Abstract
Transthyretin (TTR) is one of the many proteins that are known to misfold and aggregate (i.e., undergo amyloidogenesis) in vivo. The process of TTR amyloidogenesis causes nervous system and/or heart pathology. While several of these maladies are associated with mutations that destabilize the TTR native quaternary and/or tertiary structure, wild type TTR amyloidogenesis also leads to the degeneration of post-mitotic tissue. Over the past twenty years, much has been learned about the factors that influence the propensity of TTR to aggregate. This biophysical information led to the development of a therapeutic strategy, termed “kinetic stabilization”, to prevent TTR amyloidogenesis. This strategy afforded the drug, tafamidis (trade name: Vyndaqel®), which was recently approved by the European Medicines Agency for the treatment of Transthyretin Familial Amyloid Polyneuropathy (TTR-FAP), a common familial TTR amyloid disease. Tafamidis is the first, and currently the only, medication approved to treat TTR-FAP. Here we review the biophysical basis for the kinetic stabilization strategy and the structure-based drug design effort that led to this first-in-class pharmacologic agent.
Introduction
Transthyretin (TTR) is a tetrameric protein found in the bloodstream at a concentration of ≈ 5 µM, comprising identical 127-amino-acid β-sheet-rich subunitsin homozygotes (Figure 1A).1–3In heterozygotes, the TTR tetramers are made up of variant and/or wild type subunits, combined in a statistical fashion.4 The established function of TTR in the blood is to transport holo-retinol binding protein.5While TTR could transport up to 2 equivalents of retinol binding protein bound to retinolper TTR tetramer, the average stoichiometry of holo-retinol binding protein bound to TTR in blood is ~0.5 equivalents.5–8While TTR is the major carrier of thyroxine (T4) in the blood of rodents, utilizing binding sites that are orthogonal to those used for holo-retinol binding protein, the T4 binding sites are effectively unoccupied in humans.9There are three T4 binding proteins in human blood: TTR, albumin, and thyroid binding globulin (TBG). Of these three, albumin has the highest concentration ([albumin] = 620 µM; [TTR] = 5 µM; [TBG] = 0.3 µM) and thyroid binding globulin has the highest affinity for T4 (Kd,TBG = 0.1nM; Kd,TTR = 15 nM; Kd,albumin = 1.5 µM).10,11These facts, combined with the low T4 concentration in blood (0.1 µM), mean that very little (< 10%) of the T4 in human blood is bound to TTR, and virtually all (>99 %) of the T4 binding sites in TTR are unoccupied and available for small molecule binding. This circumstance will be taken advantage of as described below in our kinetic stabilization strategy to prevent TTR amyloidogenesis.9,12 While TTR is the primary carrier of T4 in cerebrospinal fluid (CSF), the low concentration of T4 in CSF leads to the situation being similar to that in the blood, in that the vast majority of the T4 binding sites are unoccupied.13,14
Figure 1.
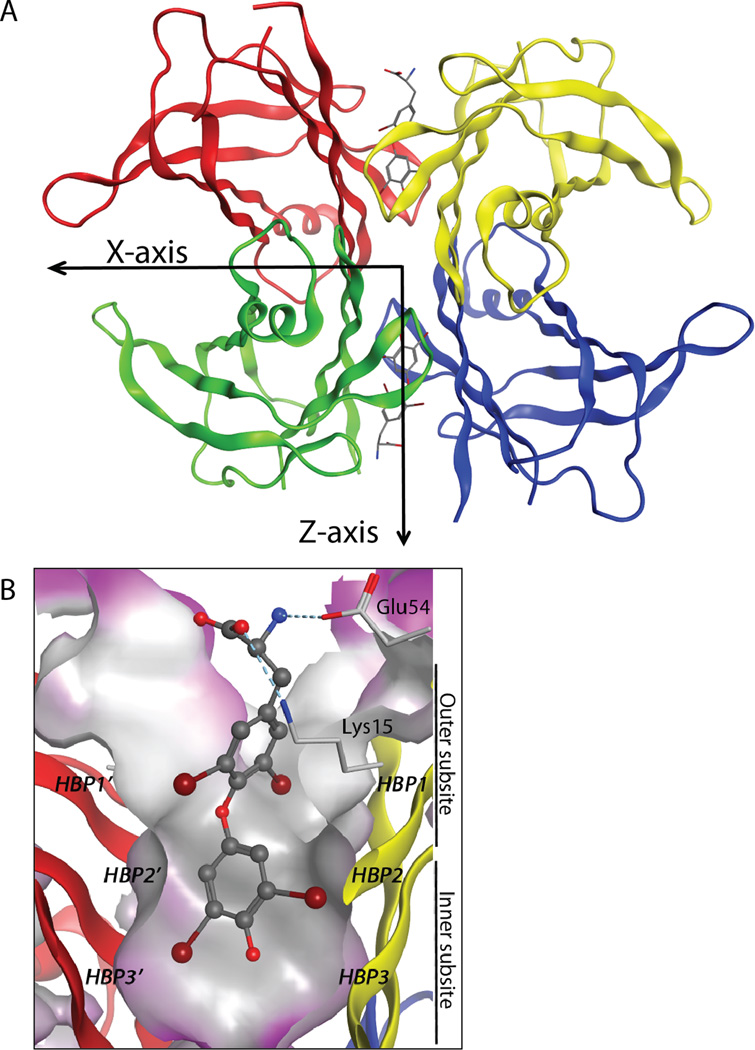
Structure of transthyretin (TTR; PDB code: 2ROX). (A) Ribbon diagram depiction of TTR with the crystallographic two-fold axis (Z-axis) bisecting the T4 binding channel comprising the weaker of TTR’s two dimer-dimer interfaces(B)B. Close-up view of onethyroid hormone binding site with T4(shown as a ball-and-stick representation) bound, showing the iodide substituents occupying symmetry-related halogen binding pockets. Primed amino acids refer to those comprising symmetry-related subunits. Hydrogen bonds are shown as light blue, dashed lines. Figure adapted from Connelly et al.18
The TTR tetramer has two distinct dimer-dimer interfaces, the less stable of which is bisected by the crystallographic 2-fold axis,15 or the Z-axis. It is this interface that creates the two identical funnel-shaped T4 binding sites (Figure 1A, B).1,12,16The largely hydrophobic T4 binding sites each have a small inner binding subsite and a larger outer subsite (Figure 1B).12,17,18 The T4 binding sites display positively (Lys 15) and negatively (Glu 54) charged side chains at their periphery, which complement the zwitterionic structure of T4 (Figure 1B).2,12,18 The three pairs of symmetric hydrophobic depressions that line the T4 binding site are referred to as the halogen binding pockets (HBPs), so named because they are occupied by the iodine atoms of T4 (Figure 1B, dark red balls).19,20
TTR is one of at least thirty different human proteins whose extracellular misfolding and/or misassembly (amyloidogenesis) into a spectrum of aggregate structures is thought to cause degenerative diseases referred to as amyloid diseases, named after the characteristic cross-β-sheet assemblies called amyloid fibrils which are one of the structures formed from the process of aggregationor amyloidogenesis.21–23Genetic, pharmacologic, pathologic and biochemical evidence all suggest that human amyloid diseases result from the process of amyloidogenesis.24–27Amyloid can form from intrinsically disordered proteins that have no defined tertiary structure (e.g., the Huntington’s-disease-related exon 1 of the Huntingtin protein, or the Alzheimer’sdisease-related amyloid-β peptide).28 In such cases, pathogenesis is often triggered by gene duplication or aberrant post-translational processing or modifications.29–31 Amyloid can also result from the partial unfolding of proteins that normally adopt a well-defined tertiary and/or quaternary structure.32–35 Substantial evidence supporting the “conformation change hypothesis” was published in the 1990s, revealing that this category of proteins must partially unfold in order to misassemble into aggregates, including amyloid fibrils.23,32–38Transthyretin is an example of a protein that has to undergo conformational changes in order to become amyloidogenic.32,33Partial unfolding exposes stretches of largely uncharged hydrophobic residues in an extended conformation that efficiently misassemble into largely unstructured spherical aggregates that ultimately undergo conformational conversion into cross-β sheet amyloid structures.39–43
Amyloid Diseases in General
Aging is the most significant risk factor for the development of both sporadic and inherited amyloidoses.44,45Aging-associated deficiencies in stress-responsive signaling probably causes or exacerbates compromised protein homeostasis (or proteostasis), leading to the demise of tissue that does not easily regenerate.46–49Mutations within the amyloidogenic proteins, while not absolutely required for human amyloid diseases, often predispose the proteins toward amyloidogenesis by destabilizing the non-amyloidogenic native state, and sometimes by making the amino acid sequences exposed in partially folded states more amyloidogenic, accelerating the onset of post-mitotic tissue degeneration.50–53 While much remains to be learned about the molecular underpinnings of why aging is the most important risk factor for the onset of amyloid diseases, it is clear that activation of the heat shock response stress-responsive signaling pathway is markedly protective in worm and mouse models of Alzheimer’s disease,46,47 and worm models of Huntington’s disease.54–56Investigations are underway to sort out the mechanism by which stress-responsive signaling pathways protect against the pathology of the amyloidoses.
Sporadic and Inherited TTR amyloidoses
In humans, both wild type TTR tetramers and mixed tetramers compromised of mutant and wild type subunits can dissociate, misfold and aggregate–with the process of amyloidogenesis leading to the degeneration of post-mitotic tissue. Deposition of wild type TTR outside and apparently, ultimately within the cardiomyocytes of the heart appears to cause senile systemic amyloidosis (SSA), a late onset sporadic cardiomyopathy, affecting as much as 15% of males>80 years of age and significant numbers as young as 60 years of age.57,58The TTR amyloidoses associated with point mutations in the TTR gene include familial amyloid polyneuropathy (FAP), familial amyloid cardiomyopathy (FAC), and the rare central nervous system selective amyloidoses (CNSA).59–65 These patients are almost always heterozygotes, meaning that their tetramers are composed of mutant and/or wild type TTR subunits, generally statistically distributed.4 These autosomal dominant, inherited (or familial) TTR amyloidoses are typically earlier onset than the sporadic disease SSA. For example, L55P FAP has an onset at around 20 years of age,67 where as V30M FAP symptoms surface around age 30 in the Portuguese population.59,65,68 More than one hundred mutations have been associated with the familial TTR amyloidosis.69,70Generally, the more destabilizing the mutant subunits are to the TTR tetramer structure, the earlier the onset of amyloid disease,50,71 although surprisingly the most destabilized TTR mutants, D18G and A25T, are not the most pathogenic.72,73 The pathogenic potential of a TTR variant is determined by a combination of its instability and its cellular secretion efficiency.71 Highly destabilized TTR mutants are subject to cellular quality control by the proteostasis network, and are thus degraded intracellularly by endoplasmic reticulum-associated degradation mediated by the proteasome.71As suggested by their names, FAP first manifests with autonomic and peripheral nervous system symptoms, while FAC presents with cardiacabnormalities—however, many patients have both nervous system and cardiac symptoms, especially late in the disease course, suggesting that these patients may have more general post-mitotic tissue dysfunction than previously thought.66 It is not currently understood why a particular mutant prefers a particular site of deposition, with the exception of the CNSA-associated TTR mutants, although tissue selective amyloid deposition probably is influenced by the extracellular matrix unique to that tissue. The CNSA-associated mutants are generally the most destabilizing and lead to central nervous system pathology because the choroid plexus, which synthesizes TTR for secretion into the CSF, is more permissive than the liver, the primary source of TTR in the bloodstream, in terms of secreting highly destabilized and extremely amyloidogenic TTR tetramers.71 This may be due to the relatively high concentration of T4 in the choroid plexus, which appears to bind to and stabilize the mutant TTR tetramer in the endoplasmic reticulum by a pharmacologic chaperoning mechanism, affording sufficient stabilization to evade endoplasmic reticulum-associated degradation.71 Once secreted, the lower extracellular T4 concentration in the CSF would then favor ligand dissociation, leaving the destabilized TTR tetramer to undergo dissociation, misfolding and amyloidogenesis.
As mentioned above, the initial pathology caused by some TTR variants comes from their selective destruction of cardiac tissue, while that from other TTR variants comes from compromising the peripheral and autonomic nervous system; the basis for this initial selectivity in clinical presentation remains unclear.59,60,65,74,75The tissue damage caused by TTR amyloidogenesis appears to stem largely from the toxicity of small, diffusible TTR aggregates,61,76although accumulation of extracellular amyloid may contribute and almost certainly compromises organ structure in the late stages of the TTR amyloidoses.77 In either case, however, we hypothesize that these post mitotic tissues are especially vulnerable to proteotoxicity because these tissues do not readily regenerate. The vulnerability of specific post-mitotic tissues to a given TTR mutation may have to do with the ineffectiveness of the extracellular matrix and the glycosaminoglycans in particular in protecting against cytotoxicity.78,79If left unchecked, amyloidogenesis of most TTR sequences leads to compromised function of both the heart (and probably other muscles) and the autonomic and peripheral nervous systems.59,60,65,74,75Ultimately, death occurs within approximately a decade after the onset of symptoms.59,74,75,80–83
Therapeutic Strategies under Development for the TTR and Other Amyloidoses
Emerging strategies to treat human amyloid diseases center on reducing the concentration of the amyloidogenic protein or peptide. Since the rate and extent of amyloidogenesis is highly dependent on the concentration of the amyloidogenic peptide and/or the population of the amyloidogenic protein conformation(s)84–86, lowering their concentration offers a potential therapeutic strategy. For example, one strategy to treat Alzheimer’s disease is to reduce the production of amyloid β (Aβ), the intrinsically disordered amyloidogenic peptide whose misassembly leads to Alzheimer's disease, by inhibiting the β- or γ-secretases that generate the Aβ from the trans-membrane amyloid precursor protein.87–89 Another strategy is to decrease the concentration of Aβ monomers and oligomers, clearing them by any one of several antibody-mediated mechanisms; several clinical trials using monoclonal anti-Aβ antibodies are ongoing (http://www.clinicaltrials.gov).90–92 In light chain amyloidosis, the clonal plasma cells in the bone marrow are eliminated with chemotherapy agents to dramatically reduce the concentration of the amyloidogenic light chain protein in the blood.93–97Light chain amyloid disease has been ameliorated in numerous individuals by eliminating the amyloidogenic light chain.93–97
The currently practiced strategy to ameliorate FAP associated with mutant TTR aggregation is liver transplantation.98,99In this surgical procedure, a patient that is heterozygous for a disease-associated TTR mutation has their liver replaced with one from a donor that is homozygous for wild type TTR.98Since TTR is mainly synthesized by the liver, this amounts to a surgical form of gene therapy. While initially effective for the ~90% of patients that survive the surgery (liver transplantation of FAP patients reduces the serum concentration of the V30M mutant to <5% of pre-transplant levels),103,104 progression of WT TTR amyloidosis after about a decadeultimately leads to cardiomyopathy.98,105,106This surgical gene therapy strategy appears to be less effective for FAP-associated with TTR variants other than V30M, probably because these non-V30M patients are generally older when they are transplanted.105,107Combined heart and liver transplantation has been employed for FAC patients, where as heart transplants have been used to ameliorate SSA.100–102It is important to realize that liver transplantation does not prevent the development of the life threatening arrhythmias in familial amyloid polyneuropathy,105 and of course liver and/or heart transplant patients require life-long immunosuppression, which creates its own challenges.
Emerging additional approaches to lower the concentration of amyloidogenic TTR include antisense oligonucleotide (Isis Pharmaceuticals) and RNA interference (Alnylam Pharmaceuticals) strategies to lower the TTR mRNA levels.108,109 Antisense oligonucleotides specific for human TTR mRNA have been shown to inhibit hepatic synthesis of TTR in mice transgenic for a human amyloid-associated TTR sequence.108Parenteral administration of a TTR-specific antisense oligonucleotide, however, had no effect on the expression of TTR by the choroid plexus, which is the source of TTR in the brain.109This is likely desirable because there are numerous reports that normal TTR levels in the brain appear to be protective against other amyloid diseases, such as Alzheimer’s disease.110,111 There is a risk that continued amyloidogenic TTR synthesis in the brain, albeit at lower levels, could put FAP patients at risk for late onset central nervous system selective TTR amyloidosis. Antisense oligonucleotides are now being tested clinically as well by Isis Pharmaceuticals (http://www.clinicaltrials.gov). Alnylam Pharmaceuticals is using double-stranded RNA (RNAi) directed against transthyretin mRNA to lower its levels,112which in turn lower the TTR plasma protein concentrations of TTR FAP-associated variants without affecting the TTR level in the CSF. The TTR-targeting RNAi, when formulated in the appropriate lipid, is quite effective at degrading hepatic TTR mRNA. This approach against the TTR amyloidosis is now being explored in human clinical trials (http://www.clinicaltrials.gov). This strategy is expected to be effective for FAC and SSA, as lowering mutant and/or wild type TTR should decrease TTR aggregation efficiency thought to cause cardiomyopathy.
The Kinetic Stabilizer Strategy to Ameliorate the TTR Amyloidoses: Leveraging an Understanding of the Molecular Mechanism of Aggregation Linked to Pathology
Another strategy for ameliorating the amyloidoses caused by the misfolding and misassembly of a protein like TTR or lysozyme, which normally adopt folded, non-amyloidogenic 3-D structures, focuses on preventing the conformational excursions from the native state or partial denaturation that renders them amyloidogenic (Figure 2).12,23,113–116Stabilizing the properly folded, non-amyloidogenic conformations of these proteins is considered to be the most conservative approach for treating these maladies, because it is still unclear which misfolded or misassembled TTR, light chain or lysozyme conformations / quaternary structures lead to proteotoxicity.61,76,117–120 There is mounting evidence that stopping the process of amyloidogenesis without necessarily clearing the deposited amyloid fibrils121is sufficient to stop the degeneration of post-mitotic tissue and disease progression.27
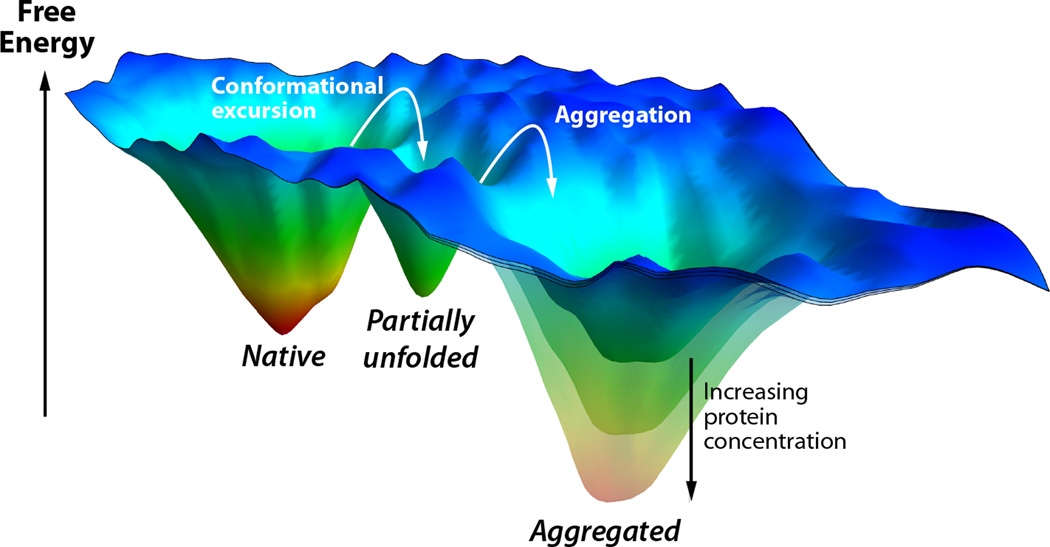
Figure 2.
Folding free energy landscape of an amyloidogenic protein that normally forms a well folded 3D structure, but can also aggregate as a consequence of a conformational change, e.g., TTR or lysozyme. Three energy wells are shown: the native state, a partially unfolded amyloidogenic intermediate, and an aggregated state. Conformational excursions convert the native state to the partially unfolded state, which can then aggregate. The stability of the aggregated state depends on the protein concentration. At low protein concentrations, it would be less stable than the native state, and therefore not substantially populated. As the protein concentration increases, it becomes increasingly stable, and will eventually become the most stable state.
In the case of TTR amyloidogenesis, the tetramer must first dissociate and then the natively folded monomer must undergo partial denaturation in order for the TTR subunits to become aggregation competent.12,24,32,33,51,114,122–124Several mechanisms have been proposed to explain the aggregation of amyloidogenic proteins.85,86In the most widely accepted mechanism, multiple chains of the amyloidogenic protein assemble into an oligomeric nucleus in the rate-limiting step, before the rate of amyloid fibril formation becomes substantial. This scenario is referred to as a nucleated polymerization (Figure 3, top).86For other proteins, e.g., Aβ whose aggregation appears to cause Alzheimer’s disease, rapid oligomerization of the amyloidogenic protein into spherical or amorphous micelle-like assemblies is observed and these undergo slow conversion into amyloid fibrils in a process associated with a high activation barrier. The latter mechanism is referred to as a nucleated conformational conversion and appears to govern Aβ aggregation, at least in vitro (Figure 3, middle panel).125TTR aggregation proceeds by yet a third mechanism, referred to as a downhill polymerization reaction.84After rate-limiting tetramer dissociation, and monomer misfolding, the partially denatured TTR monomers aggregate very efficiently because the misassembled dimer is more stable than the dimer and the misassembled trimer is more stable than the dimer, etc. TTR aggregation does not require nucleus formation, is not amenable to seeding, and is limited only by the relatively low activation barriers of the bimolecular association of misfolded TTR monomers and oligomers, thus the downhill polymerization designation (Figure 3, bottom panel).84After monomeric TTR undergoes partial denaturation, it spontaneously misassembles into a variety of aggregate morphologies, including amyloid fibrils and more structurally diverse aggregates exhibiting varying extents of cross-β-sheet structure (Figure 4).84,126,127Because TTR aggregation is very efficient once the misfolded monomer state is reached, it seems unwise to try to block TTR aggregation after rate-limiting tetramer dissociation.
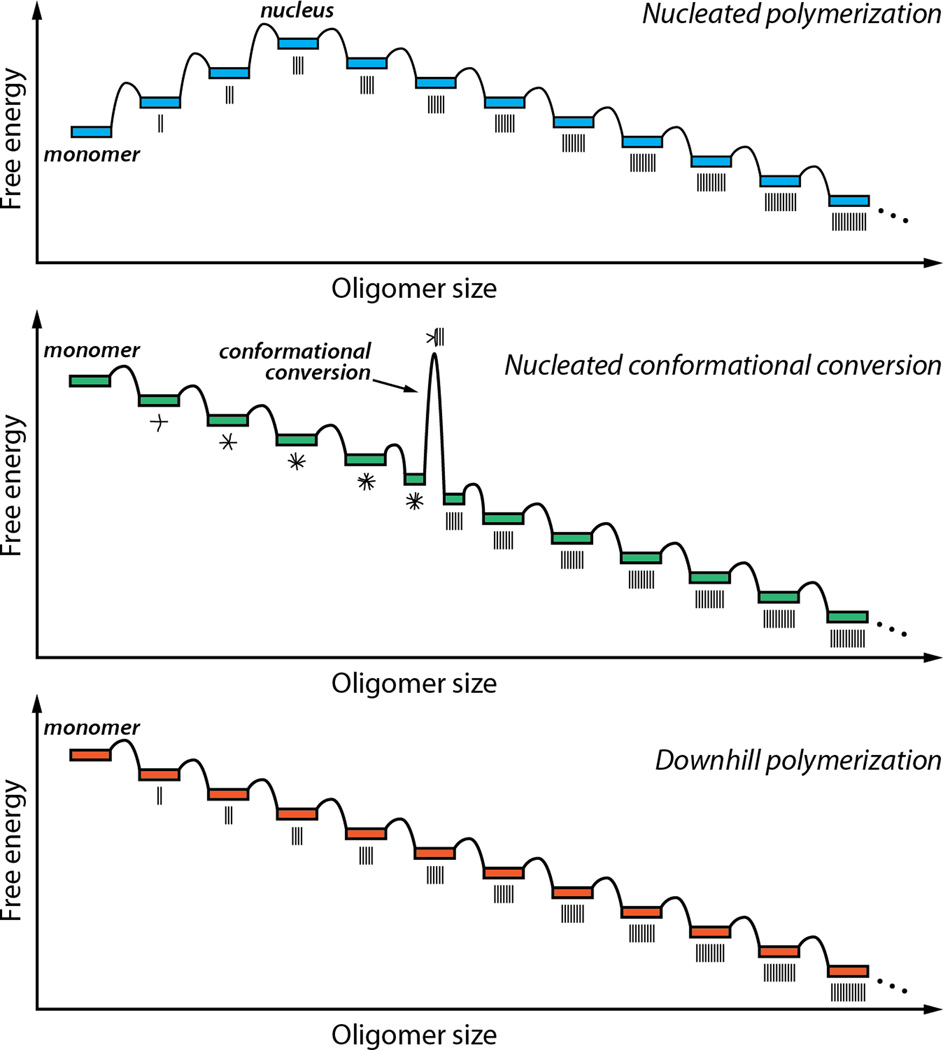
Figure 3.
Energy diagrams associated with three distinct mechanisms of protein aggregation. In a nucleated polymerization (top), the initial association events are unfavorable until a critical sizeis reached. The oligomer of this size is referred to as the nucleus. Subsequent steps are favorable, making further growth favorable for oligomers larger than the nucleus. In a nucleated conformational conversion (middle), facile initial association steps form amorphous oligomers. Oligomers of a certain size can undergo a rate-limiting conversion step, in which they change from an amorphous structure to a cross-β-sheet fibrillar state. Subsequent steps are favorable, as in the nucleated polymerization. In a downhill polymerization (bottom), the mechanism by which TTR aggregates, all of the association steps are favorable after formation of the amyloidogenic intermediate, and there is no kinetic barrier to oligomerization. The aggregates shown are ordered, but they need not be; TTR forms a collection of aggregate structures.
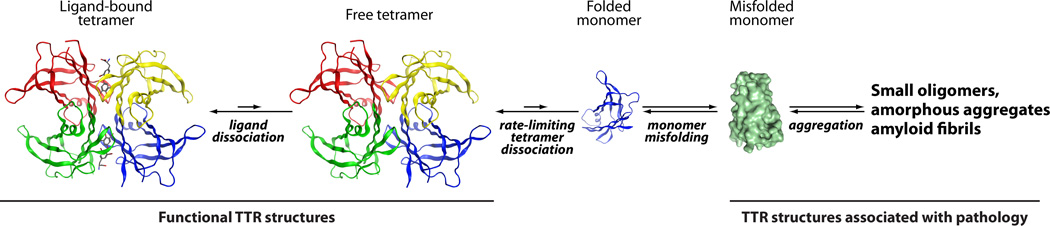
Figure 4.
TTR amyloid cascade. In order for TTR to form amyloid, the tetramer must first dissociate (the rate-limiting step) and then the natively folded monomer must undergo partial denaturation to become competent to misassemble into a variety of aggregate morphologies, including oligomers and amyloid fibrils. Ligands (such as thyroxine, shown in gray and red) stabilize the tetramer and thus prevent amyloidogenesis.
The tetramer–monomer–unfolded monomer equilibria (Figure 4) are strongly thermodynamically linked,128 thus, destabilization of either the tetramer or the monomer (and perhaps even the dimer in some mutants) can enhance TTR amyloidogenicity.50,51,53,72,73,122,127–131 Generally, the disease-associated TTR mutations characterized to date either decrease the tetrameric quaternary structural stability or the monomer’stertiary structure stability, or both.50,51,53,71–73,122,127,129–131The efficiency of TTR amyloidogenesis is dominated by the extent of thermodynamic destabilization, which determines the concentration of TTR adopting an amyloidogenic conformation. TTR tetramer dissociation kinetics, which control the maximal rate of amyloidogenesis, appear to play a less significant role–as some disease-associated mutant homotetramers dissociate more quickly and others more slowly than wild type TTR homotetramers.50,71The V122I TTR FAC variant is amyloidogenic because it forms a relatively unstable tetramer and it dissociates rapidly, but its monomers are as stable as those of wild type TTR.130 In contrast, the L55P TTR variant forms a stable tetramer, but its monomers are unstable leading to its efficient amyloidogenesis.128
It is not known what triggers TTR tetramer dissociation and monomer misfolding in vivo. We hypothesize that tetramer dissociation and monomer misfolding could occur in the acidic vesicles that transport TTR to the cell surface or in the acidified endocytic vesicles that take TTR into the cell, either by a receptor-mediated process or by macropinocytosis, or the like.32,33Tetramer dissociation does occur at physiological pH, albeit slowly, as evidenced by TTR subunit exchange occurring under physiological conditions with a t½ of ~ 1 day.4,114,126,127,132,133TTR tetramer dissociation and monomer misfolding in vitrois notably enhanced through the use of acidic denaturing conditions, supporting our hypothesis that an acidified vesicle including endosomes and lysosomes could be responsible for triggering amyloidogenesis in vivo.32,33,134–137
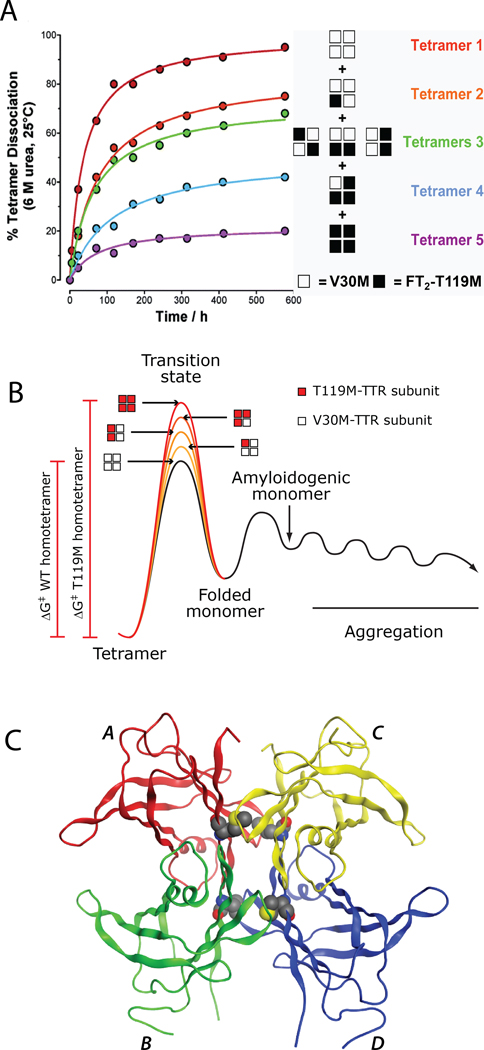
Just before the Kelly laboratory began developing small molecules that bind to the unoccupied T4 binding sites in plasma TTR to slow or prevent tetramer dissociation (Figure 4), (the rate limiting step of TTR amyloidogenesis), Coelho and colleagues-reported a Portuguese family that appeared to exhibit suppression of TTR amyloid disease phenotypes.138,139This compound heterozygous family expresses the V30M mutation associated with highly penetrant FAP on one allele, yet they do not develop polyneuropathy. Instead of expressing wild type TTR from their second allele, they express a T119M TTR variant, resulting in the formation of mixed TTR tetramers that exhibit a statistical distribution of V30M and T119M subunits.4,24
Subsequent biophysical studies by our laboratory revealed that T119M subunit incorporation into tetramers otherwise composed of V30M subunits proportionately reduces the amyloidogenesis rate under acidic conditions and the rate of tetramer dissociation at neutral pH in urea (Figure 5A).24,114Wild type and T119M TTR homotetramers have very similar thermodynamic stabilities, but differ dramatically in their dissociation kinetics (Figure 5A, B).24,50,114 The dissociation rate of the T119M TTR homotetrameris~ 25-foldslower than the dissociation rate of the wild type TTR homotetramer, demonstrating that the T119M TTR homotetramer has a much higher dissociation barrier relative to that of the wild type TTR homotetramer (Figure 5B). Hence, T119M subunit inclusion into a tetramer otherwise composed of disease-associated subunits raises the kinetic barrier of tetramer dissociation by destabilizing the dissociative transition state (Figure 5B), protecting these individuals from amyloidogenesis and disease by a process referred to as interallelic trans-suppression.24,114,138,139
Figure 5.
Kinetic stabilization through T119M TTR subunit incorporation into TTR tetramers.(A) Urea-mediated tetramer dissociation time courses of the T119M TTR homotetramer, wild type TTR homotetramer, or mixed tetramers produced by co-expression of the two different subunits, the stoichiometry being indicated on the right. (B) Free energy diagram illustrating that the increase in activation energy required for tetramer dissociation is proportional to the number of T119M subunits comprising the tetramer. (C) Ribbon diagram depiction of T119M TTR, where in the 119M side chains shown in CPK representation stabilize the weaker of TTR’s two dimer-dimer interfaces (PDB code: 1BZE). Figure adapted from Hammarstrom et al. 24.
The M119 side chains may impart kinetic stability to the TTR tetramer by increasing the surface area of the contacts between the weaker of the two dimer-dimer interfaces in the dissociative transition state: the AB/CD interface bisected by the crystallographic two-fold or Z axis (Figure 5C).15,16,140,141This interface creates the two symmetrical T4 binding sites.19,20Furthermore, perturbation of this quaternary structural interface by mutagenesis or protein engineering also kinetically stabilizes the tetramer.15,16
Collectively, these observations suggest that TTR amyloidogenesis could be suppressed and amyloid pathology ameliorated by kinetically stabilizing the weaker dimer-dimer interface of TTR. We envisioned that small molecule binding to one or both of the T4 binding sites should stabilize the AB/CD dimer-dimer interface in the dissociative transition state by simultaneously interacting with the A and C and/or B and D subunits across the weaker dimer–dimer interface of the tetramer, analogous to the hydrophobic bridging interactions enabled the T119M mutation (Figure 5C).12,114,116,132,142The human genetic data mentioned above along with the corresponding biochemistry strengthened our resolve to discover small molecules that could bind to the normally unoccupied TTR T4 binding sites in blood and prevent amyloidogenesis through kinetic stabilization of the TTR tetramer (Figures 4 and 5).12,114,116,143 This pharmacologic principle was first demonstrated with T4, a natural TTR ligand, and 2,4,6-triiodophenol, when it was found that they inhibited TTR amyloidogenesis.116 This proof-of-principle experiment justified a robust screening144–148 and structure-based drug design17,18program to find small molecule TTR ligands that bind tightly and selectively to TTR, kinetically stabilizing the native, non-amyloidogenic quaternary structure.12,114It is important that TTR kinetic stabilizers lack thyroid hormone receptor agonism or antagonism, while also exhibiting minimal to no binding to the other 4000 or more proteins found in the blood.12Poor binding selectivity to TTR would increase the concentration of the kinetic stabilizer required to inhibit TTR aggregation and possibly lead to off-target binding-associated toxicity that would derail a clinical development program.
The Discovery and Design of Kinetic Stabilizers of TTR
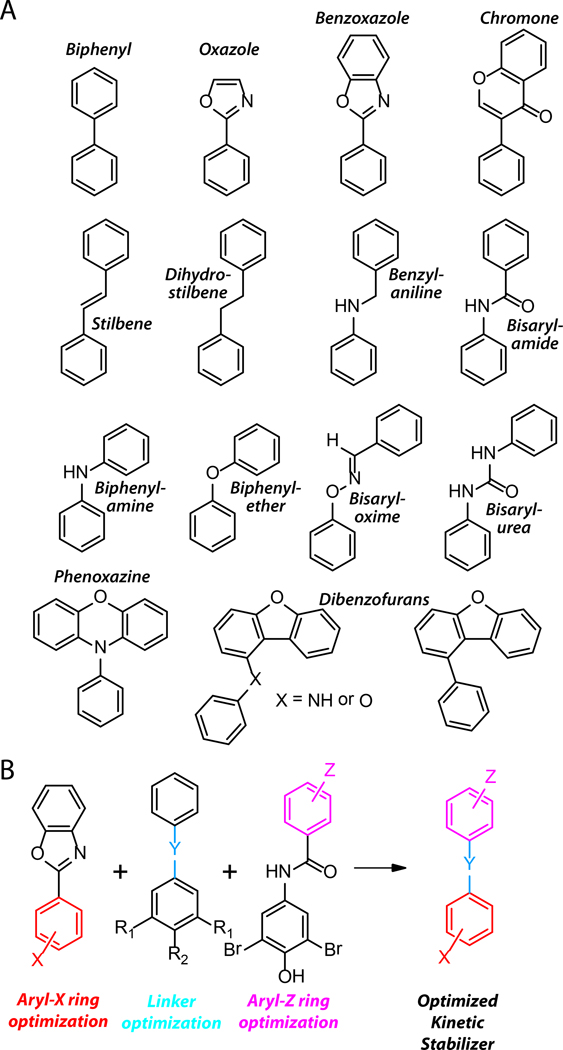
Over one thousand aromatic small molecules exhibiting structural complementarity to the T4 binding sites within TTR have been designed and synthesized by taking advantage of ligand2•TTR structural information to optimize kinetic stabilizer design.12,17,18,114,116,142,143,149–164Numerous structurally distinct TTR kinetic stabilizers were identified early in this program using screening approaches, including naturally derived flavonoid, xanthone derivatives, as well as biaryls and some of these facilitated the generation of the early ligand2•TTR structural information.144–146,148 Structure-based drug design and screening hits guided our synthetic chemistry, affording compounds in multiple structural families including: bisaryloxime ethers, biphenyls, 1-aryl-4,6-biscarboxydibenzofurans, 2-phenylbenzoxazole and biphenylamines (Figure 6A).12,17,18,114,116,142,143,149–164Potent compounds have also been identified through halogenation of nonsteroidal anti-inflammatory drugs (NSAIDs), such as salicylic acid, diflunisal, and flufenamic acid.153,165,166We also recently employed a substructure combination strategy163 to arrive at potent and selective TTR kinetic stabilizers, where in we systematically ranked the candidate substructures composing a typical TTR kinetic stabilizer, the two aromatic substructures and the linker, using fibril inhibition potency and plasma TTR binding selectivity data.159–161 Of the 92 stilbene and dihydrostilbene candidate kinetic stabilizers predicted to be potent and selective by the substructure combination strategy, nearly all potently inhibit TTR fibril formation and 17 of these exhibited a binding stoichiometry of >1.5 (out of a maximum of 2) to plasma TTR, while displaying minimal binding to the thyroid hormone receptor (<20% hormone displacement). These potent and selective TTR kinetic stabilizers also rescue cells from the cytotoxic effects of TTR amyloidogenesis.163Others have also identified TTR kinetic stabilizers using a variety of the approaches mentioned above.167–177
Figure 6.
The structural diversity of TTR kinetic stabilizer core structures. (A) Line drawings of the structural cores underpinning the 1000+ TTR kinetic stabilizers synthesized to date. Adapted from Johnson et al.12 (B) Schematic depiction of the substructure combination strategy to create potent and highly selective TTR kinetic stabilizers. Individual elements of candidate TTR kinetic stabilizers are varied and the most potent and selective substructures of the candidates are combined to create potent, highly selective TTR kinetic stabilizers. Adapted from Choi et al.163
TTR kinetic stabilizers are typically composed of three substructures: two differentially substituted aryl rings connected by a linker (Figure 6B).159–161Each ring occupies one of the two subsites of the T4 binding site in TTR.12,17,18,163Substituted aryl rings occupying the outer binding subsite form salt bridges with the Lys-15 and/or 15'ε-ammonium groups and /or engage in complementary hydrophobic interactions with halogen binding sites 1 /1' and/or 2/2', where as the functionalized aromatic rings occupying the inner binding subsite can engage in hydrogen bonds with Ser-117 and/or 117' and/or occupy the hydrophobic halogen binding pockets 2/2' and/or 3/3'.12,17,18These rings can either be linked directly, as in the case of the biphenyls,143,154 or can be connected through short hydrophobic linkers,12,160as in the case of the stilbenes (Figure 6A). The linker typically interacts with the hydrophobic side chains of Leu-17 and/or 17', Ala-108 and/or 108', Leu-110 and/or 110', and Val-121 and/or 121'.160 These complementary interactions between the kinetic stabilizer and TTR combine to differentially stabilize the ground state over the dissociative transition state, making tetramer dissociation extremely slow under physiological conditions (Figures 4 and 5).12,114
Most TTR kinetic stabilizers bind to the T4 binding sites with negative cooperativity, apparently resulting from conformational changes within the tetramer upon binding to the first T4 site.12,142,178Strikingly, occupancy of only one T4 binding site is sufficient to impart kinetic stabilization on the entire TTR tetramer.142This was demonstrated unequivocally by tethering a kinetic stabilizer via a linker to Cys10 in a single monomer of a TTR tetramer.142 Such a chemically modified TTR tetramer was found to be highly resistant to denaturation and aggregation. This data is enabling in that it allows patients to be treated with lower doses of kinetic stabilizers that are sufficient to occupy one of the two T4 binding sites.124
TTR kinetic stabilizers must exhibit high binding affinity and high binding selectivity to plasma TTR over the blood plasma proteome in order to be useful pharmacologic agents.9,12,159–161,163There are >4000 proteins in blood plasma, including albumin. Albumin binds promiscuously to many small molecules and, by doing so, could prevent TTR kinetic stabilizers from binding to TTR. In a recent review, and in the original papers, we provide a comprehensive list of recently synthesized TTR kinetic stabilizers along with their so-called efficacy scores, which integrate TTR amyloidogenesis inhibitor potency and TTR binding selectivity in plasma.18,159,160,163Furthermore, as mentioned above, candidate kinetic stabilizers must not interact with the thyroid hormone receptor, a major concern given the structural similarity of some kinetic stabilizers with triiodothyronine (T3, the primary thyroid hormone) and T4 (the prohormone). NSAID activity is also undesirable in candidate TTR kinetic stabilizers, as cyclooxygenase inhibition is contraindicated for treating TTR cardiomyopathy patients, who often have impaired renal blood flow.159–161,179
Testing the Kinetic Stabilizer Strategy in an FAP Clinical Trial
The pharmacokinetic and pharmacodynamic properties of most of the small molecules that are potent TTR kinetic stabilizers in vitro and exhibit excellent plasma TTR binding selectivity ex vivo have not been evaluated. To date, only two small molecules have been assessed in animal safety studies and in human clinical trials.27,159–161,180One of these, diflunisal, is an FDA-approved NSAID, which binds to TTR with negative cooperativity (Kd1= 75 nM, Kd2= 1100 nM).153,166,180,181Diflunisal exhibits only modest binding selectivity to TTR over all the other plasma proteins and displays modest binding affinity to TTR in vitro. Nevertheless, recent Phase I clinical trials showed that diflunisal kinetically stabilizes TTR tetramers in human plasma because of its excellent oral bioavailability and high plasma concentrations after oral dosing (250 mg BID).180,181A placebo-controlled, multicenter phase III clinical trial to test the efficacy of diflunisal for the treatment of FAP, FAC and SSA is currently fully enrolled, with the results expected within the next two years (http://www.clinicaltrials.gov/). The enhanced sensitivity of some patients to the gastrointestinal, cardiac, and renal side effects of taking 0.5 g of diflunisal a day may limit its applicability to those who can tolerate this dose.
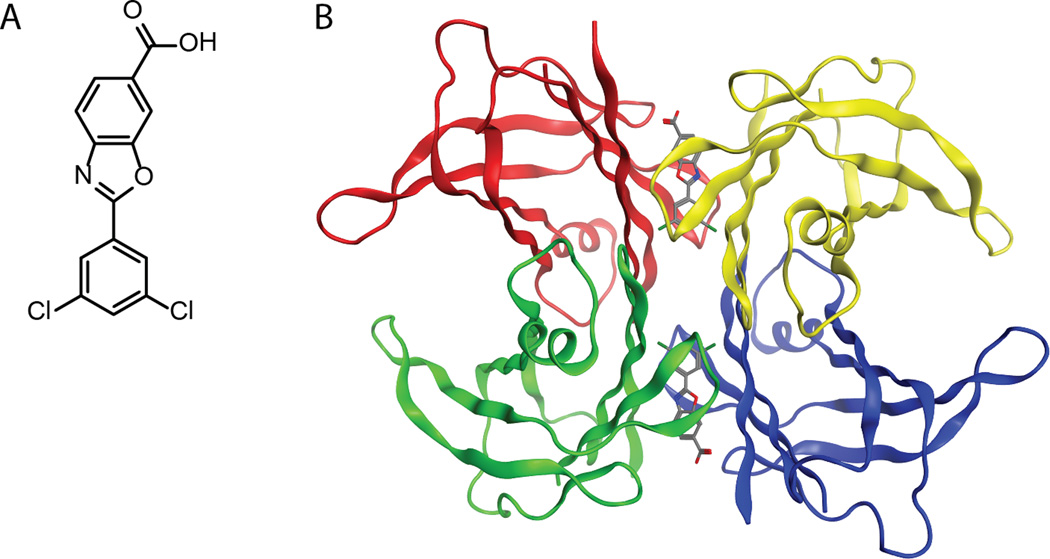
Another small molecule discovered by the Kelly laboratory143and developed by FoldRx Pharmaceuticals (acquired by Pfizer in September 2010) was recently shown to slow the progression of FAP in a placebo controlled, double-blind Phase II/III clinical trial (http://www.clinicaltrials.gov/).27Tafamidis, or 2-(3,5-dichloro-phenyl)-benzoxazole-6-carboxylic acid (Figure 7A; trade name: Vyndaqel®), was shown to bind highly selectively to TTR in human plasma and with negative cooperativity (Kds ~ 2 nM and ~200 nM) to TTR (manuscript in preparation). Tafamidis reaches its EC50 for preventing TTR fibril formation at a tafamidis: TTR tetramer ratio of <1, consistent with tafamidis effectively stabilizing TTR when it occupies only one of TTR’s two T4 binding sites. Tafamidis dose-dependently kinetically stabilizes TTR under denaturing conditions (in the presence of 6.5 M urea) and under physiologic conditions. According to structural modeling, tafamidis is envisioned to bind to TTR such that it stabilizes the weaker dimer-dimer interface through a combination of specific hydrophobic and electrostatic interactions (Figure 7B). Importantly, tafamidis kinetically stabilizes a broad spectrum of TTR variants, suggesting a broadly applicable therapeutic for all the TTR amyloidosis (manuscript in preparation).
Figure 7.
(A) Line drawing of tafamidis. (B) Structural model of how tafamidis is envisioned to bind to and kinetically stabilize TTR.
The 18-month duration phase II/III clinical trial for tafamidis enrolled > 100 patients heterozygous for V30M TTR, the clinically most important FAP-associated mutation (≈ 10,000 FAP cases world-wide).27Treatment with tafamidis (20 mg, once a day) slowed progression of peripheral and autonomic neuropathy, as measured by neurologic examination of the lower limbs, nerve conduction studies, among other measures of neuropathy; see the European Medicines Agency website (www.ema.europa.eu) for more details.27Tafamidis treatment also improved autonomic nervous system dysfunction and cachexiaas reflected by an increase in the modified body mass index (mBMI). Finally, tafamidis slowed the decline in the total quality of life, as measured by the Norfolk quality of life questionnaire used for diabetic neuropathy (QOL-DN).27Tafamidis was approved by the European Medicines Agency in November 2011 for the treatment of FAP (www.ema.europa.eu). Approval by the United States Food and Drug Administration is expected in 2012. It seems unlikely that the amyloid fibrils in the tafamidis treated V30M FAP patients were cleared over the course of this trial based on amyloid P component imaging data revealing that amyloid is rarely cleared post liver transplantation.121 What seems almost certainis that the process of TTR amyloidogenesis is stopped in the patients taking tafamidis, although further studies will be required to demonstrate this assertion. The lack of misassembly intermediates in FAP patients with slowed disease progression suggests that it is the process of TTR amyloidogenesis, and not the amyloid fibrils themselves, that cause the TTR amyloidoses.
New Directions in the Kinetic Stabilizer Approach
In addition to the above-mentioned monovalent TTR kinetic stabilizers, bivalent kinetic stabilizers that simultaneously bind to both T4 sites have also been developed (Figure 8A). Initial work by Green et al. showed that bivalent kinetic stabilizers bind with 1:1 stoichiometry to TTR during tetramer formation within the cell, but do not bind to already-formed tetramers.152 More recently, the Pepys group has developed bivalent palindromic ligands that bind with high affinity to preformed TTR tetramers under physiological conditions.167 These bivalent ligands stabilize the tetramer more potently than monovalent ligands. In addition, they preferentially bind mutant TTR over wild type TTR, perhaps because the inherent instability of mutant TTR allows for easier access to T4 binding sites in the tetramer. It is not yet clear whether these molecules have suitable TTR plasma binding selectivity, solubility, and appropriate pharmacokinetic and pharmacodynamics properties to serve as clinical candidates.
Figure 8.
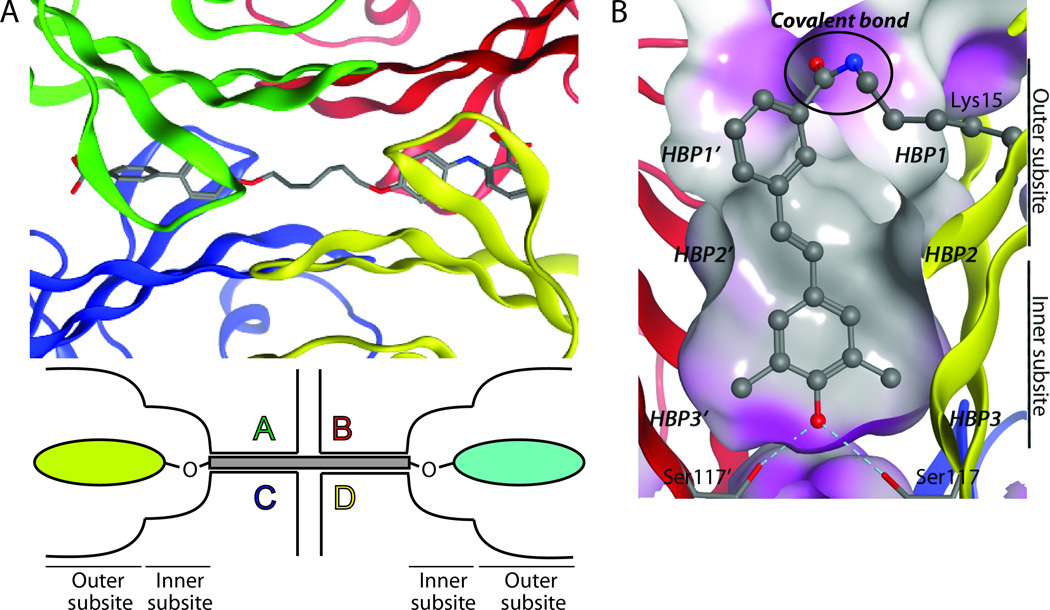
(A) Top: Close-up view of a bivalent TTR kinetic stabilizer bound to the thyroid hormone binding sites (PDB code:2FLM). Bottom: Schematic representation of a bivalent TTR kinetic stabilizer bound simultaneously to both T4 binding sites of tetrameric TTR. Figure adapted from Green et al.152 (B) Close-up view of a covalent kinetic stabilizer attached via an amide bond to Lys 15 in one thyroid hormone binding site of TTR (PDB code: 3HJ0).
Covalent kinetic stabilizers of TTR that are more potent inhibitors of amyloidogenesis than their non-covalent counterparts have also recently been reported.162Stilbenes, conceived of by structure-based design, that selectively bind to TTR in preference to the more than 4000 other human plasma proteins and then react chemo-selectively with only one of eight lysine ε-amino groups (Lys15) within transthyretin have been reported. The crystal structure confirms the expected binding orientation of the stilbene substructure and the conjugating amide bond (Figure 8B). While these covalent transthyretin kinetic stabilizers exhibit superior amyloid inhibition potency compared to their non-covalent counterpartsin vitro, and prevent cytotoxicity associated with the process of amyloidogenesis, their safety, pharmacokinetics and pharmacodynamicsremain to be evaluated.
Perspective and Conclusions
Structure-based drug design principles12,17,18enabled the efficient design of tafamidis,143 a high affinity, highly selective TTR kinetic stabilizer that slows the progression of TTR amyloid disease in FAP patients.27This kinetic stabilizer approach for halting aggregation and preventing the degeneration of post-mitotic tissue is also being explored in other amyloid diseases as well. For example, small molecules have been used to stabilize the mutant superoxide dismutase-1 associated with familial amyotrophic lateral sclerosis,182and β2-microglobulin associated with dialysis-related amyloidosis.183 In addition, camelid antibodies have been employed to stabilize lysozyme to prevent lysozymeamyloidosis.115 Thus, the kinetic stabilizer strategy, now clinically validated for TTR amyloidosis, has the potential to ameliorate additional degenerative diseases. A modification of this approach, the discovery of small molecules that bind to monomeric intrinsically disordered protein conformational ensembles, altering their aggregation propensity, is also being explored for Alzheimer’s disease. We envision a pharmacologic future, where in synergy in ameliorating the human amyloid diseases is achieved by using small molecule kinetic stabilizers (or the equivalent),12 in combination with drugs that lower the concentration of the amyloidogenic protein of interest,108,109and also in combination with drugs that enhance the capacity of the proteostasis network to achieve proteome maintenance.46,48
Highlights.
Wild type and mutant transthyretin (TTR) causes amyloid diseases in humans
Amyloid formation requires tetramer dissociation (rate limiting) and monomer misfolding
Transthyretin tetramers can be kinetically stabilized by binding small molecules
Tafamidis, a kinetic stabilizer of TTR, is the first drug approved to treat a TTR amyloidosis
Acknowledgements
Tafamidis would not have been possible without sustained NIH support (DK 046335; since 1990). Financial support from the Skaggs Institute for Chemical Biologyand the Lita Annenberg Hazen Foundation also critically accelerated progress. The creation of FoldRx was made possible by Christopher Mirabelli’s leadership (Health Care Ventures).The development of Tafamidis was ably accomplished by Richard Labaudiniere (FoldRx; President and CEO), Donna Grogan (FoldRx; Chief Medical Officer) and Jeff Packman (FoldRx; Sr. Director, Drug Development Operations). Paul S. Anderson (Vice President of Drug Discovery, Bristol-Myers Squibb (retired)) helped the FoldRx team and the founders choose Tafamidis out of the numerous appealing kinetic stabilizer candidates that FoldRx could have pursued.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blake CC, Geisow MJ, Oatley SJ, Rerat B, Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J. Mol. Biol. 1978;121:339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 2.Hornberg A, Eneqvist T, Olofsson A, Lundgren E, Sauer-Eriksson AE. A comparative analysis of 23 structures of the amyloidogenic protein transthyretin. J. Mol. Biol. 2000;302:649–669. doi: 10.1006/jmbi.2000.4078. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JA, Benson MD. Transthyretin: a review from a structural perspective. Cell Mol. Life Sci. 2001;58:1491–1521. doi: 10.1007/PL00000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider F, Hammarstrom P, Kelly JW. Transthyretin slowly exchanges subunits under physiological conditions: A convenient chromatographic method to study subunit exchange in oligomeric proteins. Protein Sci. 2001;10:1606–1613. doi: 10.1110/ps.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268:1039–1041. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- 6.Kopelman M, Cogan U, Mokady S, Shinitzky M. The interaction between retinol-binding proteins and prealbumins studied by fluorescence polarization. Biochim. Biophys. Acta. 1976;439:449–460. doi: 10.1016/0005-2795(76)90082-9. [DOI] [PubMed] [Google Scholar]
- 7.Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam. Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
- 8.White JT, Kelly JW. Support for the multigenic hypothesis of amyloidosis: the binding stoichiometry of retinol-binding protein, vitamin A thyroid hormone influences transthyretin amyloidogenicity in vitro. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13019–13024. doi: 10.1073/pnas.241406698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purkey HE, Dorrell MI, Kelly JW. Evaluating the binding selectivity of transthyretin amyloid fibril inhibitors in blood plasma. Proc. Natl. Acad. Sci. U. S. A. 2001;98:5566–5571. doi: 10.1073/pnas.091431798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartalena L, Robbins J. Variations in thyroid hormone transport proteins and their clinical implications. Thyroid. 1992;2:237–245. doi: 10.1089/thy.1992.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Bartalena L, Robbins J. Thyroid hormone transport proteins. Clin. Lab. Med. 1993;13:583–598. [PubMed] [Google Scholar]
- 12.Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW. Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc. Chem. Res. 2005;38:911–921. doi: 10.1021/ar020073i. [DOI] [PubMed] [Google Scholar]
- 13.Hagen GA, Elliott WJ. Transport of thyroid hormones in serum and cerebrospinal fluid. J Clin Endocrinol Metab. 1973;37:415–422. doi: 10.1210/jcem-37-3-415. [DOI] [PubMed] [Google Scholar]
- 14.Herbert J, Wilcox JN, Pham KT, Fremeau RT, Jr, Zeviani M, Dwork A, Soprano DR, Makover A, Goodman DS, Zimmerman EA, et al. Transthyretin: a choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell award. Neurology. 1986;36:900–911. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- 15.Foss TR, Wiseman RL, Kelly JW. The pathway by which the tetrameric protein transthyretin dissociates. Biochemistry. 2005;44:15525–15533. doi: 10.1021/bi051608t. [DOI] [PubMed] [Google Scholar]
- 16.Foss TR, Kelker MS, Wiseman RL, Wilson IA, Kelly JW. Kinetic stabilization of the native state by protein engineering: implications for inhibition of transthyretin amyloidogenesis. J. Mol. Biol. 2005;347:841–854. doi: 10.1016/j.jmb.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Sacchettini JC. Rational design of potent human transthyretin amyloid disease inhibitors. Nat. Struct. Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 18.Connelly S, Choi S, Johnson SM, Kelly JW, Wilson IA. Structurebased design of kinetic stabilizers that ameliorate the transthyretin amyloidoses. Curr. Opin. Struct. Biol. 2010;20:54–62. doi: 10.1016/j.sbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojtczak A, Cody V, Luft JR, Pangborn W. Structures of human transthyretin complexed with thyroxine at 2.0 A resolution and 3',5'-dinitro-N-acetyl-L-thyronine at 2.2 A resolution. Acta. Crystallogr. D. 1996;52:758–765. doi: 10.1107/S0907444996003046. [DOI] [PubMed] [Google Scholar]
- 20.Wojtczak A, Luft J, Cody V. Mechanism of molecular recognition. Structural aspects of 3,3'-diiodo-L-thyroxine binding to human serum transthyretin. J. Biol. Chem. 1992;267:353–357. [PubMed] [Google Scholar]
- 21.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 22.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 23.Kelly JW. Alternative conformations of amyloidogenic proteins govern their behavior. Curr. Opin. Struct. Biol. 1996;6:11–17. doi: 10.1016/s0959-440x(96)80089-3. [DOI] [PubMed] [Google Scholar]
- 24.Hammarstrom P, Schneider F, Kelly JW. Trans-suppression of misfolding in an amyloid disease. Science. 2001;293:2459–2462. doi: 10.1126/science.1062245. [DOI] [PubMed] [Google Scholar]
- 25.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav. Neurol. 2009;21:117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coelho T, Maia L, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, Lozeron P, Suhr OB, Campistol JM, Conceição I, Schmidt HH-J, Trigo P, Kelly JW, Labaudinière R, Chan J, Packman J, Wilson A, Grogan DR. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2011 doi: 10.1212/WNL.0b013e3182661eb1. under final revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 29.Bradbury J. Alpha-synuclein gene triplication discovered in Parkinson's disease. Lancet Neurol. 2003;2:715. doi: 10.1016/s1474-4422(03)00601-x. [DOI] [PubMed] [Google Scholar]
- 30.Theuns J, Van BC. alpha-Synuclein gene duplications in sporadic Parkinson disease. Neurology. 2008;70:7–9. doi: 10.1212/01.wnl.0000295508.10258.6b. [DOI] [PubMed] [Google Scholar]
- 31.Zempel H, Thies E, Mandelkow E, Mandelkow E-M. Abeta oligomers cause localized Ca2+ elevation, missorting of endogenous tau into dendrites, tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- 33.Lai Z, Colon W, Kelly JW. The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry. 1996;35:6470–6482. doi: 10.1021/bi952501g. [DOI] [PubMed] [Google Scholar]
- 34.Hurle MR, Helms LR, Li L, Chan W, Wetzel R. A role for destabilizing amino acid replacements in light-chain amyloidosis. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5446–5450. doi: 10.1073/pnas.91.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth DR, Sunde M, Bellotti V, Robinson CV, Hutchinson WL, Fraser PE, Hawkins PN, Dobson CM, Radford SE, Blake CC, Pepys MB. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature. 1997;385:787–793. doi: 10.1038/385787a0. [DOI] [PubMed] [Google Scholar]
- 36.Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4224–4228. doi: 10.1073/pnas.95.8.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiseman RL, Powers ET, Kelly JW. Partitioning conformational intermediates between competing refolding and aggregation pathways: insights into transthyretin amyloid disease. Biochemistry. 2005;44:16612–16623. doi: 10.1021/bi0511484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McParland VJ, Kad NM, Kalverda AP, Brown A, Kirwin-Jones P, Hunter MG, Sunde M, Radford SE. Partially unfolded states of beta(2)-microglobulin and amyloid formation in vitro. Biochemistry. 2000;39:8735–8746. doi: 10.1021/bi000276j. [DOI] [PubMed] [Google Scholar]
- 39.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 40.DuBay KF, Pawar AP, Chiti F, Zurdo J, Dobson CM, Vendruscolo M. Prediction of the Absolute Aggregation Rates of Amyloidogenic Polypeptide Chains. J. Mol. Biol. 2004;341:1317–1326. doi: 10.1016/j.jmb.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 41.Tartaglia GG, Pawar AP, Campioni S, Dobson CM, Chiti F, Vendruscolo M. Prediction of Aggregation-Prone Regions in Structured Proteins. J. Mol. Biol. 2008;380:425–436. doi: 10.1016/j.jmb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3487–3492. doi: 10.1073/pnas.0915166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Escamilla A-M, Rousseau F, Schymkowitz J, Serrano L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 2004;22:1302–1306. doi: 10.1038/nbt1012. [DOI] [PubMed] [Google Scholar]
- 44.Amaducci L, Tesco G. Aging as a major risk for degenerative diseases of the central nervous system. Curr. Opin. Neurol. 1994;7:283–286. doi: 10.1097/00019052-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Morimoto RI. Stress, aging, and neurodegenerative disease. New EnglJ. Med. 2006;355:2254–2255. doi: 10.1056/NEJMcibr065573. [DOI] [PubMed] [Google Scholar]
- 46.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 47.Cohen E, Paulsson JF, Blinder P, Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M, Kelly JW, Masliah E, Dillin A. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell. 2009;139:1157–1169. doi: 10.1016/j.cell.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 49.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 50.Hammarstrom P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly JW, Colon W, Lai Z, Lashuel HA, McCulloch J, McCutchen SL, Miroy GJ, Peterson SA. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv. Protein Chem. 1997;50:161–181. doi: 10.1016/s0065-3233(08)60321-6. [DOI] [PubMed] [Google Scholar]
- 52.McCutchen SL, Lai Z, Miroy GJ, Kelly JW, Colon W. Comparison of lethal and nonlethal transthyretin variants and their relationship to amyloid disease. Biochemistry. 1995;34:13527–13536. doi: 10.1021/bi00041a032. [DOI] [PubMed] [Google Scholar]
- 53.McCutchen SL, Colon W, Kelly JW. Transthyretin mutation Leu-55-Pro significantly alters tetramer stability and increases amyloidogenicity. Biochemistry. 1993;32:12119–12127. doi: 10.1021/bi00096a024. [DOI] [PubMed] [Google Scholar]
- 54.Brignull HR, Morley JF, Garcia SM, Morimoto RI. Modeling polyglutamine pathogenesis in C. elegans. Methods Enzymol. 2006;412:256–282. doi: 10.1016/S0076-6879(06)12016-9. [DOI] [PubMed] [Google Scholar]
- 55.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Westermark P, Sletten K, Johansson B, Cornwell GG., 3rd Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2843–2845. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cornwell GG, 3rd, Sletten K, Johansson B, Westermark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem. Biophys. Res. Comm. 1988;154:648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- 59.Coelho T. Familial amyloid polyneuropathy: new developments in genetics and treatment. Curr. Opin. Neurol. 1996;9:355–359. [PubMed] [Google Scholar]
- 60.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, Buxbaum JN. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. New EnglJ. Med. 1997;336:466–473. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 61.Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2817–2822. doi: 10.1073/pnas.0400062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saraiva MJ, Birken S, Costa PP, Goodman DS. Amyloid fibril protein in familial amyloidotic polyneuropathy, Portuguese type. Definition of molecular abnormality in transthyretin (prealbumin) J. Clin. Invest. 1984;74:104–119. doi: 10.1172/JCI111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saraiva MJ, Costa PP, Birken S, Goodman DS. Presence of an abnormal transthyretin (prealbumin) in Portuguese patients with familial amyloidotic polyneuropathy. Transactions of the Association of American Physicians. 1983;96:261–270. [PubMed] [Google Scholar]
- 64.Sousa MM, Saraiva MJ. Neurodegeneration in familial amyloid polyneuropathy: from pathology to molecular signaling. Prog. Neurobiol. 2003;71:385–400. doi: 10.1016/j.pneurobio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Andrade C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- 66.Buxbaum JN. Transthyretin and the transthyretin amyloidoses. In: Uversky VN, Fink AL, editors. Protein Misfolding, Aggregation, and Conformational Diseases, Part B: Molecular Mechanisms of Conformational Diseases. Springer Science; 2007. pp. 259–283. [Google Scholar]
- 67.Jacobson DR, McFarlin DE, Kane I, Buxbaum JN. Transthyretin Pro55, a variant associated with early-onset, aggressive, diffuse amyloidosis with cardiac and neurologic involvement. Hum. Genet. 1992;89:353–356. doi: 10.1007/BF00220559. [DOI] [PubMed] [Google Scholar]
- 68.Soares M, Buxbaum J, Sirugo G, Coelho T, Sousa A, Kastner D, Joao Saraiva M. Genetic anticipation in Portuguese kindreds with familial amyloidotic polyneuropathy is unlikely to be caused by triplet repeat expansions. Hum. Genet. 1999;104:480–485. doi: 10.1007/s004390050991. [DOI] [PubMed] [Google Scholar]
- 69.Saraiva MJM. Transthyretin mutations in health and disease. Hum. Mutat. 1995;5:191–196. doi: 10.1002/humu.1380050302. [DOI] [PubMed] [Google Scholar]
- 70.Connors LH, Richardson AM, Theberge R, Costello CE. Tabulation of transthyretin (TTR) variants as of 1/1/2000. Amyloid. 2000;7:54–69. doi: 10.3109/13506120009146826. [DOI] [PubMed] [Google Scholar]
- 71.Sekijima Y, Wiseman RL, Matteson J, Hammarstrom P, Miller SR, Sawkar AR, Balch WE, Kelly JW. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121:73–85. doi: 10.1016/j.cell.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 72.Hammarstrom P, Sekijima Y, White JT, Wiseman RL, Lim A, Costello CE, Altland K, Garzuly F, Budka H, Kelly JW. D18G transthyretin is monomeric, aggregation prone, and not detectable in plasma and cerebrospinal fluid: a prescription for central nervous system amyloidosis? Biochemistry. 2003;42:6656–6663. doi: 10.1021/bi027319b. [DOI] [PubMed] [Google Scholar]
- 73.Sekijima Y, Hammarstrom P, Matsumura M, Shimizu Y, Iwata M, Tokuda T, Ikeda S, Kelly JW. Energetic characteristics of the new transthyretin variant A25T may explain its atypical central nervous system pathology. Lab. Invest. 2003;83:409–417. doi: 10.1097/01.lab.0000059937.11023.1f. [DOI] [PubMed] [Google Scholar]
- 74.Benson MD. Familial Amyloidotic polyneuropathy. Trends Biochem. Sci. 1989;12:88–92. doi: 10.1016/0166-2236(89)90162-8. [DOI] [PubMed] [Google Scholar]
- 75.Ando Y, Suhr OB. Autonomic dysfunction in familial amyloidotic polyneuropathy (FAP) Amyloid. 1998;5:288–300. doi: 10.3109/13506129809007303. [DOI] [PubMed] [Google Scholar]
- 76.Sousa MM, Cardoso I, Fernandes R, Guimaraes A, Saraiva MJ. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 2001;159:1993–2000. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou X, Aguilar MI, Small DH. Transthyretin and familial amyloidotic polyneuropathy. Recent progress in understanding the molecular mechanism of neurodegeneration. FEBS J. 2007;274:1637–1650. doi: 10.1111/j.1742-4658.2007.05712.x. [DOI] [PubMed] [Google Scholar]
- 78.Bourgault S, Solomon JP, Reixach N, Kelly JW. Sulfated glycosaminoglycans accelerate transthyretin amyloidogenesis by quaternary structural conversion. Biochemistry. 2011;50:1001–1015. doi: 10.1021/bi101822y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourgault S, Choi S, Buxbaum JN, Kelly JW, Price JL, Reixach N. Mechanisms of transthyretin cardiomyocyte toxicity inhibition by resveratrol analogs. Biochem. Biophys. Res. Comm. 2011;410:707–713. doi: 10.1016/j.bbrc.2011.04.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeldenrust SR, Benson MD. Familial and senile amyloidosis caused by transthyretin. In: Ramirez-Alvarado M, Kelly JW, Dobson CM, editors. Protein Misfolding Diseases. John Wiley & Sons, Inc.; 2010. pp. 795–815. [Google Scholar]
- 81.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch. Intern. Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 82.Pepys MB. Amyloidosis. In: Samter M, editor. Immunological Diseases. Vol. 1. Boston/Toronto: Little, Brown and Company; 1988. pp. 631–674. [Google Scholar]
- 83.Westermark P, Bergstrom J, Solomon A, Murphy C, Sletten K. Transthyretin-derived senile systemic amyloidosis: clinicopathologic and structural considerations. Amyloid. 2003;10:48–54. [PubMed] [Google Scholar]
- 84.Hurshman AR, White JT, Powers ET, Kelly JW. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry. 2004;43:7365–7381. doi: 10.1021/bi049621l. [DOI] [PubMed] [Google Scholar]
- 85.Powers ET, Powers DL. The kinetics of nucleated polymerizations at high concentrations: amyloid fibril formation near and above the "supercritical concentration". Biophys. J. 2006;91:122–132. doi: 10.1529/biophysj.105.073767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferrone F. Analysis of protein aggregation kinetics. Methods Enzymol. 1999;309:256–274. doi: 10.1016/s0076-6879(99)09019-9. [DOI] [PubMed] [Google Scholar]
- 87.Wolfe MS. Structure, mechanism and inhibition of OE≥-secretase and presenilin-like proteases. Biol. Chem. 2010;391:839–847. doi: 10.1515/BC.2010.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.John V, Beck JP, Bienkowski MJ, Sinha S, Heinrikson RL. Human beta-secretase (BACE) and BACE inhibitors. J. Med. Chem. 2003;46:4625–4630. doi: 10.1021/jm030247h. [DOI] [PubMed] [Google Scholar]
- 89.Sambamurti K, Greig NH, Utsuki T, Barnwell EL, Sharma E, Mazell C, Bhat NR, Kindy MS, Lahiri DK, Pappolla MA. Targets for AD treatment: conflicting messages from gamma-secretase inhibitors. J. Neurochem. 2011;117:359–374. doi: 10.1111/j.1471-4159.2011.07213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat. Rev. Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schenk D. Opinion: Amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat. Rev. Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 92.Schenk D. Hopes remain for an Alzheimer's vaccine. Nature. 2004;431:398. doi: 10.1038/431398b. [DOI] [PubMed] [Google Scholar]
- 93.Comenzo RL. Current and emerging views and treatments of systemic immunoglobulin light-chain (AL) amyloidosis. Contrib. Nephrol. 2007;153:195–210. doi: 10.1159/000096768. [DOI] [PubMed] [Google Scholar]
- 94.Gertz MA. Immunoglobulin light chain amyloidosis: 2011 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2011;86:181–186. doi: 10.1002/ajh.21934. [DOI] [PubMed] [Google Scholar]
- 95.Kastritis E, Anagnostopoulos A, Roussou M, Toumanidis S, Pamboukas C, Migkou M, Tassidou A, Xilouri I, Delibasi S, Psimenou E, Mellou S, Terpos E, Nanas J, Dimopoulos MA. Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 2007;92:1351–1358. doi: 10.3324/haematol.11325. [DOI] [PubMed] [Google Scholar]
- 96.Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkins PN, Perfetti V, Gillmore JD, Palladini G. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J. Clin. Oncol. 2010;28:1031–1037. doi: 10.1200/JCO.2009.23.8220. [DOI] [PubMed] [Google Scholar]
- 97.Sitia R, Palladini G, Merlini G. Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica. 2007;92:1302–1307. doi: 10.3324/haematol.12136. [DOI] [PubMed] [Google Scholar]
- 98.Holmgren G, Ericzon BG, Groth CG, Steen L, Suhr O, Andersen O, Wallin BG, Seymour A, Richardson S, Hawkins PN. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet. 1993;341:1113–1116. doi: 10.1016/0140-6736(93)93127-m. [DOI] [PubMed] [Google Scholar]
- 99.Herlenius G, Wilczek HE, Larsson M, Ericzon BG. Ten years of international experience with liver transplantation for familial amyloidotic polyneuropathy: results from the Familial Amyloidotic Polyneuropathy World Transplant Registry. Transplantation. 2004;77:64–71. doi: 10.1097/01.TP.0000092307.98347.CB. [DOI] [PubMed] [Google Scholar]
- 100.Dubrey SW, Burke MM, Hawkins PN, Banner NR. Cardiac transplantation for amyloid heart disease: the United Kingdom experience. J Heart Lung Transplant. 2004;23:1142–1153. doi: 10.1016/j.healun.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 101.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 102.Ruygrok PN, Gane EJ, McCall JL, Chen XZ, Haydock DA, Munn SR. Combined heart and liver transplantation for familial amyloidosis. Intern Med J. 2001;31:66–67. doi: 10.1046/j.1445-5994.2001.00008.x. [DOI] [PubMed] [Google Scholar]
- 103.Ando Y, Tashima K, Tanaka Y, Nakazato M, Ericzon BG, Duraj FF, Sakashita N, Kimura E, Ando E, Yonehara T, et al. Treatment of a Japanese patient with familial amyloidotic polyneuropathy with orthotopic liver transplantation. Internal Med. 1994;33:730–732. doi: 10.2169/internalmedicine.33.730. [DOI] [PubMed] [Google Scholar]
- 104.Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, Andersen O, Karlberg I, Norden G, Nakazato M, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-Met30) Clin. Genet. 1991;40:242–246. doi: 10.1111/j.1399-0004.1991.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 105.Hornsten R, Wiklund U, Olofsson BO, Jensen SM, Suhr OB. Liver transplantation does not prevent the development of life-threatening arrhythmia in familial amyloidotic polyneuropathy, Portuguese-type (ATTR Val30Met) patients. Transplantation. 2004;78:112–116. doi: 10.1097/01.tp.0000133517.20972.27. [DOI] [PubMed] [Google Scholar]
- 106.Olofsson BO, Backman C, Karp K, Suhr OB. Progression of cardiomyopathy after liver transplantation in patients with familial amyloidotic polyneuropathy, Portuguese type. Transplantation. 2002;73:745–751. doi: 10.1097/00007890-200203150-00015. [DOI] [PubMed] [Google Scholar]
- 107.Liepnieks JJ, Zhang LQ, Benson MD. Progression of transthyretin amyloid neuropathy after liver transplantation. Neurology. 2010;75:324–327. doi: 10.1212/WNL.0b013e3181ea15d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, Sloop KW. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–618. doi: 10.1002/mus.20503. [DOI] [PubMed] [Google Scholar]
- 109.Benson MD, Smith RA, Hung G, Kluve-Beckerman B, Showalter AD, Sloop KW, Monia BP. Suppression of choroid plexus transthyretin levels by antisense oligonucleotide treatment. Amyloid. 2010;17:43–49. doi: 10.3109/13506129.2010.483121. [DOI] [PubMed] [Google Scholar]
- 110.Buxbaum JN, Ye Z, Reixach N, Friske L, Levy C, Das P, Golde T, Masliah E, Roberts AR, Bartfai T. Transthyretin protects Alzheimer's mice from the behavioral and biochemical effects of Abeta toxicity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2681–2686. doi: 10.1073/pnas.0712197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, Masliah E, Reixach N, Buxbaum JN. Neuronal production of transthyretin in human and murine Alzheimer's disease: is it protective? J. Neurosci. 2011;31:12483–12490. doi: 10.1523/JNEUROSCI.2417-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sah DWY. Lipid formulated compositions and methods for inhibiting expression of transthyretin (TTR) Alnylam Pharmaceuticals, Inc., USA; 2011. p. 150. (Patent, ed.) [Google Scholar]
- 113.Kelly JW. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 114.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–716. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 115.Chan PH, Pardon E, Menzer L, De Genst E, Kumita JR, Christodoulou J, Saerens D, Brans A, Bouillenne F, Archer DB, Robinson CV, Muyldermans S, Matagne A, Redfield C, Wyns L, Dobson CM, Dumoulin M. Engineering a camelid antibody fragment that binds to the active site of human lysozyme and inhibits its conversion into amyloid fibrils. Biochemistry. 2008;47:11041–11054. doi: 10.1021/bi8005797. [DOI] [PubMed] [Google Scholar]
- 116.Miroy GJ, Lai Z, Lashuel HA, Peterson SA, Strang C, Kelly JW. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc. Natl. Acad. Sci. U. S. A. 1996;93:15051–15056. doi: 10.1073/pnas.93.26.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J. Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 120.Sousa MM, Fernandes R, Palha JA, Taboada A, Vieira P, Saraiva MJ. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. AmJ. Pathol. 2002;161:1935–1948. doi: 10.1016/S0002-9440(10)64469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rydh A, Suhr O, Hietala S-O, Ahlstrom KR, Pepys MB, Hawkins PN. Serum amyloid P component scintigraphy in familial amyloid polyneuropathy: regression of visceral amyloid following liver transplantation. EurJ. Nucl. Med. 1998;25:709–713. doi: 10.1007/s002590050273. [DOI] [PubMed] [Google Scholar]
- 122.Jiang X, Smith CS, Petrassi HM, Hammarstrom P, White JT, Sacchettini JC, Kelly JW. An engineered transthyretin monomer that is nonamyloidogenic, unless it is partially denatured. Biochemistry. 2001;40:11442–11452. doi: 10.1021/bi011194d. [DOI] [PubMed] [Google Scholar]
- 123.Lai Z, McCulloch J, Lashuel HA, Kelly JW. Guanidine hydrochloride-induced denaturation and refolding of transthyretin exhibits a marked hysteresis: equilibria with high kinetic barriers. Biochemistry. 1997;36:10230–10239. doi: 10.1021/bi963195p. [DOI] [PubMed] [Google Scholar]
- 124.Hammarstrom P, Jiang X, Deechongkit S, Kelly JW. Anion shielding of electrostatic repulsions in transthyretin modulates stability and amyloidosis: insight into the chaotrope unfolding dichotomy. Biochemistry. 2001;40:11453–11459. doi: 10.1021/bi010673+. [DOI] [PubMed] [Google Scholar]
- 125.Lee J, Culyba EK, Powers ET, Kelly JW. Amyloid-beta forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 2011;7:602–609. doi: 10.1038/nchembio.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lashuel HA, Lai Z, Kelly JW. Characterization of the transthyretin acid denaturation pathways by analytical ultracentrifugation: implications for wild-type, V30M, and L55P amyloid fibril formation. Biochemistry. 1998;37:17851–17864. doi: 10.1021/bi981876+. [DOI] [PubMed] [Google Scholar]
- 127.Lashuel HA, Wurth C, Woo L, Kelly JW. The most pathogenic transthyretin variant, L55P, forms amyloid fibrils under acidic conditions and protofilaments under physiological conditions. Biochemistry. 1999;38:13560–13573. doi: 10.1021/bi991021c. [DOI] [PubMed] [Google Scholar]
- 128.Hurshman Babbes AR, Powers ET, Kelly JW. Quantification of the thermodynamically linked quaternary and tertiary structural stabilities of transthyretin and its disease-associated variants: the relationship between stability and amyloidosis. Biochemistry. 2008;47:6969–6984. doi: 10.1021/bi800636q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colon W, Lai Z, McCutchen SL, Miroy GJ, Strang C, Kelly JW. FAP mutations destabilize transthyretin facilitating conformational changes required for amyloid formation. Ciba Foundation Symposia. 1996;199:228–238. doi: 10.1002/9780470514924.ch14. discussion 239–242. [DOI] [PubMed] [Google Scholar]
- 130.Jiang X, Buxbaum JN, Kelly JW. The V122I cardiomyopathy variant of transthyretin increases the velocity of rate-limiting tetramer dissociation, resulting in accelerated amyloidosis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14943–14948. doi: 10.1073/pnas.261419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trivella DB, Bleicher L, Palmieri Lde C, Wiggers HJ, Montanari CA, Kelly JW, Lima LM, Foguel D, Polikarpov I. Conformational differences between the wild type and V30M mutant transthyretin modulate its binding to genistein: implications to tetramer stability and ligand-binding. J. Struct. Biol. 2010;170:522–531. doi: 10.1016/j.jsb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 132.Wiseman RL, Green NS, Kelly JW. Kinetic stabilization of an oligomeric protein under physiological conditions demonstrated by a lack of subunit exchange: implications for transthyretin amyloidosis. Biochemistry. 2005;44:9265–9274. doi: 10.1021/bi050352o. [DOI] [PubMed] [Google Scholar]
- 133.Quintas A, Saraiva MJ, Brito RM. The tetrameric protein transthyretin dissociates to a non-native monomer in solution. A novel model for amyloidogenesis. J. Biol. Chem. 1999;274:32943–32949. doi: 10.1074/jbc.274.46.32943. [DOI] [PubMed] [Google Scholar]
- 134.Palaninathan SK, Mohamedmohaideen NN, Snee WC, Kelly JW, Sacchettini JC. Structural insight into pH-induced conformational changes within the native human transthyretin tetramer. J. Mol. Biol. 2008;382:1157–1167. doi: 10.1016/j.jmb.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 135.Liu K, Cho HS, Hoyt DW, Nguyen TN, Olds P, Kelly JW, Wemmer DE. Deuterium-proton exchange on the native wild-type transthyretin tetramer identifies the stable core of the individual subunits and indicates mobility at the subunit interface. J. Mol. Biol. 2000;303:555–565. doi: 10.1006/jmbi.2000.4164. [DOI] [PubMed] [Google Scholar]
- 136.Liu K, Cho HS, Lashuel HA, Kelly JW, Wemmer DE. A glimpse of a possible amyloidogenic intermediate of transthyretin. Nat. Struct. Biol. 2000;7:754–757. doi: 10.1038/78980. [DOI] [PubMed] [Google Scholar]
- 137.Liu K, Kelly JW, Wemmer DE. Native state hydrogen exchange study of suppressor and pathogenic variants of transthyretin. J. Mol. Biol. 2002;320:821–832. doi: 10.1016/s0022-2836(02)00471-0. [DOI] [PubMed] [Google Scholar]
- 138.Coelho T, Carvalho M, Saraiva MJ, Alves I, Almeida MR, Costa PP. A strikingly benign evolution of FAP in an individual found to be a compound heterozygote for two TTR mutations: TTR MET 30 and TTR MET 119. J. Rheumatol. 1993;20:179. [Google Scholar]
- 139.Coelho T, Chorao R, Sausa A, Alves I, Torres MF, Saraiva MJ. Compound heterozygotes of transthyretin Met30 and transthyretin Met119 are protected from the devastating effects of familial amyloid polyneuropathy. Neuromusc. Disord. 1996;6:27. [Google Scholar]
- 140.Damas AM, Lamzin V, Domingues F, Sebastiao MP, Saraiva MJ. The crystal structure of [Met30]/[Met119] variant transthyretin - the stabilizing effects introduced by a benign mutation in an amyloidogenic variant. 8 th International Symposium on Amyloidosis; Rochester; Minn. 1998. [Google Scholar]
- 141.Schormann N, Murrell JR, Benson MD. Tertiary structures of amyloidogenic and non-amyloidogenic transthyretin variants: new model for amyloid fibril formation. Amyloid. 1998;5:175–187. doi: 10.3109/13506129809003843. [DOI] [PubMed] [Google Scholar]
- 142.Wiseman RL, Johnson SM, Kelker MS, Foss T, Wilson IA, Kelly JW. Kinetic stabilization of an oligomeric protein by a single ligand binding event. J. Am. Chem. Soc. 2005;127:5540–5551. doi: 10.1021/ja042929f. [DOI] [PubMed] [Google Scholar]
- 143.Razavi H, Palaninathan SK, Powers ET, Wiseman RL, Purkey HE, Mohamedmohaideen NN, Deechongkit S, Chiang KP, Dendle MT, Sacchettini JC, Kelly JW. Benzoxazoles as transthyretin amyloid fibril inhibitors: synthesis, evaluation, and mechanism of action. Angew. Chem. Int. Ed. 2003;42:2758–2761. doi: 10.1002/anie.200351179. [DOI] [PubMed] [Google Scholar]
- 144.Baures PW, Peterson SA, Kelly JW. Discovering transthyretin amyloid fibril inhibitors by limited screening. Bioorg. Med. Chem. 1998;6:1389–1401. doi: 10.1016/s0968-0896(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 145.Alhamadsheh MM, Connelly S, Cho A, Reixach N, Powers ET, Pan DW, Wilson IA, Kelly JW, Graef IA. Potent kinetic stabilizers that prevent transthyretin-mediated cardiomyocyte proteotoxicity. Sci. Transl. Med. 2011;3:97ra81. doi: 10.1126/scitranslmed.3002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choi S, Kelly JW. A competition assay to identify amyloidogenesis inhibitors by monitoring the fluorescence emitted by the covalent attachment of a stilbene derivative to transthyretin. Bioorg. Med. Chem. 2011;19:1505–1514. doi: 10.1016/j.bmc.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Reixach N, Adamski-Werner SL, Kelly JW, Koziol J, Buxbaum JN. Cell based screening of inhibitors of transthyretin aggregation. Biochem. Biophys. Res. Comm. 2006;348:889–897. doi: 10.1016/j.bbrc.2006.07.109. [DOI] [PubMed] [Google Scholar]
- 148.McCammon MG, Scott DJ, Keetch CA, Greene LH, Purkey HE, Petrassi HM, Kelly JW, Robinson CV. Screening transthyretin amyloid fibril inhibitors: characterization of novel multiprotein, multiligand complexes by mass spectrometry. Structure. 2002;10:851–863. doi: 10.1016/s0969-2126(02)00771-2. [DOI] [PubMed] [Google Scholar]
- 149.Baures PW, Oza VB, Peterson SA, Kelly JW. Synthesis and evaluation of inhibitors of transthyretin amyloid formation based on the non-steroidal anti-inflammatory drug, flufenamic acid. Bioorg. Med. Chem. 1999;7:1339–1347. doi: 10.1016/s0968-0896(99)00066-8. [DOI] [PubMed] [Google Scholar]
- 150.Oza VB, Petrassi HM, Purkey HE, Kelly JW. Synthesis and evaluation of anthranilic acid-based transthyretin amyloid fibril inhibitors. Bioorg. Med. Chem. Lett. 1999;9:1–6. doi: 10.1016/s0960-894x(98)00696-9. [DOI] [PubMed] [Google Scholar]
- 151.Petrassi HM, Klabunde T, Sacchettini J, Kelly JW. Structure-Based Design of N-Phenyl Phenoxazine Transthyretin Amyloid Fibril Inhibitors. J. Am. Chem. Soc. 2000;122:2178–2192. [Google Scholar]
- 152.Green NS, Palaninathan SK, Sacchettini JC, Kelly JW. Synthesis and characterization of potent bivalent amyloidosis inhibitors that bind prior to transthyretin tetramerization. J. Am. Chem. Soc. 2003;125:13404–13414. doi: 10.1021/ja030294z. [DOI] [PubMed] [Google Scholar]
- 153.Adamski-Werner SL, Palaninathan SK, Sacchettini JC, Kelly JW. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J. Med. Chem. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 154.Purkey HE, Palaninathan SK, Kent KC, Smith C, Safe SH, Sacchettini JC, Kelly JW. Hydroxylated polychlorinated biphenyls selectively bind transthyretin in blood and inhibit amyloidogenesis: rationalizing rodent PCB toxicity. Chem. Biol. 2004;11:1719–1728. doi: 10.1016/j.chembiol.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 155.Johnson SM, Petrassi HM, Palaninathan SK, Mohamedmohaideen NN, Purkey HE, Nichols C, Chiang KP, Walkup T, Sacchettini JC, Sharpless KB, Kelly JW. Bisaryloxime ethers as potent inhibitors of transthyretin amyloid fibril formation. J. Med. Chem. 2005;48:1576–1587. doi: 10.1021/jm049274d. [DOI] [PubMed] [Google Scholar]
- 156.Razavi H, Powers ET, Purkey HE, Adamski-Werner SL, Chiang KP, Dendle MT, Kelly JW. Design, synthesis, and evaluation of oxazole transthyretin amyloidogenesis inhibitors. Bioorg. Med. Chem. Lett. 2005;15:1075–1078. doi: 10.1016/j.bmcl.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 157.Petrassi HM, Johnson SM, Purkey HE, Chiang KP, Walkup T, Jiang X, Powers ET, Kelly JW. Potent and selective structure-based dibenzofuran inhibitors of transthyretin amyloidogenesis: kinetic stabilization of the native state. J. Am. Chem. Soc. 2005;127:6662–6671. doi: 10.1021/ja044351f. [DOI] [PubMed] [Google Scholar]
- 158.Green NS, Foss TR, Kelly JW. Genistein, a natural product from soy, is a potent inhibitor of transthyretin amyloidosis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14545–14550. doi: 10.1073/pnas.0501609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Johnson SM, Connelly S, Wilson IA, Kelly JW. Biochemical and structural evaluation of highly selective 2-arylbenzoxazole-based transthyretin amyloidogenesis inhibitors. J. Med. Chem. 2008;51:260–270. doi: 10.1021/jm0708735. [DOI] [PubMed] [Google Scholar]
- 160.Johnson SM, Connelly S, Wilson IA, Kelly JW. Toward optimization of the linker substructure common to transthyretin amyloidogenesis inhibitors using biochemical and structural studies. J. Med. Chem. 2008;51:6348–6358. doi: 10.1021/jm800435s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson SM, Connelly S, Wilson IA, Kelly JW. Toward optimization of the second aryl substructure common to transthyretin amyloidogenesis inhibitors using biochemical and structural studies. J. Med. Chem. 2009;52:1115–1125. doi: 10.1021/jm801347s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Choi S, Connelly S, Reixach N, Wilson IA, Kelly JW. Chemoselective small molecules that covalently modify one lysine in a non-enzyme protein in plasma. Nat. Chem. Biol. 2010;6:133–139. doi: 10.1038/nchembio.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choi S, Reixach N, Connelly S, Johnson SM, Wilson IA, Kelly JW. A substructure combination strategy to create potent and selective transthyretin kinetic stabilizers that prevent amyloidogenesis and cytotoxicity. J. Am. Chem. Soc. 2010;132:1359–1370. doi: 10.1021/ja908562q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi S, Ong DS, Kelly JW. A stilbene that binds selectively to transthyretin in cells and remains dark until it undergoes a chemoselective reaction to create a bright blue fluorescent conjugate. J. Am. Chem. Soc. 2010;132:16043–16051. doi: 10.1021/ja104999v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peterson SA, Klabunde T, Lashuel HA, Purkey H, Sacchettini JC, Kelly JW. Inhibiting transthyretin conformational changes that lead to amyloid fibril formation. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12956–12960. doi: 10.1073/pnas.95.22.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miller SR, Sekijima Y, Kelly JW. Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants. Lab. Invest. 2004;84:545–552. doi: 10.1038/labinvest.3700059. [DOI] [PubMed] [Google Scholar]
- 167.Kolstoe SE, Mangione PP, Bellotti V, Taylor GW, Tennent GA, Deroo S, Morrison AJ, Cobb AJA, Coyne A, McCammon MG, Warner TD, Mitchell J, Gill R, Smith MD, Ley SV, Robinson CV, Wood SP, Pepys MB. Trapping of palindromic ligands within native transthyretin prevents amyloid formation. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20483–20488. doi: 10.1073/pnas.1008255107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Almeida MR, Gales L, Damas AM, Cardoso I, Saraiva MJ. Small transthyretin (TTR) ligands as possible therapeutic agents in TTR amyloidosis. Curr. Drug Targets: CNS Neurol. Disord. 2005;4:587–596. doi: 10.2174/156800705774322076. [DOI] [PubMed] [Google Scholar]
- 169.Almeida MR, Macedo B, Cardoso I, Valencia G, Arsequell G, Planas A, Saraiva MJ. Effects of a new diflunisal derivative on transthyretin binding and stabilization in serum from FAP patients; 10 th International Symposium on Amyloidosis; Tours, France. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mairal T, Arsequell G, Valencia G, Dolado I, Nieto J, Planas A, Barluenga J, Ballesteros A, Almeida R, Saraiva MJ. Identification of new diflunisal derivatives as potent in vitro transthyretin fibril inhibitors; 10 th International Symposium on Amyloidosis; Tours, France. 2004. [Google Scholar]
- 171.Morais-de-Sa E, Pereira PJB, Saraiva MJ, Damas AM. The binding of diethylstilbestrol to transthyretin - a crystallographic model; 10 th International Symposium on Amyloidosis; Tours, France. 2004. [Google Scholar]
- 172.Vilaro M, Arsequell G, Valencia G, Ballesteros A, Barluenga J, Nieto J, Planas A, Almeida R, Saraiva MJ. Reengineering TTR amyloid inhibition properties of diflunisal; 11 th International Symposium on Amyloidosis; Woods Hole, MA. 2006. [Google Scholar]
- 173.Gupta S, Chhibber M, Sinha S, Surolia A. Design of Mechanism-Based Inhibitors of Transthyretin Amyloidosis: Studies with Biphenyl Ethers and New Structural Templates. J. Med. Chem. 2007;50:5589–5599. doi: 10.1021/jm0700159. [DOI] [PubMed] [Google Scholar]
- 174.Julius RL, Hawthorne MF. Amyloid disease prevention by transthyretin native state complexation with carborane derivatives lacking cyclooxygenase inhibition. Drug News Perspect. 2008;21:258–266. doi: 10.1358/dnp.2008.21.5.1219011. [DOI] [PubMed] [Google Scholar]
- 175.Pullakhandam R, Srinivas PNBS, Nair MK, Reddy GB. Binding and stabilization of transthyretin by curcumin. Arch. Biochem. Biophys. 2009;485:115–119. doi: 10.1016/j.abb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 176.Raghu P, Reddy GB, Sivakumar B. Inhibition of Transthyretin Amyloid Fibril Formation by 2,4-Dinitrophenol through Tetramer Stabilization. Arch. Biochem. Biophys. 2002;400:43–47. doi: 10.1006/abbi.2002.2779. [DOI] [PubMed] [Google Scholar]
- 177.Simoes CJ, Mukherjee T, Jackson RM, Brito RM. Functional binders for non-specific binding: Evaluation of virtual screening methods for the elucidation of novel transthyretin amyloid inhibitors; 240th ACS National Meeting; Boston, MA. 2010. [Google Scholar]
- 178.Ferguson RN, Edelhoch H, Saroff HA, Robbins J. Negative Cooperativity in the Binding of Thyroxine to Human Serum Prealbumin. Biochemistry. 1975;14:282–289. doi: 10.1021/bi00673a014. [DOI] [PubMed] [Google Scholar]
- 179.Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm. Des. 2008;14:3219–3230. doi: 10.2174/138161208786404155. [DOI] [PubMed] [Google Scholar]
- 180.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 181.Tojo K, Sekijima Y, Kelly JW, Ikeda S. Diflunisal stabilizes familial amyloid polyneuropathy-associated transthyretin variant tetramers in serum against dissociation required for amyloidogenesis. Neurosci. Res. 2006;56:441–449. doi: 10.1016/j.neures.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 182.Nowak RJ, Cuny GD, Choi S, Lansbury PT, Ray SS. Improving binding specificity of pharmacological chaperones that target mutant superoxide dismutase-1 linked to familial amyotrophic lateral sclerosis using computational methods. J. Med. Chem. 2010;53:2709–2718. doi: 10.1021/jm901062p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Woods LA, Platt GW, Hellewell AL, Hewitt EW, Homans SW, Ashcroft AE, Radford SE. Ligand binding to distinct states diverts aggregation of an amyloid-forming protein. Nat. Chem. Biol. 2011;7:730–739. doi: 10.1038/nchembio.635. [DOI] [PMC free article] [PubMed] [Google Scholar]