Abstract
Progress in understanding how a particular genotype produces the phenotype of an inborn error of metabolism, such as a mucopolysaccharidosis, in human patients has been facilitated by the study of animals with mutations in the orthologous genes. These are not just animal models, but true orthologues of the human genetic disease, with defects involving the same evolutionarily conserved genes and the same molecular, biochemical, and anatomic lesions as in human patients. These animals are often domestic species because of the individual medical attention paid to them, particularly dogs and cats. In addition, naturally occurring mouse models have also been found in breeding colonies. Within the last several decades, advances in molecular biology have allowed the production of knockout mouse models of human genetic disease, including the lysosomal storage diseases. The ability to use both inbred strains of a small, prolific species together with larger out-bred animals found because of their disease phenotype provides a powerful combination with which to investigate pathogenesis, develop approaches to therapy, and define biomarkers to evaluate therapeutic success. This has been true for the inborn errors of metabolism and, in particular, the mucopolysaccharidoses.
Conclusion
Animal models of human genetic disease continue to play an important role in understanding the molecular and physiological consequences of lysosomal storage diseases and to provide an opportunity to evaluate the efficacy and safety of therapeutic interventions.
Keywords: Animal models, Lysosomal storage diseases, Mucopolysaccharidosis
ANIMAL MODELS OF LYSOSOMAL STORAGE DISEASES (LSDs)
The genes involved in many genetic disorders have been isolated and pathogenic mechanisms understood in terms of the underlying molecular derangements. However, to fully understand and treat genetic diseases in human patients, the use of authentic animal models is required to perform studies that for ethical and practical reasons are not possible in human patients. The advent of large colonies of mice bred for research, together with a systematic approach to look for naturally occurring models of genetic disease, has led to the discovery of mouse models of human genetic diseases. Gene knockout technology has been a powerful approach that has contributed in a major way to the understanding of gene function in health and disease and can be used in cases in which the gene of interest has been cloned. The mouse and other small laboratory animals will continue to be a very valuable source of disease models and have the advantage of being available in well-characterized inbred strains, which can be bred easily to produce large numbers of affected as well as unaffected control animals with the same genetic background. For initial studies of pathogenesis and therapy, these naturally occurring LSDs and knockout mouse models have proven extremely valuable.
Clinical veterinary medicine provides a vast and sophisticated screening mechanism in which animal patients are examined individually and in detail by diagnostic methods approaching in accuracy and sensitivity those used in human patients. Furthermore, the naturally occurring genetic diseases in dogs and cats exist in various genetic isolates (breeds) maintained by members of the public. Once identified, these models of human genetic disease can be established in special research colonies that are frequently associated with veterinary schools. Dog and cat breeders have been eager to cooperate in these endeavours because the scientific knowledge gained aids in understanding and controlling animal diseases as well as contributing to human health (1). Also, because large animal models are discovered through their clinical phenotype, they are more suitable than some knockout or point mutation mouse models, which may have lesions that are lethal in utero, or may lack the full range of clinical disease, as has been the case in mice with Tay–Sachs disease, Fabry disease, cystinosis and Gaucher disease (2–6). In addition, for some knockout models, such as those for type 2 and type 3 Gaucher disease, animals die within a few days of birth, limiting their value for therapy research (6,7). Of course, not all genetic diseases have been found in large animals, and knockout mice have been essential to fill the gaps.
Perhaps due to the often progressive nature and striking clinical signs of LSDs, domesticated animals have been a rich source of models of these diseases (Fig. 1). Some of these were recognized in veterinary medicine prior to the time when LSDs were understood at the level of the specific enzymes involved. Because of the distinctive central and peripheral nervous system lesions, the first of these diseases to be described was globoid cell leukodystrophy in Cairn and West Highland white terriers in 1963 (8). Affected dogs of these two breeds are now known to have the same mutation in galactosylceramidase (9). The mutation apparently originated in the 19th century from a common ancestor of both breeds, which diverged around the beginning of the 20th century. The first LSD in animals that was identified by its deficient enzyme (β-galactosidase) was GM1 gangliosidosis in a Siamese cat in 1971 (10). Since then, naturally occurring LSDs have been recognized in cats, cattle, dogs, guinea pigs, goats, mice, pigs, rats, sheep, quail, emus and horses (11,12) (Table 1), as well as in flamingos (48). Breeding colonies of various species have been established and exist for a large number of LSDs (13,49). Larger species, such as the dog and cat, have the advantages of a heterogeneous genetic background more similar to humans, and a size and longevity more suitable for repeated sampling from an individual and assessment of the long-term consequences of therapy over many years. These advantages, along with the accumulated background of physiological and clinical veterinary knowledge in these species, make these large animal models extremely useful.
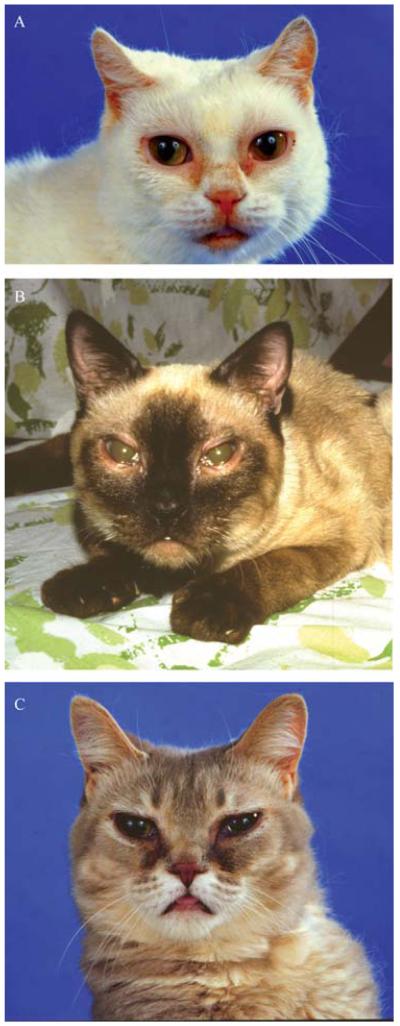
Figure 1.
Three cats with mucopolysaccharidosis (MPS) showing the typical features of this class of disease including small ears, wide-spaced eyes, thick eyelid margins, tear staining due to occluded naso-lacrimal ducts, flattened face with protuberant tongue and corneal clouding. (A) MPS I (β-l-iduronidase deficiency). (B) MPS VI (4-sulphatase deficiency). (C) MPS VII (β-glucuronidase deficiency).
Table 1.
Animal models of mucopolysaccharidoses (MPSs)
| Disease | Deficient enzyme | Species and selected references |
|---|---|---|
| MPS I | α-l-Iduronidase | Domestic cat† (14,15) Plott hound dog† (16) Rotweiler dog (unpublished) Boston terrier dog (unpublished) Knockout mouse (17) |
| MPS II | Iduronate sulphatase | Labrador retriever dog (18) Knockout mouse (19) |
| MPS IIIA | Heparan N-sulphatase | Wirehaired dachshund dog† (20,21) Mouse† (22,23) Huntaway dog† (24,25) |
| MPS IIIB | α-N-Acetylglucosaminidase | Emu† (26) Schipperke dog† (27) Cattle (J.J. Hopwood, personal communication 2006) Knockout mouse (28) |
| MPS IIID |
N-Acetylglucosamine 6-sulphatase |
Nubian goat† (29,30) |
| MPS IV |
N-Acetylgalactosamine 6-sulphate sulphatase |
Knockout mouse (31) |
| MPS VI |
N-Acetylglucosamine 4-sulphatase (arylsulphatase B) |
Siamese cat† (32–34) Domestic short-haired cat (unpublished) Miniature Pinscher dog† (35,36) Welsh corgi dog (unpublished) Chesapeake Bay retriever dog (unpublished) Miniature Schnauzer dog† (37) Rat† (38–40) Knockout mouse (41) |
| MPS VII | β-Glucuronidase | German shepherd dog† (42,43) GUS mouse† (44,45) Cat† (46,47) |
The mutation is known. (Adapted from Ref. 13 with permission.)
While much progress has been made in defining the molecular basis of genetic diseases in man, there remain large gaps in our understanding of the complex chain of events between the underlying genetic defect and the phenotypic abnormalities at various levels – from cells, tissues and organs to the whole organism. For example, while we know the genes and many of the mutations underlying the mucopolysaccharidoses (MPSs), and the clinical and pathological features of the articular cartilage lesions have been described, the pathogenesis of how the substrate storage results in the cartilage lesions is just beginning to be understood from investigations in cats and rats with MPS (50,51). For many lethal or debilitating genetic disorders in man, there are still no satisfactory means of treatment. One of the most exciting prospects for the use of animals with orthologous genetic diseases lies in testing new approaches to therapy. The monogenic inborn errors of metabolism are particularly attractive targets for the development of therapies because they constitute a significant proportion of genetic diseases, and they are usually autosomal recessive disorders involving the deficiency of a single specific protein gene product.
CLINICAL RELEVANCE TO THERAPY
The animal models of MPS have significant clinical signs and lesions in the same organ systems as human patients, including the CNS, skeleton, eye, cardiovascular system and liver. Hence, the models offer the opportunity to test systemic therapy as well as approaches limited to a single organ system such as the CNS or synovial joints. What has become clear over the last several decades is that many of the LSDs are unique and one approach to therapy will not fit them all. This is true even in subclasses such as the MPSs. Thus, having animal models for each disease is important for evaluating therapy. Testing the efficacy and safety of a therapy in both a mouse and large animal model increases the confidence that such an approach will be helpful to human patients.
The basic approach for the treatment of most LSDs, including the MPSs, relies on the phenomenon of cross correction, which permits cells to take up exogenous normal enzyme and deliver it to the lysosome, usually by a mannose 6-phosphate receptor-mediated process (52,53). Fortunately, the amount of enzyme needed in the lysosome for phenotypic correction of an individual cell is only a small percentage of normal. In general, the most difficult target tissue is the CNS, for which systemic therapy is limited by the blood–brain barrier. The three approaches to providing normal enzyme to a patient’s cells are enzyme replacement therapy (ERT), bone marrow transplantation and gene therapy.
ERT
The parenteral injection of purified recombinant enzyme (ERT) has been tested in various animal models including MPS VII mice (54–58), MPS I dogs (59) and cats (60), MPS VI cats (61–65) and MPS II mice (19). While ERT is currently the standard therapy for patients with non-neuronopathic Gaucher disease and was developed without an animal model, similar therapy has been tested in animal models and is approved or under evaluation for treatment of human patients with MPS I, MPS II and MPS VI. The major limitations of ERT remain the inability of intravenously injected enzyme to reach the CNS (and synovial joints in some diseases), the cost, an immune response in some patients and the need for frequent, life-long injections. That said, for some MPS disorders the clinical benefits are well established (66–68).
Bone marrow transplantation (BMT)
Heterologous BMT as therapy for LSDs has been performed for decades in animal models and human patients (reviewed in Refs. 69–75). This approach provides both normal bone marrow and bone marrow-derived cells that are available to release enzyme continuously for uptake by other cells. In addition, monocyte-derived cells can cross the blood–brain barrier, become microglia and secrete enzyme that is available to neurons. BMT has been performed in animals including MPS VI cats (76–80), MPS VII mice (81–83), and MPS VII dogs (84) (Fig. 2). A combination of neonatal ERT followed by BMT at 5 weeks of age in MPS VII mice had long-term positive effects (57). In humans, the central limitations of BMT include the difficulty in finding a matched donor, the mortality and morbidity of the procedure, and the variable results achieved in the CNS in some diseases. When successful, BMT has improved the quality of life of patients.
Figure 2.

Two dogs with MPS VII. The dog standing on the left was treated with heterologous bone marrow transplant at 6 weeks of age and was able to stand and walk for 6 years. The dog on the right is typical of those with the disease and was unable to stand by 6 months of age. Similar results were obtained treating affected dogs at 3 days of age with intravenous gene therapy with a retroviral vector containing the human α1-antitrypsin promoter, canine β-glucuronidase cDNA and woodchuck hepatitis post-transcriptional regulatory element.
Gene therapy
The basic premise in gene therapy for LSDs is to provide some autologous cells with the relevant normal cDNA to produce and secrete normal enzyme for uptake by abnormal cells. Thus, the phenomenon of cross correction in MPSs obviates the need to transfer the normal cDNA to all cells, as a small percentage of transduced cells can be therapeutic at the organ or whole animal level by secreting enough of the normal enzyme for mannose 6-phosphate receptor-mediated uptake by other deficient cells. Although simple in concept, and a number of human trials have been reported, the cure of inherited metabolic diseases in human patients by somatic cell gene therapy has been limited (85), and not without complications, including insertional mutagenesis (86). The major difficulties in the field at this point are obtaining adequate levels of gene product in the specific cell types in which they are needed (e.g. in the CNS), maintaining expression over long periods of time in vivo, and regulating the levels of gene expression. The necessary research requires animal models that are true orthologues of the human disease due to a defect in the homologous gene and that have the same molecular, pathological and clinical phenotype as the human disease. A series of in vitro and in vivo experiments have been performed to evaluate gene therapy in animal models of MPS. In spite of the rarity of MPS VII (< 1 in 250 000), the mouse and dog models of MPS VII have become a paradigm for LSDs in general because of the ability to detect the normal enzyme (β-glucuronidase, encoded by the gene GUSB) activity directly using a histochemical technique. Many gene transfer approaches have been used in the naturally occurring MPS VII mouse (49). Some of the most striking clinical results of gene therapy involving an MPS have been those seen in a series of neonatal gene transfer studies conducted using retroviral vectors in the canine model of MPS VII, with treated dogs now over 6 years of age (87–90). Even so, although a constant serum β-glucuronidase activity that is 50–60-fold greater than normal by 1 week of age in MPS VII dogs has been achieved, the dogs are not completely cured. Importantly, this finding has established limits to what systemic enzyme therapy can achieve in MPS VII. Other MPS disorders have also been used in gene therapy experiments with varying degrees of clinical and pathological improvement (49).
Feline and canine models are also useful for addressing gene transfer in relatively long-lived species that are more similar in size to human infants, particularly to evaluate the effect of direct injection of vectors into a relatively large CNS. Additionally, viral vector tropism in the mouse may not be the same as in other model species or humans. For example, in normal cats, human GUSB has been used as a reporter gene, due to the fact that it can be distinguished from feline GUSB by its heat stability. Recombinant adeno-associated viral (AAV) vectors of serotypes 1, 2 and 5 were injected into six areas of the brain of 8-week-old cats (91). The brains were evaluated for gene expression using in situ hybridization and enzyme histochemistry 10 weeks after injection. The AAV2/2 vector transduced cells in the grey matter, while the AAV2/1 vector surpassed both the AAV2/2 vector in grey matter transduction and also transduced cells of the white matter. The AAV2/5 vector did not result in detectable transduction in the cat brain (91). This last finding was in contrast with what had been reported in the mouse brain when using AAV2/5 (92), although the murine study utilized the AAV2/5 inverted terminal repeat for expression, making direct comparisons difficult. Following this experiment, kittens with β-mannosidosis were treated by direct injection into the brain, producing widespread correction of storage with clinical improvement (93).
Substrate reduction therapy (SRT)
Rather than treat LSDs by providing normal enzyme, an alternate approach involves limiting the amount of substrate that requires degradation. This therapy, SRT (94–97), uses pharmacological agents that are inhibitors of the enzymes responsible for the production of substrates. The aim of SRT is to limit the accumulation of substrate to a point that can be accommodated by any residual enzyme activity. Hence, SRT therapy would either require some residual enzyme activity be present or could be used as an adjunct to other therapies.
CONCLUSION
Finding spontaneous mouse models, as well as producing knockout mice with a disruption of the gene of interest, provides the ability to evaluate the effects of disease and therapy on a relatively large number of animals with a uniform genetic background. Domestic animals with spontaneous genetic disease can be of great importance in understanding pathogenesis and in the development of therapy. Veterinary medicine provides an increasingly high degree of medical scrutiny of animals, particularly the dog and cat, and new orthologues of human genetic diseases are being recognized with increasing frequency. These models provide an opportunity to monitor therapeutic efficacy and the possible development of untoward side effects in out-bred, long-lived animals that can be monitored as individuals using the same methods that are applicable to human patients. The ideal is to have a mouse, dog or cat model, together with an authentic primate model. Currently, this exists only for globoid cell leukodystrophy (Krabbe disease) with the twitcher mouse (98), the dog (9) and the rhesus monkey (99) which are all spontaneous models of the human disease.
ACKNOWLEDGEMENTS
The discovery and characterization of large animal models of human genetic disease have been supported by grants from the NIH, currently P40-RR02512. Approaches to therapy have been supported by NIH grants DK25759 and DK54481.
Footnotes
Conflict of Interest Statement MEH has declared no conflict of interest.
References
- 1.Patterson DF, Haskins ME, Jezyk PF, Giger U, Meyers-Wallen VN, Aguirre G, et al. Research on genetic diseases: reciprocal benefits to animals and man. J Am Vet Med Assoc. 1988;193:1131–44. [PubMed] [Google Scholar]
- 2.Phaneuf D, Wakamatsu N, Huang JQ, Borowski A, Peterson AC, Fortunato SR, et al. Dramatically different phenotypes in mouse models of human Tay-Sachs and Sandhoff diseases. Hum Mol Genet. 1996;5:1–14. doi: 10.1093/hmg/5.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, et al. α-Galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci USA. 1997;94:2540–4. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen-Tannoudji M, Marchand P, Akli S, Sheardown SA, Puech JP, Kress C, et al. Disruption of murine Hexa gene leadsto enzymatic deficiency and to neuronal lysosomal storage, similar to that observed in Tay-Sachs disease. Mamm Genome. 1995;6:844–9. doi: 10.1007/BF00292433. [DOI] [PubMed] [Google Scholar]
- 5.Cherqui S, Sevin C, Hamard G, Kalatzis V, Sich M, Pequignot MO, et al. Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol Cell Biol. 2002;22:7622–32. doi: 10.1128/MCB.22.21.7622-7632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003;163:2093–101. doi: 10.1016/s0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Suzuki K, Reed JD, Grinberg A, Westphal H, Hoffmann A, et al. Mice with type 2 and 3 Gaucher disease point mutations generated by a single insertion mutagenesis procedure. Proc Natl Acad Sci USA. 1998;95:2503–8. doi: 10.1073/pnas.95.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fankhauser R, Luginbuhl H, Hartley WJ. Leukodystrophie vom Typus Krabbe Beim Hund. Arch Tierheilk. 1963;105:198–207. [Google Scholar]
- 9.Victoria T, Rafi MA, Wenger DA. Cloning of the canine GALC cDNA and identification of the mutation causing globoid cell leukodystrophy in West Highland White and Cairn terriers. Genomics. 1996;33:457–62. doi: 10.1006/geno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 10.Baker HJ, Jr., Lindsey JR, McKhann GM, Farrell DF. Neuronal GM1 gangliosidosis in a Siamese cat with beta-galactosidase deficiency. Science. 1971;174:838–9. doi: 10.1126/science.174.4011.838. [DOI] [PubMed] [Google Scholar]
- 11.Url A, Bauder B, Thalhammer J, Nowotny N, Kolodziejek J, Herout N, et al. Equine neuronal ceroid lipofuscinosis. Acta Neuropathol (Berl) 2001;101:410–14. doi: 10.1007/s004010000298. [DOI] [PubMed] [Google Scholar]
- 12.Haskins ME, Giger U. Lysosomal storage diseases. Academic Press; New York: 1997. [Google Scholar]
- 13.Haskins M, Casal M, Ellinwood NM, Melniczek J, Mazrier H, Giger U. Animal models for mucopolysaccharidoses and their clinical relevance. Acta Paediatr. 2002;91(Suppl 439):88–97. doi: 10.1111/j.1651-2227.2002.tb03117.x. [DOI] [PubMed] [Google Scholar]
- 14.Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis I. Am J Pathol. 1983;112:27–36. [PMC free article] [PubMed] [Google Scholar]
- 15.He X, Li CM, Simonaro CM, Wan Q, Haskins ME, Desnick RJ, et al. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol Genet Metab. 1999;67:106–12. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- 16.Shull RM, Helman RG, Spellacy E, Constantopoulos G, Munger RJ, Neufeld EF. Morphologic and biochemical studies of canine mucopolysaccharidosis I. Am J Pathol. 1984;114:487–95. [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, et al. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet. 1997;6:503–11. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- 18.Wilkerson MJ, Lewis DC, Marks SL, Prieur DJ. Clinical and morphologic features of mucopolysaccharidosis type II in a dog: naturally occurring model of Hunter syndrome. Vet Pathol. 1998;35:230–3. doi: 10.1177/030098589803500311. [DOI] [PubMed] [Google Scholar]
- 19.Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr. 2002;91(Suppl 439):98–9. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 20.Aronovich EL, Carmichael KP, Morizono H, Koutlas IG, Deanching M, Hoganson G, et al. Canine heparan sulfate sulfamidase and the molecular pathology underlying Sanfilippo syndrome type A in Dachshunds. Genomics. 2000;68:80–4. doi: 10.1006/geno.2000.6275. [DOI] [PubMed] [Google Scholar]
- 21.Fischer A, Carmichael KP, Munnell JF, Jhabvala P, Thompson JN, Matalon R, et al. Sulfamidase deficiency in a family of Dachshunds: a canine model of mucopolysaccharidosis IIIA (Sanfilippo A) Pediatr Res. 1998;44:74–82. doi: 10.1203/00006450-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharyya R, Gliddon B, Beccari T, Hopwood JJ, Stanley P. A novel missense mutation in lysosomal sulfamidase is the basis of MPS III A in a spontaneous mouse mutant. Glycobiology. 2001;11:99–103. doi: 10.1093/glycob/11.1.99. [DOI] [PubMed] [Google Scholar]
- 23.Bhaumik M, Muller VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R, et al. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome) Glycobiology. 1999;9:1389–96. doi: 10.1093/glycob/9.12.1389. [DOI] [PubMed] [Google Scholar]
- 24.Jolly RD, Allan FJ, Collett MG, Rozaklis T, Muller VJ, Hopwood JJ. Mucopolysaccharidosis IIIA (Sanfilippo syndrome) in a New Zealand Huntaway dog with ataxia. NZVet J. 2000;48:144–8. doi: 10.1080/00480169.2000.36181. [DOI] [PubMed] [Google Scholar]
- 25.Yogalingam G, Pollard T, Gliddon B, Jolly RD, Hopwood JJ. Identification of a mutation causing mucopolysaccharidosis type IIIA in New Zealand Huntaway dogs. Genomics. 2002;79:150–3. doi: 10.1006/geno.2002.6699. [DOI] [PubMed] [Google Scholar]
- 26.Aronovich EL, Johnston JM, Wang P, Giger U, Whitley CB. Molecular basis of mucopolysaccharidosis type IIIB in emu (Dromaius novaehollandiae): an avian model of Sanfilippo syndrome type B. Genomics. 2001;74:299–305. doi: 10.1006/geno.2001.6552. [DOI] [PubMed] [Google Scholar]
- 27.Ellinwood NM, Wang P, Skeen T, Sharp NJ, Cesta M, Decker S, et al. A model of mucopolysaccharidosis IIIB (Sanfilippo syndrome type IIIB): N-acetyl-alpha-D-glucosaminidase deficiency in Schipperke dogs. J Inherit Metab Dis. 2003;26:489–504. doi: 10.1023/a:1025177411938. [DOI] [PubMed] [Google Scholar]
- 28.Li HH, Yu WH, Rozengurt N, Zhao HZ, Lyons KM, Anagnostaras S, et al. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding alpha-N-acetylglucosaminidase. Proc Natl Acad Sci USA. 1999;96:14505–10. doi: 10.1073/pnas.96.25.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friderici K, Cavanagh KT, Leipprandt JR, Traviss CE, Anson DS, Hopwood JJ, et al. Cloning and sequence analysis of caprine N-acetylglucosamine 6-sulfatase cDNA. Biochim Biophys Acta. 1995;1271:369–73. doi: 10.1016/0925-4439(95)00054-8. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JN, Jones MZ, Dawson G, Huffman PS. N-acetylglucosamine 6-sulphatase deficiency in a Nubian goat: a model of Sanfilippo syndrome type D (mucopolysaccharidosis IIID) J Inherit Metab Dis. 1992;15:760–8. doi: 10.1007/BF01800018. [DOI] [PubMed] [Google Scholar]
- 31.Tomatsu S, Gutierrez M, Nishioka T, Yamada M, Tosaka Y, Grubb JH, et al. Development of MPS IVA mouse (Galnstm (hC79S.mC76S)slu) tolerant to human N-acetylgalacto-samine6-sulfate sulfatase. Hum Mol Genet. 2005;14:3321–35. doi: 10.1093/hmg/ddi364. [DOI] [PubMed] [Google Scholar]
- 32.Jezyk PF, Haskins ME, Patterson DF, Mellman WJ, Greenstein M. Mucopolysaccharidosis in a cat with arylsulfatase B deficiency: a model of Maroteaux-Lamy syndrome. Science. 1977;198:834–6. doi: 10.1126/science.144321. [DOI] [PubMed] [Google Scholar]
- 33.Haskins ME, Jezyk PF, Patterson DF. Mucopolysaccharide storage disease in three families of cats with arylsulfatase B deficiency: leukocyte studies and carrier identification. Pediatr Res. 1979;13:1203–10. doi: 10.1203/00006450-197911000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Yogalingam G, Crawley A, Hopwood JJ, Anson DS. Evaluation of fibroblast-mediated gene therapy in a feline model of mucopolysaccharidosis type VI. Biochim Biophys Acta. 1999;1453:284–96. doi: 10.1016/s0925-4439(98)00112-4. [DOI] [PubMed] [Google Scholar]
- 35.Neer TM, Dial SM, Pechman R, Wang P, Giger U. Mucopolysaccharidosis VI (Maroteaux-Lamy Syndrome) in a miniature pinscher. J Vet Intern Med. 1992;6:124. doi: 10.1111/j.1939-1676.1995.tb03306.x. [DOI] [PubMed] [Google Scholar]
- 36.Neer TM, Dial SM, Pechman R, Wang P, Oliver JL, Giger U. Clinical vignette. Mucopolysaccharidosis VI in a miniature pinscher. J Vet Intern Med. 1995;9:429–33. doi: 10.1111/j.1939-1676.1995.tb03306.x. [DOI] [PubMed] [Google Scholar]
- 37.Berman L, Foureman P, Stieger K, van Hoeven M, Ellinwood NM, Henthorn PS, et al. Mucopolysaccharidosis type VI caused by a point mutation in the miniature schnauzer. Proceedings of the 2nd International Conference: Advances in Canine and Feline Genomics; Utrecht, The Netherlands. 14–16 October 2004. [Google Scholar]
- 38.Yoshida M, Ikadai H, Maekawa A, Takahashi M, Nagase S. Pathological characteristics of mucopolysaccharidosis VI in the rat. J Comp Pathol. 1993;109:141–53. doi: 10.1016/s0021-9975(08)80258-7. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Noguchi J, Ikadai H, Takahashi M, Nagase S. Arylsulfatase B-deficient mucopolysaccharidosis in rats. J Clin Invest. 1993;91:1099–104. doi: 10.1172/JCI116268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida M, Tachibana M, Kobayashi E, Ikadai H, Kunieda T. The locus responsible for mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) is located on rat chromosome 2. Genomics. 1994;20:145–6. doi: 10.1006/geno.1994.1147. [DOI] [PubMed] [Google Scholar]
- 41.Evers M, Saftig P, Schmidt P, Hafner A, McLoghlin DB, Schmahl W, et al. Targeted disruption of the arylsulfatase B gene results in mice resembling the phenotype of mucopolysaccharidosis VI. Proc Natl Acad Sci USA. 1996;93:8214–9. doi: 10.1073/pnas.93.16.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–4. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Ray J, Bouvet A, DeSanto C, Fyfe JC, Xu D, Wolfe JH, et al. Cloning of the canine beta-glucuronidase cDNA, mutation identification in canine MPS VII, and retroviral vector-mediated correction of MPS VII cells. Genomics. 1998;48:248–53. doi: 10.1006/geno.1997.5189. [DOI] [PubMed] [Google Scholar]
- 44.Birkenmeier EH, Davisson MT, Beamer WG, Ganschow RE, Vogler CA, Gwynn B, et al. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989;83:1258–66. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sands MS, Birkenmeier EH. A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci USA. 1993;90:6567–71. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fyfe JC, Kurzhals RL, Lassaline ME, Henthorn PS, Alur PR, Wang P, et al. Molecular basis of feline beta-glucuronidase deficiency: an animal model of mucopolysaccharidosis VII. Genomics. 1999;58:121–8. doi: 10.1006/geno.1999.5825. [DOI] [PubMed] [Google Scholar]
- 47.Gitzelmann R, Bosshard NU, Superti-Furga A, Spycher MA, Briner J, Wiesmann U, et al. Feline mucopolysaccharidosis VII due to beta-glucuronidase deficiency. Vet Pathol. 1994;31:435–43. doi: 10.1177/030098589403100405. [DOI] [PubMed] [Google Scholar]
- 48.Kolodny EH, Zeng B-J, Viner T, Torres PA, Wang Z-H, Raghavan SS. Spontaneous appearance of Tay-Sachs disease in American flamingos. Neurology. 2006;66(Suppl 2):A274. doi: 10.1016/j.ymgme.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Ellinwood NM, Vite CH, Haskins ME. Gene therapy for lysosomal storage diseases: the lessons and promise of animal models. J Gene Med. 2004;6:481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- 50.Simonaro CM, Haskins ME, Schuchman EH. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: a possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab Invest. 2001;81:1319–28. doi: 10.1038/labinvest.3780345. [DOI] [PubMed] [Google Scholar]
- 51.Simonaro CM, D’Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–7. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 52.Fratantoni JC, Hall CW, Neufeld EF. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science. 1968;162:570–2. doi: 10.1126/science.162.3853.570. [DOI] [PubMed] [Google Scholar]
- 53.Natowicz MR, Chi MM, Lowry OH, Sly WS. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci USA. 1979;76:4322–6. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sands MS, Vogler C, Kyle JW, Grubb JH, Levy B, Galvin N, et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest. 1994;93:2324–31. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS. Enzyme replacement with recombinant beta-glucuronidase in murine mucopolysaccharidosis type VII: impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth, and survival. Pediatr Res. 1996;39:1050–4. doi: 10.1203/00006450-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Vogler C, Levy B, Galvin NJ, Thorpe C, Sands MS, Barker JE, et al. Enzyme replacement in murine mucopolysaccharidosis type VII: neuronal and glial response to beta-glucuronidase requires early initiation of enzyme replacement therapy. Pediatr Res. 1999;45:838–44. doi: 10.1203/00006450-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Sands MS, Vogler C, Torrey A, Levy B, Gwynn B, Grubb J, et al. Murine mucopolysaccharidosis type VII: long term therapeutic effects of enzyme replacement and enzyme replacement followed by bone marrow transplantation. J Clin Invest. 1997;99:1596–605. doi: 10.1172/JCI119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogler C, Sands M, Higgins A, Levy B, Grubb J, Birkenmeier EH, et al. Enzyme replacement with recombinant beta-glucuronidase in the newborn mucopolysaccharidosis type VII mouse. Pediatr Res. 1993;34:837–40. doi: 10.1203/00006450-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 59.Kakkis ED, McEntee MF, Schmidtchen A, Neufeld EF, Ward DA, Gompf RE, et al. Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. Biochem Mol Med. 1996;58:156–67. doi: 10.1006/bmme.1996.0044. [DOI] [PubMed] [Google Scholar]
- 60.Kakkis ED, Schuchman E, He X, Wan Q, Kania S, Wiemelt S, et al. Enzyme replacement therapy in feline mucopolysaccharidosis I. Mol Genet Metab. 2001;72:199–208. doi: 10.1006/mgme.2000.3140. [DOI] [PubMed] [Google Scholar]
- 61.Byers S, Crawley AC, Brumfield LK, Nuttall JD, Hopwood JJ. Enzyme replacement therapy in a feline model of MPS VI: modification of enzyme structure and dose frequency. Pediatr Res. 2000;47:743–9. doi: 10.1203/00006450-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Byers S, Nuttall JD, Crawley AC, Hopwood JJ, Smith K, Fazzalari NL. Effect of enzyme replacement therapy on bone formation in a feline model of mucopolysaccharidosis type VI. Bone. 1997;21:425–31. doi: 10.1016/s8756-3282(97)00175-0. [DOI] [PubMed] [Google Scholar]
- 63.Auclair D, Hopwood JJ, Brooks DA, Lemontt JF, Crawley AC. Replacement therapy in mucopolysaccharidosis type VI: advantages of early onset of therapy. Mol Genet Metab. 2003;78:163–74. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 64.Bielicki J, Crawley AC, Davey RC, Varnai JC, Hopwood JJ. Advantages of using same species enzyme for replacement therapy in a feline model of mucopolysaccharidosis type VI. J Biol Chem. 1999;274:36335–43. doi: 10.1074/jbc.274.51.36335. [DOI] [PubMed] [Google Scholar]
- 65.Crawley AC, Brooks DA, Muller VJ, Petersen BA, Isaac EL, Bielicki J, et al. Enzyme replacement therapy in a feline model of Maroteaux-Lamy syndrome. J Clin Invest. 1996;97:1864–73. doi: 10.1172/JCI118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harmatz P, Ketteridge D, Giugliani R, Guffon N, Teles EL, Miranda MC, et al. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetyl-galactosamine 4-sulfatase. Pediatrics. 2005;115:e681–9. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- 67.Wraith JE. The first 5 years of clinical experience with laronidase enzyme replacement therapy for mucopolysaccharidosis I. Expert Opin Pharmacother. 2005;6:489–506. doi: 10.1517/14656566.6.3.489. [DOI] [PubMed] [Google Scholar]
- 68.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase inmucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–73. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 69.Krivit W, Aubourg P, Shapiro E, Peters C. Bone marrow transplantation for globoid cell leukodystrophy, adreno-leukodystrophy, metachromatic leukodystrophy, and Hurler syndrome. Curr Opin Hematol. 1999;6:377–82. doi: 10.1097/00062752-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Brochstein JA. Bone marrow transplantation for genetic disorders. Oncology (Williston Park) 1992;6:51–8. [PubMed] [Google Scholar]
- 71.Haskins M, Abkowitz JL, Aguirre G, Casal M, Evans SM, Hasson C, et al. Bone marrow transplantation in animal models of lysosomal storage diseases. In: Ringden O, Hobbs JR, Steward ACG, editors. Correction of genetic disease by transplantation IV. COGENT Press; Middlesex, UK: 1977. pp. 1–11. [Google Scholar]
- 72.Haskins M. Bone marrow transplantation therapy for metabolic disease: animal models as predictors of success and in utero approaches. Bone Marrow Transplant. 1996;18(Suppl 3):S25–7. [PubMed] [Google Scholar]
- 73.Hoogerbrugge PM, Valerio D. Bone marrow transplantation and gene therapy for lysosomal storage diseases. Bone Marrow Transplant. 1998;21(Suppl 2):S34–6. [PubMed] [Google Scholar]
- 74.O’Marcaigh AS, Cowan MJ. Bone marrow transplantation for inherited diseases. Curr Opin Oncol. 1997;9:126–30. [PubMed] [Google Scholar]
- 75.Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, et al. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase) J Pediatr. 2004;144:581–8. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 76.Dial SM, Byrne T, Haskins M, Gasper PW, Rose B, Wenger DA, et al. Urine glycosaminoglycan concentrations in mucopolysaccharidosis VI-affected cats following bone marrow transplantation or leukocyte infusion. Clin Chim Acta. 1997;263:1–14. doi: 10.1016/s0009-8981(96)06501-1. [DOI] [PubMed] [Google Scholar]
- 77.Simonaro CM, Haskins ME, Kunieda T, Evans SM, Visser JW, Schuchman EH. Bone marrow transplantation in newborn rats with mucopolysaccharidosis type VI: biochemical, pathological, and clinical findings. Transplantation. 1997;63:1386–93. doi: 10.1097/00007890-199705270-00003. [DOI] [PubMed] [Google Scholar]
- 78.Gasper PW, Thrall MA, Wenger DA, Macy DW, Ham L, Dornsife RE, et al. Correction of feline arylsulphatase B deficiency (mucopolysaccharidosis VI) by bone marrow transplantation. Nature. 1984;312:467–9. doi: 10.1038/312467a0. [DOI] [PubMed] [Google Scholar]
- 79.Norrdin RW, Simske SJ, Gaarde S, Schwardt JD, Thrall MA. Bone changes in mucopolysaccharidosis VI in cats and the effects of bone marrow transplantation: mechanical testing of long bones. Bone. 1995;17:485–9. doi: 10.1016/8756-3282(95)00333-4. [DOI] [PubMed] [Google Scholar]
- 80.Turner AS, Norrdin RW, Gaarde S, Connally HE, Thrall MA. Bone mineral density in feline mucopolysaccharidosis VI measured using dual-energy X-ray absorptiometry. Calcif Tissue Int. 1995;57:191–5. doi: 10.1007/BF00310257. [DOI] [PubMed] [Google Scholar]
- 81.Birkenmeier EH, Barker JE, Vogler CA, Kyle JW, Sly WS, Gwynn B, et al. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991;78:3081–92. [PubMed] [Google Scholar]
- 82.Sands MS, Erway LC, Vogler C, Sly WS, Birkenmeier EH. Syngeneic bone marrow transplantation reduces the hearing loss associated with murine mucopolysaccharidosis type VII. Blood. 1995;86:2033–40. [PubMed] [Google Scholar]
- 83.Sands MS, Barker JE, Vogler C, Levy B, Gwynn B, Galvin N, et al. Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab Invest. 1993;68:676–86. [PubMed] [Google Scholar]
- 84.Sammarco C, Weil M, Just C, Weimelt S, Hasson C, O’Malley T, et al. Effects of bone marrow transplantation on the cardiovascular abnormalities in canine mucopolysaccharidosis VII. Bone Marrow Transplant. 2000;25:1289–97. doi: 10.1038/sj.bmt.1702448. [DOI] [PubMed] [Google Scholar]
- 85.Cavazzana-Calvo M, Hacein-Bey S, Yates F, de Villartay JP, Le Deist F, Fischer A. Gene therapy of severe combined immunodeficiencies. J Gene Med. 2001;3:201–6. doi: 10.1002/1521-2254(200105/06)3:3<201::AID-JGM195>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 86.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 87.Wang B, O’Malley TM, Xu L, Vite C, Wang P, O’Donnell PA, et al. Expression in blood cells may contribute to biochemical and pathological improvements after neonatal intravenous gene therapy for mucopolysaccharidosis VII in dogs. Mol Genet Metab. 2006;87:8–21. doi: 10.1016/j.ymgme.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 88.Sleeper MM, Fornasari B, Ellinwood NM, Weil MA, Melniczek J, O’Malley TM, et al. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110:815–20. doi: 10.1161/01.CIR.0000138747.82487.4B. [DOI] [PubMed] [Google Scholar]
- 89.Mango RL, Xu L, Sands MS, Vogler C, Seiler G, Schwarz T, et al. Neonatal retroviral vector-mediated hepatic gene therapy reduces bone, joint, and cartilage disease in mucopolysaccharidosis VII mice and dogs. Mol Genet Metab. 2004;82:4–19. doi: 10.1016/j.ymgme.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 90.Ponder KP, Melniczek JR, Xu L, Weil MA, O’Malley TM, O’Donnell PA, et al. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA. 2002;99:13102–7. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vite CH, Passini MA, Haskins ME, Wolfe JH. Adeno-associated virus vector-mediated transduction in the cat brain. Gene Ther. 2003;10:1874–81. doi: 10.1038/sj.gt.3302087. [DOI] [PubMed] [Google Scholar]
- 92.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–32. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vite CH, McGowan JC, Niogi SN, Passini MA, Drobatz KJ, Haskins ME, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol. 2005;57:355–64. doi: 10.1002/ana.20392. [DOI] [PubMed] [Google Scholar]
- 94.Pastores GM, Barnett NL. Substrate reduction therapy: miglustat as a remedy for symptomatic patients with Gaucher disease type 1. Expert Opin Investig Drugs. 2003;12:273–81. doi: 10.1517/13543784.12.2.273. [DOI] [PubMed] [Google Scholar]
- 95.Butters TD, Mellor HR, Narita K, Dwek RA, Platt FM. Small-molecule therapeutics for the treatment of glycolipid lysosomal storage disorders. Philos Trans R Soc Lond B Biol Sci. 2003;358:927–45. doi: 10.1098/rstb.2003.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d’Azzo A, et al. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain. 2003;126:974–87. doi: 10.1093/brain/awg089. [DOI] [PubMed] [Google Scholar]
- 97.Lachmann RH, Platt FM. Substrate reduction therapy for glycosphingolipid storage disorders. Expert Opin Investig Drugs. 2001;10:455–66. doi: 10.1517/13543784.10.3.455. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi T, Yamanaka T, Jacobs JM, Teixeira F, Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease) Brain Res. 1980;202:479–83. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 99.Luzi P, Rafi MA, Victoria T, Baskin GB, Wenger DA. Characterization of the rhesus monkey galactocerebrosidase (GALC) cDNA and gene and identification of the mutation causing globoid cell leukodystrophy (Krabbe disease) in this primate. Genomics. 1997;42:319–24. doi: 10.1006/geno.1997.4744. [DOI] [PubMed] [Google Scholar]