Abstract
When B cells differentiate into plasma cells, there is a large increase in the cellular content of mRNA coding for immunoglobulin. This increase cannot be fully accounted for by the increase in rate of transcription of the genes. We have investigated the possibility that the half-life of mu heavy chain mRNA increases during B cell differentiation, by measuring the rates of decay of the same endogenous mu gene in two cell lines representing the B cell and the plasma cell. Using a pulse-chase protocol, it was found that there was a significant increase in the half-life of mu mRNA between the B cell and the plasma cell, and no detectable difference in the average half-life of total poly(A)+ RNA in the two cell lines. The reduced rate of decay of mu mRNA in the more differentiated cell type is almost sufficient to account for the difference in steady state mu mRNA levels between the two cell lines.
Full text
PDF





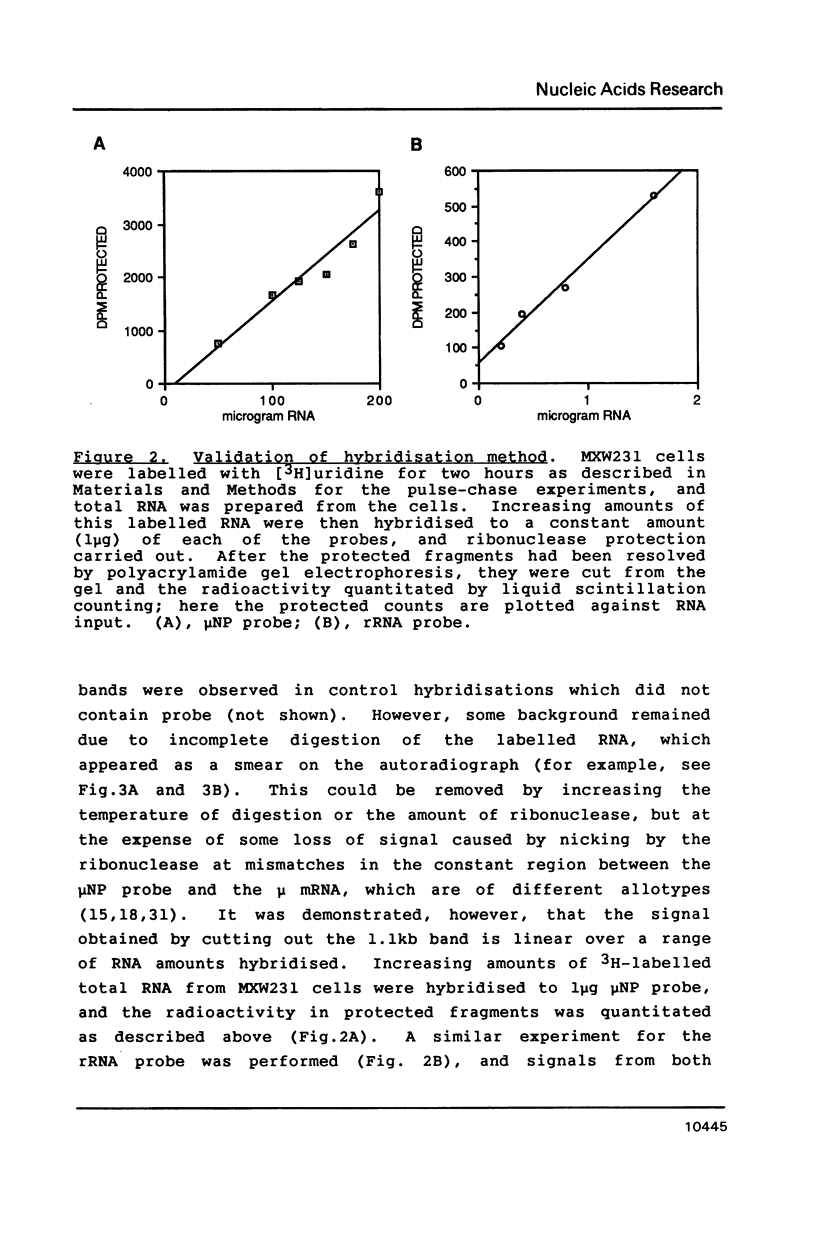
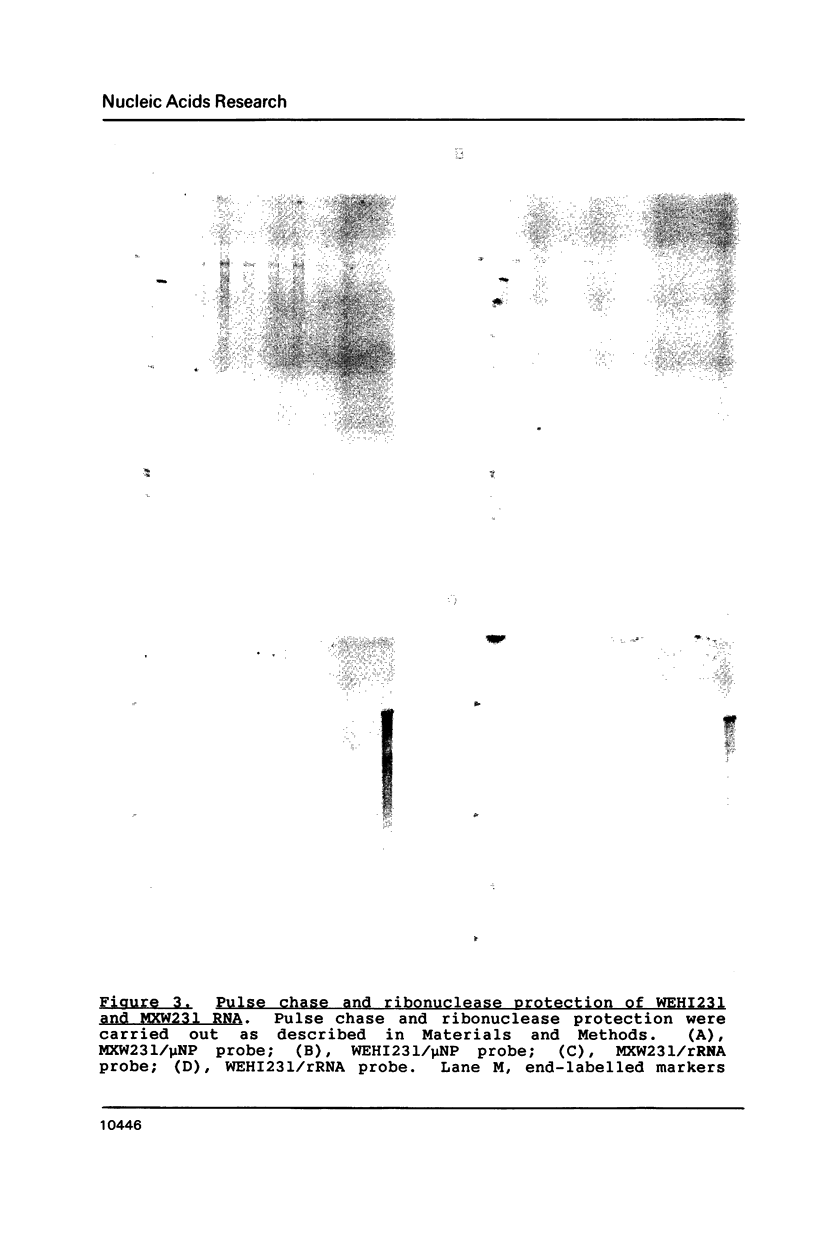

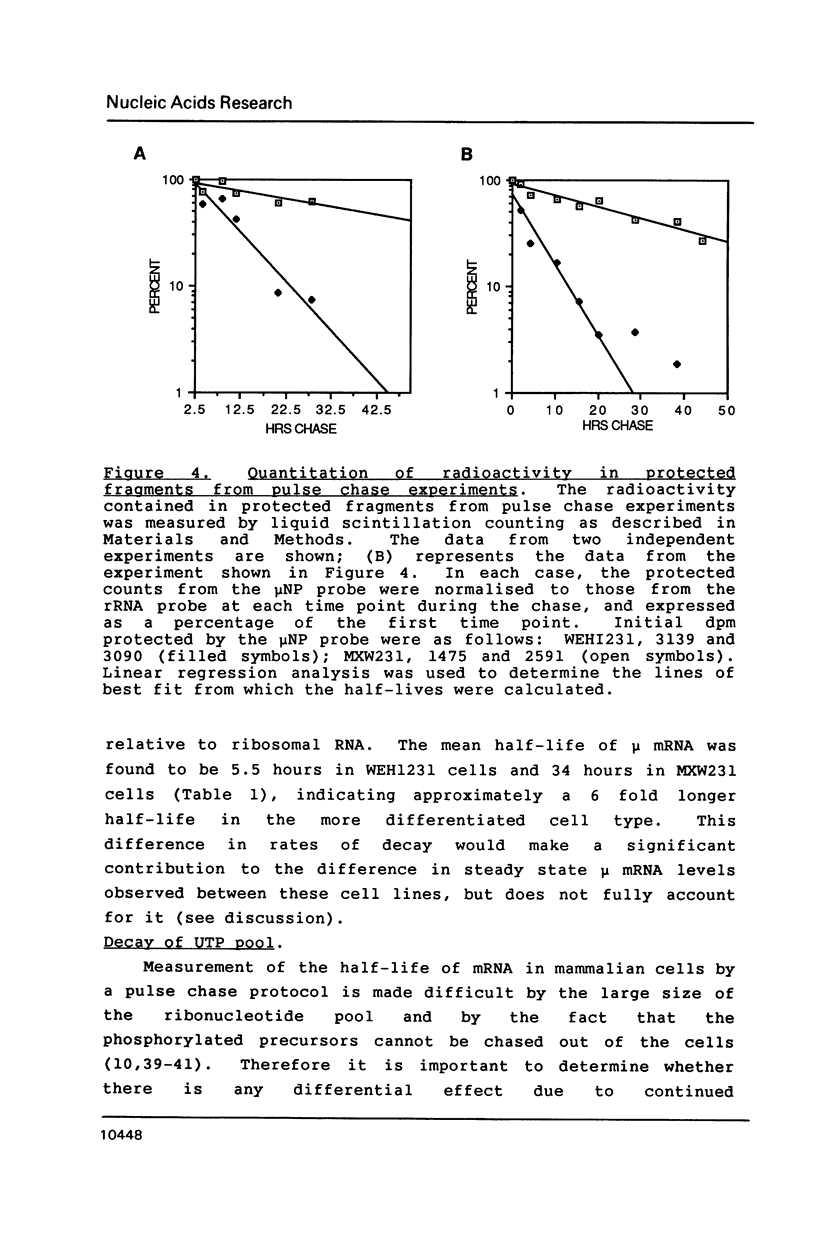

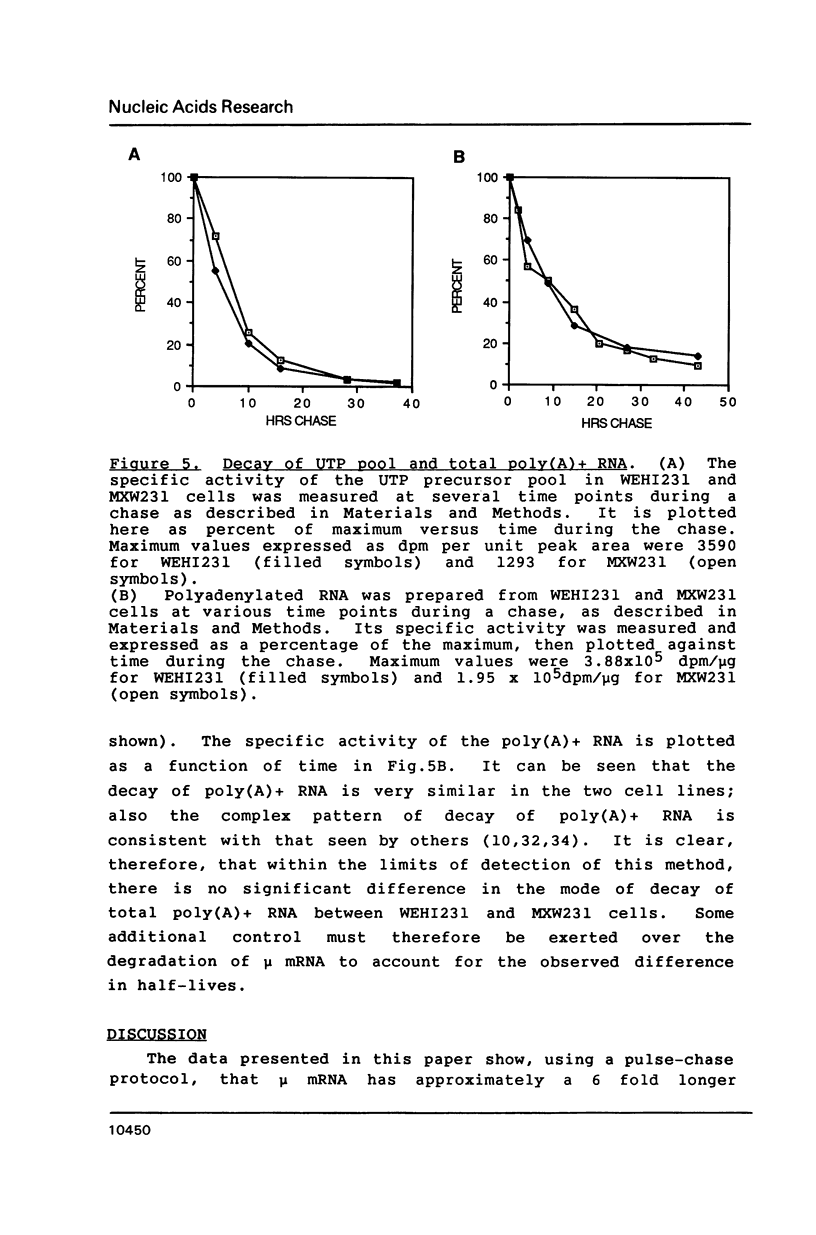




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Berger C. N. In situ hybridization of immunoglobulin-specific RNA in single cells of the B lymphocyte lineage with radiolabelled DNA probes. EMBO J. 1986 Jan;5(1):85–93. doi: 10.1002/j.1460-2075.1986.tb04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen regulates the absolute rate of transcription of the Xenopus laevis vitellogenin genes. J Biol Chem. 1983 May 10;258(9):5449–5455. [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Butler E. T., Chamberlin M. J. Bacteriophage SP6-specific RNA polymerase. I. Isolation and characterization of the enzyme. J Biol Chem. 1982 May 25;257(10):5772–5778. [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Milstein C. Stability of cytoplasmic ribonucleic acid in a mouse myeloma: estimation of the half-life of the messenger RNA coding for an immunoglobulin light chain. J Mol Biol. 1974 Feb 5;82(4):469–481. doi: 10.1016/0022-2836(74)90242-3. [DOI] [PubMed] [Google Scholar]
- Danner D., Leder P. Role of an RNA cleavage/poly(A) addition site in the production of membrane-bound and secreted IgM mRNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8658–8662. doi: 10.1073/pnas.82.24.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Emerson C. P. Regulation of the synthesis and the stability of ribosomal RNA during contact inhibition of growth. Nat New Biol. 1971 Jul 28;232(30):101–106. doi: 10.1038/newbio232101a0. [DOI] [PubMed] [Google Scholar]
- Gay D. A., Yen T. J., Lau J. T., Cleveland D. W. Sequences that confer beta-tubulin autoregulation through modulated mRNA stability reside within exon 1 of a beta-tubulin mRNA. Cell. 1987 Aug 28;50(5):671–679. doi: 10.1016/0092-8674(87)90325-4. [DOI] [PubMed] [Google Scholar]
- Gerster T., Picard D., Schaffner W. During B-cell differentiation enhancer activity and transcription rate of immunoglobulin heavy chain genes are high before mRNA accumulation. Cell. 1986 Apr 11;45(1):45–52. doi: 10.1016/0092-8674(86)90536-2. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Gutman G. A., Warner N. L., Harris A. W. Immunoglobulin production by murine B-lymphoma cells. Clin Immunol Immunopathol. 1981 Feb;18(2):230–244. doi: 10.1016/0090-1229(81)90029-5. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Abraham G., Skehel J. J., Smith J. C., Fellner P. Influenza virus messenger RNAs are incomplete transcripts of the genome RNAs. Nucleic Acids Res. 1977 Dec;4(12):4197–4209. doi: 10.1093/nar/4.12.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäck H. M., Berg J., Wabl M. Translation affects immunoglobulin mRNA stability. Eur J Immunol. 1989 May;19(5):843–847. doi: 10.1002/eji.1830190510. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. Immunoglobulin mRNA stability varies during B lymphocyte differentiation. EMBO J. 1988 Apr;7(4):1041–1046. doi: 10.1002/j.1460-2075.1988.tb02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamson G., Koshland M. E. Changes in J chain and mu chain RNA expression as a function of B cell differentiation. J Exp Med. 1984 Sep 1;160(3):877–892. doi: 10.1084/jem.160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Mason J. O., Williams G. T., Neuberger M. S. The half-life of immunoglobulin mRNA increases during B-cell differentiation: a possible role for targeting to membrane-bound polysomes. Genes Dev. 1988 Aug;2(8):1003–1011. doi: 10.1101/gad.2.8.1003. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- Michot B., Bachellerie J. P., Raynal F., Renalier M. H. Homology of the 5'-terminal sequence of 28 S rRNA of mouse with yeast and Xenopus. Implication for the secondary structure of the 5.8 S--28 S RNA complex. FEBS Lett. 1982 Apr 19;140(2):193–197. doi: 10.1016/0014-5793(82)80892-2. [DOI] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M. Purification of messenger RNA and heterogeneous nuclear RNA containing poly(a) sequences. Methods Enzymol. 1974;29:431–443. doi: 10.1016/0076-6879(74)29035-9. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. Regulated production of mu m and mu s mRNA requires linkage of the poly(A) addition sites and is dependent on the length of the mu s-mu m intron. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8883–8887. doi: 10.1073/pnas.83.23.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989 Feb;9(2):726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke W. C., Mather E. L., Koshland M. E. Assembly and secretion of pentameric IgM in a fusion between a nonsecreting B cell lymphoma and an IgG-secreting plasmacytoma. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3469–3473. doi: 10.1073/pnas.76.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Detection of an unstable RNA in chick fibroblasts after reduction of the UTP pool by glucosamine. Eur J Biochem. 1971 Dec;24(2):358–365. doi: 10.1111/j.1432-1033.1971.tb19694.x. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Sitia R., Neuberger M. S., Milstein C. Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J. 1987 Dec 20;6(13):3969–3977. doi: 10.1002/j.1460-2075.1987.tb02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurushita N., Avdalovic N. M., Korn L. J. Regulation of differential processing of mouse immunoglobulin mu heavy-chain mRNA. Nucleic Acids Res. 1987 Jun 11;15(11):4603–4615. doi: 10.1093/nar/15.11.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Ribosomal RNA turnover in contact inhibited cells. Nat New Biol. 1972 Jan 12;235(54):58–61. doi: 10.1038/newbio235058a0. [DOI] [PubMed] [Google Scholar]
- Wood C. R., Boss M. A., Patel T. P., Emtage J. S. The influence of messenger RNA secondary structure on expression of an immunoglobulin heavy chain in Escherichia coli. Nucleic Acids Res. 1984 May 11;12(9):3937–3950. doi: 10.1093/nar/12.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Regulation of IgM and IgD synthesis in B lymphocytes. I. Changes in biosynthesis of mRNA for mu- and delta-chains. J Immunol. 1984 Mar;132(3):1561–1565. [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti G., Stein J., Stein G. Targeting of a chimeric human histone fusion mRNA to membrane-bound polysomes in HeLa cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2683–2687. doi: 10.1073/pnas.84.9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]



