Abstract
Neutralizing antibodies play a central role in the prevention and clearance of viral infections, but can be detrimental to the use of viral capsids for gene delivery. Antibodies present a major hurdle for ongoing clinical trials using adeno-associated viruses (AAVs); however, relatively little is known about the antigenic epitopes of most AAV serotypes or the mechanism(s) of antibody-mediated neutralization. We developed panels of AAV mAbs by repeatedly immunizing mice with AAV serotype 1 (AAV1) capsids, or by sequentially immunizing with AAV1 followed by AAV5 capsids, in order to examine the efficiency and mechanisms of antibody-mediated neutralization. The antibodies were not cross-reactive between heterologous AAV serotypes except for a low level of recognition of AAV1 capsids by the AAV5 antibodies, probably due to the initial immunization with AAV1. The neutralization efficiency of different IgGs varied and Fab fragments derived from these antibodies were generally poorly neutralizing. The antibodies appeared to display various alternative mechanisms of neutralization, which included inhibition of receptor-binding and interference with a post-attachment step.
Introduction
Adeno-associated viruses (AAVs) are non-enveloped viruses with a 4.7 kb ssDNA genome packaged into an ~25 nm diameter icosahedral capsid (Kerr et al., 2006). Several of the 12 currently identified AAV serotypes are under investigation as vectors for therapeutic gene delivery due to features that make them potentially attractive vectors, including high capsid stability and lack of pathogenicity (Allocca et al., 2006; Dai & Rabie, 2007; Grimm & Kay, 2003; Michelfelder & Trepel, 2009; Van Vliet et al., 2008; Wu et al., 2006b). The different serotypes of AAV have viral proteins (VPs) that are ~60–90 % identical in amino acid sequence (Gao et al., 2003, 2004), with variations in capsid surface-oriented loops, resulting in alterations of antigenicity, tissue tropism and transduction efficiency (Govindasamy et al., 2006; Lerch et al., 2010; Lochrie et al., 2006; Nam et al., 2007; Ng et al., 2010). The AAV2 serotype is the best characterized and is also the capsid type with the largest number of ongoing clinical gene therapy trials (e.g. Grieger & Samulski, 2005; Marks et al., 2010; Mueller & Flotte, 2008; Scallan et al., 2003). Other serotypes such as AAV1 and AAV5 have been less well characterized, but show enhanced transduction of certain tissues such as muscle, brain and/or haematopoietic stem cells compared with AAV2 (Burger et al., 2004; Chao et al., 2000; Davidson et al., 2000; Zabner et al., 2000; Zhong et al., 2006). Both of these serotypes utilize sialic acids as their primary cellular receptors and AAV5 also binds to the platelet-derived growth factor receptor (PDGFR) as a co-receptor (Kaludov et al., 2001; Di Pasquale et al., 2003; Walters et al., 2001; Wu et al., 2006c).
Humoral immunity leading to virus neutralization has been recognized as a major barrier to clinical trials involving AAV in humans, and the role of cellular immunity is also becoming increasingly appreciated (Boutin et al., 2010; Breous et al., 2011; Calcedo et al., 2009; Mingozzi & High, 2011; van der Marel et al., 2011). Anti-AAV2 neutralizing antibodies have been detected in between 30 and 80 % of human subjects in different studies, and these antibodies are capable of neutralizing the virus and preventing transgene expression (Boutin et al., 2010; Lin et al., 2008; Petry et al., 2008; Zaiss & Muruve, 2008). The success of the trials are determined by the route, dose and serotype used for gene delivery, and by the identity, expression level and promoter of the transgene product (Breous et al., 2011; Hernandez et al., 1999; Li et al., 2008; Sun et al., 2003). In addition, anti-capsid antibodies can be formed after initial gene therapy application, and strategies for avoiding interference with those during a second round of delivery antibodies include administration of transgenes packaged by different, non-cross-reactive AAV serotypes (Halbert et al., 2000; Xiao et al., 1999). Concurrent immunosuppressant (e.g. cyclosporin) administration or plasmapheresis along with the AAV vector may also temporarily reduce immune responses to the virus capsid and/or transgene (Lorain et al., 2008; Monteilhet et al., 2011). Successful outcomes of AAV gene delivery have also been realized by direct injection into partially immune privileged tissues such as the retinal pigment epithelium (Maguire et al., 2008).
Despite the importance of antibodies in the host response to AAV, relatively little is known about the major antigenic epitopes on the capsid surface or the mechanism(s) of antibody neutralization. Antibodies may neutralize viruses by a variety of mechanisms that include capsid cross-linking, the direct or steric inhibition of receptor binding, the prevention or premature triggering of conformational changes necessary for infection, or enhancing endocytosis or complement binding (Dimmock, 1993; Klasse & Sattentau, 2001; Law & Hangartner, 2008; Mallery et al., 2010; Parren & Burton, 2001; Willey & Aasa-Chapman, 2008). The number of antibodies required for neutralization of a viral capsid varies between different viruses, and may depend on epitope accessibility and organization as well as the overall capsid size, but the number of antibodies needed to neutralize effectively is generally proportional to the viral surface area (Klasse & Sattentau, 2002; Pierson et al., 2008).
Mechanisms of antibody binding and neutralization of parvoviruses have been examined for AAV2 and for the autonomous parvoviruses canine parvovirus (CPV) and minute virus of mice (MVM) (López-Bueno et al., 2003; Murphy et al., 2009; Strassheim et al., 1994; Wistuba et al., 1997; Wobus et al., 2000). Studies of AAV2 examining a small number of mAbs or polyclonal anti-capsid antibodies show that antibody binding is affected by changes in a small number of amino acid positions within variable loops on the capsid surface (Huttner et al., 2003; Lochrie et al., 2006; Maersch et al., 2010; Moskalenko et al., 2000; Wobus et al., 2000). Mutations in some of the sites that eliminated antibody binding also altered the receptor-binding properties by reducing association with heparan sulfate proteoglycan (HSPG), the primary receptor for AAV2. Antibodies to different epitopes were characterized by peptide mapping, and it was also observed that neutralization occurred either by inhibition of receptor binding or at post-attachment steps (Wobus et al., 2000). A panel of capsid surface alanine scanning point mutants tested for antibody binding by an anti-AAV2 mAb (A20), polyclonal antibodies in three human sera or to a pool of human IgGs showed differences in neutralization efficiency as there was not always a direct relationship between the change in IgG binding and neutralization (Lochrie et al., 2006). However, when tested for HSPG binding, many of those mutants retained similar infectivity to wild-type capsids (Lochrie et al., 2006). Some mouse antibodies have also been generated against other AAV serotypes, including AAV1, AAV4–AAV6; however, their neutralization mechanism(s) and the capsid structures bound have not been defined (Kuck et al., 2007).
Here, we describe a number of mAbs (both IgGs and IgMs) produced against the capsids of AAV1 and AAV5. The reactivity of these antibodies to the homologous capsids were compared in order to define the efficiency of antibody attachment and the processes of neutralization, which may be similar to those that interfere with AAV-mediated gene therapy.
Results
Antibody production
Panels of mAbs were prepared against AAV1 and AAV5 virus-like particles (VLPs) using two different immunization strategies. The first involved repeated immunization of AAV1 VLPs 2–3 months prior to a final intravenous immunization 3 days prior to the fusion of the splenic lymphocytes. This protocol resulted in the production of three IgG- and four IgM-secreting hybridomas against AAV1. Seeking to prepare cross-reactive antibodies by the sequential administration of alternative serotypes, mice were immunized twice with AAV1 and then given a final intravenous immunization of AAV5 3 days prior to the fusion. This protocol resulted in the isolation of one IgG- and nine IgM-secreting hybridomas against AAV5. The antibodies generated are listed in Table 1.
Table 1. Mouse anti-AAV mAbs.
The immunizing antigen and isotype are indicated. na, Not applicable.
| mAb | Designation in text | Parental AAV antigen | Isotype |
| AA4E4.G7 | 4E4 | AAV1 | IgG2a* |
| AA5H7.D11 | 5H7 | AAV1 | IgG2a* |
| AA9A8.B12 | 9A8 | AAV1 | IgG1* |
| AA8B2.B10.F12 | na | AAV1 | IgG2b |
| AA6E5.A8.F9 | na | AAV1 | IgM |
| AA8B1.H10.D11 | na | AAV1 | IgM |
| AA7C8.G11.D12 | na | AAV1 | IgM |
| AA7F3.D11 | na | AAV1 | IgM |
| AA4B4.B6.E12 | na | AAV1 | IgM |
| BB3C5.F4 | 3C5 | AAV5 | IgG3* |
| BB9F7.F12 | 9F7 | AAV5 | IgM† |
| BB8F1.E8 | 8F1 | AAV5 | IgM† |
| BB10B10.D12.F11 | na | AAV5 | IgM |
| BB1C6.B9 | na | AAV5 | IgM |
| BB1H4.E12 | na | AAV5 | IgM |
| BB4H2.D12 | na | AAV5 | IgM |
| BB7C5.F12 | na | AAV5 | IgM |
| BB8A3.G12 | na | AAV5 | IgM |
Antibodies tested in neutralization assays.
IgM antibodies tested for cross-reactivity in dot-blot analysis but not further tested for neutralizing ability.
Cross-reactivity with different AAV serotypes
Cross-reactivity between the hybridoma supernatant from a subset of the AAV1 and AAV5 mAbs and capsids of several AAV serotypes was tested by ELISA (data not shown) and native dot-blot Western analysis (Fig. 1). The B1 antibody was used to confirm the presence of AAV capsid protein in the dot-blot assay after boiling; this antibody was raised against AAV2 and recognizes a linear epitope present in all AAVs tested except AAV4 at the C terminus of the VP3 common region after denaturation (Wobus et al., 2000). An antibody generated against AAV4 capsids, ADK4, was used as a positive control to verify the presence of this virus during the dot-blot Western assays (Kuck et al., 2007).
Fig. 1.
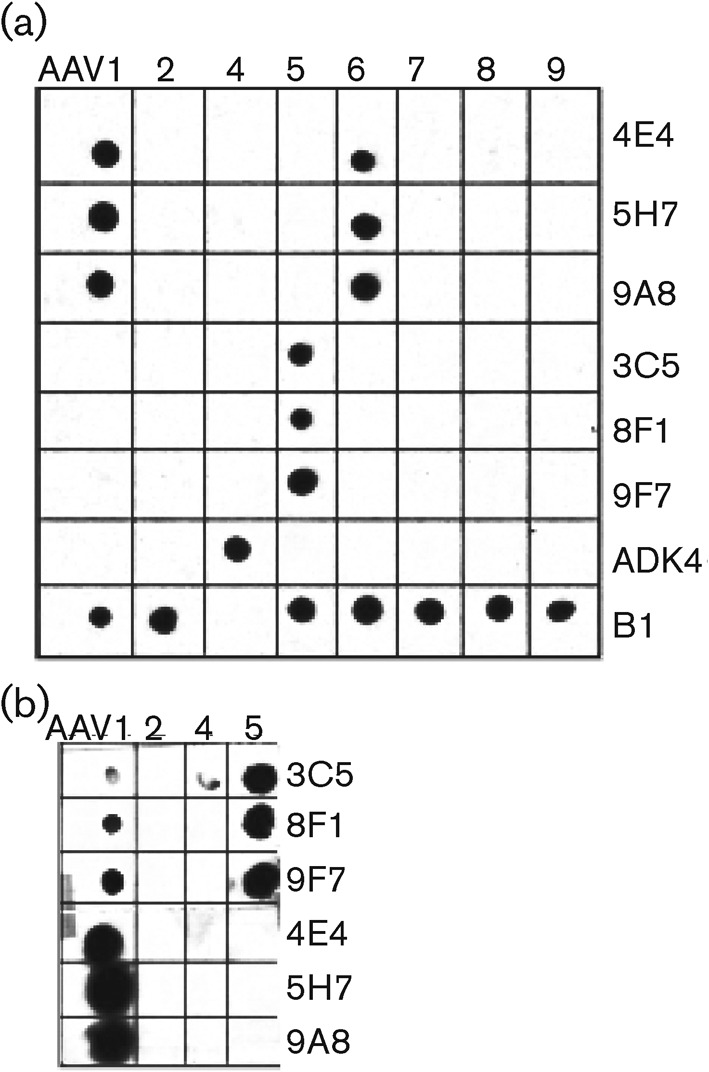
Native dot-blot analysis of AAV serotype cross-reactivity with the anti-AAV1 and anti-AAV5 mAbs. (a) At standard concentrations (hybridoma supernatant diluted 1 : 100), the anti-AAV1 antibodies (4E4, 5H7 and 9A8) cross-react only with AAV6 capsids and no cross-reactivity is seen with the anti-AAV5 antibodies (3C5, 8F1 and 9F7). The B1 and ADK4 antibodies are positive controls for the presence of VLPs. (b) Cross-reactivity of AAV5 antibodies for AAV1 capsids is observed only when tested at higher concentrations (supernatant diluted 1 : 4).
The tested mAbs recognized conformational epitopes on assembled capsids (Fig. 1a) and did not react with denatured capsids (data not shown). The eight anti-AAV1 antibodies were specific for this serotype and cross-reacted only with AAV6 capsids, which differ from AAV1 by only 6 aa. These antibodies did not react with AAV2, AAV4, AAV5, AAV7, AAV8 and AAV9. The anti-AAV5 mAbs specifically recognized this serotype and only weakly cross-reacted with AAV1 when tested at very high hybridoma supernatant concentrations (1 : 4 dilution) (Fig. 1b). The other tested serotypes were not recognized (Fig. 1a).
mAb neutralization
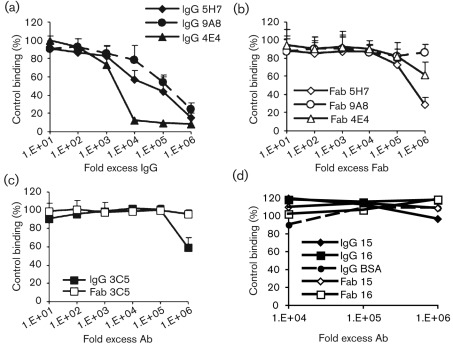
Neutralization titres were defined as the dilutions that resulted in 50 % or greater reductions of cellular transduction by AAV1 and AAV5 vectors packaging the GFP-gene, compared to a control well with no added antibody. Transduction was scored as the percentage of GFP-expressing cells 48 h post-inoculation. All of the antibodies neutralized as intact IgGs although with different efficiencies (Fig. 2a). The 4E4 IgG, generated against AAV1, was the most efficient and neutralized at 50- and 500-fold lower concentrations compared with the 5H7 and 9A8 antibodies, respectively, where the fold-excess was based on the antibody to capsid ratio. The Fabs were each less neutralizing than their IgG counterparts, with the 5H7 antibody retaining the proportionally highest level of neutralizing ability as a Fab fragment, but at a concentration that was a 100-fold higher than for the respective IgG. The 4E4 and 9A8 Fab fragments reached 50 % neutralization only at the highest concentration tested (Fig. 2b). A similar pattern was seen with the anti-AAV5 3C5 antibody, which neutralized as an intact IgG at high concentrations but was non-neutralizing as a Fab (Fig. 2c). Neither IgGs nor Fabs directed against non-homologous CPV antigens (mAbs 15 and 16) nor an anti-BSA IgG showed any neutralization against the AAVs (Fig. 2d).
Fig. 2.
Neutralization of AAV1 and AAV5 by mAb IgGs and Fabs. (a and b) Serial dilutions of the anti-AAV1 IgGs (a) or Fabs (b) were pre-incubated with rAAV1–GFP capsids, then inoculated onto Cos-1 cells and scored for infection at 48 h by flow cytometry. Data are normalized to a control with no added antibody. (c) Serial dilutions of the anti-AAV5 IgG and Fab tested for the ability to neutralize AAV5 transduction of HeLa cells as above. (d) Control IgGs and Fabs directed against canine parvovirus or BSA were non-neutralizing when added under the same conditions as in (a–c) (AAV1 data shown).
Mechanisms of neutralization
To examine virus binding and cross-linking ability of the anti-AAV antibodies, haemagglutination inhibition (HI) assays were performed for the AAV5 VLPs reacting with the 3C5, 4E4, 9A8 and 5H7 antibodies. AAV1 capsids did not haemagglutinate feline, canine, chicken or horse red blood cells and were not tested in this assay. When tested with AAV5 VLPs, the anti-AAV5 3C5 antibody had a low HI titre (1 : 4). One of the anti-AAV1 antibodies, 9A8, showed a small amount of HI (titre 1 : 2); interpreted as non-specific HI given that the antibody was not found to bind AAV5 capsids by ELISA or native Western dot-blot assays.
When tested for their ability to inhibit receptor binding, each anti-AAV1 IgG decreased the level of cellular-associated virus to <20 % of control levels, with variability in the efficiency of inhibition (Fig. 3a). Similar to the findings from the infection neutralization assay, the 4E4 antibody showed the greatest reduction in viral association with cells. The Fab fragments were less inhibitory of receptor binding than their IgG counterparts, and only the Fab of the 5H7 antibody reduced the level of cell-associated virus to <50 % of control levels (Fig. 3b). Neither the anti-AAV5 3C5 IgG nor Fab fragment reduced the level of virus binding to <80 % of control levels (Fig. 3c). Control antibodies did not inhibit the viruses’ ability to attach to cells (Fig. 3d).
Fig. 3.
Ability of the anti-AAV1 and anti-AAV5 mAbs to inhibit receptor binding. (a and b) Serial dilutions of the anti-AAV1 IgGs (a) or Fabs (b) were pre-incubated with Alexa-488 fluorescently labelled AAV1 VLPs, then inoculated onto Cos-1 cells. Flow cytometry was used to determine the level of cell-associated virus and was compared with a no-antibody control. (c) Serial dilutions of the anti-AAV5 IgG and Fab were tested for the ability to inhibit AAV5 receptor binding to HeLa cells as in (a) and (b). (d) Control IgGs and Fabs directed against canine parvovirus or BSA did not inhibit cellular attachment when added under the same conditions as in (a–c) (AAV1 data shown).
To identify potential neutralization mechanisms other than inhibition of receptor attachment, the antibodies were tested for their ability to neutralize AAV infection after the virus had already attached to cells (Fig. 4). Of the anti-AAV1 antibodies, only the 4E4 IgG gave >50 % neutralization at the highest two concentrations tested when added after virus attachment, and none of the Fabs significantly neutralized the virus (Fig. 4b). Neither the anti-AAV5 3C5 IgG nor Fab inhibited infection after the virus had bound to cells (Fig. 4c).
Fig. 4.
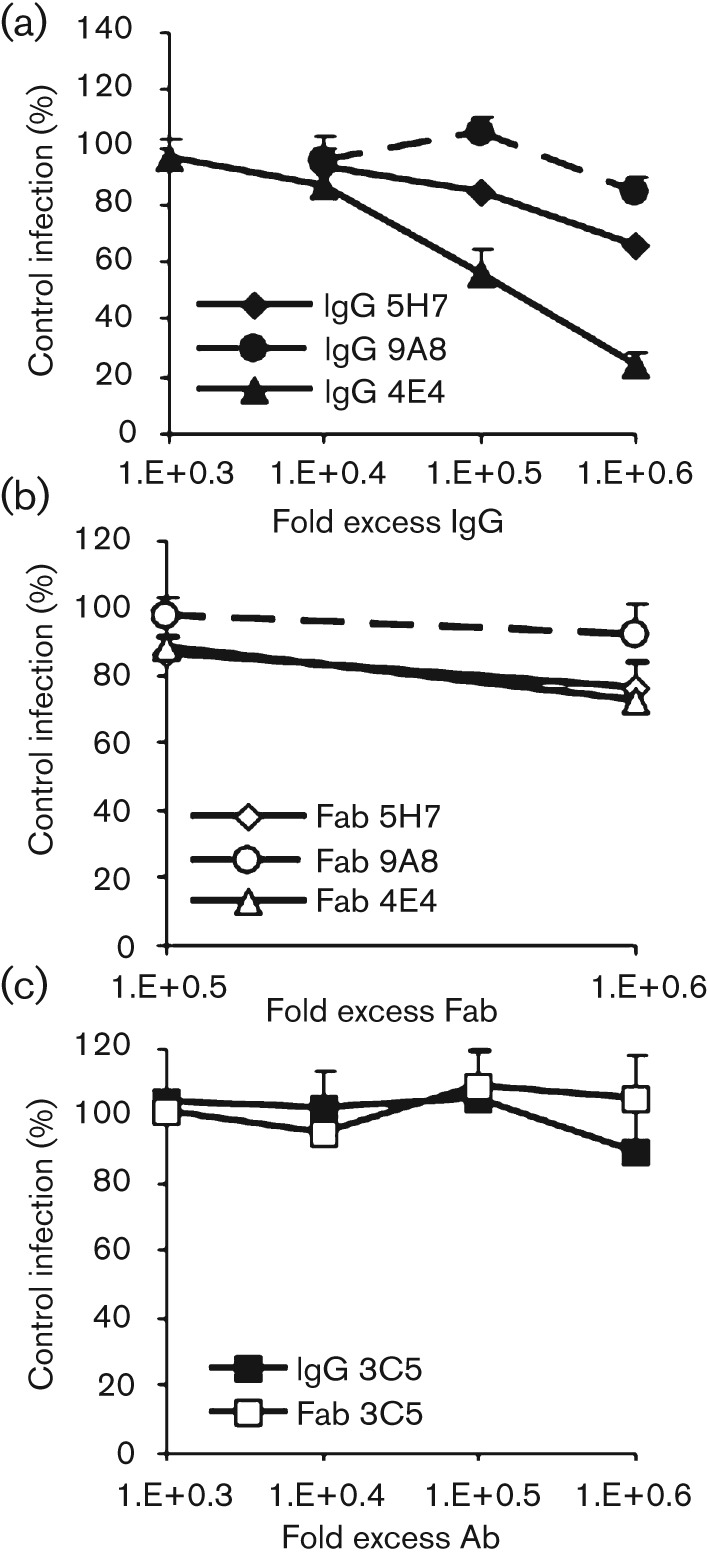
Ability of the anti-AAV1 and anti-AAV5 mAbs to neutralize at a post-attachment step. (a and b) rAAV1–GFP capsids were inoculated onto Cos-1 cells at 4 °C to allow attachment but not endocytosis. Serial dilutions of the anti-AAV1 IgGs (a) or Fabs (b) were then added for 1 h, then cells were washed prior to warming to 37 °C. Cells were scored for GFP expression after 48 h by flow cytometry and data compared to a no-antibody control. (c) Serial dilutions of the anti-AAV5 IgG and Fab were tested for the ability to neutralize rAAV5–GFP after receptor binding as in (a and b).
Discussion
Anti-AAV mAb production
Antibodies are an essential component of the immune response against parvoviruses. In this study, the AAV capsid proved to be a potent antigen for stimulating antibody production, and we were able to prepare several anti-capsid antibodies only 3 days after immunization with AAV5. Although those mice had been previously immunized with AAV1, most of those antibodies were IgMs directed against AAV5. The predominance of IgMs isolated suggests that the response to a single AAV5 VLP immunization was essentially a primary immune response. A greater proportion of anti-AAV1-specific IgGs was found following repeated administration of homologous capsid due to additional opportunities for class switching; three IgG- and four IgM-secreting hybridomas were isolated.
Cross-reactivity of the anti-AAV mAbs
These AAV1 and AAV5 mAbs provide useful reagents for the study of AAV immunogenicity and cellular entry. Most were poorly cross-reactive between serotypes, except for the anti-AAV1 antibodies that also reacted with the very similar AAV6 capsids as expected (Wu et al., 2006a, c). The anti-AAV5 antibodies showed only weak cross-reactivity with AAV1, even though the mice were immunized twice with AAV1 and then once with AAV5 shortly before the fusion. As those hybridomas were screened for reactivity with AAV5, any antibodies against only AAV1 would not be selected. These results suggest that any shared epitopes between the two serotypes are probably weak, as no antibodies that were identified by immunization and screening with only AAV1 were able to bind and neutralize the AAV5 capsid. These data support the separation of AAV1 and AAV5 as distinct serotypes, and suggest that using sequential administration of different AAVs for repeat transgene delivery in clinical trials may reduce vector neutralization.
Neutralizing ability of the anti-AAV mAbs
All the anti-AAV-specific antibodies tested neutralized the viruses as intact IgGs, but had widely different neutralization abilities. The reasons for these differences may include variability in the specific-binding site, orientation of attachment and affinity. Specifically, the most efficient anti-AAV1 neutralizing antibody, 4E4, may bind with a greater affinity to the capsid, or it may have an interaction that provides more steric hindrance to prevent interaction of the virus with the target cell than the other antibodies tested. In addition, 4E4 was the only IgG that was able to both inhibit receptor binding and act at a post-attachment step. This ability to neutralize at more than one step in the infection process may contribute to its neutralization efficacy.
The anti-AAV1 9A8 and anti-AAV5 3C5 were the least efficient neutralizing antibodies. The 3C5 antibody was produced after only 3 days of immunization with AAV5 and, given the low HI titre and high concentrations needed to neutralize, may represent an immature antibody with a relatively low affinity. These antibodies may be less able to sterically hinder receptor binding compared with the 5H7 and 4E4 anti-AAV1 antibodies. Sialic acids may be on long, flexible oligosaccharides or glycoproteins that protrude from the surface of the cell, and antibodies would probably need to bind in close proximity to the receptor-binding site and/or in high density to inhibit receptor binding. Structural studies are currently in progress to determine the specific epitopes and binding footprints of the different antibodies studied here (B. Gurda and M. Agbandje-McKenna, unpublished data).
The Fabs were poorly neutralizing compared with the intact IgGs and were also poorly inhibitory of receptor binding. This reduced efficacy of neutralization by the Fab fragments may result from the smaller degree of steric inhibition provided by the Fab fragments or from a lack of viral particle cross-linking, which would reduce their ability to prevent the viral capsid from gaining access to sialic acids on the cell surface. The fact that the 5H7 antibody retains the highest neutralizing ability as a Fab fragment and is the most able to interfere with receptor binding is suggestive of a binding footprint directly over the receptor-binding site, although this remains to be structurally confirmed. However, this antibody is not the most neutralizing as an intact IgG, suggesting that non-specific coating of the virion and cross-linking may be adequate to achieve neutralization in some cases.
For the AAV1 antibodies in any form, the profile of the neutralization curves were similar to the binding inhibition curves, suggesting that directly or indirectly preventing virus attachment is a major mechanism of neutralization. Only the anti-AAV1 4E4 IgG, and none of the Fabs, was able to significantly inhibit infection after capsids were already attached to cells. This may result from stabilization of the capsid structure by the IgG that interferes with conformational changes required during infectious entry. However, this post-attachment neutralization was not seen for the Fab fragment, so the effect may be due to a constant region (Fc)-mediated function such as the recently identified TRIM-21 dependent, intracellular viral neutralization mechanism (Mallery et al., 2010). Alternatively, the post-attachment neutralization may result from bivalent binding of the intact IgG to the capsid surface leading to cross-linking of adjacent capsid protein subunits.
The AAVs provide an important model system for examining the antigenic structure of simple capsids, as they include several genetically distinct serotypes that are under investigation for use as gene delivery vectors. The variability in neutralizing antibody efficacy and mechanism seen here echo previous results with antibodies directed against other AAV serotypes, and highlight the need to further clarify the key epitopes on the AAV capsid surface (Kuck et al., 2007; Moskalenko et al., 2000; Wobus et al., 2000). Future studies are ongoing to clarify the specific structural interactions of the Fab fragments described here with the AAV capsid and to develop antibody escape mutants that retain the receptor-binding properties of the wild-type virus, seeking to circumvent antibody interference with the effective use of AAV-derived gene therapy vectors.
Methods
Capsid production and immunizations.
Empty (no DNA) AAV1 and AAV5 VLPs were composed of VP1, VP2 and VP3 expressed from baculoviruses under the control of the polyhedron promoter, produced in Hi5 insect cells, and purified using CsCl and sucrose gradients as described previously (DiMattia et al., 2005; Miller et al., 2006; Urabe et al., 2002). Mice were immunized with 0.1 mg AAV1 capsids initially by the subcutaneous route, with Freund’s complete adjuvant in the first immunization and Freund’s incomplete adjuvant for the later immunizations. For the final booster, purified AAV1 or AAV5 capsids were administered intravenously in PBS without adjuvant in order to maximize rapid presentation of antigen to the immune system. Three days later the spleen lymphocytes were fused to mouse myeloma Sp2/0 cells according to standard methods using polyethylene glycol to create antibody-secreting hybridomas (Parrish & Carmichael, 1983). Hybrid cells were selected in the presence of hypoxanthine-aminopterin-thymidine (HAT) and screened for anti-AAV antibody production by ELISA or immunohistochemistry. Positive anti-AAV antibody secreting hybridomas were cloned three times.
Viruses and cells.
GFP-pseudopackaged AAV1 (rAAV1–GFP) and AAV5 (rAAV5–GFP) capsids were prepared as described previously (Conway et al., 1999). Briefly, 293T cells were transfected with pAAV1RC or pAAV5cap and pAAV2rep78, a plasmid containing the adenovirus helper genes, and a plasmid containing the GFP gene controlled by a cytomegalovirus immediate-early promoter and flanked by the AAV2 genome inverted terminal repeats (Halbert et al., 2000). Virus capsids were harvested 48 h after transfection and purified using a caesium chloride step gradient. The highest titre fractions were identified by qPCR and TCID50 analysis in Cos-1 or HeLa cells (Johnson et al., 2010).
AAV1 and AAV5 VLPs were fluorescently labelled with Alexa-488 dyes (Invitrogen), following the manufacturer’s instructions, with purification as described previously (Harbison et al., 2009). The degree of labelling was determined to be 10–15 dye molecules per VLP, and the VLPs were identified to be primarily single particles using the method outlined previously (Harbison et al., 2009).
Production and purification of mAb and Fab fragments.
mAb-producing hybridomas were grown in 500 ml culture bags and the IgGs were isolated from the supernatant using HiTrap Protein G columns (GE Lifesciences). To produce Fabs, the IgGs were digested with papain using a Fab preparation kit (Thermo Scientific), and the monomeric Fab were further purified by chromatography using a Sephadex G100 column (Sigma). IgG and ELISA and/or immunofluorescence confirmed Fab specificity and reactivity (Hafenstein et al., 2009).
ELISA and native dot-blot Western analysis of viral binding.
To examine the reactivity of the mAbs against different strains of AAV, both ELISA and native (non-denaturing) dot-blot Westerns of purified VLPs were performed. Purified VLPs [4 µg ml−1 in bicarbonate buffer (pH 9.6)] were adsorbed to Probind polystyrene ELISA plates (Corning). Antibodies were added and incubated for 1 h at 22 °C, followed by addition of an HRP-conjugated goat anti-mouse antibody, then an ABTS substrate (Sigma). For native dot-blot Westerns, VLPs of AAV1, AAV2 and AAV4–AAV9 were spotted onto nylon membranes, incubated with the antibody supernatant for 1 h, followed by incubation with a secondary anti-mouse HRP antibody (Sigma). Positive control (B1) samples were boiled for 10 min prior to dot blotting. The bound antibody-conjugated HRP was detected with Supersignal luminescent substrate (Thermo Scientific) and exposed to X-ray film (Anderson et al., 2000).
Neutralization assays.
rAAV1–GFP and rAAV5–GFP capsids were incubated with 10-fold dilutions of purified intact IgGs or Fab fragments as indicated for 60 min at 22 °C. This mixture was incubated with Cos-1 or HeLa cells at a fixed genome per cell ratio and incubated for 1 h at 37 °C. The cells were washed and fresh growth medium was added. The percentage of transduced cells was measured by determining the number of cells expressing GFP at 48 h after inoculation using flow cytometry and was compared to a control well with no antibody added (van der Marel et al., 2011). The results of three independent experiments are shown.
Post-attachment neutralization was examined by incubating the same concentration of virus as above with cells for 30 min at 4 °C, before adding IgG or Fab at the same dilutions as above. After an additional hour at 4 °C the cells were washed extensively, and warm growth medium was added. Cells in three independent experiments were assayed for GFP expression at 48 h post-infection as above (Sabo et al., 2010).
Assay for antibody-mediated inhibition of cellular binding.
Alexa-488-labelled AAV1 and AAV5 VLPs were incubated in solution with 10-fold dilutions of purified intact IgGs or Fab fragments for 60 min at 22 °C. This mixture was applied to Cos-1 or HeLa cells at a concentration of 25 000 particles per cell and incubated for 1 h at 4 °C. The cells were washed extensively with cold growth medium and assayed for viral binding compared with a control well with no antibody added as measured by Alexa-488 fluorescence intensity using flow cytometry (Sui et al., 2004). The results of three independent experiments are shown.
Acknowledgements
Supported by National Institutes of Allergy and Infectious Diseases, grant R21AI072341. The authors would like to thank Sandra Wainer and Beverly Handleman for excellent technical support.
References
- Allocca M., Tessitore A., Cotugno G., Auricchio A. (2006). AAV-mediated gene transfer for retinal diseases. Expert Opin Biol Ther 6, 1279–1294 10.1517/14712598.6.12.1279 [DOI] [PubMed] [Google Scholar]
- Anderson R., Macdonald I., Corbett T., Whiteway A., Prentice H. G. (2000). A method for the preparation of highly purified adeno-associated virus using affinity column chromatography, protease digestion and solvent extraction. J Virol Methods 85, 23–34 10.1016/S0166-0934(99)00150-0 [DOI] [PubMed] [Google Scholar]
- Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M. F., Masurier C. (2010). Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 21, 704–712 10.1089/hum.2009.182 [DOI] [PubMed] [Google Scholar]
- Breous E., Somanathan S., Bell P., Wilson J. M. (2011). Inflammation promotes the loss of adeno-associated virus-mediated transgene expression in mouse liver. Gastroenterology 141, 348–357.e1–e3 10.1053/j.gastro.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C., Gorbatyuk O. S., Velardo M. J., Peden C. S., Williams P., Zolotukhin S., Reier P. J., Mandel R. J., Muzyczka N. (2004). Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10, 302–317 10.1016/j.ymthe.2004.05.024 [DOI] [PubMed] [Google Scholar]
- Calcedo R., Vandenberghe L. H., Gao G., Lin J., Wilson J. M. (2009). Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 199, 381–390 10.1086/595830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H., Liu Y., Rabinowitz J., Li C., Samulski R. J., Walsh C. E. (2000). Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther 2, 619–623 10.1006/mthe.2000.0219 [DOI] [PubMed] [Google Scholar]
- Conway J. E., Rhys C. M., Zolotukhin I., Zolotukhin S., Muzyczka N., Hayward G. S., Byrne B. J. (1999). High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther 6, 986–993 10.1038/sj.gt.3300937 [DOI] [PubMed] [Google Scholar]
- Dai J., Rabie A. B. (2007). The use of recombinant adeno-associated virus for skeletal gene therapy. Orthod Craniofac Res 10, 1–14 10.1111/j.1601-6343.2007.00381.x [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Stein C. S., Heth J. A., Martins I., Kotin R. M., Derksen T. A., Zabner J., Ghodsi A., Chiorini J. A. (2000). Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A 97, 3428–3432 10.1073/pnas.050581197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMattia M., Govindasamy L., Levy H. C., Gurda-Whitaker B., Kalina A., Kohlbrenner E., Chiorini J. A., McKenna R., Muzyczka N. & other authors (2005). Production, purification, crystallization and preliminary X-ray structural studies of adeno-associated virus serotype 5. Acta Crystallogr Sect F Struct Biol Cryst Commun 61, 917–921 10.1107/S1744309105028514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. (1993). Neutralization of animal viruses. Curr Top Microbiol Immunol 183, 1–149 [DOI] [PubMed] [Google Scholar]
- Di Pasquale G., Davidson B. L., Stein C. S., Martins I., Scudiero D., Monks A., Chiorini J. A. (2003). Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med 9, 1306–1312 10.1038/nm929 [DOI] [PubMed] [Google Scholar]
- Gao G., Alvira M. R., Somanathan S., Lu Y., Vandenberghe L. H., Rux J. J., Calcedo R., Sanmiguel J., Abbas Z., Wilson J. M. (2003). Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A 100, 6081–6086 10.1073/pnas.0937739100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Vandenberghe L. H., Alvira M. R., Lu Y., Calcedo R., Zhou X., Wilson J. M. (2004). Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 78, 6381–6388 10.1128/JVI.78.12.6381-6388.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy L., Padron E., McKenna R., Muzyczka N., Kaludov N., Chiorini J. A., Agbandje-McKenna M. (2006). Structurally mapping the diverse phenotype of adeno-associated virus serotype 4. J Virol 80, 11556–11570 10.1128/JVI.01536-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J. C., Samulski R. J. (2005). Adeno-associated virus as a gene therapy vector: vector development, production and clinical applications. Adv Biochem Eng Biotechnol 99, 119–145 [PubMed] [Google Scholar]
- Grimm D., Kay M. A. (2003). From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther 3, 281–304 10.2174/1566523034578285 [DOI] [PubMed] [Google Scholar]
- Hafenstein S., Bowman V. D., Sun T., Nelson C. D., Palermo L. M., Chipman P. R., Battisti A. J., Parrish C. R., Rossmann M. G. (2009). Structural comparison of different antibodies interacting with parvovirus capsids. J Virol 83, 5556–5566 10.1128/JVI.02532-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert C. L., Rutledge E. A., Allen J. M., Russell D. W., Miller A. D. (2000). Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol 74, 1524–1532 10.1128/JVI.74.3.1524-1532.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison C. E., Lyi S. M., Weichert W. S., Parrish C. R. (2009). Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking. J Virol 83, 10504–10514 10.1128/JVI.00295-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Y. J., Wang J., Kearns W. G., Loiler S., Poirier A., Flotte T. R. (1999). Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol 73, 8549–8558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner N. A., Girod A., Perabo L., Edbauer D., Kleinschmidt J. A., Büning H., Hallek M. (2003). Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther 10, 2139–2147 10.1038/sj.gt.3302123 [DOI] [PubMed] [Google Scholar]
- Johnson J. S., Li C., DiPrimio N., Weinberg M. S., McCown T. J., Samulski R. J. (2010). Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J Virol 84, 8888–8902 10.1128/JVI.00687-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N., Brown K. E., Walters R. W., Zabner J., Chiorini J. A. (2001). Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol 75, 6884–6893 10.1128/JVI.75.15.6884-6893.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. R., Cotmore S. F., Bloom M. E., Linden R. M., Parrish C. R. (2006). Parvoviruses. London: Hodder Arnold [Google Scholar]
- Klasse P. J., Sattentau Q. J. (2001). Mechanisms of virus neutralization by antibody. Curr Top Microbiol Immunol 260, 87–108 [DOI] [PubMed] [Google Scholar]
- Klasse P. J., Sattentau Q. J. (2002). Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol 83, 2091–2108 [DOI] [PubMed] [Google Scholar]
- Kuck D., Kern A., Kleinschmidt J. A. (2007). Development of AAV serotype-specific ELISAs using novel monoclonal antibodies. J Virol Methods 140, 17–24 10.1016/j.jviromet.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Law M., Hangartner L. (2008). Antibodies against viruses: passive and active immunization. Curr Opin Immunol 20, 486–492 10.1016/j.coi.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch T. F., Xie Q., Chapman M. S. (2010). The structure of adeno-associated virus serotype 3B (AAV-3B): insights into receptor binding and immune evasion. Virology 403, 26–36 10.1016/j.virol.2010.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Miller R., Han P. Y., Pang J., Dinculescu A., Chiodo V., Hauswirth W. W. (2008). Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis 14, 1760–1769 [PMC free article] [PubMed] [Google Scholar]
- Lin J., Calcedo R., Vandenberghe L. H., Figueredo J. M., Wilson J. M. (2008). Impact of preexisting vector immunity on the efficacy of adeno-associated virus-based HIV-1 Gag vaccines. Hum Gene Ther 19, 663–669 10.1089/hum.2008.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochrie M. A., Tatsuno G. P., Christie B., McDonnell J. W., Zhou S., Surosky R., Pierce G. F., Colosi P. (2006). Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol 80, 821–834 10.1128/JVI.80.2.821-834.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bueno A., Mateu M. G., Almendral J. M. (2003). High mutant frequency in populations of a DNA virus allows evasion from antibody therapy in an immunodeficient host. J Virol 77, 2701–2708 10.1128/JVI.77.4.2701-2708.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorain S., Gross D. A., Goyenvalle A., Danos O., Davoust J., Garcia L. (2008). Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther 16, 541–547 10.1038/sj.mt.6300377 [DOI] [PubMed] [Google Scholar]
- Maersch S., Huber A., Büning H., Hallek M., Perabo L. (2010). Optimization of stealth adeno-associated virus vectors by randomization of immunogenic epitopes. Virology 397, 167–175 10.1016/j.virol.2009.10.021 [DOI] [PubMed] [Google Scholar]
- Maguire A. M., Simonelli F., Pierce E. A., Pugh E. N., Jr, Mingozzi F., Bennicelli J., Banfi S., Marshall K. A., Testa F. & other authors (2008). Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358, 2240–2248 10.1056/NEJMoa0802315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery D. L., McEwan W. A., Bidgood S. R., Towers G. J., Johnson C. M., James L. C. (2010). Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 107, 19985–19990 10.1073/pnas.1014074107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks W. J., Jr, Bartus R. T., Siffert J., Davis C. S., Lozano A., Boulis N., Vitek J., Stacy M., Turner D., Verhagen L. (2010). Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol 9, 1164–1172 10.1016/S1474-4422(10)70254-4 [DOI] [PubMed] [Google Scholar]
- Michelfelder S., Trepel M. (2009). Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet 67, 29–60 10.1016/S0065-2660(09)67002-4 [DOI] [PubMed] [Google Scholar]
- Miller E. B., Gurda-Whitaker B., Govindasamy L., McKenna R., Zolotukhin S., Muzyczka N., Agbandje-McKenna M. (2006). Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 1. Acta Crystallogr Sect F Struct Biol Cryst Commun 62, 1271–1274 10.1107/S1744309106048184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F., High K. A. (2011). Immune responses to AAV in clinical trials. Curr Gene Ther 11, 321–330 10.2174/156652311796150354 [DOI] [PubMed] [Google Scholar]
- Monteilhet V., Saheb S., Boutin S., Leborgne C., Veron P., Montus M. F., Moullier P., Benveniste O., Masurier C. (2011). A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther 19, 2084–2091 10.1038/mt.2011.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko M., Chen L., van Roey M., Donahue B. A., Snyder R. O., McArthur J. G., Patel S. D. (2000). Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol 74, 1761–1766 10.1128/JVI.74.4.1761-1766.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Flotte T. R. (2008). Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther 15, 858–863 10.1038/gt.2008.68 [DOI] [PubMed] [Google Scholar]
- Murphy S. L., Li H., Mingozzi F., Sabatino D. E., Hui D. J., Edmonson S. A., High K. A. (2009). Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol 81, 65–74 10.1002/jmv.21360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H. J., Lane M. D., Padron E., Gurda B., McKenna R., Kohlbrenner E., Aslanidi G., Byrne B., Muzyczka N. & other authors (2007). Structure of adeno-associated virus serotype 8, a gene therapy vector. J Virol 81, 12260–12271 10.1128/JVI.01304-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Govindasamy L., Gurda B. L., McKenna R., Kozyreva O. G., Samulski R. J., Parent K. N., Baker T. S., Agbandje-McKenna M. (2010). Structural characterization of the dual glycan binding adeno-associated virus serotype 6. J Virol 84, 12945–12957 10.1128/JVI.01235-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren P. W., Burton D. R. (2001). The antiviral activity of antibodies in vitro and in vivo. Adv Immunol 77, 195–262 10.1016/S0065-2776(01)77018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. (1983). Antigenic structure and variation of canine parvovirus type-2, feline panleukopenia virus, and mink enteritis virus. Virology 129, 401–414 10.1016/0042-6822(83)90179-4 [DOI] [PubMed] [Google Scholar]
- Petry H., Brooks A., Orme A., Wang P., Liu P., Xie J., Kretschmer P., Qian H. S., Hermiston T. W., Harkins R. N. (2008). Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice. Gene Ther 15, 54–60 10.1038/sj.gt.3303037 [DOI] [PubMed] [Google Scholar]
- Pierson T. C., Fremont D. H., Kuhn R. J., Diamond M. S. (2008). Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4, 229–238 10.1016/j.chom.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo M. C., Luca V., Prentoe J., Hopcraft S. E., Blight K. J., Yi M., Lemon S. M., Ball J. K., Bukh J. & other authors (2011). Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a post-attachment step. J Virol 85, 7005–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan C. D., Lillicrap D., Jiang H., Qian X., Patarroyo-White S. L., Parker A. E., Liu T., Vargas J., Nagy D. & other authors (2003). Sustained phenotypic correction of canine hemophilia A using an adeno-associated viral vector. Blood 102, 2031–2037 10.1182/blood-2003-01-0292 [DOI] [PubMed] [Google Scholar]
- Strassheim M. L., Gruenberg A., Veijalainen P., Sgro J.-Y., Parrish C. R. (1994). Two dominant neutralizing antigenic determinants of canine parvovirus are found on the threefold spike of the virus capsid. Virology 198, 175–184 10.1006/viro.1994.1020 [DOI] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L. J., Wong S. K., Moore M. J., Tallarico A. S., Olurinde M. & other authors (2004). Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci U S A 101, 2536–2541 10.1073/pnas.0307140101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. Y., Anand-Jawa V., Chatterjee S., Wong K. K. (2003). Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther 10, 964–976 10.1038/sj.gt.3302039 [DOI] [PubMed] [Google Scholar]
- Urabe M., Ding C., Kotin R. M. (2002). Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther 13, 1935–1943 10.1089/10430340260355347 [DOI] [PubMed] [Google Scholar]
- van der Marel S., Comijn E. M., Verspaget H. W., van Deventer S., van den Brink G. R., Petry H., Hommes D. W., Ferreira V. (2011). Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis 17, 2436–2442 10.1002/ibd.21673 [DOI] [PubMed] [Google Scholar]
- Van Vliet K. M., Blouin V., Brument N., Agbandje-McKenna M., Snyder R. O. (2008). The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol 437, 51–91 10.1007/978-1-59745-210-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. W., Yi S. M., Keshavjee S., Brown K. E., Welsh M. J., Chiorini J. A., Zabner J. (2001). Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem 276, 20610–20616 10.1074/jbc.M101559200 [DOI] [PubMed] [Google Scholar]
- Willey S., Aasa-Chapman M. M. (2008). Humoral immunity to HIV-1: neutralisation and antibody effector functions. Trends Microbiol 16, 596–604 10.1016/j.tim.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Wistuba A., Kern A., Weger S., Grimm D., Kleinschmidt J. A. (1997). Subcellular compartmentalization of adeno-associated virus type 2 assembly. J Virol 71, 1341–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus C. E., Hügle-Dörr B., Girod A., Petersen G., Hallek M., Kleinschmidt J. A. (2000). Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J Virol 74, 9281–9293 10.1128/JVI.74.19.9281-9293.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Asokan A., Grieger J. C., Govindasamy L., Agbandje-McKenna M., Samulski R. J. (2006a). Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol 80, 11393–11397 10.1128/JVI.01288-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Asokan A., Samulski R. J. (2006b). Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther 14, 316–327 10.1016/j.ymthe.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Wu Z., Miller E., Agbandje-McKenna M., Samulski R. J. (2006c). α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol 80, 9093–9103 10.1128/JVI.00895-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Chirmule N., Berta S. C., McCullough B., Gao G., Wilson J. M. (1999). Gene therapy vectors based on adeno-associated virus type 1. J Virol 73, 3994–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Seiler M., Walters R., Kotin R. M., Fulgeras W., Davidson B. L., Chiorini J. A. (2000). Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol 74, 3852–3858 10.1128/JVI.74.8.3852-3858.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss A. K., Muruve D. A. (2008). Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther 15, 808–816 10.1038/gt.2008.54 [DOI] [PubMed] [Google Scholar]
- Zhong L., Li W., Li Y., Zhao W., Wu J., Li B., Maina N., Bischof D., Qing K. & other authors (2006). Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Hum Gene Ther 17, 321–333 10.1089/hum.2006.17.321 [DOI] [PubMed] [Google Scholar]