Abstract
We review the evidence for fisheries-induced evolution in anadromous salmonids. Salmon are exposed to a variety of fishing gears and intensities as immature or maturing individuals. We evaluate the evidence that fishing is causing evolutionary changes to traits including body size, migration timing and age of maturation, and we discuss the implications for fisheries and conservation. Few studies have fully evaluated the ingredients of fisheries-induced evolution: selection intensity, genetic variability, correlation among traits under selection, and response to selection. Most studies are limited in their ability to separate genetic responses from phenotypic plasticity, and environmental change complicates interpretation. However, strong evidence for selection intensity and for genetic variability in salmon fitness traits indicates that fishing can cause detectable evolution within ten or fewer generations. Evolutionary issues are therefore meaningful considerations in salmon fishery management. Evolutionary biologists have rarely been involved in the development of salmon fishing policy, yet evolutionary biology is relevant to the long-term success of fisheries. Future management might consider fishing policy to (i) allow experimental testing of evolutionary responses to exploitation and (ii) improve the long-term sustainability of the fishery by mitigating unfavorable evolutionary responses to fishing. We provide suggestions for how this might be done.
Keywords: adaptation, fitness, heritability, life history, reaction norm, selection, size-selective mortality, sustainable fisheries
Introduction
Anadromous salmonids (Table 1) migrate through freshwater and marine habitats, where they grow to maturity before homing to natal rivers for reproduction (Quinn 2005). Their high nutritional quality and relative ease of capture have subjected them to substantial human exploitation, through commercial, recreational and aboriginal fisheries. Demographic and stock-recruitment relationships for salmon are often used by fisheries managers to set exploitation levels with the objective of a maximum sustainable yield (Ricker 1958, 1969; Walters and Martell 2004). But rarely are the evolutionary responses of salmon considered in the setting of exploitation levels or in the methods and timing of capture. Even though no single study has yet conclusively demonstrated fisheries-induced evolutionary changes in exploited fish in the wild, theoretical and empirical evidence for fisheries-induced selection pressures is strong (e.g. Ricker 1981; Heino 1998; Law 2000; Carlson et al. 2007), and there is a growing body of evidence suggesting that evolutionary changes in fish life histories may already be widespread (e.g. Ricker 1981; Law 2000; Kuparinen and Merilä 2007; Edeline et al. 2007; International Council for the Exploration of the Sea (ICES) 2007; Swain et al. 2007). Moreover, evolutionary changes in fish life histories could affect viability and future yield in the fisheries, which is the opposite of that desired in management (Heino 1998; Law 2000; Conover and Munch 2002; de Roos et al., 2006).
Table 1.
Prominent life history traits of the primary salmonids considered in this paper for evidence of fisheries-induced evolution. Most anadromous forms that spend more than a single season at sea are vulnerable to extensive fishing.
| Species (common names) | Scientific name | Migration | Reproduction | Age structure |
|---|---|---|---|---|
| Atlantic salmon | Salmo salar | Anadromous | Iteroparous | Variable (MSW) |
| Sea trout/brown trout | Salmo trutta | Anadromous/FW resident | Iteroparous | Variable (MSW) |
| Chinook salmon | Oncorhynchus tshawytscha | Anadromous | Semelparous | Variable (MSW) |
| Chum salmon | Oncorhynchus keta | Anadromous | Semelparous | Variable (MSW) |
| Coho salmon | Oncorhynchus kisutch | Anadromous | Semelparous | Simple (∼16 months at sea) |
| Pink salmon | Oncorhynchus gorbuscha | Anadromous | Semelparous | Fixed (2 years) |
| Sockeye salmon | Oncorhynchus nerka | Anadromous/FW resident* | Semelparous | Variable (MSW) |
| Cutthroat trout | Oncorhynchus clarki | Anadromous/FW resident† | Iteroparous | Variable |
| Steelhead/rainbow trout | Oncorhynchus mykiss | Anadromous/FW resident | Iteroparous | Variable (MSW) |
| Brook charr | Salvelinus fontinalis | Anadromous/FW resident | Iteroparous | Variable |
| Lake whitefish | Coregonus clupeaformis | FW resident | Iteroparous | Variable |
| European grayling | Thymallus thymallus | FW resident | Iteroparous | Variable |
FW, freshwater; MSW, multi-sea winter.
Freshwater resident form = kokanee.
All but the coastal subspecies exhibit the freshwater resident form only.
Concerns about the potential evolutionary effects of salmon fishing are now a century old, but relatively few studies of these effects are available, and none of these investigations provides direct evidence for fisheries-induced evolution (Table 2). Stone (1880, 1882) and Rutter (1904) appear to have been the first to speculate in the literature that salmon fisheries might enhance the representation of smaller, younger male breeders and that removal of larger adults could lead to reductions in adult size as well as yield. Smith (1920) was concerned that removal of immature salmon in ocean fisheries would reduce future yields, presumably through earlier maturation, but Miller (1957) argued that the high plasticity of salmonid growth and maturation would render inert any selection imposed by fishing.
Table 2.
Summary of studies that have evaluated trends in size and life history of exploited salmonid populations potentially affected by fishing. Putative factors are the primary ones identified by the authors. Nearly all studies evaluated phenotypic trends or estimated norms of reaction, and therefore the primary causal factors for these patterns could not be ascertained. The table does not include modeling investigations of fishing-induced evolution specific to salmonids, such as Hard (2004); Hard (in press) for Chinook salmon or Thériault et al. (in press) for brook charr.
| Species | Traits examined | Location (period) | Putative factors | Evidence for potential evolutionary response | References |
|---|---|---|---|---|---|
| Atlantic salmon | Body weight, run timing | Ireland (1926–1999) | F, E | ↓ In weight, delay in run timing | Quinn et al. (2006) |
| Body weight | Wales, UK (1907–1977) | F, E | ↓ In weight, ↓ in MSW adults, ↑ incidence of grilse | Gee and Milner (1980) | |
| Body weight, age | Quebec, Canada (1859–1983) | F | ↓ In weight, ↑ in age at maturation, ↓ in iteroparity | Bielak and Power (1986) | |
| Body weight, age | Maritime provinces, Canada (1954–1973) | F | Pop. variation in age & weight neg. correlated with fishing rate | Schaffer and Elson (1975) | |
| Body weight | North Sea – Norway and Scotland (1965–1993) | E | ↓ In weight | Friedland et al. (2000) | |
| Age at maturation | Maritime provinces, Canada (1965–1972) | F | Changes in age at maturation, ↓ in MSW adults, ↑ incidence of grilse | Ritter and Newbould (1977); Paloheimo and Elson (1974); Ritter et al. (1986) | |
| Age at maturation | Scotland, UK (1872–1993) | E | Variable trends in incidence of grilse | Summers (1995) | |
| Body weight, age | Norway and NW Russia (1980–1994) | F | Variable trends in weight & size at age, ↓ in spawner age | Jensen et al. (1999) | |
| Allele frequency | Spain (1988–2000) | F | Generally stable frequency of common MEP-2* allele | Consuegra et al. (2005) | |
| Body weight | Spain (1988–2000) | F | Trend toward ↓ spawner body weight | Consuegra et al. (2005) | |
| Age at maturation | Spain (1988–2000) | F | Trend toward ↓ sea age of spawners | Consuegra et al. (2005) | |
| Run timing | Spain (1945–2000) | F | Delays in median timing of capture | García de Leániz et al. (1992, 2001); Consuegra et al. (2005) | |
| Body length and weight | Spain (1945–2000) | F | ↓ In weight & length of harvested fish | García de Leániz et al. (1992, 2001) | |
| Degree of iteroparity | Spain (1945–2000) | F | ↓ Longevity, ↓ frequency of iteroparity | García de Leániz et al. (1992, 2001); Consuegra et al. (2005) | |
| Age at maturation | Spain (1945–2000) | F | ↑ In smolt age, ↓ in sea age, ↑ frequency of grilse | García de Leániz et al. (1992, 2001) | |
| Age at maturation | Quebec, Canada (1967–1984) | F | ↑ Frequency of mature male residents | Caswell et al. (1984); Montgomery et al. (1986) | |
| Chinook salmon | Body weight | British Columbia, Canada (1951–1975) | F | ↓ In mean weight (24 of 24 groups) | Ricker (1981) |
| Body weight | British Columbia, Canada (1951–1991) | F, E | Variable trends in mean weight, with some ↓ showing reversals | Ricker (1995) | |
| Body weight | West coast N. America (1975–1993) | E | Variable trends in mean weight, with ↓ predominant | Bigler et al. (1996) | |
| Body length | West coast N. America (1979–1993) | E | ↓ In mean weight | Bigler et al. (1996) | |
| Age at maturation | West coast N. America (1975–1993) | E | Variable trends in mean age, with ↓ predominant | Bigler et al. (1996) | |
| Spawn timing | Puget Sound, WA, USA (1960–2000) | H, E | Significant advances in spawn timing | Quinn et al. (2002) | |
| Body length and weight | British Columbia, Canada (1951–1981) | E | Predominantly negative trends in size, depending on period | Healey (1986) | |
| Body length | Yukon River, AK (1970–2004) | F or E | ↓ Trends in relative abundance of large spawners (4 of 7 groups) | Hyer and Schleusner (2005) | |
| Chum salmon | Body length | British Columbia, Canada (1951–1975) | F | ↓ In mean weight (40 of 48 groups) | Ricker (1981) |
| Body weight | British Columbia, Canada (1951–1991) | F, E | Weak, variable trends in mean weight (most groups) | Ricker (1995) | |
| Body weight | West coast N. America (1975–1993) | E | ↓ In mean weight | Bigler et al. (1996) | |
| Body length | West coast N. America (1979–1993) | E | ↓ In mean length | Bigler et al. (1996) | |
| Body length and weight | British Columbia, Canada (1951–1981) | E | Variable trends in size (mostly negative), depending on period | Healey (1986) | |
| Age at maturation | West coast N. America (1975–1993) | E | ↑ In mean age | Bigler et al. (1996) | |
| Body length | Hokkaido, Japan (1992–1997) Kurile Islands, Russia | E, H | ↓ In size at maturation & ↑ in age at maturation | Ishida et al. (1993, 1995); Kaeriyama and Katsuyama (2001); Eggers et al. (2005); Kaev (2000); Kaev and Romasenko (2003) | |
| Age at maturation, body length | Hokkaido, Japan (1962–1997) | E, H | ↓ In size at maturation & ↑ in age at maturation | Morita et al. (2005); Morita and Fukuwaka (2006, 2007) | |
| Coho salmon | Body weight | British Columbia, Canada (1951–1975) | F | ↓ In mean weight in most areas | Ricker and Wickett (1980); Ricker (1981) |
| Body weight | British Columbia, Canada (1951–1991) | E | ↓ In mean weight (56 of 60 groups) | Ricker (1981) | |
| Body weight | British Columbia, Canada (1951–1991) | F, E | ↓ In mean weight for most areas (except in north) | Ricker (1995) | |
| Body length and weight | British Columbia, Canada (1951–1981) | E | Variable trends in size (mostly negative), depending on period | Healey (1986) | |
| Body weight | West coast N. America (1975–1993) | E | ↓ In mean weight | Bigler et al. (1996) | |
| Spawn timing | Puget Sound, WA, USA (1946–2000) | H, E | Significant advances in spawn timing | Quinn et al. (2002) | |
| Pink salmon | Body weight | British Columbia, Canada (1951–1975) | F | ↓ In mean weight (even- and odd-year lines; all groups) | Ricker (1981) |
| Body weight | British Columbia, Canada (1951–1991) | F, E | ↓ In mean weight of all groups (especially southern odd-year) | Ricker (1995) | |
| Body weight | British Columbia, Canada (1953–1988) | F | ↓ In mean weight | Ricker et al. (1978), Ricker (1981) | |
| Body length and weight | British Columbia, Canada (1951–1981) | E | Variable trends in size (mostly negative), depending on period | Healey (1986) | |
| Body weight | West coast N. America (1975–1993) | E | ↓ In mean weight | Bigler et al. (1996) | |
| Allele frequency | Kamchatka, Russia (1979–1981) | F | ↑ In heterozygosity at PGM & proportion of early-maturing fish | Altukhov and Salmenkova (1991); Altukhov et al. (1991);Thorpe (2007) | |
| Sockeye salmon | Body weight | West coast N. America (1975–1993) | E | ↓ In mean weight | Bigler et al. (1996) |
| Body weight | British Columbia, Canada (1951–1991) | E | ↓ In mean weight (27 of 37 groups) | Ricker (1981) | |
| Body weight | British Columbia, Canada (1951–1991) | F, E | No sustained trend in mean weight | Ricker (1995) | |
| Body length | West coast N. America (1975–1993) | E | ↓ In mean length (selected groups) | Bigler et al. (1996) | |
| Body length and weight | British Columbia, Canada (1951–1981) | E | Variable trends in size (mostly negative), depending on period | Healey (1986) | |
| Body length at age | Fraser River, BC, Canada (1952–1993) | E | ↓ In body size correlated with sea surface temperature | Hinch et al. (1995); Cox and Hinch (1997) | |
| Body length at age | British Columbia, Canada; AK, USA (1967–1997) | E | ↓ In body size correlated with ↑ abundance & SST | Pyper and Peterman (1999) | |
| Age at maturation | West coast N. America (1975–1993) | E | ↑ In mean age (selected groups) | Bigler et al. (1996) | |
| Body girth | Bristol Bay, AK, USA (1994) | F | ↓ In girth, scaling with harvest rate | Hamon et al. (2000) | |
| Run timing | Bristol Bay, AK, USA (1969–2003) | F | Advance in river entry timing for two fishing districts | Quinn et al. (2007) | |
| Allele frequency | Kamchatka, Russia (1930s–1980s) | F | ↑ Proportion of heterozygous resident fish | Krogius (1979); Altukhov and Varnavskaya (1983); Altukhov and Salmenkova (1991) | |
| Age, growth rate | Kamchatka, Russia (1968) | F | ↑ proportion of early-maturing resident fish | Nikulin (1970) | |
| Age, size, growth rate | Kamchatka, Russia (1935–1979) | F | ↑ In proportion of early-maturing resident fish, ↓ in length | Krogius (1979); Varnavskaya and Varnavsky (1988); Altukhov (1994) | |
| Allele frequency | Kamchatka, Russia (1979–1981) | F | ↑ In heterozygosity at PGM & proportion of early-maturing fish | Altukhov and Varnavskaya (1983); Thorpe (1993) | |
| Allele frequency | Kamchatka, Russia (1979–1981) | F | ↑ In heterozygosity at PGM, LDH, PX & early-maturing males | Altukhov and Varnavskaya (1983) | |
| Brown trout | Body weight, age | Switzerland/France (1990s) | F | ↑ Larger, older, Atlantic salmon and AB hybrids in catches | Mezzera and Largiadèr (2001) |
| Lake whitefish | Growth rate, age at maturation | Alberta, Canada (1941–1975); Lake Michigan (1932–1967); Germany (1947–1997) | F | ↓ Growth rate, ↓ age at maturity | Handford et al. (1977); Taylor et al. (1992); Thomas and Eckmann (2007) |
| Size at age, fecundity | NW Territories, Canada (1971–1978) | F | ↑ Size at age and fecundity | Healey (1978, 1980) | |
| Grayling | Age and size at maturation | Norway (1900s – most of 20th century) | F | ↓ In weight, ↓ in age at maturation | Haugen and Vøllestad (2001) |
E, environment (e.g. climate, ocean conditions); F, fishing selection; H, hatchery selection (e.g. domestication); MSW, multi sea winter; SST, sea surface temperature.
In the intervening century, such general concerns have persisted (Birkeland and Dayton 2005; Law 2007; Fenberg and Roy 2008; Hutchings and Fraser 2008), but salmon fishery management seldom incorporates evolutionary considerations in practice. In this review, we discuss what is known about the evolutionary consequences of fishing for salmon and address three central questions: First, what are the likely genetic consequences for salmon exposed to fishing, and what is the evidence? Second, do these consequences matter, when considered with other factors influencing viability? Finally, what is the lesson for management – how hazardous is it to ignore evolutionary considerations in salmon fishery management?
Fishing as an agent of change for salmonid life histories
Fishing practice
Salmon are extensively exploited by fisheries. For some populations, commercial and recreational fishing for anadromous salmon kills over 80–90% of individuals (Hankin and Healey 1986; Walters 1986; Heard 1991; Hilborn and Walters 1992; Pacific Salmon Commission (PSC) 2007). Historically, anadromous salmon were intercepted in high-seas fisheries as well as in coastal and riverine fisheries both in the Pacific and in the Atlantic. High-seas salmon fisheries in the Pacific have been prohibited since the 1990s and have been strongly restricted in the Atlantic; salmon are also by-catch in other fisheries. In high-seas fisheries, both immature and maturing individuals were killed, whereas terminal fisheries in estuaries and freshwater killed maturing individuals during their spawning migrations.
In recent decades, catches of Atlantic salmon have continued to decline, reaching their lowest levels in history. Productivity in nearly all populations is limited by high rates of marine mortality (International Council for the Exploration of the Sea (ICES) 2006). For Pacific salmon, catches have generally increased since the 1980s around the northern Pacific rim, with the exception of stocks in western Alaska (declining since the 1990s) and in southern British Columbia (declining since the 1980s) and farther south (declining since the 1930s). Increases in catch have been influenced by increasing hatchery production in many areas, and improving ocean conditions in the northern regions (Eggers et al. 2005). The recent declines in salmon numbers and concerns about loss of less productive populations have resulted in killing rates now more typically capped at 40–50%, although rates vary considerably among species and populations (Walters and Cahoon 1985; Walters and Martell 2004). Most Pacific salmon populations have experienced nearly a century of intensive fishing (Walters 1986; Walters and Martell 2004; Eggers et al. 2005; Hindar et al. 2007).
Salmon fisheries can be categorized by gear types such as hook and line (e.g. recreational fishing, commercial troll fishing), net (especially gillnet and purse seine), and trap technologies, and by the locations where gear intercepts fish on migration routes. These different gear types, and timing and location of use, exert different forms of selection. In general, hook and line salmon fisheries are size selective and timing is selective through regulation (Pacific Salmon Commission (PSC) 2004; Consuegra et al. 2005). Gillnet dimensions tend to be selective for body shape and migration timing (Todd and Larkin 1971; Hamley 1975; Millar and Fryer 1999; Hamon et al. 2000; Fujimori and Tokai 2001). Purse seines scoop up fish from aggregates and are thought to be less size selective (Pope et al. 1975; Ricker 1981) but could impose selection on migration timing and schooling behavior, particularly if the fishery employs specific time or area openings.
Traits under selection
Several salmonid traits are subject to direct or indirect effects of fishing. Two that have received considerable attention are body size and migration timing (Table 2). Fishing generally targets some aspect of body size, either through regulation or gear restriction. For example, gillnets target fish of particular girths but the degree of selectivity depends on population, sex, and state of maturation (Hamon et al. 2000; Fujimori and Tokai 2001; Quinn et al. 2001). Furthermore, size is correlated, genetically as well as phenotypically (Hard 2004), with other life history traits that influence salmon fitness. Even in the absence of direct selection on body size, changes in overall mortality level are driving selection on life history traits that involve trade-offs between performance in early and later life. This is most obvious for traits that relate to timing of major life history events such as smolting and maturation (Riddell 1986; Campbell et al. 2006; Thorpe 2007), but also applies to other traits such as growth and reproductive effort.
Although fishing mortality can account for only a fraction of total salmon mortality (Healey 1986; Riddell 1986; but see Heard 1991 for a counterexample), a sufficiently high fishing mortality can result in selection that has a substantial impact on fitness variation. It is sometimes argued that because most salmon die during the early stages of life, fishing mortality cannot have a decisive effect in shaping salmon life history. However, salmon approaching maturity are those that are most likely to pass their genes to future generations, and selective mortality among them is capable of generating substantial selection differentials as well as influencing population growth rate, particularly when fishing mortality is high. The decrease in population size through fishing mortality can indirectly select against sexually selected morphologies on the spawning grounds, including investment in male kypes and humps for fighting for access to females, and female body size for fighting for quality nest sites and for increasing survival through parental care (van den Berghe and Gross 1986, 1989; Fleming and Gross 1989). It can also bias the selective advantage of alternative life histories, for example favoring ‘jack’ or early maturing precocial males at the expense of later maturing ‘hooknose’ males (Gross 1996). Fishing with nets can directly target sexually selected characters when males with larger kypes have higher probabilities of entanglement (Hamley 1975).
In addition to selective effects within populations, differential selection on mixtures of populations with distinct characteristics can alter stock composition in fisheries. For example, spawning populations often differ in their migration timing through the fishery (Quinn et al. 2007), which might affect patterns of fisheries-induced selection on size, age, or morphology among populations.
Approaches to detecting fisheries-induced evolution
Regression analyses and reaction norms
Two approaches have been used to try to disentangle genetic effects of fishing from other factors influencing phenotypes, but with mixed success for salmonids: regression-based analyses (e.g. Ricker 1981, 1995; Rijnsdorp 1993; Morita et al. 2001) and analyses using probabilistic maturation reaction norms (Heino et al. 2002; reviewed in Dieckmann and Heino 2007; see Thériault et al. in press for an application of reaction norm methodology to migratory tendency). Both approaches have considerable appeal but their limitations arise from how they deal with genetic and environmental influences on phenotypic expression of growth, size, and maturation. Maturation reaction norms may offer a powerful tool for specific situations, although there is some debate as to how cleanly they separate genetic and environmental effects acting on maturation (see below). Regression analysis is a generic but often weaker approach. However, incorporating elements of quantitative genetics (see below) to regression-based analysis can improve its power (Swain et al. 2007).
Analyses of changes in maturation likelihood as influenced by size and age (e.g. Morita and Morita 2002; Morita and Fukuwaka 2006, 2007) have tried to separate the influence of phenotypic plasticity from those of environmental variation in size and age on maturation using probabilistic maturation reaction norms (PMRN). A PMRN describes probability of maturation as a function of age and size, and potentially other explanatory variables (Heino et al. 2002). The analysis of PMRNs can help to distinguish the influences of genetic components of variation from those of phenotypic plasticity on maturation, and thereby characterize the relationship between age, size and likelihood of maturation for different levels of exploitation (Dieckmann and Heino 2007). Indeed, the PMRN approach allows removal of the influences of demography and a major source of phenotypic plasticity from analyses of trends in maturation. However, as a purely phenotypic approach, it cannot be used to unambiguously demonstrate genetic change (Dieckmann and Heino 2007; Marshall and McAdam 2007; Wright 2007); the method can also be confounded by violations of assumptions about genetic control of maturation and growth that are difficult to test.
Quantitative genetic models of response to selection
A more direct approach to determining the direction and rate of evolutionary change under fishing is through quantitative genetic analysis of phenotypic evolution (Lande 1979; McGuigan 2006). Selection requires phenotypic variation and differential reproduction or survival. With sufficient knowledge of the population’s relatedness structure, observed (i.e., phenotypic) patterns of mean trait values together with their variances and covariances can be used to estimate the genetic parameters that determine its responses to selection in a population. The framework for relating selection and its response in a particular trait relies on a simple empirical function that relates a population’s short-term evolutionary response to the selection intensity and to the amount of genetic variation present. For a single trait, the ‘breeders’ equation’ is given as
where R is the single-generation response to selection, h2 is the trait heritability, and S is the selection differential (McGuigan 2006). R represents the change in the population’s phenotypic mean for the trait from generation to generation, h2 is the trait’s heritability (i.e. the proportion of phenotypic variation that results from variation in expression of the trait’s constituent genes), and S is the difference between the phenotypic mean before selection and that of potential breeders that survive selection within the same generation.
To fully characterize the evolutionary consequences of selection, a single-trait approach is insufficient because some traits are genetically linked and therefore can respond to selection even if not directly exposed to it. A multivariate, discrete-generation form of the breeders’ equation takes these trait relationships into account (Lande 1979):
where Δz is a vector of changes in the phenotypic means for all the traits under consideration, G is the genetic covariance matrix composed of the additive genetic covariances among the traits within an individual, P−1 is the inverse of the phenotypic covariance matrix, and s is the vector of selection differentials (P−1s is a vector describing the multivariate selection gradient β). Because this equation relates phenotypic changes to the selection applied through the genetic structure underlying those phenotypes, it (together with its age-structured analogs – see Law 1991a) provides a more complete characterization of short-term phenotypic response to selection imposed by fishing (Law 1991a; Policansky 1993a; Hard 2004; McGuigan 2006).
Fisheries-induced evolution in salmonids
The critical roles of growth and maturation
Most salmonids mature over a range of ages and sizes (Hendry and Stearns 2004; Quinn 2005; Table 1). Their propensity to mature depends on growth and physiological state at any of several potentially critical points in the life history, as dictated by their developmental programs. In anadromous salmon, reproductive investment appears to depend on energy availability; in coho salmon (Oncorhynchus kisutch), for example, ovary mass, egg size, and egg number are highly correlated with growth rate during the final spring and summer prior to ovulation (Campbell et al. 2006). A positive relationship between egg size and adult body size often varies with marine growth but not size at smoltification. Fish might be expected to grow at different rates when heavily fished, for behavioral, ecological or energetic reasons (such as a reduction in density resulting from fishing mortality, or an increase in relative predator abundance; e.g. Healey 1980; Trippel 1995; Salvanes and Baliño 1998), but changes in growth and maturation will also depend on their genetic architecture, as well as on how concurrent environmental changes affect the energetics of growth and the allocation of resources to reproductive effort. Thériault et al. (in press) show that migratory and reproductive patterns in anadromous brook charr (Salvelinus fontinalis) are likely to be influenced by mortality experienced at key points in the life cycle across the marine life-history transition. Fishing may therefore alter the size or age at which allocation of resources to gonads versus somatic tissues begins to shift. This, in turn, will affect the productivity of the population as well as the biomass available for harvest. Selection for faster growth might also affect rates of natural mortality by increasing foraging intensity and risk-taking behaviors (Lee 1912; Ricker 1969; Kristiansen and Svåsand 1998; Walker et al. 1998; Mangel and Stamps 2001).
The maturation process of anadromous salmonids is complex and protracted. Salmon initiate maturation well in advance of its phenotypic expression, apparently in response to physiological state or growth rate at a particular size or developmental stage (e.g. Thorpe 2007; Wright 2007). The consequences of selective fishing for growth and maturation may affect the onset of underlying developmental processes. Analysis of these effects using a PMRN typically invokes an assumption that maturation probability can be described by age and body size and therefore by average immature growth rate, but this assumes that the actual growth trajectory leading to a particular combination of age and size is unimportant. However, this is biologically implausible for most salmonids. In chum salmon (Oncorhynchus keta Walbaum), Morita and Fukuwaka (2006) found that probability of maturing was more closely linked to recent growth history than to body size (this example also shows how the PMRN approach can be extended with additional data). If the relationship between size and age is itself heritable, then the evolutionary consequences of fishing on size and age at maturation will depend on the shape of that relationship (Kuparinen and Merilä 2007). For example, if the reaction norm describing propensity to mature as a function of age (x) and size (y) is relatively flat (approaching size-constrained maturation, wherein fish tend to mature at the same size regardless of age), then fishing is expected to lead to faster growth and earlier maturation (Fig. 1A). By contrast, if this function is relatively steep (approaching age-constrained maturation, wherein fish mature at the same age irrespective of size, e.g. pink salmon, O. gorbuscha, and coho salmon, O. kisutch), then fishing could lead to slower growth and delay maturation (Fig. 1B). For age-structured salmonids, this relationship would be relatively flat, leading to a prediction that size-selective fishing will favor faster growth and younger adults. A more complex function (Perrin and Rubin 1990; Ernande et al. 2004) would have less predictable consequences.
Figure 1.
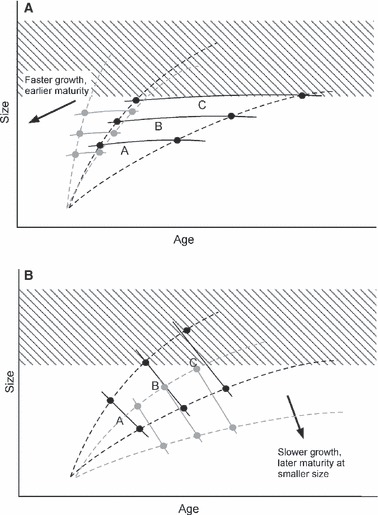
Hypothetical maturation reaction norms for size and age at maturation in salmonids under variable opportunities for growth. The dotted black curves depict hypothetical growth trajectories, from rapid (steep) to slow (shallow). In the strictest sense, reaction norms reflect phenotypic differences among distinct genotypes, although such functions are often used to evaluate patterns in other genetically differentiated groups. Here, A, B, C refer to distinct genotypes, families, or populations, with their maturation reaction norms indicated by the three solid curves in each pane. Solid black dots indicate the intersections of the growth trajectories and reaction norms for each group. (A) Maturation reaction norms corresponding to a primary influence of size on first maturation (‘size-constrained maturation’). In this case the reaction norms are relatively flat, so that size selection imposed by fishing, indicated by the hatched area, is likely to increase growth rate and reduce size and age at first maturation in an exploited population. Possible responses in the reaction norms predicted by the arrow are given by the curves and dots in grey. This scenario appears consistent with the biology and phenotypic response of several species, such as Atlantic, Chinook, chum, and sockeye salmon, and steelhead and anadromous cutthroat trout (as well as some marine species such as cod and plaice). (B) Maturation reaction norms corresponding to a primary influence of age on first maturation (‘age-constrained maturation’). In this case the reaction norms are more vertical, so that size selection imposed by fishing is likely to reduce growth rate, and perhaps increase age and reduce size at first maturation, in an exploited population. Possible responses in the reaction norms predicted by the arrow are given by the curves and dots in grey. This scenario is consistent with the biology of species with a constrained age structure, such as pink or coho salmon.
Ingredients of fisheries-induced evolution
Fishing as selection
The extent to which a population responds to fishery selection has some key prerequisites (Law 1991a; Hard 2004). First, fishing must be sufficiently strong to alter the distribution of phenotypes in the breeding population. Under constant fishing selectivity and genetic variability, higher fishing rates are more likely than lower rates to elicit an evolutionary response. If fishing selectivity is not sufficiently high to impose a detectable selection differential on size (or size at age), a short-term evolutionary response is less likely, although nonselective fishing mortality can still lead to evolution through changes in the maturation schedule (Policansky 1993a,b; Hard 2004). So too can accumulation of very small selection differentials that are repeated over the long time periods that fisheries can operate (tens or hundreds of years).
Fisheries that target maturing salmon concentrated near terminal areas are less likely to cause pronounced selection for age at maturation than those targeting immature fish migrating over ocean pathways, at least for semelparous populations or iteroparous populations with low rates of repeat spawning (Healey 1986). The primary reason for this is that fishing on semelparous individuals that have already made the physiological decision to mature will tend to have a reduced impact on age at maturation. Fisheries that target maturing fish expose all ages to the same mortality (subject to gear selectivity for size, etc.), while in fisheries that target immature fish, mortality is directly proportional to how long fish delay maturation once they become vulnerable to gear. Fisheries on immature individuals directly select for fish that mature earlier, or become vulnerable later, which might result in genetically based changes in reproductive output. Salmon fisheries in terminal areas, within rivers, or otherwise closely associated with aggregates of maturing fish are less apt to result in rapid evolutionary responses in age at maturation and correlated traits than those that are not (e.g. Kuparinen and Merilä 2007). Nevertheless, fishing on maturing individuals can alter other aspects of life history associated with size or age at maturation, including fecundity, egg size, redd size and depth, and nest defense (see van den Berghe and Gross 1989; Hamon et al. 2000; Hamon and Foote 2005).
Genetic variation in salmonid life history
Life history variation within and among populations of salmonids reflects both genetic and environmental sources of variation (Table 3; see also Carlson and Seamons in press). The genetic potential for key life history traits in salmon to respond to selection is high. However, few studies have examined specifically the genetic covariation among salmonid life history traits, which can constrain or augment selection response; virtually all studies of fisheries selection to date have focused on single characters. More recent studies that have focused on the genetic architecture of salmonid life history include analyses of growth, size and maturation (e.g. Smoker et al. 1994; Quinn et al. 2000; Kinnison et al. 2001; Hard 2004; Thrower et al. 2004), juvenile body size and shape (Kanno 1990; Hard et al. 1999), and pathogen resistance (Withler and Evelyn 1990; Fjalestad et al. 1996; Guy et al. 2006; Hard et al. 2006). Genetic correlations are difficult to estimate with precision, especially without adequate breeding designs, and such estimates are not available for most exploited populations. Nevertheless, in general these analyses suggest that the indirect responses of traits to selection depend critically on their genetic and phenotypic covariances and that these will be difficult to predict solely from phenotypic information on the trait subject to direct selection (McGuigan 2006; Law 2007).
Table 3.
Summary of heritability estimates for life history traits in anadromous salmonids likely to respond to fishing selection. With few exceptions, only studies involving narrow-sense estimates from correlation among relatives or response to selection in wild or hatchery-ranched, but not farmed, populations (i.e. considerable fraction of life cycle spent in wild and exposed to fishing mortality) are included. Data for only the species included in Table 2 are given here, and heritability estimates for disease resistance, juvenile behavior, and other traits are not included.
| Species | Trait type | Description | Range of h2 | References |
|---|---|---|---|---|
| Atlantic salmon | Body size/morphology | Juvenile length | 0.04–0.79 | Bailey and Loudenslager (1986); Garant et al. (2003); Refstie and Steine (1978) |
| Juvenile weight | 0.10–0.89 | Bailey and Loudenslager (1986); Jónasson et al. (1997) | ||
| Immature length | 0.57–0.73 | Bailey and Loudenslager (1986) | ||
| Immature weight | 0.20–0.67 | Bailey and Loudenslager (1986) | ||
| Mature weight | 0.20–0.36 | Jónasson (1993); Jónasson and Gjedrem (1997); Jónasson et al. (1997) | ||
| Survival | Marine survival | 0.01–0.24 | Jónasson et al. (1997) | |
| Chinook salmon | Body size/morphology | Juvenile length | ∼0.0–1.0 | Hard et al. (1999); Bryden and Heath (2000) |
| Juvenile weight | 0.99 | Hard et al. (1999) | ||
| Growth rate | Development rate | 0.05–0.23 | Kinnison et al. (1998) | |
| Age at maturation | 0.30–0.57 | Hankin et al. (1993); Hard (2004); Hard (1995) | ||
| Survival | Marine survival | ∼0.0–0.12 | Unwin et al. (2003) | |
| Migration or spawn timing | Maturation timing | 0.23–1.0 | Quinn et al. (2000); Hard (2004) | |
| Egg number | ∼0.0–0.76 | Kinnison et al. (2001) | ||
| Egg size | 0.5–0.78 | Kinnison et al. (2001) | ||
| Chum salmon | Body size/morphology | Juvenile length | 0.13–0.86 | Beacham (1990); Kanno (1990) |
| Survival | Enbryo/alevin survival | ∼0.0 | Beacham (1988) | |
| Coho salmon | Body size/morphology | Juvenile length | ∼0.0–0.47 | Murray et al. (1993) |
| Juvenile weight | ∼0.0–0.62 | Withler and Evelyn (1990); Murray et al. (1993) | ||
| Immature length | 0.32–0.69 | Silverstein and Hershberger (1995) | ||
| Immature weight | 0.07–0.85 | Silverstein and Hershberger (1995) | ||
| Growth rate | Juvenile/immature | 0.06–1.0 | Sato (1980); Silverstein and Hershberger (1995); Vøllestad and Quinn (2003) | |
| Age at maturation | Male precocity | 0.05–0.13 | Silverstein and Hershberger (1992) | |
| Survival | Juvenile survival | ∼0.0–0.35 | Beacham (1988); Murray et al. (1993) | |
| Pink salmon | Body size/morphology | Mature length | ∼0.0–1.0 | Smoker et al. (1994); Dickerson et al. (2005) |
| Mature weight | ∼0.0–0.66 | Smoker et al. (1994) | ||
| Survival | Embryo survival | ∼0.0–0.21 | Beacham (1988) | |
| Migration or spawn timing | Return timing | ∼0.0–1.0 | Smoker et al. (1998); Dickerson et al. (2005) | |
| Spawn timing | 0.06–0.54 | Smoker et al. (1994) | ||
| Egg number | ∼0.0 | Funk et al. (2005) | ||
| Egg size | 0.22 | Funk et al. (2005) | ||
| Sockeye salmon | Body size/morphology | Gill raker count | 0.57 | Foote et al. (1999) |
| Rainbow trout/steelhead | Body size/morphology | Immature length | 0.11–0.58 | McKay et al. (1986); Sylvén and Elvingson (1992); Thrower et al. (2004) |
| Immature weight | 0.13–0.65 | McKay et al. (1986); Sylvén and Elvingson (1992); Thrower et al. (2004) | ||
| 0.12–0.73 | McIntyre and Blanc (1973); McKay et al. (1986); Thrower et al. (2004) | |||
| Growth rate | Proportion smolting | 0.45–0.73 | Thrower et al. (2004) | |
| Age at maturation | Early male maturation | 0.02–1.0 | Sylvén and Elvingson (1992); Thrower et al. (2004) |
Few studies have provided estimates of selection differential imposed by fishing. Some of the best known estimates have been derived for body length in Atlantic cod, which varied from −1 to +2 cm for North Sea cod (Law and Rowell 1993) and from −4 to +4 cm for cod from Canadian catches (Sinclair et al. 2002). For Atlantic salmon, Hindar et al. (2007) provided estimates of selection differential on body weight for one-sea winter (1SW) grilse ranging from −0.08 to −0.52 kg, depending on the population and year. For Pacific salmon, Ricker (1981) estimated that the selection differential imposed by fishing on British Columbia coho salmon body weight between 1951 and 1975 varied from −0.50 to −0.73 kg. Hamon et al. (2000) estimated that the Bristol Bay (Alaska) gillnet fishery imposed selection differentials on body girth in sockeye salmon that ranged from −0.6 to −3.6 mm for females and −3.6 to +0.3 mm for males. Analyses by Washington Department of Fish and Wildlife (WDFW) biologists of coho salmon caught in gillnets in Washington state in recent years indicate that selection differentials on body length varied from −3.3 to +0.2 cm for females and −5.8 to +0.2 cm for males (C. Knudsen and C. Busack, WDFW, personal communication). Unfortunately, these estimates were not standardized, so direct comparisons are difficult, but from available information most selection differentials estimated for fishing appear to be in the range of ∼0 to ±0.5 phenotypic standard deviations.
The combination of selection differentials with estimates of heritability for these traits indicates that responses in salmon size, growth, and maturation age to fishing-induced selection are likely to vary considerably among populations and over time. In most cases, these responses are expected to be modest over the short term (ca. 10 or fewer generations), although they could potentially be as large as −1 cm for length and −100 g for weight on an annual basis under stable environmental conditions. That said, the estimates of selection differentials tend to be similar to, but perhaps usually lower than, estimates of selection intensity imposed by natural and sexual selection in naturally reproducing salmon populations, which can sometimes exceed 0.5 standard deviations (van den Berghe and Gross 1989; Hamon and Foote 2005).
Only a few investigations have explored the consequences of such trait architecture under selection for viability. Hankin and Healey (1986) found that selective fisheries can decrease the mean age of Chinook salmon populations and increase the probability of significant population decline. The results of simulations of fisheries-induced evolution by Hard (2004) suggest that the selective exploitation of large Chinook salmon could lead to modest reductions in size-at-age within approximately five generations; further exploratory modeling (Hard et al., unpublished data) has shown that such responses can reduce abundance and catch and produce some maladaptive changes in life history that are likely to increase risk to population viability.
Evidence for fisheries-induced evolution in salmonids
The selectivity of fishing on many fitness traits in salmonids, coupled with the ample evidence of underlying genetic variation in these traits, indicates that rapid evolutionary responses to fishing are possible. Several studies over the past quarter century have explored the potential evolutionary effects of fishing on salmon (e.g. Ricker 1981, 1995; Hankin and Healey 1986; Healey 1986; Riddell 1986; Altukhov 1994; Hard 2004; Morita et al. 2005; Quinn et al. 2007). In a recent perspective, Jørgensen et al. (2007) identified 46 studies involving six traits in 18 fish species that implied fishing-induced evolution and estimated appreciable rates of evolutionary change. For salmon, these studies involved five species, and provided evidence for evolutionary rates from less than 20 to more than 30% over 24 years (on the order of 1% change annually). However, since the design of the study by Jørgensen et al. (2007) excluded research which did not suggest evolutionary change, the overall effects of fisheries-induced evolution are likely to be less than this.
Ricker’s (1981, 1995) pioneering analysis of changes in mean weight of several Canadian species of Pacific salmon Oncorhynchus spp. (and in mean age for Chinook salmon, O. tshawytscha) caught between 1950 and 1993 raised concerns about future fishery yields. Ricker (1995) concluded that the effects of size-selective fishing were complex and difficult to disentangle from other factors affecting survival and growth in age-structured species, but that fisheries-induced genetic changes were likely, especially in pink and coho salmon (Ricker et al. 1978; Ricker and Wickett 1980). In a separate study, terminal fisheries with minimum size limits did not lead to changes in mean length in many populations of Pacific salmon in Canada, and observed changes were probably not genetic but due to environmental variation (Healey 1986; see also Riddell 1986). Summers (1995) and Friedland et al. (2000) observed changes in life history for Atlantic salmon consistent with temporal variation in marine environmental conditions. In an Asian fishery, chum salmon exhibited a decrease in the mean size at age of mature individuals, and an increase in the age at maturation, after the fishery switched from a high-seas gill-net fishery to a terminal set-net fishery (Morita et al. 2005).
Healey (1986) concluded that observed declines in the size of Pacific salmon previously attributed to selective fisheries probably also reflect changes in climate affecting marine growth and productivity of salmon, and he and Riddell (1986) identified several factors that tend to limit detection of an evolutionary response to fishing. First, the data may be inadequate or of low quality. The characteristics of many fisheries and of much of the associated catch data, such as those considered by Ricker (1981, 1995), do not lend themselves well to genetic analysis because of variable stock composition of the catch, because the data suitable for monitoring are limited, and because selection differentials are not easily quantified. Second, the environmental contribution to variation in size and age is likely to be large. Third, the genetic structure of size, age and correlated traits can constrain response to selection. Fourth, the consequences of tetraploid ancestry in salmonids for genetic variation and evolutionary dynamics are still not well understood. Fifth, response to selection can be complex for age-structured species due to variation in selection differentials for fish maturing at different ages, and specifically tailored life history models are required to adequately capture the evolutionary dynamics of salmonids (whether iteroparous or semelparous). Finally, countervailing selection in the wild (e.g. natural and sexual selection on spawners) might oppose fishing selection (Healey 1986; Riddell 1986; Carlson et al. 2007).
Hamon et al. (2000) found that selectivity in gillnet fisheries can impose strong selection on adult body morphology (girth). The magnitude and direction of this may vary as well (Miller and Kapuscinski 1994). In the Yukon River, Alaska, which historically produced appreciable numbers of large, old Chinook salmon, the numbers of very large (≥90 cm) fish have been declining in recent decades (Hyer and Schleusner 2005). Declines in body size can affect fertility (Healey and Heard 1984), mate choice and breeding behavior (Quinn and Foote 1994; Esteve 2005), and redd construction and defense and subsequent fry survivorship (van den Berghe and Gross 1989; Steen and Quinn 1999).
Some authors have also argued that fishing can affect migration timing (Quinn et al. 2007). For example, Quinn et al. (2002) demonstrated that run timing of both Chinook and coho salmon from three hatcheries in Washington has shifted in recent decades as a result of selection of brood stock which has responded to fishing patterns. For Atlantic salmon in Ireland, Quinn et al. (2006) documented a long-term delay in run timing, as well as a decline in weight, changes which they argued probably resulted from patterns of angling pressure on returning adults.
These studies point to the importance of considering selective fishing as a factor in altering salmon life history. Unfortunately, most inferences about fishing selection are based on an evaluation of selectivity or fishing mortality rate and therefore focus on only one aspect of adaptive evolution: the opportunity for directional change through an apparent measure of selection intensity. Because evolution involves change in gene frequencies, an evolutionary response requires genetic variability, and inferring evolution in response to fishing pressure in the absence of this information is far from straightforward.
What we need to know about fisheries-induced evolution
The changes in life history observed in many exploited fish populations are fueling controversy among biologists and conservationists over whether these fisheries and the populations that support them can persist (e.g. Birkeland and Dayton 2005; Kuparinen and Merilä 2007). Our review of this body of work in salmon, summarized in Table 2, suggests one reason: none of these studies provides direct evidence for evolutionary responses to fishing, or whether such responses reduce viability. Nevertheless, the collective evidence across a variety of species and environmental conditions highlights trends in size, age, and other traits – traits that have large influences on productivity and fitness – that are consistent with evolutionary responses to size-selective fishing (International Council for the Exploration of the Sea (ICES) 2007; Jørgensen et al. 2007). As Law (2007) noted, such responses may often be modest over the short term and difficult to detect without evaluating longer trends.
A concerted empirical attempt to dissect effects of fishing from those of other factors is clearly warranted. This would include careful experiments to discriminate these factors in a real-world – spatially or temporally structured but carefully monitored – evaluation of fishing effects on abundance, size, and life history of free-ranging salmon, such as the experiments suggested by McAllister and Peterman (1992) and McAllister et al. (1992). These authors emphasized the difficulty in evaluating fishing effects empirically but provided valuable guidance for how to structure the necessary experiments with adequate statistical power. Among their recommendations are to focus on species with simple life histories, such as pink salmon, and to employ adequate spatial replication; both of these recommendations can improve power considerably for relatively short-term (∼5 generation) experiments.
Large-scale manipulative experiments and evaluation of management strategies in the context of conceivable responses in the fishery (see Walters 1994) are logistically and politically challenging to implement, and would be met with resistance from fishers without adequate compensation or a clear sense of a perceived longer-term benefit. Nevertheless, such approaches, when coupled with data on trends and knowledge of selectivity and genetics, would be more convincing to scientists and more compelling to managers. As suggested by Wright (2007), additional lines of inquiry that would likely prove profitable include comparisons of patterns of reproductive investment and allocation among populations varying in exploitation history, and contrasts of state-dependent thresholds for maturation among populations that differ in exploitation history.
Implications for fisheries management
Conceptually, the simplest way of reducing fisheries-induced selection pressures and consequences of excessive exploitation in general is to reduce overall fishing pressure. However, such overall reductions are hardly ever practical, and more specific measures are probably required. Considerable discussion in the literature has focused on the merits of minimum size limits, slot limits, and other fishing strategies as means to maintain current and preserve future yields. Some researchers have argued that minimum size limits tend to lead to ‘recruitment overfishing,’ whereas practices that increase catch of smaller, younger fish tend to lead to ‘growth overfishing,’ which is often thought to have less deleterious impacts on productivity and yield (Ricker 1976; Larkin 1978).
Other management options such as fisheries moratoria, time and area closures or catch limitations, and marine reserves also merit consideration to reduce long-term effects of fishing. Baskett et al. (2005) showed in a quantitative genetic model mimicking a cod life history that marine protected areas (MPAs) could help to reduce fisheries-induced selection for size at maturation in some long-lived species. MPAs may, however, have limited utility in mitigating for evolutionary change in highly migratory fish unless reserves are very large or are carefully networked. Protecting adults on spawning grounds could favor earlier maturation for some life histories, for example (Law 2007). In practice, the boundaries of MPAs that will be effective for anadromous salmon might not be difficult to identify but they will be difficult to implement and manage.
The socioeconomic factors that maintain exploitation are unlikely to ease until a clear biological threat is identified. Because resistance to reducing fishing rates will remain high in such circumstances, reducing fishing selectivity should become a tool for management, as reducing selectivity will preserve genetic and life history variability. Even so, as Policansky (1993b) pointed out, it must be recognized that a nonselective fishery will affect a population’s evolutionary trajectory to the extent that it alters the mortality schedule. The key issue is where and how intense these pressure points are exerted by fishing on the mortality schedule relative to growth and maturation profiles.
Discussions that solely focus on productivity and yield often overlook the importance of standing genetic variation for size and associated life history traits to the resilience of an exploited population (Nelson and Soulé 1987). If exploited populations are to cope with the ecological and evolutionary pressures posed by fishing they must retain the adaptive capacity to respond. This capacity may be threatened by several of the characteristics of size-selective exploitation, especially selective removal of individuals with higher reproductive potential and elevation of the rate of stochastic genetic processes through reduction of genetic diversity (Smith et al. 1991; Harris et al. 2002).
The potential consequences of fisheries-induced evolution to viability of salmon remain poorly understood. Adaptation to fishing might reduce vulnerability of salmon to fisheries and thereby improve population viability compared to a hypothetical situation where evolution is not permitted. However, fishers may quickly adjust their capture strategies to changing fish characteristics, thereby engaging in a co-evolutionary ‘arms race’ and eradicating potential viability benefits (Heino 1998). Furthermore, when fish adapt to fishing they are likely to evolve away from configurations that natural and sexual selection alone would favor.
For example, a modest genetic influence on size at age (h2∼ 0.3) appears to permit adaptation of Chinook salmon to selection on size imposed by fishing (Hard et al., unpublished data); this adaptation is generally expressed as increased growth rate and earlier age at maturation, which tends to reduce fishery vulnerability under a fixed minimum capture size threshold. Such adaptation can take considerable time, however – several to many generations. During this period, evolution is likely to entail a period of reduced fitness, which Walsh et al. (2006) referred to as repayment of a Darwinian ‘debt.’ Thus, under some circumstances, an evolutionary response to fishing selection can negatively affect population viability. Fisheries-induced evolution might compound the demographic risk posed by overfishing, and this evolutionary trend may be difficult to reverse. Yield might decline, and vulnerability to other threats to viability during this period is likely to remain high (Hard et al., unpublished data).
The consequences of environmental variation for fish growth, size, and age at maturation might – at least in some years – overwhelm the impacts of fishing. Indeed, what cannot be determined yet is whether the selection imposed by fishing, the evolutionary response to it, and any attendant effects on viability will be sufficiently large to precipitate a fishery collapse. It is unfortunate that our knowledge of the long-term consequences of fishing in salmon has not changed appreciably since the commentaries of Larkin (1978) and the reviews by Nelson and Soulé (1987) and Policansky (1993a,b). Whether fishing selection on salmon is in most cases intense enough to pose a problem for long-term management and conservation remains unclear, but it behooves managers, in the spirit of the precautionary principle, to work with scientists to incorporate the possibility in management planning.
Given sufficient genetic variability and a stable fishing regime, salmon populations will evolve in ways that reduce fishing mortality (and yield), primarily by increasing growth rate (and, in species with complex age structure, potentially accelerating the maturation schedule). Short-term adaptation will probably not be enough to compensate for the loss of aggregate yield due to size-selective fishing. Two assumptions that are critical to recovery of exploited populations suffering from changes caused by fisheries-induced evolution are that genetic variability in size and age is not eroded by fishing selection, and that productivity is not depleted by fishing-induced changes in size and age of spawners. Quantitative genetic models indicate that aggressive reduction of fishing mortality to a fraction of initial values within several generations might be sufficient to permit an exploited salmon population to show recovery of abundance, but achieving pre-fishing maturation schedules and size distributions after adaptation to fishing mortality can take a very long time (Hard et al., unpublished data).
Salmon are unique among exploited fishes in the scale on which cultured individuals are released from hatcheries to the wild where they can be caught in fisheries or potentially spawn with naturally reproducing fish. Thus, for many stocks the hatchery and fishery regimes must be considered components of an integrated management system. To what degree selection in hatchery fish (i.e., domestication) and natural and sexual selection on spawning grounds might alter responses to fishing selection remains unclear. Understanding how hatchery and wild fish might differ in response to fishing and how domestication in hatcheries may degrade fitness of wild fish that interbreed with hatchery fish is critical to the development of sustainable hatchery production-fishing systems for salmon. For example, it is possible that fish spawned in hatcheries might have different short-term responses than fish spawning in the wild to fishing selection owing to the relaxation of natural and sexual selection on adult size in hatchery fish at time of spawning, but this issue remains unexplored (Hard 2004).
Conclusions
Do we know enough about the genetic effects of fishing on salmonids to justify reassessing current approaches to managing them? We believe so. Our survey of the literature indicates that the opportunity for fishing selection is amply demonstrated, even if it does not yet provide unambiguous evidence for rapid evolution. There are three critical uncertainties: whether trends in life history of exploited salmon are genetically based (Kuparinen and Merilä 2007), how quickly fisheries-induced evolution might occur, and whether such evolution is ‘reversible’ through management responses. Addressing these uncertainties is necessary to develop management regimes that are most effective in limiting evolutionary change caused by fishing. Meantime, a precautionary approach to fishery management that limits opportunity for adverse fishing selection is clearly warranted, and we recommend that this approach incorporate sufficient monitoring of key demographic parameters and life history traits such as run size and timing, escapement, size at age, and reproductive condition (Kuparinen and Merilä 2007). Fisheries management that promotes reduced gear selectivity with respect to size to allow sufficient larger, older individuals to breed, and focuses fishing activity on mature individuals in areas close to spawning grounds to reduce directional selection on maturation will provide some benefits to exploited populations. This is likely to be particularly important for species with restricted age structure, such as coho salmon, where variation in size of individuals vulnerable to size-selective fishing directly reflects variation in marine growth rate and high harvest rates could impose substantial negative selection differentials on growth and size at maturation.
Several researchers (e.g. Law 1991b, 2007; Heino 1998; Jørgensen et al. 2007; Kuparinen and Merilä 2007; Hutchings and Fraser 2008) are urging managers and scientists to coordinate in developing management schemes that directly account for fisheries-induced evolutionary change. The weight of evidence from the large number of studies summarized in Table 2 and the estimates of heritability in Table 3 indicate that we can be confident that evolution is being caused by fishing even if none of the individual studies is entirely conclusive. It is time to incorporate evolutionary principles into the management of salmon fisheries.
Acknowledgments
We thank Robin Waples (Northwest Fisheries Science Center), Raymond Huey (University of Washington), and Joseph Travis (Florida State University) for organizing a workshop on ‘Evolutionary consequences of anthropogenic changes on long-term viability of Pacific salmon and steelhead’ in Seattle, Washington in December 2006, where the ideas for this paper were generated. We are also grateful to the workshop participants for their contributions to the discussion of the ideas presented here, and to two anonymous reviewers for their very helpful comments on an earlier draft.
Literature cited
- Altukhov YP. Genetic consequences of selective fishing. Genetika. 1994;30:5–21. (in Russian with English abstract, tables, and figures) [Google Scholar]
- Altukhov YP, Salmenkova EA. The genetic structure of salmon populations. Aquaculture. 1991;98:11–40. [Google Scholar]
- Altukhov YP, Varnavskaya NV. Adaptive genetic structure and its connection with intrapopulation differentiation for sex, age and growth rate in sockeye salmon, Oncorhynchus nerka (Walbaum) Genetika. 1983;19:796–807. (English translation of Russian) [Google Scholar]
- Altukhov YP, Salmenkova EA, Kartavtzev YF. Relationship of allozyme heterozygosity with viability and growth rate of pink salmon. Tsitologiia Genetika. 1991;25:47–51. (English translation of Russian) [Google Scholar]
- Bailey JK, Loudenslager EJ. Genetic and environmental components of variation for growth of juvenile Atlantic salmon (Salmo salar. Aquaculture. 1986;57:125–132. [Google Scholar]
- Baskett ML, Levin SA, Gaines SD, Dushoff J. Marine reserve design and the evolution of size at maturation in harvested fish. Ecological Applications. 2005;15:882–901. [Google Scholar]
- Beacham TD. A genetic analysis of early development in pink (Oncorhynchus gorbuscha) and chum salmon (O. keta) at three different temperatures. Genome. 1988;30:89–96. doi: 10.1139/g88-015. [DOI] [PubMed] [Google Scholar]
- Beacham TD. A genetic analysis of meristic and morphometric variation in chum salmon (Oncorhynchus keta) at three different temperatures. Canadian Journal of Zoology. 1990;68:225–229. [Google Scholar]
- Van Den Berghe EP, Gross MR. Length of breeding life of coho salmon (Oncorhynchus kisutch. Canadian Journal of Zoology. 1986;64:1482–1486. [Google Scholar]
- Van Den Berghe EP, Gross MR. Natural-selection resulting from female breeding competition in a Pacific salmon (Coho: Oncorhynchus kisutch. Evolution. 1989;43:125–140. doi: 10.1111/j.1558-5646.1989.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Bielak AT, Power G. Independence of sea age and river age in Atlantic salmon (Salmo salar) from Quebec North Shore rivers. In: Meerburg DJ, editor. Salmonid Age at Maturity. Ottawa, ON: Canadian Special Publication in Fisheries and Aquatic Sciences 89; 1986. pp. 79–89. [Google Scholar]
- Bigler BS, Welch DW, Helle JH. A review of size trends among North Pacific salmon (Oncorhynchus spp.) Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:455–465. [Google Scholar]
- Birkeland C, Dayton PK. The importance in fishery management of leaving the big ones. Trends in Ecology and Evolution. 2005;20:356–358. doi: 10.1016/j.tree.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bryden CA, Heath DD. Heritability of fluctuating asymmetry for multiple traits in Chinook salmon (Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 2000;57:2186–2192. [Google Scholar]
- Campbell B, Beckman BR, Fairgrieve WT, Swanson P. Reproductive investment and growth history in coho salmon. Transactions of the American Fisheries Society. 2006;135:164–173. [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptations to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IJ, Fletcher JM, James JB, Stenseth NC. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius. Ecology Letters. 2007;10:512–521. doi: 10.1111/j.1461-0248.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Caswell H, Naiman RJ, Morin R. Evaluating the consequences of reproduction in complex salmonid life-cycles. Aquaculture. 1984;43:123–134. [Google Scholar]
- Conover DO, Munch SB. Sustaining fisheries yield over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. [DOI] [PubMed] [Google Scholar]
- Consuegra S, García de Leániz C, Serdio A, Verspoor E. Selective exploitation of early running fish may induce genetic and phenotypic changes in Atlantic salmon. Journal of Fish Biology. 2005;67(Suppl. A):129–145. [Google Scholar]
- Cox SP, Hinch SG. Changes in size at maturity of Fraser River sockeye salmon (Oncorhynchus nerka) (1952–1993) and associations with temperature. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1159–1165. [Google Scholar]
- Dickerson BR, Willson MF, Bentzen P, Quinn TP. Heritability of life history and morphological traits in a wild pink salmon, Oncorhynchus gorbuscha, population assessed by DNA parentage analysis. Transactions of the American Fisheries Society. 2005;134:1323–1328. [Google Scholar]
- Dieckmann U, Heino M. Probabilistic maturation reaction norms: their history, strengths and limitations. Marine Ecology Progress Series. 2007;335:253–269. [Google Scholar]
- Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, James JB, Haugen TO, et al. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15799–15804. doi: 10.1073/pnas.0705908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers DM, Irvine J, Fukuwaka M, Karpenko V. 2005. Catch trends and status of North Pacific salmon. North Pacific Anadromous Fisheries Commission Document 723. Revision 3, 35pp. Available at http://www.npafc.org (accessed on 15 January 2008)
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proceedings of the Royal Society of London, Series B. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve M. Observations of spawning behaviour in Salmoninae: SalmoOncorhynchus and Salvelinus. Reviews in Fish Biology and Fisheries. 2005;15:1–21. [Google Scholar]
- Fenberg PB, Roy K. Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Molecular Ecology. 2008;17:209–220. doi: 10.1111/j.1365-294X.2007.03522.x. [DOI] [PubMed] [Google Scholar]
- Fjalestad KT, Larsen HJS, Røed KH. Antibody response in Atlantic salmon (Salmo salar) against Vibrio anguillarum and Vibrio salmonicida O-antigens: Heritabilities, genetic correlations and correlations with survival. Aquaculture. 1996;145:77–89. [Google Scholar]
- Fleming IA, Gross MR. Evolution of adult female life-history and morphology in a Pacific salmon (Coho: Oncorhynchus kisutch. Evolution. 1989;43:141–157. doi: 10.1111/j.1558-5646.1989.tb04213.x. [DOI] [PubMed] [Google Scholar]
- Foote CJ, Moore K, Stenberg K, Craig KJ, Wenburg JK, Wood CC. Genetic differentiation in gill raker number and length in sympatric anadromous and nonanadromous morphs of sockeye salmon, Oncorhynchus nerka. Environmental Biology of Fishes. 1999;54:263–274. [Google Scholar]
- Friedland KD, Hansen LP, Dunkley DA, MacLean JC. Linkage between ocean climate, post-smolt growth, and survival of Atlantic salmon (Salmo salar L.) in the North Sea area. ICES Journal of Marine Science. 2000;57:419–429. [Google Scholar]
- Fujimori Y, Tokai T. Estimation of gillnet selectivity curve by maximum likelihood method. Fisheries Science. 2001;67:644–654. [Google Scholar]
- Funk WC, Tyburczy JA, Knudsen KL, Lindner KR, Allendorf FW. Genetic basis of variation in morphological and life-history traits of a wild population of pink salmon. Journal of Heredity. 2005;96:24–31. doi: 10.1093/jhered/esi009. [DOI] [PubMed] [Google Scholar]
- Garant D, Dodson JJ, Bernatchez L. Differential reproductive success and heritability of alternative reproductive tactics in wild Atlantic salmon (Salmo salar L.) Evolution. 2003;57:1133–1141. doi: 10.1111/j.0014-3820.2003.tb00322.x. [DOI] [PubMed] [Google Scholar]
- García de Leániz C, Caballero P, Valero E, Martínez JJ, Hawkins AD. Historical changes in Spanish Atlantic salmon (Salmo salar L.) rod and line fisheries: why are large multi-seawinter fish becoming scarcer? Journal of Fish Biology. 1992;41(Suppl. B):179. (Abstract) [Google Scholar]
- García de Leániz C, Serdio A, Consuegra S. Present status of Atlantic salmon in Cantabria. In: García de Leániz C, Serdio A, Consuegra S, editors. El salmón, joya de nuestros ríos. Santander (in Spanish with English summary): Consejería de Ganadería, Agricultura y Pesca; 2001. pp. 55–82. [Google Scholar]
- Gee AS, Milner NJ. Analysis of 70-year catch statistics for Atlantic salmon (Salmo salar) in the River Wye and implications for management of stocks. Journal of Applied Ecology. 1980;17:41–57. [Google Scholar]
- Gross MR. Alternative reproductive strategies and tactics: diversity within sexes. Trends in Ecology and Evolution. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- Guy DR, Bishop SC, Brotherstone S, Hamilton A, Roberts RJ, McAndrew BJ, Woolliams JA. Analysis of the incidence of infectious pancreatic necrosis mortality in pedigreed Atlantic salmon, Salmo salar L., populations. Journal of Fish Diseases. 2006;29:637–647. doi: 10.1111/j.1365-2761.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- Hard JJ. Genetic monitoring of life-history characters in salmon supplementation: problems and opportunities. American Fisheries Society Symposium. 1995;15:212–225. [Google Scholar]
- Hard JJ. Case study of Pacific salmon. In: Dieckmann U, Godø OR, Heino M, Mork J, editors. Fisheries-Induced Adaptive Change. Cambridge: Cambridge Studies in Adaptive Dynamics, Cambridge University Press; In press. [Google Scholar]
- Hamley JM. Review of gillnet selectivity. Journal of the Fisheries Research Board of Canada. 1975;32:1943–1969. [Google Scholar]
- Hamon TR, Foote CJ. Concurrent natural and sexual selection in wild male sockeye salmon, Oncorhynchus nerka. Evolution. 2005;59:1104–1118. [PubMed] [Google Scholar]
- Hamon TR, Foote CJ, Hilborn R, Rogers DE. Selection on morphology of spawning wild sockeye salmon by a gill-net fishery. Transactions of the American Fisheries Society. 2000;129:1300–1315. [Google Scholar]
- Handford P, Bell G, Reimchen TE. A gillnet fishery considered as an experiment in artificial selection. Journal of the Fisheries Research Board of Canada. 1977;34:954–961. [Google Scholar]
- Hankin DG, Healey MC. Dependence of exploitation rates for maximum yield and stock collapse on age and sex structure of Chinook salmon (Oncorhynchus tshawytscha) stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1986;43:1746–1759. [Google Scholar]
- Hankin DG, Nicholas JW, Downey TW. Evidence for inheritance of age of maturity in Chinook salmon, Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:347–358. [Google Scholar]
- Hard JJ. Evolution of Chinook salmon life history under size-selective harvest. In: Hendry A, Stearns S, editors. Evolution Illuminated: Salmon and Their Relatives. New York, NY: Oxford University Press; 2004. pp. 315–337. [Google Scholar]
- Hard JJ, Winans GA, Richardson JC. Phenotypic and genetic architecture of juvenile morphometry in Chinook salmon. Journal of Heredity. 1999;90:597–606. [Google Scholar]
- Hard JJ, Elliott DG, Pascho RG, Chase DM, Park LK, Winton JR, Campton DE. Genetic effects of ELISA-based segregation for control of bacterial kidney disease in Chinook salmon (Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2793–2808. [Google Scholar]
- Harris RB, Wall WA, Allendorf FW. Genetic consequences of hunting: what do we know and what should we do? Wildlife Society Bulletin. 2002;30:634–643. [Google Scholar]
- Haugen T, Vøllestad LA. A century of life-history evolution in grayling. Genetica. 2001;112-113:475–491. [PubMed] [Google Scholar]
- Healey MC. Fecundity changes in exploited populations of lake whitefish (Coregonus clupeaformis) and lake trout (Salvelinus namaycush. Journal of the Fisheries Research Board of Canada. 1978;35:945–950. [Google Scholar]
- Healey MC. Growth and recruitment in experimentally exploited populations of lake whitefish (Coregonus clupeaformis) populations. Canadian Journal of Fisheries and Aquatic Sciences. 1980;37:255–267. [Google Scholar]
- Healey MC. Optimum size and age at maturity in Pacific salmon and effects of size-selective fisheries. In: Meerburg DJ, editor. Salmonid Age at Maturity. Ottawa, ON: Canadian Special Publication in Fisheries and Aquatic Sciences 89; 1986. pp. 39–52. [Google Scholar]
- Healey MC, Heard WR. Inter- and intra-population variation in the fecundity of Chinook salmon (Oncorhynchus tshawytscha) and its relevance to life history theory. Canadian Journal of Fisheries and Aquatic Sciences. 1984;41:476–483. [Google Scholar]
- Heard WR. Life history of pink salmon (Oncorhynchus gorbuscha. In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. Vancouver, BC: University of British Columbia Press; 1991. pp. 121–230. [Google Scholar]
- Heino M. Management of evolving fish stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:171–182. [Google Scholar]
- Heino M, Dieckmann U, Godø OR. Measuring probabilistic reaction norms for age and size and maturation. Evolution. 2002;56:669–678. doi: 10.1111/j.0014-3820.2002.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Stearns SC, editors. Evolution Illuminated: Salmon and Their Relatives. Oxford: Oxford University Press; 2004. p. 510. [Google Scholar]
- Hilborn R, Walters CJ. Quantitative Fisheries Stock Assessment: Choice, Dynamics, and Uncertainty. New York, 570pp: Chapman and Hall; 1992. [Google Scholar]
- Hinch SG, Healey MC, Diewert RE, Thomson KA, Hourston R, Henderson MA, Juanes F. Potential effects of climate change on marine growth and survival of Fraser River sockeye salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1995;52:2651–2659. [Google Scholar]
- Hindar K, García de Leániz C, Koljonen M-L, Tufto J, Youngson AF. Fisheries exploitation. In: Verspoor E, Stradmeyer L, Nielsen JL, editors. The Atlantic Salmon: Genetics, Conservation and Management. Oxford: Blackwell Publishing; 2007. pp. 299–324. [Google Scholar]
- Hutchings JA, Fraser DJ. The nature of fisheries- and farming-induced evolution. Molecular Ecology. 2008;17:294–313. doi: 10.1111/j.1365-294X.2007.03485.x. [DOI] [PubMed] [Google Scholar]
- Hyer KE, Schleusner CJ. Chinook Salmon Age, Sex, And Length Analysis from Selected Escapement Projects on the Yukon River. Anchorage, AK, 62pp: U.S. Fish and Wildlife Service, Alaska Fisheries Technical Report 87; 2005. [Google Scholar]
- International Council for the Exploration of the Sea (ICES) 2006. p. 254. Report of the Working Group on North Atlantic Salmon (WGNAS), 4–13 April 2006, ICES Headquarters. ICES/CM ACFM:23.
- International Council for the Exploration of the Sea (ICES) 2006. p. 254. Report of the Working Group on North Atlantic Salmon (WGNAS), 4–13 April 2006, ICES Headquarters. ICES/CM ACFM:23:
- Ishida Y, Ito S, Kaeriyama M, McKinnell S, Nagasawa K. Recent changes in age and size of chum salmon (Oncorhynchus keta) in the North Pacific Ocean and possible causes. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:290–295. [Google Scholar]
- Ishida Y, Welch DW, Ogura M. Potential influence of North Pacific sea-surface temperatures on increased production of chum salmon (Oncorhynchus keta) from Japan. Canadian Special Publication in Fisheries and Aquatic Sciences. 1995;121:271–275. [Google Scholar]
- Jensen AJ, Zubchenko AV, Heggberget TG, Hvidsten NA, Johnsen BO, Kuzmin O, Loenko AA, et al. Cessation of the Norwegian drift net fishery: changes observed in Norwegian and Russian populations of Atlantic salmon. ICES Journal of Marine Science. 1999;56:84–95. [Google Scholar]
- Jónasson J. Selection experiments in salmon ranching. I. Genetic and environmental sources of variation in survival and growth in freshwater. Aquaculture. 1993;109:225–236. [Google Scholar]
- Jónasson J, Gjedrem T. Genetic correlation for body weight of Atlantic salmon grilse between fish in sea ranching and land-based farming. Aquaculture. 1997;157:205–214. [Google Scholar]
- Jónasson J, Gjerde B, Gjedrem T. Genetic parameters for return rate and body weight of sea-ranched Atlantic salmon. Aquaculture. 1997;154:219–231. [Google Scholar]
- Jørgensen C, Enberg K, Dunlop ES, Arlinghaus R, Boukal DS, Brander K, Ernande B, et al. Managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- Kaeriyama M, Katsuyama K. Increase in body size with decrease in population size of chum salmon returning to Hokkaido, Japan since late 1990s. North Pacific Anadromous Fisheries Commission Newsletter. 2001;5:6–7. [Google Scholar]
- Kaev AM. Recent reduction in chum salmon (Oncorhynchus keta) growth and its consequences for reproduction. North Pacific Anadromous Fisheries Commission Bulletin. 2000;2:247–253. [Google Scholar]
- Kaev AM, Romasenko LV. Some Results of Studying Chum Salmon in Ilyushin and Sernovodka Rivers on the Kunashir Island (Kuril Islands). North Pacific Anadromous Fisheries Commission Document 670. Yuzhno-Sakhalinsk: Sakhalin Research Institute of Fisheries and Oceanography; 2003. [Google Scholar]
- Kanno Y. Genetic parameter of chum salmon fry (Oncorhynchus keta) caught in Moheji River, Hokkaido [Japan] Bulletin of the Faculty of Fisheries Hokkaido University (Japan) 1990;41:181–190. [Google Scholar]
- Kinnison MT, Unwin MJ, Hershberger WK, Quinn TP. Egg size, fecundity and early development rate of two New Zealand Chinook salmon (Oncorhynchus tshawytscha) populations. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1946–1953. [Google Scholar]
- Kinnison MT, Unwin MJ, Hendry AP, Quinn TP. Migratory costs and the evolution of egg size and number in introduced and indigenous salmon populations. Evolution. 2001;55:1656–1667. doi: 10.1111/j.0014-3820.2001.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen TS, Svåsand T. Effect of size-selective mortality on growth of coastal cod illustrated by tagging data and an individual-based growth and mortality model. Journal of Fish Biology. 1998;52:688–705. [Google Scholar]
- Krogius FV. On the relationship of the freshwater and seawater period of life of sockeye salmon in Lake Dal’neye. Biologiya Morya. 1979;3:24–29. [Google Scholar]
- Kuparinen A, Merilä J. Detecting and managing fisheries-induced evolution. Trends in Ecology and Evolution. 2007;22:652–659. doi: 10.1016/j.tree.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lande R. Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Larkin PA. Fisheries management – an essay for ecologists. Annual Review of Ecology and Systematics. 1978;9:57–73. [Google Scholar]
- Law R. On the quantitative genetics of correlated characters under directional selection in age-structured populations. Philosophical Transactions of the Royal Society of London, Series B (Biological Sciences) 1991a;331:213–223. [Google Scholar]
- Law R. Fishing in evolutionary waters. New Scientist. 1991b;129:35–37. [Google Scholar]
- Law R. Fishing, selection, and phenotypic evolution. ICES Journal of Marine Science. 2000;57:659–669. [Google Scholar]
- Law R. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series. 2007;335:271–277. [Google Scholar]
- Law R, Rowell CA. Cohort-structured populations, selection responses, and exploitation of the North Sea cod. In: Stokes TK, McGlade JM, Law R, editors. The Exploitation of Evolving Resources. Lecture Notes in Biomathematics 99. Berlin: Springer–Verlag; 1993. pp. 155–173. [Google Scholar]
- Lee RM. An investigation into the methods of growth determination in fishes. Conseil Permanent Internationale pour l’Exploration de la Mer. Publication Circonstance. 1912;63:35. [Google Scholar]
- Mangel M, Stamps J. Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evolutionary Ecology Research. 2001;3:583–593. [Google Scholar]
- Marshall CT, McAdam BJ. Integrated perspectives on genetic and environmental effects on maturation can reduce potential for errors of inference. Marine Ecology Progress Series. 2007;335:301–310. [Google Scholar]
- McAllister MK, Peterman RM. Decision analysis of a large-scale fishing experiment designed to test for a genetic effect of size-selective fishing on British Columbia pink salmon (Oncorhynchus gorbuscha. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:1305–1314. [Google Scholar]
- McAllister MK, Peterman RM, Gillis DM. Statistical evaluation of a large-scale fishing experiment designed to test for a genetic effect of size-selective fishing on British Columbia pink salmon (Oncorhynchus gorbuscha. Canadian Journal of Fisheries and Aquatic Sciences. 1992;49:1294–1304. [Google Scholar]
- McGuigan K. Studying phenotypic evolution using multivariate quantitative genetics. Molecular Ecology. 2006;15:883–896. doi: 10.1111/j.1365-294X.2006.02809.x. [DOI] [PubMed] [Google Scholar]
- McIntyre JD, Blanc JM. A genetic analysis of hatching time in steelhead trout (Salmo gairdneri. Journal of the Fisheries Research Board of Canada. 1973;30:137–139. [Google Scholar]
- McKay LR, Ihsen PE, Friars GW. Genetic parameters of growth in rainbow trout, Salmo gairdneri, as a function of age and maturity. Aquaculture. 1986;58:241–254. [Google Scholar]
- Mezzera M, Largiadèr CR. Evidence for selective angling of introduced trout and their hybrids in a stocked brown trout population. Journal of Fish Biology. 2001;59:287–301. [Google Scholar]
- Millar RB, Fryer RJ. Estimating the size-selection curves of towed gears, traps, nets and hooks. Reviews in Fish Biology and Fisheries. 1999;9:89–116. [Google Scholar]
- Miller RB. Have the genetic patterns of fishes been altered by introduction or by selective fishing? Journal of the Fisheries Research Board of Canada. 1957;14:797–806. [Google Scholar]
- Miller LM, Kapuscinski AR. Estimation of selection differentials from fish scales: a step towards evaluating genetic alterations of fish size in exploited populations. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:774–783. [Google Scholar]
- Montgomery WL, Naiman RJ, Morin R, Caswell H, Klopfer E, Kana T. 1986. The Atlantic salmon (Salmo salar) population of the Matamek River, Quebec: 1967-1984. Woods Hole Oceanographic Institution Technical Report WHOI-86-21.
- Morita K, Fukuwaka M-a. Does size matter most? The effect of growth history on the probabilistic reaction norm for salmon maturation. Evolution. 2006;60:1516–1521. [PubMed] [Google Scholar]
- Morita K, Fukuwaka M-a. Why age and size at maturity have changed in Pacific salmon. Marine Ecology Progress Series. 2007;335:289–294. [Google Scholar]
- Morita K, Morita SH. Rule of age and size at maturity: individual variation in the maturation history of resident white-spotted charr. Journal of Fish Biology. 2002;61:1230–1238. [Google Scholar]
- Morita SH, Morita K, Sakano H. Growth of chum salmon (Oncorhynchus keta) correlated with sea surface salinity in the North Pacific. ICES Journal of Marine Science. 2001;58:1335–1339. [Google Scholar]
- Morita K, Morita SH, Fukuwaka M, Matsuda H. Rule of age and size at maturity of chum salmon (Oncorhynchus keta): implications of recent trends among Oncorhynchus spp. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:2752–2759. [Google Scholar]
- Murray CB, Beacham TD, Barner LW. Growth and survival of newly emerged and juvenile coho salmon (Oncorhynchus kisutch) reared at different salinities. Canadian Journal of Zoology. 1993;71:1230–1237. [Google Scholar]
- Nelson K, Soulé M. Genetical conservation of exploited fishes. In: Ryman N, Utter F, editors. Population Genetics & Fishery Management. Seattle, WA: University of Washington Press; 1987. pp. 345–368. [Google Scholar]
- Nikulin OA. On the relationship between the decreased absolute numbers of sockeye Oncorhynchus nerka (Walb.) and the increased relative numbers of dwarfs among young fish feeding in Lake Uyeginsk. Izvestiya TINRO. 1970;71:205–217. (English translation of Russian) [Google Scholar]
- Pacific Salmon Commission (PSC) Joint Chinook Technical Committee Report: Estimation and Application of Incidental Fishing Mortality in Chinook Salmon Management Under the 1999 Agreement to the Pacific Salmon Treaty. 2004. Report TCCHINOOK (04)-1. Vancouver, BC, Canada, 40pp. + 7 appendices. [Google Scholar]
- Pacific Salmon Commission (PSC) Joint Chinook Technical Committee Report: Annual Report on Catch, Escapement, Exploitation Rate Analysis and Model Calibration of Chinook Salmon Under Pacific Salmon Commission Jurisdiction, 2006. 2007. Report TCCHINOOK (07)-1. Vancouver, BC, Canada. 81pp. + 10 appendices. [Google Scholar]
- Paloheimo JE, Elson PF. Reduction of Atlantic salmon catches in Canada attributed to the Greenland fishery. Journal of the Fisheries Research Board of Canada. 1974;31:1467–1480. [Google Scholar]
- Perrin N, Rubin JF. On dome-shaped norms of reaction for size-to-age at maturity in fishes. Functional Ecology. 1990;4:53–57. [Google Scholar]
- Policansky D. Fishing as a cause of evolution in fishes. In: Stokes TK, McGlade JM, Law R, editors. The Exploitation of Evolving Resources. Vol. 99. Berlin: Springer-Verlag; 1993a. pp. 2–18. [Google Scholar]
- Policansky D. Evolution and management of exploited fish populations. In: Kruse G, Eggers DM, Marasco RJ, Pautzke C, Quinn TJ, editors. Management Strategies for Exploited Fish Populations. Fairbanks, AK: University of Alaska Sea Grant; 1993b. pp. 651–664. [Google Scholar]
- Pope JA, Margetts AR, Hanley JM, Akyuz EF. Manual of Methods for Fish Stock Assessment – Part III. Selectivity of Fishing Gear. 1975. FAO Fisheries Technical Paper No. 41 (Revision 1), 65pp. [Google Scholar]
- Pyper BJ, Peterman RM. Relationships among adult body length, abundance, and ocean temperature for British Columbia and Alaska sockeye salmon (Oncorhynchus nerka), 1967–1977. Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:1716–1720. [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle, 378pp: University of Washington Press; 2005. [Google Scholar]
- Quinn TP, Foote CJ. The effects of body size and sexual dimorphism on the reproductive behaviour of sockeye salmon, Oncorhynchus nerka. Animal Behaviour. 1994;48:751–761. [Google Scholar]
- Quinn TP, Unwin MJ, Kinnison MT. Evolution of temporal isolation in the wild: genetic divergence in timing of migration and breeding by introduced Chinook salmon populations. Evolution. 2000;54:1372–1385. doi: 10.1111/j.0014-3820.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Wetzel L, Bishop S, Overberg K, Rogers DE. Influence of breeding habitat on bear predation and age at maturity and sexual dimorphism of sockeye salmon populations. Canadian Journal of Zoology. 2001;79:1782–1793. [Google Scholar]
- Quinn TP, Peterson JA, Gallucci V, Hershberger WK, Brannon EL. Artificial selection and environmental change: countervailing factors affecting the timing of spawning by coho and Chinook salmon. Transactions of the American Fisheries Society. 2002;131:591–598. [Google Scholar]
- Quinn TP, McGinnity P, Cross TF. Long-term declines in body size and shifts in run timing of Atlantic salmon in Ireland. Journal of Fish Biology. 2006;68:1713–1730. [Google Scholar]
- Quinn TP, Hodgson S, Flynn L, Hilborn R, Rogers DE. Directional selection by fisheries and the timing of sockeye salmon (Oncorhynchus nerka) migrations. Ecological Applications. 2007;17:731–739. doi: 10.1890/06-0771. [DOI] [PubMed] [Google Scholar]
- Refstie T, Steine TA. Selection experiments with salmon. III. Genetic and environmental sources of variation in length and weight of Atlantic salmon in the freshwater phase. Aquaculture. 1978;14:221–234. [Google Scholar]
- Ricker WE. Maximum sustained yields from fluctuating environments and mixed stocks. Journal of the Fisheries Research Board of Canada. 1958;15:991–1006. [Google Scholar]
- Ricker WE. Effects of size-selective mortality and sampling bias on estimates of growth, mortality, production and yield. Journal of the Fisheries Research Board of Canada. 1969;261:479–541. [Google Scholar]
- Ricker WE. Review of the rate of growth and mortality of Pacific salmon in salt water, and non-catch mortality caused by fishing. Journal of the Fisheries Research Board of Canada. 1976;33:1483–1524. [Google Scholar]
- Ricker WE. Changes in the average size and age of Pacific salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1981;38:1636–1656. [Google Scholar]
- Ricker WE. Trends in the average size of Pacific salmon in Canadian catches. In: Beamish RJ, editor. Climate Change and Northern Fish Populations. Ottawa, ON: Canadian Special Publication in Fisheries and Aquatic Sciences 121; 1995. pp. 593–602. [Google Scholar]
- Ricker WE, Wickett WP. Causes of the Decrease in Size of Coho Salmon. 63pp: Canadian Technical Report of Fisheries and Aquatic Sciences 971; 1980. [Google Scholar]
- Ricker WE, Bilton HT, Aro KV. Causes of the Decline in Size of Pink Salmon (Oncorhynchus gorbuscha) 93pp: Fisheries and Oceans Canada, Fisheries and Marine Service Technical Report 820; 1978. [Google Scholar]
- Riddell BE. Assessment of selective fishing on the age at maturity in Atlantic salmon (Salmo salar): a genetic perspective. In: Meerburg DJ, editor. Salmonid Age at Maturity. Ottawa, ON: Canadian Special Publication in Fisheries and Aquatic Sciences 89; 1986. pp. 102–109. [Google Scholar]
- Rijnsdorp AD. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. [DOI] [PubMed] [Google Scholar]
- Ritter JA, Newbould K. 1977. p. 5. Relationships of Parentage and Smolt Age to Age at First Maturity of Atlantic Salmon (Salmo salar). ICES/CM M:32.
- Ritter JA, Farner GJ, Misra RK, Goff TR, Bailey JK, Baum ET. Parental influences and smolt size and sex ratio effects on sea age at first maturity of Atlantic salmon (Salmo salar. In: Meerburg DJ, editor. Salmonid Age at Maturity. Ottawa, ON: Canadian Special Publication of Fisheries and Aquatic Sciences 89; 1986. pp. 30–38. [Google Scholar]
- De Roos AM, Boukal DS, Persson L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proceedings of the Royal Society B (Biological Sciences) 2006;273:1873–1880. doi: 10.1098/rspb.2006.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter C. Natural history of the quinnat salmon. A report of investigations in the Sacramento River, 1886–1901. Bulletin of the U.S. Fisheries Commission. 1904;22:65–141. [Google Scholar]
- Salvanes AGV, Baliño BM. Productivity and fitness in a fjord cod population: an ecological and evolutionary approach. Fisheries Research. 1998;37:143–161. [Google Scholar]
- Sato R. Variations in hatchability and hatching time, and estimation of heritability for hatching time among individuals within the strain of coho salmon (Oncorhynchus kisutch. Bulletin of National Research Institute of Aquaculture. 1980;1:21–28. [Google Scholar]
- Schaffer WM, Elson PF. The adaptive significance of variations in life history among local populations of Atlantic salmon in North America. Ecology. 1975;56:577–590. [Google Scholar]
- Silverstein JT, Hershberger WK. Precocious maturation in coho salmon (Oncorhynchus kisutch): estimation of the additive genetic variance. Journal of Heredity. 1992;83:282–286. [Google Scholar]
- Silverstein JT, Hershberger WK. Genetics of size and growth rate through sexual maturity in freshwater-reared coho salmon (Oncorhynchus kisutch. Theoretical and Applied Genetics. 1995;90:733–739. doi: 10.1007/BF00222141. [DOI] [PubMed] [Google Scholar]
- Sinclair AF, Swain DP, Hanson JM. Measuring changes in the direction and magnitude of size selective mortality in a commercial fish population. Canadian Journal of Fisheries and Aquatic Sciences. 2002;59:361–371. [Google Scholar]
- Smith VE. The Taking of Immature Salmon in the Waters of the State of Washington. Olympia, WA: State of Washington, Department of Fisheries; 1920. [Google Scholar]
- Smith PJ, Francis RICC, McVeagh M. Loss of genetic diversity due to fishing pressure. Fisheries Research. 1991;10:309–316. [Google Scholar]
- Smoker WW, Gharrett AJ, Stekoll MS, Joyce JE. Genetic analysis of size in an anadromous population of pink salmon. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51(Suppl. 1):9–15. [Google Scholar]
- Smoker WW, Gharrett AJ, Stekoll MS. Genetic variation of return date in a population of pink salmon: a consequence of fluctuating environment and dispersive selection? Alaska Fishery Research Bulletin. 1998;5:46–54. [Google Scholar]
- Steen RP, Quinn TP. Egg burial depth by sockeye salmon (Oncorhynchus nerka): implications for survival of embryos and natural selection on female body size. Canadian Journal of Zoology. 1999;77:836–841. [Google Scholar]
- Stone L. Report of Operations at the United States Salmon-Hatching Station on the McCloud River, California, in 1878. Washington, DC: U.S. Commission of Fish and Fisheries; 1880. pp. 741–770. Report of the Commissioner for 1878, Appendix. [Google Scholar]
- Stone L. Report of operations at the United States salmon-breeding station on the McCloud River, California, during the season of 1879. Washington, DC: U.S. Commission of Fish and Fisheries; 1882. pp. 695–708. Report of the Commissioner for 1879 appendix, part 25. [Google Scholar]
- Summers DW. Long-term changes in sea-age at maturity and seasonal time of return of salmon, Salmo salar L., to Scottish rivers. Fisheries Management and Ecology. 1995;2:147–156. [Google Scholar]
- Swain DP, Sinclair AF, Hanson JM. Evolutionary response to size-selective mortality in an exploited fish population. Proceedings of the Royal Society Series B (Biological Sciences) 2007;274:1015–1022. doi: 10.1098/rspb.2006.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvén S, Elvingson P. Comparison of rainbow trout (Oncorhynchus mykiss) strains for body weight, length and age at maturity in different Swedish production systems. Aquaculture. 1992;104:37–50. [Google Scholar]
- Taylor WW, Smale MA, Brown RW. Evaluation of size versus age dependent maturation of lake whitefish stocks in the upper Great Lakes. Polish Archives of Hydrobiology. 1992;39:269–277. [Google Scholar]
- Thériault V, Dunlop ES, Dieckmann U, Bernatchez L, Dodson JJ. The impact of fishing-induced mortality on the evolution of alternative life-history tactics in brook charr. Evolutionary Applications. 2008;1:409–423. doi: 10.1111/j.1752-4571.2008.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Eckmann R. The influence of eutrophication and population biomass on common whitefish (Coregonus lavaretus) growth – the Lake Constance example revisited. Canadian Journal of Fisheries and Aquatic Sciences. 2007;64:402–410. [Google Scholar]
- Thorpe JE. Impacts of fishing on genetic structure of salmonid populations. In: Cloud JG, Thorgaard GH, editors. Genetic Conservation of Salmonid Fishes. New York, NY: Plenum Press; 1993. pp. 67–80. [Google Scholar]
- Thorpe JE. Maturation responses of salmonids to changing developmental opportunities. Marine Ecology Progress Series. 2007;335:285–288. [Google Scholar]
- Thrower FP, Hard JJ, Joyce JE. Genetic architecture of growth and early life history transitions in anadromous and derived freshwater populations of steelhead. Journal of Fish Biology. 2004;65:286–307. [Google Scholar]
- Todd ISP, Larkin PA. Gillnet selectivity on sockeye (Oncorhynchus nerka) and pink salmon (O. gorbuscha) of the Skeena River system, British Columbia. Journal of the Fisheries Research Board of Canada. 1971;28:821–842. [Google Scholar]
- Trippel EA. Age at maturity as a stress indicator in fisheries. BioScience. 1995;45:759–771. [Google Scholar]
- Unwin MJ, Kinnison MT, Boustead NC, Quinn TP. Genetic control over survival in Pacific salmon (Oncorhynchus spp.): experimental evidence between and within populations of New Zealand Chinook salmon (O. tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 2003;60:1–11. [Google Scholar]
- Varnavskaya NV, Varnavsky V. On the biology of dwarf forms of sockeye in Lake Dal’neye (Kamchatka) Biologiya Morya. 1988;1988:16–23. (English translation of Russian) [Google Scholar]
- Vøllestad LA, Quinn TP. Trade-off between growth rate and aggression in juvenile coho salmon, Oncorhynchus kisutch. Animal Behaviour. 2003;66:561–568. [Google Scholar]
- Walker TI, Taylor BL, Hudson RJ, Cottier JP. The phenomenon of apparent change of growth rate in gummy shark (Mustelus antarcticus) harvested off southern Australia. Fisheries Research. 1998;39:139–163. [Google Scholar]
- Walsh MR, Munch SB, Chiba S, Conover DO. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecology Letters. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. [DOI] [PubMed] [Google Scholar]
- Walters CJ. Adaptive Management of Renewable Resources. New York, 374pp: MacMillan; 1986. [Google Scholar]
- Walters CJ. Use of gaming procedures in evaluation of management experiments. Canadian Journal of Fisheries and Aquatic Sciences. 1994;51:2705–2714. [Google Scholar]
- Walters CJ, Cahoon P. Evidence of decreasing spatial diversity in British Columbia salmon stocks. Canadian Journal of Fisheries and Aquatic Sciences. 1985;42:1033–1037. [Google Scholar]
- Walters CJ, Martell SJD. Fisheries Ecology and Management. Princeton, NJ, 399pp: Princeton University Press; 2004. [Google Scholar]
- Withler RE, Evelyn TPT. Genetic variation in susceptibility to bacterial kidney disease within and between two strains of coho salmon (Oncorhynchus kisutch. Transactions of the American Fisheries Society. 1990;119:1003–1009. [Google Scholar]
- Wright PJ. Understanding the maturation process for field investigations of fisheries-induced evolution. Marine Ecology Progress Series. 2007;335:279–283. [Google Scholar]


