Abstract
Nitrogen (N) management is a promising agronomic strategy to minimize cadmium (Cd) contamination in crops. However, it is unclear how N affects Cd uptake by plants. Wild-type and iron uptake-inefficient tomato (Solanum lycopersicum) mutant (T3238fer) plants were grown in pH-buffered hydroponic culture to investigate the direct effect of N-form on Cd uptake. Wild-type plants fed NO3− accumulated more Cd than plants fed NH4+. Iron uptake and LeIRT1 expression in roots were also greater in plants fed NO3−. However, in mutant T3238fer which loses FER function, LeIRT1 expression in roots was almost completely terminated, and the difference between NO3− and NH4+ treatments vanished. As a result, the N-form had no effect on Cd uptake in this mutant. Furthermore, suppression of LeIRT1 expression by NO synthesis inhibition with either tungstate or L-NAME, also substantially inhibited Cd uptake in roots, and the difference between N-form treatments was diminished. Considering all of these findings, it was concluded that the up-regulation of the Fe uptake system was responsible for NO3− -facilitated Cd accumulation in plants.
Keywords: Ammonium, cadmium, iron uptake, nitrate
Introduction
Cadmium (Cd) is recognized as a significant pollutant due to its high toxicity (Ronald, 2000; Pan and Wang, 2011). In most instances, dietary uptake through eating crops grown in Cd-contaminated soil is the most prevalent source of environmental Cd exposure for humans. Therefore, scientists have made great efforts to identify strategies for reducing/avoiding Cd accumulation by crops grown in Cd-contaminated soils. It is known that several plant nutrients have many direct as well as indirect effects on the availability of Cd in the soil and the uptake of Cd into plants (Sarwar et al., 2010). For example, phosphate (Pi) favours the precipitation of Cd2+ (Hong et al., 2010), while ferrous iron (Fe2+) competes with Cd2+ for the same membrane transporters in plant cells (Vert et al., 2002; Kovacs et al., 2010). Growers are already applying nutrients to obtain a good crop yield. To alleviate Cd accumulation, the proper management of plant nutrients may be the only change needed due to the pre-existing interactions between Cd and plant nutrients. The use of nutrient management could be a relatively inexpensive, time-saving, and effective agronomic strategy to minimize Cd contamination in crops.
Nitrogen (N) is the main nutrient plants require as well as one of the most frequent factors limiting crop production (Daniel-Vedele et al., 2010). Therefore, management of N has become an important agronomic practice. Physiologically, when nitrate (NO3−) is taken up by plants, there is a simultaneous uptake of protons (H+), resulting in an increase in rhizosphere pH. Conversely, when ammonium (NH4+) is taken up, the H+ are released into the rhizosphere, resulting in a decrease in rhizosphere pH (Marschner, 1995). The soil pH strongly affects the availability of Cd in the soil (Grant et al., 1999). Because of this, it has often been suggested that NH4+ fertilizers could result in enhanced Cd uptake due to a decrease in soil pH, compared with the NO3− fertilizers (Sarwar et al., 2010). Numerous studies have provided evidence in support of this hypothesis. For example, a pot experiment (carried out on soils with weak buffer capacity), showed that NH4+ application clearly lowered rhizosphere pH and significantly increased Cd accumulation in sunflower plants, compared with NO3− application (Zaccheo et al., 2006). However, contrary evidence has been obtained in several other studies. In a hydroponics experiment, Xie et al. (2009) found that Thlaspi caerulesscens plants fed NO3− accumulated much more Cd than the plants supplied with NH4+, even though the solution pH was lower in plants treated with NH4+. In a soil cultivation experiment, Jalloh et al. (2009) also observed that the rice plants fed NO3− had a higher Cd concentration than the plants fed NH4+. These conflicting findings indicate that the N-form may have another effect on Cd uptake in plants besides the indirect effect, which is changing the pH of the rhizosphere.
In addition to being an essential nutrient, NO3− also serves as a signalling molecule. It is known to regulate root architecture, stimulate shoot growth, delay flowering, regulate abscisic acid-independent stomata opening, and relieve seed dormancy (Walch-Liu et al., 2005; Ho et al., 2009; Tian et al., 2009). In addition, NO3− has also been implicated in regulating the uptake of many nutrients. For instance, resupplying NO3− to tomato plants rapidly up-regulated expression of the NH4+ transporter LeAMT2, the Pi transporter LePT2, and Kdc1 (a homologue of a carrot K+ channel) (Wang et al., 2001). In addition, the Arabidopsis chl1-5 mutant, which is deficient for the NRT1.1 NO3− transporter, displays low NO3− uptake and has suppressed expression of AtIRT1 (Muños et al., 2004). IRT1 is a divalent plasma membrane cation transporter essential to the uptake of ferrous iron from the soil in non-graminaceous monocots and dicots (Vert et al., 2002; Curie and Briat, 2003; Jeong and Guerinot, 2009). Interestingly, several studies provide strong evidence that the iron transporter IRT1 is also primarily responsible for Cd2+ influx into root cells (Vert et al., 2002; Clemens, 2006; Verbruggen et al., 2009; Lux et al., 2011). This fact combined with the implication of NO3− in regulating IRT1 led us to hypothesize that NO3− may affect Cd accumulation in plants through the regulation of root cell Fe uptake system.
In this study, tomato (Solanum lycopersicum) plants were used to investigate the above hypothesis. Evidence is provided that NO3− application directly enhances Cd uptake of plants, compared with NH4+ application. This enhancement is attributed to the up-regulation of root Fe uptake systems, which require the FER protein to function.
Materials and methods
Chemicals
The chemicals used in this study were purchased as: DAF-FM DA (diaminofluorescein-FM diacetate) from Beyotime Institute of Biotechnology (http://www.beyotime.com/), L-NAME (Nω-nitro-L-arginine methyl ester hydrochloride) from the Rego Institute of Biotechnology (http://regobio.testmart.cn/), Trizol reagent from Invitrogen (http://www.invitrogen.com/), and tungstate and MES (4-morpholineethanesulfonic acid) from Sangon (http://www.sangon.com/).
Plant culture
Uniform size tomato (Solanum lycopersicum cv. Micro-Tom) seedlings were transferred to 1.0 l pots filled with aerated, full-strength complete nutrient solution. The nutrient solution had the following composition (in μM): NaH2PO4, 750; MgSO4, 500; K2SO4, 375; KNO3, 750; (NH4)2SO4, 375; CaCl2, 1000; H3BO3, 10; MnSO4, 0.5; ZnSO4, 0.5; CuSO4, 0.1; (NH4)6Mo7O24, 0.1; and Fe-EDTA, 25. The solution pH was adjusted to 5.5 using 1 M NaOH. All the plants were grown in the controlled-environment growth chamber at 70% relative humidity with a daily cycle of 14 h day at 28 °C, and 10 h night at 22 °C. The daytime light intensity was 300–350 μmol photons m−2 s−1. After 12 d of growth in the nutrient solution, plants were subjected to different N-form treatments. For the treatment of NO3− as the sole nitrogen source, 1.5 mM KNO3 was applied to the solution. For the treatment of NH4+ as the sole N source, 0.75 mM (NH4)2SO4 and 0.75 mM K2SO4 were added. For both N-form treatments, nutrient solutions were buffered with 2 mM MES at pH 5.5. Other nutrients were the same as above. Both N-form treatments were split into two sub-treatments, 0 and 2 μM Cd, added as CdCl2. For the experiments illustrated in Fig. 5, the Fe uptake-inefficient mutant, T3238fer, and its wild type, T3238 (Brown et al., 1971), were used, and the treatment methods were the same as the Cd-added treatments described above. For the experiments illustrated in Figs 6 and 7, either 0.4 mM L-NAME or 0.15 mM tungstate, were added into Cd-contained NO3− /NH4+ solutions at the start of N-form treatments. The solutions in all of the treatment containers were renewed daily. The shoots and roots of plants after 8 d of treatments were harvested for further analysis.
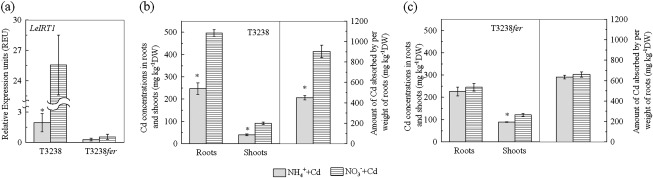
Fig. 5.
Effects of N-form on LeIRT1 expressions, Cd concentrations and Cd uptake capacities in T3238 wild-type plants and T3238fer mutants under Cd exposure condition. (a) The expression levels of LeIRT1 in roots of T3238 and T3238fer. (b) The Cd concentrations (left figure) and the amount of Cd absorbed by per weight of roots (right figure) in T3238. (c) The Cd concentrations (left figure) and the amount of Cd absorbed by per weight of roots (right figure) in T3238 fer. The T3238 wild-type plants and the T3238fer mutants were transferred to 2 μM Cd-added growth solutions with either NO3− or NH4+ as the sole nitrogen source. The pH in the all treatments was buffered at 5.5 using MES. The shoots and roots of plants after 8 d of treatments were harvested for analysis. Data are means ±SD (n=4). * Significant differences (P < 0.05) between NO3− and NH4+ treatments.
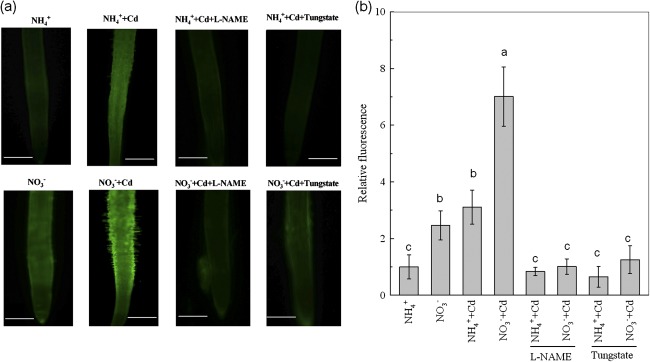
Fig. 6.
Effects of N-form on NO production in roots of Micro-Tom tomato plants under Cd-free or Cd-exposed conditions. (a) Photographs of NO production shown as green fluorescence in representative roots (bar=1 mm). (b) NO production expressed as relative fluorescence. The plants were transferred to Cd-free and 2 μM Cd-added growth solutions with either NO3− or NH4+ as the sole nitrogen source. Meanwhile, either 0.4 mM L-NAME or 0.15 mM tungstate were added to the Cd-treated solutions when the N-form treatments were started. The pH in the all treatments was buffered at 5.5 using MES. The roots of plants after 8 d of treatments were harvested for NO analysis. Data are means ±SD (n=15). Different letters indicate significant differences (P < 0.05) among the treatments.
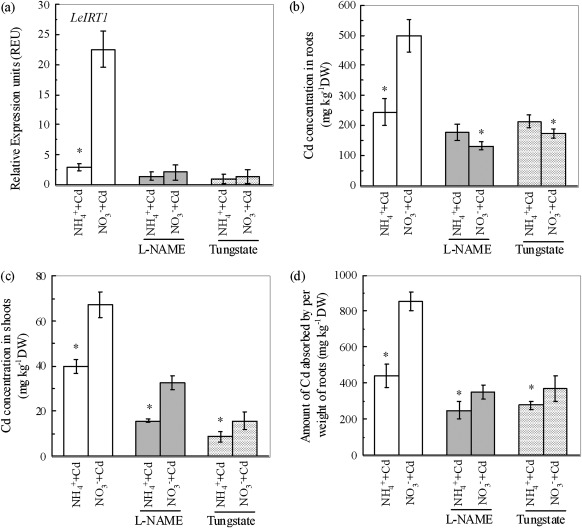
Fig. 7.
The role of NO in regulating LeIRT1 expression, Cd concentration, and Cd uptake capacity in roots of Micro-Tom tomato plants from different N-form treatment. (a) The expression levels of LeIRT1 in roots. (b) The Cd concentrations in roots. (c) The Cd concentrations in shoots. (d) The Cd uptake capacities in roots. Treatments are the same as in Fig. 6. Data are means ±SD (n=4). * Significant differences (P < 0.05) between NO3− and NH4+ treatments.
Real-time reverse transcription-PCR analyses
Root samples were frozen in liquid nitrogen immediately after collection and stored at –80 °C. About 100 mg of tissue were ground in liquid nitrogen and total RNA was extracted with TRIzol. The first-strand cDNA was synthesized with the total RNA by PrimeScript reverse transcription (RT) reagent kit (TaKaRa). All RNA samples were checked for DNA contamination before cDNA synthesis. The mRNA levels of FER, LeFRO1, and LeIRT1 were detected by the SYBR Green RT-PCR kit (TaKaRa) with the following pairs of gene-specific primers: FER fw, 5′-TGAATCTTCTGGCACAACG-3′; rev, 5′-CCAATGATGGAGGCTTTATC-3′, LeFRO1 fw, 5′-GCAAGACACCAGAAATCCTAC-3′, rev: 5′-ATCAGATGGGTTGGGCTT-3′; LeIRT1 fw, 5′-AGCACTTGGGATAGCATTG-3′; rev, 5′-ACTGACATTC CACCAGCAC-3′. The RT-PCR analysis was performed with ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) with the following cycling conditions: 10 s at 95 °C, 35 cycles of 95 °C for 5 s, 60 °C for 30 s. A pair of α-tubulin housekeeping gene primers were used for a control in the PCR: fw: 5′-CCTGAACAACTCATAAGTGGC-3′; rev, 5′-AGATTGGTGTAGGTAGGGCG-3′. Each cDNA sample was run in triplicates. Amplification of PCR products was monitored via intercalation of SYBR-Green. Relative expression units (REU) were calculated according to the equation as described previously (Jin et al., 2009).
In situ measurement of NO in the roots
Nitric oxide was imaged using DAF-FM DA (diaminofluorescein-FM diacetate). The DAF-FM DA has been successfully used to detect NO production in both plants and animals. Roots were loaded with 10 μM DAF-FM DA in 20 mM HEPES/NaOH buffer (pH 7.4) for 30 min, washed three times in fresh buffer and observed under a Nikon Eclipse E600 epifluorescence microscope equipped with a Nikon B-2A filter block (450–490 nm excitation filter, 505 nm dichroic mirror, 520 nm barrier filter). A 100 W high-pressure mercury-vapour lamp was used as a light source (HB-10103AF-Hg, Nikon). Exposure settings were constantly maintained during the fluorescence microscopy. Signal intensities of green fluorescence in the images were quantified according to the method of Guo and Crawford (2005) by using Photoshop software (Adobe Systems). Data are presented as the mean of fluorescence intensity relative to the root tips of Cd-free plants fed NH4+.
Analysis of elements’ content
The dried root and shoot samples were wet digested in the concentrated HNO3/HCl at 120 °C until there was no brown nitrogen oxide gas emitting, then further digested with HClO4 at 180 °C until the solution became transparent. Digestates were diluted by ultrapure water, and the concentrations of Cd and Fe in the digestates were analysed by ICP-OES (iCAP 6300). The concentrations of P in the digestates were evaluated by the vanadate–molybdate colorimetric method (Hesse, 1971).
Statistics
All statistical analyses were conducted with SAS software (SAS Institute, Cary, NC). Means were compared by t test or Fisher’s least significant difference test at P <0.05 in all cases.
Results
Effect of N-form on plant growth and uptake of Cd
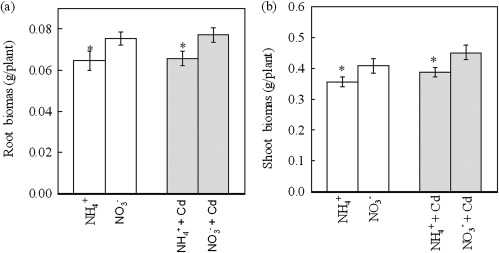
As discussed above, N-form may have a direct effect on Cd uptake in plant roots besides the indirect effect of altering rhizosphere pH. Distinguishing the ‘N-form effect’ from the ‘pH effect’ is important for understanding the mechanism of how the N-form affects Cd accumulation in plants. In this study, a pH-buffered culture solution was used to separate the two variables, so as to investigate whether N-form had a direct effect on Cd accumulation in tomato plants. In Cd-free growth solutions, after 8 d of treatment, the plants fed NO3− had a 16% greater root biomass and 17% greater shoot biomass than the plants fed NH4+. In Cd-added growth solutions, N-form had similar effects on the plant biomass (Fig. 1a, b).
Fig. 1.
Effect of N-form on growth of Micro-Tom tomato plants under Cd-free or Cd-exposed condition. (a) The root biomass. (b) The shoot biomass. The plants were pre-cultured in the growth solution contained both NO3− and NH4+ for 12 d and were then transferred to Cd-free or 2 μM Cd-added growth solutions with either NO3− or NH4+ as the sole nitrogen source. The pH in the all treatments was buffered at 5.5 using MES. The shoots and roots of plants after 8 d of treatments were harvested for biomass analysis. Data are means ±SD (n=4). * Significant differences (P < 0.05) between NO3− and NH4+ treatments.
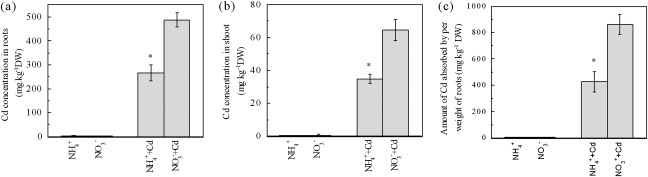
The Cd accumulation in plants was also affected by the N-form. In Cd-added growth solutions, the roots and shoots from NO3− treatment contained 83% and 85% higher Cd concentrations, respectively, than those from NH4+ treatment (Fig. 2a, b). The amount of Cd absorbed per weight of roots (CAPR) was calculated. As shown in Fig. 2c, the plants grown with NO3− had about 2-fold higher CAPR than the plants grown with NH4+, indicating that NO3− nutrition facilitates the Cd uptake of roots.
Fig. 2.
Effects of N-form on Cd concentration and Cd uptake of Micro-Tom tomato plants. (a) The root Cd concentrations. (b) The shoot Cd concentrations. (c) The amount of Cd absorbed by per weight of roots. Treatments are the same as in Fig. 1. Data are means ±SD (n=4). * Significant differences (P < 0.05) between NO3− and NH4+ treatments.
Effect of N-form on Fe uptake
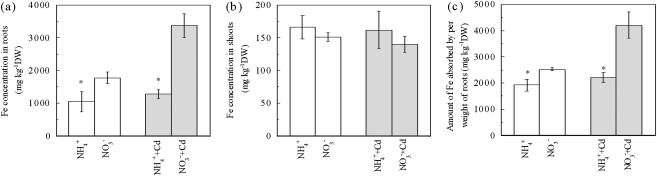
Cd uptake in plants has been linked to the Fe uptake system and, therefore, the Fe concentration in plants was checked. In Cd-free growth solutions, the Fe concentration in roots from the NO3− treatment was increased by 68% compared with those from the NH4+ treatment (Fig. 3a) while, in Cd-added growth solutions, it was increased by up to 163%. By contrast, in both Cd-free and Cd-added growth solutions, the Fe concentrations of shoots from NO3− treatments were slightly lower than those from NH4+ treatments (Fig. 3b). The amount of Fe absorbed per weight of roots (FAPR) was also calculated. As shown in Fig. 3c, in Cd-free growth solutions, FAPR in the NO3− treatment was 31% higher than that in the NH4+ treatment. Interestingly, in Cd-added growth solutions, this NO3− -enhanced FAPR was further strengthened, in some cases by up to 90%, compared with the NH4+ treatment. These results suggest that NO3− also facilitates Fe uptake in roots, particularly with Cd exposure.
Fig. 3.
Effects of N-form on Fe uptake of Micro-Tom tomato plants under Cd-free or Cd-exposed condition. (a) The root Fe concentrations. (b) The shoot Fe concentrations. (c) The amount of Fe absorbed by per weight of roots. Treatments are the same as in Fig. 1. Data are means ±SD (n=4). * Significant differences (P < 0.05) between NO3− and NH4+ treatments.
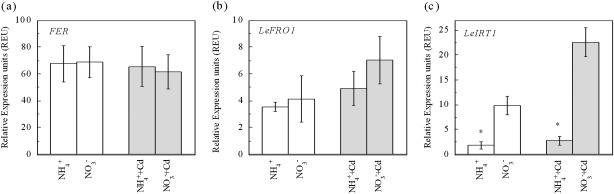
Fe (III) reduction and the transport of Fe (II) across the plasma membrane with ferric chelate reductase (FCR) and IRT1 are pivotal steps involved in Fe uptake by dicots (Curie and Briat, 2003; Jeong and Guerinot, 2009). LeFRO1 which codes for FCR and LeIRT1, which codes for IRT1 in tomato plants, both display tightly regulated expression levels by the FER protein (Ling et al., 2002; Bereczky et al., 2003; Li et al., 2004). It was found here that the expressions of FER and LeFRO1 in roots was not affected or only slightly affected by N-form (Fig. 4a, b). Interestingly, expressions of LeIRT1 were strongly affected by the N-form. In Cd-free growth solutions, the NO3− treatment had a 4.5-fold higher LeIRT1 expression than the NH4+ treatment, while in Cd-added growth solutions the NO3− treatment had a 7.2-fold increase in expression level (Fig. 4c). The results indicate that enhancement of LeIRT1 expression may be responsible for the elevation of Fe uptake under NO3− conditions.
Fig. 4.
Effects of N-form on expression levels of FER (a), LeFRO1 (b), and LeIRT1(c) in Micro-Tom tomato roots under Cd-free or Cd-exposed condition. Treatments are the same as in Fig. 1. Data are means ±SD (n=7). * Significant differences (P < 0.05) between NO3− and NH4+ treatments.
Effect of FER mutation on NO3− -enhanced Cd uptake
Loss of FER function in T3238fer tomato mutants leads to failure of Fe deficiency responses, including the expression of LeIRT1 (Ling et al., 2002). Therefore, the mutant, T3238fer, and its wild type, T3238, were used to investigate the role of Fe uptake systems in NO3− -facilitated Cd uptake. In Cd-added growth solutions, the expression of LeIRT1 in roots of T3238 was significantly higher in NO3− treatments than in NH4+ treatments (Fig. 5a). This result is similar to the Micro-Tom wild-type plants described above. However, in T3238fer the expressions of LeIRT1 in both N-form treatments were almost completely terminated compared with those in T3238. Furthermore, in this mutant strain there was not a statistically significant difference in LeIRT1 expression between the two N-form treatments (Fig. 5a).
In accordance with the findings in Micro-Tom, the Cd concentrations of both roots and shoots in T3238 were also significantly higher in the NO3− treatment than in the NH4+ treatment (Fig. 5b). In T3238fer, however, the root Cd concentration was not affected by N-form (Fig. 5c). Interestingly, the shoot Cd concentration in this mutant was still unexpectedly higher in the NO3− treatment than in the NH4+ treatment, but the difference between them was far less than that in T3238. For T3238fer, shoot Cd concentration after NO3− treatment increased by 37% compared with the NH4+ treatment, whereas for T3238, concentration was increased 128% (Fig. 5b, c). The CAPR in roots of T3238 was significantly higher in the NO3− treatment than in the NH4+ treatment (Fig. 5b), but in T3238fer there was no difference between the two N-form treatments (Fig. 5c). These results, along with the finding that the N-form fails to affect LeIRT1 expression in T3238fer mutants, indicate that the Fe uptake system is required for NO3− facilitation of Cd uptake in wild-type plants.
Effect of NO synthesis inhibition on NO3− -enhanced Cd uptake
Inhibition of nitric oxide (NO) synthesis has also been demonstrated to suppress the expression of LeIRT1 (Graziano and Lamattina, 2007; Jin et al., 2009). The nitrate reductase (NR) and the NO-synthase (NOS) enzymes have been recognized as major sources of NO generation in plants (Shapiro, 2005). Therefore, the NR inhibitor tungstate or the NOS inhibitor L-NAME was used to investigate the effect of NO synthesis inhibition on NO3− -enhanced Cd uptake. Interestingly, NO3− treatment resulted in a higher NO-associated green fluorescence in roots than did the NH4+ treatment (Fig. 6a). By quantifying the signal intensities of fluorescence, the NO contents in roots of the plants fed NO3− were increased by more than 2-fold compared with those of plants fed NH4+ in both Cd-free and Cd-added growth solutions (Fig. 6b). The presence of either tungstate or L-NAME in Cd-added growth solution substantially suppressed NO production in both N-form treatments, and eliminated any difference in NO levels between the two treatments. The NO3− -enhanced expression of LeIRT1 in roots was also completely inhibited by either inhibitor, and there was no resulting difference between the two N-form treatments (Fig. 7a). Consequently, the application of either inhibitor greatly reduced the Cd concentration in NO3− -treated roots, which was even lower than in the NH4+ -treated roots (Fig. 7b). For shoot Cd concentrations, although they were significantly reduced by either inhibitor in both N-form treatments, the NO3− treatment still had a higher value (Fig. 7c). The CAPR was then calculated. As shown in Fig. 7d, when either L-NAME or tungstate were included in the growth solutions, the NO3− treatment had only 41% or 33% higher CAPR, respectively, than the NH4+ treatment, whereas in the growth solutions containing neither L-NAME nor tungstate, the NO3− treatment had about 100% higher CAPR than the NH4+ treatment. These results suggest that inhibition of NO synthesis could diminish the difference in Cd uptake between the two N-form treatments.
Discussion
Nitrate has a direct effect on enhancing Cd uptake
In the pH-buffered growth solutions, it was observed that NO3− nutrition facilitates Cd uptake in roots compared with NH4+ nutrition (Fig. 2). The Cd availability in nutrient solutions may be unintentionally altered due to N-form treatments. However, the computer modelling by GEOCHEM-PC (Parker et al., 1995) showed that the composition of Cd species in nutrient solutions were similar between NO3− and NH4+ treatments, and all were present in soluble forms (see Supplementary Table S1 at JXB online). Furthermore, during plant growth, the pH in the pH-buffered growth solutions was kept constant, thus the variation of Cd availability in the rhizosphere due to N uptake-induced alteration of pH can be discounted. Therefore, the actions of NO3− -facilitated Cd uptake in plants should be directly related to cellular processes rather than the rhizospheric process. Nevertheless, one matter to clarify here is that NH4+ may have deleterious effects on plants when used as the sole N source for plant growth. Acidification of the rhizosphere due to NH4+ uptake is often considered to be a fundamental cause of NH4+ toxicity, particularly since relief from toxicity symptoms has often been observed when growth solutions are pH-buffered (Gigon and Rorison, 1972; Vollbrecht and Kasemir, 1992; Herbert et al., 2001). In this study, pH-buffered growth solutions were used, and therefore no visual toxic symptoms on plants were observed throughout NH4+ treatment. The biomass for the NH4+ treatment was only slightly less than the NO3− treatment (Fig. 1). Furthermore, it was observed that the concentrations of P in both shoots and roots were higher in the plants fed NH4+ than in the plants fed NO3− (see Supplementary Fig. S1 at JXB online). These results indicate that the NH4+ treatment in pH-buffered solutions did not impair the nutrient uptake systems. Therefore, it is reasonable to conclude that NO3− nutrition facilitates Cd uptake in roots and that the lower Cd uptake in NH4+ treatment is not due to deleterious effects induced by NH4+ uptake.
In contrast to our results, it has been observed that NH4+ nutrition facilitates Cd accumulation in soil-grown winter rape (Brassica napus L.) and tobacco (Nicotiana tabacum L.) plants more so than NO3− nutrition (Eriksson, 1990; Tsadilasa et al., 2005). The reason for these conflicting results may be because NH4+ has an indirect effect on increasing root Cd uptake due to a decrease of rhizosphere pH (De Roton et al., 1996; Sarwar et al., 2010). In soils with a weak buffering capacity, the effect of pH on Cd uptake due to NH4+ may be more predominant than the direct effect of NO3− facilitating Cd uptake as discussed above, whereas the opposite is probably true in soils with a strong buffer capacity. Therefore, distinguishing the indirect effects of pH from the direct effects of N-form and comprehensively considering each is a critically important step in determining whether pH amendments or N-forms should be prioritized when proposing a strategy for reducing Cd accumulation in crops grown in Cd-contaminated soils.
The system involved in Fe uptake is required for NO3− -enhanced Cd uptake
In most instances, the greater uptake of one ion can either depress the uptake of another ion with similar charge (antagonism) or stimulate the uptake of an ion with opposite charge (synergism). Therefore, the ion synergism may explain why the NO3− nutrition results in higher accumulation of Cd in the plants. However, the mechanism behind the above ion synergism remains unknown. As discussed above, reduction of Fe (III) to ferrous Fe by FCR and subsequent transport across the plasma membrane by IRT1 are pivotal steps involved in the Fe uptake of dicots (Robinson et al., 1999; Jeong and Guerinot, 2009), while IRT1 is of particular interest in this study because it is also a plasma membrane transporter of Cd2+ (Vert et al., 2002; Verbruggen et al., 2009; Lux et al., 2011). The linkage between Fe uptake and NO3− -enhanced Cd uptake was therefore analysed. It was observed here that NO3− treatment could also facilitate NO3− Fe uptake in the roots compared with the NH4+ treatment (Fig. 3). Furthermore, although the expression of LeFRO1 in roots undergoing NO3− treatment was only increased slightly, the expression of LeIRT1 NO3− treatment was greatly increased compared with the NH4+ treatment (Fig. 4b, c). Although FCR and IRT1 work together to enhance Fe uptake under Fe-deficient conditions, IRT1 seems to be more important than FCR in Fe uptake under Fe-sufficient conditions. When the plants were grown in soil, the Arabidopsis FCR-null mutant frd1-1 and the wild type had similar Fe concentrations, but the IRT1-null mutant irt1-1 contained considerably lower Fe concentrations than the wild type (Yi and Guerinot, 1996; Vert et al., 2002). Therefore, although LeFRO1 expression is not increased with the up-regulation of LeIRT1 expression, it is still reasonable to suggest that increasing Fe (II) transporter IRT1 may be responsible for increasing Fe uptake in the NO3− treatment.
The expression of LeIRT1 is tightly regulated by the FER protein (Ling et al., 2002). T3238fer tomato mutants with loss of FER function exhibit severe chlorosis and die early on unless supplied with ferrous iron or grafted onto a wild-type rootstock (Brown et al., 1971; Ling and Ganal, 2000). It was found here that the expressions of LeIRT1 in the Fe uptake-inefficient mutant T3238fer were similar between the NO3− and NH4+ treatments, and were almost completely non-existent compared with those in the wild type T3238 (Fig. 5a). Accordingly, in T3238fer, the Cd uptake in roots was not affected by the N-form, but in T3238 it was significantly higher in the NO3− treatment than in the NH4+ treatment (Fig. 5b, c). These results combined with the finding that both Fe uptake and LeIRT1 expression were increased by NO3− (Figs 3, 4b), indicate that the system involved in Fe uptake is required for the enhancement of Cd uptake by NO3− in tomato plants. Although loss of FER function resulted in the inhibition of the NO3− -induced enhancement of LeIRT1 expression and Cd uptake in the T3238fer mutant, the expression of fer in the wild-type plants was not affected by the N-form (Fig. 4a). It is speculated that FER is essential, but is not the limiting factor for the regulation of NO3− -induced enhancement of Cd uptake in tomato plants.
Several studies have demonstrated that NO is a signal controlling the Fe uptake system in roots (Graziano and Lamattina, 2007; Besson-Bard et al., 2009; Chen et al., 2010; Ramirez et al., 2010; García et al., 2010). Accordingly, in the present study, it was observed that suppression of LeIRT1 expression in roots was by the inhibition of NO synthesis. Significant decreases in the Cd concentration in plants fed NO3− were observed, which diminished the difference in Cd uptake between NO3− and NH4+ treatments (Fig. 7). The results provide more evidence for our above conclusion that the Fe uptake system is required for NO3− induction of Cd uptake. Interestingly, it was also observed here that NO3− treatment resulted in a higher NO level in roots than did the NH4+ treatment in both Cd-free and Cd-supplemented growth solutions (Fig. 6). Theoretically, the NR-dependent NO production depends on the NR activity. The increase in nitrate availability enhances NR activity (Shaner and Boyer, 1976), whereas NH4+ is an inhibitor of NR (Jin et al., 2011). Accordingly, a higher NO level in roots of NO3− treatment is probably due to activation of NR activity by NO3−. This viewpoint, combined with the fact that NO is a signal controlling the Fe uptake system in roots, allowed us to propose that NO3− -induction of NO production in roots may be the original signal causing the induction of the Fe uptake system, resulting in enhanced Cd uptake. This hypothesis will be the focus of our future research. It is interesting to note that the NOS inhibitor L-NAME could also inhibit the NO production in Cd-added NO3− treatment (Fig. 6). This may be due to the fact that accumulation of Cd in plants could also induce NO production by NOS (Besson-Bard et al., 2009).
It is worth noting that NO availability in plants also affects the expression of NRT2.1, the gene encoding a high-affinity NO3− transporter. Elevation of NO levels in roots by Cd exposure induces the expression of NRT2.1, while the opposite is true for roots treated with L-NAME (Besson-Bard et al., 2009). Therefore, it is reasonable to propose that NO3− -induced NO production may, in turn, facilitate NO3− uptake in roots, forming a positive feedback loop. In addition, because Cd in plants also induces NO production (Besson-Bard et al., 2009), the induction of IRT1 expression by NO not only may increase Cd uptake in roots, but may also enhance the production of NO. Taken together, the NO-mediated cross-talking between NO3− - and Fe-sensing pathways may take place in roots, which may aid the plants’ Cd uptake.
Overall, although previous reports have provided other evidence concerning NO3− nutrition facilitating Cd uptake in roots compared with NH4+ nutrition in different plant species, the mechanism behind this process has not previously been examined. Here, using wild-type tomato plants, Fe uptake-inefficient mutants, and NO synthesis inhibitors, it has been demonstrated that the effects of NO3− on root Cd uptake are attributed to an up-regulation of the system involved in Fe uptake. The increase of NO production may be a signalling pathway controlling the above process. To our knowledge, this is the first report to uncover why NO3− -based fertilizers result in more Cd accumulation in plants than NH4+ -based fertilizers in many cases, even though NO3− -based fertilizers are expected to decrease the Cd availability in the rhizosphere. Furthermore, this study also helped determine whether pH amendments or N-forms should be prioritized when proposing a strategy for safe crop production in contaminated soil.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Effects of N-form on P concentrations in tomato plants during Cd exposure.
Supplementary Table S1. Comparison of Cd and Fe forms between NO3− and NH4+ media.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (Grant Nos 30900920, 30900170) and the Natural Science Foundation of Ningbo City, China (Grant No. 2011A610002). We thank Dr Petra Bauer (University of Saarland, D-66041 Saarbrucken, Germany) for kindly providing T3238fer mutant seeds.
References
- Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P. Differential regulation of nramp and irt metal transporter genes in wild type and iron-uptake mutants of tomato. Journal of Biological Chemistry. 2003;278:24697–24704. doi: 10.1074/jbc.M301365200. [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Gravot A, Richaud P. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiology. 2009;149:1302–1315. doi: 10.1104/pp.108.133348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Chaney RL, Ambler JE. A new tomato mutant inefficient in the transport of iron. Physiologia Plantarum. 1971;24:48–53. [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis thaliana. Plant Physiology. 2010;154:1885–1892. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Curie C, Briat JF. Iron transport and signaling in plants. Annual Review of Plant Biology. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- Daniel-Vedele F, Krapp A, Kaiser WM. Cellular biology of nitrogen metabolism and signaling. Plant Cell Monographs. 2010;17:145–172. [Google Scholar]
- De Roton C, Tancogne J, Cadilhac JL. Effects of the soil pH and form of nitrogen fertilization on the uptake of trace-metals in Burley tobacco. 1996 CORESTA congress, Yokohama, Japan. [Google Scholar]
- Eriksson JE. Effects of nitrogen-containing fertilizers on solubility and plant uptake of cadmium. Water, Air, and Soil Pollution. 1990;49:355–368. [Google Scholar]
- García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. Journal of Experimental Botany. 2010;61:3885–3899. doi: 10.1093/jxb/erq203. [DOI] [PubMed] [Google Scholar]
- Gigon A, Rorison IH. The response of some ecologically distinct plant species to nitrate- and to ammonium-nitrogen. Journal of Ecology. 1972;60:93–102. [Google Scholar]
- Grant CA, Bailey LD, McLaughlin MJ, Singh BR. Management factors which influence cadmium concentration in crops. In: McLaughlin MJ, Singh BR, editors. Cadmium in soils and plants. Dordrecht: Kluwer; 1999. pp. 151–198. [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. The Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J, Kronzucker Dev T, Britto Romola J, Davenport Mark Tester. Ammonium toxicity and the real cost of transport. Trends in Plant Science. 2001;6:335–337. doi: 10.1016/s1360-1385(01)02022-2. [DOI] [PubMed] [Google Scholar]
- Hesse PR. Soil phosphorus: its measurements and its uptake by plants. Australian Journal of Soil Research. 1971;35:227–239. [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Hong CO, Chung DY, Lee do K, Kim PJ. Comparison of phosphate materials for immobilizing cadmium in soil. Archives of Environmental Contamination Toxicology. 2010;58:268–274. doi: 10.1007/s00244-009-9363-2. [DOI] [PubMed] [Google Scholar]
- Jalloh MA, Chen JH, Zhen FR, Zhang GP. Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. Journal of Hazardous Materials. 2009;162:1081–1085. doi: 10.1016/j.jhazmat.2008.05.146. [DOI] [PubMed] [Google Scholar]
- Jeong J, Guerinot ML. Homing in on iron homeostasis in plants. Trends in Plant Science. 2009;14:280–285. doi: 10.1016/j.tplants.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. Elevated carbon dioxide improves plant Fe nutrition through enhancing the Fe-deficiency-induced responses under Fe-limited conditions in tomato. Plant Physiology. 2009;150:272–280. doi: 10.1104/pp.109.136721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, Du ST, Shamsi IR, Luo BF, Lin XY. NO synthase-generated NO acts downstream of auxin in regulating Fe-deficiency-induced root branching that enhances Fe-deficiency tolerance in tomato plants. Journal of Experimental Botany. 2011;62:3875–3884. doi: 10.1093/jxb/err078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs K, Kuzmann E, Vertes A, Levai L, Cseh E, Fodor F. Effect of cadmium on iron uptake in cucumber roots: a Mössbauer-spectroscopic study. Plant and Soil. 2010;327:49–56. [Google Scholar]
- Li L, Cheng X, Ling HQ. Isolation and characterization of Fe(III)-chelate reductase gene LeFRO1 in tomato. Plant Molecular Biology. 2004;54:125–136. doi: 10.1023/B:PLAN.0000028774.82782.16. [DOI] [PubMed] [Google Scholar]
- Ling HQ, Bauer P, Bereczky Z, Keller B, Ganal M. The tomato fer gene encoding a bHLH protein controls iron uptake responses in roots. Proceedings of the National Academy of Sciences, USA. 2002;99:13938–13943. doi: 10.1073/pnas.212448699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling HQ, Ganal MW. Towards map-based cloning of two genes involved in iron uptake of tomato. Journal of Plant Nutrition. 2000;23:1953–1967. [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany. 2011;62:21–37. doi: 10.1093/jxb/erq281. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Muños S, Cazettes C, Fizames C, Gaymard F, Tillard P, Lepetit M, Lejay L, Gojon A. Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. The Plant Cell. 2004;16:2433–2447. doi: 10.1105/tpc.104.024380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan K, Wang WX. Trace metal contamination in estuarine and coastal environments in China. Science of Total Environment. 2011 doi: 10.1016/j.scitotenv.2011.03.013. doi:10.1016/j.scitotenv. 2011.03.013. [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In: Loeppert RH, Schwab AP, Goldberg S, editors. Chemical equilibrium and reaction models. 1995. Special Publication 42. Madison, WI: Soil Science Society of America, 253–269. [Google Scholar]
- Ramirez L, Zabaleta EJ, Lamattina L. Nitric oxide and frataxin: two players contributing to maintain cellular iron homeostasis. Annals of Botany. 2010;105:801–810. doi: 10.1093/aob/mcp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Ronald E. Handbook of chemical risk assessment: health hazards to humans, plants, and animals. Boca Raton, FL: Lewis Publishers; 2000. pp. 1–43. [Google Scholar]
- Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G. Role of mineral nutrition in minimizing cadmium accumulation by plants. Journal of the Science of Food and Agriculture. 2010;90:925–937. doi: 10.1002/jsfa.3916. [DOI] [PubMed] [Google Scholar]
- Shaner DL, Boyer JS. Nitrate reductase activity in maize (Zea mays L.) leaves. I. Regulation by nitrate flux. Plant Physiology. 1976;58:499–504. doi: 10.1104/pp.58.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AD. Nitric oxide signaling in plants. Vitamins and Hormones. 2005;72:339–398. doi: 10.1016/S0083-6729(05)72010-0. [DOI] [PubMed] [Google Scholar]
- Tian QY, Sun P, Zhang WH. Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytologist. 2005;184:918–931. doi: 10.1111/j.1469-8137.2009.03004.x. [DOI] [PubMed] [Google Scholar]
- Tsadilasa CD, Karaivazogloub NA, Tsotsolisb NC, Stamatiadisc S, Samarasa V. Cadmium uptake by tobacco as affected by liming, N form, and year of cultivation. Environmental Pollution. 2005;134:239–246. doi: 10.1016/j.envpol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Christian H, Schat H. Moleclar mechanisms of metal hyperaccumulation in plants. New Phytologist. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and plant growth. The Plant Cell. 2002;14:1223–1233. doi: 10.1105/tpc.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht P, Kasemir HI. Effects of exogenously supplied ammonium on root development of Scots Pine (Pinus sylvestris L.) seedlings. Botanica Acta. 1992;105:306–312. [Google Scholar]
- Walch-Liu P, Filleur S, Gan Y, Forde BG. Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosynthesis Research. 2005;83:239–250. doi: 10.1007/s11120-004-2080-9. [DOI] [PubMed] [Google Scholar]
- Wang HY, Garvin DF, Kochian LV. Nitrate-induced genes in tomato roots. Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiology. 2001;127:345–359. doi: 10.1104/pp.127.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie HL, Jiang RF, Zhang FS, McGrath SP, Zhao FJ. Effect of nitrogen form on the rhizosphere dynamics and uptake of cadmium and zinc by the hyperaccumulator. Thlaspi caerulescens. Plant and Soil. 2009;318:205–221. [Google Scholar]
- Yi Y, Guerinot ML. Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. The Plant Journal. 1996;10:835–844. doi: 10.1046/j.1365-313x.1996.10050835.x. [DOI] [PubMed] [Google Scholar]
- Zaccheo P, Crippa L, Pasta VD. Ammonium nutrition as a strategy for cadmium mobilisation in the rhizosphere of sunflower. Plant and Soil. 2006;283:43–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.