Abstract
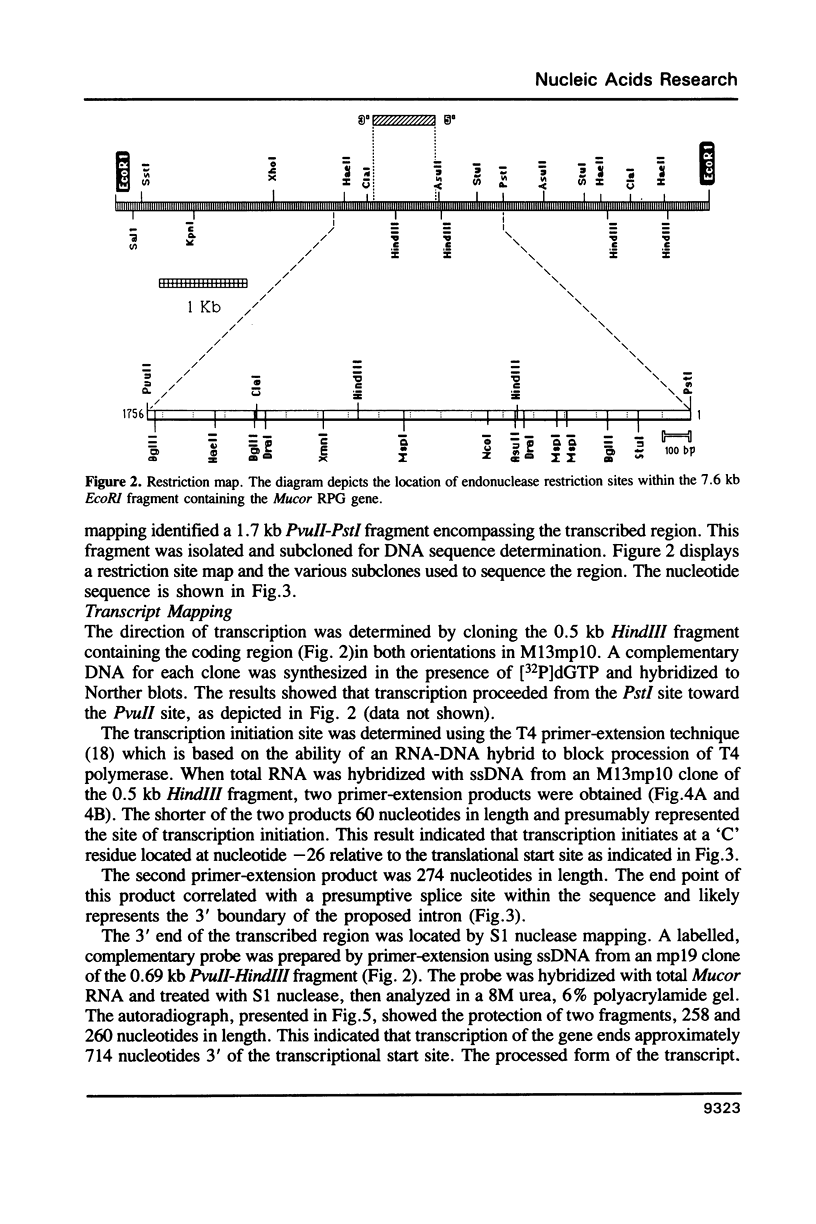
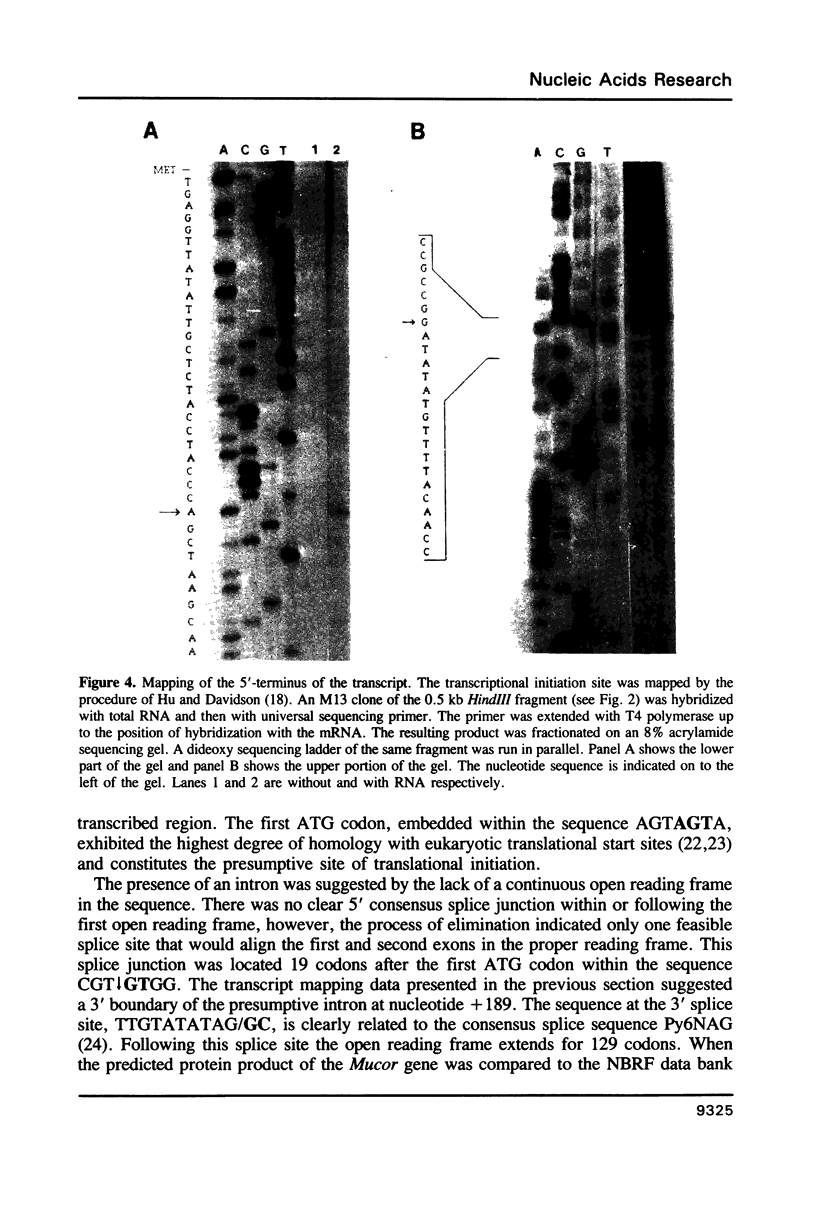
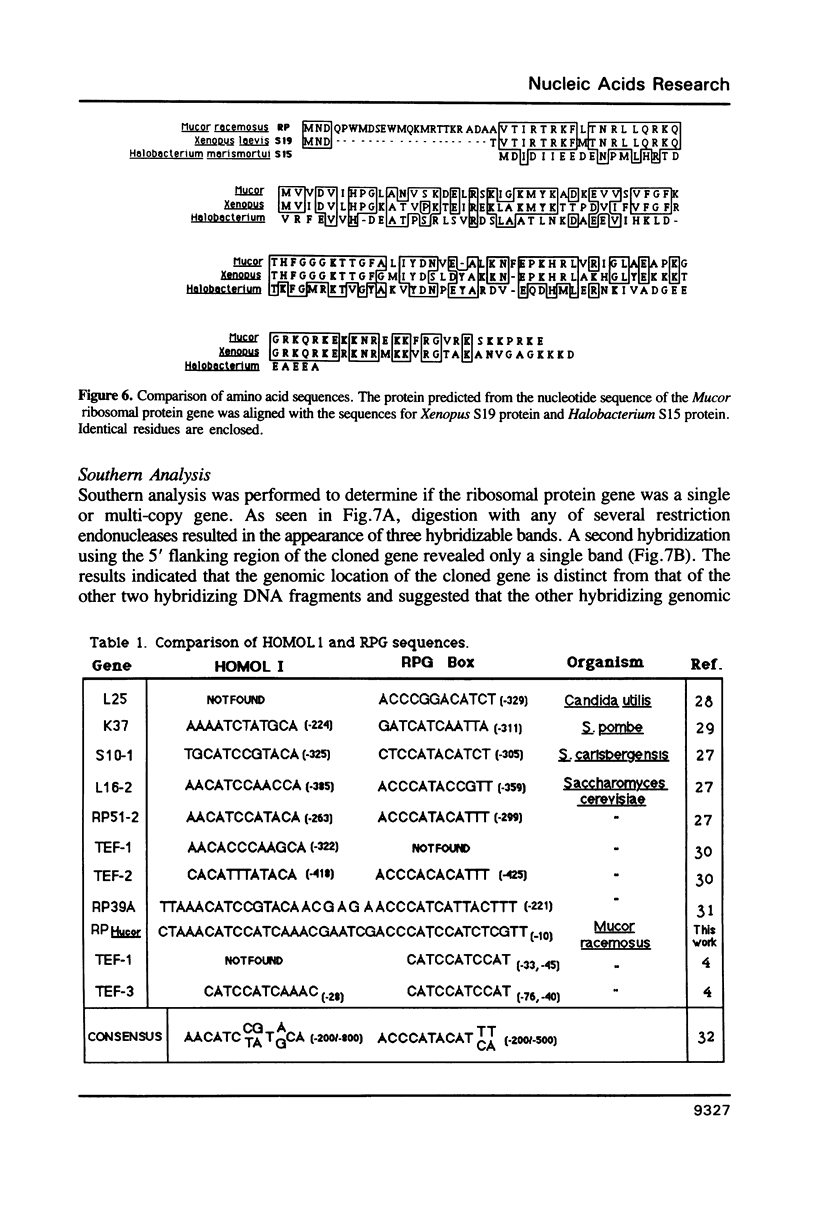
As an extension of our analysis of the translational apparatus of Mucor racemosus we have isolated a gene encoding a ribosomal protein of Mucor. Based on a method developed for S. cerevisiae, we identified by hybrid selection and in vitro translation a lambda-Charon 4A clone containing the genomic copy of a Mucor ribosomal protein. The gene consisted of two exons of 57 and 387 nucleotides. The two exons were separated by an 131 nucleotide intron. The processed transcript was 714 nucleotides in length and contained a 25 nucleotide untranscribed leader and an 114 nucleotide untranscribed 3'-end. The protein predicted from the nucleotide sequence contained 148 amino acids and exhibited 61% identity with the S19 ribosomal protein of Xenopus laevis. The promoter region of the gene contained sequences highly homologous to the RPG and Homol1 promoter elements found in S. cerevisiae. Southern blot analysis indicated that the Mucor genome contains three copies of this gene.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Beccari E., Bozzoni I., Luo Z. X., Pierandrei-Amaldi P. Nucleotide sequences of cloned cDNA fragments specific for six Xenopus laevis ribosomal proteins. Gene. 1982 Mar;17(3):311–316. doi: 10.1016/0378-1119(82)90147-0. [DOI] [PubMed] [Google Scholar]
- Bollen G. H., Molenaar C. M., Cohen L. H., van Raamsdonk-Duin M. M., Mager W. H., Planta R. J. Ribosomal protein genes of yeast contain intervening sequences. Gene. 1982 Apr;18(1):29–37. doi: 10.1016/0378-1119(82)90053-1. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Chen I. T., Dixit A., Rhoads D. D., Roufa D. J. Homologous ribosomal proteins in bacteria, yeast, and humans. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6907–6911. doi: 10.1073/pnas.83.18.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihlar R. L., Sypherd P. S. The organization of the ribosomal RNA genes in the fungus Mucor racemosus. Nucleic Acids Res. 1980 Feb 25;8(4):793–804. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fonzi W. A., Katayama C., Leathers T., Sypherd P. S. Regulation of protein synthesis factor EF-1 alpha in Mucor racemosus. Mol Cell Biol. 1985 May;5(5):1100–1103. doi: 10.1128/mcb.5.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R., Watanabe C. K., de Boer H. A. Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 1987 Apr 24;15(8):3581–3593. doi: 10.1093/nar/15.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. C., Davidson N. Mapping transcription start points on cloned genomic DNA with T4 DNA polymerase: a precise and convenient technique. Gene. 1986;42(1):21–29. doi: 10.1016/0378-1119(86)90146-0. [DOI] [PubMed] [Google Scholar]
- Huet J., Cottrelle P., Cool M., Vignais M. L., Thiele D., Marck C., Buhler J. M., Sentenac A., Fromageot P. A general upstream binding factor for genes of the yeast translational apparatus. EMBO J. 1985 Dec 16;4(13A):3539–3547. doi: 10.1002/j.1460-2075.1985.tb04114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Sentenac A. TUF, the yeast DNA-binding factor specific for UASrpg upstream activating sequences: identification of the protein and its DNA-binding domain. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3648–3652. doi: 10.1073/pnas.84.11.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Arndt E., Kimura M. Primary structures of three highly acidic ribosomal proteins S6, S12 and S15 from the archaebacterium Halobacterium marismortui. FEBS Lett. 1987 Nov 16;224(1):65–70. doi: 10.1016/0014-5793(87)80423-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Larsen A., Sypherd P. S. Physiological control of phosphorylation ribosomal protein S6 in Mucor racemosus. J Bacteriol. 1980 Jan;141(1):20–25. doi: 10.1128/jb.141.1.20-25.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A., Sypherd P. Ribosomal proteins of the dimorphic fungus, Mucor racemosus. Mol Gen Genet. 1979 Aug;175(1):99–109. doi: 10.1007/BF00267861. [DOI] [PubMed] [Google Scholar]
- Leer R. J., Van Raamsdonk-Duin M. M., Mager W. H., Planta R. J. Conserved sequences upstream of yeast ribosomal protein genes. Curr Genet. 1985;9(4):273–277. doi: 10.1007/BF00419955. [DOI] [PubMed] [Google Scholar]
- Linz J. E., Katayama C., Sypherd P. S. Three genes for the elongation factor EF-1 alpha in Mucor racemosus. Mol Cell Biol. 1986 Feb;6(2):593–600. doi: 10.1128/mcb.6.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Nagashima K., Tsunetsugu-Yokota Y., Fujimura K., Miyazaki M., Kaziro Y. Polypeptide chain elongation factor 1 alpha (EF-1 alpha) from yeast: nucleotide sequence of one of the two genes for EF-1 alpha from Saccharomyces cerevisiae. EMBO J. 1984 Aug;3(8):1825–1830. doi: 10.1002/j.1460-2075.1984.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwint R. T., Molenaar C. M., van Bommel J. H., van Raamsdonk-Duin M. M., Mager W. H., Planta R. J. The gene for yeast ribosomal protein S31 contains an intron in the leader sequence. Curr Genet. 1985;10(1):1–5. doi: 10.1007/BF00418486. [DOI] [PubMed] [Google Scholar]
- Nischt R., Gross T., Gatermann K., Swida U., Käufer N. Sequence and regulatory responses of a ribosomal protein gene from the fission yeast Schizosaccharomyces pombe. Nucleic Acids Res. 1987 Feb 25;15(4):1477–1492. doi: 10.1093/nar/15.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Sypherd P. S. Regulation of translation rate during morphogenesis in the fungus Mucor. Biochemistry. 1978 Feb 21;17(4):569–575. doi: 10.1021/bi00597a002. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Harris P. K., Woolford J. L., Jr, Teem J. L. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981 Jun;24(3):679–686. doi: 10.1016/0092-8674(81)90094-5. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Woolford J. L., Jr Tripartite upstream promoter element essential for expression of Saccharomyces cerevisiae ribosomal protein genes. Mol Cell Biol. 1986 Feb;6(2):674–687. doi: 10.1128/mcb.6.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kuwano Y., Ishikawa K., Ogata K. Nucleotide sequence of cloned cDNA specific for rat ribosomal protein S11. J Biol Chem. 1985 May 25;260(10):6329–6333. [PubMed] [Google Scholar]
- Teem J. L., Abovich N., Kaufer N. F., Schwindinger W. F., Warner J. R., Levy A., Woolford J., Leer R. J., van Raamsdonk-Duin M. M., Mager W. H. A comparison of yeast ribosomal protein gene DNA sequences. Nucleic Acids Res. 1984 Nov 26;12(22):8295–8312. doi: 10.1093/nar/12.22.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teem J. L., Rosbash M. Expression of a beta-galactosidase gene containing the ribosomal protein 51 intron is sensitive to the rna2 mutation of yeast. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4403–4407. doi: 10.1073/pnas.80.14.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Wagner M., Perry R. P. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol Cell Biol. 1985 Dec;5(12):3560–3576. doi: 10.1128/mcb.5.12.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Hereford L. M., Rosbash M. Isolation of cloned DNA sequences containing ribosomal protein genes from Saccharomyces cerevisiae. Cell. 1979 Dec;18(4):1247–1259. doi: 10.1016/0092-8674(79)90236-8. [DOI] [PubMed] [Google Scholar]
- Woudt L. P., Mager W. H., Beek J. G., Wassenaar G. M., Planta R. J. Structural and putative regulatory sequences of the gene encoding ribosomal protein L25 in Candida utilis. Curr Genet. 1987;12(3):193–198. doi: 10.1007/BF00436878. [DOI] [PubMed] [Google Scholar]
- Woudt L. P., Mager W. H., Nieuwint R. T., Wassenaar G. M., van der Kuyl A. C., Murre J. J., Hoekman M. F., Brockhoff P. G., Planta R. J. Analysis of upstream activation sites of yeast ribosomal protein genes. Nucleic Acids Res. 1987 Aug 11;15(15):6037–6048. doi: 10.1093/nar/15.15.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudt L. P., Smit A. B., Mager W. H., Planta R. J. Conserved sequence elements upstream of the gene encoding yeast ribosomal protein L25 are involved in transcription activation. EMBO J. 1986 May;5(5):1037–1040. doi: 10.1002/j.1460-2075.1986.tb04319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]