness advantage. Moreover, the female morphs were found to exhibit different fitness consequences with respect to immunocompetence responses associated with an increase in corticosterone (orange females had lower survivorship than yellow females) [204]. As a consequence of changing thermal conditions it is possible to imagine that given unpredictable climatic events, variation in the stress response between morphs initiates differences in rising corticosterone levels that may induce or exacerbate similar population cycles in a context-dependent fashion [205].
7. Conclusions
Two major themes have emerged from this review. First, we suggest that hormonal cascades, especially an interaction between the HPG and HPA axes, are likely to be critical for adjusting to rapidly changing environments. Indeed, hormones, especially glucocorticoids, can be used as an index to estimate stress levels of populations and predict individual responses to variation in climate. Second, hormonally induced maternal effects are the likely mechanism that facilitates phenotypic changes in the face of global change. Because maternal effects may be context-dependent, the benefits may shift from favouring parents to that of preparing offspring to environmental conditions based on maternal condition. Furthermore, accumulating evidence suggests a substantial role for maternal effects in modulating population dynamics.
Based on these known correlations among environment perturbation and individual hormonal level and the importance of hormonally mediated maternal effects on life-history traits previously described, the consequences of global changes can have profound impact on individuals and populations at different time scales. Measuring hormone levels (especially glucocorticoids) and their associated influence on physiological and life-history traits of organisms in response to their changed environment can be a successful tool for conservation biologists to predict the population responses to climate change [30,88]. Understanding the role of hormones in modulating offspring phenotypes via maternal effects is also critical for understanding the potential for species to adapt to changing environments. Indeed, when the link between baseline hormonal level and fitness is known [145], conservation managers can use hormonal levels to predict and anticipate future problems. But because different hormones can interact to modify life-history traits [206], it appears important also to study multiple endocrine signals.
Acknowledgements
We thank two anonymous reviewers and Craig Franklin for providing constructive comments on the manuscript. D.B.M. was supported by NSF grant IOS 1022031, a CNRS fellowship and a Université Paul Sabatier Toulouse III visiting Professorship. J.C. was supported by a Biodiversity grant (TenLamas), a FP7 grant (SCALES) and two French ANR grants (DIAME and MOBIGEN).
References
- 1.IPCC 2007. Summary for Policymakers. In Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Holmgren M., Scheffer M., Ezcurra E., Gutiérrez J. R., Mohren G. M. J. 2001. El Niño effects on the dynamics of terrestrial ecosystems. Trends Ecol. Evol. 16, 89–94 10.1016/S0169-5347(00)02052-8 (doi:10.1016/S0169-5347(00)02052-8) [DOI] [PubMed] [Google Scholar]
- 3.Visser M. E. 2008. Keeping up with a warming world: assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 10.1098/rspb.2007.0997 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt R. D. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315 10.1016/0169-5347(90)90088-U (doi:10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 5.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 6.Chown S. L., Hoffman A. A., Kristensen T. N., Angilletta M. J., Jr, Stenseth N. C., Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15 10.3354/cr00879 (doi:10.3354/cr00879) [DOI] [Google Scholar]
- 7.Huey R. B., Kearney M. R., Krockenberger A., Holtum J. A. M., Jess M., Williams S. E. 2012. Predicting organismal vulnerability to climate warming: rules of behaviour, physiology, and adaptation. Phil. Trans. R. Soc. B 367, 1665–1679 10.1098/rstb.2012.0005 (doi:10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinervo B., et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 10.1126/science.1184695 (doi:10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 9.Canale C. I., Henry P. Y. 2010. Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim. Res. 43, 135–147 10.3354/cr00897 (doi:10.3354/cr00897) [DOI] [Google Scholar]
- 10.Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 10.1126/science.1157174 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 11.Fuller A., Dawson T., Helmuth B., Hetem R. S., Mitchell D., Maloney S. K. 2010. Physiological mechanisms in coping with climate change. Physiol. Biochem. Zool. 83, 713–720 10.1086/652242 (doi:10.1086/652242) [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw W. E., Holzapfel C. M. 2008. Genetic response to rapid climate change: it's seasonal timing that matters. Mol. Ecol. 17, 157–166 10.1111/j.1365-294X.2007.03509.x (doi:10.1111/j.1365-294X.2007.03509.x) [DOI] [PubMed] [Google Scholar]
- 13.Ghalambor C. K., Reznick D. N., Walker J. A. 2004. Constraints on adaptive evolution: the functional trade-off between reproduction and fast-start swimming performance in the Trinidadian guppy (Poecilia reticulata). Am. Nat. 164, 38–50 10.1086/421412 (doi:10.1086/421412) [DOI] [PubMed] [Google Scholar]
- 14.Meyers L. A., Bull J. J. 2002. Fighting change with change: adaptive variation in an uncertain world. Trends Ecol. Evol. 17, 551–557 10.1016/S0169-5347(02)02633-2 (doi:10.1016/S0169-5347(02)02633-2) [DOI] [Google Scholar]
- 15.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 16.Chown S. L. 2012. Trait-based approaches to conservation physiology: forecasting environmental change risks from the bottom up. Phil. Trans. R. Soc. B 367, 1615–1627 10.1098/rstb.2011.0422 (doi:10.1098/rstb.2011.0422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepetz V., Massot M., Chaine A. S., Clobert J. 2009. Climate warming and the evolution of morphotypes in a reptile. Global Change Biol. 15, 454–466 10.1111/j.1365-2486.2008.01761.x (doi:10.1111/j.1365-2486.2008.01761.x) [DOI] [Google Scholar]
- 18.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 19.Walther G. R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J. M., Hoegh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 20.Chen I. C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 10.1126/science.1206432 (doi:10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 21.Forister M. L., McCall A. C., Sanders N. J., Fordyce J. A., Thorne J. H., O'Brien J., Waetjen D. P., Shapiro A. M. 2010. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl Acad. Sci. USA 107, 2088–2092 10.1073/pnas.0909686107 (doi:10.1073/pnas.0909686107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Roux P. C., McGeoch M. A. 2008. Rapid range expansion and community reorganization in response to warming. Global Change Biol. 14, 2950–2962 10.1111/j.1365-2486.2008.01687.x (doi:10.1111/j.1365-2486.2008.01687.x) [DOI] [Google Scholar]
- 23.Thomas C. D. 2010. Climate, climate change and range boundaries. Diversity Distrib. 16, 488–495 10.1111/j.1472-4642.2010.00642.x (doi:10.1111/j.1472-4642.2010.00642.x) [DOI] [Google Scholar]
- 24.Warren M. S., et al. 2001. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414, 65–69 10.1038/35102054 (doi:10.1038/35102054) [DOI] [PubMed] [Google Scholar]
- 25.Smallegange I. M., Fiedler W., Köppen U., Geiter O., Bairlein F. 2010. Tits on the move: exploring the impact of environmental change on blue tit and great tit migration distance. J. Anim. Ecol. 79, 350–357 10.1111/j.1365-2656.2009.01643.x (doi:10.1111/j.1365-2656.2009.01643.x) [DOI] [PubMed] [Google Scholar]
- 26.Gienapp P., Teplitsky C., Alho J. S., Mills J. A., Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 10.1111/j.1365-294X.2007.03413.x (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 27.Husby A., Visser M. E., Kruuk L. E. B. 2011. Speeding up microevolution: the effects of increasing temperature on selection and genetic variance in a wild bird population. PLoS Biol. 9, e1000585. 10.1371/journal.pbio.1000585 (doi:10.1371/journal.pbio.1000585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingsolver J. G., Hoekstra H. E., Hoekstra J. M., Berrigan D., Vignieri S. N., Hill C. E., Hong A., Gilbert P., Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 10.1086/319193 (doi:10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 29.Sinervo B. 1990. Evolution of thermal physiology and growth rate between populations of the western fence lizard (Sceloporus occidentalis). Oecologia 83, 228–237 10.1007/BF00317757 (doi:10.1007/BF00317757) [DOI] [PubMed] [Google Scholar]
- 30.Wikelski M., Cooke S. J. 2006. Conservation physiology. Trends Ecol. Evol. 21, 38–46 10.1016/j.tree.2005.10.018 (doi:10.1016/j.tree.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 31.Seebacher F., Franklin C. E. 2012. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Phil. Trans. R. Soc. B 367, 1607–1614 10.1098/rstb.2012.0036 (doi:10.1098/rstb.2012.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquis O., Massot M., Le Galliard J. F. 2008. Intergenerational effects of climate generate cohort variation in lizard reproductive performance. Ecology 89, 2575–2583 10.1890/07-1211.1 (doi:10.1890/07-1211.1) [DOI] [PubMed] [Google Scholar]
- 33.Mousseau T. A., Fox C. W. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 10.1016/S0169-5347(98)01472-4 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 34.Groothuis T. G. G., Schwabl H. 2008. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil. Trans. R. Soc. B 363, 1647–1661 10.1098/rstb.2007.0007 (doi:10.1098/rstb.2007.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver I. C. G., Cervoni N., Champagne F. A., D'Alessio A. C., Sharma S., Seckl J. R., Symov S., Szyf M., Meaney M. J. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 10.1038/nn1276 (doi:10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 36.Sinervo B., Clobert J., Miles D. B., McAdam A. G., Lancaster L. T. 2008. The role of pleiotropy versus signaller-receiver gene epistasis in life history trade-offs: dissecting the genomic architecture of organismal design in social systems. Heredity 101, 197–211 10.1038/hdy.2008.64 (doi:10.1038/hdy.2008.64) [DOI] [PubMed] [Google Scholar]
- 37.Lessells C. M. 2008. Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity? Phil. Trans. R. Soc. B 363, 1589–1598 10.1098/rstb.2007.0008 (doi:10.1098/rstb.2007.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 39.Groothuis T. G. G., Müller W., von Engelhardt N., Carere C., Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 40.Phoenix C. H., Goy R. W., Gerall A. A., Young W. C. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382 10.1210/endo-65-3-369 (doi:10.1210/endo-65-3-369) [DOI] [PubMed] [Google Scholar]
- 41.Nottebohm F., Arnold A. P. 1976. Sexual dimorphism in vocal control areas of the songbird brain. Science 194, 211–213 10.1126/science.959852 (doi:10.1126/science.959852) [DOI] [PubMed] [Google Scholar]
- 42.Crews D., Cantu A. R., Rhen T., Vohra R. 1996. The relative effectiveness of estrone, estradiol-17, and estriol in sex reversal in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. Gen. Comp. Endocrinol. 102, 317–326 10.1006/gcen.1996.0075 (doi:10.1006/gcen.1996.0075) [DOI] [PubMed] [Google Scholar]
- 43.Haider S., Rai U. 1986. Effects of cyproterone-acetate and flutamide on the testis and epididymis of the Indian wall lizard, Hemidactylus flaviviridis (Ruppell). Gen. Comp. Endocrinol. 64, 321–329 10.1016/0016-6480(86)90065-1 (doi:10.1016/0016-6480(86)90065-1) [DOI] [PubMed] [Google Scholar]
- 44.Sinervo B., Miles D. B. 2011. Hormones and behavior of reptiles. In Hormones and reproduction of vertebrates (eds Norris D. O., Lopez K. H.), pp. 215–246 London, UK: Academic Press [Google Scholar]
- 45.Orchinik M., Licht P., Crews D. 1988. Plasma steroid concentrations change in response to sexual behavior in Bufo marinus. Horm. Behav. 22, 338–350 10.1016/0018-506X(88)90006-2 (doi:10.1016/0018-506X(88)90006-2) [DOI] [PubMed] [Google Scholar]
- 46.Adkins E., Schlesinger L. 1979. Androgens and the social behavior of male and female lizards (Anolis carolinensis). Horm. Behav. 13, 139–152 10.1016/0018-506X(79)90053-9 (doi:10.1016/0018-506X(79)90053-9) [DOI] [PubMed] [Google Scholar]
- 47.Crews D., Traina V., Wetzel F. T., Muller C. 1978. Hormonal control of male reproductive behavior in the lizard, Anolis carolinensis: role of testosterone, dihydrotestosterone, and estradiol. Endocrinology 103, 1814–1821 10.1210/endo-103-5-1814 (doi:10.1210/endo-103-5-1814) [DOI] [PubMed] [Google Scholar]
- 48.Sinervo B., Miles D. B., Frankino W. A., Klukowski M., DeNardo D. F. 2000. Testosterone, endurance, and Darwinian fitness: natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Horm. Behav. 38, 222–233 10.1006/hbeh.2000.1622 (doi:10.1006/hbeh.2000.1622) [DOI] [PubMed] [Google Scholar]
- 49.Sapolsky R. M., Romero L. M., Munck A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 50.Dufty A. M., Jr, Danchin É. 2007. Hormones and behaviour. In Behavioural ecology: an evolutionary perspective on behaviour (eds Danchin É., Giraldeau L.-A., Cézilly F.), pp. 186–228 Oxford, UK: Oxford University Press [Google Scholar]
- 51.Miles D. B., Sinervo B., Hazard L. C., Svensson E. I., Costa D. 2007. Relating endocrinology, physiology and behaviour using species with alternative mating strategies. Funct. Ecol. 21, 653–665 10.1111/j.1365-2435.2007.01304.x (doi:10.1111/j.1365-2435.2007.01304.x) [DOI] [Google Scholar]
- 52.Ketterson E. D., Atwell J. W., McGlothlin J. W. 2009. Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr. Comp. Biol. 49, 365–379 10.1093/icb/icp057 (doi:10.1093/icb/icp057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinervo B., Svensson E. 2002. Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338 10.1038/sj.hdy.6800148 (doi:10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- 54.Besedovsky H. O., Del Rey A. 1996. Immune–neuro-endocrine interactions: facts and hypotheses. Endocr. Rev. 17, 64–102 10.1210/edrv-17-1-64 (doi:10.1210/edrv-17-1-64) [DOI] [PubMed] [Google Scholar]
- 55.Harbuz M. 2003. Neuroendocrine–immune interactions. Trends Endocrinol. Metab. 14, 51–52 10.1016/S1043-2760(03)00007-9 (doi:10.1016/S1043-2760(03)00007-9) [DOI] [PubMed] [Google Scholar]
- 56.Shanks N., Larocque S., Meaney M. J. 1995. Neonatal endotoxin exposure alters the development of the hypothalamic–pituitary–adrenal axis: early illness and later responsivity to stress. J. Neurosci. 15, 376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorrells S. F., Sapolsky R. M. 2007. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 21, 259–272 10.1016/j.bbi.2006.11.006 (doi:10.1016/j.bbi.2006.11.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spencer S. J., Heida J. G., Pittman Q. J. 2005. Early life immune challenge—effects on behavioural indices of adult rat fear and anxiety. Behav. Brain Res. 164, 231–238 10.1016/j.bbr.2005.06.032 (doi:10.1016/j.bbr.2005.06.032) [DOI] [PubMed] [Google Scholar]
- 59.Grindstaff J. L., Brodie E. D., III, Ketterson E. D. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. Lond. B 270, 2309–2319 10.1098/rspb.2003.2485 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gervasi S. S., Foufopoulos J. 2008. Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct. Ecol. 22, 100–108 10.1111/j.1365-2435.2007.01340.x (doi:10.1111/j.1365-2435.2007.01340.x) [DOI] [Google Scholar]
- 61.Berger S., Martin L. B., II, Wikelski M., Romero L. M., Kalko E. K. V., Vitousek M. N., Rödl T. 2005. Corticosterone suppresses immune activity in territorial Galápagos marine iguanas during reproduction. Horm. Behav. 47, 419–429 10.1016/j.yhbeh.2004.11.011 (doi:10.1016/j.yhbeh.2004.11.011) [DOI] [PubMed] [Google Scholar]
- 62.Loiseau C., Sorci G., Dano S., Chastel O. 2008. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen. Comp. Endocrinol. 155, 101–108 10.1016/j.ygcen.2007.03.004 (doi:10.1016/j.ygcen.2007.03.004) [DOI] [PubMed] [Google Scholar]
- 63.McEwen B. S., et al. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Rev. 23, 79–133 10.1016/S0165-0173(96)00012-4 (doi:10.1016/S0165-0173(96)00012-4) [DOI] [PubMed] [Google Scholar]
- 64.Rubolini D., Romano M., Boncoraglio G., Ferrari R. P., Martinelli R., Galeotti P., Fasola M., Saino N. 2005. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm. Behav. 47, 592–605 10.1016/j.yhbeh.2005.01.006 (doi:10.1016/j.yhbeh.2005.01.006) [DOI] [PubMed] [Google Scholar]
- 65.Apanius V. 1998. The immune system. Oxford Ornithol. Ser. 8, 203–222 [Google Scholar]
- 66.French S. S., DeNardo D. F., Moore M. C. 2007. Trade-offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction. Am. Nat. 170, 79–89 10.1086/518569 (doi:10.1086/518569) [DOI] [PubMed] [Google Scholar]
- 67.French S. S., Moore M. C., Demas G. E. 2009. Ecological immunology: the organism in context. Integr. Comp. Biol. 49, 246–253 10.1093/icb/icp032 (doi:10.1093/icb/icp032) [DOI] [PubMed] [Google Scholar]
- 68.Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 69.Munck A., Guyre P. M., Holbrook N. J. 1984. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 5, 25–44 10.1210/edrv-5-1-25 (doi:10.1210/edrv-5-1-25) [DOI] [PubMed] [Google Scholar]
- 70.Dhabhar F. S., McEwen B. S. 1997. Acute stress enhances while chronic stress suppresses immune function in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 11, 286–306 10.1006/brbi.1997.0508 (doi:10.1006/brbi.1997.0508) [DOI] [PubMed] [Google Scholar]
- 71.Dhabhar F. S. 2002. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav. Immunity 16, 785–798 10.1016/S0889-1591(02)00036-3 (doi:10.1016/S0889-1591(02)00036-3) [DOI] [PubMed] [Google Scholar]
- 72.Fleshner M., Nguyen K. T., Cotter C. S., Watkins L. R., Maier S. F. 1998. Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 275, R870–R878 [DOI] [PubMed] [Google Scholar]
- 73.Roberts M. L., Buchanan K. L., Hasselquist D., Evans M. R. 2007. Effects of testosterone and corticosterone on immunocompetence in the zebra finch. Horm. Behav. 51, 126–134 10.1016/j.yhbeh.2006.09.004 (doi:10.1016/j.yhbeh.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 74.Mills S. C., Grapputo A., Jokinen I., Koskela E., Mappes T., Poikonen T. 2010. Fitness trade offs mediated by immunosuppression costs in a small mammal. Evolution 64, 166–279 10.1111/j.1558-5646.2009.00820.x (doi:10.1111/j.1558-5646.2009.00820.x) [DOI] [PubMed] [Google Scholar]
- 75.Blaustein A. R., Gervasi S. S., Johnson P. T. J., Hoverman J. T., Belden L. K., Bradley P. W., Xie G. Y. 2012. Ecophysiology meets conservation: understanding the role of disease in amphibian population declines. Phil. Trans. R. Soc. B 367, 1688–1707 10.1098/rstb.2012.0011 (doi:10.1098/rstb.2012.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McEwen B. S., Wingfield J. C. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 10.1016/S0018-506X(02)00024-7 (doi:10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 77.Bradshaw D. 2007. Environmental endocrinology. Gen. Comp. Endocrinol. 152, 125–141 10.1016/j.ygcen.2006.12.026 (doi:10.1016/j.ygcen.2006.12.026) [DOI] [PubMed] [Google Scholar]
- 78.Wingfield J. C. 1985. Influences of weather on reproductive function in male song sparrows, Melospiza melodia. J. Zool. 205, 525–544 10.1111/j.1469-7998.1985.tb03542.x (doi:10.1111/j.1469-7998.1985.tb03542.x) [DOI] [Google Scholar]
- 79.Wingfield J. C., Ramenofsky M. 1997. Corticosterone and facultative dispersal in response to unpredictable events. Ardea 85, 155 [Google Scholar]
- 80.Wingfield J. C. 2008. Comparative endocrinology, environment and global change. Gen. Comp. Endocrinol. 157, 207–216 10.1016/j.ygcen.2008.04.017 (doi:10.1016/j.ygcen.2008.04.017) [DOI] [PubMed] [Google Scholar]
- 81.O'Reilly K. M., Wingfield J. C. 2001. Ecological factors underlying the adrenocortical response to capture stress in arctic-breeding shorebirds. Gen. Comp. Endocrinol. 124, 1–11 10.1006/gcen.2001.7676 (doi:10.1006/gcen.2001.7676) [DOI] [PubMed] [Google Scholar]
- 82.Astheimer L. B., Buttemer W. A., Wingfield J. C. 1995. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Horm. Behav. 29, 442–457 10.1006/hbeh.1995.1276 (doi:10.1006/hbeh.1995.1276) [DOI] [PubMed] [Google Scholar]
- 83.Silverin B., Arvidsson B., Wingfield J. 1997. The adrenocortical responses to stress in breeding willow warblers Phylloscopus trochilus in Sweden: effects of latitude and gender. Funct. Ecol. 11, 376–384 10.1046/j.1365-2435.1997.00097.x (doi:10.1046/j.1365-2435.1997.00097.x) [DOI] [Google Scholar]
- 84.Romero L. M., Wikelski M. 2001. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370 10.1073/pnas.131091498 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wikelski M., Romero L. M. 2003. Body size, performance and fitness in Galapagos marine iguanas. Integr. Comp. Biol. 43, 376–386 10.1093/icb/43.3.376 (doi:10.1093/icb/43.3.376) [DOI] [PubMed] [Google Scholar]
- 86.de Bruijn R., Romero L. M. 2011. Behavioral and physiological responses of wild-caught European starlings (Sturnus vulgaris) to a minor, rapid change in ambient temperature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 160, 260–266 10.1016/j.cbpa.2011.06.011 (doi:10.1016/j.cbpa.2011.06.011) [DOI] [PubMed] [Google Scholar]
- 87.Ellenberg U., Setiawan A. N., Cree A., Houston D. M., Seddon P. J. 2007. Elevated hormonal stress response and reduced reproductive output in yellow-eyed penguins exposed to unregulated tourism. Gen. Comp. Endocrinol. 152, 54–63 10.1016/j.ygcen.2007.02.022 (doi:10.1016/j.ygcen.2007.02.022) [DOI] [PubMed] [Google Scholar]
- 88.Walker B. G., Boersma P. D., Wingfield J. C. 2005. Field endocrinology and conservation biology. Integr. Comp. Biol. 45, 12–18 10.1093/icb/45.1.12 (doi:10.1093/icb/45.1.12) [DOI] [PubMed] [Google Scholar]
- 89.Bonier F., Martin P. R., Sheldon K. S., Jensen J. P., Foltz S. L., Wingfield J. C. 2007. Sex-specific consequences of life in the city. Behav. Ecol. 18, 121–129 10.1093/beheco/arl050 (doi:10.1093/beheco/arl050) [DOI] [Google Scholar]
- 90.Partecke J., Schwabl I., Gwinner E. 2006. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87, 1945–1952 10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 91.Trompeter W. P., Langkilde T. 2011. Invader danger: lizards faced with novel predators exhibit an altered behavioral response to stress. Horm. Behav. 60, 152–158 10.1016/j.yhbeh.2011.04.001 (doi:10.1016/j.yhbeh.2011.04.001) [DOI] [PubMed] [Google Scholar]
- 92.Berger S., Wikelski M., Romero L. M., Kalko E. K. V., Rödl T. 2007. Behavioral and physiological adjustments to new predators in an endemic island species, the Galápagos marine iguana. Horm. Behav. 52, 653–663 10.1016/j.yhbeh.2007.08.004 (doi:10.1016/j.yhbeh.2007.08.004) [DOI] [PubMed] [Google Scholar]
- 93.Romero L. M., Wikelski M. 2002. Severe effects of low-level oil contamination on wildlife predicted by the corticosterone-stress response: preliminary data and a research agenda. Spill Sci. Technol. Bull. 7, 309–313 10.1016/S1353-2561(02)00067-1 (doi:10.1016/S1353-2561(02)00067-1) [DOI] [Google Scholar]
- 94.Hill R. L., Janz D. M. 2003. Developmental estrogenic exposure in zebrafish (Danio rerio). I. Effects on sex ratio and breeding success . Aquat. Toxicol. 63, 417–429 10.1016/S0166-445X(02)00207-2 (doi:10.1016/S0166-445X(02)00207-2) [DOI] [PubMed] [Google Scholar]
- 95.Clouzot L., Marrot B., Doumenq P., Roche N. 2008. 17α-Ethinylestradiol: an endocrine disrupter of great concern. Analytical methods and removal processes applied to water purification. A review. Environ. Prog. 27, 383–396 10.1002/ep.10291 (doi:10.1002/ep.10291) [DOI] [Google Scholar]
- 96.Ying G. G., Kookana R. S., Ru Y. J. 2002. Occurrence and fate of hormone steroids in the environment. Environ. Int. 28, 545–551 10.1016/S0160-4120(02)00075-2 (doi:10.1016/S0160-4120(02)00075-2) [DOI] [PubMed] [Google Scholar]
- 97.Partridge C., Boettcher A., Jones A. G. 2010. Short-term exposure to a synthetic estrogen disrupts mating dynamics in a pipefish. Horm. Behav. 58, 800–807 10.1016/j.yhbeh.2010.08.002 (doi:10.1016/j.yhbeh.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 98.Bell A. M. 2001. Effects of an endocrine disrupter on courtship and aggressive behaviour of male three-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 62, 775–780 10.1006/anbe.2001.1824 (doi:10.1006/anbe.2001.1824) [DOI] [Google Scholar]
- 99.Orlando E. F., Guillette L. J., Jr 2007. Sexual dimorphic responses in wildlife exposed to endocrine disrupting chemicals. Environ. Res. 104, 163–173 10.1016/j.envres.2006.06.002 (doi:10.1016/j.envres.2006.06.002) [DOI] [PubMed] [Google Scholar]
- 100.Willingham T., Rhen T., Sakata J. T., Crews D. 2000. Embryonic treatment with xenobiotics disrupts steroid hormone profiles in hatchling red-eared turtles (Trachemys scripta elegans). Environ. Health Perspect. 108, 329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guillette L. J., Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. 1996. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen. Comp. Endocrinol. 101, 32–42 10.1006/gcen.1996.0005 (doi:10.1006/gcen.1996.0005) [DOI] [PubMed] [Google Scholar]
- 102.Guillette L. J., Crain D. A., Galle A., Gunderson M. P., Kools S. A. E., Milnes M. R., Orlando E. F., Rooney A. A., Woodward A. R. 2000. Alligators and endocrine disrupting contaminants: a current perspective. Am. Zool. 40, 438–452 10.1093/icb/40.3.438 (doi:10.1093/icb/40.3.438) [DOI] [Google Scholar]
- 103.Milnes M. R., Woodward A. R., Rooney A. A., Guillette L. J. 2002. Plasma steroid concentrations in relation to size and age in juvenile alligators from two Florida lakes. Comp. Biochem. Physiol. A 131, 923–930 10.1016/S1095-6433(02)00025-9 (doi:10.1016/S1095-6433(02)00025-9) [DOI] [PubMed] [Google Scholar]
- 104.Knapp R. 2003. Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integr. Comp. Biol. 43, 658–668 10.1093/icb/43.5.658 (doi:10.1093/icb/43.5.658) [DOI] [PubMed] [Google Scholar]
- 105.Nelson R. J. 2011. An introduction to behavioral endocrinology, 4th edn. Sunderland, MA: Sinauer [Google Scholar]
- 106.Ricklefs R. E., Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.1016/S0169-5347(02)02578-8 (doi:10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 107.Dufty A. M., Jr, Clobert J., Møller A. P. 2002. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 17, 190–196 10.1016/S0169-5347(02)02498-9 (doi:10.1016/S0169-5347(02)02498-9) [DOI] [Google Scholar]
- 108.Axelrod J., Reisine T. D. 1984. Stress hormones: their interaction and regulation. Science 224, 452–459 10.1126/science.6143403 (doi:10.1126/science.6143403) [DOI] [PubMed] [Google Scholar]
- 109.Ketterson E. D., Nolan V. 1999. Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 154, S4–S25 10.1086/303280 (doi:10.1086/303280) [DOI] [PubMed] [Google Scholar]
- 110.McGlothlin J. W., Ketterson E. D. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 10.1098/rstb.2007.0002 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Enstrom D. A., Ketterson E. D., Nolan V. 1997. Testosterone and mate choice in the dark-eyed junco. Anim. Behav. 54, 1135–1146 10.1006/anbe.1997.0555 (doi:10.1006/anbe.1997.0555) [DOI] [Google Scholar]
- 112.Ketterson E. D., Nolan V., Jr, Wolf L., Ziegenfus C. 1992. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat. 140, 980–999 [Google Scholar]
- 113.Casto J. M., Nolan V., Jr, Ketterson E. D. 2001. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis). Am. Nat. 157, 408–420 10.1086/319318 (doi:10.1086/319318) [DOI] [PubMed] [Google Scholar]
- 114.Ketterson E. D., Nolan V., Jr, Wolf L., Ziegenfus C., Dufty A. M., Jr, Ball G. F., Johnsen T. S. 1991. Testosterone and avian life histories: the effect of experimentally elevated testosterone on corticosterone and body mass in dark-eyed juncos. Horm. Behav. 25, 489–503 10.1016/0018-506X(91)90016-B (doi:10.1016/0018-506X(91)90016-B) [DOI] [PubMed] [Google Scholar]
- 115.Ketterson E. D., Nolan V., Jr, Cawthorn M. J., Parker P. G., Ziegenfus C. 1996. Phenotypic engineering: using hormones to explore the mechanistic and functional bases of phenotypic variation in nature. Ibis 138, 70–86 [Google Scholar]
- 116.Reed W. L., Clark M. E., Parker P. G., Raouf S. A., Arguedas N., Monk D. S., Snajdr E., Nolan V., Jr, Ketterson E. D. 2006. Physiological effects on demography: a long-term experimental study of testosterone's effects on fitness. Am. Nat. 167, 667–683 10.1086/503054 (doi:10.1086/503054) [DOI] [PubMed] [Google Scholar]
- 117.Mills S. C., Hazard L., Lancaster L., Mappes T., Miles D., Oksanen T. A., Sinervo B. 2008. Gonadotropin hormone modulation of testosterone, immune function, performance, and behavioral trade-offs among male morphs of the lizard Uta stansburiana. Am. Nat. 171, 339–357 10.1086/527520 (doi:10.1086/527520) [DOI] [PubMed] [Google Scholar]
- 118.Sinervo B., Licht P. 1991. Hormonal and physiological control of clutch size, egg size, and egg shape in side-blotched lizards (Uta stansburiana): constraints on the evolution of lizard life histories. J. Exp. Zool. 257, 252–264 10.1002/jez.1402570216 (doi:10.1002/jez.1402570216) [DOI] [Google Scholar]
- 119.Lancaster L. T., Hazard L. C., Clobert J., Sinervo B. R. 2008. Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J. Evol. Biol. 21, 556–565 10.1111/j.1420-9101.2007.01478.x (doi:10.1111/j.1420-9101.2007.01478.x) [DOI] [PubMed] [Google Scholar]
- 120.Arnold S. J. 1983. Morphology, performance and fitness. Am. Zool. 23, 347–361 10.1093/icb/23.2.347 (doi:10.1093/icb/23.2.347) [DOI] [Google Scholar]
- 121.Garland T., Jr, Losos J. B. 1994. Ecological morphology of locomotor performance in squamate reptiles. In Ecological morphology: integrative organismal biology (eds Wainwright P. C., Reilly S. M.), pp. 240–302 Chicago, IL: University of Chicago Press [Google Scholar]
- 122.Irschick D. J., Le Galliard J. 2008. Studying the evolution of whole-organism performance capacity: sex, selection, and haiku—an introduction. Evol. Ecol. Res. 10, 155–156 [Google Scholar]
- 123.Irschick D. J., Meyers J. J., Husak J. F., Le Galliard J. 2008. How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol. Ecol. Res. 10, 177–196 [Google Scholar]
- 124.Miles D. B. 1994. Population differentiation in locomotor performance and the potential response of a terrestrial organism to global environmental change. Am. Zool. 34, 422–436 10.1093/icb/34.3.422 (doi:10.1093/icb/34.3.422) [DOI] [Google Scholar]
- 125.Aerts P., Van Damme R., Vanhooydonck B., Zaaf A., Herrel A. 2000. Lizard locomotion: how morphology meets ecology. Neth. J. Zool. 50, 261–277 10.1163/156854200X00126 (doi:10.1163/156854200X00126) [DOI] [Google Scholar]
- 126.Miles D. B. 1994. Covariation between morphology and locomotory performance in Sceloporine lizards. In Lizard ecology: historical and evolutionary perspectives (eds Vitt L. J., Pianka E. R.), pp. 207–236 Princeton, NJ: Princeton University Press [Google Scholar]
- 127.Bennett A. F., Huey R. B. 1990. Studying the evolution of physiological performance. Oxford Surv. Evol. Biol. 7, 251–284 [Google Scholar]
- 128.Goodman B. A., Miles D. B., Schwarzkopf L. 2008. Life on the rocks: habitat use drives morphological and performance evolution in lizards. Ecology 89, 3462–3471 10.1890/07-2093.1 (doi:10.1890/07-2093.1) [DOI] [PubMed] [Google Scholar]
- 129.Robson M. A., Miles D. B. 2000. Locomotor performance and dominance in male tree lizards, Urosaurus ornatus. Funct. Ecol. 14, 338–344 10.1046/j.1365-2435.2000.00427.x (doi:10.1046/j.1365-2435.2000.00427.x) [DOI] [Google Scholar]
- 130.Johnson E. O., Kamilaris T. C., Chrousos G. P., Gold P. W. 1992. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 16, 115–130 10.1016/S0149-7634(05)80175-7 (doi:10.1016/S0149-7634(05)80175-7) [DOI] [PubMed] [Google Scholar]
- 131.Cote J., Clobert J., Meylan S., Fitze P. S. 2006. Experimental enhancement of corticosterone levels positively affects subsequent male survival. Horm. Behav. 49, 320–327 10.1016/j.yhbeh.2005.08.004 (doi:10.1016/j.yhbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 132.Bradley A. J. 1987. Stress and mortality in the Red-tailed Phascogale, Phascogale calura (Marsupialia: Dasyuridae). Gen. Comp. Endocrinol. 67, 85–100 10.1016/0016-6480(87)90208-5 (doi:10.1016/0016-6480(87)90208-5) [DOI] [PubMed] [Google Scholar]
- 133.Devenport L., Doughty D., Heiberger B., Burton D., Brown R., Stith R. 1993. Exercise endurance in rats: roles of type I and II corticosteroid receptors. Physiol. Behav. 53, 1171–1175 10.1016/0031-9384(93)90375-P (doi:10.1016/0031-9384(93)90375-P) [DOI] [PubMed] [Google Scholar]
- 134.Wolkowitz O. M. 1994. Prospective controlled studies of the behavioral and biological effects of exogenous corticosteroids. Psychoneuroendocrinology 19, 233–255 10.1016/0306-4530(94)90064-7 (doi:10.1016/0306-4530(94)90064-7) [DOI] [PubMed] [Google Scholar]
- 135.Breuner C. W., Greenberg A. L., Wingfield J. C. 1998. Noninvasive corticosterone treatment rapidly increases activity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen. Comp. Endocrinol. 111, 386–394 10.1006/gcen.1998.7128 (doi:10.1006/gcen.1998.7128) [DOI] [PubMed] [Google Scholar]
- 136.Holberton R. L. 1999. Changes in patterns of corticosterone secretion concurrent with migratory fattening in a Neotropical migratory bird. Gen. Comp. Endocrinol. 116, 49–58 10.1006/gcen.1999.7336 (doi:10.1006/gcen.1999.7336) [DOI] [PubMed] [Google Scholar]
- 137.Silverin B. 1997. The stress response and autumn dispersal behaviour in willow tits. Anim. Behav. 53, 451–459 10.1006/anbe.1996.0295 (doi:10.1006/anbe.1996.0295) [DOI] [Google Scholar]
- 138.Belliure J., Carrascal L. M., Diaz J. A. 1996. Covariation of thermal biology and foraging mode in two Mediterranean lacertid lizards. Ecology 77, 1163–1173 [Google Scholar]
- 139.Miles D. B., Calsbeek R., Sinervo B. 2007. Corticosterone, locomotor performance, and metabolism in side-blotched lizards (Uta stansburiana). Horm. Behav. 51, 548–554 10.1016/j.yhbeh.2007.02.005 (doi:10.1016/j.yhbeh.2007.02.005) [DOI] [PubMed] [Google Scholar]
- 140.Cash W. B., Holberton R. L. 1999. Effects of exogenous corticosterone on locomotor activity in the red eared slider turtle, Trachemys scripta elegans. J. Exp. Zool. 284, 637–644 10.1002/(SICI)1097-010X(19991101)284:6%3C637::AID-JEZ5%3E3.0.CO;2-N (doi:10.1002/(SICI)1097-010X(19991101)284:6<637::AID-JEZ5>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 141.Meylan S., Clobert J. 2004. Maternal effects on offspring locomotion: influence of density and corticosterone elevation in the lizard Lacerta vivipara. Physiol. Biochem. Zool. 77, 450–458 10.1086/383508 (doi:10.1086/383508) [DOI] [PubMed] [Google Scholar]
- 142.Silverin B. 1998. Stress responses in birds. Poult. Avian Biol. Rev. UK 9, 153–168 [Google Scholar]
- 143.Wikelski M., Lynn S., Breuner J. C., Wingfield J. C., Kenagy G. J. 1999. Energy metabolism, testosterone and corticosterone in white-crowned sparrows. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 185, 463–470 [Google Scholar]
- 144.Breuner C. W., Patterson S. H., Hahn T. P. 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 10.1016/j.ygcen.2008.05.017 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 145.Bonier F., Martin P. R., Moore I. T., Wingfield J. C. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 10.1016/j.tree.2009.04.013 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 146.Cabezas S., Blas J., Marchant T. A., Moreno S. 2007. Physiological stress levels predict survival probabilities in wild rabbits. Horm. Behav. 51, 313–320 10.1016/j.yhbeh.2006.11.004 (doi:10.1016/j.yhbeh.2006.11.004) [DOI] [PubMed] [Google Scholar]
- 147.Sinervo B., DeNardo D. F. 1996. Costs of reproduction in the wild: path analysis of natural selection and experimental tests of causation. Evolution 50, 1299–1313 [DOI] [PubMed] [Google Scholar]
- 148.Brown C. R., Brown M. B., Raouf S. A., Smith L. C., Wingfield J. C. 2005. Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecology 86, 1034–1046 [Google Scholar]
- 149.Angelier F., Wingfield J. C., Weimerskirch H., Chastel O. 2010. Hormonal correlates of individual quality in a long-lived bird: a test of the corticosterone–fitness hypothesis. Biol. Lett. 6, 846–849 10.1098/rsbl.2010.0376 (doi:10.1098/rsbl.2010.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meylan S., Clobert J. 2005. Is corticosterone-mediated phenotype development adaptive? Maternal corticosterone treatment enhances survival in male lizards. Horm. Behav. 48, 44–52 10.1016/j.yhbeh.2004.11.022 (doi:10.1016/j.yhbeh.2004.11.022) [DOI] [PubMed] [Google Scholar]
- 151.Adkins-Regan E. 2008. Do hormonal control systems produce evolutionary inertia? Phil. Trans. R. Soc. B 363, 819–825 10.1098/rspb.2006.3764 (doi:10.1098/rspb.2006.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zera A. J., Harshman L. G., Williams T. D. 2007. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 38, 793–817 10.1146/annurev.ecolsys.38.091206.095615 (doi:10.1146/annurev.ecolsys.38.091206.095615) [DOI] [Google Scholar]
- 153.Sinervo B., Calsbeek R. 2003. Physiological epistasis, ontogenetic conflict and natural selection on physiology and life history. Integr. Comp. Biol. 43, 419–430 10.1093/icb/43.3.419 (doi:10.1093/icb/43.3.419) [DOI] [PubMed] [Google Scholar]
- 154.Pollard I. 1986. Prenatal stress effects over two generations in rats. J. Endocrinol. 109, 239–244 10.1677/joe.0.1090239 (doi:10.1677/joe.0.1090239) [DOI] [PubMed] [Google Scholar]
- 155.Takahashi L. K., Kalin N. H., Barksdale C. M., Vanden Burgt J. A., Brownfield M. S. 1988. Stressor controllability during pregnancy influences pituitary–adrenal hormone concentrations and analgesic responsiveness in offspring. Physiol. Behav. 42, 323–329 10.1016/0031-9384(88)90273-9 (doi:10.1016/0031-9384(88)90273-9) [DOI] [PubMed] [Google Scholar]
- 156.Takahashi L. K. 1998. Prenatal stress: consequences of glucocorticoids on hippocampal development and function. Int. J. Dev. Neurosci. 16, 199–207 10.1016/S0736-5748(98)00020-3 (doi:10.1016/S0736-5748(98)00020-3) [DOI] [PubMed] [Google Scholar]
- 157.Clark M. M., Galef B. G. 1995. Prenatal influences on reproductive life-history strategies. Trends Ecol. Evol. 10, 151–153 10.1016/S0169-5347(00)89025-4 (doi:10.1016/S0169-5347(00)89025-4) [DOI] [PubMed] [Google Scholar]
- 158.Uller T., Astheimer L., Olsson M. 2007. Consequences of maternal yolk testosterone for offspring development and survival: experimental test in a lizard. Funct. Ecol. 21, 544–551 10.1111/j.1365-2435.2007.01264.x (doi:10.1111/j.1365-2435.2007.01264.x) [DOI] [Google Scholar]
- 159.Eising C. M., Eikenaar C., Schwabl H., Groothuis T. G. G. 2001. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. Lond. B 268, 839–846 10.1098/rspb.2001.1594 (doi:10.1098/rspb.2001.1594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwabl H. 1995. Individual variation of the acute adrenocortical response to stress in the white-throated sparrow. Zool. Anal. Complex Syst. 99, 113–120 [Google Scholar]
- 161.Eising C. M., Müller W., Dijkstra C., Groothuis T. G. G. 2003. Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen. Comp. Endocrinol. 132, 241–247 10.1016/S0016-6480(03)00090-X (doi:10.1016/S0016-6480(03)00090-X) [DOI] [PubMed] [Google Scholar]
- 162.Boncoraglio G., Groothuis T. G. G., von Engelhardt N. 2011. Differential maternal testosterone allocation among siblings benefits both mother and offspring in the Zebra Finch Taeniopygia guttata. Am. Nat. 178, 64–74 10.1086/660278 (doi:10.1086/660278) [DOI] [PubMed] [Google Scholar]
- 163.Eriksen M. S., Bakken M., Espmark Å., Braastad B. O., Salte R. 2006. Prespawning stress in farmed Atlantic salmon Salmo salar: maternal cortisol exposure and hyperthermia during embryonic development affect offspring survival, growth and incidence of malformations. J. Fish Biol. 69, 114–129 10.1111/j.1095-8649.2006.01071.x (doi:10.1111/j.1095-8649.2006.01071.x) [DOI] [Google Scholar]
- 164.Gagliano M., McCormick M. 2009. Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia 160, 657–665 10.1007/s00442-009-1335-8 (doi:10.1007/s00442-009-1335-8) [DOI] [PubMed] [Google Scholar]
- 165.Saino N., Romano M., Ferrari R. P., Martinelli R., Møller A. P. 2005. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J. Exp. Zool. Part A Comp. Exp. Biol. 303A, 998–1006 10.1002/jez.a.224 (doi:10.1002/jez.a.224) [DOI] [PubMed] [Google Scholar]
- 166.Mashaly M. M. 1991. Effect of exogenous corticosterone on chicken embryonic development. Poult. Sci. 70, 371. 10.3382/ps.0700371 (doi:10.3382/ps.0700371) [DOI] [PubMed] [Google Scholar]
- 167.Love O. P., Chin E. H., Wynne-Edwards K. E., Williams T. D. 2005. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 166, 751–766 10.1086/497440 (doi:10.1086/497440) [DOI] [PubMed] [Google Scholar]
- 168.Cree A., Tyrrell C. L., Preest M. R., Thorburn D., Guillette L. J. 2003. Protecting embryos from stress: corticosterone effects and the corticosterone response to capture and confinement during pregnancy in a live-bearing lizard (Hoplodactylus maculatus). Gen. Comp. Endocrinol. 134, 316–329 10.1016/S0016-6480(03)00282-X (doi:10.1016/S0016-6480(03)00282-X) [DOI] [PubMed] [Google Scholar]
- 169.Hayward L. S., Wingfield J. C. 2004. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen. Comp. Endocrinol. 135, 365–371 10.1016/j.ygcen.2003.11.002 (doi:10.1016/j.ygcen.2003.11.002) [DOI] [PubMed] [Google Scholar]
- 170.Silverin B. 1986. Corticosterone-binding proteins and behavioral effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 64, 67–74 [DOI] [PubMed] [Google Scholar]
- 171.Robert K. A., Vleck C., Bronikowski A. M. 2009. The effects of maternal corticosterone levels on offspring behavior in fast-and slow-growth garter snakes (Thamnophis elegans). Horm. Behav. 55, 24–32 10.1016/j.yhbeh.2008.07.008 (doi:10.1016/j.yhbeh.2008.07.008) [DOI] [PubMed] [Google Scholar]
- 172.Sockman K. W., Schwabl H. 2000. Yolk androgens reduce offspring survival. Proc. R. Soc. Lond. B 267, 1451–1456 10.1098/rspb.2000.1163 (doi:10.1098/rspb.2000.1163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hegyi G., Schwabl H. 2010. Do different yolk androgens exert similar effects on the morphology or behaviour of Japanese quail hatchlings Coturnix japonica? J. Avian Biol. 41, 258–265 (doi:10.1111/j.1600-048X.2009.04787.x') [Google Scholar]
- 174.Rubolini D., Romano M., Martinelli R., Saino N. 2006. Effects of elevated yolk testosterone levels on survival, growth and immunity of male and female yellow-legged gull chicks. Behav. Ecol. Sociobiol. 59, 344–352 10.1007/s00265-005-0057-0 (doi:10.1007/s00265-005-0057-0) [DOI] [Google Scholar]
- 175.Weinstock M. 2008. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 32, 1073–1086 10.1016/j.neubiorev.2008.03.002 (doi:10.1016/j.neubiorev.2008.03.002) [DOI] [PubMed] [Google Scholar]
- 176.Herrenkohl L. R. 1979. Prenatal stress reduces fertility and fecundity in female offspring. Science 206, 1097–1099 10.1126/science.573923 (doi:10.1126/science.573923) [DOI] [PubMed] [Google Scholar]
- 177.Zielinski W. J., Vandenbergh J. G., Montano M. M. 1991. Effects of social stress and intrauterine position on sexual phenotype in wild-type house mice (Mus musculus). Physiol. Behav. 49, 117–123 10.1016/0031-9384(91)90241-F (doi:10.1016/0031-9384(91)90241-F) [DOI] [PubMed] [Google Scholar]
- 178.Love O. P., Williams T. D. 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat. 172, E135–E149 10.1086/590959 (doi:10.1086/590959) [DOI] [PubMed] [Google Scholar]
- 179.Love O. P., Gilchrist H. G., Bety J., Wynne-Edwards K. E., Berzins L., Williams T. D. 2009. Using life-histories to predict and interpret variability in yolk hormones. Gen. Comp. Endocrinol. 163, 169–174 10.1016/j.ygcen.2008.10.001 (doi:10.1016/j.ygcen.2008.10.001) [DOI] [PubMed] [Google Scholar]
- 180.Crean A. J., Marshall D. J. 2009. Coping with environmental uncertainty: dynamic bet hedging as a maternal effect. Phil. Trans. R. Soc. B 364, 1087–1096 10.1098/rstb.2008.0237 (doi:10.1098/rstb.2008.0237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hoffman A. A., Parsons P. A. () 1991. Evolutionary genetics and environmental stress. Oxford, UK: Oxford University Press [Google Scholar]
- 182.Einum S., Fleming I. A. 2004. Environmental unpredictability and offspring size: conservative versus diversified bet-hedging. Evol. Ecol. Res. 6, 443–455 [Google Scholar]
- 183.Marshall D. J., Uller T. 2007. When is a maternal effect adaptive. Oikos 116, 1957–1963 10.1111/j.2007.0030-1299.16203.x (doi:10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 184.Cadby C. D., Jones S. M., Wapstra E. 2010. Are increased concentrations of maternal corticosterone adaptive to offspring? A test using a placentotrophic lizard. Funct. Ecol. 24, 409–416 10.1111/j.1365-2435.2009.01637.x (doi:10.1111/j.1365-2435.2009.01637.x) [DOI] [Google Scholar]
- 185.Meylan S., Belliure J., Clobert J., de Fraipont M. 2002. Stress and body condition as prenatal and postnatal determinants of dispersal in the Common Lizard (Lacerta vivipara). Horm. Behav. 42, 319–326 10.1006/hbeh.2002.1827 (doi:10.1006/hbeh.2002.1827) [DOI] [PubMed] [Google Scholar]
- 186.Moore I. T., Jessop T. S. 2003. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm. Behav. 43, 39–47 10.1016/S0018-506X(02)00038-7 (doi:10.1016/S0018-506X(02)00038-7) [DOI] [PubMed] [Google Scholar]
- 187.Morici L. A., Elsey R. M., Lance V. A. 1997. Effects of long-term corticosterone implants on growth and immune function in juvenile alligators, Alligator mississippiensis. J. Exp. Zool. 279, 156–162 10.1002/(SICI)1097-010X(19971001)279:2%3C156::AID-JEZ6%3E3.0.CO;2-N (doi:10.1002/(SICI)1097-010X(19971001)279:2<156::AID-JEZ6>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- 188.Räsänen K., Kruuk L. E. B. 2007. Maternal effects and evolution at ecological time-scales. Funct. Ecol. 21, 408–421 10.1111/j.1365-2435.2007.01246.x (doi:10.1111/j.1365-2435.2007.01246.x) [DOI] [Google Scholar]
- 189.Clobert J., Le Galliard J. F., Cote J., Meylan S., Massot M. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209 [DOI] [PubMed] [Google Scholar]
- 190.Tschirren B., Saladin V., Fitze P. S., Schwabl H., Richner H. 2005. Maternal yolk testosterone does not modulate parasite susceptibility or immune function in great tit nestlings. J. Anim. Ecol. 74, 675–682 10.1111/j.1365-2656.2005.00963.x (doi:10.1111/j.1365-2656.2005.00963.x) [DOI] [Google Scholar]
- 191.Tschirren B., Fitze P. S., Richner H. 2007. Maternal modulation of natal dispersal in a passerine bird: an adaptive strategy to cope with parasitism. Am. Nat. 169, 87–93 10.1086/509945 (doi:10.1086/509945) [DOI] [PubMed] [Google Scholar]
- 192.Tschirren B., Richner H., Schwabl H. 2004. Ectoparasite-modulated deposition of maternal androgens in great tit eggs. Proc. R. Soc. Lond. B 271, 1371–1375 10.1098/rspb.2004.2730 (doi:10.1098/rspb.2004.2730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Provan J., Beatty G. E., Keating S. L., Maggs C. A., Savidge G. 2009. High dispersal potential has maintained long-term population stability in the North Atlantic copepod Calanus finmarchicus. Proc. R. Soc. B 276, 301–307 10.1098/rspb.2008.1062 (doi:10.1098/rspb.2008.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hanski I., Erälahti C., Kankare M., Ovaskainen O., Sirén H. 2004. Variation in migration propensity among individuals maintained by landscape structure. Ecol. Lett. 7, 958–966 10.1111/j.1461-0248.2004.00654.x (doi:10.1111/j.1461-0248.2004.00654.x) [DOI] [Google Scholar]
- 195.Plaistow S. J., Benton T. G. 2009. The influence of context-dependent maternal effects on population dynamics: an experimental test. Phil. Trans. R. Soc. B 364, 1049–1058 10.1098/rstb.2008.0251 (doi:10.1098/rstb.2008.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Benton T. G., St Clair J. J. H., Plaistow S. J. 2008. Maternal effects mediated by maternal age: from life histories to population dynamics. J. Anim. Ecol. 77, 1038–1046 10.1111/j.1365-2656.2008.01434.x (doi:10.1111/j.1365-2656.2008.01434.x) [DOI] [PubMed] [Google Scholar]
- 197.Le Galliard J.-F., Marquis O., Massot M. 2010. Cohort variation, climate effects and population dynamics in a short-lived lizard. J. Anim. Ecol. 79, 1296–1307 10.1111/j.1365-2656.2010.01732.x (doi:10.1111/j.1365-2656.2010.01732.x) [DOI] [PubMed] [Google Scholar]
- 198.Sinervo B., Lively C. M. 1996. The rock–paper–scissors game and the evolution of alternative male strategies. Nature 380, 240–243 10.1038/380240a0 (doi:10.1038/380240a0) [DOI] [Google Scholar]
- 199.Sinervo B., Heulin B., Surget-Groba Y., Clobert J., Miles D. B., Corl A., Chaine A., Davis A. 2007. Models of density-dependent genic selection and a new rock–papers–scissors social system. Am. Nat. 170, 663–680 10.1086/522092 (doi:10.1086/522092) [DOI] [PubMed] [Google Scholar]
- 200.Chitty D. 1960. Population processes in the vole and their relevance to general theory. Can. J. Zool. 38, 99–113 10.1139/z60-011 (doi:10.1139/z60-011) [DOI] [Google Scholar]
- 201.Inchausti P., Ginzburg L. R. 1998. Small mammals cycles in northern Europe: patterns and evidence for a maternal effect hypothesis. J. Anim. Ecol. 67, 180–194 10.1046/j.1365-2656.1998.00189.x (doi:10.1046/j.1365-2656.1998.00189.x) [DOI] [Google Scholar]
- 202.Christian J. J. 1950. The adreno-pituitary system and population cycles in mammals. J. Mammal. 31, 247–259 [Google Scholar]
- 203.Sinervo B., Svensson E., Comendant T. 2000. Density cycles and an offspring quantity and quality game driven by natural selection. Nature 406, 985–988 10.1038/35023149 (doi:10.1038/35023149) [DOI] [PubMed] [Google Scholar]
- 204.Svensson E., Sinervo B., Comendant T. 2001. Density-dependent competition and selection on immune function in genetic lizard morphs. Proc. Natl Acad. Sci. USA 98, 12 561–12 565 10.1073/pnas.211071298 (doi:10.1073/pnas.211071298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Svensson E. I., Sinervo B., Comendant T. 2002. Mechanistic and experimental analysis of condition and reproduction in a polymorphic lizard. J. Evol. Biol. 15, 1034–1047 10.1046/j.1420-9101.2002.00452.x (doi:10.1046/j.1420-9101.2002.00452.x) [DOI] [Google Scholar]
- 206.Ouyang J. Q., Sharp P. J., Dawson A., Quetting M., Hau M. 2011. Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. R. Soc. B 278, 2537–2545 10.1098/rspb.2010.2490 (doi:10.1098/rspb.2010.2490) [DOI] [PMC free article] [PubMed] [Google Scholar]