Abstract
Sclerotiorin, an azaphilone polyketide, is a bioactive natural product known to inhibit 15-lipoxygenase and many other biological targets. To readily access sclerotiorin and analogs, we developed a 2–3 step semisynthetic route to produce a variety of azaphilones starting from an advanced, putative azaphilone intermediate (5) over-produced by an engineered strain of Aspergillus nidulans. The inhibitory activities of the semisynthetic azaphilones against 15-lipoxygenase were evaluated with several compounds displaying low micromolar potency.
Lipoxygenases (EC 1.13.11.12) are ubiquitous enzymes widely distributed within plants, fungi and mammals.1 They are non-heme iron dioxygenases that catalyze the addition of molecular oxygen to polyunsaturated fatty acids with a cis, cis-1,4 pentadiene to generate a hydroperoxydiene formed through a radical, regio- and stereoselective mechanism.2 Reaction products of lipoxygenases are involved in several common human disorders such as allergies, inflammation and asthma. Lipoxygenases are responsible for the oxidation lipids in foods subsequently reducing the foods’ nutritional value.3
Several natural products from microbial sources inhibit 15-lipoxgyenase (15-LOX).4 More recently the fungal pigment, (+)-sclerotiorin (1) was found to inhibit lipoxygenase-1, also known as 15-LOX.5 Sclerotiorin was first isolated from P. sclerotiorum in 1940.6 Since then 1 has been found to inhibit multiple therapeutic targets.7
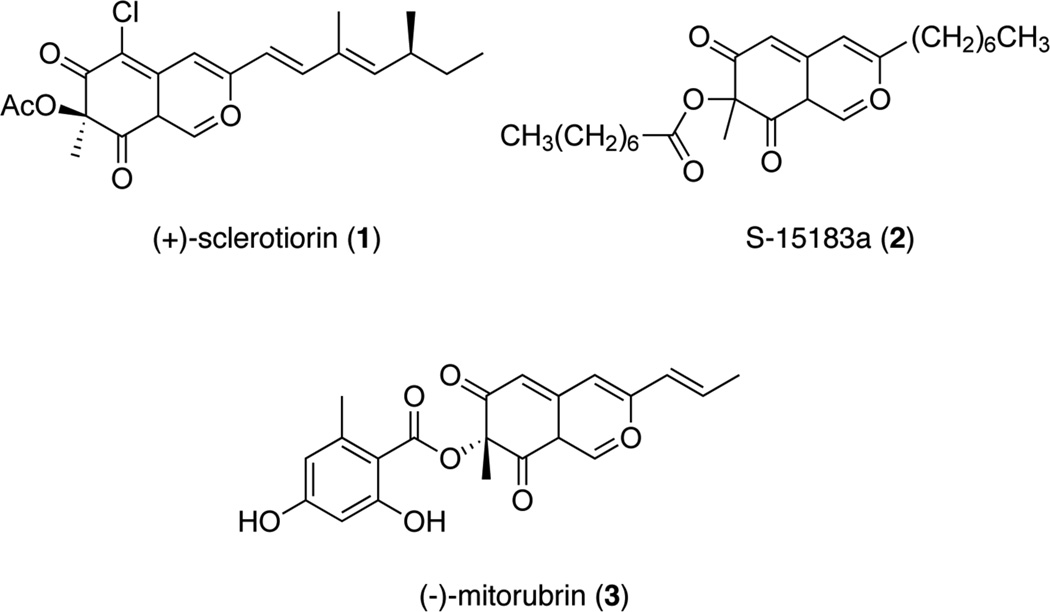
Sclerotiorin belongs to an important class of natural products called azaphilones. Azaphilones are structurally diverse polyketides that share a highly oxygenated bicyclic core and chiral quaternary center. These polyketides are known for their 4H-pyran motif, which reacts with amines to produce the corresponding vinylogous γ-pyridones.8 Early synthetic studies by Whalley and coworkers reported the total synthesis of several azaphilones, which included compound 8 prepared in 14 steps.9 Recent synthetic efforts by Porco and coworkers have shown assembly of the azaphilone core through a copper-mediated enantioselective dearomatization route. The application of their asymmetric methodology was demonstrated on (−)-S-15183a (2), (−)-mitrorubin (3), and more recently with 1 (Figure 1).10
Figure 1.
Azaphilone natural products
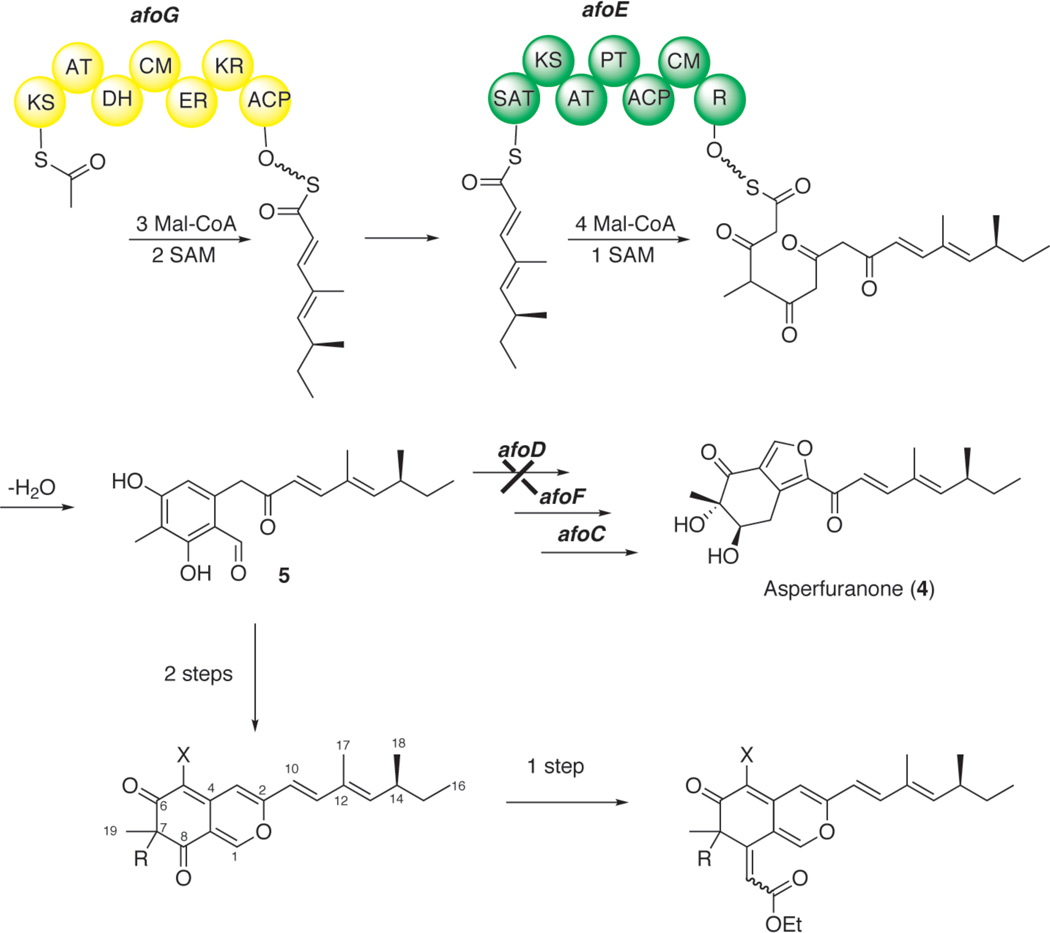
Although many azaphilones have been isolated and identified, their biosynthetic pathways remained unknown until our recent identification of the asperfuranone (4) biosynthetic pathway in Aspergillus nidulans.11 A mutant strain from the previous study provided aldehyde 5 as a stable intermediate, which has been isolated from other azaphilone-producing organisms. Our work aims to enhance the production of the putative azaphilone intermediate (5) and use synthetic chemistry to structurally diversify 5 into natural and non-natural azaphilones.
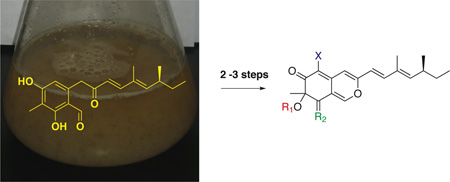
In this study, the fungal strain used to over-produce compound 5 contains two genetic alterations (Scheme 1). The native promoter of afoA, the gene that codes for the pathway-specific transcription activator of the asperfuranone pathway was replaced with the inducible alcohol dehydrogenase promoter, alcA. The afoD gene, that codes for the hydroxylase in the asperfuranone pathway, was deleted to enable the accumulation of intermediate, compound 5.11 It should be noted that the afo cluster is silent under normal laboratory growing conditions. The wild type strain, thus, did not produce detectable quantities of compound 5 and asperfuranone (4) by LC/MS analysis. The mutant strain was initially cultured in a liquid lactose minimal medium under inducing conditions (refer to Supporting Information) at 37°C for three days to produce nearly 200 mg/L of 5 without need for further purification since 5 is poorly dissolved in aqueous media.
Scheme 1.
The reengineered biosynthetic pathway for the synthesis of (+)-sclerotiorin and 7-epi-sclerotiorin (8) and non-natural azaphilone polyketides.
We altered culture conditions in several ways to optimize the titer of compound 5. First, culture time prior to the induction of alcA was investigated. Cultures were incubated from 12 to 36 hours before the chemical inducer cyclopentanone, necessary to induce the alcA promoter, was introduced. Thereafter the culture remained under inducing conditions for an additional 72 hours. The experiment revealed the production of 5 was enhanced by growth 30-36 hours before induction (Fig S1). We next examined a second parameter, the culture time post induction. The A. nidulans strain was cultured for seven days after induction and samples were collected at one-day intervals from day three through day seven (Fig S2). An increase and then decline was observed over the period, with the accumulation of 5 peaking on day five. Under optimized expression conditions our engineered strain produced the polyketide (5) abundantly, providing a titer of 900 mg/L. The elevated production of this advanced metabolite allowed us to employ it for the preparation of a small library of azaphilones.
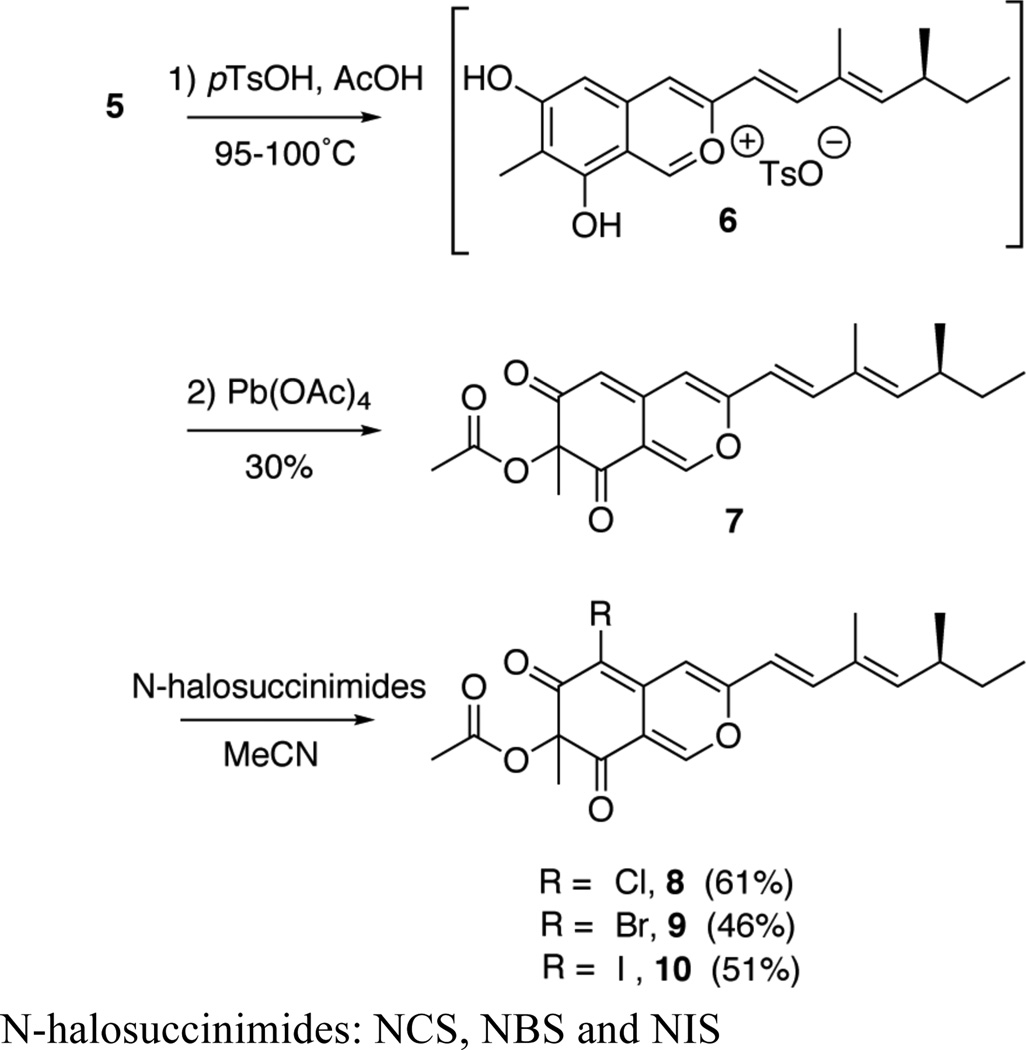
We focused on applying our semisynthetic route to prepare (+)-sclerotiorin by treating 5 with p-toluenesulfonic acid (Scheme 2) to form the 2-benzopyrilium salt (6), which is then oxidized by lead tetraacetate to generate the non-halogenated azaphilone (7).9 Although the acetoxylation at C-7 would be non-stereospecific, the diastereomers were indistinguishable (t.l.c., 1H and 13C NMR) nor could they be separated by HPLC.
Scheme 2.
Concise synthesis of (+)sclerotiorin and 7-epi-sclerotiorin (8) and analogs.
Electrophilic chlorination of azaphilone 7 introduces a chlorine atom at C-5 by using a slight excess of Nchlorosuccinimide to provide the natural product (+)-sclerotiorin and 7-epi-sclerotiorin (8) in 61% yield. Despite the recalcitrant purification of 8, the diastereomers were separated by analytical chiral HPLC to reveal close to a 1:1 ratio of (+)-sclerotiorin and 7-epi-sclerotiorin.
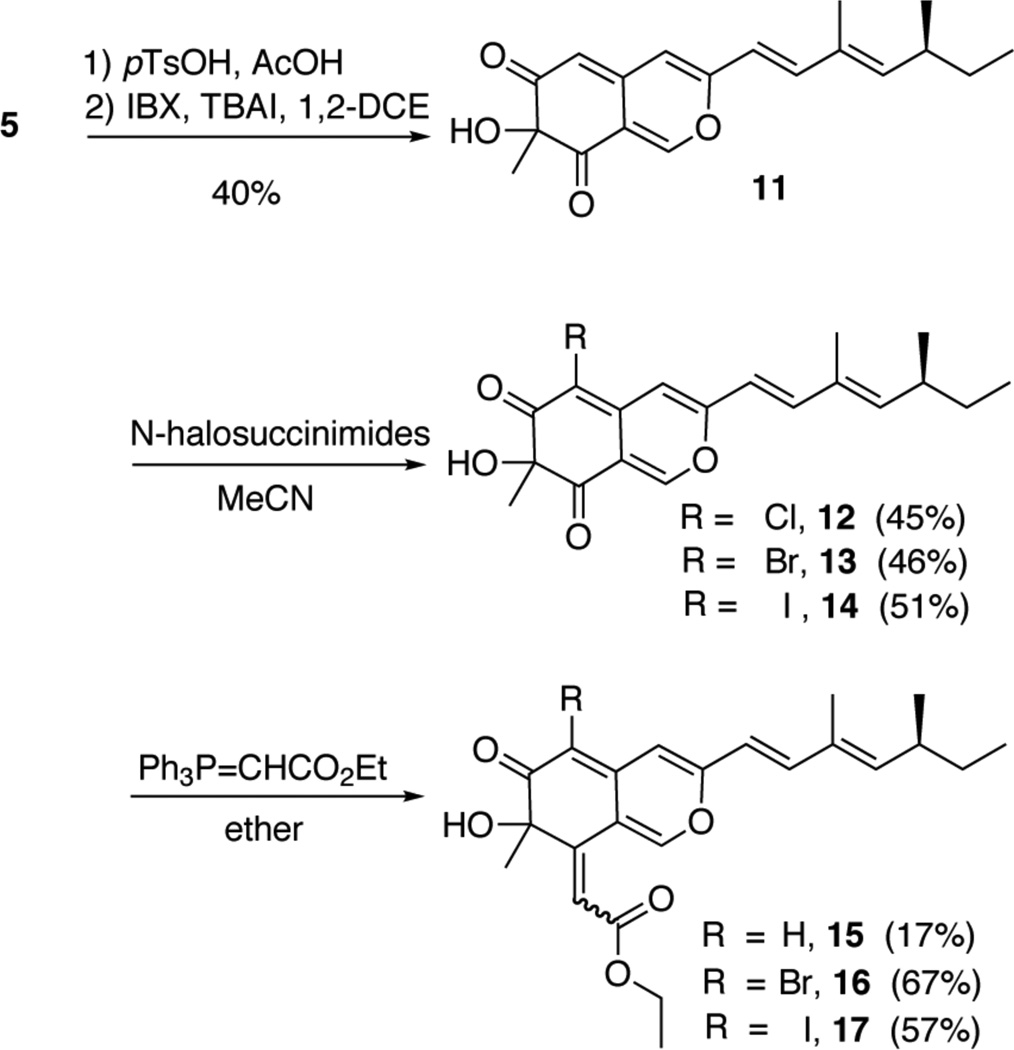
Additionally, several azaphilone analogs were also prepared from 5 (Scheme 3). To create a more efficient synthetic route, we were interested in hypervalent-iodine-mediated phenol oxidative dearomatization with o-iodoxybenzoic acid (IBX), a method developed by Pettus and coworkers.12 The reaction proceeds with the formation of 6, which subsequently is treated with IBX and catalyst Bu4NI at room temperature to form 9. We observed 14 as the major side product of the reaction. It is plausible that the generation of 14 could arise from tetrabutylammonium triiodide or IOH formed in the presence of the residual acetic acid with adventitious water.13,14 To assist in the regioselective halogenation of 11, the corresponding N-halosuccinimides were employed to produce compounds 12, 13 and 14. Then to further functionalize the scaffold of the azaphilone core, a wittig olefination was performed with carbethoxymethylenetriphenylphosphorane. It was observed the ylide selectively coupled with the less hindered ketone and produced a mixture of E:Z isomers (1:1.05) as determined by 1H NMR data. Due to the difficulties in separation, the isomeric mixtures of 15–17 were tested towards the inhibition lipoxygenase-1.
Scheme 3.
A short route to azaphilone analogs.
Based on a report that (+)-sclerotiorin has potent LOX-1 inhibition, 5 the biological activities of all semisynthetic azaphilones were evaluated for soybean LOX-1 inhibition to provide a preliminary structure-activity relationship (SAR). In screening for inhibition, azaphilones 7 and 13 displayed the highest lipoxgyenase-1 inhibition (Table 1). Azaphilones 5, 6, 8, and 9 showed very similar inhibition activities. Iodinated azaphilones displayed less inhibition toward LOX-1, which may be due, in part to putative chemical instability. The other compounds (15–17), showed no appreciable LOX-1 inhibition.
Table 1.
Lipoxygenase-1 Inhibitory Activity.
| Compound | IC50 (µM) ± s.d. |
|---|---|
| 1 | 7.8 ± 2.4 |
| 4 | >100 |
| 5 | 97.2 ± 2.0 |
| 7 | 4.9 ± 3.3 |
| 8 | 2.3 ± 0.9 |
| 9 | 6.8 ± 4.5 |
| 10 | 17.4 ± 8.1 |
| 11 | 10.7 ± 6.6 |
| 12 | 7.9 ± 3.9 |
| 13 | 3.2 ± 1.5 |
| 14 | 19.6 ± 11.9 |
| 15 | >100 |
| 16 | >100 |
| 17 | >100 |
All assays performed in triplicate with the average value reported.
The LOX-1 inhibition screening suggested that the C-8 ketone might be an important structural feature for activity against lipoxygenase targets. The azaphilone analogs also indicated that halogenation at C-5 is not essential to maintain low micromolar activity, except that when C-5 was iodinated a loss of inhibition was observed. Since (+)-sclerotiorin has similar IC50 with 8 containing both (+)-sclerotiorin and 7-epi-sclerotiorin, the chiral center C-7 is not critical for the LOX-1 inhibition.
It has been suggested that 1 inhibits LOX-1 by trapping lipid radicals formed at the active site of the enzyme-substrate complex. Although, we have not measured the reductive properties of our semisynthetic azaphilones, it is reasonable that they have similar antioxidant properties to (+)-sclerotiorin.
The metabolic engineering of a biosynthetic pathway in the filamentous fungus, Aspergillus nidulans, demonstrates the feasibility of producing copious amounts of the advanced polyketide (5). Coupled with existing synthetic methodology, this provides facile synthetic access to derivatives of the natural product sclerotiorin. Azaphilone analogs 7 and 13 were the most effective to inhibit the therapeutic target, LOX-1. Preliminary SAR indicates the importance of the C-8 ketone for inhibition of lipoxygenase. This may also provide insight into the further development of more potent LOX-1 inhibitors.
Supplementary Material
Acknowledgment
The project described was supported by Grant Number PO1GM084077 from the National Institute of General Medical Sciences to BRO and CCCW.
Footnotes
Supporting Information Available
The Aspergillus nidulans strain used in the study, in addition to experimental details and characterization of compounds 7–17 (1H NMR, 13C NMR, HRMS, and FT-IR) is provided in supporting information.
References
- 1.Brash AR. J. Biol. Chem. 1999;274:23679. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C, Pratt DA, Porter NA, Brash AR. Chem. Biol. 2007;14:473. doi: 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon M. Chapter 2. In: Pokorny J, Yanishlieva N, Gordon M, editors. Antioxidants in food, Practical applications. Woodhead Publishing Ltd.; Cambridge, England: 2001. p. 7. [Google Scholar]
- 4.(a) Komoda T, Sugiyama Y, Abe N, Imachi M, Hirota H, Hirota A. Tet. Lett. 2003;44:1659. [Google Scholar]; (b) Rao KCS, Divakar S, Rao AGA, Karanth NG, Suneetha WJ, Krishnakantha TP, Sattur AP. Biotech. Lett. 2002;24:1967. [Google Scholar]
- 5.Chidananda C, Sattur AP. J. Agric. Food Chem. 2007;55:2879. doi: 10.1021/jf062032x. [DOI] [PubMed] [Google Scholar]
- 6.Curtin TP, Reilly J. Biochem J. 1940;34:1419. doi: 10.1042/bj0341418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osmanova N, Schultze W, Ayoub N. Phytochem. Rev. 2010;9:315. [Google Scholar]
- 8.Wei W-G, Yao ZJ. J. Org. Chem. 2005;70:4585. doi: 10.1021/jo050414g. [DOI] [PubMed] [Google Scholar]
- 9.Chong R, King RR, Whalley WB. J. Chem. Soc. (C) 1971:3566. doi: 10.1039/j39710003566. [DOI] [PubMed] [Google Scholar]
- 10.(a) Zhu J, Grigoriadis NP, Lee JP, Porco JA. J. Am. Chem. Soc. 2005;127:9342. doi: 10.1021/ja052049g. [DOI] [PubMed] [Google Scholar]; (b) Zhu J, Porco JA. Org. Lett. 2006;8:5169. doi: 10.1021/ol062233m. [DOI] [PubMed] [Google Scholar]; (c) Germain AR, Bruggemeyer DM, Zhu J, Genet C, O'Brien P, Porco JA. J. Org. Chem. 2011;76:2577. doi: 10.1021/jo102448n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang Y-M, Szewczyk E, Oakley BR, Wang CCC. J. Am. Chem. Soc. 2009;131:2965. doi: 10.1021/ja8088185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsini MA, Gowin KM, Pettus TRR. Org. Lett. 2006;8:3481. doi: 10.1021/o10610993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Benito JF, Brillas E, Arias C. Can. J. Chem. 1989;68:79. [Google Scholar]
- 14.Moorthy JN, Senapati K, Kumar S. J. Org. Chem. 2009;74:6287. doi: 10.1021/jo9007892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.