Abstract
There is a growing recognition for the significance of evolutionary thinking in ecology and conservation biology. However, ecology and conservation studies often work with species-specific, fixed traits that ignore intraspecific variation. The way the habitat of a species is considered is an example of typological thinking biased by human perception. Structural habitat units (e.g., land cover types) as perceived by humans may not represent functional habitat units for other organisms. Human activity may also interfere with the environmental information used by organisms. Therefore, the Umwelt-concept from ethology needs to be integrated in the way we think about habitat and habitat selection. It states that different organisms live in different perceptual worlds dealing with specific subsamples of the environment as a result of their evolutionary and developmental history. The resource-based habitat concept is a functional habitat model based on resource distributions (consumables and conditions) and individual movements. This behavioural approach takes into account aspects that relate to the perceptual world of organisms. This approach may offer new opportunities for conservation and may help avoid failures with habitat restoration. Perceptual ability may be subject to adaptive change, but it may also constrain organisms from showing adaptive behaviours in rapidly changing environments.
Keywords: animal behaviour, conservation biology, environment, habitat concept, niche construction, niche evolution, perception
Introduction
Ecology is generally described as the study of the relationships between organisms and the (a)biotic environment. Such relationships are best understood as interactions because the environment is not only dictating the fate of organisms, but organisms also affect the environment. Organisms make use of their phenotype (behaviour, morphology, physiology and life-history traits) to deal with their environment (both opportunities and constraints). Because phenotypic traits are shaped by evolutionary processes, evolutionary history has the potential to affect ecological interactions and, hence, the prosperity of species in rapidly changing environments under human impact (e.g., Purvis et al. 2000; Jones et al. 2003). There is a growing recognition of the significance of evolutionary thinking for conservation biology (e.g., Ashley et al. 2003; Carroll and Fox 2008; Hendry et al. 2010). Although there is still a long way to go to fully develop eco-evolutionary conservation strategies in biodiversity management and policymaking, several recent papers provide stimulating perspectives (e.g., Kinnison and Hairston 2007; Mace and Purvis 2008; Lankau et al. 2011). This new wave of interest is not independent of climate change and the need to better understand (limits of) evolutionary adaptation in non-model organisms in the field (Hoffmann and Sgro 2011). The recent appreciation that evolution is not necessarily a slow process is another factor of significance in this context; there is evidence for rapid evolutionary responses of organisms, particularly in human-dominated environments (Hairston et al. 2005; Baker et al. 2011), although the relative contribution of rapid evolution on ecological dynamics may vary considerably (Ellner et al. 2011).
Using this evolutionary ecology framework for dealing with conservation issues, I will discuss the significance of an evolutionary way of thinking about what actually represents a species’ habitat. The current vision on the concept of habitat in ecology and conservation, and surprisingly even in several evolutionary studies, appears often as a remarkable example of typological, static thinking. Structural habitat (e.g., vegetation or land cover types) as it is perceived by humans and used on maps for different conservation applications as habitat units may not (closely) reflect the functional habitat of an organism. Organisms are able to pay attention to specific relevant information of the environment and screen or ignore many other signals. Therefore, habitat use is not a fixed species-specific trait, but a trait that may show significant intra-specific variation and deserves a more functional, mechanistic approach. Although there is rich literature on the adaptive significance of animal communication since the pioneer work of Tinbergen (1963), the way habitat and habitat use have been adopted in several ecological and conservation biology studies largely ignores this aspect of integrating sensory inputs and how it may deviate from human perception. An appropriate functional definition and conception of the habitat of an animal should integrate this behavioural viewpoint on the sensory interactions between the organism and its environment.
Therefore, I will emphasize in this paper the conceptual link with the Umwelt-concept from ethology and psychology and explore its significance for animal conservation. First, I will briefly discuss how human activities create anthropogenic niches that affect ecological and evolutionary responses of organisms, and I will also illustrate how such environments may interfere with information processing of organisms. Secondly, I introduce the Umwelt concept as it may help us avoid a far too human perception on how animals perceive, and hence deal with, their environment. There is evidence of adaptive changes in perceptual ability relative to changes in landscape structures. To integrate these aspects into a conservation context, I will discuss the resource-based habitat concept as a functional, organism-centred view of the interactions between the organism and its relevant environment.
Anthropogenic niche construction: constraints and opportunities for other organisms
Organisms are influenced by the environment, but they may also modify their environment, which in turn may have consequences for their own ecology and evolution, as well as for other organisms. This process is known as niche construction (Laland et al. 1999). Niche construction theory offers interesting insights for conservation biology (Boogert et al. 2006), and there is a recent synthesis on how human activities and different types of land use can be viewed as forms of stable or unstable niche construction (Rowley-Conwy and Layton 2011). Niches created by humans (e.g., several urban, industrial and agricultural land-use types) have been shown to have negative impacts on the population dynamics of many species. Human activity can have a direct impact on animals (e.g., by persecution and harvesting) or an indirect impact via effects on the quantity, quality or configuration of their habitat. However, particular human-made environments can be favourable to some organisms as surrogates of natural environments (e.g., Benes et al. 2003), or as novel environments that result from novel physical or biotic conditions (e.g., Hobbs et al. 2006; Ghalambor et al. 2007; Carroll 2008). It has, for example, been hypothesized that a considerable number of British butterflies have taken advantage of warmer man-made, early successional vegetations like grasslands, heathlands and forest clearings (Thomas 1993). A recent analysis by Møller (2010) showed elevated success of birds breeding inside human buildings (like barns) compared with outdoors as in human buildings they can easily escape nest predation. Human activities may even create opportunities for diversification of species, with the food resources provided by human activities and livestock farming suggested to provide an opportunity for an island raptor species to colonize the Canary Islands (Agudo et al. 2010). Anthropogenic environments provide interesting opportunities for studying population differentiation, adaptation and rapid evolution (Lankau et al. 2011; Sih et al. 2011). Anthropogenic environments have a strong capacity to alter phenotypic traits of wild organisms by rapid evolution (Darimont et al. 2009); however, it is more widely acknowledged that anthropogenic niches typically constrain most wild organisms.
Human interference with information processing in wild animals
Adaptive behaviour relies upon accurate information use of relevant ecological parameters (Dall et al. 2005). In environments under strong human impact, physical and biotic environmental changes can be of significance to several organisms, but the way these organisms are behaviourally operating may be affected as well. Humans are interfering with information processing and with the use of environmental cues by wild organisms to a much larger extent than is often realized. Think, for example, about sensory pollution like light and noise pollution. With industrialization, noise pollution from machinery and other human activities continues to expand in space and intensity (Barber et al. 2010). Chronic noise exposure is now widespread. The problem is not limited to terrestrial organisms as freshwater and marine species can also be affected by noise either from terrestrial sources or from traffic and other sources on or in the water (Slabbekoorn et al. 2010). Recent work has mainly focused on the impact of noise on intraspecific animal communication (e.g., Slabbekoorn and Ripmeester 2008), but Siemers and Schaub (2011) provided experimental evidence of an impact of traffic noise on foraging efficiency in acoustic predators and hence on predator–prey interactions. Light pollution is another important case (Hölker et al. 2010). Many ecologists have neglected to consider artificial night lighting as a relevant environmental factor, while conservationists have largely neglected to include the nighttime environment in conservation strategies (Longcore and Rich 2004). We are at the very beginning of understanding evolutionary effects of light pollution and how they may in turn feedback on ecological interactions. The case of light pollution illustrates that human-dominated environments do not only alter the presence and quality of habitats, but also the environmental cues organisms use to deal with their environment. For example, artificial lighting has been shown to disrupt the nocturnal movements of sea turtle hatchlings to the ocean, as artificial light will attract them landward rather than seaward (Tuxbury and Salmon 2005).
Interestingly, some minor details or even unnoticed sources of information to the human observer can be essential for habitat selection in other organisms. This observation has received much attention in dairy management practices and with respect to the well-being of farm animals (Grandin and Johnson 2005). In the same vein, we should recognize the importance for wild animals and hence several of their conservation issues. Take the case of polarized light that is used by several insects to locate water surfaces. Several human creations (including glass buildings, asphalt roads and even particular cars) appear to offer similar cues to these insects (Kriska et al. 2006, 2008; Horvath et al. 2009). Hence, they are attracted to wrongly interpret anthropogenic substrates and defend territories and lay eggs. Interestingly, some predators take advantage of such trapped insect prey in urban areas (e.g., Robertson et al. 2010), illustrating potential positive feedbacks for other species and their urban life styles. The polarized light examples bring us to the concept of ecological and evolutionary traps that have attracted much attention in anthropogenic environments (e.g., Schlaepfer et al. 2002; Gilroy and Sutherland 2007). Ecological traps occur when, by various mechanisms, low-quality habitat is more attractive and hence preferred over available good habitat (e.g., Hollander et al. 2011). Patten and Kelly (2010) argue that also the converse problem may occur: the avoidance of high-quality habitat because it is less attractive. They refer to a ‘perceptual trap’ in such a case and showed evidence with a field experiment in the Lesser Prairie-Chicken Tympanuchus pallidicinctus (Patten and Kelly 2010). However, the label ‘perceptual trap’ is somewhat confusing as perception is also much of an issue in the other cases of trapping. Ecological and evolutionary trapping is anyway a significant issue for conservation in anthropogenic landscapes, even if it often remains difficult to show empirically and in an unequivocal way that reduced breeding success in preferred, low-quality habitat is related to population decline.
Habitat and ‘Umwelt’: the far too human perspective
Organisms respond to information from the environment through their sensory systems. Sensory systems have evolved to respond to relevant functional subsamples of information from the environment. Several organisms rely on other senses than their visual system alone. Moreover, the performance and sensitivity of visual systems vary considerably among organisms as illustrated earlier with the example of light pollution. For defining and recognizing the habitat of a species, the ‘what you see, is what you get’– assumption should not be applied by default. Therefore, it is relevant to connect the habitat issue to the ‘Umwelt’-concept, a longstanding concept from ethology and psychology. From his discussion of the animal's relationship with its environment, Von Uexküll (1909) argued already at that time that different organisms live in different perceptual worlds and that we should be more sensitive to the environmental ‘carriers of significance’ that differ among species and individuals. This specific perceptual environment is referred to as the Umwelt. The analysis of information use by animals is considered a central field to organismal biology, and hence to evolutionary and behavioural ecology (Dall et al. 2005). A behavioural viewpoint on habitat recognition and selection is also of significance for conservation biology, including conservation action on the ground (e.g., species action plans).
Manning et al. (2004) discussed the relevance of the Umwelt concept for improving realism of landscape fragmentation models, as common simplifications that ignore the organism's perspective can be problematic for conservation and land management. Here, I want to emphasize and even widen this conclusion or warning for several, if not most aspects in conservation and land management that explicitly or implicitly deal with functional habitat and movements of animals.
Scanning for resources in the landscape matrix
What is perceived and used as habitat by organisms is driven by their perception and their responses to environmental cues. Dispersal is widely recognized as a key process for ecology, evolution and conservation (Ronce 2007). The issue of available information and sensitivity to cues would also need careful treatment during dispersal. Habitat use and dispersal behaviour may alter with changing landscapes, stressing the evolutionary dimension that has often been ignored in landscape ecology (Baguette and Van Dyck 2007). The spatial scale of interaction between landscape structure – or more precisely resource distributions – and the perception of the organism is referred to as the functional grain of a landscape (Baguette and Van Dyck 2007). Experimental work by Romero et al. (2009), for example, demonstrated modification of the search strategies of red flour beetles (Tribolium castaneum) in response to the scale of habitat structure, emphasizing the significance of functional grain. They concluded that spatially explicit, organism-centred studies focusing on behavioural responses to different habitat configurations are much needed to improve our ability to accurately predict space use of organisms in landscapes.
Our biased and incomplete view on how organisms perceive and use their environment can be illustrated with manipulative field experiments adding or removing particular ecological resources and conditions within and among vegetation types at the landscape level. A simple field experiment I conducted with Orange tip butterflies (Anthocharis cardamines) is illustrative in this context (H. Van Dyck, unpublished data). Potted host plants of this butterfly were placed for short periods during the adult flight period inside and outside the local patchwork of known ‘habitat’ (in the sense of vegetation type) in a local network population in a human-dominated agricultural landscape in NE-Belgium where its host plant (Cardamine pratensis) typically occurs in damp meadows. The presence and number of eggs on the host plants was recorded. This experiment allowed examination of the extent this butterfly would scan the landscape matrix outside the patches of damp meadows. Host plants were even placed at places where it was physically unable to grow and at distances up to >1300 m from the nearest occupied meadow. After a timeframe of 2 days only, I noticed that >70% of the experimental plants outside habitat patches carried eggs. Moreover, the average number of eggs per used host plant was 3.6 times higher on the host plants placed across the landscape matrix compared with plants placed in meadows (maximum number of eggs per host plant was nine outside meadows and two only within meadows). This is remarkable as it has been demonstrated that A. cardamines females tend to avoid plants that already carry eggs because of the females’ response to an oviposition-deterring pheromone that is deposited with each egg (Dempster 1992). This experiment illustrates that females were readily able to find and use host plants at different places across the landscape matrix even without any ‘suitable habitat’ from the vegetation-type-based habitat view. The landscape matrix was more intensively scanned for resources than is generally appreciated in species with a patchy host plant distribution. High egg loads outside habitat patches also suggest that movement through a resource-poor matrix may affect reproductive decisions (i.e., acceptance of already used host plants), which may signify deferred search costs of dispersal in fragmented landscape (Stamps et al. 2005). Anthocharis cardamines is often considered to be a grassland species, but most grasslands in NW-Europe have been created by human activity for agriculture. So, the butterfly most likely has a longer evolutionary history as an organism using resources in forest clearings, likely explaining why it still makes use of forest edges and hedgerows in agricultural landscapes. It would be interesting to understand how search behaviour, movements and perception of such an insect may have been altered from forested to agricultural landscapes. Comparing phenotypes and behavioural performances among different landscape types would hence be promising in this context.
A behavioural viewpoint on the interactions between an organism and its environment is essential to get a better mechanistic understanding of habitat selection and dispersal, including the costs of dispersal across anthropogenic landscapes (Baguette and Van Dyck 2007; Bonte et al. 2011). Determining the landscape structures that result in barriers or corridors for a particular species in rapidly changing landscapes may deviate from commonly made assumptions like in ‘least cost path’ analyses that have become popular in conservation and applied landscape ecology (e.g., Fahrig 2007). This is particularly so if movement mortality is taken into account. More mobile species are considered more resilient to the dynamics of anthropogenic landscapes than less mobile species. However, if more mobile organisms experience increased mortality rates as they move into more hostile anthropogenic parts of the landscape matrix, it may compromise their survival in such landscapes even more than less mobile organisms (Thomas 2000; Fahrig 2007). Individuals of the same species may show different behavioural searching strategies rather than one species-specific strategy (Heinz and Strand 2006). Despite the growing interest for studying the eco-evolutionary dynamics of dispersal, current studies rarely assess to what extent, and under which conditions, naturally selected dispersal behaviour has failed to promote population persistence (Delgado et al. 2011). Individuals can be selected to have traits that diminish population-level performance; individuals can disperse less (or more) than would be ideal for a population. Delgado et al. (2011) argue that as conservation managers typically need to take a population-level view of performance, these novel insights may necessitate their intervention if it differs from what is selected for. The potential discrepancy between individual and population level for conservation in an eco-evolutionary context will need more attention in the future as it has not been fully appreciated yet.
Movements other than those that aim for displacement (e.g., foraging movements) may also be altered in anthropogenic landscapes and should not be ignored. From a behavioural point of view, real displacement movements and routine foraging movements can be very different, and may evolve independently in changing landscapes (Van Dyck and Baguette 2005; Bonte et al. 2009). The Umwelt of organisms that decided to emigrate from a site may be different from the Umwelt of conspecifics that make local, routine movements to forage; dispersing animals may need to ignore particular cues from resources, conspecifics and other species (e.g., predators), or at least alter their responses to them compared with individuals in a different behavioural mood. If dispersers are not a random sample of the population based on their perceptual abilities (together with other phenotypic traits), they may have a different Umwelt compared with the average resident individual. This may have consequences for choosing relevant individuals for experimental studies on dispersal (e.g., release experiments).
Studies on dispersal often interpret vegetation or land-use types in terms of barriers and corridors based on a human perspective or interpretation of the landscape. Several studies suggest that such assumed relationships may not hold when they are tested. For example, a recent genetic study by Leidner and Haddad (2010) could not find any indication that existing levels of urbanization were barriers to dispersal in a coastal endemic butterfly. As the degree to which anthropogenic environments creates barriers to animal dispersal remains poorly understood, our understanding would benefit from integrating knowledge about landscape perception.
Perception under selection: adaptive perceptual ranges
It is well appreciated that species that live in different environments may need different sensory abilities. The same can be true at the intraspecific level, if populations of the same species have to contend with, for example, different levels of habitat fragmentation (Lima and Zollner 1996). Field release experiments of varying distances to a target habitat have been successful in showing variation in perceptual range (e.g., in mammals; Zollner 2000). Our work on the speckled wood butterfly (Pararge aegeria L.) in different landscapes offers an interesting case in this context. Although many butterflies in NW Europe have shown negative population trends over the last decades, P. aegeria is among the few successful butterfly species that have increased considerably in distribution and abundance (Van Dyck et al. 2009). It has recently expanded its habitat use in NW Europe from mainly forests to much more open environments, including agricultural land with some hedgerows and small woodlots. For a flying heliotherm, forested and agricultural landscapes represent very different operational worlds in terms of the biotic and abiotic environmental interactions (Karlsson and Van Dyck 2005). Our comparative work on forest and agricultural populations has already pointed to several phenotypic differences in functional morphology, behaviour and life history (e.g., Gibbs and Van Dyck 2010; Vandewoestijne and Van Dyck 2010). Pararge aegeria has not shifted but expanded its habitat use, with evidence of ecotypic, and hence phenotypic, differentiation. Interestingly, there is also evidence for evolutionary effects on their perceptual ability relative to landscape type. Field experiments with wild caught P. aegeria individuals showed that butterflies from an agricultural landscape were able to detect a target habitat from a greater distance than forest butterflies (Merckx and Van Dyck 2007). More recently, we confirmed this effect with laboratory reared males and females of the two types of landscape and replicate populations (E. Öckinger and H. Van Dyck, submitted manuscript). This is a highly relevant result within a framework of functional landscape connectivity as the same fragmented landscape will be perceived as more fragmented (less functionally connected) by speckled woods with a recent evolutionary history in forested landscapes than by conspecifics with a history in fragmented agricultural landscapes. Based on the case of P. aegeria, we have recently hypothesized that the success in dynamic anthropogenic landscapes might be related to the existence of seasonal plasticity in morphology and life history in this multivoltine species (Van Dyck et al. 2009). This idea builds upon the Baldwin effect that plasticity can be significant in the successful colonization of novel environments (Yeh and Price 2004). The idea of a link between phenotypic plasticity and conservation status now warrants a well-designed test with multiple species within a phylogenetically controlled, analytical framework. Using simulation models, Olden et al. (2004) have explored the potential for plasticity in perceptual ranges and argued that context-dependent perceptual ranges need to be carefully considered and further explored in future landscape ecological studies.
From Umwelt to functional habitat
Conservationists may wonder about the practical relevance and feasibility of detailed behavioural studies inspired by the Umwelt-concept for application in conservation. However, the issue fundamentally relates to the way we consider habitat. The habitat is one of our basic concepts in ecology and evolutionary biology as it captures where an organism lives. However, there is quite some confusion about the definition of a habitat (Hall et al. 1997). Different habitat concepts have been applied, but often without carefully considering the underlying assumptions and simplifications (Dennis et al. 2003). The way we consider the habitat of an organism is strongly biased by our own, largely visual perception of the environment. Most ecologists, evolutionary biologists and conservationists treat habitat as being synonymous with a particular vegetation or land-cover category without questioning the underlying assumptions of the adopted habitat concept. Structural units as perceived by humans do not necessarily reflect the functional units of habitat for other organisms. This point has been appreciated within the context of dispersal across the landscape matrix because structural and functional connectivity – related to structural and functional landscape heterogeneity – have been distinguished in landscape ecology (e.g., Fahrig et al. 2011). The key issue here is that structure does not always equal function. However, the same philosophy should also apply to an organism's habitat (Dennis et al. 2003). Structural variation like vegetation types (or biotopes) can at most be proxies of the functional habitat of a species in particular cases, but in several other cases such a habitat approach is unable to realistically recognize what is the habitat of a species and to predict the presence of a species (e.g., Vanreusel and Van Dyck 2007).
A functional habitat model based on resource distributions and individual movements is the resource-based habitat concept (Dennis et al. 2003, 2006) and has attracted much recent interest (e.g., Fattorini 2010; Bates et al. 2011; Jarosik et al. 2011). Hence, a habitat from this viewpoint is the intersection and union of the necessary complementary resources (i.e., consumables and conditions) for an organism at all its life stages, where resources are connected by daily movements to give functional habitats by combination and overlap (Dennis et al. 2003). Conditions will include relevant physical, but also biotic parameters (e.g., relative to predation). So, the concept reconnects to the ecological niche by being the multi-dimensional, functional space of an organism (Hutchinson's hypervolume –Whittaker et al. 1973). The resource-based habitat is then the spatial projection on the ground of this functional space (Fig 1).
Figure 1.
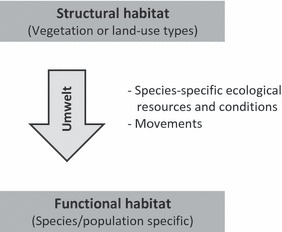
Scheme illustrating the key concepts of this paper. The Umwelt-concept refers to the observation from ethology that different organisms may perceive their environment different than do human observers. The organism's unique sensory world explains why different organisms can have different Umwelten, even though they share the same structural environment. Conservation biology often assumes that structural habitats reflect functional habitat. The resource-based habitat concept (seeDennis et al. 2003) offers a model that takes into account specific resources and conditions, as well as movements at the appropriate spatial scale and hence provides a means to translate human observed structural habitats into organism-centred functional habitat.
There are a growing number of papers stressing the importance of considering animal behaviour and the scale of habitat-use decisions when assessing landscape conditions (e.g., ‘a koala's eye view of spatial variation in habitat quality’–Moore et al. 2010; Borkin and Parsons 2011; Cardador et al. 2011; Popescu and Hunter 2011). The key point of the resource-based habitat approach is that it is an organism-centred approach that focuses on the behavioural interactions between the organism and the relevant part of its environment. This is of particular significance in rapidly changing anthropogenic landscapes because resource distributions and relevant conditions may change without obvious alteration in the general vegetation structures. These changes can be the result of (unintentional) interventions including conservation management, but also of several other human activities. Changes in resources may also occur in an indirect way through, for example, pollution effects. The combination of regional warming and atmospheric nitrogen deposition may, for example, make herbaceous vegetation grow more densely which in turn makes them too cold for larval development under spring conditions in a number of declining butterfly species (WallisDevries and van Swaay 2006).
From concepts to conservation practice
In this final section, I will discuss three issues to illustrate the practical significance for conservation of a functional habitat approach that takes into account the organism's perspective to the environment: (i) detecting additional conservation opportunities, (ii) avoiding failures of habitat restoration and (iii) implementing the functional habitat approach with Geographic Information Systems (GIS).
The fact that habitat is often perceived in a simplistic binary way as discrete patches of a particular vegetation type in a hostile landscape matrix may limit conservation opportunities (Dennis et al. 2006). Davison and Fitzpatrick (2010) provide an instructive example with the Florida Scrub-Jay (Aphelocoma coerulescens), a highly endangered habitat specialist bird that was known to be confined to remnant native scrub patches surrounded by agricultural and suburban landscapes. But against common belief among conservationists, they showed that regenerated pastures could also serve as suitable habitat (with equal breeding success), at least when adjacent to native scrub. The authors defended additional conservation strategies involving the relaxing of accepted definitions of suitable habitat for this species (Davison and Fitzpatrick 2010). The bigger issue here is that habitat definitions may constrain the opportunities for conservation and restoration in landscapes under intense human use.
Restoration actions may also deteriorate the habitat quality for threatened species that are unable to respond to altered conditions (e.g., predation risks) as a result of restoration. In other words, the structural changes in the environment do not necessarily alter the Umwelt of the threatened species. I will illustrate this with a recent study on the critically endangered desert lizard Acanthodactylus beershebensis (Hawlena et al. 2010). The lizard is an endemic of the Negev desert in Israel. The strong decline of the species occurred in parallel with a large landscape restoration project including the planting of trees. As the species disappeared from both natural and altered sites in the area, land managers argued that the decline could not have been caused by the afforestation. Hawlena et al. (2010) showed, however, experimental evidence with artificial trees that the increased structural complexity in planted patches favoured avian predation. Survival was reduced in plots with artificial trees. Natural perches are rare in the structurally simple arid habitat. It is argued that the lizards have not evolved to assess structural cues to evaluate elevated predation risks in this system. Emigrants were equally likely to settle in manipulated and unmanipulated sites. The study by Hawlena et al. (2010) demonstrates that local anthropogenic changes in habitat structure may have a considerable negative effect, even beyond the immediate area of action because it may induce ecological trapping affecting the entire network of local populations.
Conservation managers are accustomed to working with spatial information like vegetation units and land-use types in different GIS applications (i.e., Geographic Information System). However, by now it should be clear that making use of information on structural habitat may not be the same as functional habitat as it is perceived and used by species. However, we should not throw out the baby with the bath water. As we applied for heathland butterflies of conservation interest in a National park in NE-Belgium, one can make use of GIS-based data and modelling techniques to create resource-based functional habitat maps that more closely match the organism-specific environment than methods directly applying general vegetation types from land cover maps (Vanreusel and Van Dyck 2007). Moreover, the approach was found to be fruitful for ecological niche modelling as models based on a quantitative resource-based habitat approach were successfully transferred among nature reserves in the same region (Vanreusel et al. 2007). So, although it would require additional layers of information and some GIS-based calculations to combine the resource-based information and spatial scale of interaction with the environment, the final output of such an exercise will be a map with different functional habitat areas and indications of zones that lack particular resources or conditions (see Vanreusel and Van Dyck 2007 for an example). Therefore, although there can be a significant amount of ‘detailed’ autecology behind such a final map, the end product is not that different from what is currently often used by conservation practitioners. But the delineated zones based on a functional habitat approach may differ significantly from the generally adopted vegetation type or biotope approach (e.g., Turlure et al. 2010).
Evolutionary biologists rarely use the extensive environmental data and tools available from GIS and spatial land cover databases for dealing with environmental variation. For a detailed discussion and review on how evolutionary studies can integrate GIS-based data and approaches, I refer to Kozak et al. (2008). Evolutionary studies along environmental gradients and mosaics that adopt such a functional, resource-based habitat approach provide a very interesting scope for a better mechanistic understanding of the match and mismatch of different phenotypes and their environment in anthropogenic landscapes.
In summary, the way several ecological, conservation and evolutionary studies perceive and treat the habitat of organisms is a clear example of simplistic, typological thinking. Moreover, there is a strong bias towards human perception in terms of vegetation or land-use types. Such an approach can be ignorant to the specific sensory information different organisms will use (i.e., their Umwelt). A functional habitat concept based on the ecological resources and conditions a population (or species) will use offers a better perspective for the recognition of functional habitat compared with the use of vegetation or land-use types (i.e., general structural habitats) as a proxy. Such an approach may detect conservation opportunities and help avoid failures of habitat restoration for endangered species. I suggest that functional habitat approaches and the Umwelt concept need to be essential ingredients of evolutionary conservation programs, as they will help creating better conditions for this single living planet with its multiple perceptual worlds.
Acknowledgments
Thanks are due to the guest editors of this special volume for the invitation to write this paper. This work was funded by an ARC-research grant of the Académie Louvain to HVD (ARC Grant 10/15-031). Thanks are also due to three anonymous reviewers for their valuable comments and to Kate Mitchell for language editing. This is publication no BRC 236 of the Biodiversity Research Centre (UCL, Louvain-la-Neuve).
Literature cited
- Agudo R, Rico C, Vilà C, Hiraldo F, Donazar JA. The role of humans in the diversification of a threatened island raptor. BMC Evolutionary Biology. 2010;10:e384. doi: 10.1186/1471-2148-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MV, Willson MF, Pergams ORW, O'Dowd DJ, Gende SM, Brown JS. Evolutionarily enlightened management. Biological Conservation. 2003;111:115–123. [Google Scholar]
- Baguette M, Van Dyck H. Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landscape Ecology. 2007;22:1117–1129. [Google Scholar]
- Baker JA, Heins DC, King RW, Foster SA. Rapid shifts in multiple life history traits in a population of threespine stickleback. Journal of Evolutionary Biology. 2011;24:863–870. doi: 10.1111/j.1420-9101.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- Barber JR, Crooks KR, Fristrup KM. The costs of chronic noise exposure for terrestrial organisms. Trends in Ecology and Evolution. 2010;25:180–189. doi: 10.1016/j.tree.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Bates AJ, Sadler JP, Fairbrass AJ, Falk SJ, Hale JD, Matthews TJ. Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE. 2011;6:e23459. doi: 10.1371/journal.pone.0023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes J, Kepka P, Konvicka M. Limestone quarries as refuges for European xerophilous butterflies. Conservation Biology. 2003;17:1058–1069. [Google Scholar]
- Bonte D, De Clercq N, Zwertvaegher I, Lens L. Repeatability of dispersal behaviour in a common dwarf spider: evidence for different mechanisms behind short and long distance dispersal. Ecological Entomology. 2009;34:271–276. [Google Scholar]
- Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, et al. Costs of dispersal. Biological Reviews. 2011 doi: 10.1111/j.1469-185X.2011.00201.x. (in press) [DOI] [PubMed] [Google Scholar]
- Boogert NJ, Paterson DM, Laland KN. The implications of niche construction and ecosystem engineering for conservation biology. BioScience. 2006;56:1–9. [Google Scholar]
- Borkin KM, Parsons S. Home range and habitat selection by a threatened bat in exotic plantation forest. Forest Ecology and Management. 2011;262:845–852. [Google Scholar]
- Cardador L, Carrete M, Manosa S. Can intensive agricultural landscapes favour raptor species? The Marsh harrier in north-eastern Spain. Animal Conservation. 2011;14:382–390. [Google Scholar]
- Carroll SP. Facing change: forms and foundations of contemporary adaptation to biotic invasions. Molecular Ecology. 2008;17:361–372. doi: 10.1111/j.1365-294X.2007.03484.x. [DOI] [PubMed] [Google Scholar]
- Carroll SP, Fox CW. Conservation Biology: Evolution in Action. Oxford: Oxford University Press; 2008. [Google Scholar]
- Dall SRX, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. Information and its use by animals in evolutionary ecology. Trends in Ecology and Evolution. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. Human predators outpace agents of trait change in the wild. Proceedings of the National Academy of Sciences, USA. 2009;106:952–954. doi: 10.1073/pnas.0809235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison MA, Fitzpatrick JW. Role of human-modified habitat in protecting specialist species: a case study in the threatened Florida Scrub-Jay. Biological Conservation. 2010;143:2815–2822. [Google Scholar]
- Delgado MDM, Ratikainen II, Kokko H. Inertia: the discrepancy between individual and common good in dispersal and prospecting behaviour. Biological Reviews. 2011;86:717–732. doi: 10.1111/j.1469-185X.2010.00167.x. [DOI] [PubMed] [Google Scholar]
- Dempster JP. Evidence of an oviposition-deterring pheromone in the orange-tip butterfly, Anthocharis cardamines (L.) Ecological Entomology. 1992;17:83–85. [Google Scholar]
- Dennis RLH, Shreeve TG, Van Dyck H. Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos. 2003;102:417–426. [Google Scholar]
- Dennis RLH, Shreeve TG, Van Dyck H. Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodiversity & Conservation. 2006;15:1943–1966. [Google Scholar]
- Ellner SP, Geber MA, Hairston NG., Jr Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecology Letters. 2011;14:603–614. doi: 10.1111/j.1461-0248.2011.01616.x. [DOI] [PubMed] [Google Scholar]
- Fahrig L. Non-optimal animal movement in human-altered landscapes. Functional Ecology. 2007;21:1003–1015. [Google Scholar]
- Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, et al. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecology Letters. 2011;14:101–112. doi: 10.1111/j.1461-0248.2010.01559.x. [DOI] [PubMed] [Google Scholar]
- Fattorini S. Biotope prioritisation in the Central Apennines (Italy): species rarity and cross-taxon congruence. Biodiversity and Conservation. 2010;19:3413–3429. [Google Scholar]
- Ghalambor CK, McKay KK, Carroll SP, Reznick DN. Adaptive versus non-adaptive plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Gibbs M, Van Dyck H. Butterfly flight activity affects reproductive performance and longevity relative to landscape structure. Oecologia. 2010;163:341–350. doi: 10.1007/s00442-010-1613-5. [DOI] [PubMed] [Google Scholar]
- Gilroy JJ, Sutherland WJ. Beyond ecological traps: perceptual errors and undervalued resources. Trends in Ecology and Evolution. 2007;22:351–356. doi: 10.1016/j.tree.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Grandin T, Johnson C. Animals in Translation. New York: Scribner; 2005. [Google Scholar]
- Hairston NG, Jr, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecology Letters. 2005;8:1114–1127. [Google Scholar]
- Hall LS, Krausman PR, Morrison ML. The habitat concept and a plea for standard terminology. Wildlife Society Bulletin. 1997;25:173–182. [Google Scholar]
- Hawlena D, Saltz D, Abramsky Z, Bouskila A. Ecological trap for desert lizards caused by anthropogenic changes in habitat structure that favor predator activity. Conservation Biology. 2010;24:803–809. doi: 10.1111/j.1523-1739.2010.01477.x. [DOI] [PubMed] [Google Scholar]
- Heinz SK, Strand E. Adaptive patch searching strategies in fragmented landscapes. Evolutionary Ecology. 2006;20:113–130. [Google Scholar]
- Hendry AP, Lohmann LG, Conti E, Cracraft J, Crandall KA, Faith DP, Häuser C, et al. Evolutionary biology in biodiversity science, conservation, and policy: a call to action. Evolution. 2010;64:1517–1528. doi: 10.1111/j.1558-5646.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- Hobbs RJ, Arico S, Aronson J, Baron JS, et al. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography. 2006;15:1–7. [Google Scholar]
- Hoffmann AA, Sgro CM. Climate change and evolutionary adaptation. Nature. 2011;470:479–485. doi: 10.1038/nature09670. [DOI] [PubMed] [Google Scholar]
- Hölker F, Wolter C, Perkin EK, Tockner K. Light pollution as a biodiversity threat. Trends in Ecology and Evolution. 2010;25:681–682. doi: 10.1016/j.tree.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Hollander F, Van Dyck H, San Martin G, Titeux N. Maladaptive habitat selection of a migratory passerine bird in a human-modified landscape. PLoS ONE. 2011;6:e25703. doi: 10.1371/journal.pone.0025703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath G, Kriska G, Malik P, Robertson B. Polarized light pollution: a new kind of ecological photopollution. Frontiers in Ecology and the Environment. 2009;7:317–325. [Google Scholar]
- Jarosik V, Konvicka M, Pysek P, Kadlec T, Benes J. Conservation in the city: do the same principles apply to different taxa? Biological Conservation. 2011;144:490–499. [Google Scholar]
- Jones KE, Purvis A, Gittleman JL. Biological correlates of extinction risk in bats. The American Naturalist. 2003;161:601–614. doi: 10.1086/368289. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Van Dyck H. Does habitat fragmentation affect temperature-related life-history traits? A laboratory test with a woodland butterfly. Proceedings of the Royal Society B: Biological Sciences. 2005;272:1257–1263. doi: 10.1098/rspb.2005.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:444–454. [Google Scholar]
- Kozak KH, Graham CH, Wiens JJ. Integrating GIS-based environmental data into evolutionary biology. Trends in Ecology and Evolution. 2008;23:141–148. doi: 10.1016/j.tree.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Kriska G, Csabai Z, Boda P, Malik PG. Why do red and dark-coloured cars lure aquatic insects? The attraction of water insects to car paintwork explained by reflection-polarization signals. Proceedings of the Royal Society of London B. 2006;273:1667–1671. doi: 10.1098/rspb.2006.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriska G, Malik P, Szivak I, Horvath G. Glass buildings on river banks as ‘polarized light traps’ for mass-swarming polarotactic caddisflies. Naturwissenschaften. 2008;95:461–467. doi: 10.1007/s00114-008-0345-4. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee FJ, Feldman MW. Evolutionary consequences of niche construction and their implications for ecology. Proceedings of the National Academy of Sciences, USA. 1999;96:10242–10247. doi: 10.1073/pnas.96.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau R, Jorgensen PS, Harris DJ, Sih A. Incorporating evolutionary principles into environmental management and policy. Evolutionary Applications. 2011;4:315–325. doi: 10.1111/j.1752-4571.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidner AK, Haddad NM. Natural, not urban, barriers define population structure for a coastal endemic butterfly. Conservation Genetics. 2010;11:2311–2320. [Google Scholar]
- Lima SL, Zollner PA. Towards a behavioral ecology of ecological landscapes. Trends in Ecology & Evolution. 1996;11:131–135. doi: 10.1016/0169-5347(96)81094-9. [DOI] [PubMed] [Google Scholar]
- Longcore T, Rich C. Ecological light pollution. Frontiers in Ecology and the Environment. 2004;2:191–198. [Google Scholar]
- Mace GM, Purvis A. Evolutionary biology and practical conservation: bridging a widening gap. Molecular Ecology. 2008;17:9–19. doi: 10.1111/j.1365-294X.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- Manning AD, Lindenmayer DB, Nix HA. Continua and Umwelt: novel perspectives on viewing landscapes. Oikos. 2004;104:621–628. [Google Scholar]
- Merckx T, Van Dyck H. Habitat fragmentation affects habitat-finding ability of the speckled wood butterfly (Pararge aegeria L.) Animal Behaviour. 2007;74:1029–1037. [Google Scholar]
- Møller AP. The fitness benefit of association with humans: elevated success of birds breeding indoors. Behavioral Ecology. 2010;21:913–918. [Google Scholar]
- Moore BD, Lawler IR, Wallis IR, Beale CM, Foley WJ. Palatability mapping: a koala's eye view of spatial variation in habitat quality. Ecology. 2010;91:3165–3176. doi: 10.1890/09-1714.1. [DOI] [PubMed] [Google Scholar]
- Olden JD, Schooley RL, Monroe JB, Poff NL. Context-dependent perceptual ranges and their relevance to animal movements in landscapes. Journal of Animal Ecology. 2004;73:1190–1194. [Google Scholar]
- Patten MA, Kelly JF. Habitat selection and the perceptual trap. Ecological Applications. 2010;20:2148–2156. doi: 10.1890/09-2370.1. [DOI] [PubMed] [Google Scholar]
- Popescu VD, Hunter ML. Clear-cutting affects habitat connectivity for a forest amphibian by decreasing permeability to juvenile movements. Ecological Applications. 2011;21:1283–1295. doi: 10.1890/10-0658.1. [DOI] [PubMed] [Google Scholar]
- Purvis A, Agapow PM, Gittleman JL, et al. Nonrandom extinction and the loss of evolutionary history. Science. 2000;288:328–330. doi: 10.1126/science.288.5464.328. [DOI] [PubMed] [Google Scholar]
- Robertson B, Kriska G, Horvath V, Horvath G. Glass buildings as bird feeders: urban birds exploit insects trapped by polarized light pollution. Acta Zoologica Academiae Scientiarum Hungaricae. 2010;56:283–293. [Google Scholar]
- Romero S, Campbell JF, Nechols JR, With KA. Movement behavior in response to landscape structure: the role of functional grain. Landscape Ecology. 2009;24:39–51. [Google Scholar]
- Ronce O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annual Review of Ecology, Evolution and Systematics. 2007;38:231–253. [Google Scholar]
- Rowley-Conwy P, Layton R. Foraging and farming as niche construction: stable and unstable adaptations. Philosophical Transactions of the Royal Society B. 2011;366:849–862. doi: 10.1098/rstb.2010.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer MA, Runge MC, Sherman PW. Ecological and evolutionary traps. Trends in Ecology and Evolution. 2002;17:474–480. [Google Scholar]
- Siemers BM, Schaub A. Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proceedings of the Royal Society B: Biological Sciences. 2011;278:1646–1652. doi: 10.1098/rspb.2010.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Ferrari MCO, Harris DJ. Evolution and behavioural responses to human-induced rapid environmental change. Evolutionary Applications. 2011;4:367–387. doi: 10.1111/j.1752-4571.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H, Ripmeester EAP. Birdsong and anthropogenic noise: implications and applications for conservation. Molecular Ecology. 2008;17:72–83. doi: 10.1111/j.1365-294X.2007.03487.x. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Bouton N, Van Opzeeland I, Coers A, Ten Cate C, Propper AN. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends in Ecology and Evolution. 2010;25:419–427. doi: 10.1016/j.tree.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Stamps JA, Krishnan VV, Reid ML. Search costs and habitat selection by dispersers. Ecology. 2005;86:510–518. [Google Scholar]
- Thomas JA. Holocene climate change and warm man-made refugia explain why a sixth of British butterflies inhabit unnatural early successional habitats. Ecography. 1993;16:278–284. [Google Scholar]
- Thomas CD. Dispersal and extinction in fragmented landscapes. Proceedings of the Royal Society B: Biological Sciences. 2000;267:139–145. doi: 10.1098/rspb.2000.0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410–433. [Google Scholar]
- Turlure C, Choutt J, Van Dyck H, Baguette M, Schtickzelle N. Functional habitat area as a reliable proxy for population size: case study using two butterfly species of conservation concern. Journal of Insect Conservation. 2010;14:379–388. [Google Scholar]
- Tuxbury SM, Salmon M. Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biological Conservation. 2005;121:311–316. [Google Scholar]
- Van Dyck H, Baguette M. Dispersal behaviour in fragmented landscapes: routine or special movements? Basic and Applied Ecology. 2005;6:535–545. [Google Scholar]
- Van Dyck H, Van Strien AJ, Maes D, Van Swaay CAM. Declines in common, widespread butterflies in a landscape under intense human use. Conservation Biology. 2009;23:957–965. doi: 10.1111/j.1523-1739.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- Vandewoestijne S, Van Dyck H. Population genetic differences along a latitudinal cline between original and recently colonized habitat in a butterfly. PLoS ONE. 2010;5:e13810. doi: 10.1371/journal.pone.0013810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanreusel W, Van Dyck H. When functional habitat does not match vegetation types: a resource-based approach to map butterfly habitat. Biological Conservation. 2007;135:202–211. [Google Scholar]
- Vanreusel W, Maes D, Van Dyck H. Transferability of species distribution models: a functional habitat approach for two regionally threatened butterflies. Conservation Biology. 2007;21:201–212. doi: 10.1111/j.1523-1739.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- Von Uexküll J. Umwelt und Innenwelt der Tiere. Berlin: J. Springer; 1909. [Google Scholar]
- WallisDevries MF, van Swaay CAM. Global warming and excess nitrogen may induce butterfly decline by microclimatic cooling. Global Change Biology. 2006;12:1620–1626. [Google Scholar]
- Whittaker RH, Levin SA, Roost RB. Niche, habitat and ecotope. The American Naturalist. 1973;107:321–338. [Google Scholar]
- Yeh PJ, Price TD. Adaptive phenotypic plasticity and the successful colonization of a novel environment. The American Naturalist. 2004;164:531–542. doi: 10.1086/423825. [DOI] [PubMed] [Google Scholar]
- Zollner PA. Comparing the landscape level perceptual abilities of forest sciurids in fragmented agricultural landscapes. Landscape Ecology. 2000;15:523–533. [Google Scholar]


