Summary
Elevated hippocampal activation is observed in conditions that confer risk for Alzheimer's disease, including amnestic mild cognitive impairment (aMCI). Studies in relevant animal models have indicated that over-activity in selective hippocampal circuits contributes to cognitive impairment. Here we tested the effect of reducing hippocampal activation in aMCI. Under placebo treatment, hippocampal activation in the dentate gyrus/CA3 was elevated in aMCI patients compared to a healthy control group. By using a low dose of the antiepileptic levetiracetam hippocampal activation in aMCI was reduced to a level that did not differ from the control group. Compared to aMCI memory performance under placebo, performance in the scanning task was significantly improved under drug treatment. Contrary to the view that greater hippocampal activation might serve a beneficial function, these results support the view that increased hippocampal activation in aMCI is a dysfunctional condition and that targeting excess hippocampal activity has therapeutic potential.
Introduction
In studies using functional magnetic resonance imaging (fMRI), elevated hippocampal activation is observed in a number of conditions that confer risk for Alzheimer’s Disease (AD), including cognitively normal carriers of the ApoE4 allele (Bookheimer et al., 2000; Trivedi et al., 2008; Filippini et al., 2009; Dennis et al., 2010), pre-symptomatic carriers of genetic mutations for familial AD (Quiroz et al., 2010), and patients with amnestic mild cognitive impairment (aMCI) (Dickerson et al., 2004; Dickerson et al., 2005; Celone et al., 2006; Hämäläinen et al., 2007), although patients with late aMCI and early AD show reduced hippocampal activity (Celone et al., 2006; for a review see Ewers et al., 2011). In the case of early aMCI, a condition in which memory is worse than would be expected for a person’s age, such increased hippocampal activation has been suggested to serve a beneficial compensatory function by recruiting additional neural resources. An alternative view is that excess activation directly contributes to memory impairment and may be tied to widespread degenerative processes in prodromal AD (Putcha et al., 2011).
Supporting the possibility of adverse consequences, studies in a rodent model of age-related memory loss have demonstrated that abnormally elevated neural activity specifically occurs in the CA3 region of the hippocampus when those neurons are unable to encode new information (Wilson et al., 2006; Wilson et al., 2005). Additionally, treatments designed to target excess CA3 activity in that animal model, including the use of low doses of certain antiepileptic drugs, were demonstrated to improve memory performance (Koh et al., 2010). Consistent with those findings, recent evidence from high-resolution fMRI in humans indicates that greater hippocampal activation in aMCI localizes to the dentate gyrus/CA3 (DG/CA3) region (Yassa et al., 2010), suggesting similar network dysfunction. Together these findings support the concept that reducing excess activity would have potential benefit in aMCI. The current study tested that hypothesis using a FDA approved compound to lower excess hippocampal activity.
Results
Here we used low dose levetiracetam, an AED that has shown efficacy in animals with hippocampal hyperactivity (Koh et al. 2010), to examine the functional significance of this condition in aMCI. Seventeen patients with aMCI and seventeen healthy age-matched control subjects completed a baseline assessment after which they participated in two treatment phases, separated by a washout period of 4 weeks. Control subjects were given placebo during both treatment phases (single-blind) while patients with aMCI were given placebo during one treatment phase and low dose levetiracetam (125 mg BID) during the second treatment phase, with order of treatment counterbalanced (randomized, double-blind). After two weeks in each treatment phase, participants completed a high-resolution fMRI scan while performing a cognitive task designed to assess memory errors attributable to DG/CA3 dysfunction (Yassa et al., 2010; Toner et al., 2009; Stark et al., 2010). It was hypothesized that if hippocampal hyperactivity serves a compensatory role, reducing that activity would further degrade memory function. However, if the observed hyperactivity is a condition contributing to hippocampal dysfunction, reducing excess activity should improve memory performance in those patients. Here we show that greater hippocampal activation in aMCI relative to the control group was isolated to the DG/CA3 region consistent with earlier studies. Treatment with low dose levetiracetam significantly reduced that excess activity, such that hippocampal activation in patients on drug did not differ from age-matched control subjects. Additionally, drug treatment significantly improved 3-choice recognition performance. Memory errors attributable to DG/CA3 dysfunction, which differed between the groups when aMCI subjects were on placebo, were significantly reduced by levetiracetam treatment.
Diagnosis of aMCI was based on criteria proposed by Petersen et al. (1999). Patients with aMCI had a global clinical dementia rating (CDR; Morris, 1993) of 0.5 with a sum of boxes score not exceeding 2.5, scored at least 1.5 standard deviations below the norm on neuropsychological assessments of memory function, and reported a decline of memory confirmed by an informant. These aMCI subjects showed impairments in both single and multiple domains (All neuropsychological test data acquired at baseline are shown in Supplemental Table S1). Healthy control subjects had a global CDR of 0 and scored within 1 standard deviation of the norm on neuropsychological testing. Group demographics and baseline data are shown in Table 1.
Table.
| Characteristic | Controls |
MCI |
p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Subjects | 17 | 17 | - | ||
| Sex (M/F) | 9/8 | 6/11 | 0.307 | ||
| Age (years) | 69.4 | 7.0 | 72.9 | 8.9 | 0.201 |
| Education (years) | 15.9 | 2.6 | 15.8 | 2.9 | 0.951 |
| CDR | 0 | 0.5 | - | ||
| MMSE | 27.9 | 1.5 | 25.7 | 2.3 | 0.002 |
| BSRT Delayed Recall | 7.7 | 1.9 | 3.6 | 2.4 | <0.001 |
| Wechsler VPA Delayed Recall | 7.0 | 1.5 | 3.9 | 2.9 | <0.001 |
| BVRT | 5.8 | 1.8 | 4.5 | 1.5 | 0.026 |
CDR: Clinical Dementia Rating
MMSE: Mini Mental Status Exam
BSRT: Buschke Selective Reminding Test
VPA: Verbal Paired Associates
BVRT: Benton Visual Retention Test
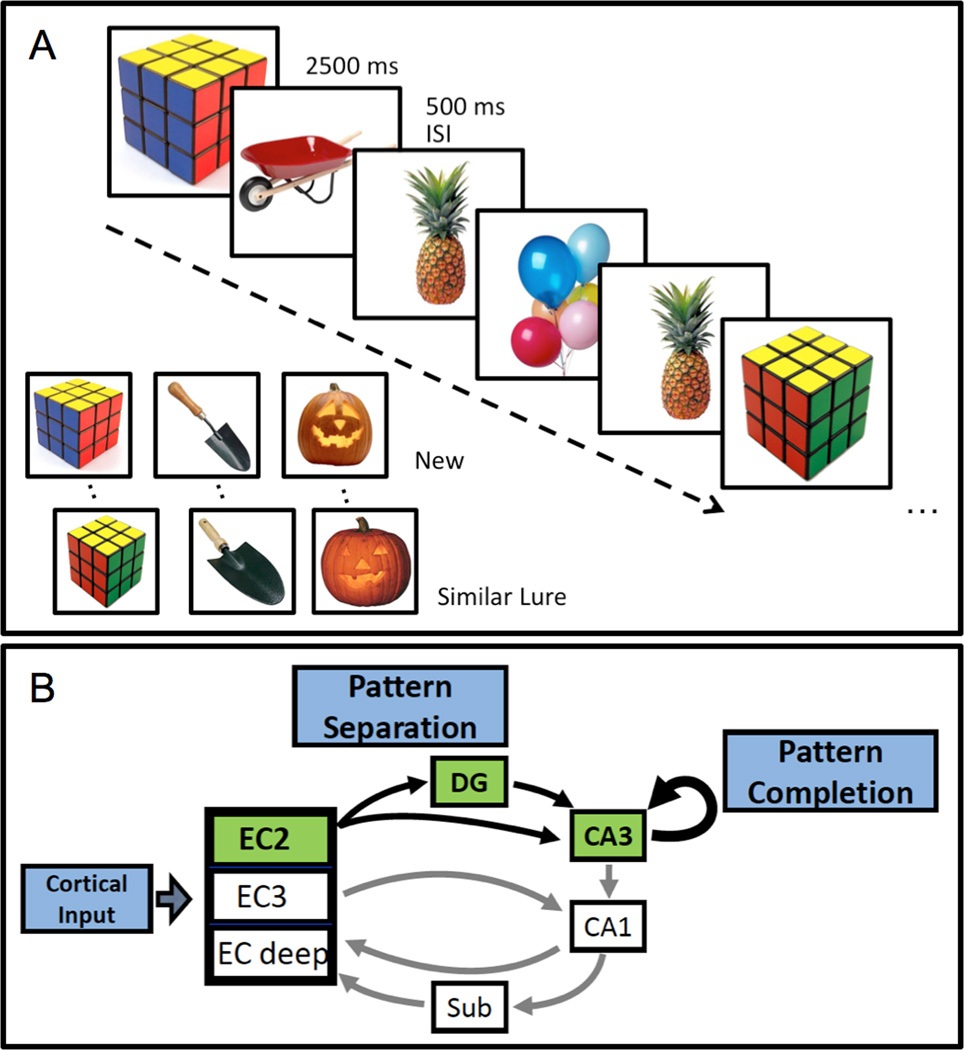
At the end of each treatment phase, participants completed a high-resolution fMRI scan while performing a memory task designed to assess the function of the DG/CA3 network (Bakker et al., 2008; Lacy et al., 2011). Subjects were presented with a series of pictures of everyday objects and asked to determine for each object if the item was 'new', 'old' or 'similar'. As in typical 2-judgment recognition memory tests, an item was correctly judged 'new' if it was seen for the first time in the context of the task and 'old' if the item was repeated. The third option of 'similar' was the correct judgment when an object only resembled an item previously seen in the task (Fig. 1a). These 'similar lures' were the critical trials for assessment of DG/CA3 contribution to memory performance. Correct identification of 'similar' items should depend on DG-mediated pattern separation, referring to the ability to encode inputs with some degree of overlapping information into distinctive representations. The CA3 and its strong autoassociative network mediates a complementary function of pattern completion, in which retrieval of previously stored information is based on commonalities between current input and prior experience (Fig. 1b). These functions of the DG/CA3 network are supported by behavioral and neurophysiological data obtained in animal studies (Leutgeb et al., 2004; Leutgeb and Leutgeb, 2007; McHugh et al., 2007), which have also shown a weakening of pattern separation and a shift to pattern completion in age-related memory loss when CA3 neurons are hyperactive (Wilson et al., 2006; Wilson et al., 2005). Consistent with those findings, behavioral data obtained in 3-choice recognition memory tasks from elderly humans show a shift in performance toward pattern completion, as reflected during lure trials by more incorrect 'old' responses and fewer correct 'similar' responses when compared to young adults (Toner et al., 2009; Stark et al., 2010). This error profile on lure trials is further worsened in aMCI patients compared to normal aging (Yassa et al., 2010).
Figure 1. Task designed to tax hippocampal DG/CA3 function.
(A) Participants were presented with pictures of everyday objects for 2500 ms each separated by a 500 ms inter stimulus interval and asked to judge if the item was new (seen for the first time), old (a repeated item) or similar (resembled a previously shown item). The similar lure items served as the critical trials for assessing performance dependent on the dentate gyrus/CA3. (B) The entorhinal cortex (EC) provides input from the cortex, via layer II (EC2) neurons, to the dentate gyrus (DG) and distal dendrites of CA3 pyramidal neurons. The CA3 afferents, in addition to innervating CA1, have autoassociative collaterals, forming the majority of CA3 synaptic input. The EC2 neurons and their targets in the hippocampus comprise a system that rapidly encodes representations that are distinct from prior memories. The sparse connections onto granule cells in DG by EC afferents promote pattern separation, while the recurrent CA3 network promote pattern completion.
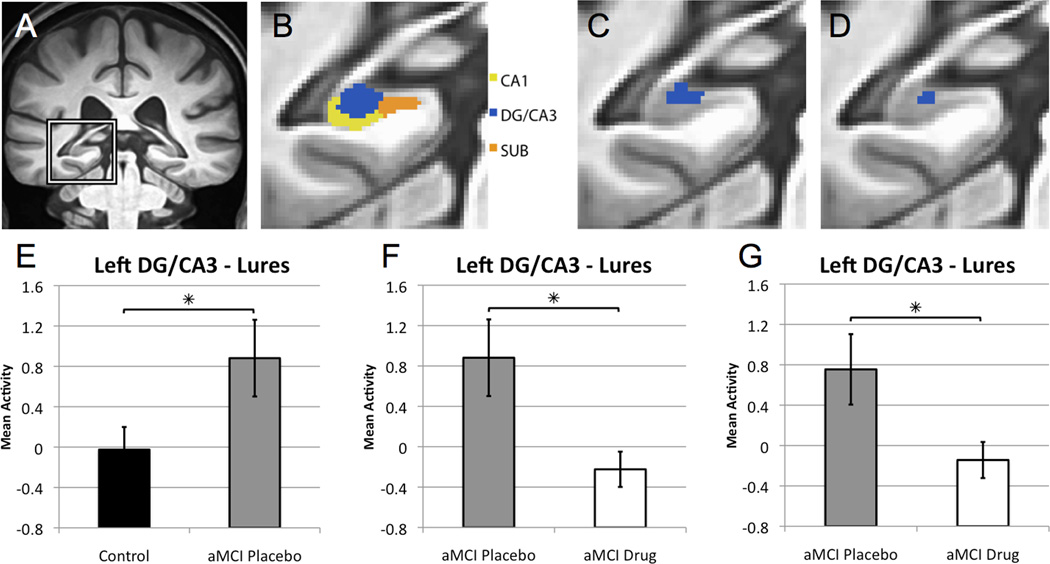
Functional neuroimaging data was first subjected to a one-way analysis of variance (ANOVA) of trial type to select voxels that showed task related activity (see experimental procedures). To avoid selection bias while acknowledging the dependence of observations in the two treatment conditions for aMCI subjects, this analysis included data from all control subjects and aMCI patients randomly selected from either their placebo or their drug condition such that both treatment conditions were equally represented (approximately half from placebo and half from the drug condition). This analysis maintains independence of observations by including each aMCI patient only once. Unrelated foil items correctly identified as 'new' were used as an implicit baseline against which all other conditions were compared. This analysis resulted in an area of task-related activity localized within the left DG/CA3 subregion of the hippocampus (Fig. 2c).
Figure 2. Levetiracetam treatment normalizes increased dentate gyrus/CA3 activity in patients with aMCI.
(A) High-resolution anatomical scan, with the left medial temporal lobe demarcated. (B) Segmentation of medial temporal lobe structures, including the CA1, dentate gyrus/CA3 (DG/CA3) and subiculum (SUB) subregions of the hippocampal formation. The boundaries between the CA3 and dentate gyrus (DG) cannot be clearly defined in structural scans and, therefore, are combined as DG/CA3 for analysis. (C) Area of task related activity based on an ANOVA of task condition in the left DG/CA3. (D) Area of task related activity in the left DG/CA3 resulting from the confirmatory analysis in which voxel selection is based on ANOVA of task condition that includes only age-matched healthy control subjects. (E) Graphs show mean activity ± SEM on lure trials correctly called similar. Patients with aMCI on placebo show increased activity in the left DG/CA3 compared to healthy control subjects during the lure trials. (F) Levetiracetam treatment significantly reduced activity in the DG/CA3 in patients with aMCI compared to the placebo condition. (G) In the confirmatory analysis for drug treatment, patients with aMCI taking low dose levetiracetam again showed significantly reduced BOLD activation during lure trials correctly identified as similar relative to the activity during those trials under placebo treatment. * p < 0.05 group comparisons using independent and paired samples t-tests respectively. See also Figure S1.
Increased DG/CA3 activation in aMCI compared to age-matched controls
To assess whether increased hippocampal activation was observed in the current study of patients with aMCI, we first compared functional activity during fMRI memory task performance between healthy control subjects and patients with aMCI on placebo within the DG/CA3 region as shown in Fig. 2c. The aMCI patients on placebo showed significantly increased BOLD activation during lure trials correctly identified as similar when compared to control subjects (t = 2.056, p = 0.048) (Fig. 2e; see also Figure S1). This finding replicates earlier findings in Yassa et al. (2010).
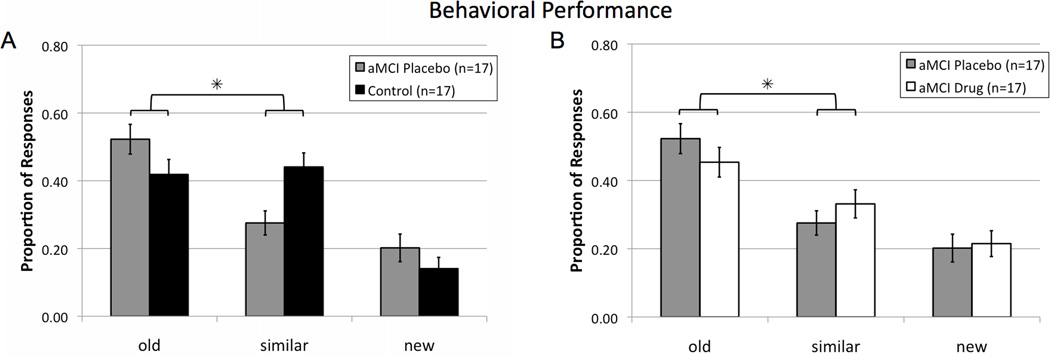
Behavioral performance of the aMCI subjects on placebo compared to healthy controls during the scanning task was assessed by the rates of each response option (old, similar, or new) on the critical lure trials. A between-groups ANOVA using only the response categories ‘old’ and ‘similar’ to maintain response independence revealed a significant effect of response type (F(1,32) = 5.357, p = 0.027), and, importantly, a significant group (control vs. aMCI placebo) × response interaction (F(1,32) = 7.687, p = 0.009) showing that aMCI patients on placebo incorrectly identified lure items as 'old' more often and gave relatively fewer correct responses of 'similar' compared to control subjects (Fig. 3a). That profile is consistent with reduced pattern separation and a shift to pattern completion in aMCI.
Figure 3. Levetiracetam treatment improves task-related memory performance on critical lure items in patients with aMCI.
(A) Patients with aMCI on placebo had impaired memory performance by more often incorrectly judging lure items as 'old' instead of 'similar' when compared to healthy control subjects. (B) Levetiracetam improved performance in aMCI patients, by reducing errors in which lures were incorrectly judged 'old', with more correct judgments of 'similar', when comparing aMCI performance on drug with placebo. * Two-way repeated measures ANOVA revealed a significant contrast for the interaction of group as a function of response type (old vs similar), p < 0.05. Values are means ± SEM. See also Figure S2.
Furthermore, a correlational analysis of activity in the left DG/CA3 and the proportion of lures called similar showed a marginal inverse relationship in patients with aMCI such that increased activity in the DG/CA3 was associated with fewer correct responses of ‘similar’ to lure items reflecting reduced pattern separation (r = −0.433, p = 0.083; Fig. S1). In contrast, such a correlation was not observed in control subjects.
Together, these results demonstrate that patients with aMCI had increased activation in the left DG/CA3 subregion of the hippocampus and performed significantly worse on trials with lures, compared to the age-matched control group. These results are consistent with earlier reported findings (Yassa et al., 2010), providing a sample of aMCI patients appropriate for testing the functional significance of hippocampal hyperactivity.
Levetiracetam reduces DG/CA3 activation in aMCI and improves memory function in the 3-choice memory task
To assess whether low dose levetiracetam treatment reduces the observed increased DG/CA3 activation, we compared functional data during the fMRI memory task in aMCI patients upon completion of the placebo and drug treatment phases. Within the neuroanatomical region of task related activity (as shown in Fig. 2c), low dose levetiracetam significantly reduced BOLD activation relative to the placebo treatment (t = 2.537, p = 0.022) (Fig. 2f). This effect was not influenced by order of treatment. Thus there was no evidence for any carry-over six weeks after drug treatment was terminated (4 week washout and placebo for two weeks before the second assessment). Further, BOLD activation in the DG/CA3 in aMCI patients after levetiracetam treatment did not differ from activity in that region observed in the normal age-matched control subjects. To confirm this finding, a separate analysis was conducted in which voxel selection was based on a one-way ANOVA of trial type only in control subjects. This analysis resulted in an area of task related activity similarly localized in the left DG/CA3 subregion of the hippocampus (Fig. 2d). The effect of drug treatment was confirmed by comparing fMRI activation in that area of task related activity in aMCI patients on placebo and levetiracetam. In that analysis, patients with aMCI taking low dose levetiracetam again showed significantly reduced BOLD activation relative to their activity under placebo treatment (t = 2.192, p = 0.044) (Fig. 2g). These effects were obtained with a drug dose well below that used clinically for the treatment of epilepsy; drug levels in patients were determined to be 4.4 mcg/ml ± 0.53 (mean and sem), as compared to typical ranges for efficacy as an antiepileptic with doses of 1000–3000 mg/day achieving levels of 10–40 mcg/ml (Lyseng-Willimason, 2011).
Treatment with low dose levetiracetam also improved behavioral performance on the lure trials. Analysis of the responses on those trials showed an overall significant effect of response type (F(1,16) = 5.992, p = 0.026), and, importantly, a significant interaction effect of treatment (drug vs. placebo) × response (F(1,16) = 5.028, p = 0.039) showing that relative to their performance on placebo, aMCI patients taking levetiracetam made fewer incorrect responses of 'old' while concomitantly increasing correct judgments of 'similar' (Fig. 3b).
Finally, in addition to the fMRI scanning session, participants completed a neuropsychological assessment after each treatment phase. Levetiracetam did not significantly alter performance as assessed by neuropsychological tests of memory or general cognitive functioning. Specifically within the memory domain, performance of patients with aMCI after taking levetiracetam for two weeks did not differ significantly compared to their performance on placebo for delayed recall on the Buschke Selective Reminding Test (Buschke and Fuld, 1974) (t = 0.145, p = 0.887), delayed recall on the Verbal Paired Associates subtest of the Wechsler Memory Scale (Wechsler, 1997) (t = 0.194, p = 0.848) and the Benton Visual Retention Test (Benton, 1974) (t = 0.251, p = 0.805), while performance of aMCI participants under both treatment conditions differed from the age-matched control group (p<0.05) (see Fig. S2).
Discussion
The functional significance of greater hippocampal activation in aMCI is addressed by the current study, which fails to support the view that such activity benefits memory performance. After levetiracetam treatment, when DG/CA3 activation was reduced in aMCI patients, no worsening of memory occurred either in the scanning task or on memory tests in a neuropsychological assessment. To the contrary, levetiracetam treatment altered performance in the 3-choice memory task in a manner consistent with improved DG/CA3 function, indicating the therapeutic potential of targeting excess hippocampal activation in aMCI.
The lure items in the memory task are designed to assess the balance of pattern separation and pattern completion mediated by the DG/CA3. In memory-impaired aged rats with excess CA3 activity, the CA3 pyramidal neurons activate representations tied to prior experiences and fail to encode distinctive representations for new information, indicating a shift in network function toward greater pattern completion and diminished pattern separation (Wilson et al., 2006). A similar condition in humans would be expected to produce more errors with lures incorrectly identified as repetitions of prior items rather than correctly identified as similar but distinctive, only sharing features with prior items in the task. We found this specific profile in the aMCI patients. Furthermore, comparing aMCI patients on placebo with drug treatment demonstrated that a low dose of levetiracetam, which attenuated DG/CA3 activation, significantly improved performance by reducing errors attributable to an overriding pattern completion process. To the extent that excess hippocampal activation does not serve a supportive memory function, such activity might not only contribute to memory impairment but also have adverse effects on vulnerable neural systems.
The current findings may have broader implications because increased hippocampal activation occurs not only in aMCI, but also in other conditions associated with risk for AD. Many studies of ApoE4 carriers have reported increased fMRI activation in the hippocampus (Dickerson et al., 2004; Dickerson et al., 2005; Celone et al., 2006; Hämäläinen et al., 2007). Contrary to the notion that such activity is compensatory, recent research has revealed a loss of hippocampal inhibitory function in animal models used to study ApoE4, and demonstrated that such loss contributes to behavioral deficits (Andrews-Zwilling et al., 2010). In those models of ApoE4, memory performance was improved by treatment with the GABAA receptor potentiator, pentobarbital. In the context of the hippocampal subsystem highlighted here, it is also noteworthy that the impact of ApoE4 on inhibitory circuits in mouse models was regionally restricted within the hippocampal formation, affecting interneurons in the hilus but not in CA1. Greater hippocampal activation is also a signature in genetic conditions for familial AD (Quiroz et al., 2010), and aberrant excitatory activity affecting hippocampal circuits occurs in mouse models of familial AD (Palop et al., 2007).
Apart from contributing to symptomatic memory impairment, there is concern that elevated activity in vulnerable neural networks could drive pathophysiology in conditions of risk for AD. In the clinical context, this concern is suggested by evidence that elevated hippocampal activation may be tied to widespread disease related degeneration in a distributed network of brain regions in prodromal AD (Putcha et al., 2011) and predicts subsequent cognitive decline and conversion to AD (Dickerson et al., 2004; Miller et al., 2008; O’Brien et al., 2010). Mechanisms tied to AD amyloid pathology demonstrate that fluctuations in neural activity dynamically regulate levels of Abeta in the interstitial fluid (Bero et al., 2011). Such findings support the regulation of neural activity as a possible therapeutic modality to modify disease progression. The current findings encourage such an approach, indicating that patients receiving levetiracetam did not lose function that might have been supported by greater recruitment of neural activity but instead exhibited a benefit as predicted by computational models and preclinical studies of animals with age-related memory loss.
Experimental Procedures
Participants and clinical characterization
Twenty-three patients with amnestic mild cognitive impairment (aMCI) and 22 healthy older adults participated. Complete data from 17 aMCI patients and 17 control participants were included in the analysis. Data from 6 aMCI patients and 5 control participants were excluded from analysis due to inability to complete the MRI session, not taking the study medication according to the instructions provided or were otherwise unable to complete the study protocol. See Table 1 with additional details in supplementary methods.
All participants underwent medical, psychiatric, neurological and neuropsychological evaluations, which included the Buschke Selective Reminding Test (Buschke and Fuld, 1974), the Verbal Paired Associates subtest of the Wechsler Memory Scale (Wechsler, 1997) and the Benton Visual Retention Test (Benton, 1974) and completed the Clinical Dementia Rating scale (Morris, 1993). All aMCI patients had global CDR scores of 0.5 with a sum of boxes score not exceeding 2.5 and met the diagnostic criteria for aMCI proposed by Petersen et al. (1999). All healthy control subjects had a global CDR score of 0. None of the aMCI patients or healthy control participants met criteria for dementia.
Study Design
All participants completed a total of four study visits. Participants in the healthy control group were assigned to placebo in both treatment phases. If a participant met criteria for the aMCI group, the participant was randomly assigned to either the placebo condition or the levetiracetam condition. Participants were provided with the study medication (either placebo or drug) and provided with instructions to take one capsule twice daily until the next visit. The second visit occurred approximately two weeks after the first visit and included a brief medical and psychiatric exam, a blood draw and a MRI. The third visit occurred approximately four weeks after the second visit and included a brief medical and psychiatric exam and a blood draw. No treatment occurred between the second and the third visit. At the third visit the participant was provided with study medication for the second treatment phase of the study. A counterbalanced design was used such that aMCI patients who received placebo for the first treatment phase received levetiracetam and aMCI patients who received levetiracetam for the first treatment phase received placebo for the second treatment phase of the study. The fourth and final visit occurred approximately two weeks after the third and was identical to the second visit. All participants were blind to their treatment status throughout the study. The study team was blind to the treatment status of the aMCI patients and levetiracetam blood levels until the completion of the study (for additional details see supplementary methods).
fMRI acquisition
The fMRI behavioral paradigm was a 3-alternative forced choice task described in detail previously (Yassa et al., 2010; Lacy et al., 2011). High-resolution functional images were collected on a 3 Tesla Phillips scanner using a T2*-weighted echo planar single shot pulse sequence with an acquisition matrix of 64 × 64, an echo time of 30 ms, flip angle of 70°, a SENSE factor of 2, an in plane resolution of 1.5 × 1.5 mm and a TR of 1.5 seconds (Kirwan et al., 2007). Each volume consisted of 19 oblique 1.5 mm thick axial slices with no gap oriented along the principal axis of the hippocampus and covered the medial temporal lobe bilaterally. Each run consisted of 96 trials and each trial was presented for 2500 ms with a 500 ms interstimulus-interval. Each subject completed 8 runs. In addition, a whole brain structural scan was acquired using a magnetization prepared rapid gradient echo (MP-RAGE) T1-weighted sequence with 231 oblique slices, 0.65 mm isotropic resolution and a field of view of 240.
Image data analysis was performed using the Analysis of Functional Neuroimages software package (Cox et al., 1996). The resulting statistical fit coefficient maps represent the difference in activity between each of the task trial types and the baseline for a given time point for a given voxel. The statistical maps were then smoothed using a Gaussian kernel of 3 mm to account for variations in individual functional anatomy. Methods used for cross-participant alignment in this study were previously described in detail (Kirwan and Stark, 2007; Yassa and Stark, 2009; Lacy et al., 2011). This method increases the power of multi-subject regional fMRI studies by focusing the alignment power to the regions of interest using a segmentation of the subject’s anatomical image. The resulting 3D vector field for each individual was then applied to the concatenated fit coefficient maps resulting from the functional analysis (for additional details see supplementary methods).
Statistical analysis
Age, education and neuropsychological and functional assessment scores between groups were compared using independent samples t-tests. The distribution of sex between groups was compared using a chi-square test.
The fMRI data was analyzed using a two-step procedure. First, a one-way ANOVA of trial type (sTH, sLS, sLO, TH, LS and LO) was used to select voxels that showed task-related activity. All control and aMCI participants were included in this analysis to avoid bias; however, to avoid the dependence arising from the aMCI patients contributing two data points, aMCI data were randomly selected from either the placebo or drug condition (approximately half from each condition). In a confirmatory analysis, voxel selection was based on a one-way ANOVA of trial type using only the healthy age-matched control subjects. The second level statistical analysis for group and treatment differences used a final alpha of .05 for tests in both the main analysis (between group and within-aMCI for treatment condition) and in the confirmatory analysis of the aMCI data.
In the first level analysis a voxel threshold of p<0.07 was used on the overall F-statistic in combination with a spatial extent threshold of 40 voxels to select areas of task related activation. Voxel selection based on trial type alone was not robust at p = 0.05 due to increased variability introduced by collapsing across groups. The voxel threshold at p < 0.07 yielded a sizable ROI for the purpose of hypothesis testing in the main second level statistical analyses and the confirmatory analysis. The resulting areas of activation in voxel selection were combined with the anatomical segmentations to only include voxels within our areas of interest. This hybrid functional/anatomical analysis resulted in clusters of voxels that showed changes with task condition within an anatomical region of interest. In the main analysis, voxels within each functional/anatomical region of interest were then collapsed for the second level ROI analysis comparing control subjects and aMCI patients on placebo using a t-test. A separate paired samples t-test was used to compare the placebo condition to the levetiracetam condition in aMCI patients for both the main and confirmatory analysis.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Jason Brandt, Dr. Paul Dash, Dr. Argye Hillis-Trupe, Dr. Majid Fotuhi and Dr. Peter Rabins for help with participant recruitment and the staff of the F.M. Kirby Center for Functional Brain Imaging and Alica Diehl, Benjamin Drapcho and Christina Li for their assistance with data collection. This work was supported by NIH grant RC2AG036419 to M.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 cause age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;41:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL. Revised Visual Retention Test 4th. New York: Psychological Corporation; 1974. [Google Scholar]
- Bero AW, Yan P, Roth JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: and independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE ε4 carriers. Alzheimers Dement. 2010;6:303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging. 2007;28:1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Hum Brain Mapp. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn. Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lyseng-Williamson KA. Levetiracetam: A review of its use in epilepsy. Drug. 2011;71:489–514. doi: 10.2165/11204490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, O’Keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74:1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Robertson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J. Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz YT, Budson AE, Celone K, Ruiz A, Newmark R, Castrillon G, Lopera F, Stern CE. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann. Neurol. 2010;68:865–875. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CEL. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn. Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn. Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, Atwood CS, Rowley HA, Asthana S, Sager MA, Johnson SC. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer’s disease. Neuropsychologia. 2008;46:1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol. Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.