Abstract
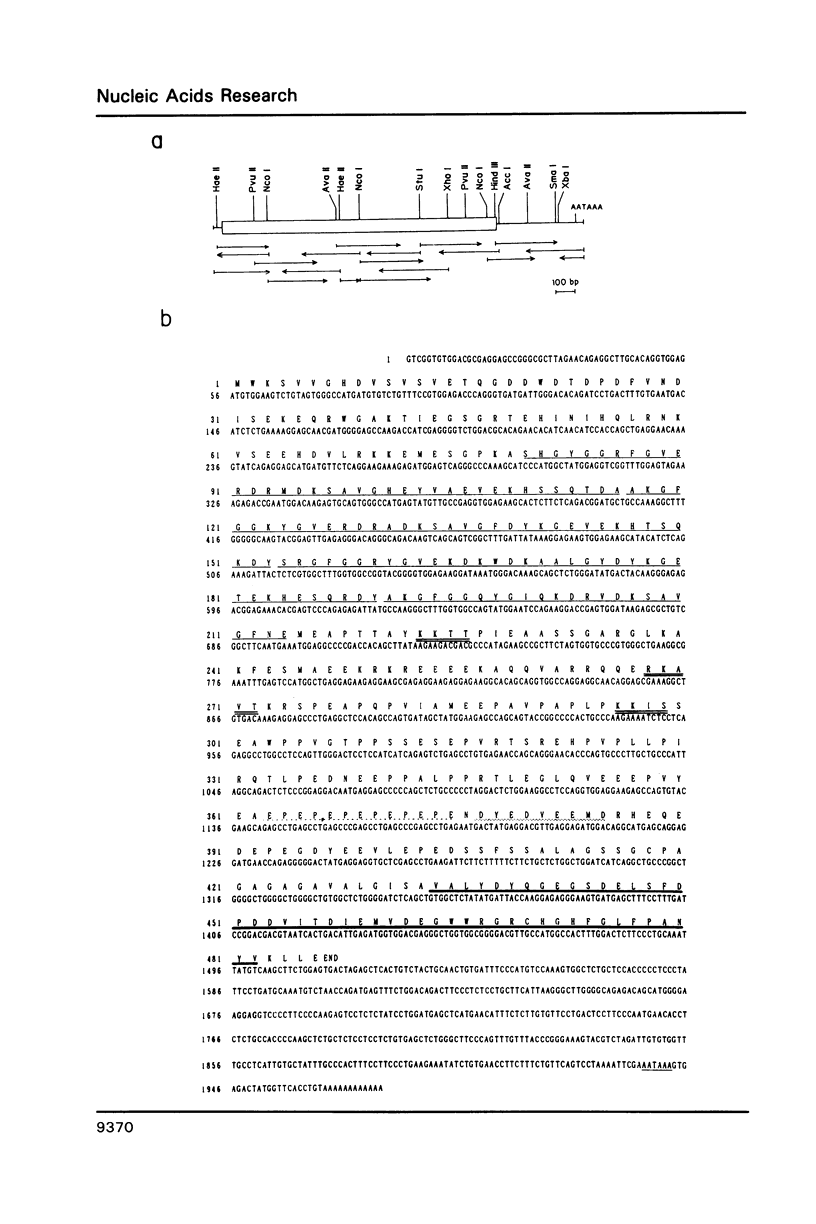
A novel cDNA clone designated as HS1, which show an expression pattern limited to human hematopoietic cells, was isolated. About 2kb mRNA of the clone was accumulated in all the mature and immature lymphoid and myeloid cell lines tested, and two of three erythroblastoid cell lines, but not in any cell lines of non-hematopoietic tissues. The same mRNA was also detected in normal lymphoid and myeloid tissues and peripheral blood lymphocytes, granulocytes and macrophages, but again not in non-hematopoietic tissues. Nucleotide sequence of the HS1 predicts a protein of 486 amino acids (Mr 53,931). N-terminal half of the protein retains unique repeating motifs, each of which shows a significant homology with the helix-turn-helix DNA-binding motif of several proteins reported previously. C-terminal half of the protein retains a region conserved between non-receptor tyrosine kinases (src family), phospholipase C(PLC)-148 and the crk oncogene product. A unique feature of HS1 suggests that the protein may be involved in signal transduction and regulation of gene expression.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Teitelbaum S. L., Reitsma P., Hall A., Pegg L. E., Trial J., Kahn A. J. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5907–5911. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S. R., Holmes K. L., Morse H. C., 3rd, Potter M. Clonal relationship of the lymphoblastic cell line P388 to the macrophage cell line P388D1 as evidenced by immunoglobulin gene rearrangements and expression of cell surface antigens. J Immunol. 1986 Jun 15;136(12):4695–4699. [PubMed] [Google Scholar]
- Bennett C. F., Balcarek J. M., Varrichio A., Crooke S. T. Molecular cloning and complete amino-acid sequence of form-I phosphoinositide-specific phospholipase C. Nature. 1988 Jul 21;334(6179):268–270. doi: 10.1038/334268a0. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fraser J. K., Berridge M. V. Induction of B-lymphocyte antigens on the chronic myeloid leukemic cell line K562 using sodium butyrate. Exp Hematol. 1987 May;15(4):406–413. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Holmes K. L., Pierce J. H., Davidson W. F., Morse H. C., 3rd Murine hematopoietic cells with pre-B or pre-B/myeloid characteristics are generated by in vitro transformation with retroviruses containing fes, ras, abl, and src oncogenes. J Exp Med. 1986 Aug 1;164(2):443–457. doi: 10.1084/jem.164.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ball C. A., Pfeffer D., Simon M. I. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci U S A. 1988 May;85(10):3484–3488. doi: 10.1073/pnas.85.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M., Kriz R. W., Totty N., Philp R., Meldrum E., Aldape R. A., Knopf J. L., Parker P. J. Determination of the primary structure of PLC-154 demonstrates diversity of phosphoinositide-specific phospholipase C activities. Cell. 1988 Jul 15;54(2):171–177. doi: 10.1016/0092-8674(88)90549-1. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Klinken S. P., Alexander W. S., Adams J. M. Hemopoietic lineage switch: v-raf oncogene converts Emu-myc transgenic B cells into macrophages. Cell. 1988 Jun 17;53(6):857–867. doi: 10.1016/s0092-8674(88)90309-1. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science. 1978 Jun 9;200(4346):1153–1154. doi: 10.1126/science.306682. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Burrows P. D., Grossi C. E., Mestecky J., Cooper M. D. Precursor B cells transformed by Epstein-Barr virus undergo sterile plasma-cell differentiation: J-chain expression without immunoglobulin. Proc Natl Acad Sci U S A. 1988 Feb;85(3):875–879. doi: 10.1073/pnas.85.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Laughon A., Scott M. P. Sequence of a Drosophila segmentation gene: protein structure homology with DNA-binding proteins. Nature. 1984 Jul 5;310(5972):25–31. doi: 10.1038/310025a0. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Maeda K., Nakashima M., Komori S., Watanabe T. Trans-acting regulatory factors for T cell antigen receptor alpha- and gamma-chain gene expression. J Immunol. 1988 Apr 15;140(8):2796–2801. [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Stone J. C., Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986 Dec;6(12):4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevitz R. W., Otwinowski Z., Joachimiak A., Lawson C. L., Sigler P. B. The three-dimensional structure of trp repressor. 1985 Oct 31-Nov 6Nature. 317(6040):782–786. doi: 10.1038/317782a0. [DOI] [PubMed] [Google Scholar]
- Seremetis S. V., Pelicci P. G., Tabilio A., Ubriaco A., Grignani F., Cuttner J., Winchester R. J., Knowles D. M., 2nd, Dalla-Favera R. High frequency of clonal immunoglobulin or T cell receptor gene rearrangements in acute myelogenous leukemia expressing terminal deoxyribonucleotidyltransferase. J Exp Med. 1987 Jun 1;165(6):1703–1712. doi: 10.1084/jem.165.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Moon K. H., Suh H. W., Rhee S. G. Cloning and sequence of multiple forms of phospholipase C. Cell. 1988 Jul 15;54(2):161–169. doi: 10.1016/0092-8674(88)90548-x. [DOI] [PubMed] [Google Scholar]
- Tetteroo P. A., Massaro F., Mulder A., Schreuder-van Gelder R., von dem Borne A. E. Megakaryoblastic differentiation of proerythroblastic K562 cell-line cells. Leuk Res. 1984;8(2):197–206. doi: 10.1016/0145-2126(84)90143-7. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Nishiyama K., Araki K., Kitamura D., Watanabe T. Purification of an octamer sequence (ATGCAAAT)-binding protein from human B cells. Nucleic Acids Res. 1987 Dec 23;15(24):10105–10116. doi: 10.1093/nar/15.24.10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yamasaki N., Komori S., Watanabe T. Complementary DNA for a human subgroup IV immunoglobulin lambda-chain. Mol Immunol. 1987 Sep;24(9):981–985. doi: 10.1016/0161-5890(87)90010-1. [DOI] [PubMed] [Google Scholar]