Background: γH2AX (H2AX S139P) is one of the most upstream and important components of the DDR.
Results: Intercellular contact increases H2AX by activating the γ-catenin pathway.
Conclusion: Cellular microenvironment might determine cellular commitment to apoptosis.
Significance: Regulation of the DDR pathway by the cadherin-catenin complex is innovative.
Keywords: Cadherins, Catenin, Checkpoint Control, DNA Damage, p53
Abstract
Deregulation of the DNA damage response (DDR) pathway could compromise genomic integrity in normal cells and reduce cancer cell sensitivity to anticancer treatments. We found that intercellular contact stabilizes histone H2AX and γH2AX (H2AX phosphorylated on Ser-139) by up-regulating N/E-cadherin and γ-catenin. γ-catenin and its DNA-binding partner LEF-1 indirectly increase levels of H2AX by suppressing the promoter of the RNF8 ubiquitin ligase, which decreases levels of H2AX protein under conditions of low intercellular contact. Hyperphosphorylation of DDR proteins is induced by up-regulated H2AX. Constitutive apoptosis is caused in confluent cells but is not further induced by DNA damage. This is conceivably due to insufficient p53 activation because ChIP assay shows that its DNA binding ability is not induced in those cells. Together, our results illustrate a novel mechanism of the regulation of DDR proteins by the cadherin-catenin pathway.
Introduction
Accumulation of mutations in the genome causes cancer development and progression. Genetic mutations can result in the activation of oncogenes or inactivation of tumor suppressors and also change the levels or functions of “modifier” proteins that determine chemosensitivity or tumor progression, thus potentially increasing an organism's susceptibility to cancer and a myriad of other diseases (1, 2). To defend cells against the deleterious effects of DNA double strand breaks (DSBs),2 an intricate DNA damage response (DDR) pathway is employed to detect DNA lesions, arrest the cell cycle until damaged DNA is repaired, and induce cell death if cells receive overwhelming DNA damage (3–5). Therefore, appropriate activation of the DDR machinery after DNA damage is crucial in cellular defenses against malignant transformation. In addition, anticancer treatments, such as ionizing radiation and chemotherapeutic reagents (e.g. neocarzinostatin (NCS) and doxorubicin), induce DSBs and consequently activate DDR-induced cell death. Thus, in addition to guarding against DNA damage-induced malignant progression, the DDR machinery also determines cancer cells' sensitivity to anticancer therapy.
One of the most important proteins in the DDR pathway is histone H2AX (6–8). Results from H2AX knock-out studies in mice indicate that the loss of one or two alleles of the H2AX gene compromises genomic integrity and DDR efficiency and increases tumor formation in a p53-null background (3, 9, 11, 12). Furthermore, H2AX phosphorylation patterns have been implicated to determine whether cells repair the damaged DNA to survive or undergo apoptosis (13). In response to DNA DSBs, ATM and/or DNA-PK phosphorylate histone H2AX at Ser-139 to form the phospho-H2AX moieties known as γH2AX (14, 15). Formation of γH2AX foci on DSB sites is the earliest event and the critical event in the DDR pathway (16, 17, 18). H2AX not only serves to indicate the localization of DNA lesions (18); its phosphorylation and subsequent ubiquitylation by the RNF8 ubiquitin ligase are required for DNA damage signal amplification and the accumulation of numerous DDR proteins at the sites of DSBs to form the so-called ionizing radiation-induced foci (16, 19–23). A recent study showed that the generation of DSBs results in potent transcriptional repression that was dependent on ATM, RNF8, and RNF168 (24), indicating that in addition to its role in γH2AX ubiquitylation, RNF8 may also regulate transcriptional silencing in response to DNA damage.
Adherens junctions are formed by complex and dynamic interactions between two well characterized families of proteins: cadherins and catenins. Classical types of adherens complexes involve E (epithelial)-, N (neural)-, P (placental)-, VE (vascular endothelial)-, R (retinal)-, and K (kidney)-cadherins, as well as α-, β-, and γ-catenin (plakoglobin) (25–31). γ-Catenin is highly homologous to β-catenin (26, 27, 30). Upon stimulation, β- or γ-catenin accumulates in the nuclei, complexing with LEF-1/TCF transcription factors and transactivating LEF-T-cell factor target genes (25, 32, 33, 34). The roles of cadherins and catenins in regulating cell adhesion and Wnt signaling have been thoroughly characterized (29, 30). However, the functions of these families of proteins in regulating cell stress pathways are largely unknown.
In this study, we describe a novel mechanism of regulating H2AX protein levels through a pathway involving RNF8, N/E-cadherin, γ-catenin, and LEF-1. We found that increased intercellular contact reduces proteasomal degradation of histone H2AX and its phosphorylated form (γH2AX). We also show that the RNF8 ubiquitin ligase down-regulates H2AX under conditions of low intercellular contact. Furthermore, we show that intercellular contact stabilizes H2AX by suppressing RNF8 expression through the N/E-cadherin·γ-catenin·LEF-1 pathway. We provide evidence that through stabilized H2AX, intercellular contact creates a state of DDR hyperphosphorylation, increases basal cell death, and increases cellular resistance to DSB-induced cell death. We demonstrate that the reduction in DSB-induced cell death occurs not through a reduction in DSBs but through a reduction in p53-mediated gene expression when confluent cell culture is exposed to DNA damage stimuli. Our results suggest that intercellular contact might induce malignant transformation or resistance to anticancer treatments by promoting H2AX-mediated deregulation of the DDR pathway and the accumulation of cells with abrogation in p53-mediated apoptosis.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
U2OS, HBL100, HeLa, and HCT116, BJ cells, and mouse embryonic fibroblasts were maintained in Dulbecco's modified Eagle's medium (Invitrogen), supplemented with 10% fetal bovine serum (Invitrogen) or Cosmic calf serum (HyClone). HCC-1937 and SNU-251 cells were maintained in RPMI (Invitrogen) supplemented with 10% Cosmic calf serum. To induce DSBs, cells were treated with 0.4–1 μg/ml neocarzinostatin (Kayaku, Tokyo, Japan) or 8.5 μm doxorubicin (Sigma). 2–20 μg/ml cycloheximide (Sigma) was used to inhibit protein synthesis, whereas 0.01–0.5 μm MG132 (Calbiochem) was used to inhibit proteasome-mediated degradation.
Analysis of Intercellular Contact
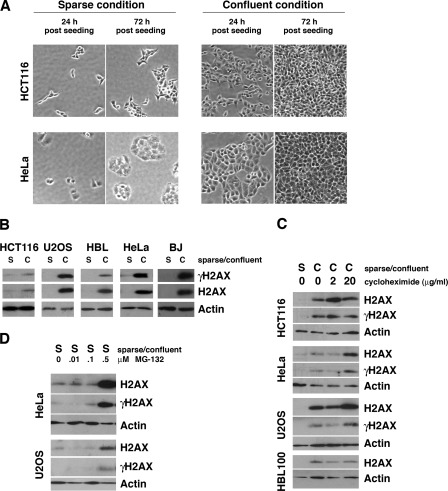
To analyze intercellular contact, cells were seeded at low and high density and cultured for 72 h (the optimal culture time to observe confluence-induced H2AX up-regulation). Confluent samples were 70–90% confluent 24 h postseeding and were 100% confluent at the time of harvest (72 h postseeding) (Fig. 1A, right). Sparse samples were 10–20% confluent 24 h postseeding and were 40–60% confluent at the time of harvest (72 h postseeding) (Fig. 1A, left). Cell culture medium was changed everyday to eliminate the effect of nutritional depletion and pH.
FIGURE 1.
Intercellular contact up-regulates levels of H2AX protein and γH2AX by reducing H2AX proteasomal degradation. A, images of sparse and confluent HeLa and HCT116 cells 24 h postseeding and at the time of harvest (72 h postseeding). B, levels of H2AX and γH2AX are significantly up-regulated by intercellular contact in the indicated cell lines. C, cycloheximide (2 or 20 μg/ml) does not abrogate the elevated levels of H2AX and γH2AX induced by intercellular contact. D, MG132 (0.5 μm) significantly increases H2AX and γH2AX levels in sparse cell culture.
Plasmids and Transfection
Plasmids expressing shRNA for RNF8, N-cadherin, and γ-catenin were purchased from Sigma. pcDNA3-RNF8 was generated by PCR cloning from pOTB7-RNF8 (Invitrogen) into the EcoRI/XhoI sites of pcDNA3. pcDNA3-N-cadherin was obtained from Dr. Rachel Hazan (Mount Sinai School of Medicine). pSingle-tTs-shRNA vector (Clontech) was used to generate plasmids expressing tetracycline-inducible H2AX shRNA with the target sequence 5′-CTGGAATTCTGCAGCTAAC-3′ or 5′-CAACAAGAAGACGCGAATC-3′ according to the manufacturer's instructions. Briefly, shRNA-encoding oligonucleotides were synthesized, annealed, and cloned into XhoI/HindIII sites of the vector. To construct the luciferase reporter gene plasmids containing the full-length promoter region of the H2AX and RNF8 genes, PCR was performed using human genomic DNA from HCT116 cells as template, LA-Taq polymerase (Takara), and the following primers: 5′-TTCTCGAGGAATTACTCTTGCTTTCTTTTCTTT-3′ (H2AX forward), 5′-TTAAGCTTCATGCTAGCGAGGTAGACCGGTGAA-3′ (H2AX reverse), 5′-TTCTCGAGTTTTTTTTTTTTGTAGATACGGTGT-3′ (RNF8 forward), and 5′-TTAAGCTTTGCATCCCCTCAGCTTCCATATCTA-3′ (RNF8 reverse). PCR products were gel-purified and cloned into pGEM-T Easy vector (Promega). After screening of colonies by restriction digest analysis and sequencing, H2AX and RNF8 promoters were subcloned into the XhoI/HindIII sites of the luciferase reporter vector pGL3 (Promega). Transfections were performed with Fugene-6 or Fugene-HD (Roche Applied Science) according to the manufacturer's instructions.
Generation of Stable Cell Lines and Inducible Knockdown
To generate stable cell lines, cells were first transfected with plasmids expressing the desired cDNA or shRNA and a puromycin or neomycin resistance gene. 48–72 h after transfection, cells were reseeded at low density, and stable transfectants were positively selected using the appropriate drug. Neomycin (1000 μg/ml for U2OS, 500 μg/ml for HCT116, and 1500 μg/ml for HBL100) was used to generate cells stably expressing pSingle-tTs-H2AX-shRNA, pcDNA3-FLAG-H2AX, pcDNA3-RNF8, and pcDNA3-N-cadherin. Puromycin (3 μg/ml for HCT-116, 1 μg/ml for U2OS and HBL100) was used to generate cells stably expressing shRNAs of RNF8, N-cadherin, and γ-catenin. Stable transfectants were cultured in neomycin- or puromycin-containing medium until colonies become visible (after ∼1–2 weeks in culture); medium (and neomycin) was changed and refreshed every 72 h. Colonies were trypsinized and transferred to 96- or 48-well plates when they became visible, and both colonies and “pools” of stable transfectants (a pool of the colonies that were not picked) were cultured for an additional 1–2 weeks. Stable transfectants were subsequently screened for H2AX overexpression and knockdown through Western blot. Inducible H2AX knockdown was performed by culturing cells stably expressing pSingle-tTS-H2AX-shRNA in medium containing 2 μg/ml doxycycline (Sigma) for 24–48 h.
Immunoblot Analysis and Densitometry
The following primary antibodies were purchased for immunoblot analysis: for H2AX protein (77635, Genetex; NB 100-638, Novus), H2AX S139P (2577, Cell Signaling), ATM (2C1, Genetex), ATM S1981P (4526, Cell Signaling), β-actin (C-11, Santa Cruz Biotechnology, Inc.), RNF-8 (N-13, Santa Cruz Biotechnology, Inc.), DNA-PK (Ab-1, Calbiochem), DNA-PK S2056P (18192, Abcam), N-cadherin (610920, BD Transduction Laboratories), E-cadherin (610181, BD Transduction Laboratories), γ-catenin (610253, BD Transduction Laboratories), α-tubulin (5286, Santa Cruz Biotechnology, Inc.), HA (Y11, Santa Cruz Biotechnology, Inc.), p21/WAF (C-19, Santa Cruz Biotechnology, Inc.). Cell extracts were prepared in EBC buffer (50 mm HEPES, pH 7.6, 250 mm NaCl, 0.1% Nonidet P-40, 5 mm EDTA, pH 8.0, with mixed protease inhibitor; Sigma). The secondary antibodies (Jackson ImmunoResearch) were peroxidase-conjugated anti-mouse IgG (H+L), anti-rabbit IgG (H+L), or anti-goat IgG (H+L). Film was developed by ECL. Densitometry analysis was performed with the ImageJ software (National Institutes of Health) using standard procedures.
Cell Death Assays
Apoptotic cell death was quantified using the Annexin-V-FITC apoptosis detection kit (Calbiochem) and a FACSCalibur flow cytometer (BD Biosciences) according to the manufacturers' instructions.
Luciferase Assay
293T and U2OS cells were transiently transfected with pGL3 luciferase reporter plasmids containing full-length H2AX, RNF8 promoters, or TopFlash LEF-1-responsive reporter vector (a gift from Dr. T. Akagi, Osaka Bioscience Institute (Osaka, Japan)) plus an empty vector or plasmids expressing γ-catenin or LEF-1 using Fugene HD transfection reagent (Roche Applied Science). In all cases, pRL-TK vector (Renilla luciferase, Promega) was cotransfected to normalize the transfection efficiency. 24 h after transfection, cells were washed with PBS and treated with passive lysis buffer (Promega). The promoter activities of H2AX, RNF8, and TopFlash reporter with or without γ-catenin or LEF-1 co-expression were measured by a Dual-Luciferase reporter assay using a GloMax 20/20 luminometer (Promega) according to the manufacturer's procedure.
Immunofluorescence
Immunofluorescence analysis of γH2AX was performed using standard procedures. Briefly, U2OS cells were seeded in Lab-tekII sterile 8-chamber slides (Nalge Nunc International), fixed in ice-cold methanol, permeabilized with 0.1% Triton X-100, PBS, and blocked with 1% BSA, 0.5% FBS, 0.5% NHS. The primary antibody for γH2AX was purchased from Millipore (catalog no. 07-164). The secondary antibody was Rabbit anti-goat IgG-FITC from Santa Cruz Biotechnology, Inc. (catalog no. 2777). Nuclear staining was performed using TO-PRO-3 iodide (T3605, Invitrogen) according to the manufacturer's instructions. Cells were image using a Leica SP-2 confocal microscope.
Chromatin Immunoprecipitation
The ChIP assay was performed based on previous results (10). Briefly, one confluent 10-cm plate or 3–4 subconfluent (50–70%) 10-cm plates of U2OS cells were used to prepare samples for ChIP analysis. After medium was removed, cells were rinsed with PBS and fixed in 1% formaldehyde for 10 min at room temperature. After formaldehyde was quenched with 0.125 m glycine for 5 min at room temperature, cells were rinsed with PBS, scraped in PBS, and pelleted in test tubes. Cells were lysed in cold lysis buffer (10 mm Tris-HCl, pH 7.5, 1 mm EDTA, pH 8.0, 400 mm NaCl, 10% glycerol, 1% Nonidet P-40) on ice for 5 min and centrifuged at 12,000 rpm for 5 min, and the pellet was resuspended in lysis buffer. Sonication was performed using a Branson sonicator at 42% output for 8 min (pulsed, 30 s on/off). Sonicated samples were centrifuged at 12,000 rpm for 10 min, and the supernatant was diluted 1:1 in dilution buffer (10 mm Tris-HCl, pH 7.5, 1 mm EDTA, pH 8.0, 10% glycerol). A small fraction of chromatin supernatant was saved as “input,” and the rest was incubated with p53 antibodies (DO-1 + Ab-1 + Ab-3) overnight at 4 °C. p53-bound chromatin was pulled down using protein A-Sepharose (Sigma) for 2 h at 4 °C. Beads were washed 4 times in 1:1 lysis buffer/dilution buffer. p53-bound chromatin was eluted by incubating beads with elution buffer (50 mm Tris, pH 8.0, 1% SDS, 10 mm EDTA, pH 8.0) at 65 °C (2 × 75-μl elutions). Pooled eluates and input were reverse-cross-linked at 65 °C for 4 h, and DNA was purified using the PCR purification kit from Qiagen. ChIP samples were analyzed using PCR under standard conditions with primers flanking the p53 response elements on the p21 promoter with the following sequences: 5′-CTGGACTGGCACTCTTGTC-3′ (forward) and 5′-CTCCTACCATCCCCTTCCTC-3′ (reverse).
RESULTS
Intercellular Contact Up-regulates both Protein Levels and Phosphorylation of Histone H2AX
We initiated studies of whether intercellular contact regulates the DDR pathway by analyzing protein levels and phosphorylation of histone H2AX, which has been implicated in the DDR pathway (6–8, 16–18, 23). Conditions of low and high intercellular contact were generated by culturing cells under “sparse” or “confluent” conditions for 72 h. To generate the sparse condition, cell lines are seeded at a low density, so that they will not become confluent within 72 h. On the other hand, to generate the confluent condition, cell lines are seeded at a higher density (see “Experimental Procedures” and Fig. 1A), so that they become 90% confluent within 24 h of seeding and continue to grow under conditions of high intercellular contact for 72 h
Five different human cell lines (HCT116 human colorectal cancer, U2OS human osteosarcoma, HBL100 human mammary epithelial cells, HeLa human cervical cancer, and BJ human normal fibroblasts) were cultured under sparse and confluent conditions for 72 h. Immunoblot analysis revealed that levels of H2AX protein and the Ser-139-phosphorylated form of H2AX (γH2AX) were significantly increased when cells were cultured under confluent conditions for 72 h (Fig. 1B), indicating that intercellular contact regulates the levels and phosphorylation of histone H2AX.
To determine whether the up-regulation of H2AX and γH2AX by intercellular contact occurs by increased protein synthesis, confluent cells were treated with a protein synthesis inhibitor, cycloheximide, at two different concentrations at 24 h postseeding and harvested at 72 h postseeding. Immunoblot and densitometric analysis (Fig. 1C and supplemental Fig. 1) demonstrated that cycloheximide rather slightly increased levels of H2AX in four different cell lines, indicating that the elevated levels of H2AX in confluent cells are not due to increased protein synthesis.
We next studied whether lower levels of H2AX in sparse conditions are due to increased protein degradation. When cells maintained at sparse density were treated with a proteasome inhibitor (MG132), levels of H2AX and γH2AX were increased at 0.5 μm MG132 (Fig. 1D and supplemental Fig. 2). These results indicate that the lower levels of H2AX in sparse cell cultures are due to increased proteasome-dependent protein degradation and suggest that intercellular contact increases levels of H2AX by reducing its turnover.
Intercellular Contact Stabilizes H2AX by Inhibiting RNF8-mediated H2AX Turnover
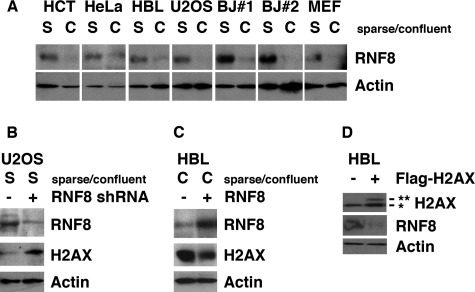
We studied whether RNF8 is involved in regulating levels of H2AX in the context of intercellular contact. In all of the seven cell lines examined, levels of RNF8 were decreased when cells were confluent (Fig. 2A). When endogenous RNF8 was knocked down by shRNA in sparse cell cultures, levels of H2AX were increased (Fig. 2B). On the other hand, when RNF8 was exogenously expressed in confluent cells, endogenous H2AX was decreased (Fig. 2C). These results strongly suggest that the decreased levels of RNF8 in confluent cells cause the increase in H2AX levels. Interestingly, levels of RNF8 were decreased when FLAG-H2AX was stably expressed in HBL100 and U2OS cells (Fig. 2D) (data not shown), suggesting that the two proteins regulate each other through a negative feedback loop. Taken together with the previous results, it is suggested that the level of H2AX is regulated by RNF8, whose expression is negatively regulated by intercellular contact.
FIGURE 2.
Intercellular contact stabilizes H2AX by inhibiting RNF8-mediated H2AX turnover. A, levels of RNF8 are significantly down-regulated by intercellular contact. HCT, HCT116 cells; HBL, HBL100 cells; BJ#1 and BJ#2, subclones of BJ human fibroblasts; MEF, mouse embryonic fibroblasts. B, stable knockdown of RNF8 increases levels of H2AX in sparse U2OS cells. C, stable overexpression of RNF8 decreases levels of H2AX in confluent HBL100 cells. D, stable overexpression of FLAG-H2AX reduces RNF8 levels, indicating the presence of a feedback loop. **, FLAG-H2AX; *, endogenous H2AX.
Intercellular Contact Stabilizes Histone H2AX through Cadherin·Catenin·LEF-1-mediated Suppression of RNF8 Gene Expression
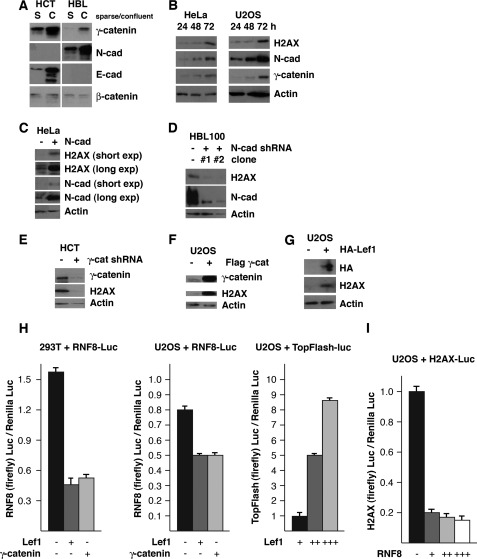
It has been well illustrated that the cadherin-catenin pathway plays crucial roles in intercellular contact (16, 29). We explored whether this pathway regulates the levels of H2AX and RNF8. The HCT116 human colorectal cancer cell line does not express detectable levels of N-cadherin; however, we found that levels of E-cadherin increase when these cells become confluent. In HBL100 human mammary epithelial cell line, we found that levels of N-cadherin increase when these cells become confluent. Of note, we found that HBL100, HeLa, U2OS, 293T (human embryonic kidney cell line), BJ (human fibroblasts), and mouse embryonic fibroblasts express N-cadherin but not E-cadherin, which is increased when cells become confluent (data not shown) (Fig. 3B). γ-Catenin, but not β-catenin, is also increased by intercellular contact (Fig. 3, A and B).
FIGURE 3.
Intercellular contact stabilizes H2AX by suppressing RNF8 expression through the cadherin·γ-catenin·LEF-1 pathway. A, intercellular contact up-regulates γ-catenin and N/E-cadherin but does not significantly affect levels of β-catenin. B, N-cadherin and γ-catenin are up-regulated as cells become confluent. C, stable overexpression of N-cadherin increases levels of H2AX protein in sparse cells. D, stable knockdown of N-cadherin decreases levels of H2AX protein. E, stable knockdown of γ-catenin by shRNA decreases levels of H2AX protein in confluent cells. F and G, transient overexpression of FLAG-γ-catenin (F) or HA-LEF-1 (G) increases levels of H2AX protein. H and I, suppression of the RNF8 promoter by LEF-1 and γ-catenin (H) and the H2AX promoter by RNF8 (I), as determined by luciferase assay. S, sparse; C, confluent; HCT, HCT116 cells.
To test whether the up-regulation of N-cadherin and γ-catenin by cell contact may function upstream of H2AX, cells were cultured under confluent conditions for 24, 48, and 72 h. We found that levels of γ-catenin and N-cadherin was increased within 48 h of culture, whereas the increase in the levels of H2AX did not become apparent until 72 h, suggesting that N-cadherin and γ-catenin may be upstream regulators of H2AX.
Next, we further examined how increased levels of cadherin or γ-catenin regulate H2AX. Although levels of N-cadherin were low in sparse HeLa cell culture, we found that the cells transfected with N-cadherin expressed high levels of H2AX in similar conditions (Fig. 3C). In line with these results, when N-cadherin was stably reduced with shRNA in HBL100 cells, levels of H2AX were concomitantly reduced (Fig. 3D).
Regulation of the levels of H2AX by γ-catenin was studied by shRNA-mediated knockdown or overexpression of γ-catenin. We found that knockdown of γ-catenin by shRNA in confluent HCT116 cells reduced the levels of H2AX (Fig. 3E), whereas exogenous expression of FLAG-γ-catenin increased the levels of H2AX in sparse cells (Fig. 3F).
γ-Catenin contains a transactivation domain, and it can bind and enhance LEF-1-directed transcription when overexpressed (33, 34). When U2OS cells were transfected with HA-LEF-1, endogenous H2AX was increased (Fig. 3G). These results suggest that increased γ-catenin in confluent cells enhances LEF-1-dependent regulation of gene expression, resulting in elevated levels of H2AX.
Recent studies indicated that RNF8 is involved in transcriptional repression (33). We performed a reporter gene assay to study the regulation of the RNF8 and H2AX promoters. About 800 bp of the human RNF8 promoter or ∼4 kb of the human H2AX promoter was subcloned upstream of the luciferase gene (supplemental Fig. 3). The reporter gene assay indicated that the RNF8 promoter is suppressed by γ-catenin and LEF-1 and that the H2AX promoter was suppressed by RNF8 (Fig. 3, H and I). As a positive control, TopFlash reporter plasmid carrying LEF-1-responsive elements upstream of the luciferase gene was activated by LEF-1 in U2OS cells in a dose-dependent manner (Fig. 3H).
Intercellular Contact Increases Basal and DNA Damage-induced Phosphorylation of DDR Proteins
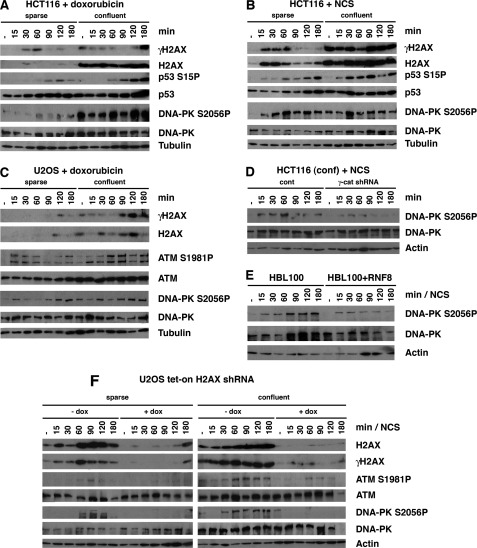
It has been well illustrated that γH2AX contributes to the assembly of various DDR proteins at the sites of DNA lesions, which is essential for the regulation of cell cycle checkpoints (6–8, 16, 20, 22, 23). We studied whether intercellular contact also affects the phosphorylation of DDR proteins. Sparse or confluent cultures of HCT116 and U2OS cells were treated with NCS (an ionizing radiation mimetic) or doxorubicin (a DNA intercalator), and the levels and phosphorylation of H2AX at Ser-139 (γH2AX), p53 at Ser-15, ATM at Ser-1981, and DNA-PK catolytic subunit at Ser-2056 were analyzed at 15, 30, 60, 90, 120, and 180 min after treatment by immunoblot followed by densitometric analysis.
We found that in HCT116 and U2OS cells, basal levels as well as NCS- and doxorubicin-induced levels of H2AX, γH2AX, ATM S1981P, and DNA-PK S2056P were significantly elevated in confluent cells (Fig. 4, A–C and supplemental Figs. 4–6). In addition, we found that NCS- and doxorubicin-induced levels of p53 S15P, but not its basal levels, were also increased in confluent cells (Fig. 4, A and B). These results suggest that intercellular contact generates a state of DDR hyperphosphorylation in the presence or absence of DNA damage.
FIGURE 4.
Intercellular contact increases the basal and DNA damage-induced phosphorylation levels of DDR proteins through H2AX. A–C, intercellular contact increases the basal and DNA damage-induced levels of H2AX, γH2AX, DNA-PK S2056P, and ATM S1981P and the NCS (0.5 μg/ml)-induced or doxorubicin (8.5 μm)-induced levels of p53 S15P. D, phosphorylation of DNA-PK at Ser-2056 is reduced by NCS (0.5 μg/ml) when γ-catenin knockdown HCT116 cells become confluent. E, phosphorylation of DNA-PK at Ser-2056 is weakly induced when sparse cultures of HBL100 cells expressing RNF8 are treated with NCS (0.5 μg/ml). F, inducible knockdown of H2AX in sparse and confluent cells stably expressing tet-on-H2AX-shRNA decrease the levels of ATM S1981P and DNA-PK S2056P. Doxocycline (dox) was added to the cell culture medium 24 h prior to the NCS (0.5 μg/ml) treatment.
Finally, we tested if H2AX is involved in the hyperphosphorylation of DDR proteins induced by intercellular contact by establishing U2OS cells expressing tetracycline-regulatable shRNA of H2AX in which endogenous H2AX is knocked down in the presence of doxycycline. We found that knockdown of H2AX in sparse and confluent U2OS cells significantly reduced NCS-induced levels of γH2AX, ATM S1981P, and DNA-PK S2056P. These results strongly support the notion that the elevated H2AX induced by intercellular contact is involved in the increased phosphorylation of DDR proteins.
Because γ-catenin positively regulates H2AX in our studies, we next studied whether γ-catenin is involved in the enhanced phosphorylation of DDR proteins in confluent conditions. We found that relative to control cells, the phosphorylation of DNA-PK at Ser-2056 was significantly weakened in HCT116 cells stably expressing γ-catenin shRNA cells (Fig. 4D). Because levels of H2AX were decreased when RNF8 is exogenously expressed, we studied whether phosphorylation of DNA-PK is inhibited in cells expressing exogenous RNF8. We found that, compared with the vector control cells, phosphorylation of DNA-PK at Ser-2056 in RNF8-expressing cells was significantly reduced (Fig. 4E). The results from the γ-catenin knockdown and RNF8 overexpression experiments strengthen the notion that H2AX is involved in the hyperphosphorylation of DDR proteins induced by intercellular contact.
Apoptosis Constitutively Induced by Intercellular Contact Is Not Further Enhanced by DNA Damage
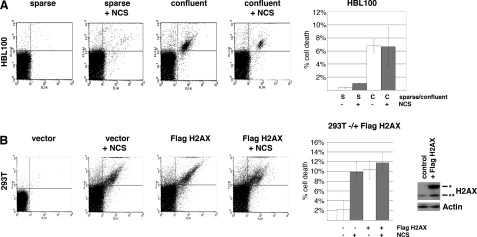
We explored whether the increased phosphorylation of DDR proteins mediated by intercellular contact-induced H2AX results in enhanced apoptosis when cells are under conditions of DNA damage. To test this, we quantified apoptosis in sparse and confluent cells using Annexin-V/propidium iodide staining followed by FACS analysis. We found that apoptosis was increased ∼3-fold when sparse HBL100 cells were treated with NCS (Fig. 5A). In confluent conditions, constitutive apoptosis was detected without NCS treatment (∼13.6-fold), but it was not further increased when cells were treated with NCS for 24 h.
FIGURE 5.
Intercellular contact increases basal cell death and confers resistance to DNA damage-induced cell death through H2AX. A, confluent HBL100 cells have much higher rates of basal cell death compared with sparse cells but display resistance to NCS (1 μg/ml)-induced cell death. B, sparse 293T cells expressing FLAG-H2AX have much higher rates of basal cell death compared with sparse control cells but display resistance to NCS (1 μg/ml)-induced cell death. Apoptosis was determined by Annexin-V-FITC/propidium iodide staining followed by FACS analyses. The significance of differences in the mean values was determined by the two-tailed unpaired Student's t test. p values of <0.05 were considered to be statistically significant. Error bars, S.D.
We tested whether overexpression of H2AX on its own can induce apoptosis without DNA damage in sparse conditions. Treatment of 293T cells with NCS for 24 h induced apoptosis about 5-fold. Transient expression of FLAG-H2AX induced apoptosis about 5-fold compared with the vector control. However, when cells transfected with FLAG-H2AX cells were treated with NCS for 24 h, only a slight increase in apoptosis was detected (Fig. 5B).
These results suggest that cells expressing increased levels of H2AX induced by intercellular contact undergo constitutive apoptosis but are insensitive to DNA-damaging agents.
Intercellular Contact Results in Inhibition of p53-mediated Gene Expression under Conditions of DNA Damage
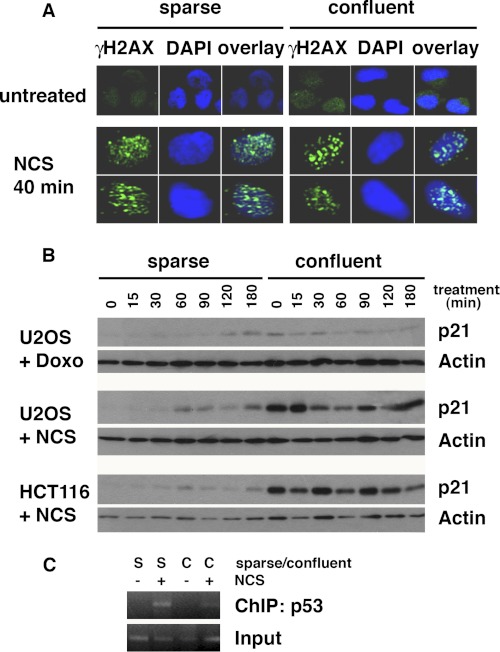
Nuclear dot signals of γH2AX are known to indicate the localization of DNA lesions (18). We studied whether intercellular contact generates endogenous DNA damage through γH2AX immunostaining in confluent U2OS cell cultures. Interestingly, in both sparse and confluent cells, γH2AX did not form dot patterns without NCS treatment, suggesting that DNA lesions are not induced by intercellular contact (Fig. 6A, top). In both sparse and confluent cell cultures, dot patterns appeared within 40 min after NCS, indicating that the mechanism to detect DNA lesions are similarly activated in sparse and confluent cells (Fig. 6A, bottom).
FIGURE 6.
Intercellular contact induces resistance to DNA damage-induced cell death by attenuating p53-mediated gene expression. A, γH2AX foci of sparse and confluent U2OS cells were studied by immunofluorescence analysis with NCS treatment (0.5 μg/ml, 40 min). B, intercellular contact increases basal levels of the p21 but abrogates DNA-damage-induced p21 up-regulation. U2OS or HCT116 cells were treated with doxorubicin (8.5 nm) or NCS (1 μg/ml), and samples were analyzed at the indicated time points. C, ChIP assay using the p21 promoter region containing the p53 response element indicates that DNA damage (NCS, 1 μg/ml) induces p53 binding to the p21 promoter in sparse but not in confluent U2OS cells. S, sparse cell culture; C, confluent cell culture.
Our results demonstrate that the basal and DNA damage-induced protein and phosphorylation levels of DDR proteins are higher in confluent cells than in sparse cells. However, DNA damage-induced apoptosis is not further enhanced by DNA damage in confluent cells. p53 is known to play a pivotal role in regulating apoptosis (35, 36). We used two cell lines, U2OS and HCT116, in which p53 is wild type, to study the activation of the p53 pathway in confluent cell cultures. Levels of p21, a well characterized target of p53, were induced after NCS or doxorubicin treatment in both cell types, when cell density was sparse (Fig. 6B). However, in confluent U2OS and HCT116 cells, basal levels of p21 were constitutively elevated but were not further induced after NCS treatment, suggesting that the p53 response to DNA damage is compromised by intercellular contact.
Of note, NCS-induced phosphorylation of p53 at Ser-15 is much higher in confluent cells than in sparse cells (Fig. 4, A and B). Therefore, we asked whether p53 is functionally activated in NCS-treated confluent cells. We performed ChIP assays to test p53 binding to the p53-binding element of the p21 promoter in sparse and confluent cells under conditions of DNA damage. p53 binding to the p21 promoter is significantly induced by NCS treatment in sparse but not in confluent conditions (Fig. 6C). These results are consistent with our p21 Western blot results, which demonstrate that treatment of confluent cells with NCS does not increase p21 expression. Taken together, these results demonstrate that intercellular contact perturbs the activation of the DNA damage checkpoint by DDR proteins.
DISCUSSION
The DDR pathway plays crucial roles in cellular defense against DSB-induced malignant transformation as well as the induction of cell death by anticancer treatments. The importance of histone H2AX and its phosphorylated form γH2AX in the DDR has been extensively studied. γH2AX proteins not only serve as markers of DSBs (30); they are also needed for the accumulation of repair proteins at DSB sites (8, 16, 20, 22, 23) and for determining whether the damaged cells survive or die (13). Although the regulation and significance of H2AX and γH2AX under conditions of DNA damage have been investigated, the roles of the protein under physiological conditions remain to be elucidated.
Our results point to a novel mechanism of H2AX regulation by which N/E-cadherin and γ-catenin, which have been implicated in intercellular contact, determine levels of H2AX. Previous studies have demonstrated that γ-catenin and its homolog, β-catenin, share dimerization partners and functions (25, 29, 33, 34, 37, 38). In particular, the ability of γ- and β-catenin to form a complex with the LEF-1/TCF family of transcription factors (33, 34) suggests that the increased γ-catenin in confluent cell cultures can directly or indirectly control levels of H2AX. Several studies have suggested that transactivation of the promoter by γ-catenin is indirectly caused by an increase in the levels of endogenous β-catenin (34, 39). However, a recent study proposed an alternative mechanism and showed that overexpression of γ-catenin induces transactivation in a β-catenin-null cell line (32). In our study, when cells became confluent, γ-catenin was up-regulated, whereas β-catenin was not (Fig. 3A). Thus, we posit that γ-catenin is directly involved in regulating levels of H2AX in confluent cell cultures, rather than activation of β-catenin. These results suggest that induction or repression of proteins under conditions of intercellular contact is also mediated by active γ-catenin·LEF-1 complex.
RNF8 has been shown to ubiquitinate γH2AX during the DDR (20, 22); however, the modification and its physiological roles remain to be detailed. We found that RNF8 induces diubiquitination of H2AX in sparse but not confluent cells (not shown), although the importance of this diubiquitination in regulating H2AX stability in sparse cell cultures remains to be investigated. Recently, RNF8 was shown to be involved in transcriptional repression (15). Consistently, our results indicate that RNF8 can suppress the H2AX promoter in the absence of DNA damage. We also found that the human RNF8 promoter that we used for the luciferase assay contains at least 15 binding sites for LEF-1 (supplemental Fig. 3), as predicted by the TRANSFAC program, and that both γ-catenin and LEF-1 suppressed the activity of the RNF8 promoter. Together, the results from our promoter studies suggest that intercellular contact and γ-catenin up-regulate H2AX by reducing RNF8 expression.
We show that DDR-associated proteins, such as H2AX, ATM, and DNA-PK, are highly phosphorylated by intercellular contact without DNA damage. Immunocytochemical analysis revealed no appreciable dot pattern of γH2AX in confluent cells without DNA damage (Fig. 6A), suggesting that although intercellular contact induces hyperphosphorylation of DDR proteins (Fig. 4, A–C), it does not generate DSBs. Bakkenist and Kastan (39) have demonstrated that chromatin-modifying conditions rapidly induce phosphorylated ATM that forms diffused nuclear localization patterns rather than dot patterns. Taken together with our results of diffused γH2AX localization in undamaged confluent cells (Fig. 6A), we postulate that intercellular contact may alter chromatin structure, leading to the induction of ATM phosphorylation at Ser-1981, DNA-PK at Ser-2056, and subsequently H2AX at Ser-139. Interestingly, when confluent cells were DNA-damaged, γH2AX started to form dot patterns, indicating that the mechanism to localize H2AX to the sites of DNA lesions was intact and competent in confluent cell cultures.
We show that, although DNA damage increases the phosphorylation of DDR proteins in confluent cultures, it does not induce apoptosis. We found that although p53 is highly phosphorylated on Ser-15 in confluent cells in response to DNA damage, its DNA binding ability is reduced, suggesting that the resistance of confluent cells to DNA damage-induced apoptosis is at least in part due to insufficient activation of p53-dependent apoptosis. Consistently, expression of p21, a major target of p53 that is widely used as a reporter of p53 transactivational activity, is not induced by NCS or doxorubicin in confluent cells (Fig. 6B). Interestingly, basal levels of p21 in confluent cells are much higher than in sparse cells (Fig. 6B, time 0). Because the DNA binding ability of p53 is not increased in undamaged confluent cells, we postulate that the increase in basal p21 levels occurs through p53-independent pathways. The mechanism of how intercellular contact suppresses p53 accessibility to its target sequences remains unclear. However, it is possible that increased intercellular contact generates a population of cells in which p53-dependent apoptosis is compromised.
These findings point to a novel mechanism of H2AX regulation by intercellular contact. The phosphorylated form of H2AX, γH2AX, has been shown to play a crucial role in recruiting DDR proteins to the sites of DNA lesions when cells are exposed to DNA stress (7, 8, 16, 20, 22, 23). The results described here show that intercellular contact activates the cadherin-catenin pathway, leading to a decrease in the levels of RNF8 and a subsequent increase in the levels of H2AX and γH2AX in the absence of DNA damage. Increased levels of H2AX and γH2AX in turn create a state of constitutive DDR hyperphosphorylation and apoptosis through an unknown mechanism. Our data support the notion that the cell microenvironment may determine cellular commitment to apoptosis when cells are exposed to chemotherapeutic agents.
Supplementary Material
Acknowledgments
We thank all of the members of the Ouchi laboratory for discussion.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA79892 and R01CA90631. This work is also supported by a Breast Cancer Research Grant from the Susan Komen Foundation (to T. O.).

This article contains supplemental Figs. 1–6.
- DSB
- double strand break
- DDR
- DNA damage response
- NCS
- neocarzinostatin
- ATM
- ataxia telangiectasia mutated
- DNA-PK
- DNA-dependent protein kinase.
REFERENCES
- 1. Hoeijmakers J. H. (2001) Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 [DOI] [PubMed] [Google Scholar]
- 2. Stucki M., Jackson S. P. (2006) γH2AX and MDC1. Anchoring the DNA damage-response machinery to broken chromosomes. DNA Repair 5, 534–543 [DOI] [PubMed] [Google Scholar]
- 3. Bassing C. H., Suh H., Ferguson D. O., Chua K. F., Manis J., Eckersdorff M., Gleason M., Bronson R., Lee C., Alt F. W. (2003) Histone H2AX. A dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114, 359–370 [DOI] [PubMed] [Google Scholar]
- 4. Ciccia A., Elledge S. J. (2010) The DNA damage response. Making it safe to play with knives. Mol. Cell 40, 179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khanna K. K., Jackson S. P. (2001) DNA double-strand breaks. Signaling, repair, and the cancer connection. Nat. Genet. 27, 247–254 [DOI] [PubMed] [Google Scholar]
- 6. Fernandez-Capetillo O., Chen H. T., Celeste A., Ward I., Romanienko P. J., Morales J. C., Naka K., Xia Z., Camerini-Otero R. D., Motoyama N., Carpenter P. B., Bonner W. M., Chen J., Nussenzweig A. (2002) DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4, 993–997 [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Capetillo O., Lee A., Nussenzweig M., Nussenzweig A. (2004) H2AX. The histone guardian of the genome. DNA Repair 3, 959–967 [DOI] [PubMed] [Google Scholar]
- 8. Stucki M., Clapperton J. A., Mohammad D., Yaffe M. B., Smerdon S. J., Jackson S. P. (2005) MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 9. Bassing C. H., Chua K. F., Sekiguchi J., Suh H., Whitlow S. R., Fleming J. C., Monroe B. C., Ciccone D. N., Yan C., Vlasakova K., Livingston D. M., Ferguson D. O., Scully R., Alt F. W. (2002) Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. U.S.A. 99, 8173–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang M. A., So E. Y., Ouchi T. (2011) Cell Death Dis., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celeste A., Petersen S., Romanienko P. J., Fernandez-Capetillo O., Chen H. T., Sedelnikova O. A., Reina-San-Martin B., Coppola V., Meffre E., Difilippantonio M. J., Redon C., Pilch D. R., Olaru A., Eckhaus M., Camerini-Otero R. D., Tessarollo L., Livak F., Manova K., Bonner W. M., Nussenzweig M. C., Nussenzweig A. (2002) Genomic instability in mice lacking histone H2AX. Science 296, 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Celeste A., Difilippantonio S., Difilippantonio M. J., Fernandez-Capetillo O., Pilch D. R., Sedelnikova O. A., Eckhaus M., Ried T., Bonner W. M., Nussenzweig A. (2003) H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell 114, 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cook P. J., Ju B. G., Telese F., Wang X., Glass C. K., Rosenfeld M. G. (2009) Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458, 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burma S., Chen B. P., Murphy M., Kurimasa A., Chen D. J. (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 [DOI] [PubMed] [Google Scholar]
- 15. Simcha I., Shtutman M., Salomon D., Zhurinsky J., Sadot E., Geiger B., Ben-Ze'ev A. (1998) Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin. J. Cell Biol. 141, 1433–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagafuchi A., Takeichi M., Tsukita S. (1991) The 102-kDa cadherin-associated protein. Similarity to vinculin and posttranscriptional regulation of expression. Cell 65, 849–857 [DOI] [PubMed] [Google Scholar]
- 17. Paull T. T., Rogakou E. P., Yamazaki V., Kirchgessner C. U., Gellert M., Bonner W. M. (2000) A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886–895 [DOI] [PubMed] [Google Scholar]
- 18. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 19. Celeste A., Fernandez-Capetillo O., Kruhlak M. J., Pilch D. R., Staudt D. W., Lee A., Bonner R. F., Bonner W. M., Nussenzweig A. (2003) Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell Biol. 5, 675–679 [DOI] [PubMed] [Google Scholar]
- 20. Huen M. S., Grant R., Manke I., Minn K., Yu X., Yaffe M. B., Chen J. (2007) RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huen M. S., Chen J. (2010) Assembly of checkpoint and repair machineries at DNA damage sites. Trends Biochem. Sci. 35, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mailand N., Bekker-Jensen S., Faustrup H., Melander F., Bartek J., Lukas C., Lukas J. (2007) RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131, 887–900 [DOI] [PubMed] [Google Scholar]
- 23. Stiff T., O'Driscoll M., Rief N., Iwabuchi K., Löbrich M., Jeggo P. A. (2004) ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 64, 2390–2396 [DOI] [PubMed] [Google Scholar]
- 24. Sadot E., Simcha I., Iwai K., Ciechanover A., Geiger B., Ben-Ze'ev A. (2000) Differential interaction of plakoglobin and β-catenin with the ubiquitin-proteasome system. Oncogene 19, 1992–2001 [DOI] [PubMed] [Google Scholar]
- 25. Behrens J., von Kries J. P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. (1996) Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382, 638–642 [DOI] [PubMed] [Google Scholar]
- 26. Butz S., Stappert J., Weissig H., Kemler R. (1992) Plakoglobin and β-catenin. Distinct but closely related. Science 257, 1142–1144 [DOI] [PubMed] [Google Scholar]
- 27. Cowin P., Kapprell H. P., Franke W. W., Tamkun J., Hynes R. O. (1986) Plakoglobin. A protein common to different kinds of intercellular adhering junctions. Cell 46, 1063–1073 [DOI] [PubMed] [Google Scholar]
- 28. Hirano S., Kimoto N., Shimoyama Y., Hirohashi S., Takeichi M. (1992) Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell 70, 293–301 [DOI] [PubMed] [Google Scholar]
- 29. Kemler R. (1993) From cadherins to catenins. Cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 9, 317–321 [DOI] [PubMed] [Google Scholar]
- 30. McCrea P. D., Turck C. W., Gumbiner B. (1991) A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254, 1359–1361 [DOI] [PubMed] [Google Scholar]
- 31. Meng W., Takeichi M. (2009) Adherens junction. Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 1, a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maeda O., Usami N., Kondo M., Takahashi M., Goto H., Shimokata K., Kusugami K., Sekido Y. (2004) Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene 23, 964–972 [DOI] [PubMed] [Google Scholar]
- 33. Shanbhag N. M., Rafalska-Metcalf I. U., Balane-Bolivar C., Janicki S. M., Greenberg R. A. (2010) ATM-dependent chromatin changes silence transcription in cis to DNA double strand breaks. Cell 141, 970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou B. B., Elledge S. J. (2000) The DNA damage response. Putting checkpoints in perspective. Nature 408, 433–439 [DOI] [PubMed] [Google Scholar]
- 35. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 36. Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362, 847–849 [DOI] [PubMed] [Google Scholar]
- 37. Rogakou E. P., Boon C., Redon C., Bonner W. M. (1999) Megabase chromatin domains involved in DNA double strand breaks in vivo. J. Cell Biol. 146, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S. H., Masiarz F. R., Munemitsu S., Polakis P. (1993) Association of the APC gene product with β-catenin. Science 262, 1731–1734 [DOI] [PubMed] [Google Scholar]
- 39. Bakkenist C. J., Kastan M. B. (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.