Abstract
Background
A low cardiovascular disease (CVD) risk profile (untreated cholesterol < 200 mg/dl, untreated blood pressure < 120/<80 mmHg, never smoking, and no history of diabetes and myocardial infarction) in middle age is associated with markedly better health outcomes in older age, but few middle aged adults have this low risk profile. We examined whether adopting a healthy lifestyle throughout young adulthood is associated with presence of the low CVD risk profile in middle age.
Methods and Results
The CARDIA study sample consisted of 3,154 black and white participants aged 18 to 30 years at Year 0 (Y0, 1985-86) who attended the Year 0, 7 and 20 (Y0, Y7 and Y20) examinations. Healthy lifestyle factors (HLFs) defined at Y0, Y7 and Y20 included: 1) Average BMI < 25 kg/m2; 2) No or moderate alcohol intake; 3) higher healthy diet score; 4) higher physical activity score; and 5) Never smoking. Mean age (25 years) and percentage of women (56%) were comparable across groups defined by number of HLFs. The age-, sex- and race-adjusted prevalences of low CVD risk profile at Y20 were 3.0%, 14.6%, 29.5%, 39.2% and 60.7% for people with 0 or 1, 2, 3, 4, and 5 HLFs, respectively (p-trend <0.0001). Similar graded relationships were observed for each sex-race group (all p-trend<0.0001).
Conclusions
Maintaining a healthy lifestyle throughout young adulthood is strongly associated with low CVD risk profile in middle age. Public health and individual efforts are needed to improve adoption and maintenance of healthy lifestyles in young adults.
Keywords: epidemiology, follow-up studies, risk factors, prevention
Introduction
The concept of a low cardiovascular disease (CVD) risk profile, encompassing optimal levels of all established, modifiable CVD risk factors, was proposed by Stamler et al. in the late 1990s.1 Recently, there has been significant interest in the potential power of the low risk profile, if maintained into middle age, for substantially reducing the burden of CVD in the population. Research has shown that individuals with the low risk profile in middle age have dramatically lower total, cardiovascular and non-cardiovascular mortality rates, greater longevity, and substantially lower rates and remaining lifetime risks for CVD events compared with individuals without the profile.1-4 In addition, a low risk profile in middle age has been associated with a higher quality of life and lower Medicare charges in older age.2,5-6 Capewell and colleagues estimated that if all American adults had achieved the low risk profile by 2010, there would have been 95% fewer coronary heart disease deaths than expected in 2010.7 Based on the strength of all of these data, the low risk profile serves as the foundation for the American Heart Association’s 2020 Strategic Impact Goal8 and its focus on “ideal cardiovascular health.” However, the prevalence of the low risk profile in general is remarkably low. Using National Health and Nutrition Examination Survey (NHANES) 1999 to 2004 data, Ford et al. estimated that the prevalence of a low risk profile (the same criteria stated above with an addition of BMI < 25 kg/m2) in the United States was only 7.5% overall among individuals ages 25-74 years, and it declined with age.9 It is unclear whether a low risk profile can be achieved in middle age by adopting healthy lifestyles. Longitudinal data from the Coronary Artery Risk Development in (Young) Adults (CARDIA) Study10 provide an important opportunity to assess whether adopting and maintaining a healthy lifestyle from young adulthood to middle age can help achieve a low CVD risk profile in middle age.
Methods
The CARDIA Study is a multicenter longitudinal study sponsored by the National Heart, Lung and Blood Institute of the National Institutes of Health. The initial cohort consisted of 5,115 black and white men and women ages 18 to 30 years and free of CVD, who were recruited in 1985-86 (Y0) from 4 urban centers: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. The cohort was roughly balanced within each center by sex, race, ages ≤ 24 or 25 to 30 years, and education ≤ high school or > high school.
Sample
A detailed description of the study design has been published previously.10 Seven follow-up examinations have occurred at Years (Y) 2, 5, 7, 10, 15, 20, and 25. Analyses for this study were based on a sample consisting of 3,154 participants (1420 black, 1734 white, 1392 men, 1762 women) who attended the Y0, Y7, and Y20 examinations at which dietary intake was assessed. Women who were pregnant at the time of the Y0, Y7 or Y20 examinations were excluded from these analyses. From the sample of 3154 participants who were included in the primary analysis, 818 were missing data on variables used in either the healthy lifestyle or the low risk profile definitions. Specifically, 33, 114, and 372 participants were missing data on healthy lifestyle variables measured at Y0, Y7 and Y20, respectively, and this was mainly due to missing diet data. Another 117 participants had extreme reported caloric intake at Y0, Y7 or Y20 (>8000 kcal or <800 kcal for men and >6000 kcal or <600 kcal for women); their nurient values were recoded as missing. Finally, of the remaining 182 who were missing data on low risk variables measured at Y20, 20 were missing cholesterol or blood pressure data and 162 were missing glucose; glucose values were recoded as missing for participants who did not fast at least 8 hours. This left 2336 participants with complete data for all of the variables used to define low risk profile, healthy lifestyle factors and covariates.
Data Collection
All measurements were obtained by trained and certified technicians. Participants were asked to fast for 12 hours and to refrain from smoking and heavy physical activity for 2 hours prior to each examination. At Y0 and Y7, following a 5-minute rest period in a quiet room, a random zero sphygmomanometer was used on each participant’s right arm to take three systolic and fifth-phase diastolic blood pressure measurements at 1-minute intervals. The average of the second and third blood pressure measurements was used in the analysis. At Y20, the Omron model HEM907XL (Omron Healthcare, Inc) was used to measure blood pressure. A calibration study was conducted (n=800) to convert Y20 values to their Y0 equivalents. Total cholesterol and HDL cholesterol were measured enzymatically by the Northwest Lipid Laboratory.11-12 The Friedewald equation was used to calculate LDL cholesterol.13 Fasting glucose was measured by Linco Research, Inc. using the hexokinase ultraviolet method. Height and weight were measured with the participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Sex, race, and years of education were self-reported. Alcohol intake (ml/day) was computed from the self-reported frequency of beer, wine, and liquor consumed per week.14 Smoking was self reported using a tobacco use questionnaire previously validated by a study using serum cotinine levels.15 The physical activity score was derived from the CARDIA Physical Activity History previously validated by Jacobs, et al.16 Dietary intake was assessed using the CARDIA dietary history, an interviewer-administered quantitative food frequency questionnaire.17 Nutrient data, which included calcium, potassium, fiber, and saturated fat, were calculated using the University of Minnesota Nutrition Coordinating Center (NCC) food composition database (Version 10 for Y0, version 20 for Y7 and Version 36 for Y20). Reliability and validity studies were previously performed on the questionnaire.18 Medication use was self-reported and recorded at Y0 and Y7; at Y20, participants were asked to bring their medications for verification. Participants provide informed written consent at each examination, and the data collection and cohort follow-up protocols have been approved by the Institutional Review Board of each field center and the Coordinating Center.
Statistical Analysis
The primary analysis examined the relationship between the number of healthy lifestyle factors defined based on the average values from Y0, Y7 and Y20, and the prevalance of low CVD risk profiles at Y20. This low CVD risk profile at Y20 (henceforth referred to as “low risk profile”) is defined as the absence of pre-existing CVD and the simultaneous presence of untreated total cholesterol < 200 mg/dL, untreated blood pressure < 120/<80 mmHg, no diabetes, and never smoking. We selected 5 healthy lifestyle factors (HLFs) based on the data from Y0, Y7 and Y20 : 1) average BMI < 25kg/m2; 2) average alcohol intake from 0 to 15 g/day for women and 0 to 30g/day for men; 3) within the highest 40% on a dietary score based on higher average intake of potassium, calcium and fiber and lower intake of saturated fat (see below); 4) within the highest 40% of the sex-specific distribution of average physical activity score (Y0, Y7 and Y20 exams); and 5) never smoking. These factors were selected to coincide with the healthy lifetsyle pattern described by Stampfer et al,19 which has been associated with lower CVD event rates and diabetes incidence in multiple cohorts.20-22 For the physical activity score, described in detail elsewhere,23 the highest 40% cutpoints were 480.0 and 307.3 exercise units for men and women, respectively. Roughly 300 exercise units approximates the recommendation by the American College of Sports Medicine24 for the amount of exercise needed to support weight loss (i.e. energy expenditure equal to 1260 kJ (300 kcal) per session at 5 sessions/week).23 The dietary score was designed to be consistent with the DASH (Dietary Approaches to Stop Hypertension) eating pattern.25 The dietary scores were computed separately for each sex as follows. The average intakes from food sources only of potassium (mg), calcium (mg), fiber (g), and saturated fat (g) from the Y0, Y7, and Y20 exams were calculated for each participant. A quintile score (low=1 to high=5) for each mean intake of potassium, calcium and fiber was assigned to each participant and a quintile score (high=1 to low=5) was assigned for saturated fat intake. The 4 quintile scores were summed to create a total score ranging from 4 to 20. The highest 40% on this total score were defined as having a healthy diet. For sensitivity analyses, a score based on the highest sex-specific 40% of the Alternate Healthy Eating Index (AHEI), proposed by McCullough and Willett,26 was also computed. The AHEI consists of nine components representing intakes of vegetables, fruit, fish and poultry, non-meat protein (e.g., nuts and soy products), whole grains, ratio of polyunsaturated to saturated fats, low trans fat intake, moderate alcohol consumption, and a long-term multivitamin component. The two last components (i.e. alcohol consumption and multivitamin) were not included in the score for the present study because our HLF score includes an alcohol component, and data on long-term vitamin useage were not available at all three visits. Participants were stratified a priori into groups based on their total number of healthy lifestyle factors (0-1, 2, 3, 4, or 5 ) from young adulthood to middle age.
The primary data analysis was based on the sample of 3,154 participants who attended all three examinations at Y0, Y7 and Y20. The adjusted prevalence rate of low CVD risk profile for each number of HLFs through young adulthood at Y20 was estimated using multiple linear regression. Multiple logistic regression was used to test the significance of the trend in the prevalence rate of low risk with higher number of HLFs adjusting for age, sex and/or race when appropriate. Multiple imputation (5 times) was used to impute missing values using the method of Raghunathan et al.27 Three sensitivity analyses were done. Data from 2,336 participants with complete information at Y0, Y7 and Y20 were also analyzed. Another analysis, which based the number of healthy lifestyle factors on the average Y0 and Y7 values, was conducted to address the issue of reverse causation. The third analysis substituted the diet score with one based on the AHEI (described above).
Results
Baseline (Year 0) characteristics of the 3,154 included participants, stratified by the number of HLFs, are presented in Table 1. The baseline average ages were slightly younger with greater numbers of HLFs, and the percentages of women were similar across HLF groups. The percentage of black participants was lower and the average level of education was higher among those with greater numbers of HLFs. Overall mean levels of SBP, DBP, LDL-C, fasting glucose, and HDL-C levels were generally favorable for all five groups at baseline, when the participants were on average 25 years old. However, despite the overall favorable levels, all risk factors except for DBP were associated with the number of HLFs and the trends were in the expected direction. At baseline, 1379 (43.7%) participants had the low risk profile. For the majority of participants not classified as low risk at baseline, the main reason for non-low risk status was smoking, although elevated blood pressure or cholesterol contributed as well (data not shown).
Table 1.
Baseline characteristics (mean or percent) by Number of Healthy Lifestyle factors (HLF), Y0 to Y20 among CARDIA Study participants (n=3154).
| Variable | Number of HLFs | |||||
|---|---|---|---|---|---|---|
| 0-1 | 2 | 3 | 4 | 5 | p-trend | |
| Number (%) | 485 (15.4%) |
956 (30.3%) |
959 (30.4%) |
566 (17.9%) |
189 (6.0%)† |
|
| Age (yr) | 25.4 | 25.5 | 24.9 | 25.0 | 25.1 | .006 |
| Women (%) | 59.6% | 55.0% | 54.0% | 54.6% | 63.0% | 0.85 |
| Black (%) | 59.4% | 53.4% | 42.2% | 32.5% | 16.9% | <0.0001 |
| Education (yr) | 14.4 | 15.3 | 15.8 | 16.3 | 17.2 | <0.0001 |
| SBP (mmHg) | 111.7 | 110.6 | 109.9 | 109.6 | 108.0 | <0.0001 |
| DBP (mmHg) | 68.5 | 69.1 | 68.5 | 68.3 | 68.3 | 0.33 |
| BP med (%) | 1.4% | 1.2% | 0.4% | 0.2% | 0 | 0.003 |
| LDL-C (mg/dL) | 112.6 | 113.0 | 108.0 | 107.6 | 99.6 | <0.0001 |
| HDL-C (mg/dL) |
52.0 | 52.1 | 54.0 | 55.3 | 54.0 | <0.0001 |
| Fasting glucose (mg/dL) |
83.0 | 82.7 | 81.9 | 81.5 | 82.4 | 0.04 |
| Current Smoker at Y0 |
68.3% | 30.1% | 16.1% | 8.3% | 0 | <0.0001 |
| Low Risk at Y0 (%)* |
16.5% | 36.7% | 52.5% | 55.5% | 69.3% | <0.0001 |
The overall unadjusted prevalence rate of low risk at baseline is 43.7%
SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol
Sums to 3155 due to roundoff.
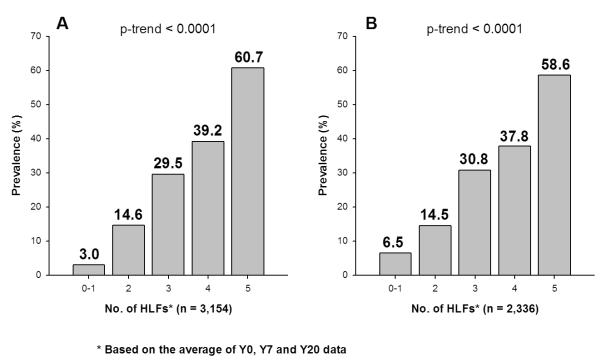
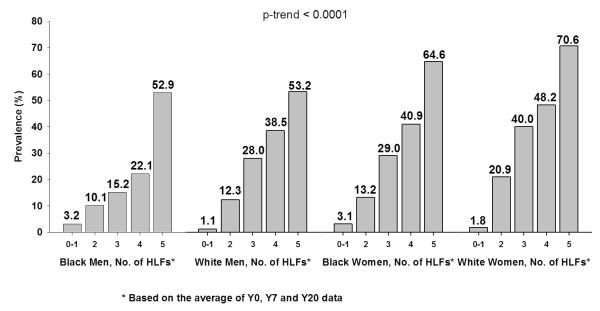
At Y20, 774 (24.5%) participants had the low risk profile (Table 2). The age-, sex-, and race-adjusted prevalence rates of the low risk profile stratified by the number of HLFs, as defined by the average Y0, Y7, and Y20 values, are shown in Figure 1A. The prevalence of the low risk profile in middle age was significantly and substantially higher with increasing numbers of HLFs during the period from young adulthood to middle age. The prevalence rates were 3.0%, 14.6%, 29.5%, 39.2%, and 60.7% for people with 0-1, 2, 3, 4 and 5 HLFs, respectively. Based on these prevalence rates, the attributable risk for not having a low risk profile at Y20 that could be explained by not having all 5 HLFs was 48%. Compared with people who only have 0-1 HLF, the age-, sex- and race-adjusted odds ratios for having a low risk profile at Y20 were 8.1, 20.5, 31.4 and 74.8 for people with 2, 3, 4 and 5 HLFs, respectively (Table 2). Since never smoking was considered as a HLF and as a low risk factor, the prevalence rates of low risk profile were estimated among never smokers and the results were consistent (Table 2). Similar results were also obtained for the sample of 2,336 young adults who had complete data (Figure 1B); again, the relationship was graded and highly significant (p-trend <0.0001). When using the HLFs based only on the Y0 and Y7 average, the age-, sex- and race-adjusted prevalence rates of low risk profile at Y20 were still strongly graded: 2.6%, 13.5%, 26.9%, 36.8% and 50.1% for the group with 0-1, 2, 3, 4, and 5 HLFs, respectively (data not shown). Figure 2 displays the age-adjusted prevalence of the low risk profile at Y20 according to the number of HLFs, stratified by sex and race. For all four sex and race groups, the prevalence rate was higher as the number of the HLFs increased (p-trend <0.0001 for all sex and race groups).
Table 2.
Low risk status at Y20 by Number of Healthy Lifestyle Factors (HLFs), Y0 to Y20 among CARDIA Study participants (n=3154).
| Number of HLFs† |
||||||
|---|---|---|---|---|---|---|
| 0-1 | 2 | 3 | 4 | 5 | p-trend | |
| Number (%) | 485 (15.4%) |
956 (30.3%) |
959 (30.4%) |
566 (18.0%) |
189‡ (6.0%) |
|
| Low Risk at Y20 |
2.1% | 13.6% | 29.8% | 40.1% | 64.0% | <0.0001 |
| Odds Ratio§ (95% CI) for Low Risk at Y20 |
1 | 8.1 (3.1, 21.0) |
20.5 (8.0, 52.7) |
31.4 (12.4, 79.9) |
74.8 (27.5, 204.0) |
|
| Total No. of Never Smokers through Year 20* |
558 | 729 | 492 | 189 | - | |
| Low Risk at Y20 among Never Smokers (%) |
25.1% | 39.2% | 46.1% | 64.0% | - | <0.0001 |
| Odds Ratio§ (95% CI) for Low Risk at Y20 among Never Smokers |
1 | 1.8 (1.4, 2.4) |
2.4 (1.8, 3.2) |
4.3 (2.9, 6.3) |
||
Never smokers have at most 4 HLFs.
HLFs are based on the average of Y0, Y7, and Y20 data.
Sums to 3155 because of roundoff.
Adjusted for age, sex, and race.
Figure 1.
Age-, Sex- and Race-Adjusted Prevalence of Low Risk Profile at Y20 by Healthy Lifestyle Factors (HLF) during Y0-Y20 among CARDIA Study Participants. Left figure (Figure 1A) is based on multiple imputation and right figure (Figure 1B) is based on complete data. P-trend is computed using logistic regression.
Figure 2.
Age-Adjusted Prevalence of Low Risk Profile at Y20 by Healthy Lifestyle Factors (HLF) during Y0-Y20 among CARDIA Study Participants by Race-Sex Group. P-trend is computed using logistic regression.
Table 3 shows the prevalence of the low risk profile for people who had at least 4 HLFs at various combinations of the Y0, Y7 and Y20 exams. The age-, sex-, and race-adjusted prevalence ranges from 53.8% for those who had 4 or 5 HLFs at all three time points to 13.6% for those who did not have 4+ HLFs at all three time points. Among those who adopted 4+ HLFs at only one exam, the prevalence of a low risk profile is highest for those who adopted 4+ HLFs at Y20. Similarly, among those who adopted 4+ HLFs at two exams, the prevalence of a low risk profile is highest for those who adopted 4+ HLFS at Y7 and Y20 exams.
Table 3.
Age-, race-, and sex- adjusted prevalence of Low Risk status at Year 20 by presence (denoted by a +) or absence (denoted by a −) of 4+ Healthy Lifestyle Factors at Years 0, 7, and 20 (using imputed data).
| Year 0 | Year 7 | Year 20 | n* | Prevalence of Low Risk at Y20 (%) requiring 4+ HLFs |
|---|---|---|---|---|
| − | − | − | 1648 | 13.6 |
| + | − | − | 471 | 23.9 |
| − | + | − | 174 | 29.8 |
| − | − | + | 105 | 37.5 |
| + | + | − | 284 | 37.7 |
| + | − | + | 103 | 39.0 |
| − | + | + | 86 | 52.2 |
| + | + | + | 285 | 53.8 |
indicates presence of 4+ HLFs at the examination,
indicates absence of 4+ HLFs at the examination.
sums to 3156 due to rounding
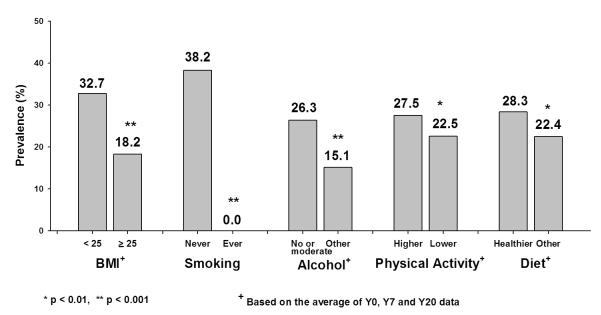
The Y20 prevalence of the low risk profile by individual HLFs over 20 years was analyzed after adjusting for age, sex and race (Figure 3). The prevalence of the low risk profile was significantly higher for those with average BMI <25 kg/m2 than those with average BMI ≥ 25 kg/m2 (p <0.001). The low risk profile was significantly more prevalent among never smokers than among ever smokers (p <0.001). Low risk prevalence was significantly higher in those who had no or moderate alcohol intake on average compared with higher alcohol intake (p <0.001). Higher average levels of physical activity were associated with significantly higher prevalence of the low risk profile when compared to lower levels of physical activity (p <0.01). Similar results were found for a healthier diet compared with less healthy diet based on the original diet score (28.3% for a healthier diet vs. 22.4% for a less healthy one, p <.01) as well as the diet score based on the AHEI (27.8% for a healthier diet vs. 22.3% for a less healthier, p <.01; data not shown).
Figure 3.
Age-, Sex- and Race-Adjusted Prevalence of Low Risk Profile at Y20 by Individual Healthy Lifestyle Factors (HLF) among CARDIA Study Participants. P-value is computed using logistic regression.
When “not currently smoking” (i.e., not smoking at least 5 cigarettes per week almost every week in the last 3 months prior to the exam) replaced “never smoking” in the definitions of the low risk profile and the HLF, similar results were observed (Figure 4).
Figure 4.
Age-, Sex- and Race-Adjusted Prevalence of Low Risk Profile at Y20 by Healthy Lifestyle Factors (HLF) During Y0-Y20 among CARDIA Study Participants. In the Low Risk Profile, “not a current smoker” replaces “never smoker”. Left figure is based on multiple imputation and right figure is based on complete data. P-trend is computed using logistic regression.
In addition, it is unclear whether the prospective association between HLFs and the low risk profile is also seen among people with family history of myocardial infarction (MI). In CARDIA, we collected data on whether each parent had a history of MI at Y0, Y5 and Y10. Among the 1220 participants with a positive parental history of MI, the age-, race-, and sex-adjusted prevalence of the low risk profile was 2.6%, 11.5%, 25.7%, 31.9%, and 53.8% in the 0-1, 2, 3, 4, and 5 HLF groups, respectively.
In sensitivity analyses substituting the AHEI-based diet score, the prevalence of the low-risk profile over the 5 HLF groups was still graded (Supplemental Table) for all participants and when stratified by the four sex and race groups. However, the prevalence of the low risk profile was slightly lower in the group with 5 HLFs when compared to the analysis based on the original diet score.
Discussion
Results of this study demonstrate a strong, graded relationship between maintenance of healthy lifestyles from young adulthood to middle age and the presence of the low CVD risk profile in middle age. Three-fourths of white and black women and over half of black and white men who adopted and maintained a healthy lifestyle with all five HLFs had a lower CVD risk profile after 20 years of follow up, when the average age was 45 years. Our results also indicate that for each sex and race group, as well as for the entire cohort and in all sensitivity analyses, people with a higher number of HLFs have a significantly higher prevalence of the low CVD risk profile, and vice versa. Moreover, among participants with a parental history of MI, the graded relationship between higher number of HLFs and presence of low CVD risk profile was consistent as well. These findings, which have significant public health implications, suggest that the low CVD risk profile in middle age can be achieved by adopting and maintaining a healthy lifestyle pattern early in adulthood.
Benefits of having the low CVD risk profile in middle age were first demonstrated by Stamler et al., utilizing 22-year follow-up data of the Chicago Heart Association Detection Project in Industry (CHA) and 16-year follow-up data of the Multiple Risk Factor Intervention Trial (MRFIT) Screenees Study.1 In both cohorts, mortality rates from coronary heart disease (CHD), CVD, and all causes in people with the low CVD risk profile were substantially lower than in others. Daviglus et al. analyzed Medicare data and reported that middle-aged participants with the low CVD risk profile in the CHA study had much lower rates of various chronic diseases at older ages.2 Morbidity rates decreased as the number of low CVD risk factors increased. More recently, Lloyd-Jones et al. used Framington Heart Study data to show that for those with the low CVD risk profile at age 50 the remaining lifetime risk of CVD was substantially lower than for people with two or more CVD risk factors.4 Furthermore, Capewell et al. estimated that if all U.S. adults had a low risk profile between 2000 and 2010, 372,000 (95%) fewer CHD deaths would have occurred in 2010.7 Benefits of the low CVD risk profile are not limited only to morbidity and mortality. Daviglus et al. showed in the CHA study that a low CVD risk profile in middle age was associated with significantly higher health related quality of life at older ages,2 and significantly lower Medicare charges, both average annual charges and those in the last year of life.5-6
Unfortunately, the prevalence of the low CVD risk profile is very low in the US population. In the CHA and MRFIT Screenees studies, baseline prevalence rates of the low risk profile range from 5 to 8%.1 In the recent report by Ford et al. using NHANES data, prevalence in the U.S. population was only 7.5%.9 However, as in our study, Ford et al. noted that prevalence is higher in younger age groups and declines dramatically with age. It has been unclear from these cross-sectional data whether the low risk profile can be achieved or maintained by adopting a healthy lifestyle.
In this study, five HLFs (never smoking, habitual moderate to vigorous physical activity, BMI<25 kg/m2, modest or no alcohol drinking, and a healthy diet) were selected to address this question. The selection of these lifestyle factors was based on the study by Stampfer et. al, which clearly demonstrated that nurses with similar healthy lifestyle factors had much lower risk of coronary heart disease. In addition, Vita et al.28 demonstrated that people with low risk profile defined based on normal BMI, no cigarette smoking and vigorous exercise, had much lower risk for disability than others. The justification for inclusion of each of these is important. Obesity has been associated with high blood pressure and high serum cholesterol and is the major risk factor for metabolic syndrome and type 2 diabetes.29-30 Cigarette smoking has been established as the most important risk factor for lung cancer and is one of the major risk factors for CHD.31-32 Excessive alcohol consumption has been associated with hypertension, breast cancer and liver disease and mortality.33-36 The American Cancer Society and the American Heart Association recommend no or moderate alcohol consumption.37-38 Unfortunately in CARDIA, due to limitations of dietary data, we cannot directly define a healthy diet that is the same as the diet suggested by Stampfer et al.19 or as the DASH (Dietary Approaches to Stop Hypertension) diet. Instead, we used higher intake of potassium, calcium and fiber and lower saturated fat to define a healthy diet that is consistent with the DASH eating pattern. For example, high calcium intakes reflect higher intakes of dairy products, higher potassium and fiber intakes reflect higher fruit, vegetable and whole grain intakes, and lower saturated fat intakes reflect lower intake of red meat and butter. Habitual exercise has been shown to prevent CVD risk factors and CVD mortality and morbidity.39-41 In this study, pursuit of each of these HLF individually through young adulthood was significantly associated with the prevalence of low risk profile during middle age. We also performed a sensitivity analysis based on AHEI. The results are also similar.
Across the 4 race and sex groups, the prevalence of low risk profile in middle age in the group with all 5 HLFs were much higher than the prevalence in the 0-1 HLF group. Although a very high percentage of participants in the group with 5 HLFs attained low risk status in middle age, there were still approximately 40% who did not. Further, the attributable risk of not having low risk profile due to lack of 5 HLFs was 48%. These results are subject to some limitations. Diet, physical activity, alcohol and smoking data are self-reported and subject to measurement errors. Misclassification, if anything, may attenuate the associations with the low risk profile. Classifications of healthy diet and higher physical activity level were not based on well-established threshold levels but were selected relative to peers. In addition, due to lack of data, low sodium intake was not included as part of the healthy diet. For these reasons, the role of HLF on the low CVD risk profile is likely underestimated.
The finding that the prevalence of low risk profiles at Y20 tends to be lower in those who adopted HLFs but did not maintain them over time, as opposed to those who did not adopt HLFs at baseline but adopted and maintained them later, is important. It suggests that people will benefit from improving their lifestyle. However, people benefit the most if they can adopt and maintain a healthy lifestyle throughout the period from young adulthood to middle age. These data also suggest that genetic factors may not be very important in determining a low risk profile. For example, despite the attenuation of association due to misclassification, for those who had only 0-1 HLF, only 3% had a low risk profile at Y20; on the other hand, 61% of those who adopted and maintained 5 HLFs had low risk profiles. The prevalence of low risk profile increases as the number of HLFs increases. If genetic factors play an important role, we would expect to see a much higher prevalence of low risk profile with 0-1 HLFs. Likewise, our data showing the association of HLFs with low risk among those with a family history further support the notion that lifestyle may play a more prominent role than genetics.
Despite the steady decline of CVD mortality rates over the past forty years, it remains the number one killer in the United States.42 Recent data suggest a flattening of these downward mortality trends, particularly among younger adults ages 35 to 54 years, with evidence for an increase in CHD death rates among women 35 to 44 years of age. Such data suggest that the decades-long improvements in CVD mortality rates may be on the verge of reversing, perhaps as a result of the effects of the obesity epidemic in the US.43 As noted above, a large array of data indicate the potency of the low risk profile as a means for substantially avoiding CVD across the lifespan, and for improving healthy longevity. Recently, the American Heart Association announced its 2020 Strategic Impact Goal,8 with the aim of improving the cardiovascular health of all Americans by 20% by the year 2020. The low risk profile (termed “ideal cardiovascular health factors”) as well as many of the lifestyle factors (termed “ideal health behaviors”) studied in the present analysis form the cornerstone of the AHA’s new definition of cardiovascular health, and the number of these factors in middle age has been shown to be strongly, inversely associated with prospective CVD events.44 The recommendations of Healthy People 2020 use similar definitions and focus on improving cardiovascular health and its components. Clearly, a broad array of public health and public policy strategies involving schools, communities, state and governmental agencies, healthcare systems and private organizations will be needed to address the societal problems underlying the loss of the low risk profile from young adulthood to middle age. Such policies should be designed to improve the likelihood that individuals can make healthier choices regarding lifestyles that are associated with long-term improvements in healthy longevity and reductions in healthcare costs.8
In order to achieve these goals, it will be critical to implement public health and individualized approaches to drastically increase the prevalence of the low CVD risk profile in the population. To our knowledge, this is the first study to clearly demonstrate that the pattern of the low CVD risk profile (e.g., ideal levels of cardiovascular health factors, including blood pressure, cholesterol, fasting glucose and never smoking status) in middle age is strongly associated with practicing a healthy lifestyle in young adulthood and maintaining it into middle age. To accomplish the goal of expanding the prevalence of the low risk profile, more emphasis should be placed on primordial prevention by encouraging the adoption of healthy lifestyles from young ages.
Supplementary Material
Acknowledgments
Funding Sources: This research was funded by contracts N01-HC-48047 through 48050 and N01-HC-95095 from the National Heart, Lung, and Blood Institute, National Institutes of Health
Footnotes
Conflict of Interest Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 2.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Feinglass J, Guralnik JM, Greenland P, Stamler J. Favorable cardiovascular risk profile in middle age and health-related quality of life in older age. Arch Intern Med. 2003;163:2460–2468. doi: 10.1001/archinte.163.20.2460. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry) Am J Cardiol. 2007;99:535–540. doi: 10.1016/j.amjcard.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risk of cardiovascular disease. N Engl J Med. 2012;366:321–29. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daviglus ML, Liu K, Greenland P, Dyer AR, Garside DB, Manheim L, Lowe LP, Rodin M, Lubitz J, Stamler J. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339:1122–1129. doi: 10.1056/NEJM199810153391606. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Greenland P, Manheim LM, Dyer AR, Wang R, Lubitz J, Manning WG, Fries JF, Stamler J. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Arch Intern Med. 2005;165:1028–1034. doi: 10.1001/archinte.165.9.1028. [DOI] [PubMed] [Google Scholar]
- 7.Capewell S, Ford ES, Croft JB, Critchley JA, Greenlund KJ, Labarthe DR. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in United States of America. Bull World Health Organ. 2010;88:120–130. doi: 10.2471/BLT.08.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Li C, Zhao G, Pearson WS, Capewell S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120:1181–1188. doi: 10.1161/CIRCULATIONAHA.108.835728. [DOI] [PubMed] [Google Scholar]
- 10.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 11.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–123. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 12.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Dyer AR, Cutter GR, Liu K, Armstrong MA, Friedman GD, Hughes GH, Dolce JJ, Raczynski J, Burke G, Manolio T. Alcohol intake and blood pressure in young adults: the CARDIA Study. J Clin Epidemiol. 1990;43:1–13. doi: 10.1016/0895-4356(90)90050-y. [DOI] [PubMed] [Google Scholar]
- 15.Wagenknecht LE, Cutter GR, Haley NJ, Sidney S, Manolio TA, Hughes GH, Jacobs DR. Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am J Public Health. 1990;80:1053–1056. doi: 10.2105/ajph.80.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs DR, Jr., Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D, Jr, Liu K, Hubert H, Gernhofer N. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–1112. [PubMed] [Google Scholar]
- 18.Liu K, Slattery ML, Jacobs DR, Jr, Cutter GR, McDonald A, Van Horn L, Hilner JE, Caan BJ, Bragg C, Dyer AR, Havlik R. A study of the reliability and comparative validity of the CARDIA dietary history. Ethn Dis. 1994;4:15–27. [PubMed] [Google Scholar]
- 19.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: The Cardiovascular Health Study. Arch Intern Med. 2009;169:798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–954. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 23.Parker ED, Schmitz KH, Jacobs DR, Jr, Dengel DR, Schreiner PJ. Physical activity in young adults and incident hypertension over 15 years of follow-up: the CARDIA study. Am J Public Health. 2007;97:703–709. doi: 10.2105/AJPH.2004.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Position stand of the American College of Sports Medicine. Schweiz Z Sportmed. 1993;41:127–137. [PubMed] [Google Scholar]
- 25.Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chim Acta. 2011;412:1493–1514. doi: 10.1016/j.cca.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutrition. 2006;9:152–157. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 27.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- 28.Vita AJ, Terry RB, Hubert HB, Fries JF. Aging, health risks, and cumulative disability. N Engl J Med. 1998;338:1035–1041. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 29.Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30:1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 30.Hardoon SL, Morris RW, Thomas MC, Wannamethee SG, Lennon LT, Whincup PH. Is the recent rise in type 2 diabetes incidence from 1984 to 2007 explained by the trend in increasing BMI? Evidence from a prospective study of British men. Diabetes Care. 2010;33:1494–1496. doi: 10.2337/dc09-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guideline. Chest. (2nd edition) 2007;132(3 Suppl):29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 32.Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23–32. doi: 10.1016/j.atherosclerosis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol consumption and the risk of hypertension in women and men. Hypertension. 2008;51:1080–1087. doi: 10.1161/HYPERTENSIONAHA.107.104968. [DOI] [PubMed] [Google Scholar]
- 34.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiuve SE, Rimm EB, Mukamal KJ, Rexrode KM, Stampfer MJ, Manson JE, Albert CM. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm. 2010;7:1374–1380. doi: 10.1016/j.hrthm.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol consumption and cardiovascular mortality among U.S. adults, 1987 to 2002. J Am Coll Cardiol. 2010;55:1328–1335. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, Gansler T, Andrews KS, Thun MJ, American Cancer Society 2006 Nutrition and Physical Activity Guidelines Advisory Committee American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 38.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 39.Lee C-D, Jacobs DR, Jr, Hankinson A, Iribarren C, Sidney S. Cardiorespiratory fitness and coronary artery calcification in young adults: the CARDIA Study. Atherosclerosis. 2009;203:263–268. doi: 10.1016/j.atherosclerosis.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu S, Yarnell JWG, Sweetnam PM, Murray L. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;89:502–506. doi: 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743–752. doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 42.National Heart Lung and Blood Institute . Morbidity & Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. National Institutes of Health; MD: 2009. [Google Scholar]
- 43.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 44.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD, ARIC Study Investigators Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.