Abstract
Background
Distinct receptors likely exist for leukotriene(LT)E4, a potent mediator of airway inflammation. Purinergic receptor P2Y12 is needed for LTE4-induced airways inflammation, and P2Y12 antagonism attenuates house dust mite-induced pulmonary eosinophilia in mice. Although experimental data support a role for P2Y12 in airway inflammation, its role in human asthma has never been studied.
Objective
To test for association between variants in the P2Y12 gene (P2RY12) and lung function in human subjects with asthma, and to examine for gene-by-environment interaction with house dust mite exposure.
Methods
19 single nucleotide polymorphisms (SNPs) in P2RY12 were genotyped in 422 children with asthma and their parents (n=1266). Using family-based methods, we tested for associations between these SNPs and five lung function measures. We performed haplotype association analyses and tested for gene-by-environment interactions using house dust mite exposure. We used the false discovery rate to account for multiple comparisons.
Results
Five SNPs in P2RY12 were associated with multiple lung function measures (P values 0.006–0.025). Haplotypes in P2RY12 were also associated with lung function (P values 0.0055–0.046). House dust mite exposure modulated associations between P2RY12 and lung function, with minor allele homozygotes exposed to house dust mite demonstrating worse lung function than those unexposed (significant interaction P values 0.0028–0.040).
Conclusions and clinical relevance
P2RY12 variants were associated with lung function in a large family-based asthma cohort. House dust mite exposure caused significant gene-by-environment effects. Our findings add the first human evidence to experimental data supporting a role for P2Y12 in lung function. P2Y12 could represent a novel target for asthma treatment.
Keywords: Purinergic receptor, leukotriene, asthma, house dust mite, lung function
INTRODUCTION
Leukotrienes are eicosanoid lipid mediators that contribute to inflammation in asthma and atopic disorders. The cysteinyl leukotrienes LTC4, LTD4, and LTE4 cause inflammation, smooth muscle contraction, and bronchoconstriction that characterize asthma [1].
In contrast to LTC4 and LTD4, LTE4 is stable and abundant in biologic fluid such as bronchoalveolar fluid and urine of asthmatic individuals [2]. Although LTE4 is a weak agonist at the classical receptors for cysteinyl leukotrienes (CysLT1 and CysLT2), LTE4 potently induces airways hyperresponsiveness and mucosal eosinophilia [3], and urinary levels of LTE4 are used to gauge therapeutic response in asthmatic patients [4]. Asthmatic airways exhibit selective responsiveness to LTE4, in contrast to LTC4 and LTD4 [5]. Although evidence points to LTE4 playing a key role in airway inflammation, our understanding of its potential receptor(s) and their biology remain limited [1]. The modest effect of current leukotriene receptor antagonists, which block only CysLT1, could be due to uninhibited effects of LTE4 in asthmatics [6].
Purinergic receptor P2Y12 is a G-protein coupled receptor that may play a key role in LTE4-induced airway inflammation. Better known for its role as the receptor for adenosine diphosphate that is targeted by thienopyridine antiplatelet agents such as clopidogrel and prasugrel, P2Y12 has mostly been studied in cardiovascular and cerebrovascular disease [7–11]. However, in silico data highlighted P2Y12 as a candidate LTE4 receptor [12], with subsequent in vitro and murine data supporting a role for P2Y12 in LTE4-induced mast cell activation, goblet cell metaplasia, eosinophilia, and IL13 production [3]. However, the role of P2Y12 in human asthma has not been explored, and there have been no genetic studies of the P2Y12 gene (P2RY12) in asthmatic subjects.
In this study, we report a genetic epidemiologic analysis of P2RY12 in a large family-based cohort of 422 children with asthma and their parents (1266 total subjects). We found that genetic variants in P2RY12 are associated with lung function. Given the likelihood that asthma and lung function are governed by both genetic and environmental forces, we were also interested in examining gene-by-environment effects for P2RY12. As experimental data demonstrate house dust mite allergen to be a potent stimulator of cysteinyl leukotriene production [13], and P2Y12 antagonism attenuates pulmonary inflammation induced by intranasal house dust mite allergen [3], we specifically examined for interactions with house dust mite exposure. We found that the effects of P2RY12 variants on lung function are modulated by subjects’ house dust mite exposure.
METHODS
Study Population
The Childhood Asthma Management Program (CAMP) is a multicenter North American clinical trial designed to investigate the long-term effects of inhaled anti-inflammatory medications in children with mild to moderate asthma [14]. Participating children had asthma defined by symptoms greater than 2 times per week, use of an inhaled bronchodilator at least twice weekly or use of daily medication for asthma, and increased airway responsiveness to methacholine (PC20 ≤ 12.5 mg/ml). Children with severe asthma or other clinically significant medical conditions were excluded. Of the 1041 children enrolled in the original clinical trial, 968 children and 1518 of their parents contributed DNA samples to the CAMP Genetics Ancillary Study [15]. Selection criteria for genotyping were (a) self-described non-Hispanic white ethnicity and (b) availability of sufficient DNA for microarray hybridization; 422 children and their parents (n=1266) met these criteria. Written informed consent was obtained from study participants. The institutional review boards of Brigham & Women’s Hospital and the eight CAMP Study Centers approved the study protocols.
Measures
Phenotypic data were collected from each child in CAMP at randomization. A complete description of the trial design and methodology of CAMP has been previously published [14]. Participating children stopped all asthma medications except for as needed albuterol 6 weeks prior to randomization. Methacholine challenge was performed 2 weeks prior to randomization, and spirometry was performed at randomization. Relevant to this study, spirometry (including measurements for forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), bronchodilator response (BDR)), methacholine challenge, and skin prick tests were performed on all children. BDR was expressed as the percentage change in FEV1 as follows: BDR = (Post-FEV1 - pre-FEV1)/pre-FEV1. House dust samples were collected by vacuuming the patient’s mattress, bedroom floor, family room floor, kitchen floor, and upholstered furniture.
Genotyping
SNP genotyping for CAMP subjects and their parents was performed on Illumina Human-Hap550 Genotyping BeadChip (Illumina, Inc., San Diego, CA). Duplicate genotyping was performed on 5% of the sample to assess genotype reproducibility. Genotype quality control was assessed by <1% discordance rate, <5 Mendelian inconsistencies, and genotype completion rates >98% for all loci.
19 SNPs in P2RY12 and its 20kb flanks with minor allele frequency >5% were available and selected for analysis. All SNPs were in Hardy-Weinberg equilibrium (p > 0.01). Of the 422 nuclear families in CAMP, 25 were excluded from this analysis because of Mendelian inconsistencies (n= 6) or missing >5% of genotypic data (n = 19), leaving 397 families for analysis. Linkage disequilibrium (LD) patterns were plotted using Haploview [16].
Statistical Analyses
Statistical calculations for the baseline characteristics of subjects were performed using R v.2.9.0 [17]. We performed family-based testing for association between SNPs in P2RY12 and five lung function measures (BDR, FEV1, FVC, FEV1/FVC, PC20) under a recessive genetic model using the Pedigree-Based Association Test (PBAT) [18] implemented in Helix Tree v6.4.3 (Golden Helix, Bozeman, MT). Analyses were adjusted for age, sex, and height. Standard phenotypic residuals were used as the offset to maximize power. An advantage of family-based association testing is that it is robust against population stratification and population admixture [19]. To account for potential false positives from multiple testing, we used the false discovery rate method [20], a strategy considered to be more powerful than Bonferroni correction [21].
Haplotypes in P2RY12 were defined using the confidence interval method [22]. Family-based haplotype association analyses were conducted in PBAT [18] using the FBAT Generalized Estimating Equations test parameter [23].
We tested for gene-by-environment interaction between house dust mite exposure and P2RY12 associations with lung function measures using the family-based association test of interaction (FBAT-I) implemented in PBAT [18]. Exposure to house dust mite allergen (Dermatophagoides pteronyssinus) was defined in two ways: dichotomized at the 4th quartile of measured Der p level (1.85 µg/g) and dichotomized at 10 µg/g or greater. Previous studies have shown that 10µg/g of house dust mite exposure influences asthma exacerbations [24,25], and this level has been used in recent studies of gene-by-environment interactions [26,27]. However, given the skewed distribution of measured Der p levels in our cohort and the relatively small number of subjects exposed to 10µg/g of Der p or greater, we also employed the quartile definition to augment power.
RESULTS
The baseline characteristics of the index children in CAMP are shown in Table 1. Consistent with the known gender distribution of asthma in children, there were more boys than girls. CAMP children were highly atopic, with 88% of subjects exhibiting a positive skin test reaction to at least one allergen, and 47% positive to house dust mite in particular. There were no significant differences in physical characteristics or lung function between subjects exposed and unexposed to house dust mite using either definition of house dust mite exposure. As would be expected, there was a higher prevalence of skin test reaction to D. pteronyssinus and D. farinae among subjects exposed to house dust mite.
Table 1.
Baseline characteristics of index children in the Childhood Asthma Management Program
| All subjects (n=422) |
Quartile definition of Der p exposure* | 10µg/g definition of Der p exposure* | |||||
|---|---|---|---|---|---|---|---|
| Exposed (n=100) |
Unexposed (n=300) |
P value^ | Exposed (n=44) |
Unexposed (n=356) |
P value^ | ||
| Age (years) | 8.7 (2.1) | 8.6 (2.0) | 8.8 (2.1) | 0.37 | 8.7 (2.0) | 8.8 (2.1) | 0.87 |
| Sex (female) | 156 (37%) | 37 (0.37) | 113 (0.38) | 0.91 | 16 (36%) | 134 (38%) | 0.87 |
| Height (cm) | 132.5 (13.5) | 131.4 (14.0) | 132.9 (13.6) | 0.34 | 131.7 (13.5) | 132.7 (13.7) | 0.65 |
| Weight (cm) | 31.9 (10.6) | 31.7 (11.0) | 32.0 (10.5) | 0.79 | 30.9 (9.1) | 32.0 (10.8) | 0.45 |
| FEV1 (L) | 1.63 (0.46) | 1.63 (0.48) | 1.63 (0.46) | 0.99 | 1.58 (0.44) | 1.64 (0.47) | 0.42 |
| FVC (L) | 2.07 (0.61) | 2.08 (0.66) | 2.08 (0.60) | 0.96 | 2.00 (0.64) | 2.09 (0.62) | 0.38 |
| FEV1/FVC | 79.4 (8.5) | 79.9 (8.4) | 79.3 (8.4) | 0.50 | 80.7 (8.8) | 79.3 (8.4) | 0.32 |
| lnPC20 (mg/ml) | 0.025 (1.15) | −0.053 (1.14) | 0.057 (1.15) | 0.41 | −0.016 (1.11) | 0.035 (1.15) | 0.78 |
| BDR (%) | 10.8 (10.4) | 9.7 (8.6) | 11.1 (10.7) | 0.18 | 10.6 (8.9) | 10.8 (10.4) | 0.88 |
| Physician visit for asthma in past year | 394 (98%) | 97 (0.97) | 279 (0.93) | 0.08 | 43 (98%) | 333 (94%) | 0.11 |
| Any positive STR | 370 (88%) | 83 (0.83) | 268 (0.89) | 0.13 | 38 (86%) | 313 (88%) | 0.78 |
| Der p STR | 197 (47%) | 68 (0.68) | 122 (0.41) | 1.5×10−6 | 33 (75%) | 157 (44%) | 5.7×10−5 |
| Der f STR | 195 (46%) | 64 (0.64) | 124 (0.41) | 7.8×10−5 | 31 (70%) | 157 (44%) | 8.1×10−4 |
| Der p level in house dust (µg/g) | 3.67 (9.31) | 13.69 (14.63) | 0.32 (0.36) | 8.4×10−15 | 25.15 (15.69) | 1.01 (1.85) | 4.6×10−13 |
Numbers are mean (standard deviation) or number (percent)
House dust mite exposure dichotomized at 4th quartile of measured Der p levels (1.85 µg/g) for quartile definition of exposure. House dust mite exposure dichotomized at Der p level of 10µg/g for 10µg/g definition. Levels not available for 22 subjects.
P value for comparison between house dust mite exposed and unexposed
STR = skin test reaction
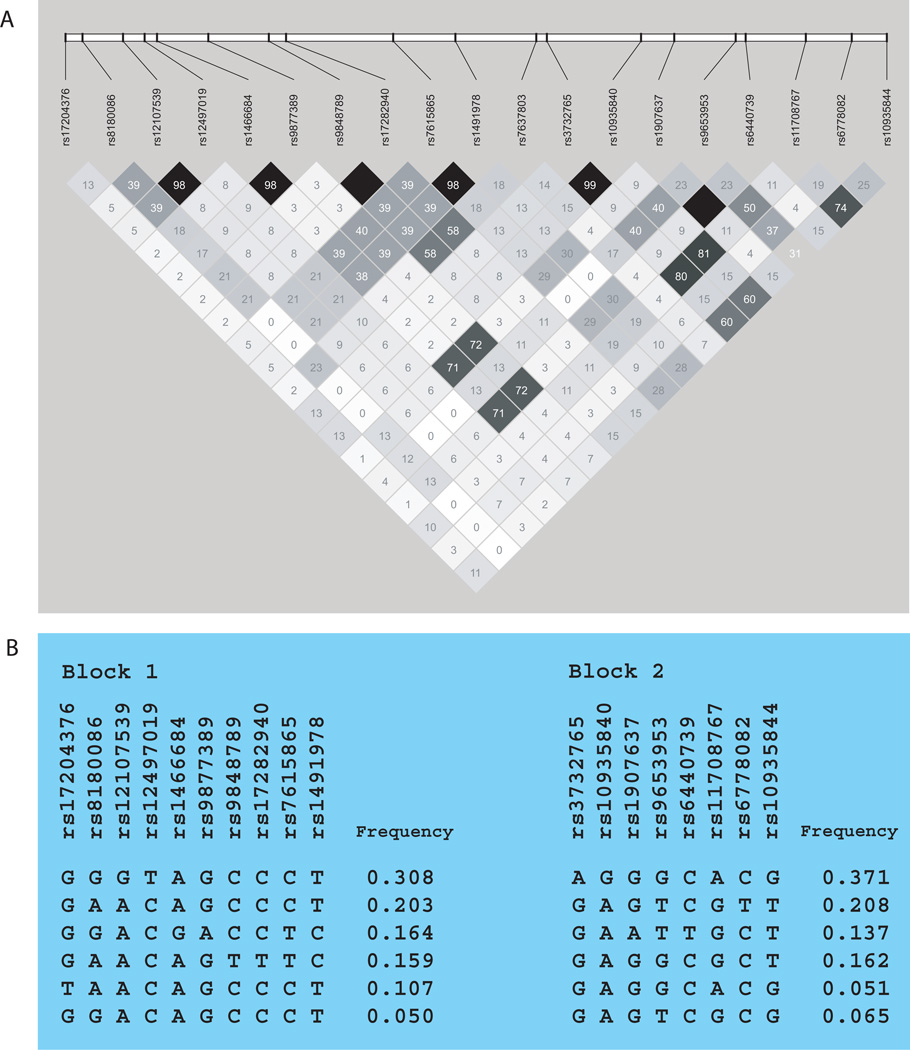
The linkage disequilibrium (LD) patterns for the P2RY12 SNPs genotyped are shown in Fig. 1a. There was high LD between a few SNP pairs, but no large LD blocks within the gene and its 20kb flanks.
Fig. 1.
(A) Linkage disequilibrium plot for single nucleotide polymorphisms (SNPs) in P2RY12 and its 20kb flanks. r2 values for pairwise correlation are shown. (B) Haplotype blocks for P2RY12. Two blocks were defined using the confidence interval method. Haplotypes for each block are shown in order of frequency.
The results for association testing between P2RY12 SNPs and lung function measures BDR, FEV1, and FEV1/FVC are shown in Table 2. The region between 152541653 base pairs and 152571916 base pairs contained five SNPs (rs9848789, rs17282940, rs7615865, rs1491978, rs7637803) that were each associated with lung function. Minor allele homozygotes for these SNPs had better lung function, with lower BDR and higher FEV1 and FEV1/FVC. There was high LD only between the first two SNPs in this region (rs9848789 and rs17282940, r2 = 1.0) and between the second two SNPs (rs7615865 and rs14919878, r2 = 0.98) (Fig. 1a). The Q values for these associations ranged from 0.11 to 0.12, suggesting that about one in ten of these associations could be false discoveries due to multiple comparisons. Additionally, there was a suggestion for association between rs7637803 and PC20 (P value 0.04) and between rs9653953 and FVC (P value = 0.03). Association tests for PC20, FVC and other SNPs were otherwise nonsignificant.
Table 2.
Association between P2RY12 SNPs and lung function measures
| Marker | Allele | Frequency | BDR | FEV1 | FEV1/FVC | |||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | |||
| rs17204376 | T | 0.09 | −0.015 (0.017) | 0.82 | 0.047 (0.045) | 0.74 | −0.37 (1.589) | 0.24 |
| rs8180086 | A | 0.46 | −0.011 (0.014) | 0.90 | 0.024 (0.022) | 0.47 | 0.63 (0.975) | 0.81 |
| rs12107539 | G | 0.32 | 0.011 (0.013) | 0.07 | −0.001 (0.021) | 0.07 | −0.97 (0.867) | 0.018 |
| rs12497019 | T | 0.31 | 0.011 (0.013) | 0.07 | −0.003 (0.021) | 0.07 | −0.89 (0.871) | 0.021 |
| rs1466684 | G | 0.18 | 0.005 (0.013) | 0.92 | −0.036 (0.028) | 0.13 | 0.16 (0.974) | 0.97 |
| rs9877389 | A | 0.17 | 0.004 (0.013) | 0.92 | −0.034 (0.028) | 0.14 | 0.23 (0.984) | 0.96 |
| rs9848789 | T | 0.16 | −0.044 (0.024) | 0.006 | 0.071 (0.039) | 0.025 | 2.68 (1.675) | 0.012 |
| rs17282940 | T | 0.16 | −0.044 (0.024) | 0.006 | 0.070 (0.038) | 0.025 | 2.68 (1.656) | 0.012 |
| rs7615865 | T | 0.33 | −0.020 (0.013) | 0.019 | 0.017 (0.023) | 0.64 | 1.53 (0.934) | 0.009 |
| rs1491978 | C | 0.33 | −0.019 (0.013) | 0.023 | 0.019 (0.023) | 0.71 | 1.59 (0.939) | 0.007 |
| rs7637803 | T | 0.21 | −0.020 (0.018) | 0.008 | 0.039 (0.032) | 0.017 | 1.23 (1.274) | 0.058 |
| rs3732765 | A | 0.36 | −0.002 (0.009) | 0.91 | 0.017 (0.020) | 0.96 | −0.42 (0.768) | 0.33 |
| rs10935840 | G | 0.36 | −0.002 (0.009) | 0.92 | 0.018 (0.020) | 0.96 | −0.30 (0.768) | 0.36 |
| rs1907637 | A | 0.14 | 0.006 (0.012) | 0.75 | −0.015 (0.031) | 0.22 | 0.37 (0.998) | 0.91 |
| rs9653953 | T | 0.42 | 0.011 (0.016) | 0.29 | −0.029 (0.027) | 0.047 | −0.13 (0.932) | 0.36 |
| rs6440739 | T | 0.14 | 0.007 (0.012) | 0.75 | −0.016 (0.031) | 0.22 | 0.32 (0.997) | 0.91 |
| rs11708767 | A | 0.41 | −0.002 (0.009) | 0.89 | 0.014 (0.020) | 0.86 | −0.45 (0.739) | 0.69 |
| rs6778082 | T | 0.20 | 0.007 (0.017) | 0.96 | −0.023 (0.030) | 0.33 | 0.42 (1.277) | 0.99 |
| rs10935844 | G | 0.49 | 0.007 (0.010) | 0.56 | −0.003 (0.020) | 0.58 | −1.32 (0.714) | 0.27 |
Significant associations are bolded
SE = Standard Error
Haplotype blocks for P2RY12 are shown in Fig. 1b. Table 3 shows the results for association testing between the haplotype blocks and lung function measures. There were significant associations between both haplotype blocks and BDR, FEV1, and FEV1/FVC.
Table 3.
Association between P2RY12 haplotypes and lung function measures
| Haplotype | BDR | FEV1 | FEV1/FVC | |||
|---|---|---|---|---|---|---|
| GSS | P value | GSS | P value | GSS | P value | |
| Block 1 | 7.53 | 0.030 | 4.78 | 0.0055 | 4.78 | 0.046 |
| Block 2 | 2.90 | 0.017 | 3.44 | 0.032 | 3.44 | 0.037 |
Significant associations are bolded
GSS = Global Score Statistic
Table 4 shows the results for gene-by-environment analyses. The effect of house dust mite exposure at the fourth quartile or greater on the association between P2RY12 SNPs and lung function measures was significant for six SNPs (P values 0.0028–0.044). This level of house dust mite exposure modified the relationship between four SNPs in P2RY12 (rs8180086, rs3732765, rs10935840, rs11708767) and airway hyperresponsiveness (PC20), and between two SNPs in P2RY12 (rs7615865, rs1491978) and BDR. The effect of house dust mite exposure dichotomized at 10µg/g on the association between P2RY12 SNPs and lung function measures was significant for five SNPs (P values 0.018–0.040). House dust mite exposure modified the relationship between three SNPs in P2RY12 (rs3732765, rs10935840, rs11708767) and airway hyperresponsiveness (PC20), and between two SNPs in P2RY12 (rs10935844, rs8180086) and FVC. The association between 3 SNPs (rs3732765, rs10935840, rs11708767) and airway hyperresponsiveness was modified by house dust mite exposure regardless of house dust mite definition exposure used. The overall and exposure stratified results for association testing are additionally shown in Table 4. For ease of viewing, SNPs and phenotypes without significant interaction P values are not listed in this table.
Table 4.
Gene-by-environment effect of house dust mite exposure on associations between P2RY12 SNPs and lung function measures
| Measure | Marker | Allele | Quartile definition of house dust mite exposure^ | 10 µg/g definition of house dust mite exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Association test P values | Interaction | Association test P values | Interaction | ||||||||
| All | Exposed | Unexposed | β(SE) | P value | Exposed | Unexposed | β (SE) | P value | |||
| Airway responsiveness* | rs8180086 | A | 0.50 | 0.012 | 0.49 | 0.89(0.33) | 0.0088 | 0.16 | 0.93 | 0.59 (0.40) | 0.15 |
| Airway responsiveness | rs3732765 | A | 0.57 | 0.0091 | 0.32 | 1.24(0.45) | 0.0035 | 0.058 | 0.89 | 1.39 (0.71) | 0.033 |
| Airway responsiveness | rs10935840 | G | 0.54 | 0.0058 | 0.35 | 1.25 (0.45) | 0.0028 | 0.028 | 0.94 | 1.47 (0.70) | 0.022 |
| Airway responsiveness | rs11708767 | A | 0.22 | 0.019 | 0.83 | 0.75 (0.39) | 0.042 | 0.038 | 0.44 | 1.07 (0.62) | 0.039 |
| BDR | rs7615865 | T | 0.019 | 0.88 | 0.018 | 0.07 (0.03) | 0.042 | 0.44 | 0.07 | 0.002 (0.02) | 0.94 |
| BDR | rs1491978 | C | 0.023 | 0.88 | 0.021 | 0.07 (0.03) | 0.044 | 0.44 | 0.08 | 0.02 (0.88) | 0.14 |
| FVC | rs10935844 | G | 0.61 | 0.83 | 0.14 | 0.09 (0.07) | 0.21 | 0.011 | 0.86 | 0.20 (0.07) | 0.018 |
| FVC | rs8180086 | A | 0.15 | 0.07 | 0.92 | 0.05 (0.10) | 0.56 | 0.17 | 0.024 | 0.23 (0.11) | 0.040 |
House dust mite exposure dichotomized at 4th quartile of measured Der p levels (1.85 µg/g) for quartile definition of exposure. House dust mite exposure dichotomized at Der p level of 10µg/g for 10µg/g definition.
As reflected by methacholine challenge response (PC20)
Significant associations are bolded
SE = Standard Error
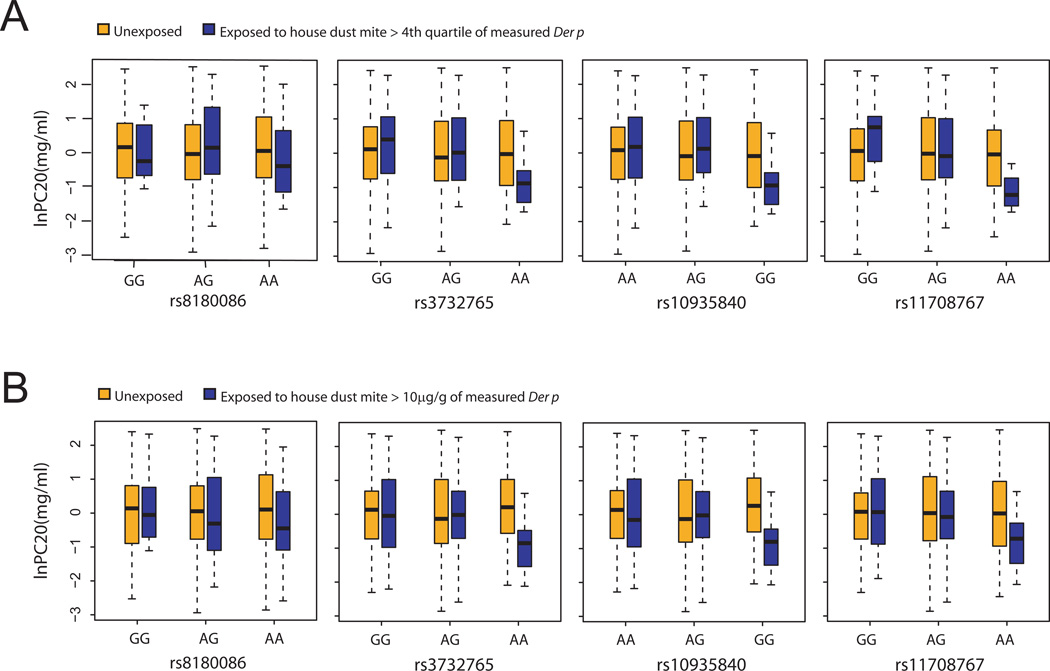
To illustrate the gene-by-environment interactions we found, Fig. 2 shows the relationship between P2RY12 and airway responsiveness stratified by house dust mite exposure. Fig. 2A shows the results when house dust mite exposure was dichotomized at the fourth quartile of measured Der p level, and Fig. 2B shows the results when house dust mite exposure was dichotomized at 10 µg/g. PC20 was low for those exposed to house dust mite and carrying two copies of the minor allele of each of these SNPs. Minor allele homozygotes not exposed to house dust mite had similar PC20 values to subjects with one or two copies of the major allele and variable house dust mite exposure. BDR and FVC were higher for those exposed to house dust mite and carrying two copies of the minor allele of each of the identified SNPs, while minor allele homozygous not exposed to house dust mite had similar BDR and FVC to subjects with one or two copies of the major allele and variable house dust mite exposure.
Fig. 2.
Relationship between P2RY12 SNPs and airways responsiveness stratified by house dust mite exposure. (A) House dust mite exposure dichotomized at 4th quartile of measured Der p level, and (B) House dust mite exposure dichotomized at 10µg/g of measured Der p level. With both definitions of house dust mite exposure, subjects with two copies of the minor allele and dust exposure had greater airway responsiveness (lower PC20) than minor allele homozygotes without dust exposure. Tests for gene-by-environment interaction were significant.
DISCUSSION
This is the first study to examine P2RY12 in human subjects with asthma. We found evidence for association between variants in P2RY12 and lung function in a large cohort of families with asthma, and evidence that house dust mite modulates these associations through gene-by-environment effects. We used family based methods, thus avoiding bias from population stratification [19]. Our work establishes a human context for previous in silico, in vitro, and murine data suggesting a role for P2Y12 in LTE4-modulated airway inflammation.
Our findings add to a growing literature on potential receptors and receptor modulators of LTE4 [3, 28]. As the most abundant and stable cysteinyl leukotriene, LTE4 is more potent than other leukotrienes in causing airways hyperresponsiveness to histamine in asthmatics and tracheal constriction in guinea pigs [2]. It has received relatively little attention since it binds poorly to CysLT1 (the target of leukotriene receptor antagonist medications such as montelukast) [29] and CysLT2 (the other well-characterized cysteinyl leukotriene receptor) [30]. Interest in a separate LTE4 receptor recently rekindled based on murine studies in which LTE4 caused 64-fold more vascular permeability in mice lacking CysLT1 and CysLT2 [28]. The identification and characterization of receptors and modulators of LTE4 could lead to new therapeutic targets for asthma and atopic disorders.
The P2RY12 SNPs that we found to be associated with BDR, FEV1, and FEV1/FVC were in proximity to one another (Table 2, Fig. 1a), suggesting that this region of P2RY12 may be of particular functional interest. The fact that many of these SNP were associated with bronchodilator response as well as FEV1 and FEV1/FVC would be consistent with these SNPs influencing not only baseline lung volumes, but also airway reversibility. The associated SNPs are intronic [31], with rs7615865 marking a potential splice site [32]. Polymorphic variation at these loci could lead to altered splicing and RNA production, resulting in aberrant P2Y12 protein structure and causing downstream effects on receptor function, inflammation, and lung function. Alternatively, these regions could have regulatory variants that are in transcription factor binding sites.
Meta-analyses of genome-wide studies have been performed to identify genes associated with pulmonary function [33, 34]. P2RY12 was not among the top genes reported, but these studies were performed in general populations unselected for asthma. It is likely that findings would be different if the contributing genome-wide studies had been performed in populations of asthmatic subjects, as there may be genes important in determining lung function in subjects with asthma that may be distinct from those influencing lung function in subjects without respiratory disease. A subsequent study replicated some of the gene associations from the general population meta-analyses in subjects with asthma [35]. However, this study focused on candidate genes drawn from the general population study results, and thus never tested for P2RY12. Again, it is likely findings would be distinct had the initial genome-wide studies for lung function been performed in populations selected for asthma. A large, consortium-based genome wide association study of asthma was also recently performed, but the study focused on asthma phenotypes and IgE, and it did not test for associations with lung function measures [36].
Our finding that several SNPs and haplotypes in P2RY12 are associated with lung function in children with asthma is supported by in vitro and murine data that provide potential mechanisms for this effect. Experiments using shRNA-mediated knockdown of P2Y12 in LAD2 cells, a well-differentiated human mast cell sarcoma line, show that P2Y12 is needed for LTE4-mediated mast cell activation [3]. Mast cells populate the upper and lower respiratory tract epithelia, unequivocally play a role in allergic reactions, and their hyperplasia is observed in the inflamed mucosal epithelium and adjacent smooth muscle of asthmatic subjects [37]. P2Y12 knock out mice do not exhibit LTE4-induced goblet cell metaplasia, IL13 production, and eosinophilia [3], phenomena that are also observed in asthmatic subjects. Further, treatment of mice with the P2Y12 antagonist clopidogrel leads to decreased airways inflammation and decreased goblet cell hyperplasia [3].
P2Y12 and its gene had not been studied in asthmatic subjects prior to this investigation. Given the known role of P2Y12 as the receptor for ADP-mediated platelet aggregation, previous studies of P2RY12 focused on cardiovascular and cerebrovascular disease. These studies identified haplotype variants in P2RY12 that were inconsistently associated with platelet aggregation, peripheral arterial disease, venous thromboembolism, myocardial infarction, and cerebrovascular accidents [7–11]. One study identified a haplotype in P2RY12, H2, as a gain-of-function haplotype with regards to platelet aggregation [7]. Our first haplotype block includes this H2 haplotype. We tested for association between the previously defined H2 haplotype and lung function, and these results did not differ significantly from the results for our first haplotype block. The P2RY12 haplotypes that we tested were associated with lung function, consistent with our results from single SNP association testing.
House dust mite exposure modulated the effect of P2RY12 SNPs on lung function. With house dust mite exposure, subjects recessive for the minor allele of identified SNPs exhibited worse lung function measures. Specifically, homozygotes for the minor allele of the identified SNPs who were also exposed to house dust mite had lower PC20 levels (Fig. 2). These gene-by-environment effects for airway responsiveness were significant with either definition of house dust mite exposure. The risk for worse lung function with recessive status and house dust mite exposure was further supported by our gene-by-environment tests with BDR, where we observed higher BDR in subjects recessive for the minor allele of identified SNP and exposed to dust. The interaction effect with BDR was significant with exposure to the highest quartile of house dust mite and not significant when the more stringent threshold of 10µg/g Der p was employed. This may have been due to limited power with the more stringent threshold of Der p exposure, given only 44 index subjects met this strict exposure criterion. Conversely, subjects recessive for the minor allele of identified SNPs and exposed to house dust mite demonstrated higher FVC volumes, with interaction effects significant with the 10µg/g Der p exposure model but not with the more liberal 4th quartile house dust mite exposure model. It is possible that gene-by-environment interactions for lung volumes are more sensitive to high levels of environmental exposure, thus resulting in the significant effects seen only with high levels of house dust mite exposure. Children with asthma have been observed to have higher FVC volumes than children without asthma [38, 39]. The SNPs contributing to the gene-by-environment interactions we observed were distinct for the lung function measure being tested, suggesting variable gene-by-environment effects dependent on both SNP and phenotype being tested. Gene-by-environment effects were most pronounced in subjects recessive for the minor alleles of each SNP. Given the relative high minor allele frequencies for these variants (33–49%), a substantial proportion of this population (10–24%) would be recessive for these variants and influenced by these gene-by-environment effects.
There were some variants in P2RY12 that were associated with lung function measures without gene-by-environment effects (Table 2), and other variants that exhibited associations with lung function measures only if gene-by-environment effects were included (Table 4). The former tended to be for BDR and lung volume phenotypes, while the latter for airway hyperresponsiveness as well as BDR. This could reflect a greater tendency for airway hyperresponsiveness to be influenced by environmental exposures. The fact that there was no overall association between many of these SNPs and lung function if house dust mite exposure was not considered corroborates a role for house dust mite in gene-by-environment interactions for these P2RY12 SNPs. Since house dust mite allergen has been observed to stimulate rapid and robust production of cysteinyl leukotrienes [13], it is possible that P2Y12 receptors mediate the LTE4-driven components of this effector response in vivo.
As the first study of P2RY12 in human subjects with asthma, our findings add an important human dimension to existing in silico, in vitro, and murine studies of P2Y12 and airway inflammation. We genotyped multiple markers in the gene and found associations with lung function measures. Our study additionally offers the strengths of family-based association findings free from population stratification bias, examination of single SNP as well as haplotype associations, and formal tests of gene-by-environment interactions for a common environmental allergen that impacts asthma severity in the population at large. Despite the strengths of our study, we acknowledge several limitations. Our analyses were limited to CAMP, a single multi-center study. While we have identified polymorphisms and haplotypes in P2RY12 that are associated with lung function, we have not defined precise mechanisms for their effect, although existing experimental studies of P2Y12 lend clues to potential mechanisms as discussed. It is also possible that more comprehensive genotyping within the P2RY12 gene could further define the functional variants involved. Finally, some of our findings could be due to false positive associations from multiple testing, but our adjustments for multiple comparisons suggested only a one in ten chance of false discovery among the many associations we identified.
In summary, we found that SNPs and haplotypes in P2RY12 were associated with lung function in a large cohort of children with asthma and their parents. Further, house dust mite exposure modulated the effect of these genetic variants on airway responsiveness. Our data contribute to a growing literature on gene-by-environment interactions in asthma [40]. Our findings add human evidence to existing in silico, in vitro, and murine data supporting a role for P2Y12 in LTE4-mediated airway inflammation and asthma.
ACKNOWLEDGMENTS
We thank all subjects for their participation. We acknowledge the CAMP research team, supported by NHLBI, for collection of CAMP Genetic Ancillary Study data. This work was supported by the National Institute of Health grants U01HL075419, P01HL083069, R01AI078908, R0152353, T32HL007427, and K12HL089990.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lee TH, Woszczek G, Farooque SP. Leukotriene E4: perspective on the forgotten mediator. J Allergy Clin Immunol. 2009;124:417–421. doi: 10.1016/j.jaci.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Austen KF, Maekawa A, Kanaoka Y, Boyce JA. The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J Allergy Clin Immunol. 2009;124:406–14. doi: 10.1016/j.jaci.2009.05.046. quiz 15–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paruchuri S, Tashimo H, Feng C, et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovitch N, Graber NJ, Chinchilli VM, et al. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. J Allergy Clin Immunol. 2010;126:545–551. e1–e4. doi: 10.1016/j.jaci.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arm JP, O'Hickey SP, Hawksworth RJ, et al. Asthmatic airways have a disproportionate hyperresponsiveness to LTE4, as compared with normal airways, but not to LTC4, LTD4, methacholine, and histamine. Am Rev Respir Dis. 1990;142:1112–1118. doi: 10.1164/ajrccm/142.5.1112. [DOI] [PubMed] [Google Scholar]
- 6.Scadding GW, Scadding GK. Recent advances in antileukotriene therapy. Curr Opin Allergy Clin Immunol. 2010;10:370–376. doi: 10.1097/ACI.0b013e32833bfa20. [DOI] [PubMed] [Google Scholar]
- 7.Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108:989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 8.Fontana P, Gaussem P, Aiach M, et al. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003;108:2971–2973. doi: 10.1161/01.CIR.0000106904.80795.35. [DOI] [PubMed] [Google Scholar]
- 9.Zee RY, Michaud SE, Diehl KA, et al. Purinergic receptor P2Y, G-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008;197:694–699. doi: 10.1016/j.atherosclerosis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Rudez G, Pons D, Leebeek F, et al. Platelet receptor P2RY12 haplotypes predict restenosis after percutaneous coronary interventions. Hum Mutat. 2008;29:375–380. doi: 10.1002/humu.20641. [DOI] [PubMed] [Google Scholar]
- 11.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 12.Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–288. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 13.Barrett NA, Maekawa A, Rahman OM, et al. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 15.Himes BE, Hunninghake GM, Baurley JW, et al. Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. Am J Hum Genet. 2009;84:581–593. doi: 10.1016/j.ajhg.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 17.Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R: Foundation for Statistical Computing; 2010. [Google Scholar]
- 18.Lange C, DeMeo D, Silverman EK, et al. PBAT: tools for family-based association studies. Am J Hum Genet. 2004;74:367–369. doi: 10.1086/381563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird NM, Lange C. Family-based designs in the age of large-scale gene-association studies. Nat Rev Genet. 2006;7:385–394. doi: 10.1038/nrg1839. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annals of Statistics. 2001;29:1165–1188. [Google Scholar]
- 21.Rice TK, Schork NJ, Rao DC. Methods for handling multiple testing. Adv Genet. 2008;60:293–308. doi: 10.1016/S0065-2660(07)00412-9. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 23.Vansteelandt S, Demeo DL, Lasky-Su J, et al. Testing and estimating gene-environment interactions in family-based association studies. Biometrics. 2008;64:458–467. doi: 10.1111/j.1541-0420.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 24.Platts-Mills TA, Hayden ML, Chapman MD, Wilkins SR. Seasonal variation in dust mite and grass-pollen allergens in dust from the houses of patients with asthma. J Allergy Clin Immunol. 1987;79:781–791. doi: 10.1016/0091-6749(87)90211-9. [DOI] [PubMed] [Google Scholar]
- 25.Sporik R, Platts-Mills TA, Cogswell JJ. Exposure to house dust mite allergen of children admitted to hospital with asthma. Clin Exp Allergy. 1993;23:740–746. doi: 10.1111/j.1365-2222.1993.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Raby BA, Hunninghake GM, et al. Variants in TGFB1, dust mite exposure, and disease severity in children with asthma. Am J Respir Crit Care Med. 2009;179:356–362. doi: 10.1164/rccm.200808-1268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunninghake GM, Soto-Quiros ME, Lasky-Su J, et al. Dust mite exposure modifies the effect of functional IL10 polymorphisms on allergy and asthma exacerbations. J Allergy Clin Immunol. 2008;122:93–98. 8, e1–e5. doi: 10.1016/j.jaci.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maekawa A, Kanaoka Y, Xing W, Austen KF. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A. 2008;105:16695–16700. doi: 10.1073/pnas.0808993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch KR, O'Neill GP, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 30.Heise CE, O'Dowd BF, Figueroa DJ, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Biotechnology Information (NCBI) [accessed on February 8, 2011];Entrez SNP. http://www.ncbi.nlm.nih.gov.ezp-prod1.hul.harvard.edu/projects/SNP/snp_ref.cgi?rs=20541.
- 32.Conde L, Vaquerizas JM, Dopazo H, et al. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Res. 2006;34:W621–W625. doi: 10.1093/nar/gkl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42:36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hancock DB, Eijgelsheim M, Wilk JB, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42:45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Howard TD, Moore WC, et al. Importance of hedgehog interacting protein and other lung function genes in asthma. J Allergy Clin Immunol. 2011;127:1457–1465. doi: 10.1016/j.jaci.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffatt M, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M. Cookson WOCM, and the GABRIEL consortium. A GABRIEL consortium large-scale genome-wide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyce JA. The role of mast cells in asthma. Prostaglandins Leukot Essent Fatty Acids. 2003;69:195–205. doi: 10.1016/s0952-3278(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 38.Weiss ST, Tosteson TD, Segal MR, et al. Effects of asthma on pulmonary function in children. A longitudinal population-based study. Am Rev Respir Dis. 1992;145:58–64. doi: 10.1164/ajrccm/145.1.58. [DOI] [PubMed] [Google Scholar]
- 39.Gold DR, Wypij D, Wang X, et al. Gender- and race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. Am J Respir Crit Care Med. 1994;149:1198–1208. doi: 10.1164/ajrccm.149.5.8173760. [DOI] [PubMed] [Google Scholar]
- 40.London SJ, Romieu I. Gene by environment interaction in asthma. Annu Rev Public Health. 2009;30:55–80. doi: 10.1146/annurev.publhealth.031308.100151. [DOI] [PubMed] [Google Scholar]