Abstract
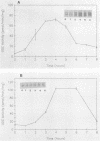
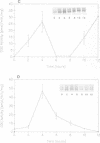
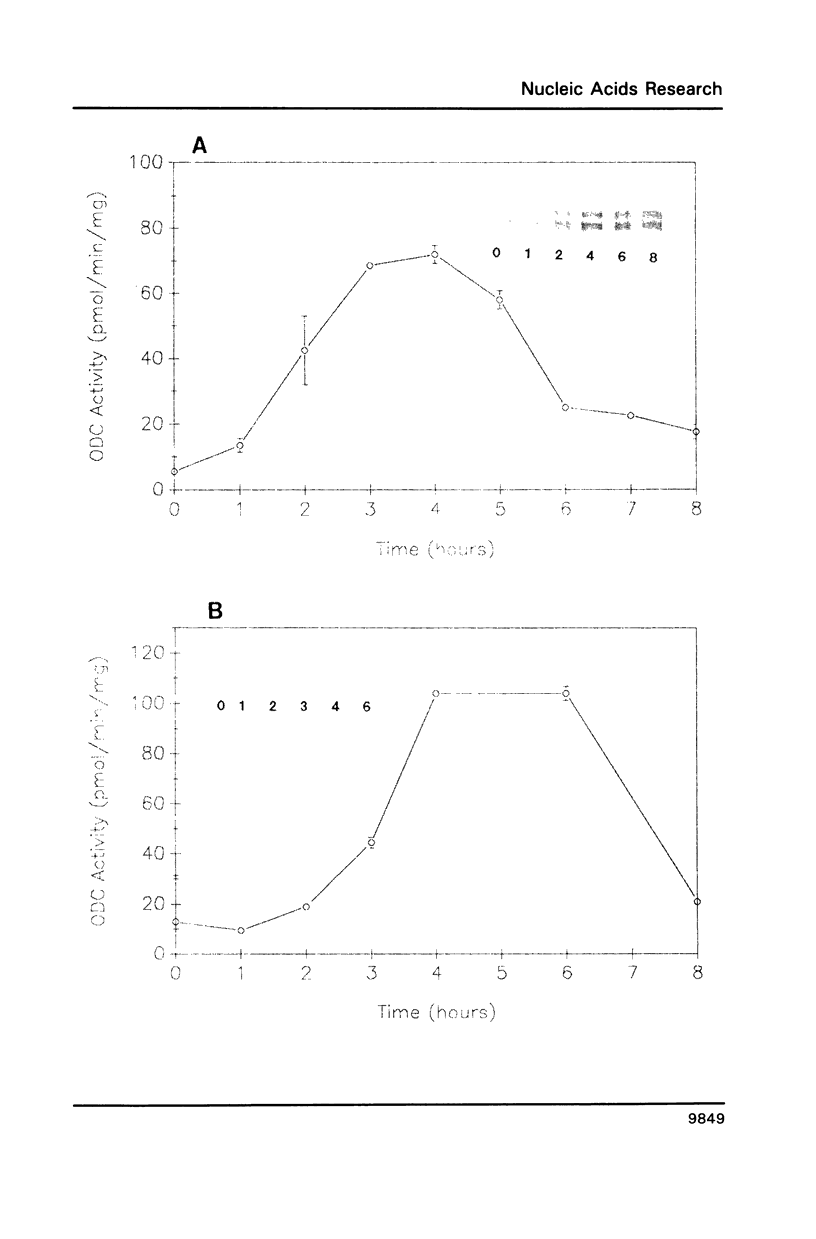
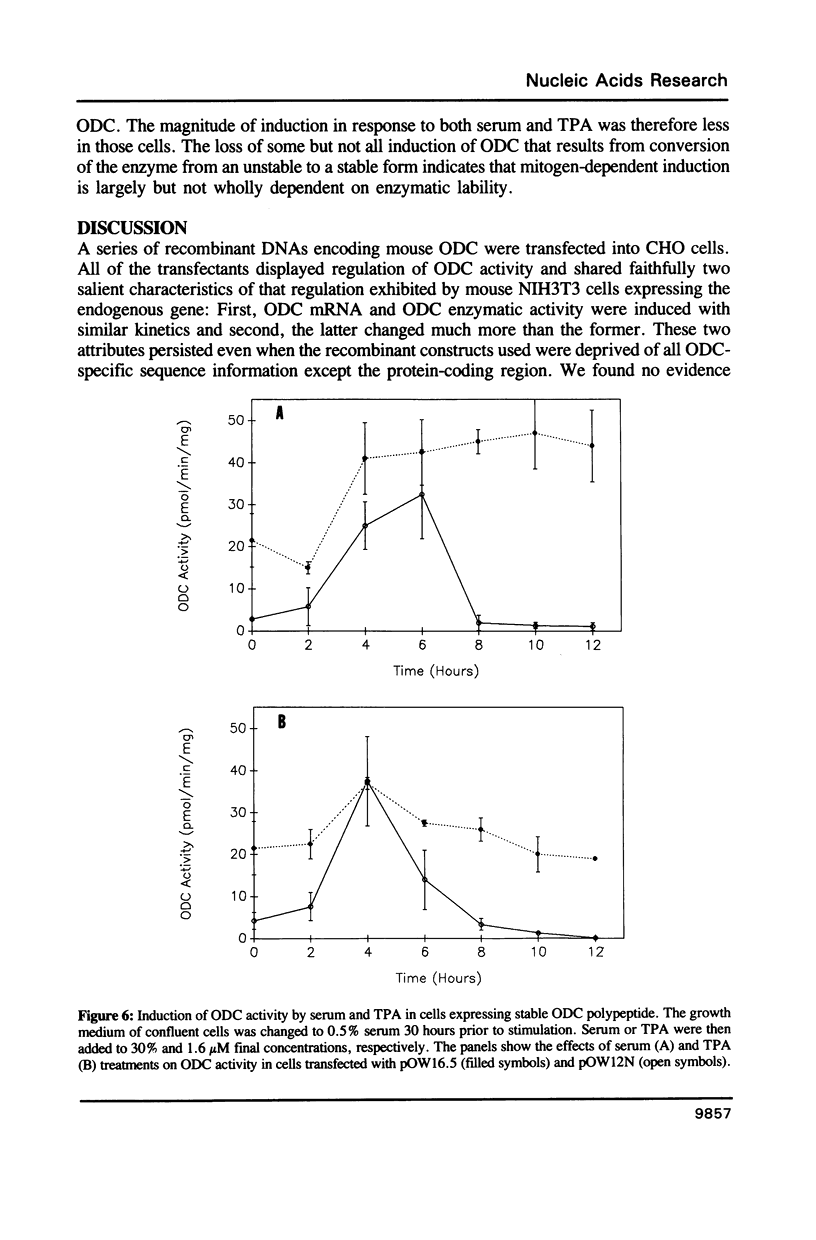
To determine the genetic elements required for modulation of ornithine decarboxylase (ODC) activity in response to cell growth or treatment with serum or with tetradecanoyl phorbol acetate, ODC-deficient cells were transfected with a series of recombinant DNAs encoding mouse ODC. All of the transfected cells expressing an intact mouse ODC protein displayed regulation of ODC activity, including those expressing a construct deprived of all ODC-specific sequence information except the protein-coding region. ODC mRNA changed much less than enzymatic activity. A mutation of the protein-coding region that converted ODC from an unstable to a stable intracellular protein attenuated the regulatory response. We conclude that post-transcriptional events associated with ODC degradation dominate the response to these stimuli.
Full text
PDF









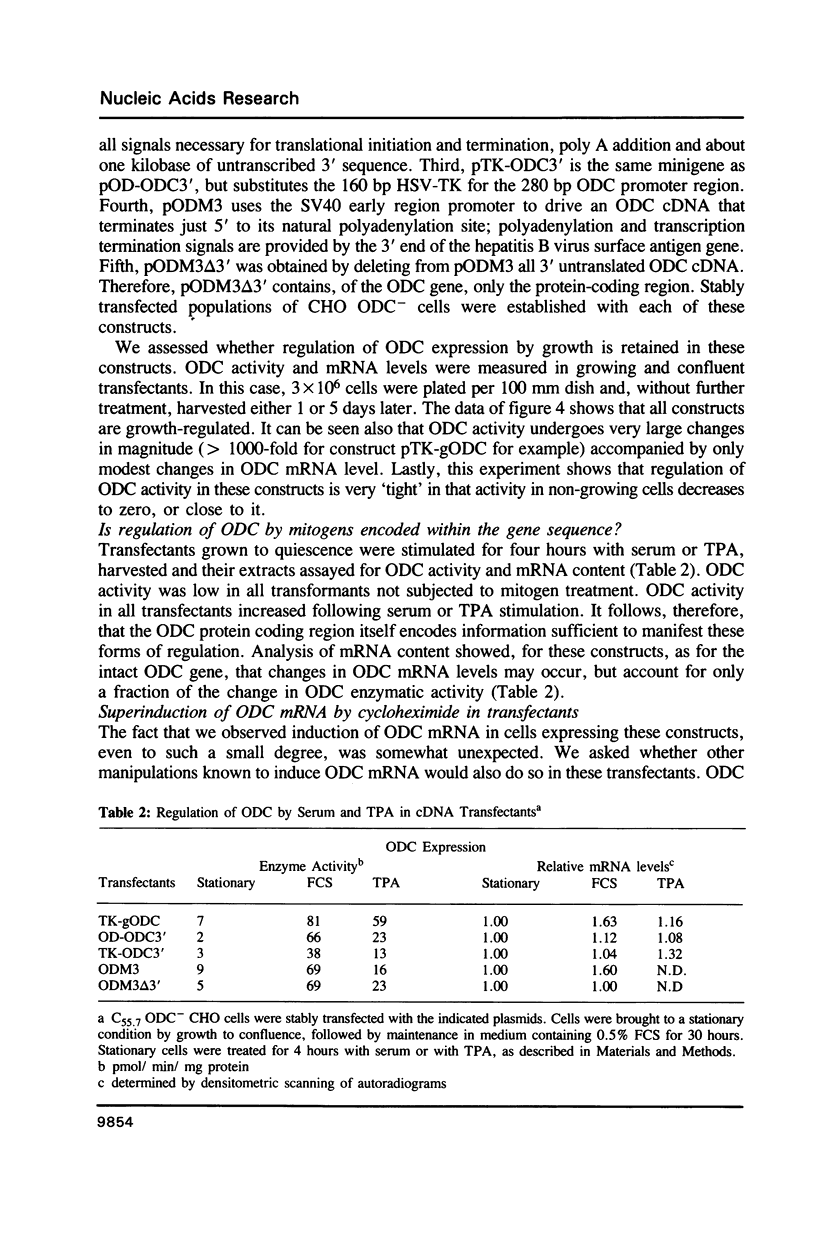





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Loose D., Meisner H., Watson G. Androgen induction of messenger RNA concentrations in mouse kidney is posttranscriptional. Biochemistry. 1986 Mar 11;25(5):1170–1175. doi: 10.1021/bi00353a034. [DOI] [PubMed] [Google Scholar]
- Berger F. G., Szymanski P., Read E., Watson G. Androgen-regulated ornithine decarboxylase mRNAs of mouse kidney. J Biol Chem. 1984 Jun 25;259(12):7941–7946. [PubMed] [Google Scholar]
- Brabant M., McConlogue L., van Daalen Wetters T., Coffino P. Mouse ornithine decarboxylase gene: cloning, structure, and expression. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2200–2204. doi: 10.1073/pnas.85.7.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A. P., McDonald F. F. Transient induction of ornithine decarboxylase mRNA in rat hepatoma cells treated with 12-O-tetradecanoylphorbol-13-acetate. Biochem Biophys Res Commun. 1987 Sep 15;147(2):809–817. doi: 10.1016/0006-291x(87)91002-3. [DOI] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Dana S. L., McConlogue L., Shooter E. M., Coffino P. Nerve growth factor rapidly induces ornithine decarboxylase mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5761–5765. doi: 10.1073/pnas.82.17.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoda L., van Daalen Wetters T., Macrae M., Ascherman D., Coffino P. Prevention of rapid intracellular degradation of ODC by a carboxyl-terminal truncation. Science. 1989 Mar 17;243(4897):1493–1495. doi: 10.1126/science.2928784. [DOI] [PubMed] [Google Scholar]
- Gilmour S. K., Avdalovic N., Madara T., O'Brien T. G. Induction of ornithine decarboxylase by 12-O-tetradecanoylphorbol 13-acetate in hamster fibroblasts. Relationship between levels of enzyme activity, immunoreactive protein, and RNA during the induction process. J Biol Chem. 1985 Dec 25;260(30):16439–16444. [PubMed] [Google Scholar]
- Goodman S. A., Esau B., Koontz J. W. Insulin and phorbol myristic acetate induce ornithine decarboxylase in Reuber H35 rat hepatoma cells by different mechanisms. Arch Biochem Biophys. 1988 Nov 1;266(2):343–350. doi: 10.1016/0003-9861(88)90266-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gross M. K., Merrill G. F. Regulation of thymidine kinase protein levels during myogenic withdrawal from the cell cycle is independent of mRNA regulation. Nucleic Acids Res. 1988 Dec 23;16(24):11625–11643. doi: 10.1093/nar/16.24.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., Coffino P. Mouse ornithine decarboxylase. Complete amino acid sequence deduced from cDNA. J Biol Chem. 1985 Mar 10;260(5):2941–2944. [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hsieh J. T., Verma A. K. Involvement of protein kinase C in the transcriptional regulation of ornithine decarboxylase gene expression by 12-O-tetradecanoylphorbol-13-acetate in T24 human bladder carcinoma cells. Arch Biochem Biophys. 1988 Apr;262(1):326–336. doi: 10.1016/0003-9861(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Kahana C. Isolation and characterization of the mouse ornithine decarboxylase gene. J Biol Chem. 1988 Jun 5;263(16):7604–7609. [PubMed] [Google Scholar]
- Katz A., Kahana C. Transcriptional activation of mammalian ornithine decarboxylase during stimulated growth. Mol Cell Biol. 1987 Jul;7(7):2641–2643. doi: 10.1128/mcb.7.7.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontula K. K., Torkkeli T. K., Bardin C. W., Jänne O. A. Androgen induction of ornithine decarboxylase mRNA in mouse kidney as studied by complementary DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):731–735. doi: 10.1073/pnas.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Matkovich D. A. Genetic determinants of growth phase-dependent and adenovirus 5-responsive expression of the Chinese hamster thymidine kinase gene are contained within thymidine kinase mRNA sequences. Mol Cell Biol. 1986 Jun;6(6):2262–2266. doi: 10.1128/mcb.6.6.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman H. B., Lin P. F., Yeh D. B., Ruddle F. H. Transcriptional and posttranscriptional mechanisms regulate murine thymidine kinase gene expression in serum-stimulated cells. Mol Cell Biol. 1988 Dec;8(12):5280–5291. doi: 10.1128/mcb.8.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L. C., Marton L. J., Coffino P. Growth regulatory effects of cyclic AMP and polyamine depletion are dissociable in cultured mouse lymphoma cells. J Cell Biol. 1983 Mar;96(3):762–767. doi: 10.1083/jcb.96.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L., Coffino P. Ornithine decarboxylase in difluoromethylornithine-resistant mouse lymphoma cells. Two-dimensional gel analysis of synthesis and turnover. J Biol Chem. 1983 Jul 10;258(13):8384–8388. [PubMed] [Google Scholar]
- McConlogue L., Dana S. L., Coffino P. Multiple mechanisms are responsible for altered expression of ornithine decarboxylase in overproducing variant cells. Mol Cell Biol. 1986 Aug;6(8):2865–2871. doi: 10.1128/mcb.6.8.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L., Gupta M., Wu L., Coffino P. Molecular cloning and expression of the mouse ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 1984 Jan;81(2):540–544. doi: 10.1073/pnas.81.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Mitogens and protein synthesis inhibitors induce ornithine decarboxylase gene transcription through separate mechanisms in the BC3H1 muscle cell line. Mol Cell Biol. 1986 Aug;6(8):2792–2799. doi: 10.1128/mcb.6.8.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J Biol Chem. 1983 Feb 25;258(4):2496–2500. [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Shurtleff S. A., McElwain C. M., Taffet S. M. Rapid expression of ornithine decarboxylase mRNA in a macrophage-like cell line: cAMP repression of the requirement for prior protein synthesis. J Cell Physiol. 1988 Mar;134(3):453–459. doi: 10.1002/jcp.1041340317. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Srinivasan P. R., Tonin P. N., Wensing E. J., Lewis W. H. The gene for ornithine decarboxylase is co-amplified in hydroxyurea-resistant hamster cells. J Biol Chem. 1987 Sep 15;262(26):12871–12878. [PubMed] [Google Scholar]
- Steglich C., Grens A., Scheffler I. E. Chinese hamster cells deficient in ornithine decarboxylase activity: reversion by gene amplification and by azacytidine treatment. Somat Cell Mol Genet. 1985 Jan;11(1):11–23. doi: 10.1007/BF01534730. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Watson G., Paigen K. mRNA synthesis rates in vivo for androgen-inducible sequences in mouse kidney. Mol Cell Biol. 1988 May;8(5):2117–2124. doi: 10.1128/mcb.8.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]