Abstract
Understanding the relative toxicities of different modes of nanoparticle exposure as compared with their dissolved metal ions are emerging areas in ecotoxicology. Here we report on bioavailability, toxicity, and bioaccumulation of carboxyl-functionalized CdSe/ZnS quantum dots (QDs) to the amphipod Leptocheirus plumulosus exposed to equivalent Cd concentrations via dissolved Cd, QDs in water or QDs in algal food. Both modes of QD exposure were accumulated to greater extent than dissolved Cd. Exposure to QDs via algae resulted in high amphipod mortality. Cadmium and Se in amphipods exposed to QDs in water were highly correlated and spatially localized within the amphipod. In contrast, when exposed to QDs via algae the metals were more disperse and not highly correlated suggesting QD dissolution and resultant metal ion toxicity. This study suggests QDs are accumulated to a greater extent than the dissolved ion and could lead to trophic transfer. QDs ingested with algae are bioavailable and result in toxicity which is not observed in the absence of algae.
Introduction
The technological advances associated with nanomaterials and their many applications have resulted in the introduction of metal contaminants in the form of nanoparticles with unknown consequences to natural ecosystems. Understanding the physical and ecotoxicological properties of nanoparticles in natural aquatic systems is critical to developing adequate regulatory structures to protect aquatic ecosystems from their detrimental effects. An increasing number of studies have demonstrated both sub-lethal and toxic effects of engineered nanoparticles (ENPs) to bacteria,1–4 invertebrates,5,6 fish7 and mammals.8 Fewer studies have investigated the bioavailability and effects of ENPs on primary and secondary consumers in aquatic food webs. Moreover, the extent to which benthic vs. pelagic organisms will be exposed to ENPs is a critical knowledge gap.9 Evaluation of potential exposures of ecosystems to ENPs involves aspects of biological fate as well as toxicity. The biological fate of contaminants in aquatic ecosystems results from uptake and assimilation from both food and water by pelagic and benthic organisms.10–15 Our own studies and those of others have shown that Cd, As, and Hg are taken up by Leptocheirus plumulosus, and Fundulus heteroclitus, via food and water.16–18 Uptake via food accounts for up to 85% of uptake of Cd by L. plumulosus and toxicity increases with bioaccumulation. Ingestion of food and sediments was also found to be the principal uptake of Ag by marine invertebrates.19 Past studies of metal contaminants in lake food webs have shown that certain metals biomagnify (Hg, Zn), others biodiminish (Pb, As) while Cd increases from algae to zooplankton and decreases to fish.20–23
To date few studies have investigated trophic transfer of metal-based ENPs.9,24 In a planktonic food web, Holbrook et al.24 studied uptake and trophic transfer of carboxylated and biotinylated QDs to bacteria (E. coli), ciliates (T. pyriformis) and rotifers (B. calyciflorus). Trophic transfer of QDs between bacteria and ciliates was not observed but ciliates did accumulate QDs from the water column. Trophic transfer of QDs between ciliates and rotifers occurred but no biomagnification by the rotifers was observed. Bouldin et al.6 studied trophic transfer of QDs from the algae P. subcapitata to C. dubia. An increase in characteristic fluorescence for C. dubia fed with the highest dosed algal treatment indicated uptake of the QDs; however, it was not clear whether the QDs were assimilated by the higher organism. QDs biomagnified in a microbial food chain to a greater extent than an equivalent concentration of dissolved Cd.25 Additionally, QDs remained intact in the predator and therefore available to higher trophic levels.
Uptake of ENPs by aquatic organisms will be largely dependent on the feeding mode of the organism (e.g. filter feeding, grazing, predation) as well as the ultimate form of ENPs in the aqueous medium resulting from processes of dissolution or aggregation. The extent to which these particles will be available to benthic feeders has not been investigated.9 For filter feeders the distance between the setae of filtering appendages determines the range of particle size which are most efficiently captured.26 In most cases, the larger the particle size the more readily it is consumed. Thus, conditions which favor aggregation of ENPs may favor ingestion by particle feeders. Ward and Kach5 have recently shown that suspension feeders such as mussels and oysters ingest aggregated ENPs to a much greater extent than single ENPs. Aquatic invertebrates themselves may influence the particle size distribution of inorganic colloids in the water column27 which may influence the bioavailability of ENPs.
In this study we report on the bioavailability of QDs to the amphipod L. plumulosus by comparing the effects of exposure via algal food (algae, Isochrysis galbana) with exposure directly from water. We hypothesize that uptake of QDs by the algae may result in an increase in bioavailability to L. plumulosus due to either ingestion of algal cells containing QDs or aggregation of QDs into ingestible particles. This would result in increasing body burden and, potentially, toxicity.
Materials and Methods
Quantum Dots
Quantum dots were purchased from Invitrogen (Eugene, OR) in 250 µl amounts. We used the 655 nm (florescence emission wavelength) QDs surface functionalized by carboxyl polymers which results in an overall particle size (including the polymer coating) of 15–20 nm. We were unable to learn more specific details on the nature of this carboxyl polymer. These QDs are CdSe cores with a ZnS outer layer. The QDs are shipped in 50mM borate solution at a stated concentration of 8 µM QDs (particle number) and 2mM total Cd concentration. As shown in Table 1 and discussed later, dissolved Cd is essentially zero in the QDs as supplied by the manufacturer. We diluted these QDs by 1000X and measured zeta potential (Malvern NanoZS, Malvern, UK) as −29 ± 7 mV.
Table 1.
Concentration of Cd, Se, Zn in QDs suspended in DI water, in 20 ppt saline with algae and 20 ppt saline without algae after 24 hrs and in the amphipod exposures at 96 hrs.
| QDs suspended in DI | QDs and algae in saline @ 24 hrs | QDs in saline @ 24 hrs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn (mg/l) |
Se (mg/l) |
Cd (mg/l) |
Zn (mg/l) |
Se (mg/l) |
Cd (mg/l) |
Zn (mg/l) |
Se (mg/l) |
Cd (mg/l) |
|
| Unfiltered | 0.77 | 0.04 | 3.26 | 0.30 | 0.29 | 1.04 | 0.57 | 0.10 | 2.55 |
| < 650nm | 0.73 | 0.02 | 3.55 | 0.14 | 0.03 | 0.05 | 0.55 | 0.07 | 2.57 |
| <100nm | 0.71 | 0.02 | 3.50 | 0.14 | 0.03 | 0.03 | 0.55 | 0.07 | 2.52 |
| < 3KDa | 0.00 | 0.00 | 0.00 | 0.13 | 0.03 | 0.02 | 0.20 | 0.04 | 0.18 |
| amphipod exposures @ 96 hrs | |||||||||
| QDs and Algae in saline | QDs in saline | ||||||||
| Zn (mg/l) |
Se (mg/l) |
Cd (mg/l) |
Zn (mg/l) |
Se (mg/l) |
Cd (mg/l) |
||||
| Unfiltered | 0.03 | 0.01 | 0.03 | 0.04 | 0.01 | 0.05 | |||
| < 650nm | 0.03 | 0.00 | 0.01 | 0.03 | 0.00 | 0.02 | |||
| <100nm | 0.03 | 0.00 | 0.01 | 0.05 | 0.00 | 0.02 | |||
| < 3KDa | 0.03 | 0.00 | 0.01 | 0.02 | 0.01 | 0.02 | |||
Exposures
We conducted a number of exposure assays over the course of this study which all followed the same basic procedure. All treatment exposures were performed in triplicate. Leptocheirus plumulosus were cultured in the laboratory using standard methods.28 Isochrysis galbana was cultured in f/2 medium.29 All cultures and tests used 20 parts per thousand (ppt) artificial seawater prepared with Instant Ocean® (20 g of Instant Ocean salts dissolved in 1 L of deionized water) and were housed in a temperature controlled room at 20 °C with a 16:8 h light:dark photoperiod. pH of these waters was 7.4 – 8.0, hardness was 3660 ppm CaCO3 and ionic strength was 0.34 M. For each test, adult amphipods were harvested by passing mixed-aged cultures through a 2 mm sieve and collecting animals retained on a 1 mm sieve. These animals were allowed to depurate for 4 h before being added to test chambers. A small volume (50 µl in all instances except the ‘LC50’ test which used a range of volumes) of the QD suspension was added to 100 ml of I. galbana culture and allowed to incubate overnight. An equivalent volume of QD suspension was added to 100 mL of 20 ppt seawater. Ten mL of these algal-QD or aqueous-QD suspensions were added to glass test chambers containing 150 mL 20 ppt seawater and 20 L. plumulosus individuals (except the LC50 test which used 10 individuals); this 10 mL addition was repeated at 24 and 48 hours. For some exposure assays, a concentration of dissolved Cd (as CdCl2) equivalent to the total Cd of the QD exposures was included as a treatment. Exposure tests included equivalent control exposures (i.e. algae or no algae but no QDs or aqueous Cd) and survival in these treatments was > 90%. All exposure assays were assessed after 96 h; mortality was defined as inability to move when prodded. Except for the first exposure assay, surviving amphipods were removed from test chambers, rinsed in 3 successive baths of 20 ppt seawater, and transferred to glass chambers containing 20 ppt seawater for a 24 h depuration period before being freeze-dried for further analytical characterization. LC50 values with 95% confidence intervals were calculated for pooled survival data by probit analysis using U.S. EPA probit (Ver 1.5).
We calculated free Cd2+ activity in the exposures using major cation and anion data for Instant Ocean (www.instantocean.com) at the 20 ppt salinity in our exposures. We used the chemical equilibrium modeling software Visual Minteq (http://www2.lwr.kth.se/english/). We assume the calculated activity can be expressed as a molarity and convert this to an equivalent µg l−1 as these units are often used to express LC50 values.
Particle size analysis of algal-QD and aqueous-QD solutions
Aliquots of the algal-QD and aqueous-QD solutions were filtered through centrifuge filters of 0.65 µm, 0.1 µm and 3KDa (Millipore, Billerica, MA). After acid digestion, the concentration of Cd, Zn and Se in the filtrates and the unfiltered solution were determined by ICP-MS (Agilent 7500cx, Santa Clara, CA). We tested retention of ions by the spin filters by running true dissolved solutions of Zn, Se and Cd ions, prepared from dilution of single element ICP-MS calibration standards to 1, 1 and 4 mg L−1, respectively, through each of the individual filters. 100% recovery for each ion was recorded in the filtrate from each spin filter.
Dissolved organic carbon (Tekmar Dohrmann Apollo 9000, Teledyne, Tekmar, Mason, OH) was measured in selected algal and amphipod treatments at the beginning and end of the amphipod exposures.
Analytical characterization was conducted for total concentration and spatial distribution of QDs in L. plumulosus. Total body burden of QDs was assessed by determining the Cd, Zn and Se concentration of the organisms. The organisms were acid digested in open vessels at 105°C followed by analysis of the diluted digestate by ICP-MS. Spatial distribution of Cd, Zn, Se and Ca was assessed by 2D elemental mapping of these elements within the whole organism using laser ablation (New Wave UP213, Freemont CA) coupled to ICP-MS (Agilent 7500cx, Santa Clara, CA). Multiple exposed organisms were formalin fixed, alcohol dehydrated and embedded in paraffin at the Dartmouth Medical School Histology laboratory. The samples were then sectioned at 20 µm and mounted on microscope slides for LA-ICP-MS analysis. For LA-ICP-MS an approximately 1 cm2 area of the section was analyzed and was selected to include sections from at least four individuals from each treatment. The analysis was conducted as a series of line scans 40 µm apart. The laser spot size was 40 µm and scan speed was 80 µm sec−1 for each line scan. The ICP-MS was operated in reaction mode with H2 as the reaction gas at a flow rate of 2.5 ml min−1; this is optimum for Se analysis and was not detrimental to the Cd or Zn signal response. A data file was collected for each line scan in time resolved mode with time points at 500 msec intervals. Data was exported as time vs. response files which were then concatenated and edited to include Y positional information and replace the time variable with the equivalent X positional variable (each time data point corresponded to 40 µm in the X direction). The data was then opened in the SMAK software package30 for image processing and data analysis.
Statistical analysis
All statistical analysis was performed in JMP ver 9.0.2 (SAS Institute, Inc, Cary, NC). Standard means comparisons were conducted by ANOVA and Tukeys HSD test.
Results and Discussion
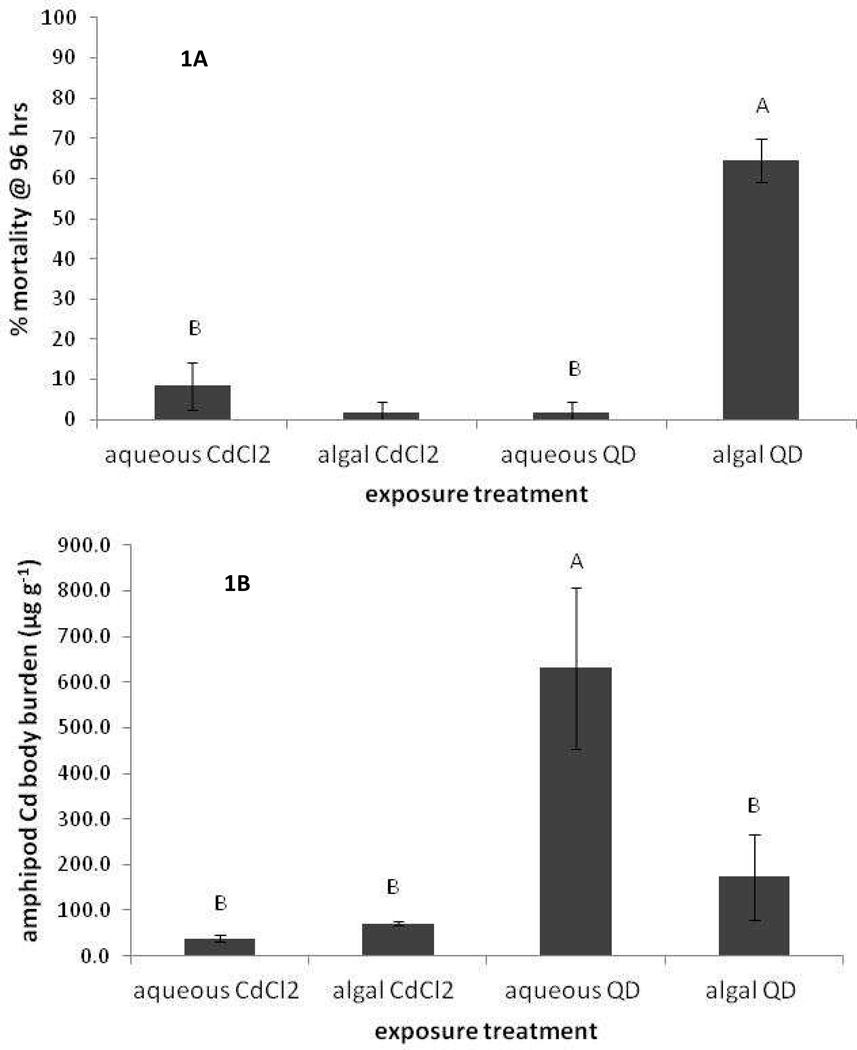
Our initial exposure assay was conducted at one exposure concentration of QDs equivalent to 3.6 ppm Cd in the algal and aqueous (saline) suspensions, which would result in a final exposure concentration of 543 µg l−1 total Cd to the amphipods (30 ml of algal or aqueous suspension added to the initial 150 ml volume for the amphipod exposure) assuming all the QDs remained in suspension in the algal and aqueous treatments. Our algal exposure concentrations are similar in total Cd concentration to another recent study focused on CdTe/CdS bioaccumulation to Chlamydomonas reinhardti.31 While all the metal(oid)s, Cd, Zn and Se, which constitute the QD are known to be toxic to aquatic organisms in the dissolved form, we focus on Cd because it is the most acutely toxic. Additionally, Cd concentration in the QDs is 58mM, compared with 23 mM Zn and 1 mM Se. Dissolved Cd LC50 values for L. plumulosus in 15 ppt salinity water have been reported as 880 µg l−1 which we calculate equates to a free Cd2+ activity of 26 µg l−1, and our exposure concentration was below this LC50 for Cd2+ activity. We contrasted the QD exposures via algal and aqueous solution with equivalent dissolved Cd treatments via algal and aqueous exposures, assuming the ‘worst case scenario’ that all Cd in the QDs would be bioavailable. The mortality and Cd body burden results are shown in Figure 1A and 1B, respectively. For mortality a significant difference between the treatments was observed (p < 0.001) with the algal-QD treatment having greater mortality than the others. When amphipods were exposed to equivalent QD concentrations without the algae or aqueous Cd2+ with or without algae essentially zero mortality was observed (Figure 1A). For the aqueous Cd2+ treatments the high Cl levels of saline test solutions would lead to significant ion pairing of Cd, thus a reduction in free Cd2+ concentrations. Additionally the high ionic strength of the test solutions would further reduce the free Cd2+ activity; we calculate the Cd2+ activity in these exposures to be ca. 11.5 µg l−1, less than half the value calculated above for the LC50 at 15 ppt salinity. Hence, it is perhaps unsurprising that no mortality was observed for the Cd2+ treatments. The difference in mortality between the aqueous-QD (no mortality) and algal-QD treatments (64% mortality) was unexpected and clearly points to a difference in toxicity between these two treatments. It also seems clear that the toxicity of the algal-QD treatment is not related to dissolved Cd (or Se or Zn) as the free Cd2+ activity in the water column is < 0.5 µg l−1 based on the total dissolved Cd shown in table 1.
Figure 1.
1A L. plumulosus mortality; and 1B, Cd concentration in L. plumulosus (µg g−1 DW) after 96 hr exposures. Error bars are one standard deviation. Treatments with the same letter are not significantly different. There were 3 replicate exposures per treatment and 20 animals per replicate.
The mortality results are not explained simply by uptake as the body burden of Cd in amphipods is significantly higher (p < 0.0003) in the aqueous-QD exposure (630 µg g−1) than the algal-QD treatments (Figure 1B). Previous work by our group has shown that when D. magna are exposed to QDs in aqueous solution the QDs are not assimilated within the organism but are concentrated in the gut.33 Hence we repeated the amphipod exposures but allowed the animals to depurate for 24 hours and monitored Cd body burden over this time period (supplemental Figure 1). Again in this assay ‘Cd uptake’ by organisms exposed to aqueous-QDs was greater than the algal-QD treatment and both of these were much greater than their aqueous Cd2+ equivalents. Body burdens in the aqueous-QD treatment decreased rapidly and by 24 hrs they were not significantly different than the algal-QD treatment although the aqueous-QD treatment remained significantly higher than the Cd2+ exposures at 24 hrs.
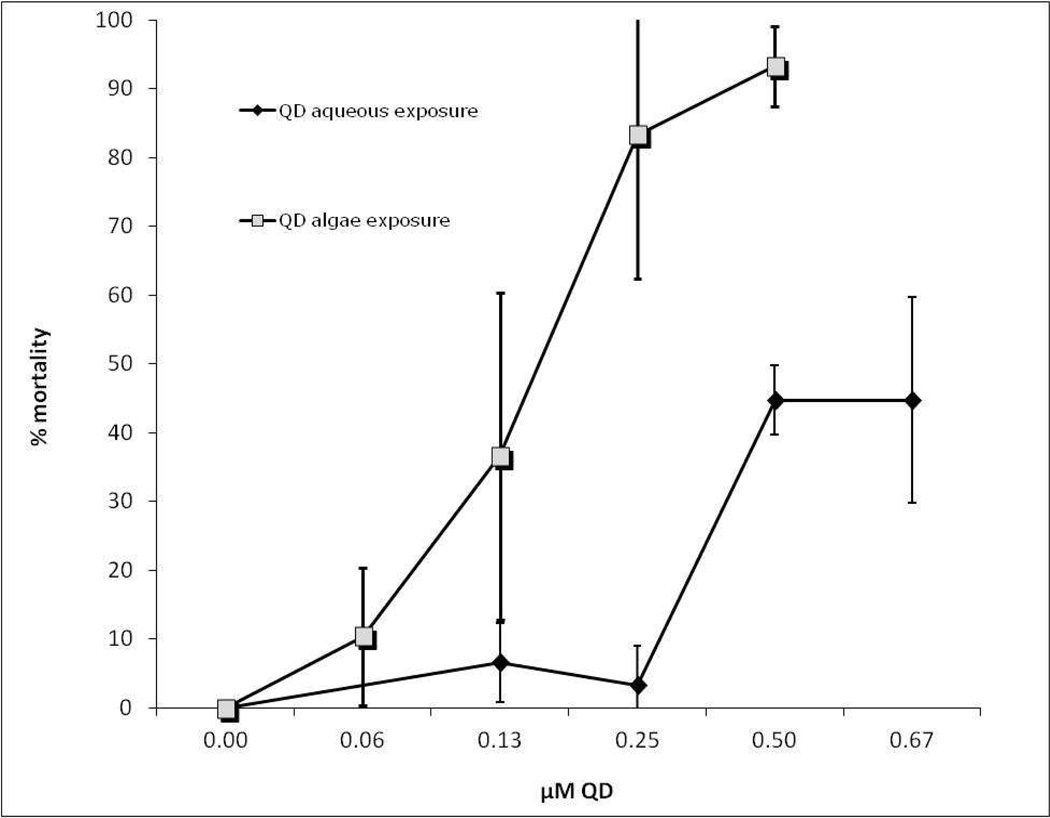
The difference in toxicity between the two exposure treatments is clearly evidenced in Figure 2 where the dose response of L. plumulosus to QD concentration was measured and the LC50 for the algal and aqueous exposures calculated. LC50 values were calculated based on the manufacturer provided concentration of QDs of 8 µM (particle number) in the stock suspension. The LC50 values calculated by the EPA probit software (http://www.epa.gov/eerd/stat2.htm), for the algal and aqueous exposures were statistically different from each other and were 0.61 nM (95% CI, 0.48 – 0.77) for algal exposure and 2.93 nM (95% CI, 2.2 – 5.45) for aqueous exposure, although it should be noted that 50% mortality was not achieved in the aqueous-QD treatment and the confidence intervals around the calculated LC50 are quite large. However, there is clear a dose/response relationship for both treatments and difference in toxicity between the treatments.
Figure 2.
Mortality data for L. plumulosus exposed to increasing concentrations of QD via algae and in the absence of algae. There were 3 replicate exposures per treatment and 10 animals per replicate.
The acute toxicity data indicate a clear difference in toxicity between the QD exposure via algae and exposure via the water column to L. plumulosus. However, it is not clear that this is a result of trophic transfer as body burden of QDs is greater in the aqueous than the algal exposure. We hypothesized that differences in particle size could be affecting the observed difference in mortality between the two treatments. ENPs have been shown to bind the surface of algal cells34 and the resultant particle size of these algal cell/QD aggregates may cause them to be effectively assimilated compared to dispersed QDs.5 However, because the exposure assays were conducted in solutions of 20 ppt salinity it is possible that aggregation may also have been favored in the aqueous-QD exposures.
We examined the effective particle size range in each of the treatment exposures by filtering aliquots through 0.65 µm, 0.1 µm or 3 KDa centrifuge filters. This was done for the algal-QD exposure and the aqueous-QD (saline) exposure and, for comparison with a truly dispersed system, an equivalent volume of QDs dispersed in deionized water. We also determined this size fractionation scheme in the water column for the two L. plumulosus QD exposure treatments at 96 hrs. Results for the filtration at different particle sizes are presented in Table 1. For QDs suspended in deionized water essentially no ions passed through the 3KDa filter while 100% passed through the 100 nm (and 650 nm) filter. Clearly the QDs stay dispersed in DI water over 24 hrs and do not dissolve. Interestingly, the levels of Se and Cd we measured for these QDs are much different that the 1:1 stoichiometry predicted for the CdSe core. In fact, the Cd:Se molar ratio is 60, and the Cd concentration in the QD suspension is 58 mM rather than the 2 mM specified by the manufacturer. Nevertheless, this excess Cd is part of the nanoparticle as none passes through the 3KDa filter; it may be present as CdS, with the ZnS layer or bound to the polymer carboxyl functional groups coating the inorganic core.
Comparing the concentrations of Cd in the unfiltered samples for algal-QD treatment with an equal amount of QDs dispersed in DI water, there is a 66% loss of Cd from the water column for the algal-QD indicating the QDs were bound to large aggregates that immediately settled. For the Cd remaining in the water column in the algal-QD only 5% of Cd was < 650 nm and 2.5% of this was dissolved Cd (< 3KDa). Clearly QDs in the algal treatment were aggregated, presumably bound to the algal cells. A recent study presented evidence, based on intracellular Cd and Te concentrations, that CdTe/CdS QDs cross the cell membrane of algae and are internalized.31 However, attempts at imaging or fluorescence of intracellular intact QDs were not successful and the possibility that the internalized QDs had dissolved could not be discounted. Similarly for our study, strong autofluorescence of the algae hindered spectroscopic examination of whether the QDs were internal or external to the cell. Other studies have shown that ENPs bind to the algae cell surface but are not internalized.34,35 Although not measured here, our previous studies have shown that only 22% of initially dissolved Cd2+ partitions to the algae in 20 ppt saline solutions.36
For the QD suspension in 20 ppt saline the Cd concentration in the unfiltered samples was 22% less than the fully dispersed DI QD sample, again indicating some large aggregation. However, for the QDs remaining in suspension 100 % were < 100 nm while only 6% were < 3KDa, so there was some QD dissolution leading to free Cd, but 94% of the Cd in the sample appears to be in the form of dispersed QDs or small aggregates thereof. It would appear that the surface charge generated by the carboxyl polymer functional groups is high enough to keep the QDs dispersed despite the high ionic strength of the saline solution. The difference in aggregation between these two treatments was confirmed by TEM where individual QDs could be discerned in the aqueous-QD treatment while clustering and aggregation was apparent in the algal-QD treatment (supplemental Figure 2). In the two L. plumulosus exposure solutions tested at the end of the 96 hr exposure period, there was very low metal concentration in the unfiltered water column sample, hence aggregation and particle settling had removed all QDs from the water column.
Dissolved organic carbon measurements in the algal-QD and aqueous QD treatments were 6.4 and 4.5 mg l−1, respectively at the beginning of the amphipod exposures and 12.7 and 3.5 mg l−1, respectively at the end of the amphipod exposure period. The greater DOC in the algal treatment was presumably due to either lysed cells or algal exudates. The DOC in the amphipod exposures at 96 hours were 5.82 and 0.56 mg l−1 for the algal-QD and aqueous-QD treatments respectively. For the aqueous-QD amphipod exposure, the 0.56 mg l−1 DOC is in good agreement with the simple dilution of the aqueous-QD stock as it is added to the L plumulosus exposure solution (30 ml of stock solution to a final volume of 180 ml for the exposure solution). However, the DOC in the algal-QD amphipod exposure is approximately twice the highest value it could be through dilution of the algal-QD treatment. We postulate that DOC may be higher in this treatment because the amphipods are feeding on the algae and excreting during the duration of the test whereas they are not feeding in the aqueous-QD exposure. It is also possible that dead amphipods in the algal-QD exposure release soluble organic contents which increase DOC.
The results of all the filtration measurements suggest that the QDs either stayed intact and dispersed or aggregated but did not dissolve to an appreciable extent. This further suggests that uptake by the amphipod is by feeding/intake of particulates not uptake of dissolved ions in the water column. Furthermore the DOC measurements suggest amphipods may be actively feeding in the algal-QD exposure but not in the aqueous-QD exposure.
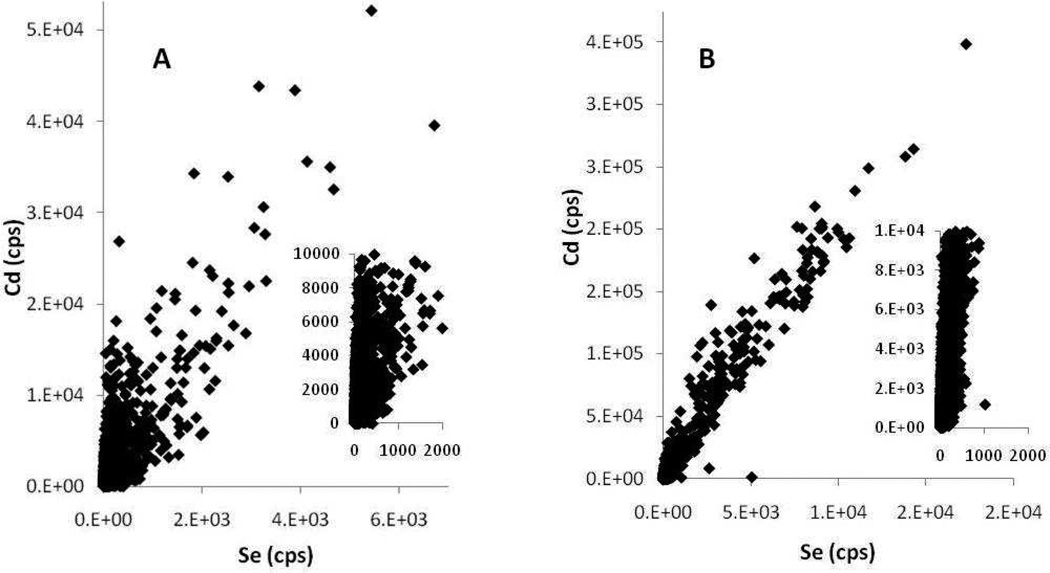
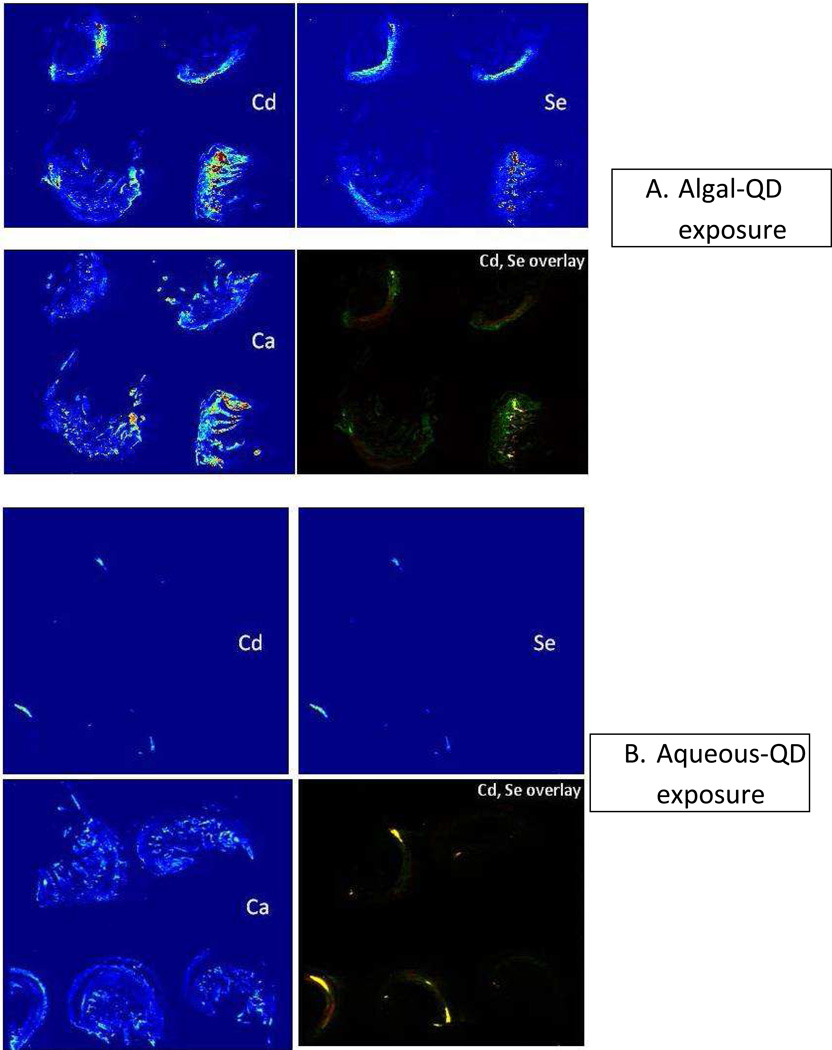
The mechanism of toxicity of the algal-QD exposures was still not clear. It is possible that the greater particle size of QDs in the algal treatment facilitates their ingestion and assimilation by the amphipod. Digestion with algae may lead to dissolution of the QDs and toxicity of Cd or Se. We investigated this hypothesis by determining the spatial distribution of Cd, Zn, Se and Ca in exposed organisms using LA-ICP-MS of 20 µm thick sections of exposed amphipods. The elemental distributions are shown in Figure 3A,B. Multiple individual organisms from each treatment were embedded and sectioned and an area (ca. 1 cm2) that covered 4 organisms was selected for LA-ICP-MS analysis. The Zn elemental map is not shown because, as well as being present in the QDs, it is a constituent element of the amphipod and its elemental distribution is not informative as to QD location or dissolution. Instead, the elemental map for Ca is shown as Ca is present throughout the whole organism and is a good proxy for an optical image of the orientation of organisms on the section. While these types of false color elemental images are somewhat subjective, clear differences between the two treatments are apparent. Cadmium and Se are more dispersed, and assimilated, in the organisms exposed to QDs via algae whereas Cd and Se remain highly localized in the aqueous-QD exposure and appear to be located exclusively in the gut. This is a similar elemental distribution as we observed by synchrotron XRF for D. magna exposed to QDs in the water column.33 Furthermore, the Cd, Se elemental overlay image clearly shows co-localization of Cd and Se for the aqueous exposures and, more definitively, areas of high Se, low Cd and vice versa for the algal-QD exposures. The pixel size for the laser ablation analysis was 40µm, clearly too large to be conclusive about the integrity of individual QDs of ca. 5 nm for the aqueous exposure, i.e. Cd and Se could appear in the same pixel even though the QD was no longer intact and Cd and Se ions where no longer present as the CdSe core. However, the observation of spatially distinct areas of high Cd and low Se at this pixel scale for the algal-QD exposures indicate some measure of dissolution of QDs in this treatment; i.e. there is no way to have high Cd and low Se except for the QD to have broken down in some fashion releasing either Cd ions, Se ions or both. The differences in Cd and Se distribution between the two treatments are more quantitatively presented in Figure 4 where the Cd vs. ICP-MS response for each pixel is plotted. The Cd and Se ICP-MS responses are highly correlated for the aqueous-QD exposures (R2= 0.95, Figure 4B) whereas the correlation is not strong for the algae-QD exposure (R2= 0.6, Figure 4A) which again supports the observation QD dissolution in the algal exposure but not in the aqueous exposure.
Figure 4.
Cadmium vs Se intensity scatter plots from laser ablation ICP-MS data for algal-QD exposed amphipods (A) and aqueous-QD exposed amphipods (B) Inserts show data around the x,y origin and substantiate the greater spread of Cd, Se data in the QD-algae treatments.
The LA-ICP-MS data suggests assimilation of the QDs in the algal-QD treatment and not the aqueous-QD treatment which would explain the much greater toxicity observed for the QD exposure. We hypothesize that exposure via algae stimulates a digestive response leading breakdown of the QDs. Metal assimilation has been shown to be related to food quality, with particle retention time within the organism being positively related to particle nutritional value.37,38 As discussed above, the Cd concentration of these QDs is much greater (58mM) the 2 mM stated by the manufacturer and 60X in excess of the 1:1 molar stoichiometry with The excess Cd may be present in the ZnS coat or bound to the polymer carboxyl outer layer. This carboxyl polymer may also stimulate digestion and subsequent Cd assimilation as has documented for Cd bound to bacterial extracellular polymeric coatings.39,40 It is also possible that the polymer coating itself might cause a toxic response in L. plumulosus, While surface coatings have been shown to modulate the toxicity (arising from Cd ions) of CdSe QDs,41 toxicity arising from ENP surface coatings has been reported in other instances.42
We have shown large differences in bioaccumulation (of Cd) in the amphipod L. plumulosus, when exposed to CdSe QDs via algae or the water column compared with an equivalent exposure to ionic Cd, indicating a greater potential for QDs to be trophically transferred. Furthermore, amphipods exposed to QDs via algal food exhibited much greater mortality than when exposed to QDs in the water column or to an equivalent exposure of ionic Cd (via algae dissolved). These results confirm the potential for QD bioaccumulation in benthic and pelagic foodwebs. This is one of the first studies to show a dramatic difference in toxicity of a metallic ENP compared with an equivalent concentration of dissolved metal ions.
Supplementary Material
Figure 3.
Elemental distribution of Cd, Se, Ca and Cd/Se overlay in algal-QD exposed amphipods (top four images) or aqueous-QD exposed amphipods (bottom four images).
Acknowledgements
This work was partially supported by US EPA RD-83332401-0 and by NIH Grant Number P42 ES007373 from the National Institute of Environmental Health Sciences. Dr Sam Webb is kindly thanked for modifying his SMAK software to accept LA-ICP-MS generated data.
Footnotes
Supporting Information Available.
Supplemental figures 1 and 2 are provided to accompany this manuscript. This information is available free of charge via the Internet at http://pubs.acs.org/
References
- 1.Slaveykova VI, Startchev K, Roberts J. Amine- and Carboxyl- Quantum Dots Affect Membrane Integrity of Bacterium Cupriavidus metallidurans CH34. Environ. Sci. Technol. 2009;43:5117–5122. doi: 10.1021/es900526r. [DOI] [PubMed] [Google Scholar]
- 2.Muhling M, Bradford A, Readman JW, Somerfield PJ, Handy RD. An investigation into the effects of silver nanoparticles on antibiotic resistance of naturally occurring bacteria in an estuarine sediment. Mar. Environ. Res. 2009;68:278–283. doi: 10.1016/j.marenvres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Fabrega J, Fawcett SR, Renshaw JC, Lead JR. Silver Nanoparticle Impact on Bacterial Growth: Effect of pH, Concentration, and Organic Matter. Environ. Sci. Technol. 2009;43:7285–7290. doi: 10.1021/es803259g. [DOI] [PubMed] [Google Scholar]
- 4.Mahendra S, Zhu HG, Colvin VL, Alvarez PJ. Quantum Dot Weathering Results in Microbial Toxicity. Environ. Sci. Technol. 2008;42:9424–9430. doi: 10.1021/es8023385. [DOI] [PubMed] [Google Scholar]
- 5.Ward JE, Kach DJ. Marine aggregates facilitate ingestion of nanoparticles by suspension-feeding bivalves. Mar. Environ. Res. 2009;68:137–142. doi: 10.1016/j.marenvres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Bouldin JL, Ingle TM, Sengupta A, Alexander R, Hannigan RE, Buchanan RA. Aqueous toxicity and food chain transfer of quantum Dots (TM) in freshwater algae and Ceriodaphnia dubia. Environ. Toxicol. Chem. 2008;27:1958–1963. doi: 10.1897/07-637.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong SWY, Leung PTY, Djurisic AB, Leung KMY. Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010;396:609–618. doi: 10.1007/s00216-009-3249-z. [DOI] [PubMed] [Google Scholar]
- 8.Geys J, Nemmar A, Verbeken E, Smolders E, Ratoi M, Hoylaerts MF, Nemery B, Hoet PHM. Acute Toxicity and Prothrombotic Effects of Quantum Dots: Impact of Surface Charge. Environ. Health Perspect. 2008;116:1607–1613. doi: 10.1289/ehp.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber DS, Denslow ND, Griffitt RJ, Martyniuk CJ. Sources, Fate and Effects of Engineered Nanomaterials in the Aquatic Environment. In: Sahu SC, Casciano DA, editors. Nanotoxicity. From In Vivo and In Vitro Models to Health Risks. Chichester, UK: John Wiley and Sons; 2009. [Google Scholar]
- 10.Chapman PM, Wang FY, Janssen CR, Goulet RR, Kamunde CN. Conducting ecological risk assessments of inorganic metals and metalloids: Current status. Hum Ecol Risk Assess. 2003;9:641–697. [Google Scholar]
- 11.Hare L, Tessier A, Borgmann U. Metal sources for freshwater invertebrates: Pertinence for risk assessment. Human and Ecological Risk Assessment. 2003;9:779–793. [Google Scholar]
- 12.Liu XJ, Ni IH, Wang WX. Trophic transfer of heavy metals from freshwater zooplankton Daphnia magna to zebrafish Danio reiro. Water Res. 2002;36:4563–4569. doi: 10.1016/s0043-1354(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 13.Schlekat CE, Lee B, Luoma SN. Chapter 7: Dietary Metals Exposure and Toxicity to Aquatic Organisms: Implications for Ecological Risk Assessment. In: Newman MC, RJ MH, Hale RC, editors. Coastal and Estuarine Risk Assessment. Lewis Publishers CRC Press; 2002. pp. 151–188. [Google Scholar]
- 14.Wang WX, Fisher NS. Assimilation efficiencies of chemical contaminants in aquatic invertebrates: A synthesis. Environ. Toxicol. Chem. 1999;18:2034–2045. [Google Scholar]
- 15.Lee BG, Griscom SB, Lee JS, Koh CH, Luoma SN, Fisher NS. Influences of dietary uptake and reactive sulfides on metal bioavailability from aquatic sediments. Science. 2000;287:282–284. doi: 10.1126/science.287.5451.282. [DOI] [PubMed] [Google Scholar]
- 16.King CK, Simpson SL, Smith SV, Stauber JL, Batley GE. Short-term accumulation of Cd and Cu from water, sediment and algae by the amphipod Melita plumulosa and the bivalve Tellina deltoidalis. Mar. Ecol. Prog. Ser. 2005;287:177–188. [Google Scholar]
- 17.Schlekat CE, Decho AW, Chandler TG. Dietary assimilation of cadmium associated with bacterial exopolymer sediment coatings by the estuarine amphipod Leptocheirus plumulosus: effects of Cd concentration and salinity. Mar. Ecol. Prog. Ser. 1999;183:205–216. [Google Scholar]
- 18.Yu R, Fleeger JW. Effects of nutrient enrichment, depuration substrate, and body size on the trophic transfer of cadmium associated with microalgae to the benthic amphipod Leptocheirus plumulosus. Environ. Toxicol. Chem. 2006;25:3065–3072. doi: 10.1897/06-029r.1. [DOI] [PubMed] [Google Scholar]
- 19.Yoo H, Lee JS, Lee BG, Lee IT, Schlekat CE, Luoma SN. Uptake pathway for Ag bioaccumulation in three benthic invertebrates exposed to contaminated sediments. Mar. Ecol. Prog. Ser. 2004;270:141–152. [Google Scholar]
- 20.Chen CY, Folt CL. Bioaccumulation and diminution of arsenic and lead in a freshwater food web. Environ. Sci. Technol. 2000;34:3878–3884. [Google Scholar]
- 21.Chen CY, Stemberger RS, Klaue B, Blum JD, Pickhardt PC, Folt CL. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol. Oceanogr. 2000;45:1525–1536. [Google Scholar]
- 22.Stemberger RS, Chen CY. Fish tissue metals and zooplankton assemblages of northeastern US lakes. Can. J. Fish. Aquat. Sci. 1998;55:339–352. [Google Scholar]
- 23.Mathews T, Fisher NS. Evaluating the trophic transfer of cadmium, polonium, and methylmercury in an estuarine food chain. Environ. Toxicol. Chem. 2008;27:1093–1101. doi: 10.1897/07-318.1. [DOI] [PubMed] [Google Scholar]
- 24.Holbrook RD, Murphy KE, Morrow JB, Cole KD. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nature Nanotechnol. 2008;3:352–355. doi: 10.1038/nnano.2008.110. [DOI] [PubMed] [Google Scholar]
- 25.Werlin R, Priester JH, Mielke RE, Kramer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E, Holden PA. Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nature Nanotechnol. 2011;6:65–71. doi: 10.1038/nnano.2010.251. [DOI] [PubMed] [Google Scholar]
- 26.Way CM. Dynamics of Filter-Feeding in Musculium transversum (Bivalvia:Sphaeriidae) J North Amer. Benthol. Soc. 1989;8:243–249. [Google Scholar]
- 27.Filella M, Rellstab C, Chanudet V, Spaak P. Effect of the filter feeder Daphnia on the particle size distribution of inorganic colloids in freshwaters. Wat. Res. 2008;42:1919–1924. doi: 10.1016/j.watres.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Methods for assessing the toxicity of sediment-associated contaminants with estuarine and marine amphipods, USEPA. Office of Research and Development, EPA/600/R-94/025/ 1994
- 29.Guillard RRL, Ryther JH. Studies on marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Can. J. Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 30.Webb SM. The MicroAnalysis Toolkit: Xray Fluorescence Image Processing. AIP Conference Proceedings. 2011;1365:196–199. [Google Scholar]
- 31.Domingos RF, Simon DF, Hauser C, Wilkinson KJ. Bioaccumulation and Effects of CdTe/CdS Quantum Dots on Chlamydomonas reinhardtii - Nanoparticles or the Free Ions? Environ. Sci. Technol. 2011;45:7664–7669. doi: 10.1021/es201193s. [DOI] [PubMed] [Google Scholar]
- 32.McGee BL, Wright DA, Fisher DJ. Biotic factors modifying acute toxicity of aqueous cadmium to estuarine amphipod Leptocheirus plumulosus. Arch. Environ. Contam. Toxicol. 1998;34:34–40. doi: 10.1007/s002449900283. [DOI] [PubMed] [Google Scholar]
- 33.Jackson BP, Pace HE, Lanzirotti A, Smith R, Ranville JF. Synchrotron X-ray 2D and 3D elemental imaging of CdSe/ZnS quantum dot nanoparticles in Daphnia magna. Anal. bioanal. Chem. 2009;394:911–917. doi: 10.1007/s00216-009-2768-y. [DOI] [PubMed] [Google Scholar]
- 34.Van Hoecke K, De Schamphelaere KAC, Van der Meeren P, Lucas S, Janssen CR. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: Importance of surface area. Environ. Sci. Technol. 2008;27:1948–1957. doi: 10.1897/07-634.1. [DOI] [PubMed] [Google Scholar]
- 35.Lin S, Bhattacharya P, Rajapakse NC, Brune DE, Ke PC. Effects of Quantum Dots Adsorption on Algal Photosynthesis. J. Phys. Chem. C. 2009;113:10962–10966. [Google Scholar]
- 36.Williams JJ, Dutton J, Chen CY, Fisher NS. Metal (As Cd, Hg, and CH(3)Hg) Bioaccumulation From Water and Food by the Benthic Amphipod Leptochirus Plumulosus. Environ. Toxicol. Chem. 2010;29:1755–1761. doi: 10.1002/etc.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagnon C, Fisher NS. Bioavailability of sediment-bound methyl and inorganic mercury to a marine bivalve. Environ. Sci. Technol. 1997;31:993–998. [Google Scholar]
- 38.Wang WX, Fisher NS. Assimilation of trace elements and carbon by the mussel Mytilus edulis: Effects of food composition. Limnol. Oceanogr. 1996;41:197–207. [Google Scholar]
- 39.Schlekat CE, Decho AW, Chandler GT. Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ. Toxicol. Chem. 1998;17:1867–1874. [Google Scholar]
- 40.Schlekat CE, Decho AW, Chandler GT. Dietary assimilation of cadmium associated with bacterial exopolymer sediment coatings by the estuarine amphipod Leptocheirus plumulosus: effects of Cd concentration and salinity. Mar. Ecol. Prog. Ser. 1999;183:205–216. [Google Scholar]
- 41.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarantola M, Pietuch A, Schneider D, Rother J, Sunnick E, Rosman C, Pierrat S, Soennichsen C, Wegener J, Janshoff A. Toxicity of gold-nanoparticles: Synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology. 2011;5:254–268. doi: 10.3109/17435390.2010.528847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.