Abstract
Human adipose-derived stem cells (hASCs) have been successfully used in treating numerous diseases. However, several aspects need to be considered, particularly in the context of allogeneic cell therapy. To better understand hASCs-host interactions, we studied the phenotype of hASCs and their modulatory effect on natural killer (NK) cells by using bone marrow-mesenchymal stem cells (hBM-MSCs) as a reference. The hASCs displayed a lower susceptibility to NK cell-mediated lysis and a lower expression of ligands for DNAM-1 when compared with hBM-MSCs. Moreover, here we demonstrated that hASCs and hBM-MSCs can modulate NK cells through the action of soluble factors such as indoleamine 2,3-dioxygenase. Altogether, these results suggest that for an adoptive cell therapy based on the transfer of allogeneic hASCs, the NK-hASCs crosstalk will not result in an immediate recognition of the transferred cells. Thus, hASCs may remain in the tissue long enough to balance the immune response before being cleared.
Introduction
Mesenchymal stem cells (MSCs) are multipotent adult stem cells capable of differentiating into a variety of cell types [1]. MSCs have been traditionally isolated from bone marrow [2,3], but recent reports have performed the isolation and in vitro expansion from a variety of tissues including fetal liver and lung [4], adipose tissue [5], skeletal muscle [6], amniotic fluid [7], synovium [8,9], dental pulp [10], and skin [11,12]. MSCs are thought to have tissue regenerative properties, in the first place, via their multilineage differentiation capacity and, more importantly, via the secretion of trophic factors that may activate local progenitor cells [13]. MSCs also have potent immunomodulatory capacities [3], inhibiting the proliferation and cytotoxic potential of natural killer (NK) cells [14], T lymphocytes [15], γδ T cells [16], and invariant NKT cells [16]. Moreover, MSCs have a limited efficiency of antigen processing and presentation [17] and influence host immunity by modulating dendritic cell function [18,19].
Human adipose-derived MSCs (hereafter referred to as hASCs) are obtained from liposuction procedures and yield a clinically useful number of cells with characteristics of stem cells. These cells can be expanded over a long time in culture for clinical practice, being an interesting tool for cellular therapy [20]. Applications of the hASCs are being explored, and several clinical trials have been started for graft-versus-host disease [21], Crohn's disease [22,23], and urinary incontinence [24]. The preclinical research activity of hASCs is currently being focused on diseases as diverse as diabetes [25], spinal cord injury [26], Huntington disease [27], multiple sclerosis [28], ischemia [29], rheumatoid arthritis [30], skin regeneration [31], glioblastoma [32], and colitis [33].
Although hASCs and bone marrow-MSCs (hereafter referred to as hBM-MSCs) come from different sources, they share functional similarities in their differentiation potential and their immunosuppressive mechanisms [34].
Despite the low immunogenicity of MSCs in vitro, one still has to be cautious in using MSCs in an allogeneic setting without immunosuppression of the patient [35]. Given the significant role of MSCs over the adaptive immune system, for therapeutic applications, it is interesting to clarify whether the immune privilege of the stem cells is maintained in the context of the innate response, particularly in an allogeneic setting. In this sense, it has been reported by several groups that hBM-MSCs and dental pulp-derived MSC (DP-MSC) can be lysed by cytotoxic immune effectors such as NK cells [13]. The NK susceptibility of these cells may be due to the expression of ligands for activating receptors involving multiple interactions between NK and target cells. The recognition and lysis of allogeneic MSCs by NK cells have implications in safety (side effect associated with immune rejection) and efficacy (reduced persistence of the cells in the patient); for this, understanding the interaction of MSCs with NK cells is crucial to optimize their potential therapeutic use.
NK cells are a subset of lymphoid cells that have the capability of killing target cells without previous sensitization [36,37]. The NK cell activation is mediated through specific interactions between activating receptors and their respective ligands. These activating receptors, once engaged, induce the lysis and cytokine release. On the contrary, to shift the balance toward NK cell inhibition, the activation of NK cells is prevented by inhibitory NK cell receptors [38–40].
Ligands for activating receptors such as DNAX accessory molecule-1 (DNAM-1) and NKG2D have been identified on the surface of hBM-MSCs cells, and both receptors are involved in killing hBM-MSCs [14,41,42]. Moreover, it has been demonstrated that hBM-MSCs inhibit interleukin 2 (IL-2)-induced proliferation of NK cells and suppress the NK cytolytic activity which may be beneficial for transplantation and autoimmune diseases [14,41,42].
Although interactions between MSCs and NK cells have been previously studied in hBM-MSCs, little information is available regarding hASCs and their effect on NK cells. We and others have previously reported that the capability of hASCs to modulate immune responses relies on both cell contact-dependent mechanisms and soluble factors secreted in response to cytokines [20,30]. The crosstalk between NK and hASCs is of significant interest in the context of their allogeneic use; however, we have no evidence whether NK cells are modulated by hASCs and whether or not they are mediated by ligand-receptor interaction or soluble factors.
Here, we analyze the phenotype of ligands for NK-activating receptors in hASCs, their susceptibility to NK cell-mediated activity, and their capacity to modulate NK cell activation.
Our results demonstrated several phenotypic differences when comparing hASCs with hBM-MSCs. The hASCs had a lower expression of histocompatible locus antigen (HLA) class I and a negative expression of ligands for NK activating receptors. Our functional data have demonstrated a higher resistance of hASCs to NK-mediated activation when compared with hBM-MSCs. Moreover, similar to hBM-MSCs, the hASCs impaired NK cell function in both contact and transwell conditions. Finally, our results indicate that indoleamine 2,3-dioxygenase (IDO) activity can be induced after cross-talk between hASCs and NK cells.
Material and Methods
Culture of hASCs and hBM-MSCs
The hASCs were isolated from the stromal vascular fraction of human lipoaspirates. The lipoaspirates were obtained by liposuction from healthy adult donors, washed twice with phosphate-buffered saline (PBS), and digested at 37°C for 30 min with 18 U/mL of collagenase type I in PBS. The digested sample was washed with 10% of fetal bovine serum (FBS), treated with ammonium chloride 160 mM, suspended in culture medium [Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS], and filtered through a 40-μm nylon mesh. Cells were seeded onto tissue culture flasks and expanded at 37°C and 5% CO2, changing the culture medium every 7 days. Cells were passed to a new culture flask when cultures reached 90% of confluence. A pool from 6 healthy donors (3 men and 3 women, aged between 35 and 47) was used in the study. Cells were used at passages 4–6. The hASCs were obtained after informed consent under the auspices of the appropriate Research and Ethics Committees.
The hBM-MSCs were used as an MSC reference cell source. hBM-MSCs were purchased from Lonza, Inc. (Walkersville, MD) and cultured according to the supplier's recommendations in DMEM containing 10% FBS.
Phenotypic analysis of hASCs by fluorescence-activated cell sorting
For flow cytometric analysis of hASC, cells were stained with the following mAbs: anti-HLA-ABC (G46-2.6), anti-HLA-DR (L243), anti-CD48 (TÜ145), anti-CD112 (R2.525), anti-CD155 (300907), and anti-MICA/B (6D4) from BD Biosciences (San Jose, CA). The UL16-binding proteins (ULBPs) were evaluated with anti-ULBP-1 (170818), anti-ULBP-2 (165903), and anti-ULBP-3 (166510) from R&D systems (Minneapolis, MN) by indirect immunofluorescence using an appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibody. The analysis of recombinant human protein chimeras [NKp30-, NKp44-, and NKp46-IgG1 fragment crystallizable region of IgG (Fc)] from R&D Systems was performed as follows: 2×105 hASCs were incubated with 100 μL of Fc Receptor Blocker (Innovex Biosciences, Richmond, VA) for 10 min at room temperature. After being washed twice with wash buffer and resuspended in PBS containing 2% FCS, cells were then incubated with 5 μg/mL of protein chimera or human IgG for 30 min at 4°C. After 3 washes at 4°C in PBS containing 2% FSC, cells were incubated for 30 min at 4°C with a human IgG-Fc fragment-specific, FITC-conjugated goat antibody. After 2 washes, the cells were resuspended in PBS and analyzed by flow cytometry. In addition, the hASCs were prepared by collagenase digestion of lipoaspirates. The isolated hASCs and stromal vascular fractions were tested by flow cytometry by using specific surface markers being negative for CD14, CD31, CD34, CD45, FCR1a, and 1B10 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd) and positive for CD29, CD59, CD73, CD90, and CD105 (Supplementary Fig. S2).
The flow cytometric analysis was performed on an FACScan cytometer (BD Biosciences) after acquisition of 105 events. Viable cells were selected by using forward and side-scatter characteristics and analyzed using CellQuest software (BD Biosciences). Isotype-matched negative control antibodies were used in all the experiments. The mean relative fluorescence intensity (MRFI) was calculated by dividing the mean fluorescent intensity (MFI) by the MFI of its negative control.
NK cell expansion and isolation
Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained by centrifugation over Histopaque-1077 (Sigma, St. Louis, MO) and washed with PBS. PBMCs were grown for 5 days in RPMI 1640 supplemented with 10% human serum, 1% glutamine, 1% penicillin, 1% nonessential amino acids, 1% Sodium Pyruvate from Cambrex Bio Science (Walkersville, MD), and 500 U/mL of rhIL-2 obtained from the National Cancer Institute (Frederick, MD). NK cells were purified in a fluorescence-activated cell sorting (FACS) Vantage Diva cell sorter (BD Biosciences) by using Peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (SK1) and phycoerythrin (PE)-conjugated anti-CD56 (NCAM16.2) from BD Biosciences. The typical purity of the sorted NK cell population defined as CD56+CD3 was 95%–98%. Purified NK cells were subsequently assayed for degranulation and interferon γ (IFN-γ) assays or tested in co-culture experiments.
NK cell degranulation assay
The surface expression of CD107a/b was analyzed after 4 h following activation of purified NK cells with target cells at ratio 1:1 in the presence of BD GolgiStop™ (BD Biosciences) and a mixture of FITC-labelled CD107a/b. NK cells were stained with PE labelled anti-CD56 (NCAM16.2) from BD Biosciences and analyzed by flow cytometry measuring the frequency of CD107a/b expression.
IFN-γ assay
IFN-γ assay was performed by using purified NK cells co-cultured with target cells at 1:1 ratio in the presence of BD GolgiStop. After 8 h of co-incubation, purified NK cells were stained with PE labelled anti-CD56 from BD Biosciences, fixed, and permeabilized using BD Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences). Finally, cells were stained with FITC-labelled anti-IFN-γ mAb (eBioscience, San Diego, CA), and flow cytometry analysis was performed by measuring the frequency of IFN-γ expression.
Co-culture of NK cells with hASCs and hBM-MSCs
To determine the inhibitory effect of hASCs and hBM-MSCs on NK cells, purified NK cells were co-cultured in the presence or absence of hASCs or hBM-MSCs by using direct co-culture or in a transwell plate system with a 0.4 μm pore size membrane (Corning Costar, Schiphol-Rijk, The Netherlands). After 72 h with rhIL-2 at 100 U/mL, NK cells were harvested and subsequently tested in a degranulation assay against the NK cell-susceptible target cell line K562. The analysis by flow cytometry was performed by measuring the frequency of CD107a/b expression as just described. The supernatants from co-cultures were collected, spun down to remove cells or cell debris, frozen, and stored at −20°C. The detection of soluble IFN-γ in supernatants from purified NK cells and co-cultured NK cells at ratio1:1 for 72 h was performed by using multiplexed beads. The cytokine was determined by using the FlowCytomix human Th1/Th2 11plex kit (Bender MedSystem, Vienna, Europe) according to manufacturer's protocol.
Phenotypic analysis of NK cells
Purified NK cells were co-cultured at a ratio 1:1 with hASCs or hBM-MSCs in direct contact or in a transwell co-culture system for 72h. For flow cytometric analysis, NK cells were washed twice in PBS and stained with the appropriate combination of fluorescent-labeled mAbs. The following mAbs were used in this study: Peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (SK1); FITC-conjugated anti-CD56 (NCAM16.2), anti-CD94 (HP-3D9) and PE-conjugated anti-CD69 (HP-4B3), anti-NKp44 (p44-8.1), anti-NKp46 (9E2/NKp46), anti-CD226 (DX11), anti-NKG2D (1D11), anti-CD69 (HP-4B3), anti-CD16 (NKP15) from BD Biosciences, anti-NKp30 (p30-15) from Miltenyi Biotec (Auburn, CA), and anti-CD244 (C1.7) from e-Bioscience (San Diego, CA).
For intracellular staining, after surface-marker labeling, cells were fixed and permeabilized by using BD Cytofix/Cytoperm fixation/permeabilization kit. Cells were stained with anti-Perforin (SG9), anti-Granzyme A (CB9), and anti-Granzyme B (GB11) from BD Biosciences.
Flow cytometric analysis was performed on an FACScan cytometer (BD Bioscience) after acquisition of 105–106 events. Viable cells were selected by using forward and side-scatter characteristics, and NK cells were gated on CD56+CD3–phenotype and analyzed by using CellQuest software (BD Biosciences).
IDO activity
IDO activity was measured by determining both tryptophan (Trp) and Kynurenine (Kyn) concentrations in supernatants from co-cultures of NK cells with hASCs or hBM-MSCs. About 200 μL of supernatants was added to 50 μL of trichloroacetic acid 2M, vortexed, spun down (10 min at 13,000 rpm), and analyzed by high-performance liquid chromatography (HPLC) (Waters 717plus Autosampler, Milford, MA).
Statistical analysis
The differences in the means of measures were compared by the Student's t-test and paired test for 2-way analysis of variance using SPSS software. A P value ≤0.05 was considered significant.
Results
Expression of ligands for NK activating receptors in hASCs and hBM-MSCs
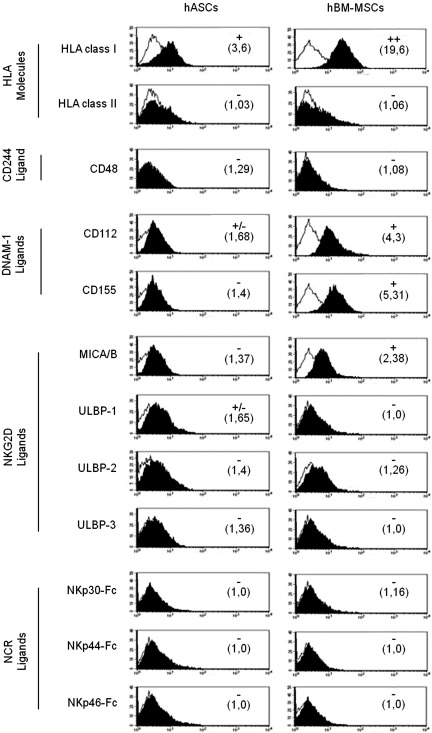
Ligands for activating or inhibitory receptors have been studied in hBM-MSCs; however, no information is found regarding hASCs profile. Here, we have performed a phenotypic analysis of ligands for NK activating receptors in hASCs, thus taking as a reference hBM-MSCs isolated from healthy donors.
Our results showed that hASCs expressed lower levels of HLA class-I molecule compared with hBM-MSCs and negative expression of HLA class-II. In both cases, they did not express CD48 molecule (ligand for CD244). The phenotypic analysis of ligands for NK activating receptors in hASCs showed a lower expression of CD112 and CD155 (ligands for DNAM-1) compared with hBM-MSCs.
Finally, we found that the expression of MICA/B and ULBPs (ligands for NKG2D) was very low or negative in hASCs and hBM-MSCs (Fig. 1).
FIG. 1.
Expression of HLA molecules and ligands for NK activating receptors in hASCs and hBM-MSCs. hASCs and hBM-MSCs obtained from healthy donors were phenotypically characterized by multicolor flow cytometry. Quantification of HLA molecules and ligands for NK activating receptors is presented as mean relative fluorescence intensity calculated by dividing the mean fluorescent intensity by its negative control (numbers in brackets). The normalization scores are determined from the fluorescence intensity according to the following keys: values<1.5=negative,>1.5<2=+ /-, 2–10=+ , >10<100=++,>100=+++ . A representative histogram of each marker is represented in both the left column (hASCs) and right column (hBM-MSCs). Values represent the mean±SD of 4–6 independently performed experiments. Black bold histograms show the marker expression, and empty lines represent the negative control abbreviations: HLA-ABC, histocompatible locus antigen-ABC; HLA-DR, histocompatible locus antigen-DR; P, passage; ULBP, UL16-binding protein; NCR, natural cytotoxicity receptor; Fc, fragment crystallizable region of IgG; DNAM, DNAX accessory molecule-1; hASCs, human adipose-derived stem cells; hBM-MSCs, human bone marrow-mesenchymal stem cells; NK, natural killer; SD, standard deviation.
For the identification of ligands to natural cytotoxicity receptors (NCRs) by flow cytometry, chimeric NCRs were used to identify their surface expression in hASCs and hBM-MSCs. Our results showed a negative expression of these ligands in both cell types (Fig. 1).
It is interesting to note that hASCs were negative for CD45, CD14, CD31, CD34, FCR1a, and 1B10 demonstrating the absence of potential contaminant cells that might be found in the stromal vascular fraction (Supplementary Fig. S1).The hASCs were positive for CD29, CD59, CD73, CD90, and CD105 with no significant differences in the phenotype of pooled hASCs samples from different donors and individual samples (data not shown) and no significant differences after different passages (Supplementary Figs. S1 and S2). Moreover, the hBM-MSCs were phenotypically characterized by flow cytometry using the mentioned marker profile of cellular impurities and stemness stable markers demonstrating no significant differences between hASCs and hBM-MSCs (data not shown).
Finally, to determine the expression levels of ligands for NK activating receptors in an inflammatory setting, hASCs and hBM-MSCs were stimulated with IFN-γ for 72h (1, 10, and 100 U/mL). Three independent experiments showed that the expression level was not significantly increased in CD112, CD155, MICA/B, CD48, or ULBPs (Supplementary Fig. S3).
As expected, the expression of HLA class I and class II molecules was enhanced when these cells were treated with IFN-γ. After 72h at 100 U/mL, the HLA class I expression (MRFI) increased in hASCs until 38.47±20.71 and 48.74±38.5 in hBM-MSCs. Moreover, the HLA class II in hASCs and hBM-MSCs switched to positive value reaching a MRFI of 2.44±1.21 and 2.21±1.32, respectively (Supplementary Fig. S3).
xs
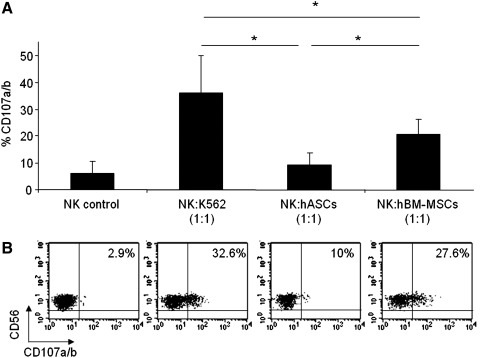
In order to compare the susceptibility of hASCs and hBM-MSCs to NK cell-mediated lysis, degranulation assays were performed by the detection of CD107a/b surface molecule as previously described [43]. The degranulation assay is a highly sensitive method, and its results are strictly correlated with NK cell cytotoxicity [44].
For these experiments, allogeneic NK cells were previously stimulated with rhIL-2 to increase their cytotoxic potential and sorted on the basis of CD56+CD3–phenotype. Both hASCs and hBM-MSCs were used as target cells in a degranulation assay. As negative and positive controls, NK cells alone and NK cells against the NK-susceptible target cell line K562 were used.
Although other ratios were used, to achieve maximal degranulation activity, the optimal effector:target (E:T) cell ratio in the degranulation assays was 1:1 (data not shown). Our results demonstrated that degranulation rates in response to hASCs were very low and not statistically significant compared with degranulation in unstimulated NK cells (NK control). In contrast, hBM-MSCs induced a significantly enhanced NK cell degranulation response when compared with hASCs (Fig. 2).
FIG. 2.
hASCs induce a low degranulation in IL-2-expanded NK cells. Allogeneic PBMCs cells were prestimulated for 5 days with rhIL-2 and then sorted on the basis of CD56+CD3- phenotype. To quantify cytotoxic granule exocytosis, the surface expression of CD107a/b was analyzed after activation of purified NK cells co-cultured with target cells (hASCs, hBM-MSCs, K562, or none) at a 1:1 (NK:Target) ratio. Graph A represents mean and SD of the percentage of CD107a/b on CD56 positive cells in 5 different experiments. A representative dot plot of each condition is shown in B. The numbers within the quadrants represent the percentage of CD107a/b in purified NK cells. *Significant at P≤0.05. PBMC, peripheral blood mononuclear cell; IL-2, interleukin 2.
To exclude the role of HLA class I-specific inhibitory receptors, HLA class I blocking was performed by pretreating the target cells with the HLA-class I specific monoclonal antibody W6/32 before co-culture. The results showed that antibody mediated masking of HLA class I did not increase NK cell degranulation against hASCs (data not shown).
hASCs and hBM-MSCs induce IFN-γ production by NK cells
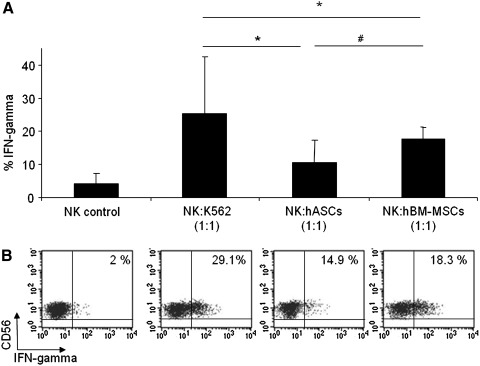
In addition to degranulation assays, we examined the NK cell cytokine response to hASCs or hBM-MSCs. Similar to degranulation assays, IFN-γ production was analyzed in purified NK cells against hASCs, hBM-MSCs, and K562 (positive control). As negative controls, purified NK cells were cultured in the absence of target cells.
The intracellular staining technique was used to assess IFN-γ production by NK cells. The Fig. 3 showed that IFN-γ production was observed when hASCs or hBM-MSCs were used as target cells. Moreover, it is interesting to note that IFN-γ response was not statistically different when comparing hBM-MSCs with hASCs. A representative dot plot with the expression of IFN-γ over the NK cell population is also depicted in Fig. 3.
FIG. 3.
hASCs induce IFN-gamma production in IL-2-expanded NK cells. Allogeneic PBMCs cells were prestimulated for 5 days with rhIL-2 and then sorted on the basis of CD56+CD3- phenotype. After activation of purified NK cells with target cells (hASCs, hBM-MSCs, K562, or none) at a 1:1 (NK:Target) ratio, intracellular staining was performed on NK cells by using anti-human IFN-γ antibody. Graph A represents mean±SD of 6 independent experiments. A representative dot plot of each condition is represented in B, and numbers in the quadrants indicate the percentage of IFN-γ in purified NK cells. *Significant at P≤0.05. #Nonsignificant at P≥0.05. IFNγ, IFN-gamma.
The cytokine IFN-γ was also detected in supernatants from purified NK cells co-cultured with hASCs at a ratio1:1 for 72 h by using the FlowCytomix human Th1/Th2 11plex kit (Bender MedSystem). The IFN-γ was secreted in NK/hASCs co-cultures (40±32.5 pg/mL) and not secreted by unstimulated NK cells or unstimulated hASCs (data not shown).
hASCs and hBM-MSCs impair NK cell function over other target cells
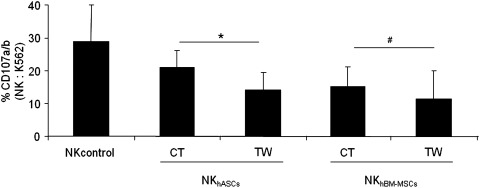
To further study the possible modulatory effect of hASCs and hBM-MSCs on NK cells, co-culture experiments were carried out in direct contact co-cultures or by using transwell inserts. These experiments were designed to quantify the potential contribution of soluble factors and cell-to-cell contact in the modulation of NK activity.
Purified NK cells were co-cultured with hASC or hBM-MSCs at a ratio 1:1 for 72 h and subsequently harvested and tested for degranulation capacity against a susceptible target cell line. Our results first revealed that the preincubation of NK cells with hASCs and hBM-MSCs were able to significantly decrease the NK degranulation capacity. Second, our results showed that precultured NK cells in transwell condition with hASCs were significantly more inhibitory than cell-to-cell contact. Finally, with regard to the effect of hBM-MSCs over NK cells, comparable results to hASCs were obtained. Interestingly, no significant differences were observed between contact and transwell conditions in NK cells preincubated with hBM-MSCs (Fig. 4).
FIG. 4.
hASCs and hBM-MSCs decrease NK cell activity through contact-dependent and independent mechanisms. Allogeneic NK cells sorted on the basis of CD56+CD3- phenotype were co-cultured at a ratio 1:1 in the presence or absence of hASCs or hBM-MSCs for 72 h in transwell or direct contact. These cells were subsequently tested in a degranulation assay against a NK-susceptible target cell line (K562 cells). The graph represents degranulation of natural killer cultured alone (NKcontrol), degranulation of NK cells preincubated with hASCs (NKhASCs), and degranulation of NK cells pre-incubated with hBM-MSCs (NKhBM-MSCs). Values represent the mean±SD of 4 independently performed experiments. NKhASCs, natural killer presensitized with hASCs; NKhBM-MSCs, natural killer presensitized with hBM-MSCs. *Significant at P≤0.05. #Nonsignificant at P≥0.05.
hASCs and hBM-MSCs induce phenotypic changes in NK cell
Once we demonstrated the low capacity of hASCs cells to induce NK cell degranulation, we analyzed the modifications over the NK cell phenotypic profile and found out whether cell-to-cell contact or only soluble factors were involved. For this purpose, we have analyzed activating receptors, NK cell markers, and effector molecules on NK cells co-cultured in the presence of hASC or BM-MSCs either in contact or in transwell conditions.
Three independent experiments were performed, and the statistical analysis revealed that NK cells co-cultured in contact with hBM-MSCs showed a significantly reduced expression of DNAM-1, and very similar results (although not statistically significant) were obtained in NK cells co-cultured in contact with hASCs (Table 1).
Table 1.
Phenotypic Analysis of Natural Killer Presensitized with Human Adipose-Derived Stem Cells or Human Bone Marrow-Mesenchymal Stem Cells in Contact and in Transwell System
| Molecules (MRFI) | NK control | NKhASCs Co-culture CT | NKhASCs Co-culture TW | NKhBM-MSCs Co-culture CT | NKhBM-MSCs Co-culture TW | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD244 | 2.73 | ±0.82 | 2.64 | ±0.93 | 2.18 | ±0.63 | 2.21 | ±0.32 | 1.98 | ±0.30 |
| DNAM-1 | 7.59 | ±1.70 | 4.05 | ±1.82 | 7.52 | ±1.31 | 3.89 | ±1.36 | 6.60 | ±2.42 |
| NKG2D | 12.53 | ±5.16 | 14.52 | ±3.53 | 17.21 | ±6.94 | 11.24 | ±2.88 | 16.98 | ±9.71 |
| NKp30 | 16.63 | ±13.68 | 9.28 | ±7.02 | 10.41 | ±6.15 | 8.50 | ±6.46 | 12.41 | ±10.84 |
| NKp44 | 1.21 | ±1.00 | 1.59 | ±0.64 | 1.76 | ±0.83 | 1.54 | ±0.82 | 1.84 | ±1.12 |
| NKp46 | 6.89 | ±4.16 | 9.63 | ±7.09 | 9.79 | ±6.43 | 10.00 | ±7.29 | 8.25 | ±5.77 |
| CD16 | 81.99 | ±43.81 | 59.76 | ±13.05 | 80.80 | ±50.25 | 55.77 | ±7.31 | 74.92 | ±41.24 |
| CD69 | 5.82 | ±1.89 | 7.26 | ±2.08 | 6.10 | ±1.72 | 6.72 | ±3.33 | 6.10 | ±2.15 |
| CD94 | 24.5 | ±22.4 | 24.48 | ±18.08 | 25.63 | ±22.4 | 27.42 | ±20.8 | 24.99 | ±22.6 |
| Perforin | 31.98 | ±9.74 | 29.96 | ±8.51 | 30.65 | ±8.87 | 32.43 | ±10.31 | 31.16 | ±8.60 |
| Granzyme A | 10.47 | ±2.96 | 7.07 | ±2.83 | 8.49 | ±3.29 | 8.90 | ±4.81 | 9.45 | ±3.97 |
| Granzyme B | 4.77 | ±0.70 | 5.19 | ±0.92 | 4.67 | ±0.35 | 5.79 | ±1.31 | 4.50 | ±0.64 |
The table represents mean±standard deviation of 3 independent experiments. Bold numbers indicate a statistically significant difference referred to the control.
MRFI, mean relative fluorescence intensity; NK, natural killer; hASC, human adipose-derived stem cells; hBM-MSC, human bone marrow-mesenchymal stem cells.
The activating receptor NKG2D was increased in NK cells co-cultured in transwell with hASC and hBM-MSCs (this change was statistically significant only in the case of hASCs). Moreover, in direct-contact co-cultures, we did not observe any difference (Table 1).
In the analysis of NCR repertoire, we have found some changes (not statistically significant). A reduced NKp30 surface expression was observed when NK cells were co-cultured in contact with hASCs and hBM-MSCs. In contrast, NKp46 expression was slightly increased (Table 1).
Data revealed that NK cells in contact with hASCs and hBM-MSCs had a significant decrease of CD16; however, CD69 and CD94 expression was maintained in all the experimental conditions.
Finally, intracellular molecules perforin, granzyme A, and granzyme B were also studied, and no changes were found for perforin and granzyme B; however, granzyme A was significantly reduced in NK cells co-cultured with hASCs both in contact and in transwells (Table 1).
IDO is induced by hASCs and hBM-MSCs in response to NK cells
Expression of IDO, a Trp catabolizing enzyme, contributes to the immunoregulatory functions of MSCs and is known to be involved in immunosuppression of effector cells. IDO expression is induced after IFN-γ stimulation. As previously shown in Fig. 3, hASCs and hBM-MSCs induced IFN-γ production by NK cells. We hypothesized that soluble factors released by NK cells could trigger IDO expression in hASCs and hBM-MSCs, which could play a role in the modulation of NK activity. Therefore, we measured by HPLC, IDO activity after co-culture.
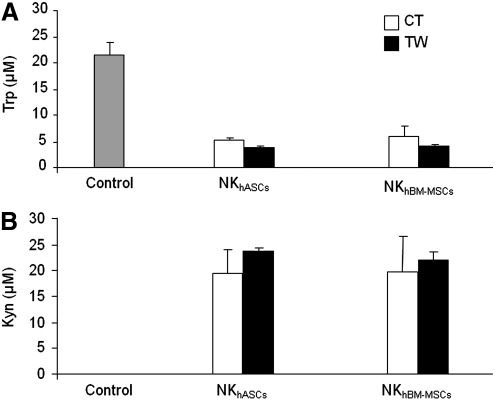
As shown in Fig. 5, NK cells did not show IDO activity (measured as degradation of Trp and accumulation of its catabolic product Kyn, However, we found that concentrations of Trp gradually decreased, with the concomitant accumulation of Kyn when NK cells were co-cultured with hASCs or hBM-MSCs in contact or transwell conditions.
FIG. 5.
NK cells promote the immunomodulatory activity of hASCs through indoleamine 2,3-dioxygenase induction. Supernatants from co-cultured NK/hASCs or NK/hBM-MSCs at a 1:1 ratio for 72 h were collected. Conditioned supernatants were measured by high-performance liquid chromatography method. Graph A shows Trp concentration (μM), and graph B shows Kyn concentration (μM). Black bars indicate mean and SD of contact conditions, and white bars indicate mean and SD of transwell conditions. Values shown in the bars represent mean±SD of 4 independently performed experiments. Trp, tryptophan; Kyn, kynurenine.
Discussion
hASCs can be harvested from the adult liposuctioned tissue and have been successfully used in treating numerous diseases [25–33]. The establishment of a cell bank of hASCs to be used as an “off-the-shelf” cellular product is an important advance in the cell therapy field. In this regard, Cellerix S.A. has developed a platform for producing hASCs under good manufacturing practices conditions that are currently used for clinical trials. The cellular products have been approved by regulatory agencies fulfilling all the release criteria for experimental cellular therapies. According to these criteria, no differences were found in the phenotypic profile of these cells (including markers for potential cell impurities and stable stemness markers) when low or high passages were used. Moreover, according to regulatory agencies, the absence of differences between pooled hASCs samples from different donors and individual samples has been demonstrated (data not shown).
It is important to note that the stromal vascular fraction from liposuctioned tissue contains, apart from hASCs, a mixture population of blood cells, mature adipocytes, stromal cells, endothelial cells, and others. These hASCs can be selected by plastic adhesion and screened by flow cytometry according to International Society for Cell Therapy guidelines. Although the hASCs used in this work are perfectly characterized and fulfill all the required release criteria for being used in the clinic, several aspects need to be addressed to better understand the underlying mechanisms of hASCs-host interactions for an allogeneic adult stem cell therapy.
The low immunogenicity of adult stem cells as hBM-MSCs has been extensively investigated [35,45–47]; however, an important aspect for preclinical and clinical application of hASCs is how the host immune system responds to allogeneic stem cells.
In this study, we have focused on the analysis of the interactions between NK cells and hASCs in an allogeneic setting: first, by analyzing the expression of ligands for activating NK receptors in hASCs and second, by studying the effect of NK-hASCs interactions on the phenotype and functionality of NK cells. In this work, we have used hBM-MSCs as a reference of a well-established source of MSCs [48–50].
It is well known that NK cell function results from a delicate balance between inhibitory and activating signals mediated by surface molecules. The inhibitory receptors recognize HLA class I molecules, and activating receptors requires the presence of ligands for these molecules on the surface of target cells. In this study, we have explored the possible role of inhibiting receptors in the low susceptibility of hASCs and hBM-MSCs to NK cell response. By using monoclonal antibodies to block these interactions, we found that, when HLA class I was neutralized, no changes were found in degranulation, thus demonstrating that HLA class I did not mediate the inhibition of NK cells (data not shown). Supporting these results, Poggi et al. have previously demonstrated that antibody–mediated masking of HLA class I in hBM-MSCs did not increase the lysis by ex vivo-isolated NK cells [41].
In this work, we have performed degranulation assays to address the susceptibility of hASCs and hBM-MSCs to NK cell response. The degranulation assays demonstrated that the proportion of NK cells expressing CD107a/b was very low when hASCs were used as targets. In contrast, NK cell degranulation against hBM-MSCs was significantly higher in comparison with hASCs.
Together with degranulation experiments, we have observed that NK/hASCs crosstalk stimulate IFN-γ production. We and others have demonstrated that the presence of IFN-γ is necessary to activate the modulatory activity of stem cells [20,51,52]. Here, we hypothesized that IFN-γ (among other soluble factors) released by NK/hASCs crosstalk could activate immunomodulatory mechanisms in hASCs and further modulate the phenotype and functionality of NK cells.
Another explanation to justify the lower NK cell degranulation against hASCs compared with that observed against hBM-MSCs could be the lower expression of ligands for activating receptors as DNAM-1 ligands on the surface of hASCs. The experimental evidence supporting this hypothesis is based on antibody-mediated blocking experiments performed by Spaggiari et al., where it was demonstrated that NKp30, NKG2D, and DNAM-1 are the major activating NK receptors involved in the cytotoxicity against hBM-MSCs [42]. Indeed, it has been demonstrated by others that NKG2D is responsible for triggering lysis of hBM-MSCs by activated NK cells [41].
To better understand the differences found at a functional level, we analyzed the ligands for NK receptors. The phenotypic analysis of hASCs clearly presents a significantly lower expression of HLA class I molecule (ligand for several inhibitory receptors) in comparison with hBM-MSCs. In addition, ligands for DNAM-1 receptors (CD112, CD155) in hASCs are expressed at a lower level when compared with hBM-MSCs. These results are in agreement with other authors who demonstrated the expression of ligands DNAM-1 in hBM-MSCs from 15 donors [42]. It is important to note that, in relation to the expression of ULBPs, we show that both hASCs and hBM-MSCs had a very low or negative expression of ULBPs; in contrast, Spaggiari et al. have shown that in the case of ULBP3, it was expressed in all 15 hBM-MSCs analyzed [42]. We consider that these differences could be the consequence of slight differences in isolation/expansion protocols and monoclonal antibodies used, which complicates a comparative study. Our results have demonstrated that, in the context of ligands for NK activating receptors, adult stem cells isolated from different sources showed several differences in terms of expression level. Therefore, we would suggest that the lower expression of ligands for activating receptors could render hASCs more resistant to NK activation than hBM-MSCs.
Another important aspect to consider related to the NK/hASCs crosstalk is whether the NK cells presensitized with hASCs modify their functionality over other target cells. In this regard, we have demonstrated that the functionality of NK cells is significantly reduced, and similar data were found for hBM-MSCs, thus supporting the hypothesis that NK cell activity is impaired after general MSC exposure.
In this work, we demonstrate that hASCs and hBM-MSCs co-cultured in transwell exerted the highest inhibitory effect on their degranulation activity in comparison with control values. In the case of hASCs, the inhibitory effect showed significant differences in comparison with the contact condition. These experiments also revealed that the phenotype of NK cells co-cultured in contact with hASCs or hBM-MSCs showed marked differences when compared with transwell co-culture. These results underlay again that soluble factors released by hASCs and hBM-MSCs were potent enough to modulate NK cell function. In these experiments, high-standard deviation values were found for CD16, CD94, and NCRs, which is probably due to a donor-to-donor variability [53,54].
Finally, in order to identify which soluble factors could be involved in the hASC modulation of the NK cell activity, and taking into account that the IDO enzyme is a key factor in the hASC immunomodulatory role over lymphocytes [20], here we demonstrate that NK/hASCs and NK/hBM-MSCs crosstalk can cause the induction of IDO expression in both contact and transwell.
Our results demonstrating the presence of IFN-γ on supernatants from NK/hASCs and NK/hBM-MSCs co-cultures suggest that IDO induction could be mediated through an IFN-γ–dependent mechanism. This idea is based on our previous findings published by delaRosa et al. by using hASC-IDOsi (with siRNA to knock down IDO expression) that demonstrated the crucial role for IFN-γ in inducing the IDO enzyme [20]. However, we should not exclude that other soluble factors such as PGE2 [55], IL-10 [33], or TGF-β1 [56] might be involved in mediating the modulation over NK cell function. Thus, Spaggiari et al. have suggested that IDO together with PGE2 may exert a synergistic effect in the immunosuppressive activity of MSCs [55].
Our results suggest that lower levels of ligands for NK activating receptors could make hASCs more resistant to NK activity than hBM-MSCs; however, we cannot exclude that differences in the isolation technique and culture conditions used for the expansion of these cells may alter their phenotypes and functions. We propose that for adoptive cell therapy based on the transfer of allogeneic hASCs, the initial crosstalk between hASCs and NK cells will not result in an immediate recognition and lysis of transferred cells, at least in a short term period. In consequence, we can speculate that hASCs will remain in the tissue long enough to balance the NK activity before being eliminated.
Conclusion
Our in vitro results demonstrate that hASCs display optimal characteristics for adoptive cell therapy in an allogeneic setting. First, the most important consequence of the lower expression of ligands for NK activating receptors would be their increased resistance to NK-mediated recognition. In comparison to hBM-MSCs, hASCs were more protected from allogeneic NK lysis. This would allow them to remain in the host for an extended period of time. Second, we suggest that mechanisms of hASCs for inducing tolerance in NK cells can be mediated by soluble factors. The IFN-γ secreted by NK cells during NK/MSCs crosstalk may induce IDO expression and other factors as PGE2 that may exert a synergistic effect in the immunosuppressive activity [55].
In summary, this study provides a biological and therapeutic significance for increasing our understanding on the interactions between NK cells and adoptively transferred hASCs. However, many aspects of the mechanistic basis for MSC-mediated immune modulation remain uncovered, and further investigation in this line needs to be performed.
Supplementary Material
Acknowledgments
This work was supported by grants PRI09A029, GRU09156 (to RT) from Junta de Extremadura (Spain) and SAF09/09711 (to RT), CEN-20091016, PSE-010000-2009-3 (to Cellerix S.A.) from Spanish Ministry of Science and Innovation and FEDER; PIE/204/2010 (to Cellerix S.A.) from Community of Madrid and FEDER; and grants to INPATT research group from University of Extremadura, co-financed by European Regional Development Fund (ERDF), CDTI, and MICINN. The authors acknowledge P. Mancheño, RG Roncero for the hASCs and hBM-MSCs handling, and J.J. Gordillo for cell sorting and cytometry. They also thank the National Cancer Institute's Biological Resources Branch (Frederick, MD) for providing human IL-2.
Author Disclosure Statement
O.D., E.L., B.R., C.R., and R.M. are employees of Cellerix S.A. A potential conflict of interest may exist. Data are original and unbiased.
References
- 1.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Ghannam S. Bouffi C. Djouad F. Jorgensen C. Noel D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uccelli A. Moretta L. Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;9:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 4.Campagnoli C. Roberts IA. Kumar S. Bennett PR. Bellantuono I. Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuznetsov SA. Mankani MH. Gronthos S. Satomura K. Bianco P. Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In 't Anker PS. Scherjon SA. Kleijburg-van der KC. Noort WA. Claas FH. Willemze R. Fibbe WE. Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 8.De BC. Dell'Accio F. Vandenabeele F. Vermeesch JR. Raymackers JM. Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De BC. Dell'Accio F. Tylzanowski P. Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Jewett A. Arasteh A. Tseng HC. Behel A. Arasteh H. Yang W. Cacalano NA. Paranjpe A. Strategies to rescue mesenchymal stem cells (MSCs) and dental pulp stem cells (DPSCs) from NK cell mediated cytotoxicity. PLoS One. 2010;5:e9874. doi: 10.1371/journal.pone.0009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toma JG. McKenzie IA. Bagli D. Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 12.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shabbir A. Zisa D. Lin H. Mastri M. Roloff G. Suzuki G. Lee T. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol. 2010;299:H1428–H1438. doi: 10.1152/ajpheart.00488.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotiropoulou PA. Perez SA. Gritzapis AD. Baxevanis CN. Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson I. Uhlin M. Le BK. Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 16.Prigione I. Benvenuto F. Bocca P. Battistini L. Uccelli A. Pistoia V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells. 2009;27:693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 17.Morandi F. Raffaghello L. Bianchi G. Meloni F. Salis A. Millo E. Ferrone S. Barnaba V. Pistoia V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–1287. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L. Zhang W. Yue H. Han Q. Chen B. Shi M. Li J. Li B. You S. Shi Y. Zhao RC. Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells Dev. 2007;16:719–731. doi: 10.1089/scd.2007.0065. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R. Fazekasova H. Lam EW. Soeiro I. Lombardi G. Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 20.DelaRosa O. Lombardo E. Beraza A. Mancheno-Corvo P. Ramirez C. Menta R. Rico L. Camarillo E. Garcia L, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 21.Fang B. Song Y. Zhao RC. Han Q. Lin Q. Using human adipose tissue-derived mesenchymal stem cells as salvage therapy for hepatic graft-versus-host disease resembling acute hepatitis. Transplant Proc. 2007;39:1710–1713. doi: 10.1016/j.transproceed.2007.02.091. [DOI] [PubMed] [Google Scholar]
- 22.Taxonera C. Schwartz DA. Garcia-Olmo D. Emerging treatments for complex perianal fistula in Crohn's disease. World J Gastroenterol. 2009;15:4263–4272. doi: 10.3748/wjg.15.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Olmo D. Herreros D. Pascual I. Pascual JA. Del-Valle E. Zorrilla J. De-La-Quintana P. Garcia-Arranz M. Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T. Gotoh M. Hattori R. Toriyama K. Kamei Y. Iwaguro H. Matsukawa Y. Funahashi Y. Periurethral injection of autologous adipose-derived stem cells for the treatment of stress urinary incontinence in patients undergoing radical prostatectomy: report of two initial cases. Int J Urol. 2010;17:75–82. doi: 10.1111/j.1442-2042.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin G. Wang G. Liu G. Yang LJ. Chang LJ. Lue TF. Lin CS. Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev. 2009;18:1399–1406. doi: 10.1089/scd.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu HH. Lim JH. Byeon YE. Park JR. Seo MS. Lee YW. Kim WH. Kang KS. Kweon OK. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;10:273–284. doi: 10.4142/jvs.2009.10.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee ST. Chu K. Jung KH. Im WS. Park JE. Lim HC. Won CH. Shin SH. Lee SK. Kim M. Roh JK. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009;66:671–681. doi: 10.1002/ana.21788. [DOI] [PubMed] [Google Scholar]
- 28.Riordan NH. Ichim TE. Min WP. Wang H. Solano F. Lara F. Alfaro M. Rodriguez JP. Harman RJ, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo K. Shintani S. Shibata R. Murakami H. Murakami R. Imaizumi M. Kitagawa Y. Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Rey E. Gonzalez MA. Varela N. O'Valle F. Hernandez-Cortes P. Rico L. Buscher D. Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 31.Trottier V. Marceau-Fortier G. Germain L. Vincent C. Fradette J. IFATS collection: using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells. 2008;26:2713–2723. doi: 10.1634/stemcells.2008-0031. [DOI] [PubMed] [Google Scholar]
- 32.Josiah DT. Zhu D. Dreher F. Olson J. McFadden G. Caldas H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol Ther. 2010;18:377–385. doi: 10.1038/mt.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Rey E. Anderson P. Gonzalez MA. Rico L. Buscher D. Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 34.Patel SA. Sherman L. Munoz J. Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz.) 2008;56:1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 35.Poncelet AJ. Vercruysse J. Saliez A. Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 36.Tarazona R. Casado JG. DelaRosa O. Torre-Cisneros J. Villanueva JL. Sanchez B. Galiani MD. Gonzalez R. Solana R. Pena J. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–183. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 37.Tarazona R. DelaRosa O. Casado JG. Torre-Cisneros J. Villanueva JL. Galiani MD. Pena J. Solana R. NK-associated receptors on CD8 T cells from treatment-naive HIV-infected individuals: defective expression of CD56. AIDS. 2002;16:197–200. doi: 10.1097/00002030-200201250-00008. [DOI] [PubMed] [Google Scholar]
- 38.Casado JG. Pawelec G. Morgado S. Sanchez-Correa B. Delgado E. Gayoso I. Duran E. Solana R. Tarazona R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immunother. 2009;58:1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solana R. Casado JG. Delgado E. DelaRosa O. Marin J. Duran E. Pawelec G. Tarazona R. Lymphocyte activation in response to melanoma: interaction of NK-associated receptors and their ligands. Cancer Immunol Immunother. 2007;56:101–109. doi: 10.1007/s00262-006-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarazona R. Casado JG. Soto R. DelaRosa O. Peralbo E. Rioja L. Pena J. Solana R. Expression of NK-associated receptors on cytotoxic T cells from melanoma patients: a two-edged sword? Cancer Immunol Immunother. 2004;53:911–924. doi: 10.1007/s00262-004-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poggi A. Prevosto C. Massaro AM. Negrini S. Urbani S. Pierri I. Saccardi R. Gobbi M. Zocchi MR. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–6360. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 42.Spaggiari GM. Capobianco A. Becchetti S. Mingari MC. Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 43.Tarazona R. Delgado E. Guarnizo MC. Roncero RG. Morgado S. Sanchez-Correa B. Gordillo JJ. Dejulian J. Casado JG. Human prostasomes express CD48 and interfere with NK cell function. Immunobiology. 2011;216:41–46. doi: 10.1016/j.imbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Alter G. Malenfant JM. Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Huang XP. Sun Z. Miyagi Y. McDonald KH. Zhang L. Weisel RD. Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 46.Koppula PR. Chelluri LK. Polisetti N. Vemuganti GK. Histocompatibility testing of cultivated human bone marrow stromal cells - a promising step towards pre-clinical screening for allogeneic stem cell therapy. Cell Immunol. 2009;259:61–65. doi: 10.1016/j.cellimm.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Nauta AJ. Westerhuis G. Kruisselbrink AB. Lurvink EG. Willemze R. Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kastrinaki MC. Papadaki HA. Mesenchymal stromal cells in rheumatoid arthritis: biological properties and clinical applications. Curr Stem Cell Res Ther. 2009;4:61–69. doi: 10.2174/157488809787169084. [DOI] [PubMed] [Google Scholar]
- 49.Pontikoglou C. Delorme B. Charbord P. Human bone marrow native mesenchymal stem cells. Regen Med. 2008;3:731–741. doi: 10.2217/17460751.3.5.731. [DOI] [PubMed] [Google Scholar]
- 50.Jones E. McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 51.Krampera M. Cosmi L. Angeli R. Pasini A. Liotta F. Andreini A. Santarlasci V. Mazzinghi B. Pizzolo G, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 52.Ren G. Zhang L. Zhao X. Xu G. Zhang Y. Roberts AI. Zhao RC. Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Hayhoe RP. Henson SM. Akbar AN. Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol. 2010;71:676–681. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Markel G. Seidman R. Besser MJ. Zabari N. Ortenberg R. Shapira R. Treves AJ. Loewenthal R. Orenstein A. Nagler A. Schachter J. Natural killer lysis receptor (NKLR)/NKLR-ligand matching as a novel approach for enhancing anti-tumor activity of allogeneic NK cells. PLoS One. 2009;4:e5597. doi: 10.1371/journal.pone.0005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spaggiari GM. Capobianco A. Abdelrazik H. Becchetti F. Mingari MC. Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 56.Kim BS. Kang KS. Kang SK. Soluble factors from ASCs effectively direct control of chondrogenic fate. Cell Prolif. 2010;43:249–261. doi: 10.1111/j.1365-2184.2010.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.