Abstract
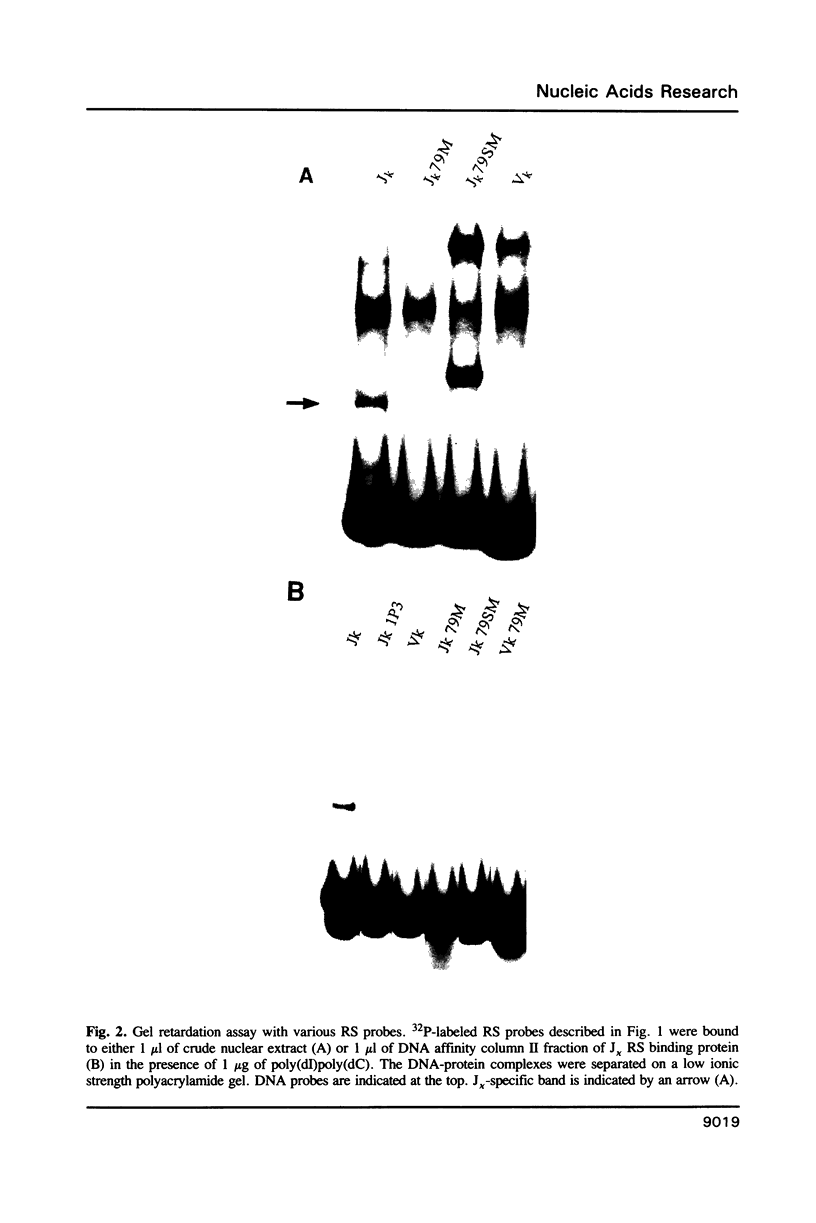
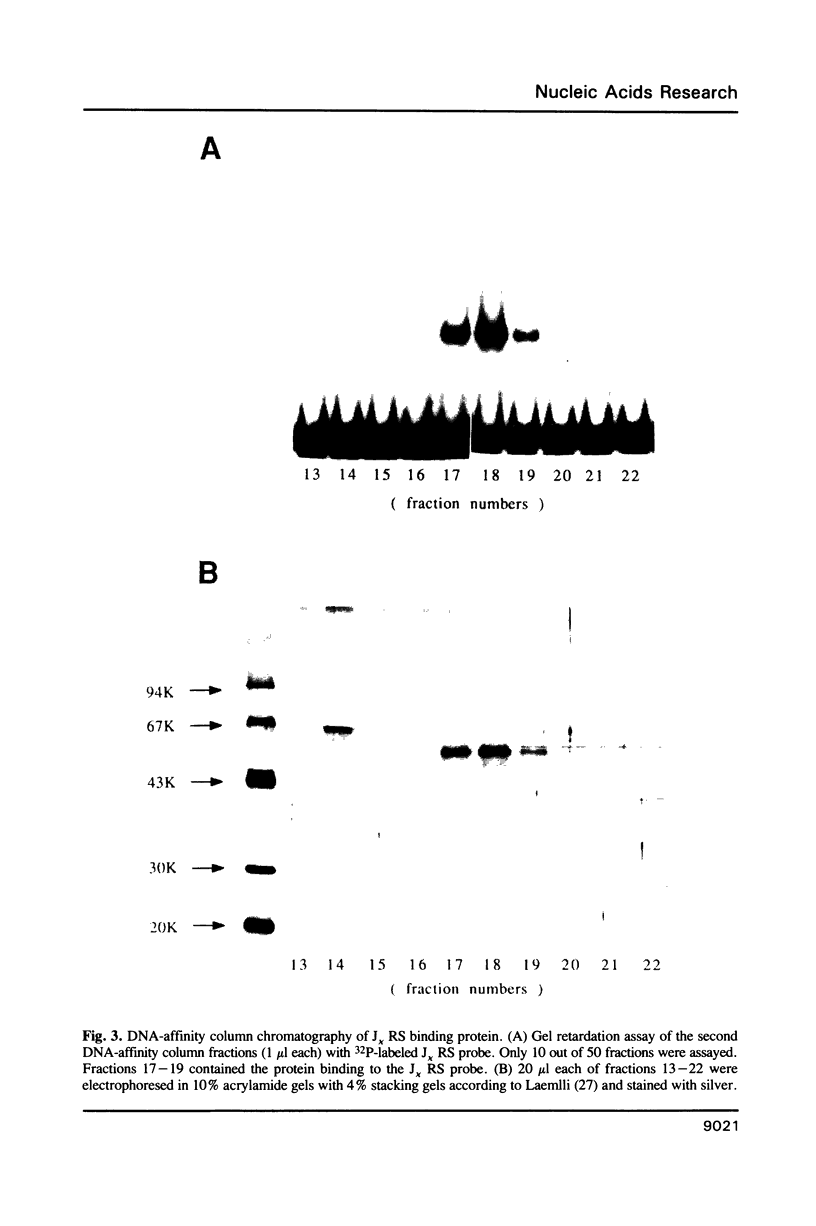
A protein that binds to the recombination signal sequence (RS) of the immunoglobulin J kappa segment was purified almost to homogeneity from the nuclear extract of a murine pre-B cell line 38B9. A similar binding protein was found in lymphoid cell lines but not in non-lymphoid cell lines. The binding activity was associated with a polypeptide with a molecular weight of 60,000. DNase I footprinting analysis demonstrated that this binding protein interacted with the heptamer and several 3' bases close to the heptamer. The Kd value of the J kappa RS binding protein to the J kappa RS was 1 nM. One base substitution in the heptamer of the J kappa RS greatly reduced the affinity of the J kappa RS binding protein. The high specificity of the binding site of the J kappa RS binding protein suggests that this protein may be involved in V-J recombination.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera R. J., Akira S., Okazaki K., Sakano H. A pre-B cell nuclear protein that specifically interacts with the immunoglobulin V-J recombination sequences. Cell. 1987 Dec 24;51(6):909–917. doi: 10.1016/0092-8674(87)90578-2. [DOI] [PubMed] [Google Scholar]
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Alt F. W. Site-specific recombination between immunoglobulin D and JH segments that were introduced into the genome of a murine pre-B cell line. Cell. 1984 May;37(1):105–112. doi: 10.1016/0092-8674(84)90305-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Ferrier P., Covey L. R., Suh H., Winoto A., Hood L., Alt F. W. T cell receptor DJ but not VDJ rearrangement within a recombination substrate introduced into a pre-B cell line. Int Immunol. 1989;1(1):66–74. doi: 10.1093/intimm/1.1.66. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Desiderio S. V. Identification of a DNA binding protein that recognizes the nonamer recombinational signal sequence of immunoglobulin genes. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7019–7023. doi: 10.1073/pnas.84.20.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Honjo T., Habu S. Origin of immune diversity: genetic variation and selection. Annu Rev Biochem. 1985;54:803–830. doi: 10.1146/annurev.bi.54.070185.004103. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. DNA elements are asymmetrically joined during the site-specific recombination of kappa immunoglobulin genes. Science. 1985 May 10;228(4700):677–685. doi: 10.1126/science.3158075. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gifford A., Baltimore D. Joining of V kappa to J kappa gene segments in a retroviral vector introduced into lymphoid cells. 1984 Mar 29-Apr 4Nature. 308(5958):425–428. doi: 10.1038/308425a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pelkonen J., Sideras P., Rammensee H. G., Karjalainen K., Palacios R. Thymocyte clones from 14-day mouse embryos. I. State of T cell receptor genes, surface markers, and growth requirements. J Exp Med. 1987 Nov 1;166(5):1245–1258. doi: 10.1084/jem.166.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]