Abstract
Naegleria fowleri and Naegleria lovaniensis are closely related free-living amoebae found in the environment. N. fowleri causes primary amoebic meningoencephalitis (PAM), a rapidly fatal disease of the central nervous system, while N. lovaniensis is non-pathogenic. N. fowleri infection occurs when the amoebae access the nasal passages, attach to the nasal mucosa and its epithelial lining, and migrate to the brain. This process involves interaction with components of the host extracellular matrix (ECM). Since the ability to invade tissues can be a characteristic that distinguishes pathogenic from non-pathogenic amoebae, the objective of this study was to assess adhesion to, and invasion of, the ECM by these two related but distinct Naegleria species. N. fowleri exhibited a higher level of adhesion to the ECM components laminin-1, fibronectin and collagen I. Scanning electron microscopy revealed that N. fowleri attached on ECM substrata exhibited a spread-out appearance that included the presence of focal adhesion-like structures. Western immunoblotting revealed two integrin-like proteins for both species, but one of these, with a molecular mass of approximately 70 kDa, was detected at a higher level in N. fowleri. Confocal microscopy indicated that the integrin-like proteins co-localized to the focal adhesion-like structures. Furthermore, anti-integrin antibody decreased adhesion of N. fowleri to ECM components. Finally, N. fowleri disrupted 3D ECM scaffolds, while N. lovaniensis had a minimal effect. Collectively, these results indicate a distinction in adhesion to, and invasion of, ECM proteins between N. fowleri and N. lovaniensis.
Introduction
The genus Naegleria consists of species of free-living amoebae that are found worldwide in soil, and freshwater lakes and ponds (Marciano-Cabral & Cabral, 2007; Martinez & Visvesvara, 1997). Although numerous species from this genus have been identified in the environment and in domestic water supplies (Anderson & Jamieson, 1972; Craun et al., 2005; Gyori, 2003; Jamerson et al., 2009; Marciano-Cabral et al., 2003; Yoder et al., 2004), only one species, Naegleria fowleri, has been linked to disease in humans (Carter, 1968; Cerva & Novăk, 1968; Martinez, 1985). N. fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), a rapidly fatal disease of the central nervous system (CNS) that occurs in individuals who have been engaged recently in activities such as swimming or diving in warm freshwater bodies (Carter, 1968; Martinez, 1985). Infection occurs when amoebae enter the nasal passages and attach to the nasal mucosa (Carter, 1972; Martinez, 1985). Following contact with the nasal epithelium, amoebae penetrate the underlying basement membrane and migrate to the brain, wherein they multiply and destroy tissues, resulting in haemorrhagic necrosis and death (Jarolim et al., 2000; Martinez et al., 1973; Martinez, 1985). While the attachment process may induce an innate immune response, N. fowleri appears to be resistant. For example, it has been reported that it activates the complement system but is capable of evading complement-mediated lysis (Marciano-Cabral & Cabral, 2007).
A pivotal step during infection by N. fowleri is its interaction with the host basement membrane, a complex layer of specialized extracellular matrix (ECM) glycoproteins and proteoglycans that serves to separate the epithelium from stromal tissues (Jarolim et al., 2000; LeBleu et al., 2007). During migration to the brain, N. fowleri must transverse the epithelium and contact specific ECM components, such as laminin-1, an important component of the basement membrane (Yurchenco & Schittny, 1990), collagen I, a prevalent ECM component in connective tissues (Nimni, 1983), and fibronectin, an adhesive glycoprotein found in connective tissues and the blood (Hynes & Yamada, 1982).
It has been reported previously that protozoa that are pathogenic recognize components of the ECM (Gordon et al., 1993; Han et al., 2004; Rocha-Azevedo et al., 2007, 2009; Shibayama et al., 2003). For example, Entamoeba histolytica binds to ECM components, an interaction that may play an important role in its penetration of the intestinal mucosa (de Lourdes Muñoz et al., 2001; Li et al., 1995; Talamás-Rohana & Meza, 1988). Species of Acanthamoeba have been reported to bind to laminin-1, collagen IV and fibronectin (Gordon et al., 1993). However, pathogenic Acanthamoeba culbertsoni has been shown to exhibit a higher level of attachment to ECM components than non-pathogenic Acanthamoeba astronyxis (Rocha-Azevedo et al., 2009). A 39 kDa surface-associated fibronectin-binding protein has been identified for Trichomonas vaginalis and linked to colonization and persistence of infection (Alderete et al., 2002; Lama et al., 2009). Also, a 67 kDa laminin-binding protein has been identified in Leishmania donovani, the causative agent of visceral leishmaniasis (Bandyopadhyay et al., 2001). Consistent with these observations, it has been reported that N. fowleri binds to immobilized fibronectin in a concentration-dependent manner through the mediation of a 60 kDa fibronectin-binding protein (Han et al., 2004).
In the present study the binding and invasive properties of thermotolerant pathogenic N. fowleri were compared with those of a thermotolerant non-pathogenic species, Naegleria lovaniensis. Adhesion to ECM components was shown to differ between the two species, with a higher level of adhesion observed for N. fowleri. Scanning electron microscopy (SEM) revealed differences in morphology of N. fowleri when compared with N. lovaniensis. N. fowleri exhibited a spread-out appearance that was associated with the presence of focal adhesion-like extensions. Western immunoblots revealed the presence of two protein species that were immunoreactive with an anti-integrin antibody. The larger of these, a 70 kDa integrin-like protein, was found at higher levels for N. fowleri as compared with N. lovaniensis. Confocal microscopy revealed that integrin-like proteins co-localized with the focal adhesion-like structures on N. fowleri. In addition, an anti-integrin antibody decreased adhesion of N. fowleri to ECM components. Finally, using an in vitro model of the ECM, N. fowleri exhibited enhanced transmigration as compared with N. lovaniensis. These results, while limited in scope to assessment of two species within the same genus, suggest that the ability to adhere to, and invade, ECM scaffolds serves as a phenotypic marker for differentiating pathogenic from non-pathogenic Naegleria.
Methods
Naegleria cultures.
N. fowleri (ATCC 30894) and N. lovaniensis (ATCC 30569) were obtained from the American Type Culture Collection and were grown at 37 °C for 24 h in Oxoid medium in 75 cm2 plastic flasks (Cline et al., 1983). A mouse-passaged strain of N. fowleri was utilized in these studies to maintain their virulence (Toney & Marciano-Cabral, 1992). For experiments, amoebae were detached from flasks by bumping and washed two times in 0.01 M PBS, pH 7.2.
ECM surface coating.
Twenty-four-well plates or glass coverslips were coated with Sigmacote (Sigma), washed once with deionized water and air-dried overnight at room temperature. The surfaces of plates or coverslips were then incubated (2 h, 37 °C) with fibronectin from human plasma (Sigma), collagen I from rat tails (Sigma) or laminin-1 from Engelbreth-Holm Swarm mouse sarcoma (Invitrogen) at 50 µg ml−1 diluted in PBS. Before addition of amoebae, wells or coverslips were washed gently once with PBS to remove unattached ECM glycoproteins.
Attachment assay.
N. fowleri and N. lovaniensis were radiolabelled (24 or 48 h) with 60 µCi (2.22 MBq) [3H]uridine [specific activity: 35.6 Ci mmol−1 (1317.2 GBq mmol−1)] (Toney & Marciano-Cabral, 1992). These labelled amoebae then were detached from flasks, washed two times with PBS, and counted using a haemocytometer. Amoebae (2×105) then were added to ECM-coated wells (50 µg µl−1) for 50 min at 37 °C. Following incubation, wells were washed once with PBS to remove non-adherent amoebae. Wells coated with BSA were used as a control for non-specific attachment. Attached amoebae were solubilized using 2 % (v/v) Triton X-100 to release the incorporated radiolabel, which was quantified by liquid scintillation counting (Packard 2200CA Tri-Carb Liquid Scintillation Analyzer, Packard Instrument). c.p.m. were converted to percentage attachment, where 100 % represented the amount of radioactivity present in 2×105 amoebae. Percentage attachment was defined using the following formula: percentage attachment = (c.p.m. of attached amoebae/c.p.m. of 2×105 amoebae)×100 %.
Amoeba whole-cell lysates.
Amoebae grown for 24 h were detached from tissue culture flasks, washed twice in PBS, and disrupted by three cycles of freezing in liquid nitrogen and thawing at 37 °C in lysis buffer containing protease inhibitors (50 mM Tris/HCl, pH 7.4, 1 mM PMSF, 1.5 mM pepstatin A and 1.5 mM leupeptin). Cell lysates were used for Western immunoblotting analysis.
Amoeba plasma membrane isolation.
Amoebae grown for 24 h were detached from tissue culture flasks and washed twice in PBS, and membrane proteins were isolated using the Mem-PER eukaryotic membrane protein extraction kit (Pierce), according to the manufacturer’s instructions.
Western immunoblot analysis.
Protein concentrations in amoeba whole-cell lysates (30 µg) and membrane fractions (5 µg) were quantified by the Bradford method (Bradford, 1976) and the RC/DC protein assay (Bio-Rad), respectively. These then were subjected to 12 % polyacrylamide SDS-PAGE. Following electrophoresis, proteins were transferred to nitrocellulose membranes (Towbin et al., 1979). The membranes were rinsed (5 min) in Tris-buffered saline containing 0.1 % tween 20 (TBST) and treated for 1 h at room temperature with blocking buffer consisting of 5 % (w/v) non-fat dry milk in TBST. Nitrocellulose membranes were then rinsed and incubated overnight with chicken polyclonal antibody directed against human β1 integrin subunit (GW22754, Sigma) diluted in blocking buffer (whole-cell lysates 1 : 500, membrane fractions 1 : 1000). Membranes were washed six times (5 min each) in TBST and incubated with a peroxidase-conjugated rabbit anti-chicken antibody (diluted 1 : 10 000). Protein bands were visualized using a chemiluminescence detection kit (Perkin Elmer), according to the manufacturer’s instructions. A U937 human monocyte lymphoma (ATCC CRL-1593.2) whole-cell homogenate (10 µg) or membrane fraction (5 µg) served as a positive control for integrin protein. The chicken polyclonal antibody directed against the human β1 integrin subunit used in Western immunoblots was pre-adsorbed with U937 cells (5×106) prior to use as a negative control. Image analysis of immunoreactive bands was performed using Quantity One version 4.5 software (Bio-Rad Laboratories).
Confocal microscopy.
N. fowleri and N. lovaniensis amoebae were incubated on uncoated, collagen-I-coated, laminin-1-coated or fibronectin-coated coverslips (25 min, 37 °C), fixed in 4 % paraformaldehyde (60 min, 37 °C) and rinsed with Dulbecco’s PBS (DPBS; containing Ca2+ and Mg2+). Cells were permeablized (20 min) with 0.1 % Triton X-100 in DPBS, blocked (60 min) with 5 % BSA in DPBS, and incubated (2 h, room temperature) with a FITC-conjugated monoclonal anti-β1 integrin subunit antibody (1 : 250, P4G11, Millipore). Coverslips were washed with DPBS (1 % BSA), incubated with Alexafluor 594 phalloidin (1 : 500, A12381, Invitrogen) and washed with DPBS. Coverslips were incubated with DAPI (1 : 20 000) to identify nuclei. Additionally, surface staining was assessed with the omission of the permeabilization step. Amoebae were fixed in 4 % paraformaldehyde (60 min, 37 °C) and rinsed with DPBS. Cells were blocked (60 min) with 5 % BSA and 10 % normal goat serum in DPBS, and incubated (2 h, room temperature) with a FITC-conjugated monoclonal anti-β1 integrin subunit antibody (1 : 250, P4G11, Millipore). Amoebae also were probed with a FITC-conjugated monoclonal anti-human IgG antibody (1 : 250, 054211, Invitrogen) as a negative control. Images were acquired by spinning-disk confocal microscopy using a BX51 microscope (Olympus) affixed with an Olympus disk spinning unit and an Orca-R2 CCD camera (Hamamatsu). Images were processed using the Slidebook software package (Intelligent Imaging Innovations).
Competitive attachment assays.
N. fowleri amoebae were radiolabelled (24 h) with 60 µCi (2.22 MBq) [3H]uridine [specific activity: 35.6 Ci mmol−1 (1317.2 GBq mmol−1)] (Thong & Ferrante, 1986). Following labelling, amoebae were pre-incubated (20 min) with the peptide sequence Gly-Arg-Gly-Asp-Thr-Pro (500 µg ml−1, MP Biomedicals), which contains an integrin recognition site, to determine whether the peptide could inhibit attachment (Ruoslahti, 1996). Amoebae were also incubated (20 min) with the peptide sequence Ser-Asp-Gly-Arg-Gly (Sigma), which represents a non-specific control. Amoebae were then used in the attachment assay using collagen I. c.p.m. were converted to percentage attachment, where 100 % represented the amount of radioactivity present in 2×105 amoebae. Percentage attachment was defined using the formula: percentage attachment = (c.p.m. of attached amoebae)/(c.p.m. of 2×105 amoebae)×100 %. Additionally, a second competitive attachment assay was performed using a blocking antibody. N. fowleri (2×105) was preincubated (10 min) with a monoclonal anti-β1 integrin subunit antibody (P4C10) (1 : 100) or with the irrelevant antibody anti-keyhole limpet haemocyanin (KLH; KLH12B4.G3.A8, Abcam) (1 : 100) used as a control, prior to addition to ECM components for 50 min to assess for blocking of attachment. At the end of the incubation period, unattached amoebae were collected, fixed with glutaraldehyde and counted using a haemocytometer to determine whether the anti-β1 integrin antibody inhibited attachment.
Invasion assays.
Tissue culture inserts (Greiner BioOne) with a pore-size of 8 µm were coated (100 µl, 10 min) with either type I collagen (1.7 mg ml−1) or Matrigel (BD Biosciences) (1.7 mg ml−1), a reconstituted basement membrane solution which forms a matrix that has been used extensively for in vitro invasion studies (Kleinman & Jacob, 2001; Kleinman & Martin, 2005). Following the coating period, residual ECM solutions were removed, and the inserts were allowed to dry (2 h) at room temperature. The coated inserts were placed in 24-well plates and were used as an upper chamber. Oxoid medium was added to the bottom chamber of the tissue culture well to serve as an amoeba attractant. N. fowleri or N. lovaniensis (2×105 amoebae) suspended in PBS were added to the upper chamber, and the plates were incubated for 4 h at 37 °C. Amoebae that passed through the ECM-coated inserts and into the bottom chamber were collected and counted using a haemocytometer.
SEM.
To examine for attachment to ECM components, N. fowleri and N. lovaniensis were incubated (50 min) on coated glass coverslips as described in the attachment assay and then fixed with 2.5 % glutaraldehyde. To examine for invasion of the ECM matrix, N. fowleri and N. lovaniensis were incubated (2 h) at 37 °C on collagen I or Matrigel (5 mg ml−1)-coated tissue culture inserts. Inserts then were immersed (1 h) in 2.5 % (v/v) glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, washed four times with PBS, and treated (40 min in the dark) with 2 % (w/v) osmium tetroxide buffered in 0.1 M cacodylate buffer, pH 7.2. The inserts were washed with PBS, dehydrated in a graded series of ethanol solutions, subjected to critical-point drying with CO2 as the transitional fluid, mounted on stubs and coated with gold (30 nm) (Rocha-Azevedo et al., 2007). Samples were examined with a Zeiss EVO 50XVP scanning electron microscope operating at an accelerating speed of 15 kV.
Statistical analysis.
Data were expressed as the mean±sd of the mean. To determine statistical significance (P<0.05), a two-tailed, unpaired Student’s t test and one-way analysis of variance (ANOVA) tests were used.
Results
Naegleria attachment on ECM glycoproteins
The attachment of Naegleria on collagen I, fibronectin and laminin-1 was assessed. N. fowleri and N. lovaniensis demonstrated a differential pattern of adhesion (Fig. 1). While a low level of attachment on BSA-coated wells was observed, N. fowleri exhibited a significantly higher level (P<0.05, ANOVA and Student’s t test) of attachment when exposed to laminin-1, collagen I or fibronectin. N. lovaniensis exhibited minimal differences in attachment to collagen I and laminin-1 compared with the BSA control. However, a slight increase (P<0.05, ANOVA and Student’s t test) in attachment of N. lovaniensis to fibronectin was observed. While both species exhibited attachment to fibronectin, attachment for N. fowleri was 30 % higher than that to the BSA control, while that of the non-pathogen N. lovaniensis was 10 % higher than that to the BSA control (Fig. 1a, b). Similarly, attachment to collagen I and laminin-1 was increased by approximately 25 and 20 %, respectively, for N. fowleri as compared with BSA (Fig. 1a). However, attachment of N. lovaniensis to laminin-1 and collagen I did not differ from the level for the BSA control (Fig. 1b). These results indicate a significantly higher level of adhesion to ECM components by N. fowleri as compared with N. lovaniensis.
Fig. 1.
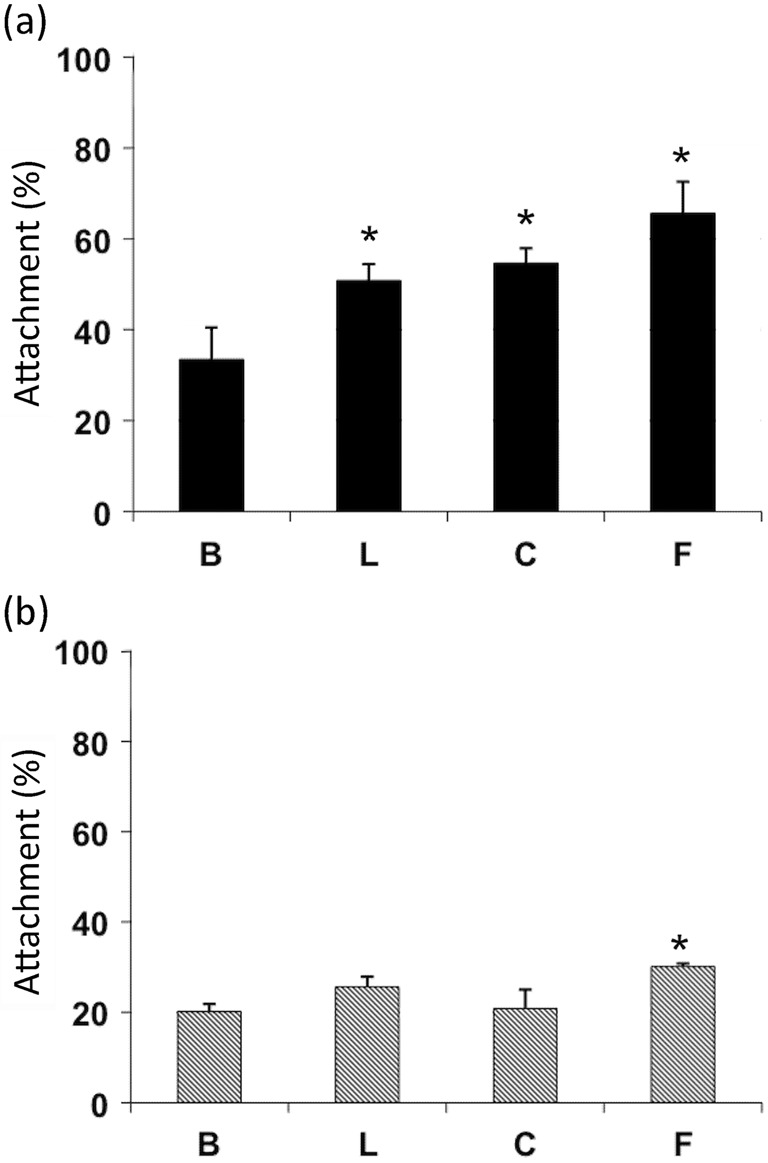
Attachment of (a) N. fowleri and (b) N. lovaniensis on ECM glycoproteins. Radiolabelled amoebae were added to tissue culture wells coated with 50 µg ml−1 laminin-1 (L), collagen I (C) or fibronectin (F). Tissue culture wells treated with BSA (B) served as a control. The number of amoebae added to each well was 2×105, which represents 100 %. Experiments were performed twice in triplicate. Asterisks represent P<0.05 by ANOVA with Student’s t test as compared with BSA.
Morphological assessment of Naegleria on ECM substrata
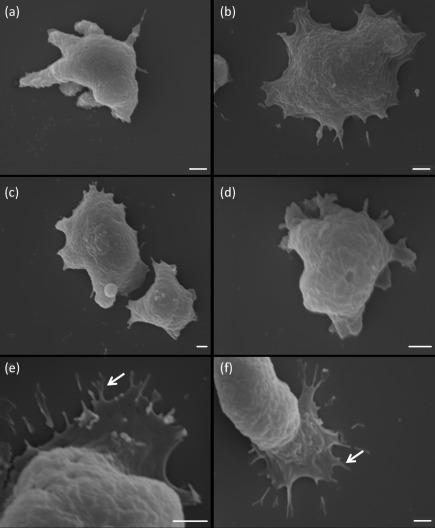
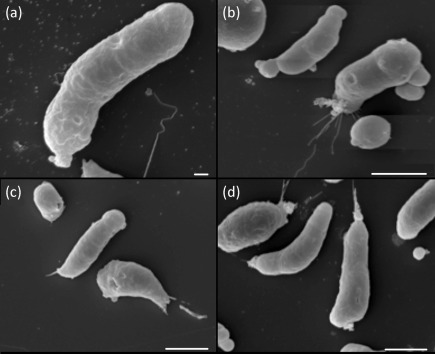
SEM demonstrated morphological differences between the two species upon exposure to ECM components. N. fowleri demonstrated a spread-out appearance on collagen I, laminin-1 and fibronectin (Fig. 2b–d). Furthermore, these amoebae when exposed to ECM components exhibited lamellipodia and morphological features that were similar to those of focal adhesions (Fig. 2e, f, arrows). These features were not observed when N. fowleri was subjected to adherence onto non-coated glass surfaces (Fig. 2a). In contrast, N. lovaniensis exhibited an elongated form when placed on all substrata (Fig. 3a–d). Focal adhesion-like structures were not observed for N. lovaniensis when placed on any of the substrata (Fig. 3).
Fig. 2.
Scanning electron micrographs of N. fowleri attached to various substrata. Amoebae interacting with (a) glass or on ECM glycoproteins, (b) collagen I, (c) fibronectin and (d) laminin-1. (e, f) Higher magnification of the focal adhesion-like structures on laminin-1 (arrows). Bars, 2 µm.
Fig. 3.
Scanning electron micrographs of N. lovaniensis placed on various substrata. Amoebae interacting on (a) glass or on ECM glycoproteins, (b) collagen I, (c) fibronectin and (d) laminin-1. Bars: (a), 1 µm; (b–d), 10 µm.
Detection of integrin-like proteins
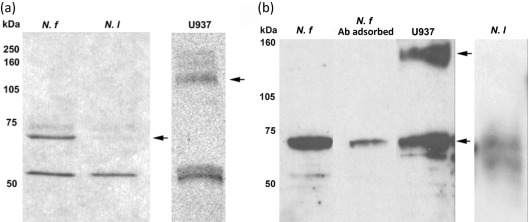
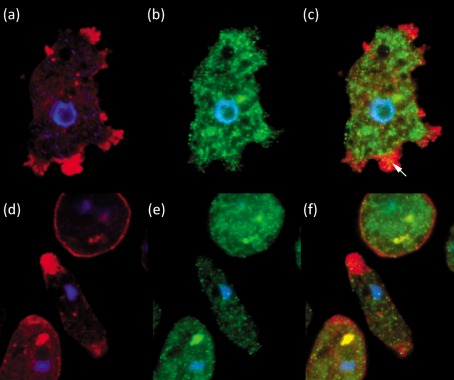
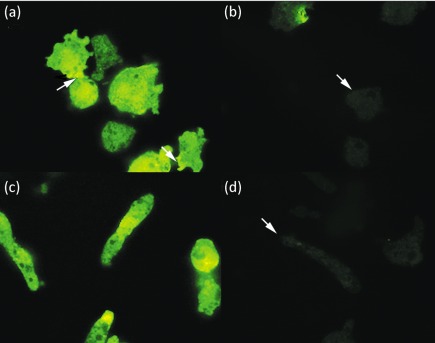
Western immunoblot analysis was performed on whole-cell lysates of N. fowleri and N. lovaniensis using a polyclonal antibody directed to a human β1 integrin subunit. A band of approximately 53 kDa was detected for both N. fowleri and N. lovaniensis (Fig. 4a). Image analysis of the band yielded optical density units of 166 and 144 for N. fowleri and N. lovaniensis, respectively. However, an additional band of approximately 70 kDa was observed at a greater than fourfold higher level for N. fowleri (Fig. 4). For N. fowleri, approximately 149 optical density units were obtained as compared with 34 optical density units for N. lovaniensis. Additionally, Western immunoblot analysis performed on membrane fractions of N. fowleri and N. lovaniensis yielded protein bands of approximately 53 and 70 kDa (Fig. 4b). The protein band of approximately 70 kDa was observed at a greater than 1.55-fold higher level for N. fowleri (Fig. 4b). Pre-adsorption of the anti-β1 integrin subunit antibody was performed prior to Western immunoblot analysis on membrane fractions using whole U937 cells. Pre-adsorption resulted in a 1.5-fold decrease in the 70 kDa protein band and total abolition of the 53 kDa protein band (Fig. 4b). Confocal microscopy confirmed the presence of integrin-like proteins on N. fowleri and N. lovaniensis. Images shown (Fig. 5) are for interaction with collagen I, but are representative of Naegleria placed on all three ECM components. Both species exhibited β1 integrin reactivity within the cell body that was dispersed in a punctate pattern (Fig. 5b, e). However, a distinctive pattern of distribution was observed for N. fowleri. These amoebae exhibited a co-localization of actin filaments and β1 integrin-like protein at their leading edge (arrow) and at focal adhesion-like structures (Fig. 5a, c). This pattern of co-localization was not observed for N. lovaniensis (Fig. 5d, f). Additionally, confocal microscopy was performed in the absence of the permeabilization step (0.1 % Triton X-100) to determine whether the integrin-like molecules were present on the surface of the amoebae. Both species exhibited surface β1 integrin reactivity (Fig. 6a, c), consistent with integrin-like molecules being located on the cell surface. However, this surface reactivity indicative of the presence of β1 integrin-like proteins was found to be concentrated at the focal adhesion-like structures of N. fowleri. A lack of reactivity was observed when the amoebae were probed with a mouse anti-human IgG antibody, which served as a negative control (Fig. 6b, d). Since Western immunoblot analysis and confocal microscopy suggested the presence of an integrin-like protein on amoebae, an RGD-containing peptide (Gly-Arg-Gly-Asp-Thr-Pro) with an integrin binding site was used to assess its ability to inhibit adhesion to collagen I. This peptide, when used in a competitive binding assay, however, did not inhibit adhesion of N. fowleri to collagen I (results not shown). To further investigate whether an integrin-like protein was linked functionally to N. fowleri attachment, a blocking antibody was utilized in N. fowleri attachment inhibition studies (Table 1). Similar studies were not performed using N. lovaniensis, since this species exhibited minimal attachment to laminin-1, collagen I and fibronectin. Adhesion of N. fowleri on laminin-1, collagen I and fibronectin was inhibited by the β1 integrin antibody. In the absence of β1 integrin antibody, 75 % of input N. fowleri were found to bind to laminin-1 and collagen I. Also, 80 % of input N. fowleri were found to bind to fibronectin. In contrast, in the presence of anti-β1 antibody, 40 and 30 % of input N. fowleri, respectively, were found to bind to laminin-1 and collagen I. Of input N. fowleri, 50 % were found to bind to fibronectin in the presence of anti-β1 integrin. Thus, in the presence of the anti-β1 integrin antibody, 1.4- to 2.2-fold less binding was obtained as compared with that obtained in the presence of the integrin-irrelevant anti-KLH antibody.
Fig. 4.
Western immunoblot analysis of (a) N. fowleri (N. f) and N. lovaniensis (N. l) whole-cell lysates and (b) N. fowleri and N. lovaniensis membrane fractions for the detection of integrin-like proteins. Amoebic extracts were separated by 12 % SDS-PAGE and transferred to a nitrocellulose membrane (left panel). A human leukaemic monocyte lymphoma (U937) cell extract was used as a positive integrin control. Membranes then were incubated with a polyclonal chicken anti-human β1 integrin subunit antibody followed by horseradish peroxidase-conjugated rabbit anti-chicken antibody. The chicken polyclonal antibody directed against the human β1 integrin subunit used in Western immunoblotting was pre-adsorbed with U937 cells prior to use to serve as a negative control (N. f Ab adsorbed). Arrows indicate the 70 kDa (N. fowleri and N. lovaniensis) and 140 kDa (U937) major immunoreactive bands.
Fig. 5.
Confocal micrographs of N. fowleri and N. lovaniensis placed on collagen I. N. fowleri was probed with Alexafluor 594 phalloidin (a) or FITC-conjugated monoclonal anti-β1 integrin antibody (b). The merged fluorescent image is shown in (c). N. lovaniensis was probed with Alexafluor 594 phalloidin (d) or FITC-conjugated monoclonal anti-β1 integrin antibody (e). The merged fluorescent image is shown in (f). Nuclear localization is depicted in all panels by DAPI staining (a–f). All images are magnified ×100.
Fig. 6.
Confocal micrographs of surface localization on N. fowleri and N. lovaniensis placed on collagen I. N. fowleri was probed with FITC-conjugated monoclonal anti-β1 integrin antibody (a) or FITC-conjugated monoclonal anti-human IgG antibody (b). N. lovaniensis was probed with FITC-conjugated monoclonal anti-β1 integrin antibody (c) or FITC-conjugated monoclonal anti-human IgG antibody (d). All images are magnified ×100. The arrows in (a) indicate concentrated localization of β1 integrin immune reactivity at focal adhesion-like structures of N. fowleri. The arrows in (b) and (d) indicate the location of amoebae in the negative control that was probed with a FITC-conjugated monoclonal anti-human IgG antibody.
Table 1. Inhibition of attachment of N. fowleri to ECM components.
N. fowleri (2×105) was preincubated (10 min) with anti-KLH or monoclonal anti-β1 integrin antibody prior to addition to ECM components for 50 min. Unattached amoebae were then collected, fixed with glutaraldehyde and counted using a haemocytometer. The percentage of bound amoebae was calculated using the following formula: percentage bound = [(2×105 amoebae)−(number of amoebae recovered)/(2×105 amoebae)]×100 %. One hundred per cent bound would indicate no amoebae recovered.
| ECM component | No antibody | Anti-KLH | Anti-β1 integrin | |||
| Amoebae recovered | Percentage bound | Amoebae recovered | Percentage bound | Amoebae recovered | Percentage bound | |
| Laminin-1 | 5.0×104 | 75 % | 7.0×104 | 65 % | 1.2×105 | 40 % |
| Collagen I | 5.0×104 | 75 % | 7.0×104 | 65 % | 1.4×105 | 30 % |
| Fibronectin | 4.0×104 | 80 % | 6.0×104 | 70 % | 1.0×105 | 50 % |
Invasion on 3D collagen I or Matrigel
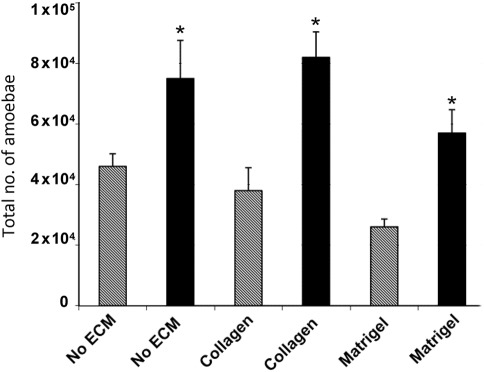
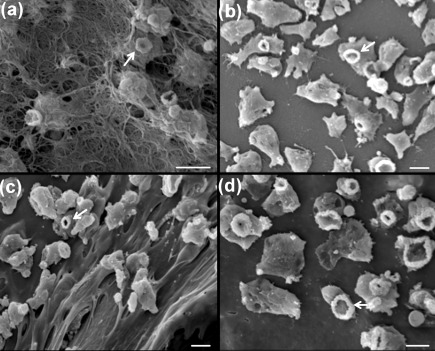
SEM indicated that N. fowleri amoebae passed through collagen I and Matrigel (Fig. 7). Evidence for penetration of these matrices was obtained as early as after a 2 h incubation period. In contrast, a very low number of N. lovaniensis was observed to have disrupted the ECM and, then, only after 4 h of incubation. SEM revealed a remodelling of collagen I fibres, with overt extensive damage to the matrix following 2 h of exposure to N. fowleri (Fig. 8a). Large gaps in the matrix were observed that appeared to provide a passage for N. fowleri through the collagen I layer. No observable perturbation of the matrix was observed in the presence of N. lovaniensis (Fig. 8b). However, both Naegleria species exhibited the presence of ‘food-cups’ when in the presence of the collagen I matrix (Fig. 8). Similar results were obtained for N. fowleri versus N. lovaniensis when placed on Matrigel. N. fowleri exerted extensive damage to the Matrigel matrix as evidenced by the presence of large holes or gaps (Fig. 8c). In contrast, the Matrigel layer remained relatively intact when exposed (2 h) to N. lovaniensis (Fig. 8d).
Fig. 7.
Invasion of N. fowleri and N. lovaniensis through ECM scaffolds (collagen I and Matrigel). Amoebae that invaded were counted using a haemocytometer. Black bars, N. fowleri; grey bars, N. lovaniensis. Experiments were performed twice in triplicate. Asterisks represent P<0.05 by Student’s t test.
Fig. 8.
Interaction (2 h) of N. fowleri and N. lovaniensis with collagen I and Matrigel matrices. (a) N. fowleri emerging through the bottom side of the collagen I scaffold and (b) N. lovaniensis interacting with collagen I on the surface of the scaffold. (c) N. fowleri interacting with the Matrigel surface. (d) N. lovaniensis interacting with the Matrigel surface. Note the presence of ‘food-cups’ (arrows) on both N. fowleri and N. lovaniensis. Bars, 10 µm.
Discussion
N. fowleri is the only species of the genus Naegleria that has been isolated from fatal CNS infections in humans (Martinez, 1985; Yoder et al., 2010). Other species, including Naegleria italica and Naegleria australiensis, have been shown to cause disease in experimentally infected mice but have never been linked to human infections (De Jonckheere, 2004). The ability to elicit cytopathic effects on cells in culture does not appear to define pathogenicity for Naegleria. That is, while species of Naegleria such as N. lovaniensis and Naegleria gruberi destroy mammalian cells in vitro (Marciano-Cabral et al., 1982; Marciano-Cabral & Fulford, 1986), they have yet to be shown to be causative of human disease. Also, the ability to tolerate temperatures of 37 °C or higher does not appear to be a defining marker of pathogenicity for Naegleria. Thermotolerance is not seen with N. gruberi, and it has been proposed that this lack of heat tolerance results in humans and other mammals, such as mice, being non-permissive hosts (Marciano-Cabral & Fulford, 1986). Furthermore, N. lovaniensis is thermotolerant and can survive at temperatures of 37 °C and higher (Stevens et al., 1980), yet has not been associated with human infection.
The interaction of cells with ECM components plays an important role in mediating cell adhesion and migration (Berrier & Yamada, 2007), and promoting invasion of host tissue. Binding to, and passage through, ECM components by micro-organisms have been implicated in the pathogenesis of bacterial, viral, protozoan and fungal infections (Alderete et al., 2002; Boshuizen et al., 2004; de Bentzmann et al., 2004; Gozalbo et al., 1998; Hostetter, 1999; Kottom et al., 2008; Lama et al., 2009; Casta e Silva Filho et al., 1988). In this context, the ability of N. fowleri to adhere to and invade ECM components may serve as a hallmark of pathogenicity. For example, attachment to the nasal epithelium by N. fowleri has been reported to be an important early event in the pathogenesis of PAM (Martinez et al., 1973). Studies characterizing experimentally induced PAM in mice have shown that deep invasion occurs after N. fowleri amoebae penetrate the basement membrane (Martinez et al., 1973).
In the present study, two species within the genus Naegleria, one linked to pathogenicity in humans (Carter, 1968; Cerva & Novăk, 1968; Martinez, 1985) and the other a non-pathogen (Stevens et al., 1980; Marciano-Cabral & Fulford, 1986), were selected for assessment of differences in attachment to, and invasion of, ECM components. A differential level of binding to laminin-1, collagen I and fibronectin was observed for N. fowleri versus N. lovaniensis. Whereas N. lovaniensis exhibited a level of attachment to the different ECM components that did not differ in large measure from that noted for the BSA control, a significant level of attachment on all matrices was observed for N. fowleri when compared with the BSA control. These observations indicate that, in contrast to N. lovaniensis, N. fowleri ‘recognizes’ select binding motifs on the target ECM proteins. Thus, in order to gain further insight into the mode by which N. fowleri attaches to ECM components, SEM was performed. N. fowleri exhibited a spread-out appearance on ECM composites and displayed numerous focal adhesion-like extensions that were in contact with, and apparently attached to, sites on collagen I, fibronectin and laminin-1. Confocal microscopy demonstrated that β1 integrin and actin immunoreactivity co-localized at these amoebic extensions, consistent with their designation as focal adhesion-like structures (Burridge & Chrzanowska-Wodnicka, 1996). These morphological features were not observed for N. lovaniensis. Additionally, confocal microscopy and SEM demonstrated that N. lovaniensis maintained a rounded appearance when placed on ECM composites and did not exhibit focal adhesion-like structures. It has been reported that mammalian cell attachment triggers signalling that leads to formation of focal adhesions (Burridge & Chrzanowska-Wodnicka, 1996). Such focal adhesions, or cell matrix adhesions, as large macromolecular aggregates, appear to serve both as anchorage points for cells and as ‘sensors’ of ECM components (Riveline et al., 2001). Consistent with these observations, it has been shown that pathogenic amoebae, such as N. fowleri and E. histolytica, form actin plates upon exposure to ECM components that are involved in adhesion (Han et al., 2004; Talamás-Rohana et al., 1994).
Formation of focal adhesions generally involves integrins that typically are found on mammalian cells and are involved in recognition of an ECM motif (Ruoslahti, 1996). In this structural rearrangement model, integrins link the extracellular environment with the actin cytoskeleton (Burridge & Chrzanowska-Wodnicka, 1996). Many microbial pathogens express integrin-like molecules on their surface. For example, a 140 kDa β1 integrin-like molecule has been identified in E. histolytica and has been linked to attachment to fibronectin (Sengupta et al., 2001; Talamás-Rohana & Meza, 1988; Talamás-Rohana et al., 1994). Furthermore, binding of this β1 integrin-like molecule to fibronectin has been demonstrated to trigger a signal transduction cascade in E. histolytica involving tyrosine protein kinase, which is similar to that observed for mammalian cells (Hernández-Ramírez et al., 2000; Meza, 2000; Flores-Robles et al., 2003). Han et al. (2004) have reported that N. fowleri possesses an integrin-like molecule, which binds to immobilized fibronectin. This molecule was designated as an α integrin subunit and has been demonstrated to play a role in cytotoxicity. In addition, a recently published sequence of the N. gruberi genome has revealed the presence of a von Willebrand factor type A (VWA) domain-containing protein (Fritz-Laylin et al., 2010). It has been suggested that all integrin beta subunits contain VWA domains (Tuckwell, 1999; Whittaker & Hynes, 2002).
In the present study, Western immunoblot analysis using a human β1 integrin antibody demonstrated that N. fowleri and N. lovaniensis exhibited a similar level of immune reactivity for a 53 kDa protein. However, a 70 kDa immunoreactive protein species was found at a higher level for N. fowleri. While the antibody used in the present study was directed at the human β1 integrin subunit, the results are consistent with reports that amoebae express integrin-like proteins. Additionally, Western immunoblot analysis using membrane fractions demonstrated that these proteins localized to the surface of the amoebae, as has been demonstrated for mammalian integrins and other eukaryotes. For example, an adhesion molecule with β integrin features has been identified in free-living Dictyostelium amoebae (Cornillon et al., 2006). However, the mere presence of cell surface integrin-like molecules may not be a distinction that discriminates pathogenic from non-pathogenic amoebae. For example, both E. histolytica, a pathogen, and Entamoeba dispar, a non-pathogen, have been reported to express integrin-like proteins on their surface (Pillai & Kain, 2005). Similarly, Candida spp. have been reported to express integrin-like proteins that mediate binding to fibronectin (Santoni et al., 1995). Thus, the context of integrin-like protein compartmentalization within the cell may be a critical element related to a pathogenic phenotype. That is, while both N. fowleri and N. lovaniensis contained integrin-like proteins, only N. fowleri exhibited a co-localization with actin filaments at focal adhesion-like structures.
In order to garner additional insight into whether integrin-like proteins played a functionally relevant role in binding to the ECM, a peptide harbouring an integrin recognition site was used in binding inhibition experiments. The peptide selected contained an arginine-glycine-aspartic acid (RGD) motif, an acidic amino acid domain found in the integrin interaction site of many ECM proteins (Ruoslahti, 1996). However, the RGD peptide selected did not inhibit adhesion of N. fowleri to collagen I. One possible explanation for the lack of inhibition is that it has been reported that RGD-dependent attachment of cells to collagen I occurs when this protein is in the denatured state. Thus, in the native conformation of collagen I, the RGD motif may not be accessible to binding of integrins (Barczyk et al., 2010). In this context, a prerequisite for RGD-mediated binding to collagen I would be a prior degradative step exerted on the part of the amoebae. That is, proteases secreted from N. fowleri could serve to ‘unmask’ RGD tripeptide motifs on the target collagen 1. An alternative possibility is that the 53 kDa and/or 70 kDa proteins expressed on N. fowleri may be linked to binding to collagen 1 in an RGD-independent fashion. Indeed, two-thirds of integrins have been found to bind ligands in an RGD-independent manner (Barczyk et al., 2010). To explore further the possibility of an integrin-like protein on N. fowleri as linked to binding to the ECM, an anti-integrin monoclonal antibody that inhibits attachment of mammalian cells to collagen I, fibronectin and laminin-1 was used in binding inhibition studies. This antibody decreased attachment of N. fowleri to ECM components. When an irrelevant antibody, namely anti-KLH antibody, was used in experiments, a 10 % decreased level of binding to collagen I, fibronectin or laminin-1 was obtained. In contrast, when the anti-integrin monoclonal antibody was used, a 30–45 % decreased level of binding to the ECM components was observed. This greater than twofold difference in decreasing the level of binding to ECM proteins implies a specificity of action of the anti-integrin antibody.
Finally, the ability of N. fowleri versus N. lovaniensis to invade collagen I or Matrigel matrices was assessed. Fibronectin and laminin-1 were not used for the invasion studies, as these components do not allow for formation of a 3D matrix structure. Invasion assays have been used to measure the ‘invasiveness’ of tumour cells (Kleinman & Jacob, 2001) and Acanthamoeba spp. (Rocha-Azevedo et al., 2009). Microscopic examination of tissue has demonstrated that N. fowleri passes through the nasal epithelium and then penetrates the basement membrane as it migrates to the brain (Jarolim et al., 2000). In addition, electron microscopic analysis of infected mouse brain has revealed amoebae apparently engulfing collagen fibrils during the invasion process (Martinez et al., 1973). In the present study, N. fowleri were shown to more readily penetrate collagen I and Matrigel constructs. This apparent enhanced capability to penetrate the ECM 3D composite could be due to enhanced motility on the part of the amoeba. For example, Thong & Ferrante (1986) compared the migration patterns of Naegleria species under agarose and reported that their locomotive ability correlated with pathogenic potential. In addition, N. fowleri, but not N. lovaniensis, has been shown to exhibit enhanced motility when placed in proximity to rat B103 neuroblastoma cells (Cline et al., 1986), suggesting that N. fowleri selectively ‘senses’ neurotropic factors as compared with N. lovaniensis and other free-living amoebae that lack neuropathogenic potential. Alternatively, the focal adhesion-like protuberances may serve as anchor points to the ECM and, in that capacity, exert tensional forces (Hersen & Ladoux, 2011) that disrupt the ECM. In this context, disruption of ECM scaffolds through the mediation of tensional forces has been observed for fibroblasts (Grinnell, 2008). The extent to which proteases released from N. fowleri (Aldape et al., 1994) or tensional forces created by the amoebae–matrix interaction are involved in invasion of the ECM scaffold remains to be elucidated. Finally, ‘food-cups’ on amoebae do not appear to play a vital role in invasion of the ECM, since SEM revealed their presence on both species. It has been proposed that these structures are used by amoebae to ingest bacteria in the environment (Marciano-Cabral & Cabral, 2007).
In summary, two free-living amoebae from the genus Naegleria, one a pathogen in humans and the other a non-pathogen in humans, were compared for their ability to attach to, and invade, ECM proteins in vitro. N. fowleri exhibited a higher level of attachment to ECM components than N. lovaniensis. A distinctive morphological feature of N. fowleri was that it displayed focal adhesion-like structures to which β1 integrin-like molecules co-localized with actin filaments. The role of the focal-like adhesions in the ECM attachment process, the functional relevance of integrin-like molecules that co-localize with actin filaments in this process, and the linkage of amoebic attachment to the ECM in induction of a signal transductional cascade that promotes invasion of the ECM, are under investigation.
Acknowledgements
This work was supported, in part, with funds provided through the Immunotoxicology Foundation of Richmond. We would like to thank Drs Jason Carlyon and Bernice Huang of the Department of Microbiology and Immunology, Virginia Commonwealth University (VCU) School of Medicine for assistance with confocal microscopy. SEM was performed at the VCU Microscopy Facility supported, in part, by NIH awards NINDS 5P30NS047463 and NIH-NCRR-1S10RR022495.
Abbreviations:
- CNS
central nervous system
- ECM
, extracellular matrix
- KLH
keyhole limpet haemocyanin
- PAM
primary amoebic meningoencephalitis
- SEM
scanning electron microscopy
References
- Aldape K., Huizinga H., Bouvier J., McKerrow J. (1994). Naegleria fowleri: characterization of a secreted histolytic cysteine protease. Exp Parasitol 78, 230–241. 10.1006/expr.1994.1023 [DOI] [PubMed] [Google Scholar]
- Alderete J. F., Benchimol M., Lehker M. W., Crouch M. L. (2002). The complex fibronectin–Trichomonas vaginalis interactions and trichomonosis. Parasitol Int 51, 285–292. 10.1016/S1383-5769(02)00015-6 [DOI] [PubMed] [Google Scholar]
- Anderson K., Jamieson A. (1972). Primary amoebic meningoencephalitis. Lancet 299, 902–903. 10.1016/S0140-6736(72)90772-6 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay K., Karmakar S., Ghosh A., Das P. K. (2001). Role of 67 kDa cell surface laminin binding protein of Leishmania donovani in pathogenesis. J Biochem 130, 141–148. [DOI] [PubMed] [Google Scholar]
- Barczyk M., Carracedo S., Gullberg D. (2010). Integrins. Cell Tissue Res 339, 269–280. 10.1007/s00441-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier A. L., Yamada K. M. (2007). Cell–matrix adhesion. J Cell Physiol 213, 565–573. 10.1002/jcp.21237 [DOI] [PubMed] [Google Scholar]
- Boshuizen J. A., Rossen J. W., Sitaram C. K., Kimenai F. F., Simons-Oosterhuis Y., Laffeber C., Büller H. A., Einerhand A. W. (2004). Rotavirus enterotoxin NSP4 binds to the extracellular matrix proteins laminin-beta3 and fibronectin. J Virol 78, 10045–10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. (1996). Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12, 463–519. 10.1146/annurev.cellbio.12.1.463 [DOI] [PubMed] [Google Scholar]
- Carter R. F. (1968). Primary amoebic meningo-encephalitis: clinical, pathological and epidemiological features of six fatal cases. J Pathol Bacteriol 96, 1–25. 10.1002/path.1700960102 [DOI] [PubMed] [Google Scholar]
- Carter R. F. (1972). Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg 66, 193–208. 10.1016/0035-9203(72)90147-2 [DOI] [PubMed] [Google Scholar]
- Casta e Silva Filho F., de Souza W., Lopes J. D. (1988). Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc Natl Acad Sci U S A 85, 8042–8046. 10.1073/pnas.85.21.8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerva L., Novăk K. (1968). Amoebic meningoencephalitis: 16 fatalities. Science 160, 92. 10.1126/science.160.3823.92 [DOI] [PubMed] [Google Scholar]
- Cline M., Marciano-Cabral F., Bradley S. G. (1983). Comparison of Naegleria fowleri and Naegleria gruberi cultivated in the same nutrient medium. J Protozool 30, 387–391. [DOI] [PubMed] [Google Scholar]
- Cline M., Carchman R., Marciano-Cabral F. (1986). Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J Protozool 33, 10–13. [DOI] [PubMed] [Google Scholar]
- Cornillon S., Gebbie L., Benghezal M., Nair P., Keller S., Wehrle-Haller B., Charette S. J., Brückert F., Letourneur F., Cosson P. (2006). An adhesion molecule in free-living Dictyostelium amoebae with integrin β features. EMBO Rep 7, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craun G. F., Calderon R. L., Craun M. F. (2005). Outbreaks associated with recreational water in the United States. Int J Environ Health Res 15, 243–262. 10.1080/09603120500155716 [DOI] [PubMed] [Google Scholar]
- de Bentzmann S., Tristan A., Etienne J., Brousse N., Vandenesch F., Lina G. (2004). Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J Infect Dis 190, 1506–1515. 10.1086/424521 [DOI] [PubMed] [Google Scholar]
- De Jonckheere J. F. (2004). Molecular definition and the ubiquity of species in the genus Naegleria. Protist 155, 89–103. [DOI] [PubMed] [Google Scholar]
- de Lourdes Muñoz M., Das P., Tovar R. (2001). Entamoeba histolytica trophozoites activated by collagen type I and Ca2+ have a structured cytoskeleton during collagenase secretion. Cell Motil Cytoskeleton 50, 45–54. 10.1002/cm.1040 [DOI] [PubMed] [Google Scholar]
- Flores-Robles D., Rosales C., Rosales-Encina J. L., Talamás-Rohana P. (2003). Entamoeba histolytica: a β1 integrin-like fibronectin receptor assembles a signaling complex similar to those of mammalian cells. Exp Parasitol 103, 8–15. 10.1016/S0014-4894(03)00062-6 [DOI] [PubMed] [Google Scholar]
- Fritz-Laylin L. K., Prochnik S. E., Ginger M. L., Dacks J. B., Carpenter M. L., Field M. C., Kuo A., Paredez A., Chapman J. & other authors (2010). The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 140, 631–642. 10.1016/j.cell.2010.01.032 [DOI] [PubMed] [Google Scholar]
- Gordon V. R., Asem E. K., Vodkin M. H., McLaughlin G. L. (1993). Acanthamoeba binds to extracellular matrix proteins in vitro. Invest Ophthalmol Vis Sci 34, 658–662. [PubMed] [Google Scholar]
- Gozalbo D., Gil-Navarro I., Azorín I., Renau-Piqueras J., Martínez J. P., Gil M. L. (1998). The cell wall-associated glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is also a fibronectin and laminin binding protein. Infect Immun 66, 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. (2008). Fibroblast mechanics in three-dimensional collagen matrices. J Bodyw Mov Ther 12, 191–193. 10.1016/j.jbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyori E. (2003). December 2002: 19-year old male with febrile illness after jet ski accident. Brain Pathol 13, 237–239. [PubMed] [Google Scholar]
- Han K. L., Lee H. J., Shin M. H., Shin H. J., Im K. I., Park S. J. (2004). The involvement of an integrin-like protein and protein kinase C in amoebic adhesion to fibronectin and amoebic cytotoxicity. Parasitol Res 94, 53–60. 10.1007/s00436-004-1158-9 [DOI] [PubMed] [Google Scholar]
- Hernández-Ramírez V. I., Anaya-Ruiz M., Rios A., Talamás-Rohana P. (2000). Entamoeba histolytica: tyrosine kinase activity induced by fibronectin through the β1-integrin-like molecule. Exp Parasitol 95, 85–95. 10.1006/expr.2000.4522 [DOI] [PubMed] [Google Scholar]
- Hersen P., Ladoux B. (2011). Biophysics: push it, pull it. Nature 470, 340–341. 10.1038/470340a [DOI] [PubMed] [Google Scholar]
- Hostetter M. K. (1999). Integrin-like proteins in Candida spp. and other microorganisms. Fungal Genet Biol 28, 135–145. 10.1006/fgbi.1999.1165 [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. (1982). Fibronectins: multifunctional modular glycoproteins. J Cell Biol 95, 369–377. 10.1083/jcb.95.2.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamerson M., Remmers K., Cabral G., Marciano-Cabral F. (2009). Survey for the presence of Naegleria fowleri amebae in lake water used to cool reactors at a nuclear power generating plant. Parasitol Res 104, 969–978. 10.1007/s00436-008-1275-y [DOI] [PubMed] [Google Scholar]
- Jarolim K. L., McCosh J. K., Howard M. J., John D. T. (2000). A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J Parasitol 86, 50–55. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Jacob K. (2001). Invasion assays. Curr Protoc Cell Biol 12, 12.2.1–12.2.5. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R. (2005). Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15, 378–386. 10.1016/j.semcancer.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Kottom T. J., Kennedy C. C., Limper A. H. (2008). Pneumocystis PCINT1, a molecule with integrin-like features that mediates organism adhesion to fibronectin. Mol Microbiol 67, 747–761. 10.1111/j.1365-2958.2007.06093.x [DOI] [PubMed] [Google Scholar]
- Lama A., Kucknoor A., Mundodi V., Alderete J. F. (2009). Glyceraldehyde-3-phosphate dehydrogenase is a surface-associated, fibronectin-binding protein of Trichomonas vaginalis. Infect Immun 77, 2703–2711. 10.1128/IAI.00157-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu V. S., MacDonald B., Kalluir R. (2007). Structure and function of basement membrane. Exp Biol Med 232, 1121–1129. 10.3181/0703-MR-72 [DOI] [PubMed] [Google Scholar]
- Li E., Yang W. G., Zhang T., Stanley S. L., Jr (1995). Interaction of laminin with Entamoeba histolytica cysteine proteinases and its effect on amebic pathogenesis. Infect Immun 63, 4150–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F., Cabral G. A. (2007). The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol Med Microbiol 51, 243–259. 10.1111/j.1574-695X.2007.00332.x [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. M., Fulford D. E. (1986). Cytopathology of pathogenic and nonpathogenic Naegleria species for cultured rat neuroblastoma cells. Appl Environ Microbiol 51, 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F. M., Patterson M., John D. T., Bradley S. G. (1982). Cytopathogenicity of Naegleria fowleri and Naegleria gruberi for established mammalian cell cultures. J Parasitol 68, 1110–1116. 10.2307/3281100 [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F., MacLean R., Mensah A., LaPat-Polasko L. (2003). Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl Environ Microbiol 69, 5864–5869. 10.1128/AEM.69.10.5864-5869.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. J. (1985). Free-Living Amoebas: Natural History, Prevention, Diagnosis, Pathology, and Treatment of Disease. Boca Raton, FL: CRC Press. [Google Scholar]
- Martinez A. J., Visvesvara G. S. (1997). Free-living, amphizoic and opportunistic amebas. Brain Pathol 7, 583–598. 10.1111/j.1750-3639.1997.tb01076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Duma R. J., Nelson E. C., Moretta F. L. (1973). Experimental Naegleria meningoencephalitis in mice. Penetration of the olfactory mucosal epithelium by Naegleria and pathologic changes produced: a light and electron microscope study. Lab Invest 29, 121–133. [PubMed] [Google Scholar]
- Meza I. (2000). Extracellular matrix-induced signaling in Entamoeba histolytica: its role in invasiveness. Parasitol Today 16, 23–28. 10.1016/S0169-4758(99)01586-0 [DOI] [PubMed] [Google Scholar]
- Nimni M. E. (1983). Collagen: structure, function, and metabolism in normal and fibrotic tissues. Semin Arthritis Rheum 13, 1–86. 10.1016/0049-0172(83)90024-0 [DOI] [PubMed] [Google Scholar]
- Pillai D. R., Kain K. C. (2005). Entamoeba histolytica: identification of a distinct β2 integrin-like molecule with a potential role in cellular adherence. Exp Parasitol 109, 135–142. 10.1016/j.exppara.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Riveline D., Zamir E., Balaban N. Q., Schwarz U. S., Ishizaki T., Narumiya S., Kam Z., Geiger B., Bershadsky A. D. (2001). Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153, 1175–1186. 10.1083/jcb.153.6.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Azevedo B., Jamerson M., Cabral G. A., Silva-Filho F. C., Marciano-Cabral F. (2007). The interaction between the amoeba Balamuthia mandrillaris and extracellular matrix glycoproteins in vitro. Parasitology 134, 51–58. 10.1017/S0031182006001272 [DOI] [PubMed] [Google Scholar]
- Rocha-Azevedo B. D., Jamerson M., Cabral G. A., Silva-Filho F. C., Marciano-Cabral F. (2009). Acanthamoeba interaction with extracellular matrix glycoproteins: biological and biochemical characterization and role in cytotoxicity and invasiveness. J Eukaryot Microbiol 56, 270–278. 10.1111/j.1550-7408.2009.00399.x [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. (1996). RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12, 697–715. 10.1146/annurev.cellbio.12.1.697 [DOI] [PubMed] [Google Scholar]
- Santoni G., Birarelli P., Hong L. J., Gamero A., Djeu J. Y., Piccoli M. (1995). An α 5 β 1-like integrin receptor mediates the binding of less pathogenic Candida species to fibronectin. J Med Microbiol 43, 360–367. 10.1099/00222615-43-5-360 [DOI] [PubMed] [Google Scholar]
- Sengupta K., Hernández-Ramírez V. I., Rios A., Mondragón R., Talamás-Rohana P. (2001). Entamoeba histolytica: monoclonal antibody against the β1 integrin-like molecule (140 kDa) inhibits cell adhesion to extracellular matrix components. Exp Parasitol 98, 83–89. 10.1006/expr.2001.4621 [DOI] [PubMed] [Google Scholar]
- Shibayama M., Serrano-Luna J. J., Rojas-Hernández S., Campos-Rodríguez R., Tsutsumi V. (2003). Interaction of secretory immunoglobulin A antibodies with Naegleria fowleri trophozoites and collagen type I. Can J Microbiol 49, 164–170. 10.1139/w03-023 [DOI] [PubMed] [Google Scholar]
- Stevens A. R., De Jonckheere J., Willaert E. (1980). Naegleria lovaniensis new species: isolation and identification of six thermophilic strains of a new species found in association with Naegleria fowleri. Int J Parasitol 10, 51–64. 10.1016/0020-7519(80)90064-8 [DOI] [PubMed] [Google Scholar]
- Talamás-Rohana P., Meza I. (1988). Interaction between pathogenic amebas and fibronectin: substrate degradation and changes in cytoskeleton organization. J Cell Biol 106, 1787–1794. 10.1083/jcb.106.5.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamás-Rohana P., Hernández V. I., Rosales-Encina J. L. (1994). A β1 integrin-like molecule in Entamoeba histolytica. Trans R Soc Trop Med Hyg 88, 596–599. 10.1016/0035-9203(94)90179-1 [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A. (1986). Migration patterns of pathogenic and nonpathogenic Naegleria spp. Infect Immun 51, 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney D. M., Marciano-Cabral F. (1992). Alterations in protein expression and complement resistance of pathogenic Naegleria amoebae. Infect Immun 60, 2784–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76, 4350–4354. 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckwell D. (1999). Evolution of von Willebrand factor A (VWA) domains. Biochem Soc Trans 27, 835–840. [DOI] [PubMed] [Google Scholar]
- Whittaker C. A., Hynes R. O. (2002). Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell 13, 3369–3387. 10.1091/mbc.E02-05-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder J. S., Blackburn B. G., Craun G. F., Hill V., Levy D. A., Chen N., Lee S. H., Calderon R. L., Beach M. J. (2004). Surveillance for waterborne-disease outbreaks associated with recreational water–United States, 2001–2002. MMWR Surveill Summ 53, 1–22. [PubMed] [Google Scholar]
- Yoder J. S., Eddy B. A., Visvesvara G. S., Capewell L., Beach M. J. (2010). The epidemiology of primary amoebic meningoencephalitis in the USA, 1962–2008. Epidemiol Infect 138, 968–975. 10.1017/S0950268809991014 [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. (1990). Molecular architecture of basement membranes. FASEB J 4, 1577–1590. [DOI] [PubMed] [Google Scholar]