Background: Citrobacter rodentium is an enteric bacterial pathogen of mouse intestinal tract.
Results: Mice lacking Nlrp3, Nlrc4, and caspase-1 are hypersusceptible to C. rodentium-induced gastrointestinal inflammation.
Conclusion: The Nlrp3 and Nlrc4 inflammasomes play a critical role in host defense against enteric infection caused by C. rodentium.
Significance: Our study establishes a critical role of inflammasomes in host defense against Citrobacter rodentium infection.
Keywords: Colitis, Host-Pathogen Interactions, Immunology, Inflammation, Innate Immunity, Citrobacter rodentium, Inflammasome
Abstract
Citrobacter rodentium is an enteric bacterial pathogen of the mouse intestinal tract that triggers inflammatory responses resembling those of humans infected with enteropathogenic and enterohemorrhagic Escherichia coli. Inflammasome signaling is emerging as a central regulator of inflammatory and host responses to several pathogens, but the in vivo role of inflammasome signaling in host defense against C. rodentium has not been characterized. Here, we show that mice lacking the inflammasome components Nlrp3, Nlrc4, and caspase-1 were hypersusceptible to C. rodentium-induced gastrointestinal inflammation. This was due to defective interleukin (IL)-1β and IL-18 production given that il-1β−/− and il-18−/− mice also suffered from increased bacterial burdens and exacerbated histopathology. C. rodentium specifically activated the Nlrp3 inflammasome in in vitro-infected macrophages independently of a functional bacterial type III secretion system. Thus, production of IL-1β and IL-18 downstream of the Nlrp3 and Nlrc4 inflammasomes plays a critical role in host defense against enteric infections caused by C. rodentium.
Introduction
Bacterial infections of the gastrointestinal tract represent a major cause of mortality worldwide and continue to threaten global health (1). Enteric bacterial pathogens such as enterohemorrhagic Escherichia coli and enteropathogenic E. coli are responsible for a large number of cases of diarrhea, which cause significant morbidity and mortality among infants and children in the developing world (2). Much of our knowledge on the mechanisms by which the host immune system controls infection by enteric pathogens stems from experimental studies with Citrobacter rodentium, a naturally occurring pathogen that infects the rodent gastrointestinal tract (3). As with enterohemorrhagic and enteropathogenic E. coli in humans, C. rodentium invasion of murine colonic epithelial cells results in the formation of lesions and mucosal hyperplasia (4).
Both innate immune cells and T cells, along with C. rodentium-specific antibody responses, contribute to containment and eradication of C. rodentium infection (5). At the molecular level, extracellular immune surveillance by members of the Toll-like receptor family is known to play a major role in host defense against C. rodentium infection (6–8). In addition, the role of the intracellular NOD-like receptor family members NOD1 and NOD2 in protection against C. rodentium infection has recently been characterized. NOD1 and NOD2 promote clearance of C. rodentium infection by inducing enteric innate T helper type 17 (iT(H)17) and T(H)1 responses in the gastrointestinal tract (9, 10). Unlike Nod1 and Nod2, which regulate MAP kinase and NF-κB signaling, a subset of NOD-like receptors that includes Nlrp3 and Nlrc4 controls innate immune responses by assembling inflammasomes. These multiprotein complexes are responsible for the activation of caspase-1 and subsequent production of bioactive interleukin IL-1β and IL-18 (11). Notably, C. rodentium was recently shown to induce noncanonical activation of the Nlrp3 inflammasome in in vitro-infected macrophages (12). Here, we analyzed inflammasome activation during in vivo infection with C. rodentium and characterized the physiological role of inflammasome signaling in host defense against C. rodentium. Mice lacking the inflammasome components Nlrp3, Nlrc4, and caspase-1 were hypersusceptible to C. rodentium-induced gastrointestinal inflammation and failed to control C. rodentium as a consequence of defective production of IL-1β and IL-18. Concurrently, il-1β−/− and il-18−/− mice suffered from increased bacterial burden and presented with more inflammatory lesions in the gut. Notably, C. rodentium specifically required Nlrp3, but not Nlrc4 or Aim2, to induce activation of caspase-1 and secretion of IL-1β from in vitro-infected macrophages. Moreover, the bacterial type III secretion system was dispensable for Nlrp3 inflammasome activation. In conclusion, we show that Nlrp3 and Nlrc4 inflammasome-mediated IL-1β and IL-18 production is required for efficiently clearing C. rodentium infections in the gastrointestinal tract.
EXPERIMENTAL PROCEDURES
Mice
Nlrp3−/− (13), Casp1−/− (14), Nlrc4−/− (15), Aim2−/− (16), and il-1β−/− (17) mice were all described before. il-18−/− mice were purchased from Jackson Laboratory. Mice were housed in a pathogen-free facility, and all animal procedures followed protocols approved by the St. Jude Children's Research Hospital Committee on Use and Care of Animals.
Citrobacter Infection
C. rodentium (ATCC 51459) was grown in LB broth overnight with shaking at 37 °C. For in vivo experiments, mice were infected with 4 × 109 cfu by oral gavage. Food and water intake were stopped 8 h prior to infection and allowed to resume 1 h after infection. To determine bacterial counts, serial dilutions of homogenized feces or colon tissues were plated on MacConkey agar plates and incubated at 37 °C for 24 h.
For in vitro experiments, bone marrow-derived macrophages (BMDMs)5 were prepared as described before (18). Cells were stimulated with or without LPS (500 ng/ml) for 5 h and then infected at 20 multiplicity of infection WT and/or type III secretion system mutants C. rodentium (ΔescC and ΔescN) for 1 h. Then gentamycin (50 μg/ml) was added to kill extracellular bacteria. After that, cells were cultured in CO2 incubator at 37 ºC for 19 h. The cell culture supernatants were collected for cytokine analysis. The cells were lysed for further Western blotting analysis.
Histological Analysis
Colons were fixed in 10% formalin and embedded in paraffin. Serial 5-μm sections were cut and stained with hematoxylin and eosin (H&E). Bacteria in colon mucosa were stained by the Gram staining method. For immunostaining of macrophages and neutrophils, sections were immunostained with anti-F4/80 and anti-Gr-1 antibodies, respectively. Histological scores were evaluated blindly by a pathologist (Dr. Peter Vogel) at St. Jude Research Hospital. The parameters used for histological scoring included inflammation, edema, hyperplasia, and the extent of colonic damage.
Cytokine Analysis
Colon homogenates were prepared mechanically in PBS containing 1% Nonidet P-40 and complete protease inhibitor mixture (Roche Applied Science). The concentrations of mouse cytokines and chemokines in colon homogenates or cell supernatants were determined using multiplex ELISA (Millipore), IL-1β ELISA kit (eBioscience), or IL-18 ELISA kit (MBL International).
Serum Antibody ELISA
Serum antibody analysis methods were modified as described previously (19). Briefly, heated-killed C. rodentium were coated in ELISA plates overnight. After wash, plates were blocked with 5% milk for 1 h. Serum samples were assayed in triplicate and incubated for 2 h at room temperature. Then Ig isotypes were detected with peroxidase-conjugated goat-anti-mouse IgG and IgM (Jackson Immunoresearch). After that, plates were developed with 3,3′, 5,5″-tetramethylbenzidine (TMB) and read at A450 after stopping reaction with 1 n H2SO4.
Flow Cytometry Analysis
Single cell suspensions were made from mouse spleen. Cells were stained with anti-CD11b (M1/70), CD11c (N418), Gr-1 (RB6–8C5), CD4 (RM4–5), and CD19 (6D5) (all from Biolegend) and CD8 (53–6.7) (from eBioscience). After staining, cells were analyzed on a FACSCalibur (BD Biosciences).
Western Blotting
For analysis of caspase-1 activation, tissue homogenate or BMDMs were denatured with SDS plus 100 mm DTT and boiled for 5 min. SDS-PAGE separated proteins were transferred to PVDF membranes and immunoblotted with rabbit antibody against caspase-1.
Statistics
Data are represented as mean ± S.E. or S.D. Statistical significance was determined by Student's t test; p < 0.05 was considered statistically significant.
RESULTS
Deficiency in Nlrp3, Nlrc4, and Caspase-1 Increases Host Susceptibility to C. rodentium Infection
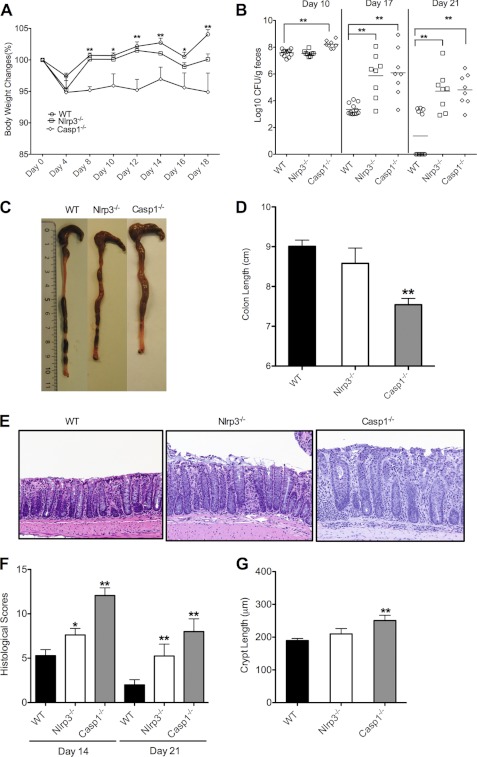
To examine the in vivo importance of inflammasome signaling in intestinal inflammation caused by C. rodentium infection, wild-type, Nlrp3−/−, and Casp1−/− mice were infected with C. rodentium at 4 × 109 cfu/mouse. Clinical manifestation of colitis was monitored during a course of 3 weeks by monitoring changes in body weight and bacterial burden in the stool. Casp1−/− mice were highly susceptible to C. rodentium infection as they lost significantly more body weight than wild-type mice from day 4 onward (Fig. 1A). Nlrp3−/− mice initially presented with a milder phenotype that gradually worsened in the course of the 3rd week (Fig. 1A). To further understand the roles of inflammasome signaling in C. rodentium infection, cfu in feces of Nlrp3−/− and Casp1−/− mice were determined at days 10, 17, and 21 after infection. In agreement with the differential changes in body weight at day 10 after infection, C. rodentium counts were initially comparably elevated in wild-type and Nlrp3−/− mice (∼107 cfu/g feces) (Fig. 1B). In contrast, cfu in Casp1−/− mice were significantly higher at this time point (∼108 cfu/g feces). At day 17, bacterial burden in wild-type mice markedly dropped to 103–104 cfu/g feces, whereas cfu remained elevated in Nlrp3−/− and Casp1−/− mice (106–109 cfu/g feces). This suggests that wild-type mice successfully started clearing the C. rodentium infection at this time point, whereas defective inflammasome signaling in Nlrp3−/− and Casp1−/− mice hampered this process. To obtain additional evidence of increased susceptibility to C. rodentium-induced colitis in inflammasome-deficient mice, we examined the gross anatomy and histology of the colon at days 14 and 21 after infection. Shortening and thickening of the colon are hallmark features of C. rodentium-induced colitis (4). Consistent with the increased body weight loss and higher bacterial burden in inflammasome-deficient mice, colons of Casp1−/− mice were significantly shorter and thicker than those of wild-type mice (Fig. 1, C and D). A tendency toward slightly shorter colons was observed in Nlrp3−/− mice, although these differences failed to reach statistical significance (Fig. 1D). The delayed onset of body weight loss and increases in bacterial burden in Nlrp3−/− mice (Fig. 1, A and B) might be explained by redundant mechanisms contributing to caspase-1 activation in response to infection with C. rodentium. Indeed, Nlrc4−/− mice that were infected with C. rodentium as described above resembled Nlrp3−/− mice in that C. rodentium counts in the stool were normal at day 10, but markedly increased at days 17 and 21 after infection (supplemental Fig. S1).
FIGURE 1.
Deficiency in inflammasome components increases host susceptibility to C. rodentium infection. WT, Nlrp3−/−, and Casp1−/− mice were orally infected with C. rodentium at a dose of 4 × 109 cfu/mouse. A, body weight changes monitored until day 18. *, p < 0.05; **, p < 0.01 WT versus Casp1−/− mice. B, C. rodentium load in feces measured by colony assay at days 10, 17, and 21. C. rodentium-infected mice were killed at days 14 and 21 to examine colons macroscopically. C, representative gross images of colons from C. rodentium-infected WT, Nlrp3−/−, and Casp1−/− mice at day 21. D, colon length at day 21. E, representative photomicrographs of H&E-stained colon sections at day 21. F, histological lesion scores at days 14 and 21. G, crypt length at day 21. *, p < 0.05; **, p < 0.01 versus WT. Data represent means ± S.E. (error bars) (WT, n = 12; Nlrp3−/−, n = 8; Casp1−/−, n = 8) from three independent experiments.
The clinical manifestation of increased colon inflammation in Nlrp3−/− and Casp1−/− mice was confirmed by histological analysis. H&E-stained colon sections of C. rodentium-infected Nlrp3−/− and Casp1−/− mice showed increased inflammation in the lamina propria along with elongated crypts and hyperplasia in inflammasome-deficient mice (Fig. 1E). Semiquantitative scoring of inflammation, edema, hyperplasia, and the extent of colonic damage confirmed that colitis severity was significantly higher in the Nlrp3−/− and Casp1−/− cohorts relative to the levels seen in wild-type mice (Fig. 1, F and G).
Increased Inflammatory Cytokine Production and Immune Invasion in Colons of C. rodentium-infected Casp1−/− Mice
Cytokines and chemokines play central roles in shaping inflammatory and host defense responses against microbial pathogens. To gain a better understanding of the mechanisms contributing to increased colitis in the absence of a functional inflammasome, we measured the local production of a variety of inflammatory cytokines and chemokines in C. rodentium-infected wild-type and Casp1−/− mice. Compared with wild-type mice, colon homogenates of Casp1−/− mice contained markedly higher levels of proinflammatory cytokines and chemokines such as IL-6, MIP2, KC, and MCP-1 at both days 14 (Fig. 2A) and 21 (Fig. 2B) after infection. These results suggest that defective inflammasome activation leads to increased local production of proinflammatory cytokines and chemokines in the gastrointestinal tract of C. rodentium-infected animals.
FIGURE 2.
Defects in inflammasome activation are associated with increased inflammatory responses in the colon. WT and Casp1−/− mice were orally infected with C. rodentium at a dose of 4 × 109 cfu/mouse. The infected mice were killed at days 14 and 21 to examine colons. At days 14 and 21 after infection, proteins were extracted from mouse colons, and the amounts of proinflammatory cytokines were measured. A, IL-6, MIP2, KC, and MCP-1 at 14 days after infection. B, IL-6, MIP2, KC, and MCP-1 at 21 days after infection. *, p < 0.05 versus WT. Data represent means ± S.E. (error bars) (WT, n = 12; Casp1−/−, n = 8) from two independent experiments.
To characterize the cell types contributing to production of these inflammatory mediators, we analyzed the immune cell populations migrating to the colon and secondary lymphoid tissues of C. rodentium-infected wild-type and Casp1−/− mice. Immunohistochemical analysis of micrographs stained for F4/80 and Gr-1 showed a markedly increased infiltration of macrophages and neutrophils in colons of Casp1−/− mice at day 21 after infection (Fig. 3A). This was associated with splenomegaly in Casp1−/− mice as evidenced by the doubled splenic size and weight (supplemental Fig. S2, A and B) compared with wild-type mice at day 14 after infection. Analysis of splenic immune cell populations showed that both the total myeloid cell (CD11b+) population as well as the subpopulations of neutrophils (CD11b+Gr1+) and dendritic cells (CD11b+CD11c+) were significantly elevated in Casp1−/− mice at day 14 (Fig. 3B). The elevated proinflammatory cytokine production and immune cell infiltration in C. rodentium-infected Casp1−/− colons were accompanied by a markedly increased bacterial colonization of the gastrointestinal tract (Fig. 3C). In situ Gram staining of wild-type and Casp1−/− colon sections confirmed an augmented bacterial presence in the mucosal tissue of Casp1−/− colons relative to wild-type colons at day 14 after C. rodentium infection (Fig. 3D), suggesting that the increased inflammatory responses in Casp1−/− colons may be due to the uncontrolled proliferation of C. rodentium in the absence of a functional inflammasome.
FIGURE 3.
Increased inflammatory cell infiltration correlates with high colonic bacterial burdens in inflammasome-deficient mice after C. rodentium infection. WT and Casp1−/− mice were orally infected with C. rodentium at a dose of 4 × 109 cfu/mouse. At days14 and 21, mice were killed. A, photomicrographs of macrophage (F4/80) and neutrophil (Gr-1) immunostained colon sections collected at day 21 after C. rodentium infection. B, spleens collected at day 14 (WT, n = 8; Casp1−/−, n = 7) and day 21(WT, n = 10; Casp1−/−, n = 6). Splenocytes were analyzed for myeloid cells (CD11b+), neutrophils (CD11b+Gr1+), and dendritic cells (CD11b+CD11c+) by flow cytometry. *, p < 0.05; **, p < 0.01 versus WT. Data represent means ± S.E. (error bars) from two independent experiments. C, C. rodentium burdens in colon tissues of WT (n = 8) and Casp1−/− (n = 8) mice at 21 days after infection. *, p < 0.05; **, p < 0.01 versus WT. Data represent means ± S.E. of two independent experiments. D, representative photomicrographs of bacterial Gram staining of colons from infected WT and Casp1−/− mice at day 14. Arrowheads point to Gram-negative bacteria colonies on colon mucosa.
Increased Lymphocyte Infiltration and Humoral Immunity in C. rodentium-infected Casp1−/− Mice
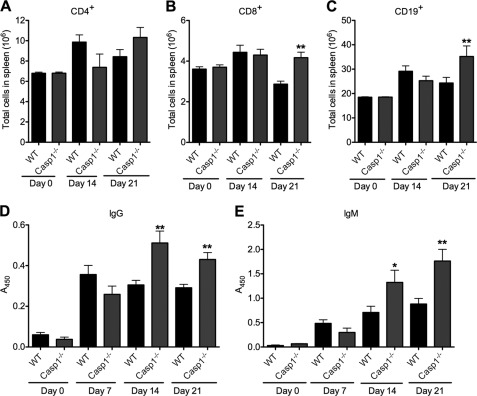
T cell and humoral responses have previously been implicated in host defense against C. rodentium (19, 20). To investigate whether caspase-1 deficiency affects C. rodentium-induced adaptive immune responses, we first determined CD4+, CD8+, and CD19+ cell numbers in the spleen of infected wild-type and Casp1−/− mice at days 14 and 21 after infection. Whereas T and B lymphocyte numbers in the spleens were comparable in the two genotypes at early stages of infection (days 0 and 14), Casp1−/− mice had significantly more CD8+ and CD19+ cells in the spleen at day 21 after infection (Fig. 4, A–C), suggesting that defective inflammasome activation is associated with enhanced adaptive immune responses. Concurrently, serum IgG and IgM levels were also higher in C. rodentium-infected Casp1−/− mice than the levels detected in circulation of wild-type mice (Fig. 4, D and E).
FIGURE 4.
Increased adaptive immune responses in caspase-1-deficient mice infected with C. rodentium. A–C, WT and Casp1−/− mice were orally infected with C. rodentium at a dose of 4 × 109 cfu/mouse. Mice were killed at days 0 (WT, n = 3; Casp1−/−, n = 3), 7 (WT, n = 10; Casp1−/−, n = 7), 14 (WT, n = 8; Casp1−/−, n = 7), and 21 (WT, n = 10; Casp1−/−, n = 6). Splenic lymphoid cells were analyzed by flow cytometry in splenic CD4+ cells (A), splenic CD8+ cells (B), and splenic CD19+ cells (C). D and E, serum IgG and IgM production were measured by ELISA. *, p < 0.05; **, p < 0.01 versus WT. Data represent means ± S.E. (error bars) from two independent experiments.
IL-1β and IL-18 Production Downstream of Inflammasomes Is Critical for Controlling C. rodentium Infection
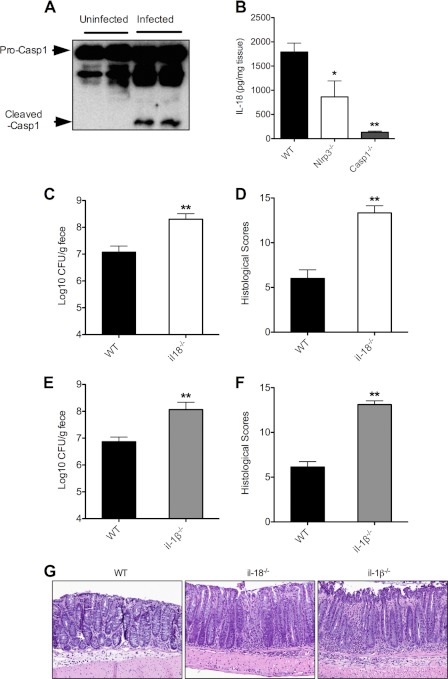
To confirm that the host responds to infection with C. rodentium by activating inflammasomes in the gastrointestinal tract, we monitored caspase-1 maturation by Western blotting in colon samples of wild-type (C57BL/6) mice that had been infected orally with C. rodentium for 14 days. As expected, procaspase-1 was matured into a 20-kDa fragment in colon samples of C. rodentium-infected wild-type mice, but not in colon samples of uninfected animals (Fig. 5A). The antibody failed to detect immunoreactive bands in lysates of Casp1−/− mice, demonstrating the specificity of these results (data not shown).
FIGURE 5.
IL-1β and IL-18 production in the downstream of inflammasomes is critical for controlling C. rodentium infection. A and B, WT, Nlrp3−/−, and Casp1−/− mice were infected with C. rodentium for 14 days. Proteins were extracted from colon. Caspase-1 activation was determined by Western blotting (A), and colonic IL-18 levels were measured by ELISA (B). Data represent means ± S.E. (error bars) (WT, n = 8; Nlrp3−/−, n = 5; Casp1−/−, n = 7) from two independent experiments. *, p < 0.05; **, p < 0.01 versus WT. C and D, WT (n = 10) and il-18−/− (n = 7) mice were infected with C. rodentium. C. rodentium counts (cfu) in stool (C) and colonic histology scores (D) were determined at day 14 after infection. Data represent means ± S.E. (error bars) from two independent experiments. **, p < 0.01 versus WT. E and F, WT (n = 10) and il-1β−/− (n = 9) mice were infected with C. rodentium. C. rodentium counts (cfu) in stool (E) and colonic histology scores (F) were determined at day 14 after infection. Data represent means ± S.E. from two independent experiments. **, p < 0.01 versus WT. G, representative photomicrographs of H&E-stained colon sections of C. rodentium-infected WT, il-18−/−, and il-1β−/− mice at day 14.
Inflammasomes are responsible for the proteolytic maturation and secretion of bioactive IL-1β and IL-18 (11). To examine whether the increased susceptibility and elevated cytokine production in Casp1−/− mice could be attributed to defective production of IL-1β and IL-18, we measured colonic levels of these inflammasome-dependent cytokines. Wild-type mice produced high levels of IL-18 at day 14 after infection (Fig. 5B), whereas IL-1β production in the colon was not easily detected (data not shown). Unlike wild-type mice, IL-18 levels were markedly reduced in colons of C. rodentium-infected Casp1−/− mice, whereas Nlrp3−/− mice had intermediate levels of IL-18 (Fig. 5B), suggesting a critical role for this cytokine in regulating susceptibility to C. rodentium.
To examine the role of IL-18 in additional detail, wild-type and il-18−/− mice were infected with C. rodentium to determine bacterial burden and histological lesions as described above. At day 14 after infection, C. rodentium counts and histological lesions were significantly higher in il-18−/− mice than in wild-type mice (Fig. 5, C and D), confirming the role of IL-18 in host defense against C. rodentium. We next examined the potential role of IL-1β in C. rodentium infection. Similar to il-18−/− mice, mice lacking IL-1β had significantly higher C. rodentium cfu in the stool (Fig. 5E) and markedly more colonic histological lesions than the control group (Fig. 5F). Analysis of H&E-stained colon sections of C. rodentium-infected WT, il-1β−/−, and il-18−/− mice confirmed that mice lacking either of the inflammasome-dependent cytokines displayed increased histopathology and hyperplasia (Fig. 5G). These data support the notion that both IL-1β and IL-18 production downstream of inflammasomes regulates replication of C. rodentium in the colon.
C. rodentium Type III Secretion System Is Dispensable for Nlrp3 Inflammasome Activation in Macrophages
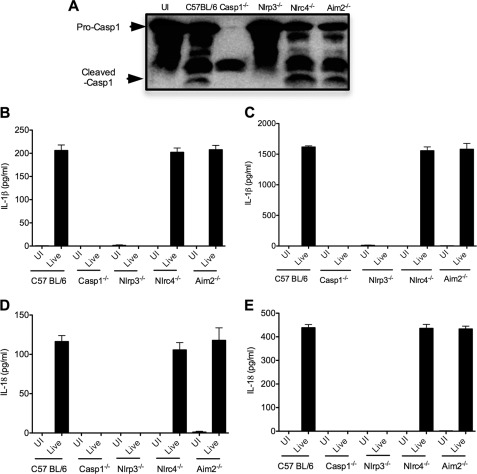
C. rodentium infection of BMDMs was recently shown to induce noncanonical caspase-1 activation downstream of Nlrp3 and caspase-11 (12). In agreement, C. rodentium induced caspase-1 maturation in wild-type BMDMs, but not in macrophages lacking Nlrp3 (Fig. 6A). In contrast, the Nlrc4 and AIM2 inflammasomes were dispensable for C. rodentium-induced caspase-1 activation because caspase-1 processing was not affected in Nlrc4−/− and Aim2−/− macrophages (Fig. 6A). Concurrently, significant production of IL-1β (Fig. 6, B and C) and IL-18 (Fig. 6, D and E) was observed in wild-type, Nlrc4−/−, and Aim2−/− macrophages, but not in Nlrp3−/− and Casp1−/− BMDMs. Together, these results suggest that C. rodentium activates caspase-1 specifically through the Nlrp3 inflammasome in infected macrophages.
FIGURE 6.
C. rodentium induces caspase-1 activation through Nlrp3 inflammasome in cultured macrophages. Bone marrow-derived macrophages from WT, Casp1−/−, Nlrp3−/−, Nlrc4−/−, and Aim2−/− mice were primed with or without LPS for 5 h and then infected with C. rodentium at 20 multiplicity of infection as described under “Experimental Procedures.” A, cells were lysed, and proteins were used to determine caspase-1 activation by Western blotting. B–E, cell culture supernatants were collected, and the cytokines IL-1β and IL-18 were measured by ELISA: IL-1β levels without LPS priming (B), IL-1β levels with LPS priming (C), IL-18 levels without LPS priming (D), and IL-18 levels with LPS priming (E). Data represent means ± S.D. (error bars) of triplicate wells from three independent experiments. UI, uninfected; Live, live bacteria.
Effector proteins secreted through type III secretion systems (T3SS) are critical for bacterial virulence. For instance, C. rodentium T3SS mediates translocation of multiple effector proteins that are required for bacterial virulence in mice (21, 22). To examine whether C. rodentium T3SS is implicated in inflammasome activation, BMDMs were infected with wild-type C. rodentium or the ΔescC and ΔescN mutants lacking essential structural components that render the T3SS inactive (22). Notably, BMDMs infected with wild-type C. rodentium secreted levels of IL-1β (Fig. 7, A and B) and IL-18 (Fig. 7 C, and D) in the culture supernatants similar to those infected with the ΔescC and ΔescN mutants. In agreement, caspase-1 processing in BMDMs infected with the mutant bacterial strains was similar to the levels seen in BMDMs infected with wild-type C. rodentium (Fig. 7E). These results suggest that C. rodentium activates the Nlrp3 inflammasome independently of T3SS effectors, raising the possibility that the Nlrp3 inflammasome may detect C. rodentium indirectly through some other non-type III bacterial component (e.g. LPS, flagellin, or peptidoglycan).
FIGURE 7.
C. rodentium type III secretion system is dispensable for inflammasome activation in infected macrophages. BMDMs from WT and Casp1−/− mice were primed with or without LPS for 5 h and then infected with C. rodentium, ΔescC (Mutant 1, M1) and ΔescN (Mutant 2, M2) at 20 multiplicity of infection as described under “Experimental Procedures.” The cytokine IL-1β and IL-18 in culture supernatants were measured by ELISA. A, IL-1β levels without LPS priming. B, IL-1β levels with LPS priming. C, IL-18 levels without LPS priming. D, IL-18 levels with LPS priming. E, Western blotting analysis of caspase-1 activation in cell lystates. Data represent means ± S.D. (error bars) of triplicate wells from three independent experiments.
DISCUSSION
Enteric bacterial pathogens represent a major public health problem in the developing world because of the significant morbidity and mortality associated with severe diarrhea in infants and children (2). Previous studies demonstrated that innate immune cells, T cell responses, and antibody production all contribute to containment and eradication of enteric infections (5). For example, extracellular immune surveillance by members of the Toll-like receptor family was shown to be critical for host defense against C. rodentium infection (6–8). More recently, the role of the intracellular NOD-like receptor family members NOD1 and NOD2 in protection against C. rodentium infection has been demonstrated (9, 10). However, the importance of inflammasome signaling in protection against enteric infections caused by C. rodentium has not been described. Our data demonstrated that mice lacking the inflammasome components Nlrp3, Nlrc4, and caspase-1 are hypersusceptible to C. rodentium-induced gastrointestinal inflammation because of their failure to produce normal levels of IL-1β and IL-18. Defective inflammasome-mediated cytokine production was associated with increased destruction of the colonic epithelium, higher bacterial burden, and elevated immune cell infiltration and production of proinflammatory cytokines and chemokines. The importance of IL-1β and IL-18 in this process was confirmed by the observation that il-1β−/− and il-18−/− mice suffered from increased bacterial burden and colon pathology. In cultured macrophages, C. rodentium activated caspase-1 and induced secretion of IL-1β and IL-18 through noncanonical activation of the Nlrp3 inflammasome. Recent work showed that available caspase-1-deficient mouse lines fail to express caspase-11 and implicated both inflammatory caspases in C. rodentium-induced IL-1β and IL-18 secretion from infected macrophages (12). Our results indicated that the bacterial T3SS and the Nlrc4 inflammasome were dispensable for C. rodentium-induced caspase-1 activation in macrophages. However, given the importance of Nlrc4 in in vivo resistance to C. rodentium infection, the Nlrc4 inflammasome may nevertheless contribute to caspase-1 activation and inflammasome-dependent cytokine secretion in other cell types. Alternatively, the complex microbial context of the gastrointestinal tract may provide an explanation. Damage to the epithelial layer due to colonic infection with C. rodentium may allow opportunistic infections with commensal bacteria. Thus, during in vivo infections with C. rodentium, host inflammasome activation may not solely depend on C. rodentium, but multiple other bacterial species. Consequently, both the Nlrp3 and Nlrc4 inflammasomes may be activated during C. rodentium infection in vivo. Regardless, our results suggest that therapies aimed at modulating inflammasome activation may provide novel approaches for controlling inflammation and infections of the gastrointestinal tract.
Supplementary Material
Acknowledgments
We thank Anthony Coyle, Ethan Grant, John Bertin (Millennium Pharmaceuticals), Gabriel Nuñez (University of Michigan), Richard Flavell (Yale), and Vishva Dixit (Genentech) for a generous supply of mutant mice.
This work was supported, in whole or in part, by National Institutes of Health Grant R01AR056296. This work was also supported by the American Lebanese Syrian Associated Charities (ALSAC) (to T.-D.K.).

This article contains supplemental Figs. S1 and S2.
- BMDM
- bone marrow-derived macrophage
- T3SS
- type 3 secretion system
- MIP2
- macrophage inflammatory protein 2
- KC
- keratinocyte-derived chemokine
- MCP-1
- monocyte chemotactic protein-1.
REFERENCES
- 1. Kappelman M. D., Rifas-Shiman S. L., Porter C. Q., Ollendorf D. A., Sandler R. S., Galanko J. A., Finkelstein J. A. (2008) Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology 135, 1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaper J. B., Nataro J. P., Mobley H. L. (2004) Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 [DOI] [PubMed] [Google Scholar]
- 3. Borenshtein D., McBee M. E., Schauer D. B. (2008) Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 24, 32–37 [DOI] [PubMed] [Google Scholar]
- 4. Mundy R., MacDonald T. T., Dougan G., Frankel G., Wiles S. (2005) Citrobacter rodentium of mice and man. Cell. Microbiol. 7, 1697–1706 [DOI] [PubMed] [Google Scholar]
- 5. Eckmann L. (2006) Animal models of inflammatory bowel disease: lessons from enteric infections. Ann. N.Y. Acad. Sci. 1072, 28–38 [DOI] [PubMed] [Google Scholar]
- 6. Gibson D. L., Ma C., Bergstrom K. S., Huang J. T., Man C., Vallance B. A. (2008) MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 618–631 [DOI] [PubMed] [Google Scholar]
- 7. Gibson D. L., Ma C., Rosenberger C. M., Bergstrom K. S., Valdez Y., Huang J. T., Khan M. A., Vallance B. A. (2008) Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 388–403 [DOI] [PubMed] [Google Scholar]
- 8. Lebeis S. L., Bommarius B., Parkos C. A., Sherman M. A., Kalman D. (2007) TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179, 566–577 [DOI] [PubMed] [Google Scholar]
- 9. Geddes K., Rubino S. J., Magalhaes J. G., Streutker C., Le Bourhis L., Cho J. H., Robertson S. J., Kim C. J., Kaul R., Philpott D. J., Girardin S. E. (2011) Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17, 837–844 [DOI] [PubMed] [Google Scholar]
- 10. Kim Y. G., Kamada N., Shaw M. H., Warner N., Chen G. Y., Franchi L., Núñez G. (2011) The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamkanfi M., Walle L. V., Kanneganti T. D. (2011) Deregulated inflammasome signaling in disease. Immunol. Rev. 243, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W. P., Roose-Girma M., Dixit V. M. (2011) Noncanonical inflammasome activation targets caspase-11. Nature 479, 117–121 [DOI] [PubMed] [Google Scholar]
- 13. Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Núñez G. (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 14. Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. (1995) Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267, 2000–2003 [DOI] [PubMed] [Google Scholar]
- 15. Franchi L., Amer A., Body-Malapel M., Kanneganti T. D., Ozören N., Jagirdar R., Inohara N., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Núñez G. (2006) Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7, 576–582 [DOI] [PubMed] [Google Scholar]
- 16. Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O'Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M., Dixit V. M., Monack D. M. (2010) Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U.S.A. 107, 9771–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horai R., Asano M., Sudo K., Kanuka H., Suzuki M., Nishihara M., Takahashi M., Iwakura Y. (1998) Production of mice deficient in genes for interleukin (IL)-α IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 187, 1463–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ozören N., Masumoto J., Franchi L., Kanneganti T. D., Body-Malapel M., Ertürk I., Jagirdar R., Zhu L., Inohara N., Bertin J., Coyle A., Grant E. P., Núñez G. (2006) Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176, 4337–4342 [DOI] [PubMed] [Google Scholar]
- 19. Bry L., Brenner M. B. (2004) Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172, 433–441 [DOI] [PubMed] [Google Scholar]
- 20. Simmons C. P., Clare S., Ghaem-Maghami M., Uren T. K., Rankin J., Huett A., Goldin R., Lewis D. J., MacDonald T. T., Strugnell R. A., Frankel G., Dougan G. (2003) Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71, 5077–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng W., Puente J. L., Gruenheid S., Li Y., Vallance B. A., Vázquez A., Barba J., Ibarra J. A., O'Donnell P., Metalnikov P., Ashman K., Lee S., Goode D., Pawson T., Finlay B. B. (2004) Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. U.S.A. 101, 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickham M. E., Lupp C., Vázquez A., Mascarenhas M., Coburn B., Coombes B. K., Karmali M. A., Puente J. L., Deng W., Finlay B. B. (2007) Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes. Infect. 9, 400–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.