Background: Artificial reduction of internal ecdysteroid titer is very difficult to achieve in insects.
Results: Injection of Nomuraea rileyi ecdysteroid-22-oxidase (E22O) or forced expression of the E22O gene reduced ecdysteroid titer and manipulated embryogenesis, molting, metamorphosis, and diapause in a number of insects.
Conclusion: E22O is the first versatile ecdysteroid titer-decreasing tool.
Significance: E220 will be used to answer various ecdysteroid-associated developmental and physiological questions.
Keywords: Development, Enzyme Purification, Insect, Oxidase, Physiology, Steroid Hormone, Diapause, Ecdysone, Ecdysteroid, Metamorphosis
Abstract
Steroid hormones ecdysteroids regulate varieties of developmental processes in insects. Although the ecdysteroid titer can be increased experimentally with ease, its artificial reduction, although desirable, is very difficult to achieve. Here we characterized the ecdysteroid-inactivating enzyme ecdysteroid-22-oxidase (E22O) from the entomopathogenic fungus Nomuraea rileyi and used it to develop methods for reducing ecdysteroid titer and thereby controlling insect development. Km and Kcat values of the purified E22O for oxidizing ecdysone were 4.4 μm and 8.4/s, respectively, indicating that E22O can inactivate ecdysone more efficiently than other ecdysteroid inactivating enzymes characterized so far. The cloned E22O cDNA encoded a FAD-dependent oxidoreductase. Injection of recombinant E22O into the silkworm Bombyx mori interfered with larval molting and metamorphosis. In the hemolymph of E22O-injected pupae, the titer of hormonally active 20-hydroxyecdysone decreased and concomitantly large amounts of inactive 22-dehydroecdysteroids accumulated. E22O injection also prevented molting of various other insects. In the larvae of the crambid moth Haritalodes basipunctalis, E22O injection induced a diapause-like developmental arrest, which, as in normal diapause, was broken by chilling. Transient expression of the E22O gene by in vivo lipofection effectively decreased the 20-hydroxyecdysone titer and blocked molting in B. mori. Transgenic expression of E22O in Drosophila melanogaster caused embryonic morphological defects, phenotypes of which were very similar to those of the ecdysteroid synthesis deficient mutants. Thus, as the first available simple but versatile tool for reducing the internal ecdysteroid titer, E22O could find use in controlling a broad range of ecdysteroid-associated developmental and physiological phenomena.
Introduction
In insects, the steroid hormones ecdysteroids, primarily 20-hydroxyecdysone (20E),5 play important roles in the regulation of various developmental and physiological processes such as embryogenesis, molting, metamorphosis, reproduction, and diapause (1–4). Titers of ecdysteroids are precisely controlled by a combination of synthetic reactions, most of which proceed in the prothoracic gland, whereas they are inactivated in various peripheral organs (5). In the last decade, eight ecdysteroid synthesis enzymes were identified, mainly from the fruit fly Drosophila melanogaster and silkworm Bombyx mori (6–16). Five of these enzymes were cytochrome P450 encoded by the so-called Halloween genes of D. melanogater and mutants of which were all embryonic lethal (17). In contrast, the ecdysteroid inactivation process has received relatively less attention. So far, four ecdysteroid inactivation enzymes have been identified in insects (18–23). Mutations in or knockdown of those genes interfered with insect metamorphoses (21–23), indicating that both synthesis and inactivation of ecdysteroids are essential for the normal development of insects.
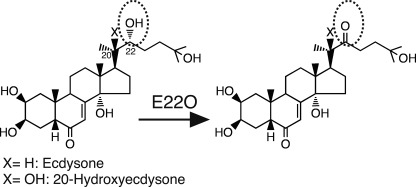
Some insect pathogens also use ecdysteroid inactivation enzymes. The best-known example is the baculoviruses, which express the ecdysteroid UDP-glucosyltransferase that inactivates ecdysteroids via sugar conjugation at position C22 (24). Another example is the entomopathogenic fungus Nomuraea rileyi that secretes the ecdysteroid-22-oxidase (E22O) that oxidizes the hydroxyl group at C22 to a carbonyl group (25) (Fig. 1). These pathogens inhibit molting of their hosts by inactivating the host ecdysteroid hormones using these enzymes, presumably to maintain a good physiological condition for growth (25, 26)
FIGURE 1.
Enzymatic reactions of E22O. E22O oxidizes the hydroxyl group at position C22 of ecdysone and 20-hydroxyecdysone to a carbonyl group. The oxidized ecdysteroids lose hormonal activity.
These ecdysteroid inactivation enzymes could potentially serve as powerful tools in ecdysteroid research, as techniques for both increasing and decreasing internal titers of hormones are essential for analyzing their actions in vivo. Indeed, increases in the ecdysteroid titer experimentally, by injecting or feeding exogenous ecdysteroid, have revealed various biological functions and modes of action of this hormone in a number of insect species (1–4). A reduction in the ecdysteroid titer using artificial means is, however, very difficult to achieve in most insects, because the prothoracic glands are intricately-shaped organs and are difficult to surgically remove completely. Furthermore, pharmacological agents that could inhibit synthesis or actions of ecdysteroids in a wide spectrum of insects have not yet been developed, and knockdown of genes encoding the ecdysteroid synthesis enzymes or forced expression of genes encoding the ecdysteroid inactivation enzymes is still difficult, except in D. melanogaster. Consequently, we do not know exactly what happens when the internal ecdysteroid titer is artificially decreased in most insects. The ecdysteroid inactivating enzymes could potentially solve this longstanding challenge. Particularly, E22O of N. rileyi is a good candidate for this purpose, because the B. mori larvae that were injected with the N. rileyi conditioned culture medium, which contained E22O activity, showed a decreased level of ecdysteroid titer (25).
In this study, we characterized E22O and used it for developing methods to reduce the ecdysteroid titer. Injection of recombinant E22O protein or forced expression of the E22O gene reduced the titer of 20E and blocked embryogenesis, larval molting, larval-pupal, or pupal (nymphal)-adult metamorphoses in a number of insects. In addition, injection of E22O induced a diapause-like developmental arrest in the crambid moth Haritalodes basipunctalis that was indistinguishable from the normal diapause. To the best of our knowledge, this is the first report indicating that a reduction in the ecdysteroid titer is a sufficient endocrinological stimulus to induce diapauses in insects. Thus, our results suggest that E22O, by reducing the ecdysteroid titer, could serve as a powerful tool for researching a variety of ecdysteroid-dependent phenomena during insect development.
EXPERIMENTAL PROCEDURES
Insects and Fungus
The F1 hybrid strain C145 × N140 of B. mori maintained at the National Institute of Agrobiological Sciences and Lucilia sericata purchased from a fishing-tackle store were reared on an artificial diet for silkworm (Nihon Nosan Kogyo). Naxa seriaria and Riptortus clavatus were collected in Tsukuba, Japan, and reared on Ligustrum obtusifolium leaves and soybeans, respectively. Tenebrio molitor was purchased from a pet shop and reared on powdered bird food. These insects were reared at 25 °C under a 12-h light, 12-h dark (12L:12D) photoperiod. H. basipunctalis was collected in Tsukuba and reared on Firmiana simplex leaves at 25 °C under 16L:8D photoperiod (nondiapausing condition) or at 17 °C under 8L:16D photoperiod (diapause-inducing condition). D. melanogaster were reared on a standard agar-cornmeal medium at 25 °C under 12L:12D photoperiod. Mutant strain hsp70-GAL4 was obtained from the Drosophila Genetic Resource Center at Kyoto Institute of Technology. Mutant strains engrailed-GAL4 and Actin5C-GAL4 were obtained from the Bloomington Stock Center. The Actin5C-GAL4 strain was originally established by Dr. Yasushi Hiromi at the National Institute for Genetics, Japan. N. rileyi, maintained at the National Institute of Agrobiological Sciences, was cultured in a medium containing 20 g of maltose, 5 g of tryptone, and 5 g of yeast extract in 1 liter of water. Conditioned media for N. rileyi were prepared as described previously (25).
Ecdysteroids and Brassinosteroids
Ecdysone (Sigma) and 20-hydroxyecdysone (Mitaka Pharmaceutical Co.) were purified using HPLC (LC-10AT, Shimadzu). Each ecdysteroid was applied to a C18 reverse-phase column (TSK gel ODS-80Ts, 4.6 × 150 mm, TOSOH) and then eluted with a 20–30% linear gradient of acetonitrile using a flow rate of 0.6 ml/min. Fractions corresponding to each ecdysteroid were pooled, methanol was evaporated off, and dried ecdysteroid was weighed and then dissolved in ethanol. 22-Dehydroecdysone and 22-dehydro-20-hydroxyecdysone were synthesized by reacting ecdysone and 20-hydroxyecdysone, respectively, with the conditioned media of Sf9-E22O cells. They were then purified by HPLC as above, weighed, and dissolved in ethanol. HPLC analysis confirmed that these ecdysteroids were >99% pure. Ponasterone A (Invitrogen), brassinolide (Wako), and castasterone (Wako) were dissolved in ethanol. The ecdysteroid and brassinosteroid solutions were diluted with distilled water and reacted with E22O-containing solutions.
E22O Activity Assay
An aliquot of E22O-containing solution was mixed with an equal volume of 200 μm ecdysone and incubated at 25 °C. Ten min later, a double volume of ethanol was added to stop the reaction and the mixture was centrifuged at 18,000 × g for 10 min. Ecdysone and synthesized 22-dehydroecdysone remaining in the supernatant were separated by HPLC and the amount of 22-dehydroecdysone was calculated from the ratios of peak areas of the two ecdysteroids. Km and Vmax values were calculated using the GraphPad Prism 5 program (GraphPad Software).
Purification of E22O
N. rileyi-conditioned medium (152 ml) was mixed with a protease inhibitor mixture and solid ammonium sulfate was then added to achieve 50% saturation, and the mixture was continuously stirred at 4 °C overnight. After centrifugation at 10,000 × g for 20 min, E22O was purified from the resulting supernatant by HPLC (Model Bio-HPLC system, TOSOH). The crude extract was applied to a HiTrap Phenyl-Sepharose HP column (Amersham Biosciences) equilibrated with buffer A (20 mm Tris-HCl (pH 7.6)) containing 50% ammonium sulfate. The column was eluted using a linear gradient (0–50%) of ammonium sulfate in buffer A. Fractions containing E22O were pooled, dialyzed against buffer A containing 0.3 m NaCl, and concentrated using an Ultrafree Biomax-5 Centrifugal filter (Millipore). The concentrated solution was then applied to a Superdex 200 pg (1.6 × 60 cm) column equilibrated with buffer A containing 0.3 m NaCl. Fractions containing E22O were pooled and concentrated using the Ultrafree Biomax-5 Centrifugal filter again. The concentrated E22O was further purified using a HiTrap Q column (Amersham Biosciences). Fractions containing E22O (0.58 mg) were pooled and used for biochemical characterization. The molecular mass of the purified E22O was estimated by running it on a 15% polyacrylamide gel containing 0.1% SDS along with molecular weight marker proteins as standards. The molecular mass of E22O was also estimated by comparing its retention time on a gel-filtration column (Superdex 200 prep grade, 10 mm × 30 cm column, equilibrated with buffer a containing 0.3 m NaCl) with the retention times of the molecular weight marker proteins.
Amino Acid Sequencing
The N-terminal sequence of the purified E22O was determined using a gas-phase protein sequencer (model LF-3400 DT, Beckman). Phenylthiohydantoin derivatives of individual amino acids were identified by reverse phase HPLC. The purified E22O was subjected to in-gel digestion by V8 protease and the products were separated on SDS-PAGE. The N-terminal sequence of one of the protease-digested products was also determined as above.
cDNA Cloning of E22O
Total RNA was extracted from the N. rileyi mycelia using TRIzol (Invitrogen) and reverse-transcribed using Ready-To-Go T-Prime First-strand Beads (Amersham Biosciences). A partial E22O cDNA was cloned from the cDNA pool by PCR. Forward and reverse PCR primers were designed on the basis of the N-terminal sequences of the purified E22O and its limited V8-proteolysis product, respectively. The first PCR was carried out using the E22O-dF1 (5′-TICCICARGGIGGITGYAG-3′) and E22O-dR1 (5′-CAIGCITTITTIACRTTRTG-3′) primers. The second nested PCR was carried out using the E22O-dF2 (5′-TGYAGRTGYATICCIGGIGA-3′) and E22O-dR2 (5′-TTIACITTITGICCYTGRTC-3′) primers. The full-length E22O cDNA was obtained by combining 5′-RACE and 3′-RACE reactions with primers that were designed based on the sequences of the partial E22O cDNA using SMART RACE cDNA Amplification Kit (Clontech). The nucleotide sequence of the full-length E22O cDNA was deposited in the GenBankTM/EMBL/DDBJ databases (accession number AB675078). A putative secretion signal of E22O was predicted using the SignalP 3.0 program (27).
Expression of E22O in Cultured Cell
The entire open reading frame of E22O was amplified by RT-PCR from the N. rileyi cDNA pool and cloned into the pIZT/V5-His expression vector (Invitrogen), and the resultant plasmid was called pIZT-E22O. The moth Spodoptera frugiperda Sf9 cells were transfected with pIZT-E22O using FuGENE HD (Roche Diagnostics). The Sf9 cells transfected with pIZT-E22O were subcultured continuously in IPL-41 medium containing 10% FBS and 300 μg/ml of zeocin. Three months later, all cells acquired resistance against zeocin. We assumed that plasmid pIZT-E22O was integrated into the genome of the Sf9 cells (designated hereafter as Sf9-E22O cells) and maintained them in IPL-41 medium with 10% FBS and 10 μg/ml of zeocin. When they reached 80–90% confluence, the medium was replaced with IPL-41 without FBS and zeocin. Two weeks later, the conditioned culture medium, which contained a large amount of E22O (supplemental Fig. S1A) but little FBS and zeocin, was collected. Because the activity of E22O in the medium remained high for a long period at 4 °C (supplemental Fig. S1C), we stored it in the refrigerator until used later in physiological experiments. Control conditioned medium was similarly prepared from the parental Sf9 cells. In physiological experiments, the conditioned medium of Sf9-E22O or Sf9 cells (5–30 μl) was injected into the hemocoel of insects using a microsyringe (Hamilton) with a 31-gauge ponit-4 needle.
Transient Expression of E22O Gene in Silkworm
The E22O gene was expressed transiently in B. mori by in vivo lipofection following a slightly modified version of our published procedure (28). Briefly, 2 μg of pIZT-E22O was mixed with 5 μl of Transfast (Promega) in 10 μl of 10 mm Tris-HCl buffer (pH 8.0), incubated for 10 min at room temperature, and then injected into the hemocoel of the silkworm larvae. As the control, the empty pIZT/V5-His plasmid was similarly lipofected into the silkworm larvae. After spinning larvae were lipofected with either of the plasmids, they were returned into cocoons. Larvae that did not resume spinning were excluded from further analyses because they did not emerge as adults normally.
Transgenic Expression of E22O Gene in Fruit Fly
The entire coding region of E22O cDNA from pIZT-E22O was cloned into the EcoRI/BglII site of the pUAST vector (29). The embryos used as recipients for DNA injection to generate transgenic lines were yellow white (y ac w1118) flies. Transgenic flies carrying UAS-E22O constructs (UAS-E22O line) were generated as described previously (30). The UAS-E22O line was crossed with different GAL4 driver lines and F1 individuals were used for experiments.
Quantification of Ecdysteroid Titer
Hemolymph samples were individually collected from B. mori pupa or H. basipunctalis larvae, mixed vigorously with a triple volume of methanol, and centrifuged at 18,000 × g for 5 min. D. melanogaster embryos were collected as batches 7–12 h after egg laying, a time when embryonic ecdysteroid titer is the highest (31), homogenized in methanol, and then centrifuged. Total ecdysteroid content in the supernatant of these samples were measured by radioimmunoassay using 20E as a standard (32). Supernatant from the B. mori sample was run on HPLC and a fraction containing ecdysone, 20E, 22-dehydroecdysone, or 22-dehydro-20-hydroxyecdysone was collected separately. The fractions were dried, dissolved in methanol again, and subjected to radioimmunoassay using each ecdysteroid as a standard. The affinities of the antibody used in radioimmunoassay to ecdysone, 20E, 22-dehydroecdysone, and 22-dehydro-20-hydroxyecdysone were ∼1:3:0.3:1.
Statistical Analysis
Student's t test was conducted to detect statistically significant differences between E22O treatments and controls using the JMP7 software (SAS Institute).
RESULTS
Purification, Kinetic Analysis, and cDNA Cloning of E22O
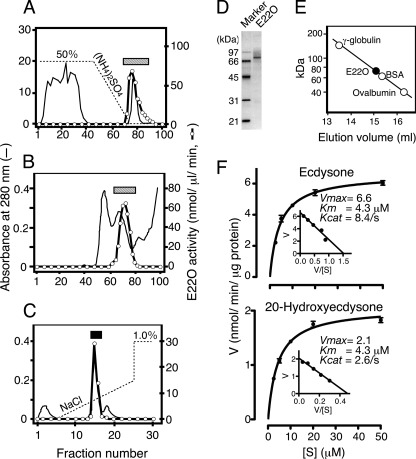
As previously shown by us (25), the conditioned culture media of N. rileyi exhibited strong E22O activity (supplemental Fig. S1A). We therefore purified E22O from the N. rileyi-conditioned medium by HPLC, sequentially using hydrophobic interaction chromatography, gel-filtration chromatography, and ion exchange chromatography, and at the end obtained E22O as a single peak (Fig. 2, A-C). The molecular mass of the purified E22O was estimated to be 76 kDa by both SDS-PAGE and gel-filtration analyses (Fig. 2, D and E), suggesting that E22O is a monomeric protein.
FIGURE 2.
Purification and biochemical characterization of E22O. A-C, E22O was purified from the N. rileyi-conditioned medium by sequential use of phenyl-Sepharose HP hydrophobic interaction chromatography (A), Superdex 200 pg gel-filtration chromatography (B), and HiTrap Q column chromatography (C). Fractions indicated using the hatched bars in A and B were pooled and purified further in B and C, respectively. Fractions indicated using the closed bar in C contained pure E22O; they were pooled and then used for further biochemical analyses. D and E, molecular mass of E22O was determined by SDS-PAGE (D) and Superdex 200 pg gel-filtration chromatography (E). F, kinetic analysis. Various concentrations of ecdysone or 20-hydroxyecdysone were incubated with 2.5 ng/50 μl of purified E22O in phosphate buffer (pH 7.0) and amounts of 22-dehydroecdysteroids formed were measured after 10 min. Kcat values were calculated using the molecular mass of E22O as 76 kDa.
E22O exhibited the highest catalytic activity for ecdysone at pH 10.5, and the activity decreased to one-third of this value at neutral pH (supplemental Fig. S2). Because the endogenous E22O normally works at neutral pH in the insect hemolymph, we measured the kinetic properties of the purified E22O at pH 7.0. The Kcat of the purified E22O for ecdysone was 8.4/s, which was three times higher than that for 20E; in contrast, the Km values were the same (i.e. 4.3 μm) for both ecdysteroids (Fig. 2F). This observed Kcat of the purified E22O for ecdysone was much higher than those of the ecdysteroid UDP-glucosyltransferase of the Autographa californica nucleopolyhedrovirus (Kcat = 0.069/s) and ecdysone oxidase of the cotton leafworm Spodoptera littoralis (Kcat = 0.11–0.12/s, calculated assuming that the molecular mass of the enzyme is 190 kDa), whereas the Km values of all three enzymes for ecdysone were similar (19, 33). These results suggest that E22O inactivates ecdysone much more efficiently than the two other well characterized ecdysteroid inactivation enzymes. E22O also oxidized and inactivated other ecdysteroids that contain the hydroxyl group at C22, for example, ponasterone A, however, had no effect on plant steroid hormones brassinosteroids, such as brassinolide and castasterone, even though they also have the hydroxyl group at C22 (data not shown).
Next, to clone the E22O cDNA, we first sequenced the N termini of the purified E22O protein and one of the peptides produced by limited hydrolysis of E22O using V8 protease (supplemental Fig. S3). Using degenerated primers, designed on the basis of these amino acid sequences, a partial E22O cDNA fragment was cloned by RT-PCR. The 5′ and 3′ ends of the E22O cDNA were then cloned using the 5′- and 3′-RACE techniques. The full-length E22O cDNA encoded a novel FAD-binding oxidoreductase comprised of 594 amino acids (supplemental Fig. S3). Consistent with the prediction that E22O is a flavoprotein, purified E22O had a brownish color with an absorbance at 454 nm. The N-terminal end of E22O contained a putative signal peptide, and the amino acid sequence following it matched the N-terminal sequence of the purified protein as determined above, suggesting that E22O is processed after the signal peptide and secreted from the cells. The amino acid sequence of E22O was up to 55% identical to those of the alcohol oxidases identified from various fungi (supplemental Fig. S3). Although some of these oxidases are involved in the biosynthesis of biologically active agents, such as Hypomyces subiculosus alcohol oxidase involved in hypothemycin synthesis and Fusarium incarnatum APS9 in apicidin synthesis (34, 35), none of them are known to be involved in the modification of steroids or hormones.
Activity of Recombinant E22O
When the E22O cDNA was transiently expressed in Sf9 cells, high ecdysone-oxidizing activity was found in the culture media (supplemental Fig. S1A). Remarkably, 50 μl of the media completely oxidized an equal volume of 200 μm (100 μm final concentration) ecdysone within 10 min (supplemental Fig. S1B); this final concentration of ecdysone was 100 times higher than the maximal titer found in the B. mori pupal hemolymph (Fig. 5C). This activity was comparable with or even higher than that observed in the N. rileyi conditioned media. In contrast, hardly any activity was observed in the cellular lysates (supplemental Fig. S1A). These results confirmed that the cloned cDNA encodes E22O and that E22O is a secretory protein.
FIGURE 5.
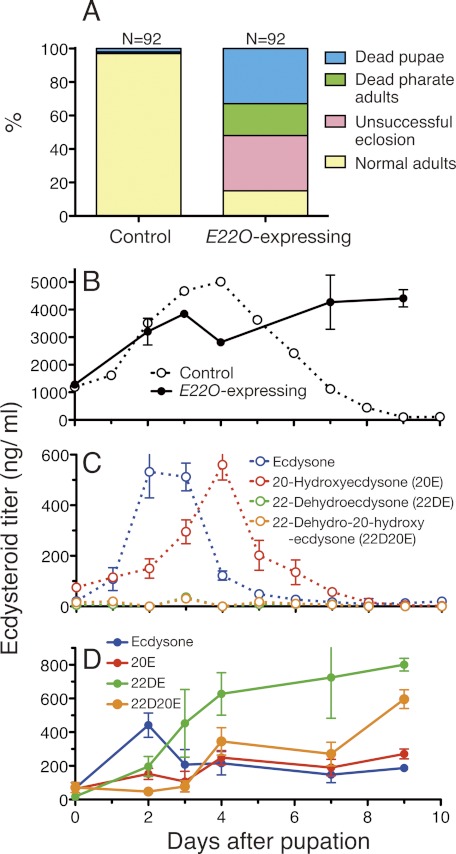
Effects of transient E22O expression in spinning silkworm on the growth and ecdysteroid titer. A, effects of E22O expression on the pupal development. Plasmid pIZT-E22O and control plasmid pIZT/V5-his were introduced separately by in vivo lipofection into the spinning last instar larvae and developments of normally pupated individuals were observed. B, changes in the total ecdysteroid titer (20E equivalent) in the hemolymph of nonlipofected control and E22O-expressing pupae. C and D, changes in the titer of each ecdysteroid in the hemolymph of the nonlipofected control (C) and E22O-expressing pupae (D). Error bars represent S.E. (n = 3–8).
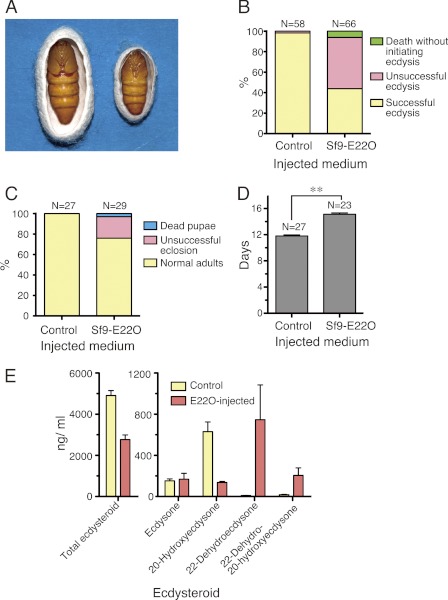
Next, we generated an Sf9 cell line that stably expressed the E22O cDNA (Sf9-E22O cell line) and injected its conditioned medium into B. mori individuals to test whether it would decrease the hemolymph ecdysteroid titer and affect their growth. We have previously shown that injection of N. rileyi-conditioned medium into the midpenultimate instar larvae of B. mori induced precocious pupation (25). Consistent with this observation, some of the larvae that were injected with Sf9-E22O-conditioned medium at the beginning of the penultimate instar ate food 3–4 days longer than the control, started spinning during the instar, and pupated precociously (Fig. 3A). When the conditioned medium was injected into the late-penultimate instar larvae, the last larval ecdysis was inhibited (Fig. 3B). Injection of Sf9-E22O-conditioned medium into B. mori pupae 2 days after pupation prolonged the pupal period and interfered with adult emergence (Fig. 3, C and D). In the hemolymph of the E22O-injected pupae, the 20E titer decreased and instead large amounts of 22-dehydroecdysone and 22-dehydro-20-hydroxyecdysone were present on day 4 when the 20E titer is the highest in the control (Figs. 3E and 5C). These results indicate that the conditioned media of Sf9-E22O cells can also be used to reduce the 20E titer and thereby manipulate the growth of B. mori.
FIGURE 3.
Effects of E22O injection on growth and hemolymph ecdysteroid titer of B. mori. A, precocious pupation induced by injection of 30 μl of Sf9-E22O-conditioned medium into newly molted penultimate (4th) instar larvae. Left, a control pupa metamorphosed from an intact 5th instar larva; right, a miniature precocious pupa induced by E22O injection. 10 % of the E22O-injected larvae started spinning a week later and half of them pupated precociously. B, molting of the penultimate instar larvae after injecting 30 μl of the conditioned medium of either Sf9 (control) or Sf9-E22O cells just before the head capsule slippage (day 3.5). C, pupal development after injecting 30 μl of the conditioned medium of either Sf9 (control) or Sf9-E22O 2 days after pupation. D, pupal period of control and E22O-injected pupae. Only pupae that eclosed as adults normally were included in the calculation. Asterisks (**) indicate that the values are significantly different (p < 0.01) by Student's t test. E, ecdysteroid titers of control and E22O-injected pupae on day 4 (2 days after the medium injection). Error bars represent S.E. (n = 4).
Molt Inhibition by E22O in Various Insects
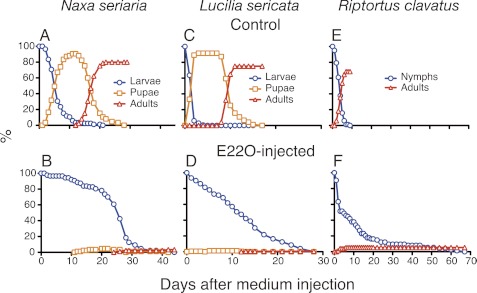
We next examined how E22O would affect the developmental programs in insects other than B. mori. E22O-injected last instar larvae of a geometrid moth Naxa seriaria (Lepidoptera) remained as larvae for a much longer period than the controls, and eventually died without initiating pupation (Fig. 4, A and B). Similar results were obtained with the last instar larvae of the blowfly L. sericata (Diptera) and bean bug R. clavatus (Hemiptera). In both species, the larval-pupal or nymphal-adult metamorphosis was rarely observed after E22O injection, whereas larvae or nymphs injected with the control medium completed metamorphosis within 2 weeks (Fig. 4, C-F). Injection of E22O into the penultimate or last instar larvae of the yellow mealworm T. molitor (Coleoptera) completely suppressed larval molting and 70% of them died as pharate pupae (Table 1). Furthermore, 70% of E22O-injected prepupae died without completing pupation. E22O injection into T. molitor pupae interfered with the normal adult eclosion and many deformed adults with folded or heavily curled wings emerged. Thus, E22O blocked molting and metamorphosis of 4 additional species belonging to different orders.
FIGURE 4.
Effects of E22O injection on the growth of three insects. Last instar larvae or nymphs were injected with 30 (for N. seriaria) or 5 μl (for L. sericata and R. clavatus) of conditioned culture medium of either the control Sf9 cell cells (A, C, and E) or Sf9-E22O cells (B, D, and F), and subsequently their growth was observed. Eighty to 160 individuals were used for each experiment.
TABLE 1.
Effects of injection of Sf9-E22O conditioned medium into T. molitor
| Injected stage | Injected medium | n | Total number of larval molts | Dead larvaa | Dead prepupa | Unsuccessful pupation | Dead pupa | Dead pharate adult | Unsuccessful eclosion | Deformed adultb | Normal adult |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | |||||||||||
| Larvaec | Control medium | 84 | 45 | 33 | 0 | 0 | 2 | 0 | 0 | 0 | 64 |
| E22O medium | 83 | 0 | 27 | 43 | 28 | 0 | 0 | 0 | 0 | 2 | |
| Prepupae | Control medium | 111 | 3 | 8 | 2 | 1 | 0 | 0 | 86 | ||
| E22O medium | 79 | 49 | 24 | 4 | 8 | 1 | 14 | 0 | |||
| Pupae | Control medium | 43 | 2 | 0 | 5 | 0 | 93 | ||||
| E22O medium | 79 | 13 | 10 | 0 | 73 | 4 | |||||
a Individuals that died within 3 days of the injection were excluded from data.
b This category includes adults with folded or heavily curled wings.
c This includes both penultimate and last instar larvae.
Transient Expression of E22O Gene in Silkworm
We have recently established an in vivo lipofection method to express foreign genes in B. mori larvae (28). Using this technique, the E22O gene was expressed in B. mori 3rd instar larvae. Four days after the lipofection, 0.098 pmol/μl/min (46 ng/ml/min, n = 2) of ecdysone-oxidizing activity was observed in the plasma of the E22O-expessing larvae, indicating that the E22O gene was successfully expressed in larval tissues and expressed E22O protein was secreted into hemolymph. The E22O-expressing larvae continued eating in the instar and grew much bigger than the control 3rd instar larvae (Table 2 and supplemental Fig. S4A). Some of them pupated precociously after ecdysis to the 4th instar larvae and feeding food for a week, 3–4 days longer than the control 4th instar larvae (supplemental Fig. S4B). Similar results were obtained by expressing E22O in the 4th instar larvae (supplemental Fig. S4C).
TABLE 2.
Effects of E22O gene expression on the growth of B. mori larvae
The E22O cDNA was introduced into B. mori by in vivo lipofection at the beginning of 3rd larval instar, and larval growth was subsequently monitored.
| Plasmid | n | Average 3rd instar period (day)a | Death during 3rd instar |
Death during 4th instar |
Precocious spinning | Ecdysis into last instar | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Larval death (1–5 days after injection) | Larval death (6–10 days after injection) | Larval death (>10 days after injection) | Unsuccessful ecdysis | Larval death | Unsuccessful ecdysis | |||||
| % | % | |||||||||
| Control plasmid | 110 | 4.7 | 1 | 0 | 0 | 0 | 5 | 1 | 0 | 93 |
| pIZT-E22O | 110 | 7.1 | 2 | 6 | 19 | 6 | 25 | 2 | 7 | 34 |
a Only larvae that molted normally into the 4th instar were included in the calculation. The two values are significantly different (p < 0.01) by Student's t test.
Next, the E22O-expressing plasmid was lipofected into the spinning last instar larvae. Whereas most of these larvae pupated normally, 85% of the pupae could not complete the pupal-adult metamorphosis (Fig. 5A). Half of these pupae grew to pharate adults but could not eclose from the pupal case normally (supplemental Fig. S4D).
We compared the ecdysteroid titers in the hemolymph of the E22O-expressing and control pupae. In controls, the total ecdysteroid titer increased after pupation, reached a peak (∼5 μg/ml) on day 4, and then decreased to the basal level (Fig. 5B); the ecdysone and 20E titers on the other hand peaked at around 500 ng/ml on days 2–3 and 4, respectively, and then decreased rapidly (Fig. 5C). These temporal changes of total ecdysteroid, ecdysone, and 20E were similar to those observed in Manduca sexta pupae (36). The sum of the ecdysone and 20E titers was 800 ng/ml at the maximum, suggesting that the hemolymph contained much more amounts of other ecdysteroids that could react with the antibody used in RIA. In contrast, the total ecdysteroid titer continued to increase in the hemolymph of the E22O-expressing pupae (Fig. 5B). The maximal ecdysone titer (∼400 ng/ml) was observed on day 2, as in the controls, but thereafter the titer maintained a level of more than 100 ng/ml (Fig. 5D). The 20E titer did not show any obvious peak, but 100–300 ng/ml of 20E was present throughout the pupal period. The maximal 20E titer in the E22O-expressing pupae was thus half of that in the controls. Although 22-dehydroecdysone and 22-dehydro-20-hydroxyecdysone were not detected in the controls, large amounts of 22-dehydroecdysteroids, particularly 22-dehydroecdysone, accumulated in the hemolymph of the E22O-expressing pupae (Fig. 5D). The affinity of the antibody used in RIA was 10 times lower to 22-dehydroecdysone than to 20E (see “Experimental Procedures”) and therefore the contribution of 22-dehydroecdysone expressed in the 20E equivalent was expected to be very small, suggesting that large amounts of unidentified ecdysteroids were also present in the hemolymph of the E22O-expressing pupae. Taken together, these results indicate that transient expression of E22O dramatically altered the temporal patterns of the ecdysteroid titers, and affected both larval molting and metamorphosis in B. mori. An injection of purified 22-dehydroecdysone or 22-dehydro-20-hydroxyecdysone into late pupae had no effects (data not shown), suggesting reduction of 20E at the peak time or its sustained presence, not accumulation of those 22-dehydroecdysteroids, caused the developmental abnormalities.
Transgenic Expression of E22O Gene in Fruit Fly
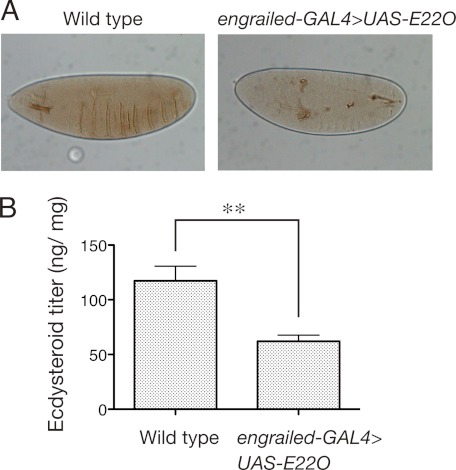
We also examined the effects of overexpression of the E22O gene in the fruit fly D. melanogaster using the GAL4/UAS gene expression system (29). When the UAS-E22O line was crossed with the engrailed-GAL4 line, most (99.8%) of the F1 individuals were embryonic lethal. They developed to around stage 14 and completed segmentation, but then stopped further differentiation. The denticle belt was not formed in 75% of them (Fig. 6A). Similar results were obtained when the UAS-E22O line was crossed with the hsp70-GAL4 line even without heat shock or crossed with the Actin5C-GAL4 line (data not shown). These phenotypes were very similar to those of the ecdysteroid biosynthesis-deficient mutants of Drosophila, such as disembodied, shadow, phantom, spook, and shroud (17), suggesting that the ecdysteroid contents in the E22O-expressing embryos were reduced. In fact, at the peak time of the embryonic ecdysteroid titer in wild type (7–12 h after egg laying, corresponding to stages 12–15) (31), the total ecdysteroid titer in the engrailed-GAL4>UAS-E22O embryos was around half that of wild type embryos (Fig. 6B).
FIGURE 6.
Effects of transgenic E22O expression in D. melanogaster embryo. A, a typical phenotype of engrailed-GAL4>UAS-E22O embryo. Left, wild type; right, engrailed-GAL4>UAS-E22O. Note that even though the segmentation was completed, the denticle belt was not formed in the transgenic fly. B, comparison of the total ecdysteroid titer (20E equivalent) between the wild type and engrailed-GAL4>UAS-E22O embryos. The ecdysteroid titer was measured in batches of eggs collected 7–12 h after the oviposition. Error bars represent S.E. (n = 6–8). Asterisks (**) indicate that the values are significantly different (p < 0.01) by Student's t test.
Induction of Diapause in H. basipunctalis by E22O Injection
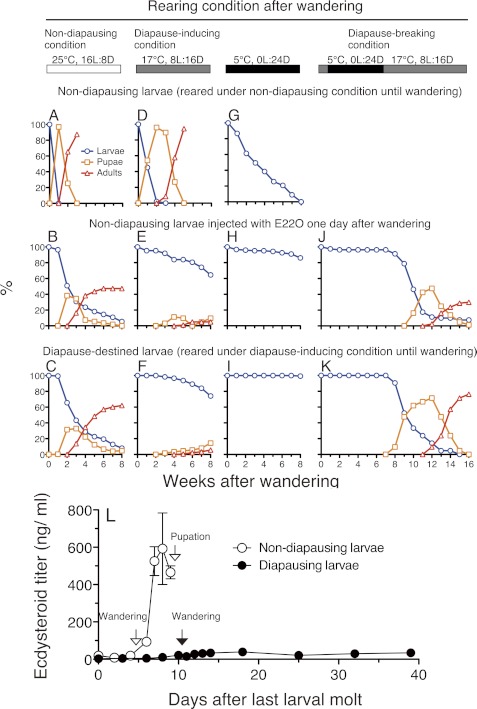
Although it is widely accepted that low ecdysteroid titer is important for the maintenance of larval and pupal diapause (4), it is still unclear whether a reduction of ecdysteroid titer is sufficient to induce diapause. To address this question, we examined the effects of E22O injection on larval diapause of the crambid moth H. basipunctalis.
H. basipunctalis larvae entered diapause in the last larval instar after the wandering behavior when reared at 17 °C under a short day length (diapausing-inducing condition), whereas they pupated and then emerged as adults when reared at 25 °C under a long day length (nondiapausing condition) (Fig. 7, A and F). First, we verified that the hemolymph ecdysteroid titer is kept low in the diapausing H. basipunctalis larvae. In the nondiapausing last instar larvae, the total ecdysteroid was less than 20 ng/ml during the feeding period (Fig. 7L). It increased drastically after the wandering behavior and peaked at around 600 ng/ml 1 day before pupation. In the diapausing last instar larvae, the ecdysteroid titer was less than 20 ng/ml during the feeding period as in the nondiapausing larvae (Fig. 7L). It increased slightly after the wandering behavior but did not exceed 40 ng/ml for the subsequent 4 weeks. Thus, the hemolymph ecdysteroid titer was kept at a much lower level in the diapausing larvae than in the nondiapausing larvae as expected. Those differences in the ecdysteroid titers between the nondiapausing and diapausing H. basipunctalis larvae were very similar to those observed in other insects that enter diapause facultatively, such as larvae of Pimpla instigator (37) and Ostrinia nubilalis (38, 39) and pupae of M. sexta (40), Sarcophaga argyrostoma (41), Boettcherisca peregrina (42), and Mamestra brassicae (43).
FIGURE 7.
Growth, survival, and ecdysteroid titer of H. basipunctalis larvae under different conditions. Nondiapausing larvae reared under nondiapausing conditions (A, D, and G), nondiapausing larvae injected with 30 μl of the conditioned culture medium of the Sf9-E22O cells 1 day after wandering (B, E, and H), and diapause-destined larvae reared under diapause-inducing conditions (C, F, and I) were reared under nondiapausing conditions (A-C), diapause-inducing conditions (D-F), or at 5 °C (G-I) after wandering, and their subsequent growth and survival were monitored. The E22O-injected nondiapausing larvae (J) and intact diapause-destined larvae (K) were reared also under diapause-breaking conditions and their growth was monitored. Sixty to 300 larvae were used for each experiment. L, changes in the total ecdysteroid titer (20E equivalent) in the hemolymph of nondiapausing and diapausing last instar larvae. Larvae were reared under the nondiapausing and diapause-inducing conditions continuously. Their growth are shown in A and F, respectively. Error bars represent S.E. (n = 2–17).
When the conditioned medium of Sf9-E22O cells was injected into the nondiapausing last instar larvae 1 day after the wandering behavior, pupation was delayed and some individuals remained at the larval stage for very long periods (Fig. 7B). When those larvae were transferred to the diapause-inducing condition or kept at 5 °C, in both cases most of them lived longer than 2 months as larvae without eating anything (Fig. 7, E and H). These developmental characteristics were very similar to those of the diapause-destined larvae (Fig. 7, C, F, and I) but distinct from those of the intact nondiapausing larvae (Fig. 7, A, D, and G). Particularly, all of intact nondiapausing larvae transferred to 5 °C died within 2 months, indicating that E22O injection rapidly imparted high cold hardiness to H. basipunctalis larvae.
When the E22O-injected larvae were reared under diapause-breaking conditions (5 °C for 6 weeks and then transferred to diapause-inducing condition), they pupated and then emerged as adults, as did diapausing larvae (Fig. 5, J and K). The E22O-injected nondiapausing larvae were thus in a physiologically similar state to that of the diapausing larvae, which strongly suggest that E22O injection induced diapause or a diapause-like state in the N. basipunctalis larvae. As far as we know, these results are the first evidence indicating that a reduction in the ecdysteroid titer is a sufficient endocrinological stimulus to induce diapause in insects.
DISCUSSION
We have characterized the ecdysteroid inactivation enzyme E22O from an entomopathogenic fungus N. rileyi and have shown that both injection of the recombinant E22O protein and forced expression of the E22O gene reduce the internal ecdysteroid titer and affect the development and physiology of several insect taxa. The E22O-modified phenomena included embryogenesis, larval-larval molt, larval-pupal metamorphosis, pupal- or nymphal-adult metamorphosis, and diapause. Thus, E22O influenced most of the major ecdysteroid-regulated events during insect development.
Injection of recombinant E22O protein or transient expression of the E22O gene inhibited molting and metamorphosis in various insect species. In N. seriaria, L. sericata, and R. clavatus, the E22O-injected last instar larvae or nymphs remained at the stage they were injected for a long period until they died without initiating molt. It is most likely that the injected E22O maintained the titers of ecdysteroids in the hemolymph, particularly that of 20E, below the thresholds to induce metamorphosis in these species. In contrast, more than half of E22O-injected B. mori and T. molitor larvae eventually initiated molting responses although many of them could not complete the molting, suggesting that E22O suppressed the rise of ecdysteroid titer incompletely or changed its temporal profile and thereby interfered with molting and metamorphosis. In fact, a lower but significant level of 20E was continuously present in B. mori pupae that were forced to express the E22O gene. These differences imply that detailed mechanisms underlying the molt inhibition caused by E22O may vary according to the insect species.
Reduction of ecdysteroid titers either by injection of E22O protein or by expression of the E22O gene induced precocious metamorphosis in B. mori. These results may appear strange because precocious metamorphosis is widely known to be a typical phenotype of the lack of juvenile hormone (1). However, it was also reported that the B. mori 4th instar larvae, in which the ecdysteroid synthesis was suppressed by using the imidazole compound KK-42, pupated precociously (44). In addition, there are some reports showing that the ecdysteroid deficiency causes precocious metamorphosis also in D. melanogaster (45–47). The precocious pupation in D. melanogaster, induced as a result of lowered ecdysteroid titer, was explained as follows: due to the low ecdysteroid titer, the penultimate instar larvae were unable to initiate the last larval-larval molt and kept on feeding, and consequently grew to the point where they surpassed the “critical weight for precocious pupation,” although what eventually initiated the precocious pupation still remains unknown (46). Because the 4th larval period of the E22O-treated B mori larvae was also prolonged, a similar mechanism may underlie in the induction of precocious pupation in B. mori.
Among the multiple applications of E22O tested here, transgenic insects carrying the E22O gene seem to be an ideal system for controlling the ecdysteroid titer at any stage of the development. The E22O-expressing transgenic D. melanogaster were, however, all embryonic lethal irrespective of the GAL4 driver lines used. These results were in contrast to those of the ecdysteroid 26-hydroxylase (cyp18A1)-expressing flies, where the time of death changed from the embryonic stage to the last larval instar stage depending on the GAL4 driver line used for crossing (21). Thus, contrary to our expectation, the E22O gene appears too strong to use in transgenic flies, suggesting that mutations to moderate the enzymatic activity of E22O may be necessary for its transgenic use.
Using E22O, we not only confirmed the importance of ecdysteroids in embryogenesis, larval molting, and metamorphoses, but also answered an unsolved question associated with diapause. E22O-injected H. basipunctalis last instar larvae remained as larvae for a long period as found with N. seriaria, L. sericata, and R. clavatus. We assumed that this was not a simple developmental arrest, but an artificially induced diapause or a diapause-like state based on the following three reasons. First, the E22O-injected H. basipunctalis larvae survived much longer than the above three species without eating anything. Second, they acquired high cold-hardiness, a characteristic often observed in insects in diapause (4). Finally, and more importantly, they resumed development after being exposed to chilling conditions, which is the environmental stimulus to break diapause in many insect species (4). These developmental and survival characteristics were indistinguishable from those of the normal diapause-destined larvae. Many researches suggested that larval and pupal diapauses are maintained by ecdysteroid deficiency (37–43). The hemolymph ecdysteroid titer was kept at a low level also in diapausing H. basipunctalis larvae. However, the endocrinological cue to trigger diapause is still not very clear, whereas a low ecdysteroid titer is obviously necessary for insects to enter diapause. Our results strongly suggest that a reduction in the ecdysteroid titer is a key signal to coordinately induce multiple diapause-associated physiological changes in N. basipunctalis including developmental arrest, enhancement of cold hardiness, and programming to resume development, although more detailed measurements of ecdysteroid titer and examination of other physiological properties would be necessary to achieve the final goal. It would also be interesting to use E22O to examine whether ecdysteroids play a similar role in inducing larval or pupal diapause in other insects.
In conclusion, the potent ecdysteroid inactivation enzyme E22O could be used as an artificial ecdysteroid titer-reducing tool to manipulate multiple ecdysteroid-regulated phenomena. Particularly, injection of the recombinant E22O protein, a relatively simple technique, is potentially applicable to all insects and other arthropods. Judicious applications of methods utilizing E22O could obtain answers to a wide range of ecdysteroid-associated developmental and physiological questions, including uncovering new functions of ecdysteroids.
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center and the Drosophila Genetic Resource Center at the Kyoto Institute of Technology for providing the fly strains, Dr. Seiji Tanaka, National Institute of Agrobiological Sciences (NIAS), for critically reading the manuscript, and Yuka Ito, NIAS, for maintaining the cell lines.
This work was supported by a grant-in-aid (Bio Design Program) from the Ministry of Agriculture, Forestry and Fisheries, Grants-in-aid for Scientific Research 21580070 and 22380039, and the Special Coordination Funds for Promotion Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japanese government.

This article contains supplemental Figs. S1–S4.
The nucleotide sequence(s) reported in this paper has been submitted to the DDBJ/GenBankTM/EBI Data Bank with accession number(s) AB675078.
- 20E
- 20-hydroxyecdysone
- E22O
- ecdysteroid-22-oxidase.
REFERENCES
- 1. Riddiford L. M. (1985) in Comprehensive Insect Physiology, Biochemistry, and Pharmacology (Kerkut G. A., Gilbert L. I., eds) pp. 37–84, Pergamon Press, Oxford [Google Scholar]
- 2. Sonobe H., Yamada R. (2004) Ecdysteroids during early embryonic development in silkworm Bombyx mori. Metabolism and functions. Zool. Sci. 21, 503–516 [DOI] [PubMed] [Google Scholar]
- 3. Raikhel A. S., Brown M. R., Belles X. (2005) in Comprehensive Molecular Insect Science (Gilbert L. I., Iatrou K., Gill S. S., eds) pp. 433–491, Elsevier, Oxford [Google Scholar]
- 4. Denlinger D. L., Yocum G. D., Rinehart J. P. (2005) in Comprehensive Molecular Insect Science (Gilbert L. I., Iatrou K., Gill S. S., eds) pp. 615–650, Elsevier, Oxford [Google Scholar]
- 5. Lafont R., Dauphin-Villemant C., Warren J. T., Rees H. (2005) in Comprehensive Molecular Insect Science (Gilbert L. I., Iatrou K., Gill S. S., eds) pp. 125–195, Elsevier, Oxford [Google Scholar]
- 6. Warren J. T., Petryk A., Marques G., Jarcho M., Parvy J. P., Dauphin-Villemant C., O'Connor M. B., Gilbert L. I. (2002) Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 99, 11043–11048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petryk A., Warren J. T., Marqués G., Jarcho M. P., Gilbert L. I., Kahler J., Parvy J. P., Li Y., Dauphin-Villemant C., O'Connor M. B. (2003) Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. U.S.A. 100, 13773–13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niwa R., Matsuda T., Yoshiyama T., Namiki T., Mita K., Fujimoto Y., Kataoka H. (2004) CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J. Biol. Chem. 279, 35942–35949 [DOI] [PubMed] [Google Scholar]
- 9. Warren J. T., Petryk A., Marqués G., Parvy J. P., Shinoda T., Itoyama K., Kobayashi J., Jarcho M., Li Y., O'Connor M. B., Dauphin-Villemant C., Gilbert L. I. (2004) Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori. A P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 34, 991–1010 [DOI] [PubMed] [Google Scholar]
- 10. Namiki T., Niwa R., Sakudoh T., Shirai K., Takeuchi H., Kataoka H. (2005) Cytochrome P450 CYP307A1/Spook, a regulator for ecdysone synthesis in insects. Biochem. Biophys. Res. Commun. 337, 367–374 [DOI] [PubMed] [Google Scholar]
- 11. Ono H., Rewitz K. F., Shinoda T., Itoyama K., Petryk A., Rybczynski R., Jarcho M., Warren J. T., Marqués G., Shimell M. J., Gilbert L. I., O'Connor M. B. (2006) Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 298, 555–570 [DOI] [PubMed] [Google Scholar]
- 12. Yoshiyama T., Namiki T., Mita K., Kataoka H., Niwa R. (2006) Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development 133, 2565–2574 [DOI] [PubMed] [Google Scholar]
- 13. Maeda S., Nakashima A., Yamada R., Hara N., Fujimoto Y., Ito Y., Sonobe H. (2008) Molecular cloning of ecdysone 20-hydroxylase and expression pattern of the enzyme during embryonic development of silkworm Bombyx mori. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 149, 507–516 [DOI] [PubMed] [Google Scholar]
- 14. Niwa R., Namiki T., Ito K., Shimada-Niwa Y., Kiuchi M., Kawaoka S., Kayukawa T., Banno Y., Fujimoto Y., Shigenobu S., Kobayashi S., Shimada T., Katsuma S., Shinoda T. (2010) Nonmolting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the “Black Box” of the ecdysteroid biosynthesis pathway. Development 137, 1991–1999 [DOI] [PubMed] [Google Scholar]
- 15. Yoshiyama-Yanagawa T., Enya S., Shimada-Niwa Y., Yaguchi S., Haramoto Y., Matsuya T., Shiomi K., Sasakura Y., Takahashi S., Asashima M., Kataoka H., Niwa R. (2011) The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J. Biol. Chem. 286, 25756–25762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ou Q., Magico A., King-Jones K. (2011) Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 9, e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chávez V. M., Marqués G., Delbecque J. P., Kobayashi K., Hollingsworth M., Burr J., Natzle J. E., O'Connor M. B. (2000) The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development 127, 4115–4126 [DOI] [PubMed] [Google Scholar]
- 18. Takeuchi H., Chen J. H., O'Reilly D. R., Rees H. H., Turner P. C. (2000) Regulation of ecdysteroid signaling. Molecular cloning, characterization, and expression of 3-dehydroecdysone 3α-reductase, a novel eukaryotic member of the short-chain dehydrogenases/reductases superfamily from the cotton leafworm, Spodoptera littoralis. Biochem. J. 349, 239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeuchi H., Chen J. H., O'Reilly D. R., Turner P. C., Rees H. H. (2001) Regulation of ecdysteroid signaling. Cloning and characterization of ecdysone oxidase. A novel steroid oxidase from the cotton leafworm, Spodoptera littoralis. J. Biol. Chem. 276, 26819–26828 [DOI] [PubMed] [Google Scholar]
- 20. Sonobe H., Ohira T., Ieki K., Maeda S., Ito Y., Ajimura M., Mita K., Matsumoto H., Wilder M. N. (2006) Purification, kinetic characterization, and molecular cloning of a novel enzyme, ecdysteroid 22-kinase. J. Biol. Chem. 281, 29513–29524 [DOI] [PubMed] [Google Scholar]
- 21. Guittard E., Blais C., Maria A., Parvy J. P., Pasricha S., Lumb C., Lafont R., Daborn P. J., Dauphin-Villemant C. (2011) CYP18A1, a key enzyme of Drosophila steroid hormone inactivation, is essential for metamorphosis. Dev. Biol. 349, 35–45 [DOI] [PubMed] [Google Scholar]
- 22. Rewitz K. F., Yamanaka N., O'Connor M. B. (2010) Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev. Cell 19, 895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang H. J., Wang M. X., Zhang P., Sabhat A., Malik F. A., Bhaskar R., Zhou F., Li X. H., Hu J. B., Sun C. G., Niu Y. S., Miao Y. G. (2011) Cloning and characterizatio of the Bombyx mori ecdysone oxidase. Arch. Insect Biochem. Physiol. 78, 17–29 [DOI] [PubMed] [Google Scholar]
- 24. O'Reilly D. R. (1995) Baculovirus-encoded ecdysteroid UDP-glucosyltransferase. Insect Biochem. Mol. Biol. 25, 541–550 [Google Scholar]
- 25. Kiuchi M., Yasui H., Hayasaka S., Kamimura M. (2003) Entomogenous fungus Nomuraea rileyi inhibits host insect molting by C22-oxidizing inactivation of hemolymph ecdysteroids. Arch. Insect Biochem. Physiol. 52, 35–44 [DOI] [PubMed] [Google Scholar]
- 26. Cory J. S., Wilson K. R., Hails R. S., O'Reilly D. R. (2001) in Endocrine interactions of insect parasites and pathogens (Edwards J. P., Weaver R. J., eds) pp. 233–244, BIOS Scientific Publishers, Oxford [Google Scholar]
- 27. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004) Improved prediction of signal peptides, SignalP 3.0. J. Mol. Biol. 340, 783–795 [DOI] [PubMed] [Google Scholar]
- 28. Kanamori Y., Hayakawa Y., Matsumoto H., Yasukochi Y., Shimura S., Nakahara Y., Kiuchi M., Kamimura M. (2010) A eukaryotic (insect) tricistronic mRNA encodes three proteins selected by context-dependent scanning. J. Biol. Chem. 285, 36933–36944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brand A. H., Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- 30. Rubin G. M., Spradling A. C. (1982) Genetic transformation of Drosophila with transposable element vectors. Science 218, 348–353 [DOI] [PubMed] [Google Scholar]
- 31. Sullivan A. A., Thummel C. S. (2003) Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol. Endocrinol. 17, 2125–2137 [DOI] [PubMed] [Google Scholar]
- 32. Takeda S., Kiuchi M., Ueda S. (1986) Preparation of anti-20-hydroxyecdysone serum and its application for radioimmunoassay of ecdysteroids in silkworm hemolymph. Bull. Seric. Exp. Stn. 30, 361–374 (in Japanese with English summary) [Google Scholar]
- 33. Evans O. P., O'reilly D. R. (1998) Purification and kinetic analysis of a baculovirus ecdysteroid UDP-glucosyltransferase. Biochem. J. 330, 1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reeves C. D., Hu Z., Reid R., Kealey J. T. (2008) Genes for the biosynthesis of the fungal polyketides hypothemycin from Hypomyces subiculosus and radicicol from Pochonia chlamydosporia. Appl. Environ. Microbiol. 74, 5121–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin J. M., Lee S., Lee J., Baek S. R., Kim J. C., Yun S. H., Park S. Y., Kang S., Lee Y. W. (2010) Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. Mol. Microbiol. 76, 456–466 [DOI] [PubMed] [Google Scholar]
- 36. Warren J. T., Gilbert L. I. (1986) Ecdysone metabolism and distribution during the pupal-adult development of Manduca sexta. Insect Biochem. 16, 65–82 [Google Scholar]
- 37. Claret J., Porcheron P., Dray F. (1978) The content in circulating ecdysones during the last larval stage of the hymenopteran endoparasite Pimpla instigator and the entry into diapause. C. R. Acad. Sci. D 286, 639–641 (in French) [Google Scholar]
- 38. Bean D. W., Beck S. D. (1983) Hemolymph ecdysteroid titres in diapause and nondiapause larvae of the European corn borer, Ostrinia nubilalis. J. Insect Physiol. 29, 687–693 [Google Scholar]
- 39. Gelman D. B., Woods C. W. (1983) Hemolymph ecdysteroid titres of diapause and nondiapause larvae fifth instars and pupae of the European corn borer, Ostrinia nubilalis. Comp. Biochem. Physiol. A 76, 367–375 [Google Scholar]
- 40. Bowen M. F., Bollenbacher W. E., Gilbert L. I. (1984) In vitro studies on the role of the brain and prothoracic glands in the pupal diapause of Manduca sexta. J. Exp. Biol. 108, 9–24 [Google Scholar]
- 41. Richard D. S., Warrent J. T., Saunders D. S., Gilbert L. I. (1987) Hemolymph ecdysteroid titres in diapause- and nondiapause-destined larvae and pupae of Sarcophaga argyrostoma. J. Insect Physiol. 33, 115–122 [Google Scholar]
- 42. Moribayashi A., Kurahashi H., Ohtaki T. (1988) Different profiles of ecdysone secretion and it metabolism between diapause- and nondiapause-destined cultures of the fleshfly, Boettcherisca peregrina. Comp. Biochem. Physiol. A 91, 157–164 [Google Scholar]
- 43. Endo K., Fujimoto Y., Kondo M., Yamanaka A., Watanabe M., Weihua K., Kumagai K. (1997) Stage-dependent changes of the prothoracicotropic hormone (PTTH) activity of brain extracts and PTTH sensitivity of the prothoracic glands in the cabbage armyworm, Mamestra brassicae, before and during winter and aestival pupal dispause. Zool. Sci. 14, 127–133 [Google Scholar]
- 44. Kadono-Okuda K., Kuwano E., Eto M., Yamashita O. (1987) Inhibitory action of an imidazole compound on ecdysone synthesis in prothoracic glands of the silkworm, Bombxy mori. Dev. Growth Differ. 29, 527–533 [DOI] [PubMed] [Google Scholar]
- 45. Bialecki M., Shilton A., Fichtenberg C., Segraves W. A., Thummel C. S. (2002) Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell 3, 209–220 [DOI] [PubMed] [Google Scholar]
- 46. Zhou X., Zhou B., Truman J. W., Riddiford L. M. (2004) Overexpression of broad. A new insight into its role in the Drosophila prothoracic gland cells. J. Exp. Biol. 207, 1151–1161 [DOI] [PubMed] [Google Scholar]
- 47. Gibbens Y. Y., Warren J. T., Gilbert L. I., O'Connor M. B. (2011) Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/activin signaling. Development 138, 2693–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.