Abstract
To address the mechanisms underlying hatha yoga’s potential stress-reduction benefits, we compared adiponectin and leptin data from well-matched novice and expert yoga practitioners. These adipocytokines have counter-regulatory functions in inflammation; leptin plays a proinflammatory role, while adiponectin has anti-inflammatory properties. Fifty healthy women (mean age=41.32, range=30-65), 25 novices and 25 experts, provided fasting blood samples during three separate visits. Leptin was 36% higher among novices compared to experts, P = .008. Analysis of adiponectin revealed a borderline effect of yoga expertise, P = .08; experts’ average adiponectin levels were 28% higher than novices across the three visits. In contrast, experts’ average adiponectin to leptin ratio was nearly twice that of novices, P = .009. Frequency of self-reported yoga practice showed significant negative relationships with leptin; more weeks of yoga practice over the last year, more lifetime yoga sessions, and more years of yoga practice were all significantly associated with lower leptin, with similar findings for the adiponectin to leptin ratio. Novices and experts did not show even marginal differences on behavioral and physiological dimensions that might represent potential confounds, including BMI, central adiposity, cardiorespiratory fitness, and diet. Prospective studies addressing increased risk for type II diabetes, hypertension, and cardiovascular disease have highlighted the importance of these adipocytokines in modulating inflammation. Although these health risks are clearly related to more extreme values then we found in our healthy sample, our data raise the possibility that longer-term and/or more intensive yoga practice could have beneficial health consequences by altering leptin and adiponectin production.
Keywords: adiponectin, leptin, yoga, inflammation, psychoneuroimmunology, complementary medicine
1. Introduction
Yoga has been used in the treatment of such diverse health problems as asthma [1], type II diabetes [2], fatigue in breast cancer survivors [3], irritable bowel syndrome [4], sleep [5-7], depression [8], and anxiety [9]. Mechanistic explanations for yoga’s mental and physical health benefits have highlighted reductions in sympathetic nervous system (SNS) tone [10, 11], and increases in parasympathetic (vagal) activity [11], both of which could have favorable immune and endocrine consequences by reducing stress-related responses. However, surprisingly few studies have attempted to relate endocrine or immune function to yoga practice, even though some hatha yoga postures are characterized as immune enhancing or restorative [12].
To address yoga’s impact on inflammation, one key facet of immune function, we compared novice and expert yoga practitioners’ inflammatory responses [13]. Despite the fact that novices and experts did not differ on key dimensions including age, abdominal adiposity, and cardiorespiratory fitness, novices’ serum interleukin 6 (IL-6) levels were 41% higher than those of experts, and the odds of a novice having detectable C-reactive protein (CRP) were 4.75 times as high as that of an expert. Differences in stress responses between the groups provided one plausible mechanism for their divergent inflammatory data; experts produced less lipopolysaccharide (LPS)-stimulated IL-6 in response to laboratory stressors than novices.
Inflammation is a robust and reliable predictor of all-cause mortality in older adults [14]. Systemic inflammation plays a role in the development of atherosclerosis, type 2 diabetes, and a number of age-related diseases [15, 16]. Stressors, anxiety, and depression can all raise proinflammatory cytokine production [17]. Larger, more frequent, or more persistent stress-related changes in inflammation can have negative consequences for health. If yoga dampens or limits stress-related inflammatory changes, then regular practice could have substantial health benefits. Accordingly, we were interested in a broader assessment of yoga’s potential anti-inflammatory actions.
Recent evidence has implicated leptin and adiponectin as mediators of inflammatory responses [18]. These adipocytokines have counter-regulatory functions in inflammation; leptin plays a proinflammatory role, while adiponectin has anti-inflammatory properties [19].
Monocytes and T-cells have receptors that allow leptin to stimulate expression and release of IL-6 and tumor necrosis factor alpha (TNF-α ) [20]. Leptin also activates macrophages [21]. Direct relationships have been reported between leptin and CRP, consistent with the hypothesis that leptin may promote CRP production independent of cytokines [22]. Leptin can enhance vascular inflammation and oxidative stress, and these actions are thought to contribute to the pathogenesis of type II diabetes, hypertension, and coronary heart disease [23]. SNS activity may be an important determinant of leptin secretion [24], suggesting one potential pathway through which yoga might modulate production.
Adiponectin enhances production of anti-inflammatory cytokines including IL-10 and the IL-1 receptor antagonist, part of the evidence for its anti-inflammatory role [21]. Furthermore, adiponectin can indirectly decrease CRP and IL-6, as well as TNF-α [25]. Because of its anti-inflammatory properties, adiponectin is important in metabolic disorders including obesity, type II diabetes, coronary heart disease, and metabolic syndrome [25]. Importantly, higher adiponectin levels have been associated with a lower risk for type II diabetes [26].
The relative balance between leptin and adiponectin may also be important. It has been suggested that the ratio of leptin to adiponectin can be used as an index of insulin resistance [27]. Similarly, a high adiponectin/leptin ratio was associated with lower insulin resistance, high triglycerides, and enhanced inflammation in women with polycystic ovary syndrome [28].
In this study we addressed the question of whether well-matched novice and expert yoga practitioners differed in their production of leptin and adiponectin using our well-characterized sample [13]. We hypothesized that expert practitioners would have higher adiponectin, lower leptin, and higher adiponectin:leptin ratios than novices, and these differences would be inversely related to IL6 and CRP, two measurements of inflammation that were associated with yoga expertise previously [13].
2. Methods
2.1 Participants
Women who had participated in some form of hatha yoga were recruited through online ads and notices posted in yoga studios. We excluded women who were taking medications with obvious immunological or endocrinological consequences, as well as individuals who reported chronic health problems with implications for these systems (e.g., cancer, recent surgeries, diabetes, etc.). Additional exclusion criteria included smoking, use of statins, beta blockers, psychoactive drugs, excessive alcohol use, convulsive disorders, or a BMI ≥ 30. The average age of the final sample of 50 women who completed all 3 visits was 41.32 (SD=10.33, range=30-65); 44 were white, 3 were African American, 2 were Native American, 1 was Asian, and all had at least some college education.
2.2 Screening and experimental sessions
Participants were screened and characterized as novices versus experts using a two-step process. First, participants completed an online screening questionnaire assessing the type, frequency, and duration of yoga practice over the past year and over their lifetimes. Women were classified as novices if they had participated in yoga classes or home practice with yoga videos for 6 - 12 sessions. Experts had practiced yoga regularly 1-2 times per week (75-90 min sessions) for at least 2 years, and at least 2 times per week for the past year. Others were rated as intermediate and deemed not eligible for further participation. Each participant was classified by two raters. Raters conferred when classifications were discrepant, obtaining additional information as needed to reach consensus.
Yoga skills, flexibility, and cardiovascular fitness were assessed during the screening session. Participants performed 8 selected poses under the guidance of an experienced instructor, blind to their reported experience, who evaluated their form to assure that novices and experts had skills commensurate with their self-reports as previously described [13]. To further objectively characterize hamstring and low back elasticity, participants completed the sit-and-reach test, a common flexibility test [29].
Sagittal abdominal diameter (SAD) measurements provided data on the total amount of abdominal fat. Validational studies using computerized axial tomography and dual-energy X-ray absorptiometry have demonstrated its utility as a noninvasive central adiposity measure [30].
Cardiopulmonary endurance was evaluated during a maximal graded cycle ergometry exercise test, starting at 25 watts and increasing by 25 watts every two minutes, with continuous monitoring via 12-lead EKG (MedGraphics Cardio2, Cardio Perfect). Maximum oxygen consumption (VO2max) was calculated from 10-second averages of breath-by-breath expired air (MedGraphics Cardio2, Breeze Suite).
Each participant completed three sessions at the Clinical Research Center (CRC), a hospital research floor, scheduled at least 2 weeks apart as described in detail previously [13]. The leptin and adiponectin data were collected as part of the fasting blood draw at the beginning of each of the three sessions.
2.3 Self-report measures
During the screening session participants completed the version of the Food Frequency Questionnaire (FFQ) validated for the Women’s Health Initiative [31]. Participants reported the type, frequency, and quantity of foods and beverages consumed in the past 90 days.
The Pittsburgh Sleep Quality Index assessed sleep quality and disturbances over a one-month interval; it has good diagnostic sensitivity and specificity in distinguishing good and poor sleepers [32]. Completed during the screening session, we also assessed sleep prior to each visit.
Evidence suggests that the scales of the Mood and Anxiety Symptom Questionnaire (MASQ) measure anxiety and depression well, with limited overlap, compared with other self-report measures [33, 34]. The MASQ was administered during the screening session and at the beginning of each of the three admissions.
2.4 Leptin and adiponectin
All blood samples for a subject were collected via a catheter and frozen after collection and analyzed within the same assay run. Determinations for leptin and adiponectin were made using the respective RIA kits per kit instructions (Millipore Corporation, St. Charles, MO 63304). For leptin, the intra-assay coefficient of variation is 4.2% and inter-assay coefficient of variation is 4.5%; sensitivity is 0.5 ng/ml. For adiponectin, the intra-assay coefficient of variation is 3.8% and inter-assay coefficient of variation is 8.5%; sensitivity is 1 ng/ml. IL-6 and hsCRP were measured as reported previously[13].
2.5 Statistical analyses
Mixed effects models were used to analyze differences between novices and experts in adiponectin, leptin, and their ratio. This type of model treats the responses from each subject across the three visits as repeated measures, accounting for the within-subject correlation. A compound-symmetric variance-covariance structure was used to estimate error variance, using the PROC MIXED procedure in SAS 9.1 (SAS Institute Inc., Cary, NC). Models included the fixed effects of expertise, visit, and their interaction, and body mass index was included as a potential confounder. All tests used a two-sided, alpha = 0.05 significance level. One yoga expert did not have leptin measured at any visit and thus was excluded from the leptin and adiponectin/leptin ratio models. In correlation analyses, skewed measures (IL-6, CRP) were log (base 10) transformed before calculation of Pearson’s correlations. For count measures (practice time), log transformation did not improve the fit of correlation models and thus rank-based Spearman’s correlation was used to describe associations.
3. Results
3.1 Study population and health behaviors
As shown in Table 1, novice and expert practitioners did not differ on key variables that have been associated with inflammation. As previously reported, we did not find dietary differences on the FFQ when we examined nutrients and energy (including energy intake in kcal, and daily intake of fats, carbohydrates, and protein), vitamins (E, C, and D), or the number of daily fruit and vegetable servings [13]. Seven women in each of the groups were postmenopausal. As a consequence of our stringent exclusion criteria, overall medication use was low; novices and experts did not differ in the proportion reporting use of aspirin, ibuprofen, or other over-the-counter analgesics, ps > .39, birth control pills, hormone replacement therapy, omega-3 supplements, or a daily multivitamin, ps > .23.
Table 1.
Mean (SD) demographic, physiological, and behavioral data for novice and expert yoga practitioners
| Univariate MANOVA | ||||||
|---|---|---|---|---|---|---|
| Novice (n=25) | Expert (n=25) | p-value | p-value | |||
| Age | 39.96 | (10.51) | 42.68 | (10.18) | 0.36 | |
| Education (Hollingshead categories) | 5.36 | (0.64) | 5 .36 | (0.76) | 1.00 | |
| Adiposity measurements | 0.75 | |||||
| Body mass index (BMI) | 23.56 | (2.83) | 22.85 | (2.71) | ||
| Sagittal abdominal diameter | 17.91 | (2.29) | 17.69 | (2.52) | ||
| Cholesterol, mg/dL | 179.28 | (29.13) | 176.60 | (33.74) | 0.12 | |
| Fasting glucose, mg/dL | 88.92 | (8.13) | 86.80 | (8.02) | 0.44 | |
| Cardiorespiratory fitness | 0.13 | |||||
| VO2 peak | 27.43 | (5.54) | 28.36 | (6.62) | ||
| Maximal workload | 139.56 | (25.25) | 148.24 | (30.12) | ||
| Maximum heart rate | 173.88 | (13.12) | 166.04 | (15.88) | ||
| Baseline heart rate/blood pressure | 0.28 | |||||
| Heart rate | 69.88 | (9.23) | 67.04 | (9.07) | ||
| Systolic blood pressure, mmHg | 111.44 | (15.73) | 108.12 | (12.02) | ||
| Diastolic blood pressure, mmHg | 68.08 | (12.66) | 68.20 | (11.52) | ||
| Alcohol, drinks/week | 2.32 | (2.85) | 1.92 | (1.91) | 0.56 | |
| Pittsburgh Sleep Questionnaire | 4.28 | (2.32) | 4.20 | (2.08) | 0.90 | |
| Mood/Affect | 0.17 | |||||
| PANAS (positive mood) | 26.73 | (6.88) | 28.43 | (6.23) | ||
| MASQ-depressive symptoms | 19.08 | (5.66) | 21.08 | (8.94) | ||
| MASQ-anxiety symptoms | 16.93 | (2.98) | 18.79 | (5.55) | ||
3.2 Yoga Expertise
Mean ratings of novices’ (21.92, SD=4.93) and experts’ (31.86, SD=4.83) ability to perform common yoga poses, assessed during the screening session, were clearly different, F(1,48) = 51.82, P < .000. Similarly, novices (M=31.88, SD=7.77) had substantially less hamstring and low back flexibility than experts (M=41.81, SD=5.19), producing the expected differences on the sit-and-reach test, F(1,49) = 27.91, P < .001.
3.3 Adiponectin and leptin
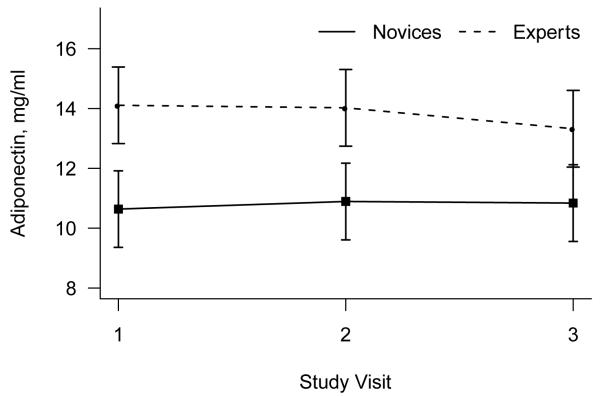
Analysis of adiponectin revealed a borderline effect of yoga expertise, F(1,47) = 3.16, P = .08. Effects of visit and the interaction of expertise and visit were non-significant (both p > .6). Experts’ average adiponectin levels were 28% higher than novices across the three visits (Figure 1).
Figure 1.
Mean (+/− standard error of the mean) adiponectin as a function of novice versus expert yoga practitioner status.
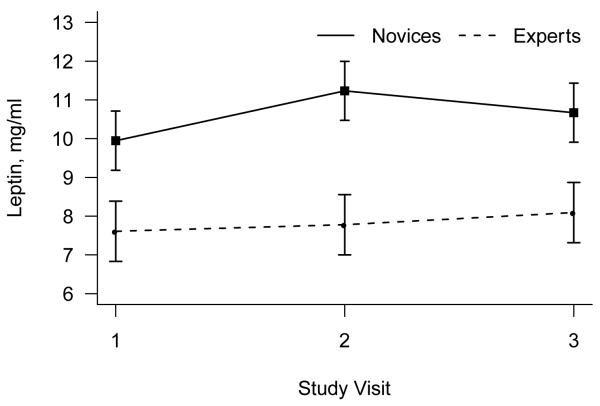
There was a significant difference between novices and experts in leptin levels, F(1,46) = 7.80, P = .008, with novices having 36% higher leptin levels on average than experts (Figure 2). The effects of visit and the interaction of visit with expertise were non-significant (both p > .13).
Figure 2.
Mean (+/− standard error of the mean) leptin as a function of novice versus expert yoga practitioner status.
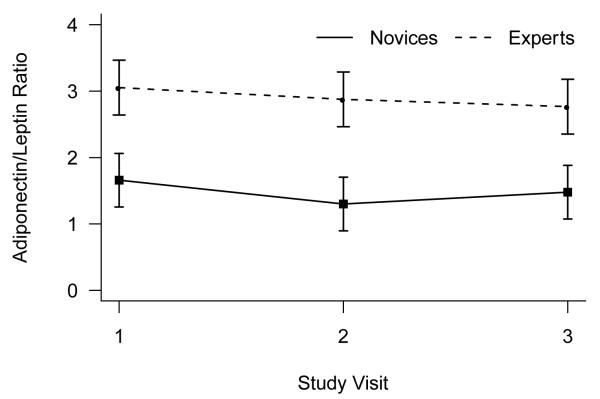
Analysis of adiponectin to leptin ratios showed a significant effect of yoga expertise, F(1,46) = 7.49, P = .009, and no effects of visit or the interaction of visit with expertise (both p > .4). Experts’ average adiponectin to leptin ratio was nearly twice that of novices (Figure 3).
Figure 3.
Mean (+/− standard error of the mean) adiponectin to leptin ratio as a function of novice versus expert yoga practitioner status.
Table 2 displays pairwise correlations among adiponectin, leptin, IL-6, and CRP using subjects’ average values across the three visits. IL-6 values ranged from 0.17 to 5.0 pg/mL (median=0.59), and CRP values ranged from 0.3 to 6.5 mg/dL (median=0.42). Higher adiponectin levels were associated with lower leptin levels, r = −0.32 (p = 0.03). Significant negative associations were seen between adiponectin and both IL-6 (r = −0.44, p = 0.002) and CRP (r = −0.28, p = 0.05). A significant positive association existed between leptin and IL-6 (r = 0.39, p = 0.006), while the association between leptin and CRP was positive but nonsignificant (r = 0.19, p = 0.20).
Table 2.
Correlations (p-values) between adiponectin, leptin, IL-6, and CRP, using individuals’ values averaged across the three study visits.
| Leptin | Adi/Lep Ratio | log(IL6) | log(CRP) | |
|---|---|---|---|---|
| Adiponectin | −0.32 (0.03) |
0.69 (<0.001) |
−0.44 (0.002) |
−0.28 (0.05) |
| Leptin | −0.68 (<0.001) |
0.39 (0.006) |
0.19 (0.20) |
|
| Adi/Lep Ratio | −0.50 (<0.001) |
−0.34 (0.02) |
||
| ln(IL6) | 0.33 (0.02) |
IL-6 and CRP are log (base 10) transformed.
A secondary analysis was performed to examine the association between self-reported yoga practice time and adiponectin, leptin, and their ratio. Participants reported the number of yoga sessions per week in the past year, the number of weeks they practiced in the past year, and the total number of yoga sessions and years practiced over their lifetime. Since these measures were skewed, Spearman’s rank-based correlation was used to describe the strength of the association between these measures and average outcomes across the three visits (Table 3). Correlations between adiponectin and practice time were positive but non-significant, however there were strong negative associations between leptin and practice time. Longer practice time was associated with lower leptin, and accordingly there were significant positive correlations between the adiponectin/leptin ratio and practice time, confirming the results of the ANOVA analysis.
Table 3.
Spearman rank-based correlations (p-values) between adiponectin, leptin, and their ratio with self-reported measures of yoga practice time, using individuals’ adipocytokine values averaged across the three study visits.
| Adiponectin | Leptin | Adi/Lep Ratio | |
|---|---|---|---|
| Weeks practiced, past year (median: 40; range: 0-52) |
0.26 (0.07) |
−0.37 (0.008) |
0.40 (0.004) |
| Years practiced, lifetime (median: 3.3; range: 0-25) |
0.08 (0.58) |
−0.39 (0.01) |
0.31 (0.03) |
| Sessions per week, past year (median: 2; range: 0-15) |
0.12 (0.39) |
−0.24 (0.10) |
0.25 (0.08) |
| Sessions total, lifetime* (median: 4) |
0.14 (0.33) |
−0.40 (0.005) |
0.36 (0.01) |
Ordinal scale: 1=0-4; 2=5-24; 3=25-99; 4=100-149; 5=150-299; 6=300-499; 7=500+
In order to rule out the possibility of confounding by dietary intake, analyses were repeated controlling for subjects’ responses to the FFQ. The addition of these controlling variables did not influence the main findings of the paper; all conclusions remained the same and magnitude of group differences were not substantially changed.
4. Discussion
4.1 Adipocytokine differences and implications
We found sizeable differences between our well-matched novice and expert yoga practitioner groups. Leptin was 36% higher among novices compared to experts. In contrast, experts’ average adiponectin to leptin ratio was nearly twice that of novices. Furthermore, frequency of self-reported yoga practice showed significant negative relationships with leptin; more weeks of yoga practice over the last year, more lifetime yoga sessions, and more years of yoga practice were all significantly associated with lower leptin, with similar findings for the adiponectin to leptin ratio.
These two adipocytokines are mainly produced by adipose tissue [21]; in general, leptin increases with increasing obesity, while adiponectin decreases [35, 36]. In addition, exercise training and detraining can alter both leptin and adiponectin, with changes dependent on concurrent alterations in BMI as well as training intensity [25, 37]. Accordingly, we assessed a number of behavioral and physiological dimensions that might represent potential confounds including cardiorespiratory fitness, BMI, central adiposity, mood/affect, and diet [38]. Novices and experts did not show even marginal differences on any of these dimensions. Thus, we have no evidence that extraneous factors unrelated to regular yoga practice are responsible for the differences.
As we previously reported, novices’ serum IL-6 levels were 41% higher than those of experts, and the odds of a novice having detectable CRP were 4.75 times as high as that of an expert [13]. Adiponectin can indirectly decrease CRP and IL-6 [25], and it has been hypothesized that leptin may promote CRP production independent of cytokines [22]. Thus, these new data suggest potential mechanistic pathways for broader alterations in inflammation.
Data from RCTs show that yoga can reduce depression [8], and anxiety [9]. Several lines of work have linked production of these adipocytokines with depression and anxiety, as well as severe stressors. Two laboratories reported that patients with major depression had lower plasma adiponectin concentrations compared to controls, and adiponectin was inversely related to the severity of depressive symptoms [39, 40]. In a large epidemiological cohort, women who had a history of dysthymia or major depressive disorder had higher levels of leptin than women who had never met syndromal criteria; furthermore, high leptin levels predicted development of a depressive disorder in longitudinal data [41]. Leptin levels were substantially higher among individuals who had persistent posttraumatic symptoms following a major earthquake compared to those who were not symptomatic [42]. Higher levels of phobic anxiety were associated with leptin, but not adiponectin, in 984 women with type 2 diabetes from the Nurses’ Health Study [43]. We did not find group differences in mood or affect in our data. However, if yoga practice enhances positive moods and decreases negative moods as other reports suggest [8, 9], it might have downstream effects on adiponectin and leptin.
This study is cross-sectional, so we cannot infer causality, one obvious limitation. We only studied females, and do not have data on males, another limitation. The results may not be generalizable to individuals who do not make a choice to practice yoga; however, realistically, it is an intervention that is typically self-selected.
4.2 Conclusions
Prospective studies addressing increased risk for type II diabetes, hypertension, and cardiovascular disease have highlighted the importance of these adipocytokines [23, 25, 26]. Although these health risks are clearly related to more extreme values then we found in our healthy sample, our data raise the possibility that longer-term and/or more intensive yoga practice could have beneficial health consequences by altering production of leptin and/or adiponectin.
Highlights.
We compared adiponectin and leptin data from novice and expert yoga practitioners.
Leptin plays a proinflammatory role, adiponectin has anti-inflammatory properties.
Leptin was 36% higher among novices compared to experts.
Experts’ average adiponectin to leptin ratio was nearly twice that of novices
Intensive yoga practice may benefit health by altering leptin and adiponectin production.
Acknowledgments
This research was supported by grant NIH grants AT00297 and CA126857 (J.K-G, W.B.M., C.F.E., R.G.; J.K-G, PI), NIH Training Grant AI55411 (L.C.; Virginia Sanders, PI), NCRR Grant UL1RR025755 which funds the Clinical Research Center, and by Ohio State Comprehensive Cancer Center Core Grant CA16058. The funding sources did not did not influence the design, implementation, interpretation or publication of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sabina AB, Williams AL, Wall HK, Bansal S, Chupp G, Katz DL. Yoga intervention for adults with mild-to-moderate asthma: A pilot study. Annals of Allergy Asthma and Immunology. 2005;94:543–8. doi: 10.1016/s1081-1206(10)61131-3. [DOI] [PubMed] [Google Scholar]
- [2].Aljasir B, Bryson M, Al-shehri B. Yoga practice for the management of type II diabetes mellitus in adults: A systematic review. Evidence-Based Complementary and Alternative Medicine. 2010;7:399–408. doi: 10.1093/ecam/nen027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bower JE, Garet D, Sternlieb B. Yoga for persistent fatigue in breast cancer survivors: Results of a pilot study. Evid Based Complement Alternat Med. 2011;2011:623168. doi: 10.1155/2011/623168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Taneja I, Deepak KK, Poojary G, Acharya IN, Pandey RM, Sharma MP. Yogic versus conventional treatment in diarrhea-predominant irritable bowel syndrome: A randomized control study. Appl. Psychophysiol. Biofeedback. 2004;29:19–33. doi: 10.1023/b:apbi.0000017861.60439.95. [DOI] [PubMed] [Google Scholar]
- [5].Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–60. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- [6].Vera FM, Manzaneque JM, Maldonado EF, Carranque GA, Rodriguez FM, Blanca MJ, et al. Subjective sleep quality and hormonal modulation in long-term yoga practitioners. Biol. Psychol. 2009;81:164–8. doi: 10.1016/j.biopsycho.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [7].Khalsa SB. Treatment of chronic insomnia with yoga: A preliminary study with sleep-wake diaries. Appl. Psychophysiol. Biofeedback. 2004;29:269–78. doi: 10.1007/s10484-004-0387-0. [DOI] [PubMed] [Google Scholar]
- [8].Uebelacker LA, Epstein-Lubow G, Gaudiano BA, Tremont G, Battle CL, Miller IW. Hatha yoga for depression: Critical review of the evidence for efficacy, plausible mechanisms of action, and directions for future research. Journal of Psychiatric Practice. 2010;16:22–33. doi: 10.1097/01.pra.0000367775.88388.96. [DOI] [PubMed] [Google Scholar]
- [9].Streeter CC, Whitfield TH, Owen L, Rein T, Karri SK, Yakhkind A, et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: A randomized controlled MRS study. J. Altern. Complement. Med. 2010;16:1145–52. doi: 10.1089/acm.2010.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Riley D. Hatha yoga and the treatment of illness. Altern. Ther. Health Med. 2004;10:20–1. [PubMed] [Google Scholar]
- [11].Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, et al. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: Comparative study. Br. Med. J. 2001;323:1446–9. doi: 10.1136/bmj.323.7327.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iyengar BKS. Light on yoga. Schocken Books; New York: 1995. [Google Scholar]
- [13].Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF, et al. Stress, inflammation, and yoga practice. Psychosom. Med. 2010;72:113–21. doi: 10.1097/PSY.0b013e3181cb9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- [15].Kiecolt-Glaser JK, McGuire L, Robles TR, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annu. Rev. Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- [16].Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- [18].Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taub DD. Neuroendocrine interactions in the immune system. Cell. Immunol. 2008;252:1–6. doi: 10.1016/j.cellimm.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- [22].Ble A, Windham BG, Bandinelli S, Taub DD, Volpato S, Bartali B, et al. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study) Am. J. Cardiol. 2005;96:991–5. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- [23].Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease - response to therapeutic interventions. Circulation. 2008;117:3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Flanagan DE, Vaile JC, Petley GW, Phillips DI, Godsland IF, Owens P, et al. Gender differences in the relationship between leptin, insulin resistance and the autonomic nervous system. Regul. Pept. 2007;140:37–42. doi: 10.1016/j.regpep.2006.11.009. [DOI] [PubMed] [Google Scholar]
- [25].Simpson KA, Singh MAF. Effects of exercise on adiponectin: A systematic review. Obesity. 2008;16:241–56. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- [26].Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- [27].Oda N, Iniamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57:268–73. doi: 10.1016/j.metabol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- [28].Xita N, Papassotiriou I, Georgiou I, Vounatsou M, Margeli A, Tsatsoulis A. The adiponectin-to-leptin ratio in women with polycystic ovary syndrome: Relation to insulin resistance and proinflammatory markers. Metabolism. 2007;56:766–71. doi: 10.1016/j.metabol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [29].Lemmink KAPM, Kemper HCG, de Greef MHG, Rispens P, Stevens M. The validity of the sit-and-reach test and the modified sit-and-reach test in middle-aged to older men and women. Res. Q. Exerc. Sport. 2003;74:331–6. doi: 10.1080/02701367.2003.10609099. [DOI] [PubMed] [Google Scholar]
- [30].Clasey JL, Bouchard C, Teates CD, Riblett JE, Thorner MO, Hartman ML, et al. The use of anthropometric and dual-energy x-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes. Res. 1999;7:256–64. doi: 10.1002/j.1550-8528.1999.tb00404.x. [DOI] [PubMed] [Google Scholar]
- [31].Patterson RE, Kristal AR, Carter RA, Fels-Tinker LF, Bolton MP, Argurs-Collins T. Measurement characteristics of women’s health initiative food frequency questionnaire. Ann. Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- [32].Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [33].Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnorm. Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- [34].Ruth S, Mehrotra S. Differentiating depression and anxiety: Psychometric implications of the tripartite model of affect. Journal of Personality and Clinical Studies. 2001;17:9–18. [Google Scholar]
- [35].Matsuzawa Y. White adipose tissue and cardiovascular disease. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19:637–47. doi: 10.1016/j.beem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [36].Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: Effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J. Clin. Endocrinol. Metab. 2004;89:2672–7. doi: 10.1210/jc.2003-031931. [DOI] [PubMed] [Google Scholar]
- [37].Fatouros IG, Tournis S, Leontsini D, Jamurtas AZ, Sxina M, Thomakos P, et al. Leptin and adiponectin responses in overweight inactive elderly following resistance training and detraining are intensity related. J. Clin. Endocrinol. Metab. 2005;90:5970–7. doi: 10.1210/jc.2005-0261. [DOI] [PubMed] [Google Scholar]
- [38].Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll. Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- [39].Leo R, Di Lorenzo G, Tesauro M, Cola C, Fortuna E, Zanasi M, et al. Decreased plasma adiponectin concentration in major depression. Neurosci. Lett. 2006;407:211–3. doi: 10.1016/j.neulet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- [40].Cizza G, Nguyen VT, Eskandari F, Duan Z, Wright EC, Reynolds JC, et al. Low 24-hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: The premenopausal, osteopenia/osteoporosis, women, alendronate, depression (power) study. J. Clin. Psychiatry. 2010;71:1079–87. doi: 10.4088/JCP.09m05314blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Leptin in depressed women: Cross-sectional and longitudinal data from an epidemiologic study. J. Affect. Disord. 2008;107:221–5. doi: 10.1016/j.jad.2007.07.024. [DOI] [PubMed] [Google Scholar]
- [42].Liao SC, Lee MB, Lee YJ, Huang TS. Hyperleptinemia in subjects with persistent partial posttraumatic stress disorder after a major earthquake. Psychosom. Med. 2004;66:23–8. doi: 10.1097/01.psy.0000106880.22867.0e. [DOI] [PubMed] [Google Scholar]
- [43].Brennan AM, Fargnoli JL, Williams CJ, Li T, Willett W, Kawachi I, et al. Phobic anxiety is associated with higher serum concentrations of adipokines and cytokines in women with diabetes. Diabetes Care. 2009;32:926–31. doi: 10.2337/dc08-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]