Abstract
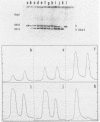
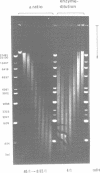
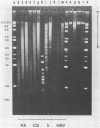
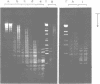
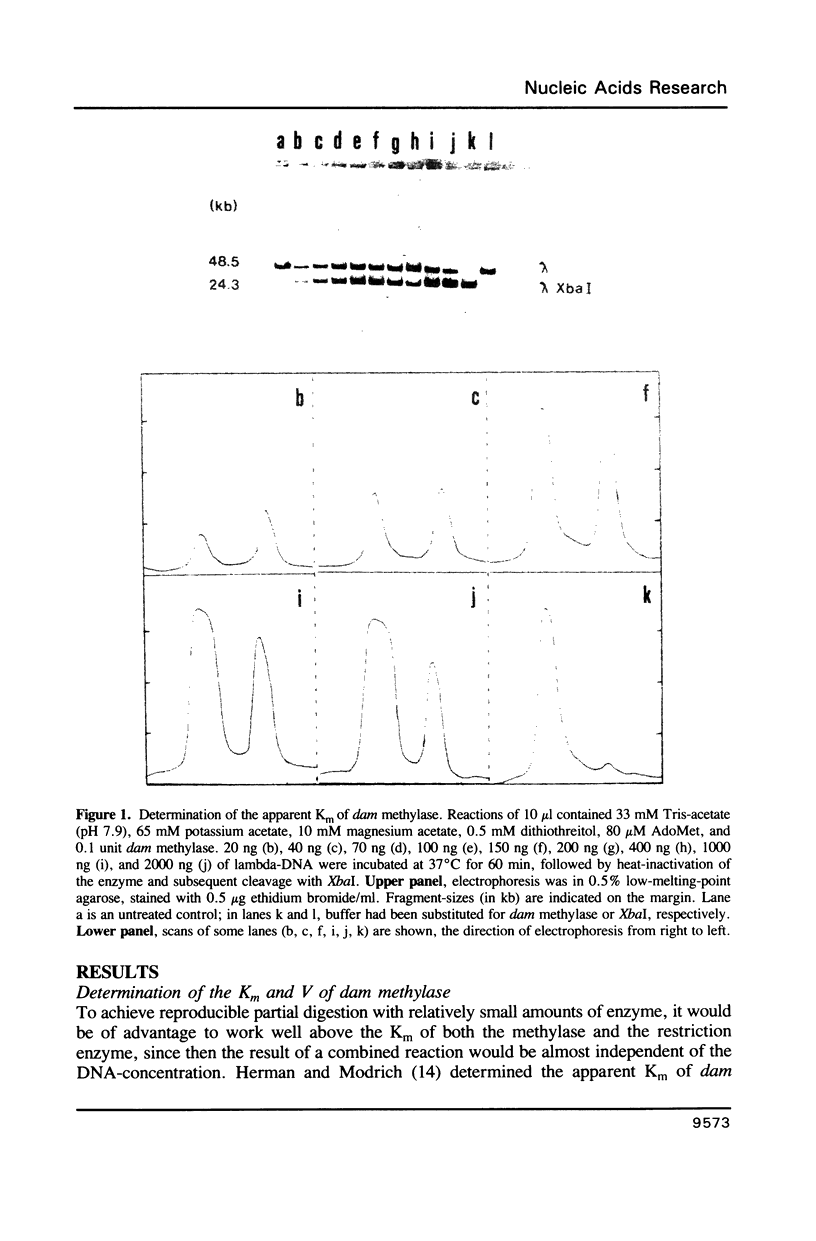
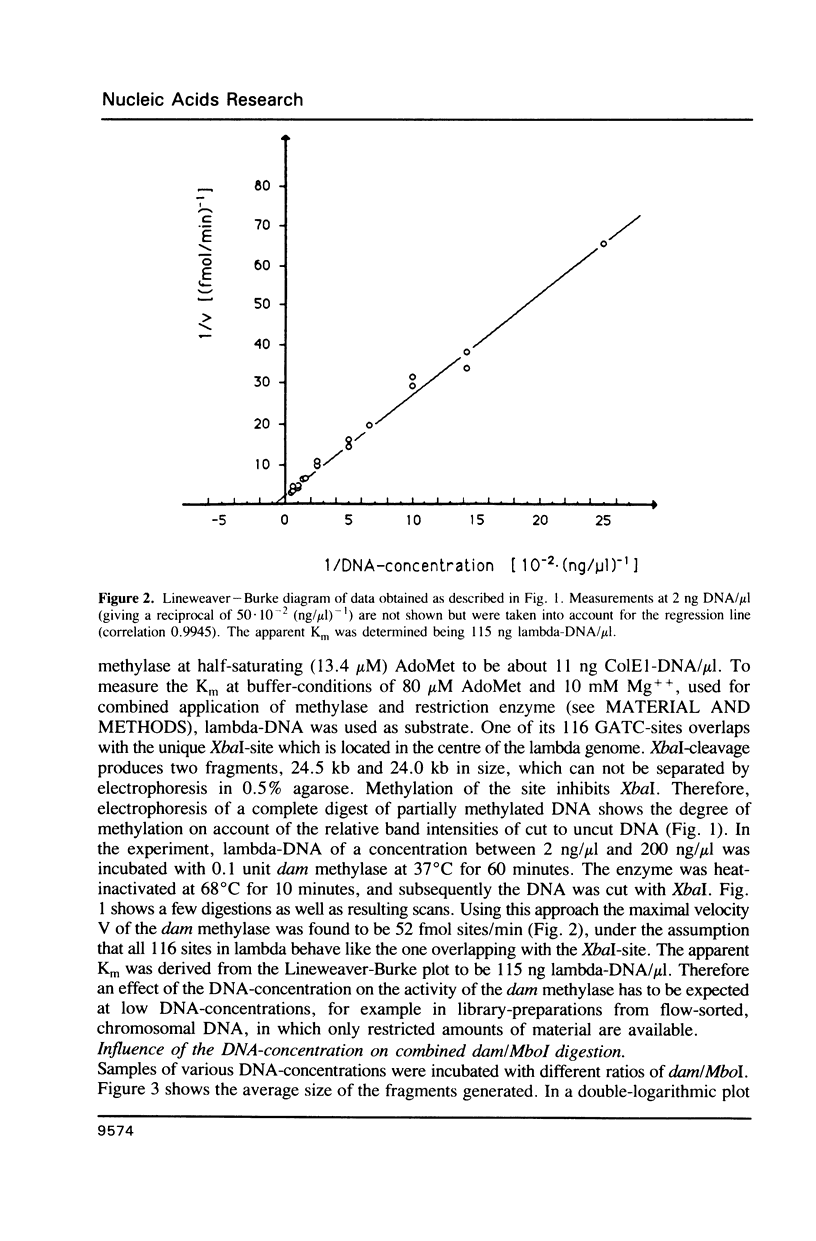
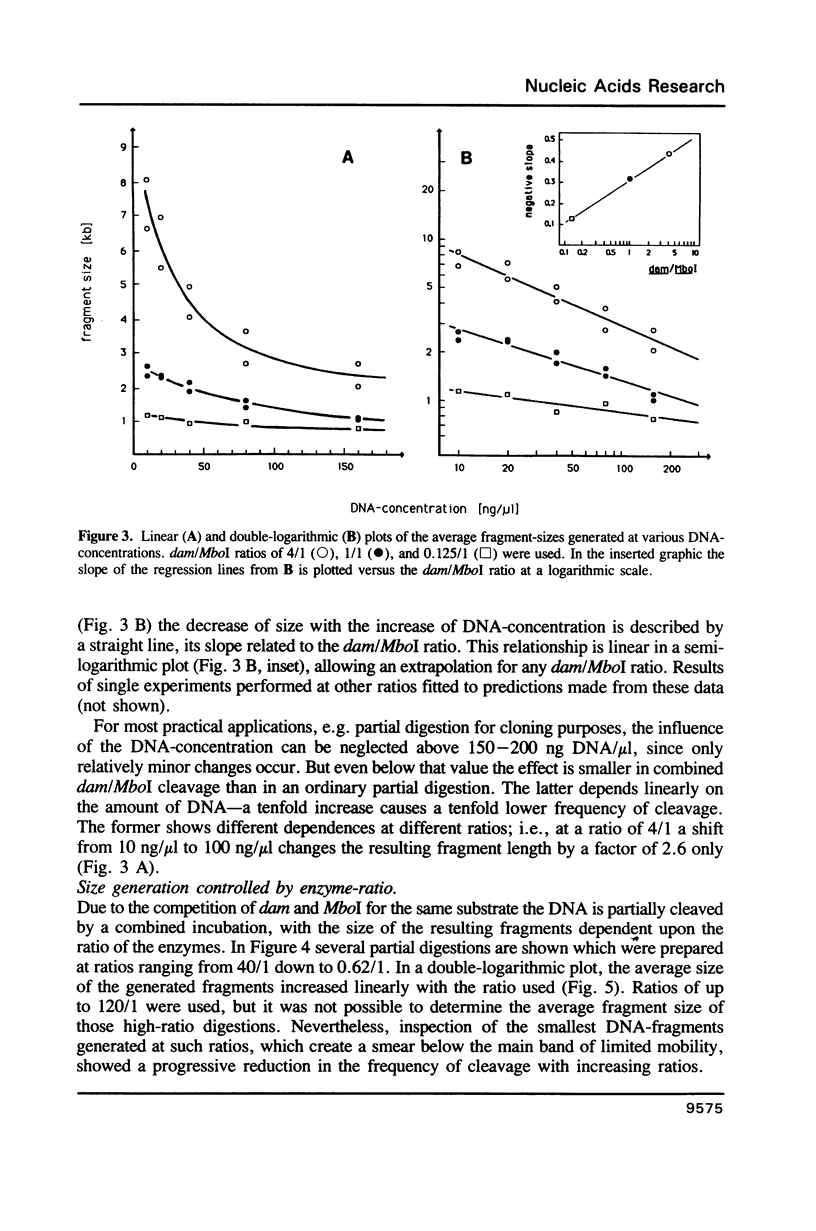
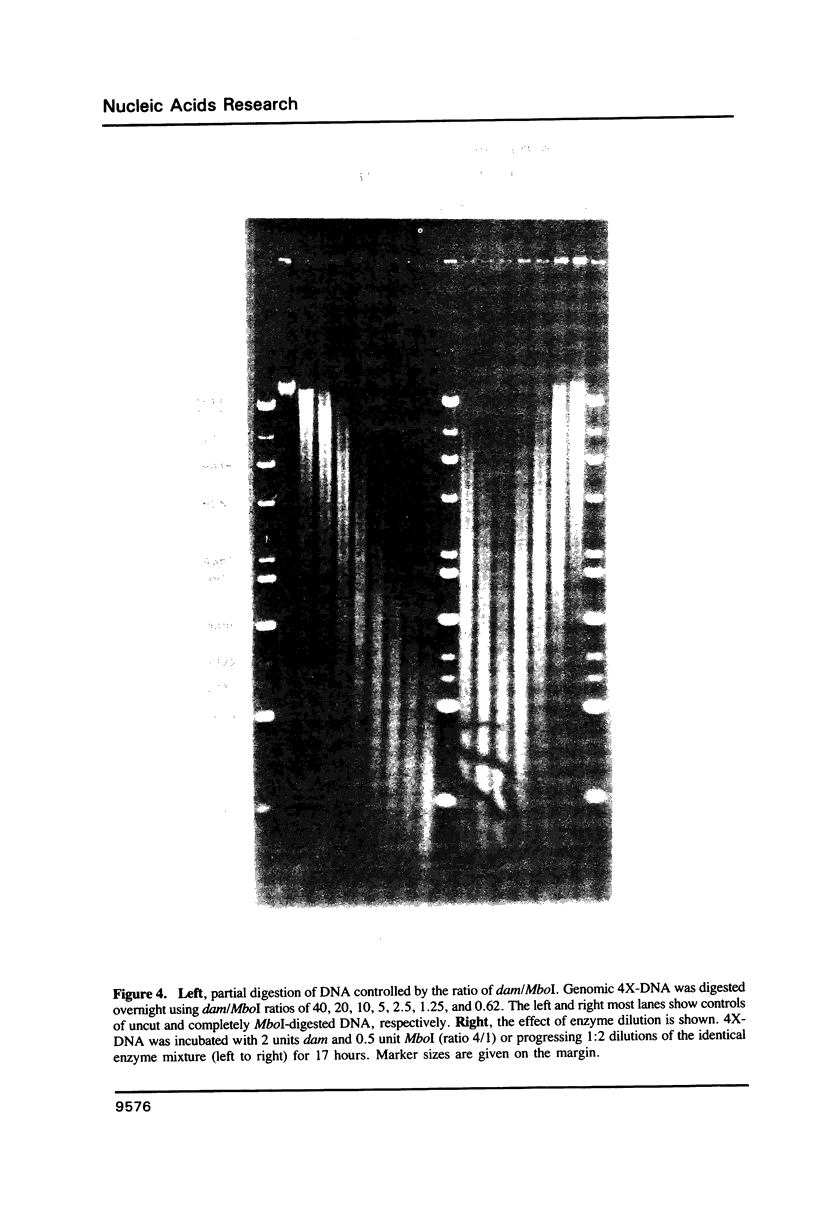
A method is described which allows the preparation of reproducible partial digests without previous establishment of the incubation conditions. It is based on a combined application of dam methylase and the restriction endonuclease MboI, both recognizing the sequence 5'-GATC-3' but MboI unable to cut the methylated site. Due to their competition for the same substrate the DNA is partially digested, with the size of the resulting fragments strongly dependent on the ratio of enzymes. The Km of the dam methylase was determined to be 115 ng DNA/microliters indicating a variance in fragment sizes generated at low DNA-concentrations. This effect is minimized above 150 ng/microliters. Any influence of digestion time is avoided, because the reaction runs until complete modification of all sites. The dependence on enzyme concentration and presence of agarose was checked. Knowledge of these parameters allows an accurate prediction of fragment sizes generated at different conditions. The technique was successfully used to construct libraries from different sources, in particular chromosome-specific libraries from small amounts of flow-sorted material.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreiseikelmann B., Eichenlaub R., Wackernagel W. The effect of differential methylation by Escherichia coli of plasmid DNA and phage T7 and lambda DNA on the cleavage by restriction endonuclease MboI from Moraxella bovis. Biochim Biophys Acta. 1979 May 24;562(3):418–428. doi: 10.1016/0005-2787(79)90105-9. [DOI] [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Hanish J., McClelland M. Controlled partial restriction digestions of DNA by competition with modification methyltransferases. Anal Biochem. 1989 Jun;179(2):357–360. doi: 10.1016/0003-2697(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Herman G. E., Modrich P. Escherichia coli dam methylase. Physical and catalytic properties of the homogeneous enzyme. J Biol Chem. 1982 Mar 10;257(5):2605–2612. [PubMed] [Google Scholar]
- Herrmann B. G., Frischauf A. M. Isolation of genomic DNA. Methods Enzymol. 1987;152:180–183. doi: 10.1016/0076-6879(87)52018-3. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Lee J. S., Pulleyblank D. E., Murray N. L., Evans D. H. Review: ethidium fluorescence assays. Part 1. Physicochemical studies. Nucleic Acids Res. 1979 Oct 10;7(3):547–569. doi: 10.1093/nar/7.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Thomae R., Karst A. Temperature dependence of the activity of DNA-modifying enzymes: endonucleases and DNA ligase. Eur J Biochem. 1982 Mar;123(1):141–152. doi: 10.1111/j.1432-1033.1982.tb06510.x. [DOI] [PubMed] [Google Scholar]
- Poustka A., Pohl T. M., Barlow D. P., Frischauf A. M., Lehrach H. Construction and use of human chromosome jumping libraries from NotI-digested DNA. Nature. 1987 Jan 22;325(6102):353–355. doi: 10.1038/325353a0. [DOI] [PubMed] [Google Scholar]
- Poustka A., Pohl T., Barlow D. P., Zehetner G., Craig A., Michiels F., Ehrich E., Frischauf A. M., Lehrach H. Molecular approaches to mammalian genetics. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):131–139. doi: 10.1101/sqb.1986.051.01.016. [DOI] [PubMed] [Google Scholar]
- Whittaker P. A., Southern E. M. Ultraviolet irradiation of DNA: a way of generating partial digests for rapid restriction site mapping. Gene. 1986;41(1):129–134. doi: 10.1016/0378-1119(86)90276-3. [DOI] [PubMed] [Google Scholar]