Abstract
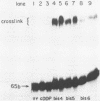
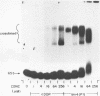
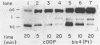
Bis(platinum) complexes [[cis-PtCl2(NH3)]2H2N(CH2)nNH2] are a novel series of potential anticancer agents in which two cis-diamine(platinum) groups are linked by an alkyldiamine of variable length. These complexes are potentially tetrafunctional, a unique feature in comparison with known anticancer agents. Studies of DNA interactions of bis(platinum) complexes in comparison with cisplatin demonstrate significant differences. Investigations of interstrand crosslink formation in which crosslinking of a short DNA fragment is detected by gel electrophoresis under denaturing conditions demonstrate that interstrand crosslinks are 250 fold more frequent among bis(platinum) adducts than among cisplatin-derived adducts under the conditions examined. These investigations indicate that bis(platinum) adducts contain a high frequency of structurally novel interstrand crosslinks formed through binding of the two platinum centers to opposite DNA strands. Unlike cisplatin, bis(platinum) complex binding does not unwind supercoiled DNA. Studies with the E. coli UvrABC nuclease complex demonstrate that both linear and supercoiled DNA containing bis(platinum) adducts are subject to incision by the repair enzyme complex. Initial studies using UvrABC nuclease as a probe to define the base and sequence specificity for bis(platinum) complex binding suggest that the specificity of the bis(platinum)s is similar, but not identical, to that of cisplatin.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck D. J., Popoff S., Sancar A., Rupp W. D. Reactions of the UVRABC excision nuclease with DNA damaged by diamminedichloroplatinum(II). Nucleic Acids Res. 1985 Oct 25;13(20):7395–7412. doi: 10.1093/nar/13.20.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchenal J. H., Kalaher K., O'Toole T., Chisholm J. Lack of cross-resistance between certain platinum coordination compounds in mouse leukemia. Cancer Res. 1977 Sep;37(9):3455–3457. [PubMed] [Google Scholar]
- Canetta R., Bragman K., Smaldone L., Rozencweig M. Carboplatin: current status and future prospects. Cancer Treat Rev. 1988 Jun;15 (Suppl B):17–32. doi: 10.1016/0305-7372(88)90031-x. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Harrap K. R. Preclinical studies identifying carboplatin as a viable cisplatin alternative. Cancer Treat Rev. 1985 Sep;12 (Suppl A):21–33. doi: 10.1016/0305-7372(85)90015-5. [DOI] [PubMed] [Google Scholar]
- Jones B. K., Yeung A. T. Repair of 4,5',8-trimethylpsoralen monoadducts and cross-links by the Escherichia coli UvrABC endonuclease. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8410–8414. doi: 10.1073/pnas.85.22.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Lydall D. A., Roberts J. J. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986 Apr;46(4 Pt 2):1972–1979. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reaction of nucleic acids and cis-diamminedichloroplatinum(II) in the presence of intercalating agents. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6317–6321. doi: 10.1073/pnas.83.17.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Schwartz A., Leng M. Characterization of the ternary complexes formed in the reaction of cis-diamminedichloroplatinum (II), ethidium bromide and nucleic acids. Nucleic Acids Res. 1987 Feb 25;15(4):1779–1797. doi: 10.1093/nar/15.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Myles G. M., Van Houten B., Sancar A. Utilization of DNA photolyase, pyrimidine dimer endonucleases, and alkali hydrolysis in the analysis of aberrant ABC excinuclease incisions adjacent to UV-induced DNA photoproducts. Nucleic Acids Res. 1987 Feb 11;15(3):1227–1243. doi: 10.1093/nar/15.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera M. F., Jr, Rawlings C. J., Roberts J. J. The role of DNA repair in the recovery of human cells from cisplatin toxicity. Chem Biol Interact. 1981 Oct;37(1-2):245–261. doi: 10.1016/0009-2797(81)90181-2. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Binding of the antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA. Biochim Biophys Acta. 1985;780(3):167–180. doi: 10.1016/0304-419x(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Pinto A. L., Lippard S. J. Sequence-dependent termination of in vitro DNA synthesis by cis- and trans-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1985 Jul;82(14):4616–4619. doi: 10.1073/pnas.82.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoff S. C., Beck D. J., Rupp W. D. Repair of plasmid DNA damaged in vitro with cis- or trans-diamminedichloroplatinum(II) in Escherichia coli. Mutat Res. 1987 Mar;183(2):129–137. doi: 10.1016/0167-8817(87)90055-1. [DOI] [PubMed] [Google Scholar]
- Rahmouni A., Leng M. Reaction of nucleic acids with cis-diamminedichloroplatinum(II): interstrand cross-links. Biochemistry. 1987 Nov 17;26(23):7229–7234. doi: 10.1021/bi00397a005. [DOI] [PubMed] [Google Scholar]
- Rahmouni A., Leng M. Reaction of nucleic acids with cis-diamminedichloroplatinum(II): interstrand cross-links. Biochemistry. 1987 Nov 17;26(23):7229–7234. doi: 10.1021/bi00397a005. [DOI] [PubMed] [Google Scholar]
- Rice J. A., Crothers D. M., Pinto A. L., Lippard S. J. The major adduct of the antitumor drug cis-diamminedichloroplatinum(II) with DNA bends the duplex by approximately equal to 40 degrees toward the major groove. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4158–4161. doi: 10.1073/pnas.85.12.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. Quantitative estimation of cisplatin-induced DNA interstrand cross-links and their repair in mammalian cells: relationship to toxicity. Pharmacol Ther. 1987;34(2):215–246. doi: 10.1016/0163-7258(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Friedlos F. The frequency of interstrand cross-links in DNA following reaction of cis-diamminedichloroplatinum(II) with cells in culture or DNA in vitro: stability of DNA cross-links and their repair. Chem Biol Interact. 1982 Mar 15;39(2):181–189. doi: 10.1016/0009-2797(82)90120-x. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Gordon L. K., Haseltine W. A. Use of exonuclease III to determine the site of stable lesions in defined sequences of DNA: the cyclobutane pyrimidine dimer and cis and trans dichlorodiammine platinum II examples. Nucleic Acids Res. 1981 Sep 25;9(18):4595–4609. doi: 10.1093/nar/9.18.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G., Tang M. S. Repair of psoralen and acetylaminofluorene DNA adducts by ABC excinuclease. J Mol Biol. 1985 Aug 20;184(4):725–734. doi: 10.1016/0022-2836(85)90316-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Scovell W. M., Collart F. Unwinding of supercoiled DNA by cis- and trans-diamminedichloroplatinum(II): influence of the torsional strain on DNA unwinding. Nucleic Acids Res. 1985 Apr 25;13(8):2881–2895. doi: 10.1093/nar/13.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby C. P., Sancar A. ABC excinuclease incises both 5' and 3' to the CC-1065-DNA adduct and its incision activity is stimulated by DNA helicase II and DNA polymerase I. Biochemistry. 1988 Sep 20;27(19):7184–7188. doi: 10.1021/bi00419a004. [DOI] [PubMed] [Google Scholar]
- Sladek F. M., Munn M. M., Rupp W. D., Howard-Flanders P. In vitro repair of psoralen-DNA cross-links by RecA, UvrABC, and the 5'-exonuclease of DNA polymerase I. J Biol Chem. 1989 Apr 25;264(12):6755–6765. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tang M. S., Lee C. S., Doisy R., Ross L., Needham-VanDevanter D. R., Hurley L. H. Recognition and repair of the CC-1065-(N3-adenine)-DNA adduct by the UVRABC nucleases. Biochemistry. 1988 Feb 9;27(3):893–901. doi: 10.1021/bi00403a009. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R. B., Pierce J., Case R., Tang M. S. Recognition of the DNA helix stabilizing anthramycin-N2 guanine adduct by UVRABC nuclease. J Mol Biol. 1988 Oct 20;203(4):939–947. doi: 10.1016/0022-2836(88)90119-2. [DOI] [PubMed] [Google Scholar]
- Yeung A. T., Jones B. K., Capraro M., Chu T. The repair of psoralen monoadducts by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1987 Jun 25;15(12):4957–4971. doi: 10.1093/nar/15.12.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]
- den Hartog J. H., Altona C., van Boom J. H., Marcelis A. T., van der Marel G. A., Rinkel L. J., Wille-Hazeleger G., Reedijk J. cis-Platinum induced distortions in DNA. Conformational analysis of d(GpCpG) and cis-pt(NH3)2[d(GpCpG)], studied by 500-MHz NMR. Eur J Biochem. 1983 Aug 15;134(3):485–495. doi: 10.1111/j.1432-1033.1983.tb07593.x. [DOI] [PubMed] [Google Scholar]