Abstract
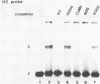
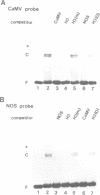
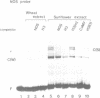
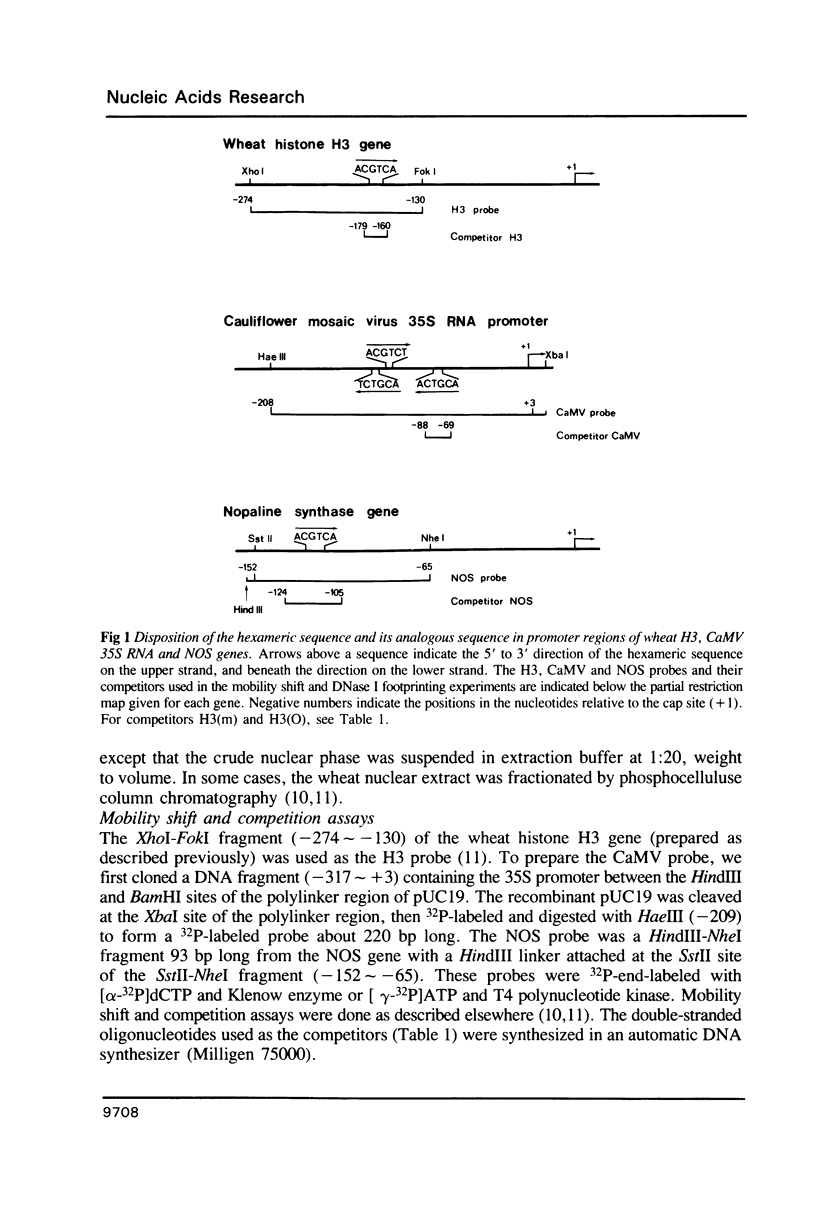
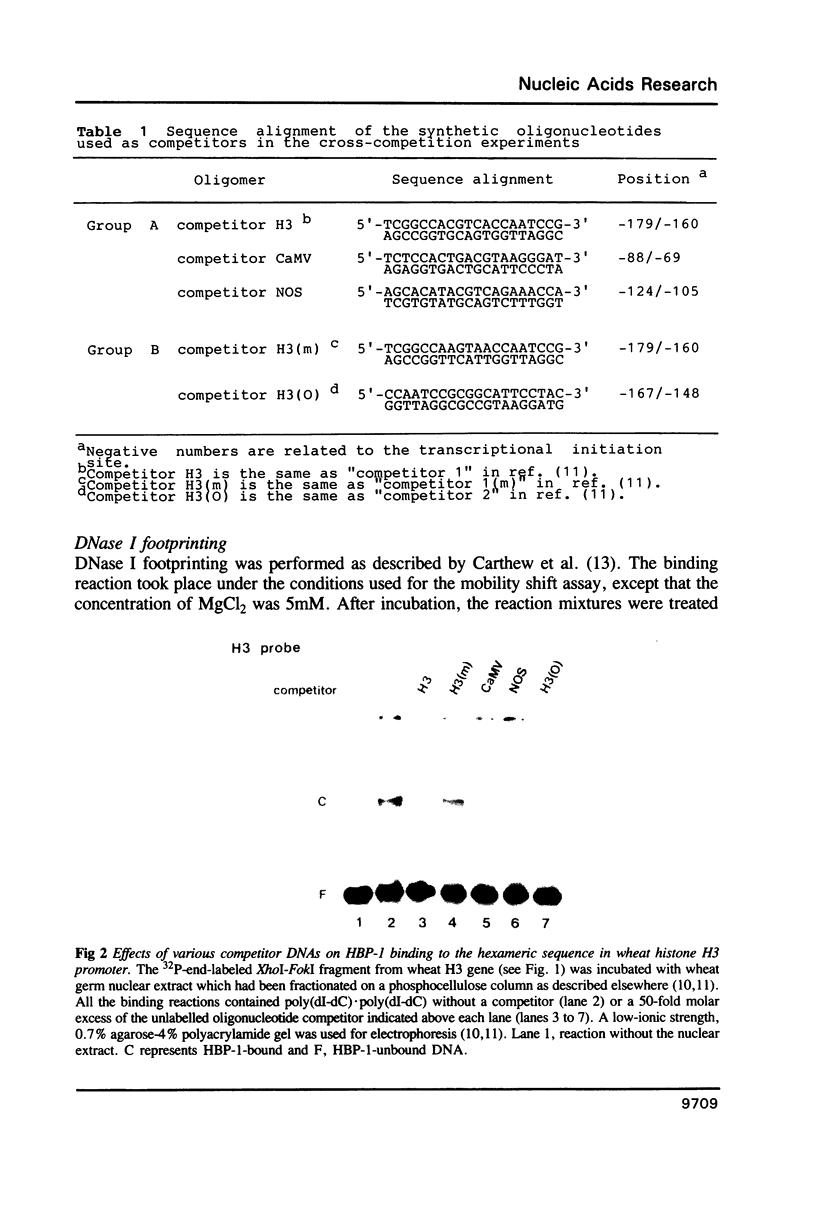
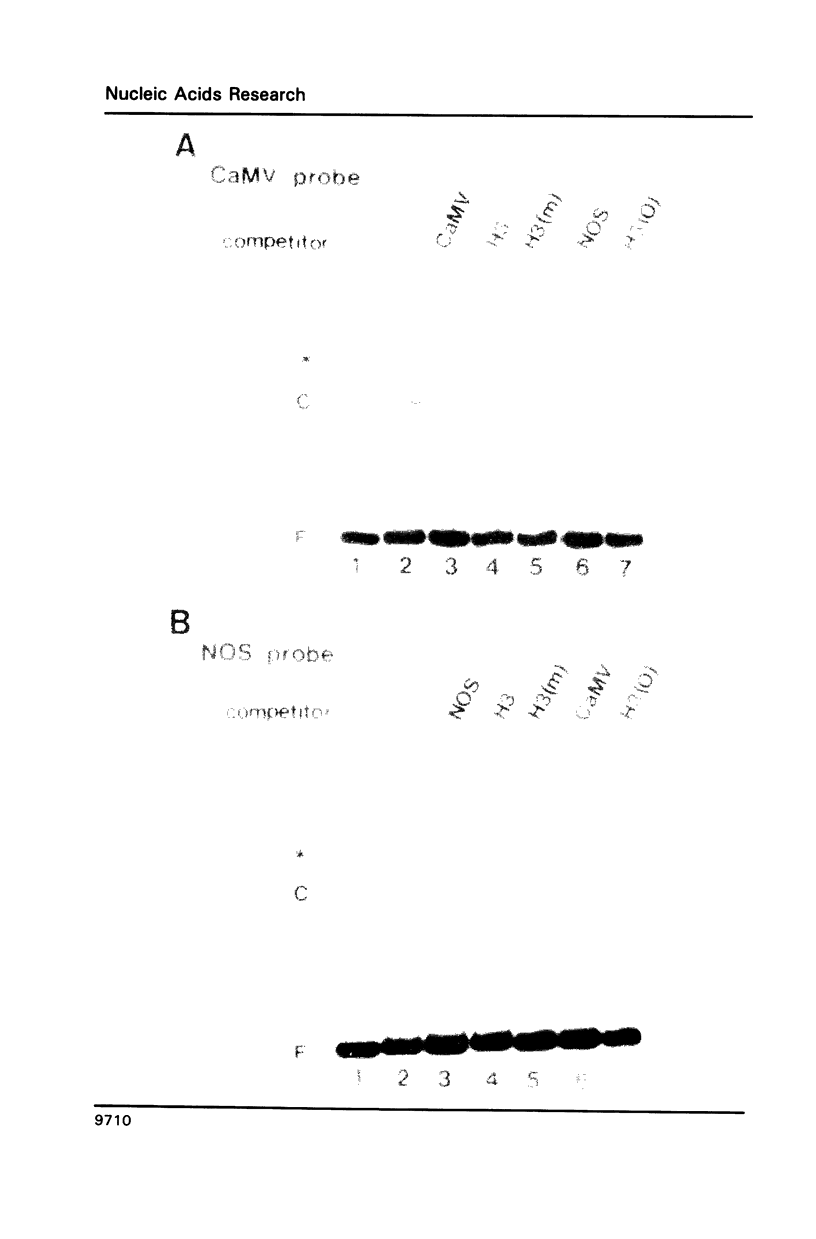
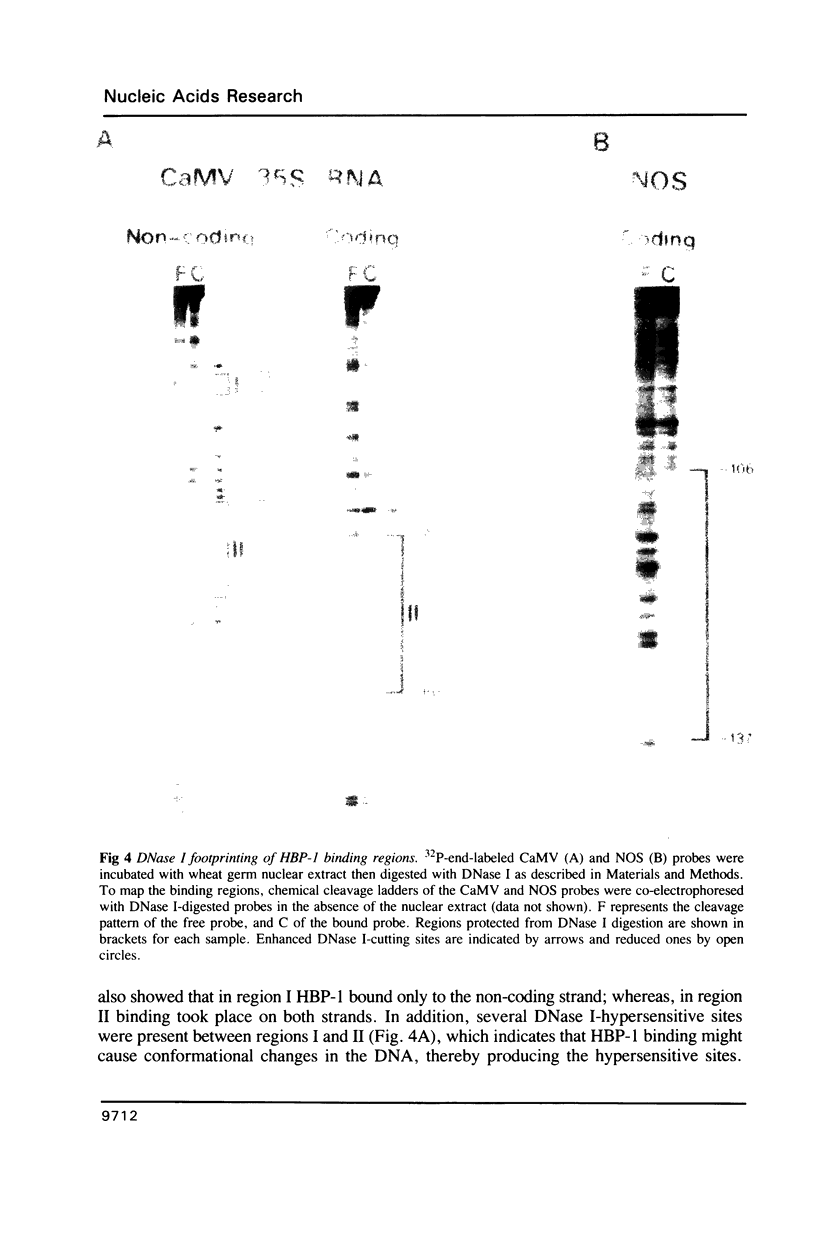
HBP-1 is a sequence-specific DNA-binding protein that interacts with the hexameric sequence ACGTCA, the putative cis-acting element of the wheat histone H3 gene. Gel mobility shift and DNase I footprint analyses showed that this protein interacts with homologous sequences in the regulatory regions for the transcription of the cauliflower mosaic virus (CaMV) 35S RNA and nopaline synthase (NOS) genes, evidence that HBP-1 may bind to hexameric sequences in the regulatory regions of various genes. An HBP-1-like protein, indistinguishable from wheat HBP-1 in its the DNA-binding specificity, is present in sunflower nuclear extract, an indication that HBP-1-like DNA-binding proteins also exist in dicots.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alliotte T., Tiré C., Engler G., Peleman J., Caplan A., Van Montagu M., Inzé D. An Auxin-Regulated Gene of Arabidopsis thaliana Encodes a DNA-Binding Protein. Plant Physiol. 1989 Mar;89(3):743–752. doi: 10.1104/pp.89.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Ellis J. G., Llewellyn D. J., Walker J. C., Dennis E. S., Peacock W. J. The ocs element: a 16 base pair palindrome essential for activity of the octopine synthase enhancer. EMBO J. 1987 Nov;6(11):3203–3208. doi: 10.1002/j.1460-2075.1987.tb02636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R. X., Nagy F., Sivasubramaniam S., Chua N. H. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell. 1989 Jan;1(1):141–150. doi: 10.1105/tpc.1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1781–1785. doi: 10.1073/pnas.86.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Jones R. H., Moreno S., Nurse P., Jones N. C. Expression of the SV40 promoter in fission yeast: identification and characterization of an AP-1-like factor. Cell. 1988 May 20;53(4):659–667. doi: 10.1016/0092-8674(88)90581-8. [DOI] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Lin W., Odell J. T., Schreiner R. M. Soybean protoplast culture and direct gene uptake and expression by cultured soybean protoplasts. Plant Physiol. 1987 Jul;84(3):856–861. doi: 10.1104/pp.84.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mikami K., Tabata T., Kawata T., Nakayama T., Iwabuchi M. Nuclear protein(s) binding to the conserved DNA hexameric sequence postulated to regulate transcription of wheat histone genes. FEBS Lett. 1987 Nov 2;223(2):273–278. doi: 10.1016/0014-5793(87)80303-4. [DOI] [PubMed] [Google Scholar]
- Mikami K., Takase H., Tabata T., Iwabuchi M. Multiplicity of the DNA-binding protein HBP-1 specific to the conserved hexameric sequence ACGTCA in various plant gene promoters. FEBS Lett. 1989 Oct 9;256(1-2):67–70. doi: 10.1016/0014-5793(89)81719-3. [DOI] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Ou-Lee T. M., Turgeon R., Wu R. Expression of a foreign gene linked to either a plant-virus or a Drosophila promoter, after electroporation of protoplasts of rice, wheat, and sorghum. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6815–6819. doi: 10.1073/pnas.83.18.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Jacobs J. D., Howell S. H. Functional regions of the cauliflower mosaic virus 35S RNA promoter determined by use of the firefly luciferase gene as a reporter of promoter activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4870–4874. doi: 10.1073/pnas.84.14.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Dutta A., Cromlish J. A., Roeder R. G. Phosphorylation of serum response factor, a factor that binds to the serum response element of the c-FOS enhancer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7206–7210. doi: 10.1073/pnas.85.19.7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Tokuhisa J. G., Dennis E. S., Peacock W. J. Saturation mutagenesis of the octopine synthase enhancer: correlation of mutant phenotypes with binding of a nuclear protein factor. Proc Natl Acad Sci U S A. 1989 May;86(10):3733–3737. doi: 10.1073/pnas.86.10.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Tabata T., Takase H., Takayama S., Mikami K., Nakatsuka A., Kawata T., Nakayama T., Iwabuchi M. A protein that binds to a cis-acting element of wheat histone genes has a leucine zipper motif. Science. 1989 Sep 1;245(4921):965–967. doi: 10.1126/science.2772648. [DOI] [PubMed] [Google Scholar]