Abstract
Critical physiological roles of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) include the regulation glucose and lipid homeostasis, cellular differentiation, and modulation of inflammation. The potential for targeting PPARβ/δ for the prevention and treatment of metabolic diseases or cancer, is compromised because of major inconsistencies in the literature. This is due primarily to uncertainty regarding the effect of PPARβ/δ and its activation on cell proliferation, apoptosis and cell survival. This review summarizes both the confirmed and conflicting mechanisms that have been described for PPARβ/δ and the potential for targeting this nuclear receptor for the prevention and treatment of colon cancer.
Introduction
There are three PPARs, PPARα, PPARβ/δ (also referred to as PPARβ or PPARδ) and PPARγ (Fig. 1). The fibrate class of hypolipidemic drugs used for the treatment of dyslipidemias was the first chemicals found to target a PPAR, namely PPARα, to elicit their pharmacological effects. Interestingly, these drugs were developed without a priori knowledge of the actual molecular target, which was determined years later after PPARα was discovered [1]. Fibrates effectively lower serum lipids by binding to and activating PPARα, which causes transcriptional upregulation of target genes encoding proteins that mobilize fatty acids from adipose and increase β-oxidation of fatty acids in liver and extra-hepatic tissues [2]. Fibrates have been used for more than forty years with a relatively good safety profile (Fig. 1). The thiazolidinedione class of insulin sensitizing drugs is the second class of chemicals that targeted another PPAR, PPARγ, for the treatment and management of type II diabetes (Fig. 1). Similar to fibrates that act as agonists of PPARα, thiazolidinediones are PPARγ agonists. However, while it is known that thiazolidinedione require PPARγ to elicit the hypoglycemic effect, the mechanism of action of thiazolidinediones is less clear. In contrast to fibrates, the safety of thiazolidinedione has recently been called into question as increased heart failure and other cardiovascular risk have been found in patients being treated with these drugs [3]. Agonists for PPARβ/δ have also been examined clinically due to preclinical evidence showing anti-inflammatory activities, weight loss, increased HDL cholesterol and improved insulin sensitivity in response to these ligands [4]. Because PPARα, PPARβ/δ and PPARγ agonists have been shown improve clinical indices associated with metabolic syndrome, there remains strong interest in developing new selective and pan agonists as therapeutic agents (Fig. 1). A number of PPARβ/δ agonists have also been developed including L165041 [5], GW501516 and GW0742 [6]. However, the development of PPARβ/δ agonists as therapeutic drugs has been hampered because of the conflicting data in the literature describing different effects of activating this PPAR isoform on cancer.
Figure 1.
Targeting PPARs for the treatment and prevention of diseases. The fibrate class of hypolipidemic drugs activate PPARα causing increased expression of proteins that facilitate hepatic uptake and catabolism of fatty acids. Fibrates have been used for decades for the effective treatment of dyslipidemias. The thiazolidinediones drugs activate PPARγ and through still undefined mechanisms, reduce serum glucose and improve insulin sensitivity in diabetic patients. Strong evidence also supports the targeting of PPARγ for the prevention of cancer because PPARγ ligands can inhibit cell proliferation, promote terminal differentiation, promote apoptosis and inhibit inflammatory signaling. Clinical and preclinical evidence shows that PPARβ/δ has anti-inflammatory activities, promotes terminal differentiation, increases fatty acid catabolism in skeletal muscle, may promote weight loss, increases HDL cholesterol, improves insulin sensitivity and clinical indices associated with metabolic syndrome. Whether PPARβ/δ agonists can be developed for the treatment of diabetes, metabolic syndrome or cancer is under evaluation.
Controversial role of PPARβ/δ in colon cancer
There are eight reports from four independent laboratories that have examined the role of PPARβ/δ on colon cancer using mouse models. Three different conclusions were drawn from these reports thus leading to uncertainty surrounding the role of this receptor in cancer. Barak and colleagues found that intestinal tumorigenesis was unchanged in APCmin+/- mice crossed with Pparβ/δ-null mice as compared to control APCmin+/- mice [7]. This is the first report to suggest that expression of PPARβ/δ had no influence on colon cancer incidence in a mouse model. In contrast, Gupta et al. were the first to show that administration of GW501516 caused an increase in the number and size of small intestinal tumors in APCmin+/- mice, but no change in colon tumors, as compared to controls [8]. Consistent with this finding, the same laboratory went on to show that the effect of GW501516 on small intestinal tumorigenesis was mediated by PPARβ/δ since the observed increase in tumorigenesis was not found in APCmin+/- mice crossed with Pparβ/δ-null mice [9]. However, in a later study [9], GW501516 caused an increase in colon tumor multiplicity not found in the former study [8]. Wang and colleagues also reported that prostaglandin E2 could activate PPARβ/δ and promote intestinal tumorigenesis through a PPARβ/δ-dependent mechanism [10]. In addition to genetically-dependent intestinal tumorigenesis, another group has shown that azoxymethane-induced colon tumorigenesis requires PPARβ/δ in mice on an FVB genetic background [11]. While these studies suggest that activating PPARβ/δ promotes intestinal tumorigenesis, other studies suggest otherwise. Harman found that genetic (APCmin+/-) and chemically-induced (azoxymethane) colon tumorigenesis is exacerbated in the absence of PPARβ/δ expression [12]. Consistent with this finding, ligand activation of PPARβ/δ modestly inhibited colon tumorigenesis in azoxymethane-treated wild-type mice but not in Pparβ/δ-null mice, demonstrating that PPARβ/δ is required for this chemopreventive effect [13]. In contrast to the report from Gupta [8], ligand activation of PPARβ/δ had no influence on intestinal tumor multiplicity or size in APCmin+/- mice [13]. Interestingly, in another study, inhibition of azoxymethane-induced colon tumorigenesis as a result of ligand activation of PPARβ/δ with GW0742 was also found to be PPARβ/δ-dependent whereas inhibition of azoxymethane-induced colon tumorigenesis by COX2 inhibition was found to be PPARβ/δ-independent [14]. Collectively, results from these eight reports using in vivo models of colon cancer have led to opposing views and raises the question of how and why some studies find protumorigenic effects of activating PPARβ/δ while other studies find inhibitory, or no effect, of activating PPARβ/δ. Some of these discrepancies may be explained in part by the role of PPARβ/δ in the immune system, which could affect different stages of tumorigenesis. Therefore, the experimental conditions including whether PPARβ/δ ligands were administered prior to or after initiation of DNA damage, ligand specificity, and the time frame between ligand treatments could theoretically lead to different outcomes. The remainder of this review will focus on three critical areas and illustrate the strengths and weaknesses of each viewpoint: expression of PPARβ/δ in colon cancer, the role of PPARβ/δ in inflammation, and the effects of PPARβ/δ on apoptosis and cell survival/proliferation.
Expression
One of the first reports to suggest that PPARβ/δ was pro-tumorigenic was based on the hypotheses that PPARβ/δ was upregulated by the adenomatous polyposis coli (APC)/β-CATENIN/transcription factor 4 (TCF4) pathway during colon carcinogenesis, and that activating PPARβ/δ facilitated tumor growth by modulating a group of yet-to-be identified target genes [15]. This hypothesis was based on the inverse correlation of decreased PPARβ/δ expression and increased APC expression in a human colon cancer cell line and the reported increase in PPARβ/δ mRNA expression in four human colon tumors as compared to normal tissue [15]. At the time this hypothesis was proposed, quantitative expression patterns of PPARβ/δ in the intestine and other tissues had not been reported. This is important to note because it is now clear that expression of PPARβ/δ is highest in epithelium including small intestine and colon, and this pattern of expression is found in both human and mouse models [16-18]. The fact that PPARβ/δ is also found in the nucleus with its heterodimerization partner, retinoid X receptor-α (RXRα), also suggests that PPARβ/δ likely has a critical constitutive role in tissues such as colon and small intestine [16], possibly mediated by an endogenous ligand. Additionally, results from a number of studies that have subsequently examined expression of PPARβ/δ in human and experimental models of colon cancer have failed to provide support for the view that PPARβ/δ is increased in colon cancer cells or that PPARβ/δ is up-regulated by the APC/β-CATENIN/TCF4 pathway [19], (reviewed in [20]). For example, expression of PPARβ/δ is unchanged in human colon cancer cell lines with gain-of-function mutations in APC/β-catenin signaling, while expression of CYCLIN D1 is increased, as compared to cells with wild-type APC/β-catenin signaling [19]. More recently, the most convincing evidence to date examining expression of PPARβ/δ in human and mouse colon tumors is the finding that expression of PPARβ/δ protein is markedly lower, while expression of CYCLIN D1 is higher, in a cohort of nineteen human colon tumors as compared to matched non-transformed tissue [21]. Similarly, expression of PPARβ/δ protein is markedly lower and expression of CYCLIN D1 is higher in a cohort of nine colon tumors as compared to colonic epithelium from Apc+/Min-FCCC mice [21]. This is consistent with a report showing lower expression of mRNA encoding PPARβ/δ in colon polyps from APCmin+/- mice as compared to non-transformed colon [22], as well as many other studies (reviewed in [20,23,24]). Recent analysis of 141 colorectal cancer patients revealed markedly longer survival in patients with relatively higher expression of PPARβ/δ in primary tumors as compared to colorectal cancer patients with relatively lower of PPARβ/δ in primary tumors [25]. In fact, colorectal cancer patients with relatively lower expression of PPARβ/δ in primary cancers were ~4X as likely to die of this disease as compared to colorectal cancer patients with relatively higher expression of PPARβ/δ in primary cancers [25]. Given the number of human patients examined in this study, and the length of this retrospective study (more than 15 years), this is by far the most compelling data to date to support the hypothesis that PPARβ/δ protects, rather than promotes, colorectal carcinogenesis in humans. These combined observations have led to a stronger body of evidence indicating that expression of PPARβ/δ is reduced in colon tumors in both humans and mouse models of colon cancer (Fig. 2), as compared to the body of evidence suggesting that PPARβ/δ expression is increased during colon carcinogenesis.
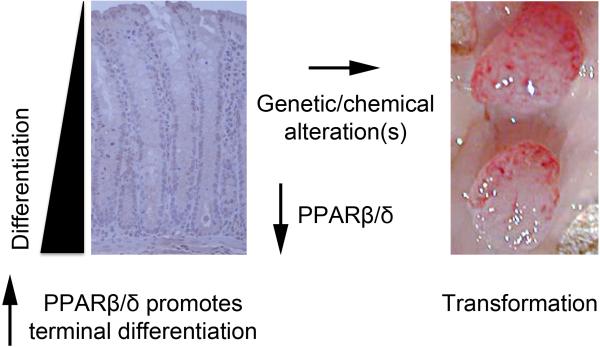
Figure 2.
Expression and function of PPARβ/δ in colon. In human and mouse colon PPARβ/δ expression is high, and found primarily in the nucleus. Nuclear PPARβ/δ in the colon can be co-immunoprecipitated with RXRα, suggesting that PPARβ/δ has an important constitutive function in the colon, likely mediated by the presence of an endogenous ligand. Based on similar evidence from multiple models, it is likely that PPARβ/δ promotes terminal differentiation of colonocytes. Expression of PPARβ/δ is reduced in transformed colon tumors and this could prevent cell cycle withdrawal associated with the induction of terminal differentiation leading to dysregulation of cell proliferation. Recent evidence showing that colorectal cancer patients with relatively low levels of PPARβ/δ are ~4X as likely to die from this disease as compared to patients expressing relatively higher levels of PPARβ/δ in primary tumors strongly support this mode of action.
Given the facts that expression of PPARβ/δ is now known to be decreased in colon tumors and that relatively lower expression of PPARβ/δ in primary colorectal tumors is associated with a higher incidence of death due to colorectal cancer, these observations support previous work showing enhanced tumorigenesis in the absence of PPARβ/δ expression [12-14,26], and inhibition of colon tumorigenesis by ligand activation of PPARβ/δ [12,14]. The possibility exists that studies suggesting tumor promoting effects of PPARβ/δ in colon cancer could also reflect a species difference. Since PPARβ/δ expression is reduced in human and mouse colon tumors, it is also curious to note that some have suggested that one mechanism by which non-steroidal anti-inflammatory drugs (NSAIDs) inhibit colon tumorigenesis is through down-regulation of PPARβ/δ expression (reviewed in [20,23,24]). This hypothesis requires considerable re-evaluation given the fact that PPARβ/δ expression is reduced in human and mouse colon tumors. However, increased expression of PPARβ/δ has also been found in human colon cancer cell lines treated with NSAIDs [21]. This suggests that increased expression and/or activation of PPARβ/δ may contribute to the chemopreventive properties associated with NSAID treatment during colon cancer.
Combined, the weight of evidence is becoming larger that PPARβ/δ is lower in human and mouse colon tumors and that relatively higher expression correlates well with better disease prognosis. These conclusions are in line with the fact that expression of PPARβ/δ is relatively high in normal colonic epithelium.
Inflammation
PPARβ/δ, much like PPARα and PPARγ, has a significant role in the function and balance of the immune system. It is no surprise that the suitability of PPARβ/δ as a pharmacological target is currently being investigated in experimental models of multiple sclerosis (e.g., experimental allergic encephalitis), diabetes, psoriasis, arthritis, and inflammatory bowel disease. Multiple studies have shown that high affinity PPARβ/δ agonists, in particular GW501516 and GW0742, attenuate innate inflammatory responses mediated by cytokines such as interleukin 1β (IL1β), tumor necrosis factor α (TNFα), and interleukin 6 (IL6) (reviewed in [27]). This results in a net decrease of downstream effector molecules such as interleukin 8 (IL8), vascular cell adhesion molecule 1/cluster of differentiation 106 (VCAM-1/CD106), intercellular adhesion molecule (ICAM), and monocyte chemotactic protein 1 (MCP1) thus reducing recruitment of immune infiltrates to areas where tissue homeostasis has been compromised. The anti-inflammatory effects of PPARβ/δ ligands are thought to be mediated through at least three different mechanisms (Fig. 3). Ligand-bound PPARβ/δ is thought to interfere with the p65 subunit of NF-κB [28-32], inhibit signal transducer and activator of transcription 3 (STAT3) signaling [33], and/or directly inhibit myeloperoxidase activity associated with neutrophils [34]. Some studies have also shown a net increase in interleukin 4 (IL4) and interleukin 10 (IL10) production after ligand activation of PPARβ/δ, suggesting additional mechanisms by which PPARβ/δ may modulate innate inflammation [35-38].
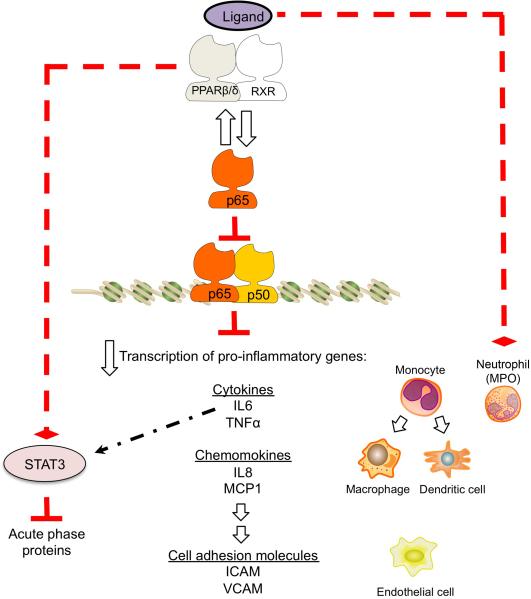
Figure 3.
Regulation of innate inflammation by PPARβ/δ. In the presence of pro-inflammatory signaling, ligand activation of PPARβ/δ can interfere with the p65 subunit of NF-κB leading to reduced expression of cytokines and chemokines that leads to reduced expression of cell adhesion molecules associated with innate inflammation. These changes are collectively found in endothelial cells, neutrophils, and monocyte-derived macrophages and dendritic cells. PPARβ/δ can also interfere with STAT3 activation, which is downstream of IL6 signaling, causing reduced expression of acute phase proteins. PPARβ/δ ligands can directly inhibit myeloperoxidase (MPO) activity associated with neutrophils typically recruited to sites of innate inflammation.
Collectively, there are more than 70 studies demonstrating beneficial effects of PPARβ/δ ligands in various inflammatory disease models (reviewed in [27]) with at least 11 reports alone published in 2010 on this subject [30,35,36,39-47]. Given the well-characterized role of PPARβ/δ in inflammation, it is somewhat surprising that to date, no studies have specifically examined the effect of PPARβ/δ-dependent modulation of inflammation on colon carcinogenesis. However, exacerbated dextran sodium sulfate (DSS)-induced colitis is found in Pparβ/δ-null mice as compared to wild-type mice [48]. While this shows that PPARβ/δ protects against inflammatory colitis, activating PPARβ/δ did not protect against DSS-induced colitis [48]. This suggests a ligand-independent anti-inflammatory effect that is mediated by PPARβ/δ interacting with other transcription factors such as NF-κB. Alternatively, it also possible that this phenotype is due in part to impaired healing or altered cellular differentiation of epithelium, since PPARβ/δ is important for modulation of the signaling pathways required for both of these processes (reviewed in [20,24]). Despite the overwhelming amount of studies demonstrating anti-inflammatory effects of PPARβ/δ and PPARβ/δ ligands, there is one report showing enhanced colitis in Il10-null mice after oral administration of GW0742 [49]. However, it is possible that the beneficial effects of GW0742 and PPARβ/δ could be mechanistically distinct and relies on both IL4 and IL10 production [38]. Collectively, there is strong evidence from multiple laboratories in many different models establishing that PPARβ/δ and PPARβ/δ ligands inhibit innate inflammation in both human and animal models, but this has not been extensively examined in inflammation-associated colon cancer models.
Apoptosis, cell survival and cell proliferation
The hypothesis that PPARβ/δ promotes anti-apoptotic activities is based on an original study using primary keratinocytes that is limited due to the experimental conditions used. Di-Poi and colleagues showed that activation of PPARβ/δ with L165041 caused decreased expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN) and increased expression of 3-phosphoinositide-dependent-protein kinase 1 (PDPK1) and integrin-linked kinase (ILK) [50]. These changes were associated with increased phosphorylation of protein kinase B (AKT) causing inhibition of apoptosis and increased cell survival [50]. However, the mouse primary keratinocyte cultures used to suggest this anti-apoptotic activity by PPARβ/δ exhibited atypical keratinocyte morphology [50] and did not express keratin 6 (K6) [51], a well-characterized marker of hyperproliferative mouse primary keratinocytes [52,53]. Using mouse primary keratinocytes that expressed K6 and exhibited normal keratinocyte morphology, it was later shown that activation of PPARβ/δ does not alter expression of PTEN, PDPK1, ILK or phosphorylated AKT [54]; an observation that was also confirmed in human keratinocyte cell lines [55]. These observations demonstrate that the putative PTEN/PDPK1/ILK/AKT anti-apoptotic signaling induced by activating PPARβ/δ is not functional in normal mouse and human keratinocytes. It is thus of interest to note that differences in the expression of these and related markers of apoptosis have also been reported in colon cancer models. Wang showed that administration of the PPARβ/δ ligand GW501516 caused increased phosphorylation of AKT in colon tumors that was associated with decreased TUNEL staining and went on to hypothesize that this was mediated by direct upregulation of vascular endothelial growth factor (VEGF) by PPARβ/δ [9]. These observations are consistent with several other studies showing increased expression of VEGF or phosphorylation of AKT, and/or inhibition of induced apoptosis in human colon cancer cell lines [8-10]. In contrast, phosphorylation of AKT and expression of VEGF is unchanged in colon polyps from mice treated with the PPARβ/δ ligand GW0742 (structurally similar to GW501516) [56]. Similarly, expression of PTEN, PDPK1, ILK and phosphorylated AKT is unchanged in colonic epithelium from wild-type or APCmin+/- mice [13]. Ligand activation of PPARβ/δ had no effect on expression of VEGF or phosphorylation of AKT in HCT116, LS174T or HT29 human colon cancer cell lines [56]. Increased measures of apoptosis (TUNEL staining) and decreased cell proliferation (BrdU labeling) were also found in mouse colonic epithelium following ligand activation of PPARβ/δ [13,14]. This is consistent with inhibition of cell proliferation observed in human colon cancer cell lines cultured in the presence of the PPARβ/δ ligands GW0742 and GW501516 [56], and enhanced cell proliferation found in HCT116, KM12C, KM12SM and KM12L4a cells when PPARβ/δ expression is knocked down [25,57]. Moreover, recent evidence from clinical colorectal tumor samples demonstrated that expression of Ki67 was markedly lower in primary cancers with relatively higher expression of PPARβ/δ as compared to primary cancers with relatively lower expression of PPARβ/δ [25]. While Ki67 expression was inversely correlated with PPARβ/δ expression, no association between PPARβ/δ expression and SURVIVIN, an anti-apoptotic protein was observed in human colorectal primary cancers [25].
The effect of PPARβ/δ on apoptosis and cell survival remains unclear because of conflicting evidence in the literature. Whereas one hypothesis suggests that activating PPARβ/δ will lead to anti-apoptotic signaling, alternative data exists suggesting that activating PPARβ/δ may promote apoptosis (reviewed in [20,23,24]). If PPARβ/δ promotes anti-apoptotic signaling in colon cancer cells, then activating PPARβ/δ when apoptosis is induced should lead to attenuated apoptotic markers (e.g. PARP cleavage, caspase activity, etc) and more importantly, an increase in the number of viable cells. This was recently examined in several human colon cancer cell lines treated with hydrogen peroxide to induce apoptosis [21]. Interestingly, ligand activation of PPARβ/δ in DLD1 cells caused a dose-dependent decrease in the number cells in the early stages of apoptosis as assessed by flow cytometry (e.g. annexin V positive, propidium iodide negative), but this change was associated with a decrease in the number of viable cells and an increase in the number cells that were either necrotic or in the late stages of apoptosis [21]. Ligand activation of PPARβ/δ did not cause an increase in the number of viable human colon cancer cells (RKO, DLD1 or HT29) when the cells were triggered to undergo apoptosis. This suggests that previous reports suggesting that PPARβ/δ promotes anti-apoptotic signaling in colon cancer cells did not effectively examine this idea because while there can be a reduction in the number of cells undergoing early stages of apoptosis, this change was associated with more cells undergoing later stages of apoptosis, but not an increase in the number of viable cells. Collectively, this raises the distinct possibility that earlier reports suggesting that PPARβ/δ promotes anti-apoptotic signaling are misleading. This also illustrates the need for studies to include more comprehensive analysis of apoptosis including the number of viable cells.
Conclusions
Preclinical and clinical data demonstrates that PPARβ/δ can promote weight loss due in part to increased oxidation of fatty acids in skeletal muscle, improve dyslipidemias by increasing serum HDL cholesterol and decreasing serum LDL and triglycerides, improve insulin resistance by reducing hyperglycemia, promote terminal differentiation in multiple cell types, and inhibit many pro-inflammatory signaling pathways [20,24,27,58-60]. These features of PPARβ/δ ligands are the basis for the interest in developing agonists for the treatment or prevention of metabolic syndrome. Since many of the phenotypes associated with metabolic syndrome including obesity, dyslipidemias, insulin insensitivity and chronic inflammation are negatively associated with colon cancer [61-65], and ligand activation of PPARβ/δ can clearly inhibit or prevent these phenotypes based on evidence from preclinical and clinical studies, it is curious why it has been hypothesized that activating PPARβ/δ promotes colon tumorigenesis.
The hypothesis that PPARβ/δ is upregulated by the APC/β-CATENIN/TCF4 pathway during colon carcinogenesis, and that activating PPARβ/δ facilitates tumor growth by modulating a group of unknown target genes as proposed more than ten years ago [15] is not supported strongly by many reports published since this time. For example, PPARβ/δ expression is not increased in human colon cancer cell lines when the activity of APC/β-CATENIN/TCF4 is altered [19], and many studies failed to observe increased expression of PPARβ/δ in colon cancer models (reviewed in [20,24]). The evidence is becoming more convincing that expression of PPARβ/δ is lower in mouse and human colon tumors [17,18,21]. Moreover, the most compelling evidence to date is the study showing that colorectal cancer patients with relatively lower expression of PPARβ/δ in primary cancers were ~4X as likely to die of this disease as compared to colorectal cancer patients with relatively higher expression of PPARβ/δ in primary cancers [25]. While the evidence is becoming stronger that relatively higher expression of PPARβ/δ is important for colorectal cancer patient survival and that activating PPARβ/δ may prevent colon tumorigenesis, there remains a need for future studies to quantitatively examine PPARβ/δ expression at the protein level. The related hypothesis that NSAIDs down-regulate PPARβ/δ expression allowing for increased apoptosis has not been verified by independent laboratories and is inconsistent with the known expression pattern of PPARβ/δ in normal and diseased colon. Since the PPARβ/δ-dependent changes in 14-3-3ε signaling hypothesized by one laboratory to explain the chemopreventive effects of NSAIDs [66-69], have not been verified [21], this shows why other laboratories should critically evaluate this hypothesis. Indeed, NSAIDs have also been shown to increase expression of PPARβ/δ in human colon cancer cell lines [21], which supports the hypothesis that NSAIDs may function as chemopreventive agents because of this change, an hypothesis that is also supported by the observed decrease in PPARβ/δ expression found in mouse and human colon tumors.
It has also been suggested that PPARβ/δ antagonists may be suitable for chemoprevention of colon cancer based in large part on results from mouse models showing PPARβ/δ-dependent enhancement of colon tumorigenesis [9,11]. However, this idea is not supported by the recent findings that expression of PPARβ/δ is down-regulated in human and mouse colon tumors [21] or that survival of colorectal cancer patients is markedly improved in patients with relatively higher expression of PPARβ/δ in primary cancers as compared to patients with relatively lower expression of PPARβ/δ in primary cancers [25]. Additionally, many studies have also observed decreased expression of PPARβ/δ at the mRNA and/or protein level, or that ligand activation of PPARβ/δ inhibits colon tumorigenesis in mouse models and/or proliferation of human colon cancer cell lines (reviewed in [20,24]). It remains unclear why some studies show that PPARβ/δ promotes colon tumorigenesis while others do not. However, it is important to note that a specific PPARβ/δ antagonist does not modulate proliferation of SW480, HCT116, DLD1 or RKO human colon cancer cell lines at concentrations that specifically antagonize PPARβ/δ [70]. This suggests that targeting PPARβ/δ antagonists for colon cancer chemoprevention is not a viable strategy. Further, since PPARβ/δ has so many critical functional roles in the regulation of glucose/lipid homeostasis, inflammation and cellular differentiation, the notion that a PPARβ/δ antagonist would be suitable for colon cancer chemoprevention is likely not feasible.
In summary, significant progress has been made in the last ten years since the original hypothesis that PPARβ/δ promotes colon tumorigenesis was postulated. While some studies continue to report results that support this hypothesis, many others do not (reviewed in [20,24]). Given the great potential of targeting PPARβ/δ for the treatment/prevention of metabolic disease, there is a need to determine whether this is possible. Until a consensus can be reached on the role of PPARβ/δ in colon cancer, the development of small molecule agonists of PPARβ/δ as therapeutics will be hampered. However, recent clinical findings showing greatly enhanced survival of colorectal cancer patients with relatively higher expression of PPARβ/δ in primary cancers as compared colorectal patients with relatively lower expression of PPARβ/δ provides the most compelling evidence to date that PPARβ/δ prevents, rather than promotes, colorectal cancer and that targeting this receptor may be feasible. Based on this and many other findings in the past ten years has led to a larger body of evidence supporting the hypothesis that PPARβ/δ prevents colon cancer.
Acknowledgements
Our research is supported by the National Institutes of Health (CA124533, CA126826, CA141029, CA140369, AA018863) J.M.P., and the National Cancer Institute Intramural Research Program (ZIABC005561, ZIABC005562, ZIABC005708) F.J.G..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Peters JM, et al. Peroxisome proliferator-activated receptor-α and liver cancer: where do we stand? J Mol Med. 2005;83:774–785. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 3.Chaggar PS, et al. Review article: Thiazolidinediones and heart failure. Diab Vasc Dis Res. 2009;6:146–152. doi: 10.1177/1479164109338772. [DOI] [PubMed] [Google Scholar]
- 4.Billin AN. PPAR-β/δ agonists for Type 2 diabetes and dyslipidemia: an adopted orphan still looking for a home. Expert Opin Investig Drugs. 2008;17:1465–1471. doi: 10.1517/13543784.17.10.1465. [DOI] [PubMed] [Google Scholar]
- 5.Berger J, et al. Novel peroxisome proliferator-activated receptor (PPAR) γ and PPARδ ligands produce distinct biological effects. J Biol Chem. 1999;274:6718–6725. doi: 10.1074/jbc.274.10.6718. [DOI] [PubMed] [Google Scholar]
- 6.Sznaidman ML, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor δ (PPARδ)-synthesis and biological activity. Bioorg Med Chem Lett. 2003;13:1517–1521. doi: 10.1016/s0960-894x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 7.Barak Y, et al. Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RA, et al. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nat Med. 2004;10:245–247. doi: 10.1038/nm993. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, et al. Crosstalk between peroxisome proliferator-activated receptor δ and VEGF stimulates cancer progression. Proc Natl Acad Sci U S A. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Zuo X, et al. Targeted genetic disruption of peroxisome proliferator-activated receptor-δ and colonic tumorigenesis. J Natl Cancer Inst. 2009;101:762–767. doi: 10.1093/jnci/djp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harman FS, et al. Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nat Med. 2004;10:481–483. doi: 10.1038/nm1026. [DOI] [PubMed] [Google Scholar]
- 13.Marin HE, et al. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) inhibits colon carcinogenesis. Cancer Res. 2006;66:4394–4401. doi: 10.1158/0008-5472.CAN-05-4277. [DOI] [PubMed] [Google Scholar]
- 14.Hollingshead HE, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) and inhibition of cyclooxygenase 2 (COX2) attenuate colon carcinogenesis through independent signaling mechanisms. Carcinogenesis. 2008;29:169–176. doi: 10.1093/carcin/bgm209. [DOI] [PubMed] [Google Scholar]
- 15.He TC, et al. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girroir EE, et al. Quantitative expression patterns of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) protein in mice. Biochem Biophys Res Commun. 2008;371:456–461. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berglund L, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–2027. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Uhlen M, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4:1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Foreman JE, et al. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol Carcinog. 2009;48:942–952. doi: 10.1002/mc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters JM, et al. Role of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foreman JE, et al. Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer. Mol Carcinog. 2011;50:884–900. doi: 10.1002/mc.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LC, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 23.Burdick AD, et al. The role of peroxisome proliferator-activated receptor-β/δ in epithelial cell growth and differentiation. Cell Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–241. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, et al. Biological Function and Prognostic Significance of Peroxisome Proliferator-Activated Receptor δ in Rectal Cancer. Clin Cancer Res. 2011;17:3760–3770. doi: 10.1158/1078-0432.CCR-10-2779. [DOI] [PubMed] [Google Scholar]
- 26.Reed KR, et al. PPARδ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004;23:8992–8996. doi: 10.1038/sj.onc.1208143. [DOI] [PubMed] [Google Scholar]
- 27.Kilgore KS, Billin AN. PPARβ/δ ligands as modulators of the inflammatory response. Curr Opin Investig Drugs. 2008;9:463–469. [PubMed] [Google Scholar]
- 28.Alvarez-Guardia D, et al. PPARβ/δ activation blocks lipid-induced inflammatory pathways in mouse heart and human cardiac cells. Biochim Biophys Acta. 2011;1811:59–67. doi: 10.1016/j.bbalip.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Barroso E, et al. The peroxisome proliferator-activated receptor β/δ (PPARβ/δ) agonist GW501516 prevents TNF-α-induced NF-κB activation in human HaCaT cells by reducing p65 acetylation through AMPK and SIRT1. Biochem Pharmacol. 2011;81:534–543. doi: 10.1016/j.bcp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Coll T, et al. Activation of peroxisome proliferator-activated receptor-δ by GW501516 prevents fatty acid-induced nuclear factor-kB activation and insulin resistance in skeletal muscle cells. Endocrinology. 2010;151:1560–1569. doi: 10.1210/en.2009-1211. [DOI] [PubMed] [Google Scholar]
- 31.Planavila A, et al. Peroxisome proliferator-activated receptor β/δ activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;65:832–841. doi: 10.1016/j.cardiores.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Calvo R, et al. Activation of Peroxisome Proliferator-Activated Receptor β/δ (PPARβ/δ) Inhibits LPS-induced Cytokine Production in Adipocytes by Lowering NF-κB Activity via ERK1/2. Diabetes. 2008;57:2149–2157. doi: 10.2337/db08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kino T, et al. The PPARδ agonist GW501516 suppresses interleukin-6-mediated hepatocyte acute phase reaction via STAT3 inhibition. Eur J Clin Invest. 2007;37:425–433. doi: 10.1111/j.1365-2362.2007.01796.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim DJ, et al. PPARβ/δ selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- 35.Dunn SE, et al. Peroxisome proliferator-activated receptor δ limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J Exp Med. 2010;207:1599–1608. doi: 10.1084/jem.20091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanakasabai S, et al. Peroxisome proliferator-activated receptor δ agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology. 2010;130:572–588. doi: 10.1111/j.1365-2567.2010.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanakasabai S, et al. PPARδ deficient mice develop elevated Th1/Th17 responses and prolonged experimental autoimmune encephalomyelitis. Brain Res. 2011;1376:101–112. doi: 10.1016/j.brainres.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 38.Mukundan L, et al. PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collino M, et al. Peroxisome proliferator-activated receptor β/δ agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic Biol Med. 2011;50:345–353. doi: 10.1016/j.freeradbiomed.2010.10.710. [DOI] [PubMed] [Google Scholar]
- 40.Di Paola R, et al. GW0742, a high-affinity PPAR-β/δ agonist, inhibits acute lung injury in mice. Shock. 2010;33:426–435. doi: 10.1097/SHK.0b013e3181b8f2fb. [DOI] [PubMed] [Google Scholar]
- 41.Di Paola R, et al. GW0742, a selective PPAR-β/δ agonist, contributes to the resolution of inflammation after gut ischemia/reperfusion injury. J Leukoc Biol. 2010;88:291–301. doi: 10.1189/jlb.0110053. [DOI] [PubMed] [Google Scholar]
- 42.Glatz T, et al. Peroxisome-proliferator-activated receptors γ and peroxisomeproliferator-activated receptors β/δ and the regulation of interleukin 1 receptor antagonist expression by pioglitazone in ischaemic brain. J Hypertens. 2010;28:1488–1497. doi: 10.1097/HJH.0b013e3283396e4e. [DOI] [PubMed] [Google Scholar]
- 43.Kapoor A, et al. Activation of peroxisome proliferator-activated receptor-β/δ attenuates myocardial ischemia/reperfusion injury in the rat. Shock. 2010;34:117–124. doi: 10.1097/SHK.0b013e3181cd86d6. [DOI] [PubMed] [Google Scholar]
- 44.Kapoor A, et al. Protective Role of Peroxisome Proliferator-Activated Receptor-β/δ in Septic Shock. Am J Respir Crit Care Med. 2010;182:1506–1515. doi: 10.1164/rccm.201002-0240OC. [DOI] [PubMed] [Google Scholar]
- 45.Liang YJ, et al. Peroxisome proliferator-activated receptor δ agonists attenuated the C-reactive protein-induced pro-inflammation in cardiomyocytes and H9c2 cardiomyoblasts. Eur J Pharmacol. 2010;643:84–92. doi: 10.1016/j.ejphar.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Stockert J, et al. Reverse crosstalk of TGFβ and PPARβ/δ signaling identified by transcriptional profiling. Nucleic Acids Res. 2011;39:119–131. doi: 10.1093/nar/gkq773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingarelli B, et al. Peroxisome proliferator-activated receptor δ regulates inflammation via NF-kB signaling in polymicrobial sepsis. Am J Pathol. 2010;177:1834–1847. doi: 10.2353/ajpath.2010.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollingshead HE, et al. PPARβ/δ Protects Against Experimental Colitis Through a Ligand-Independent Mechanism. Dig Dis Sci. 2007;52:2912–2919. doi: 10.1007/s10620-006-9644-9. [DOI] [PubMed] [Google Scholar]
- 49.Lee JW, et al. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133:108–123. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 50.Di-Poi N, et al. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Molecular Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 51.Tan NS, et al. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roop DR, et al. Regulated expression of differentiation-associated keratins in cultured epidermal cells detected by monospecific antibodies to unique peptides of mouse epidermal keratins. Differentiation. 1987;35:143–150. doi: 10.1111/j.1432-0436.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 53.Tyner AL, Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J Cell Biol. 1986;103:1945–1955. doi: 10.1083/jcb.103.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burdick AD, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007;19:1163–1171. doi: 10.1016/j.cellsig.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borland MG, et al. Ligand Activation of Peroxisome Proliferator-Activated Receptor-β/δ (PPARβ/δ) Inhibits Cell Proliferation in Human HaCaT Keratinocytes. Mol Pharmacol. 2008;74:1429–1442. doi: 10.1124/mol.108.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hollingshead HE, et al. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007;28:2641–2649. doi: 10.1093/carcin/bgm183. [DOI] [PubMed] [Google Scholar]
- 57.Yang L, et al. RNA Interference Against Peroxisome Proliferator-Activated Receptor δ Gene Promotes Proliferation of Human Colorectal Cancer Cells. Dis Colon Rectum. 2008;51:318–328. doi: 10.1007/s10350-007-9145-8. [DOI] [PubMed] [Google Scholar]
- 58.Grimaldi PA. Regulatory functions of PPARβ in metabolism: implications for the treatment of metabolic syndrome. Biochim Biophys Acta. 2007;1771:983–990. doi: 10.1016/j.bbalip.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Lee CH, et al. PPARδ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi S, et al. New therapeutic target for metabolic syndrome: PPARδ. Endocr J. 2007;54:347–357. doi: 10.1507/endocrj.kr-99. [DOI] [PubMed] [Google Scholar]
- 61.Pais R, et al. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol. 2009;15:5141–5148. doi: 10.3748/wjg.15.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prizment AE, et al. Survival of women with colon cancer in relation to precancer anthropometric characteristics: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2229–2237. doi: 10.1158/1055-9965.EPI-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terzic J, et al. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 64.Tsugane S, Inoue M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. 2010;101:1073–1079. doi: 10.1111/j.1349-7006.2010.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolin KY, et al. Obesity and cancer. Oncologist. 2011;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liou JY, et al. Nonsteroidal anti-inflammatory drugs induce colorectal cancer cell apoptosis by suppressing 14-3-3ε. Cancer Res. 2007;67:3185–3191. doi: 10.1158/0008-5472.CAN-06-3431. [DOI] [PubMed] [Google Scholar]
- 67.Liou JY, et al. Protection of endothelial survival by peroxisome proliferator-activated receptor-δ mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481–1487. doi: 10.1161/01.ATV.0000223875.14120.93. [DOI] [PubMed] [Google Scholar]
- 68.Liou JY, et al. Nonsteroidal anti-inflammatory drugs induced endothelial apoptosis by perturbing peroxisome proliferator-activated receptor-δ transcriptional pathway. Mol Pharmacol. 2008;74:1399–1406. doi: 10.1124/mol.108.049569. [DOI] [PubMed] [Google Scholar]
- 69.Wu KK, Liou JY. Cyclooxygenase inhibitors induce colon cancer cell apoptosis Via PPARδ --> 14-3-3ε pathway. Methods Mol Biol. 2009;512:295–307. doi: 10.1007/978-1-60327-530-9_16. [DOI] [PubMed] [Google Scholar]
- 70.Shearer BG, et al. Identification and Characterization of 4-Chloro-N-(2-{[5-trifluoromethyl)-2-pyridyl]sulfonyl}ethyl)benzamide (GSK3787), a Selective and Irreversible Peroxisome Proliferator-Activated Receptor δ (PPARδ) Antagonist. J Med Chem. 2010;53:1857–1861. doi: 10.1021/jm900464j. [DOI] [PubMed] [Google Scholar]