Abstract
Compulsive drug-seeking and drug-taking are important substance-abuse behaviors that have been linked to alterations in dopaminergic neurotransmission and to impaired inhibitory control. Evidence supports the notions that abnormal D2 receptor-mediated dopamine transmission and inhibitory control may be heritable risk factors for addictions, and that they also reflect drug-induced neuroadaptations. To provide a mechanistic explanation for the drug-induced emergence of inhibitory-control deficits, this study examined how a chronic, escalating-dose regimen of methamphetamine administration affected dopaminergic neurochemistry and cognition in monkeys. Dopamine D2-like receptor and dopamine transporter (DAT) availability and reversal-learning performance were measured before and after exposure to methamphetamine (or saline), and brain dopamine levels were assayed at the conclusion of the study. Exposure to methamphetamine reduced dopamine D2-like receptor and DAT availability and produced transient, selective impairments in the reversal of a stimulus–outcome association. Furthermore, individual differences in the change in D2-like receptor availability in the striatum were related to the change in response to positive feedback. These data provide evidence that chronic, escalating-dose methamphetamine administration alters the dopamine system in a manner similar to that observed in methamphetamine-dependent humans. They also implicate alterations in positive-feedback sensitivity associated with D2-like receptor dysfunction as the mechanism by which inhibitory control deficits emerge in stimulant-dependent individuals. Finally, a significant degree of neurochemical and behavioral variation in response to methamphetamine was detected, indicating that individual differences affect the degree to which drugs of abuse alter these processes. Identification of these factors ultimately may assist in the development of individualized treatments for substance dependence.
Introduction
Difficulties with exerting inhibitory control over prepotent behaviors has been proposed as a central feature of substance dependence (Jentsch and Taylor, 1999). Support for this hypothesis comes from observations that individuals dependent on illicit substances exhibit inhibitory control deficits (Fortier et al., 2008; Ersche et al., 2011; Ghahremani et al., 2011), suggesting that these impairments represent a dimension of behavioral dysfunction common to many forms of addiction. Although pre-existing inhibitory control deficits may be a risk factor for the development of substance dependence (Dalley et al., 2007; Romer et al., 2009), there is evidence that these deficits can result from drug exposure (Jentsch et al., 2002; Stalnaker et al., 2009; Izquierdo et al., 2010; Porter et al., 2011). However, the neural adaptations consequent to drug use that elicit inhibitory control deficits remains an open research question.
Dysregulation of the dopamine D2-like (D2 and D3) receptor system has emerged as a candidate mechanism underlying difficulties with inhibitory control in addictions (Groman and Jentsch, 2011; Izquierdo and Jentsch, 2012). Stimulant-dependent individuals exhibit abnormally low striatal D2-like receptor availability (Volkow et al., 1993, 2001b; Lee et al., 2009), and these deficits may result from drug exposure itself (Nader et al., 2006). Outside the context of experience with drugs, pharmacological blockade of D2-like receptors results in inhibitory control deficits (Lee et al., 2007; Herold, 2010), and variation in D2-like receptor availability is correlated with individual differences in measures of inhibitory control (Groman et al., 2011). Therefore, dysfunction of D2-like receptor transmission may underlie the deficits in inhibitory control that emerge in drug-dependent individuals.
Some of the alterations in dopaminergic markers observed in methamphetamine-dependent individuals (McCann et al., 1998; Lee et al., 2009) can be modeled with high-dose methamphetamine administration (Wagner et al., 1980; Villemagne et al., 1998). However, much of the long-term impact of realistic patterns of slow, escalating-dose exposure to methamphetamine, which produces tolerance that protects against drug-induced neurotoxicity (Segal et al., 2003), on the integrity of the dopamine system remains unknown. Additionally, inconsistent results have been found regarding the characteristics of cognitive dysfunction in methamphetamine-dependent persons (Price et al., 2011), results that may relate to variable drug histories and individual differences. Finally, measures of inhibitory control have not been combined with biochemical and neuroimaging assessments within the same subjects, limiting our understanding of the complex relationships between these measures.
To bridge these gaps, the current study used positron emission tomography (PET) to quantify D2-like receptor and dopamine transporter (DAT) availability in monkeys tested for their abilities to acquire, retain, and reverse visual discriminations before and after a 31 d escalating dose-regimen of methamphetamine (or saline). The in vivo phase of this experiment was followed by ex vivo measurements of dopamine and dopamine-related metabolites in brain regions involved in inhibitory control. Based on the available evidence, we hypothesized that chronic methamphetamine would reduce striatal D2-like receptor and DAT availability, and dopamine levels, and would produce behavioral deficits specific to the reversal phase of the discrimination task.
Materials and Methods
Subjects
Fourteen male vervet monkeys [Chlorocebus aethiops sabaeus from the University of California, Los Angeles (UCLA) Vervet Research Colony], ranging from 5 to 9 years of age, were included in this study. Monkeys were individually housed in a climate-controlled vivarium, where they had unlimited access to water and received twice-daily portions of standard monkey chow in amounts that exceeded their nutritional needs (Teklad, Harlan Laboratories). All of the subjects were able to see, hear, and communicate with other individuals in the room. After behavioral testing was conducted in the morning, the monkeys received half of their daily portion of allotted chow (at ∼1100 h); their second half was provided in the afternoon (at ∼1500 h). The total amount of chow received was never reduced during the experiment to increase motivation for task performance. Two monkeys were excluded from some aspects of the study for unrelated reasons: one exhibited anxiety that compromised task performance, and was only used for the imaging and biochemical assays, and one did not receive a baseline PET scan for D2-like receptor availability due to technical problems.
All monkeys were maintained in accordance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85–23, revised 1996. Research protocols were approved by the UCLA Chancellor's Animal Research Committee.
Drugs
Methamphetamine hydrochloride was purchased from Sigma-Aldrich. Doses of methamphetamine were prepared fresh daily in sterile normal saline, and the solution was filtered through 22 μm Millex syringe filters (Millipore). All injections were administered intramuscularly at a volume of 0.1 ml/kg.
Dosing regimen
The dosing regimen was designed to model the escalation in both frequency of intake and cumulative daily dose reported by human users of methamphetamine (Han et al., 2011). Table 1 provides details of the 31 d regimen used. The initial dose was administered at 0830 h. During week 2, a second dose was administered at 1630 h. During weeks 3 through 5, the second dose was administered at 1330 h, and a third was given at 1630 h. For the last 2 weeks of treatment, the second dose was administered at 1100 h, the third at 1330 h, and a fourth at 1600 h.
Table 1.
Dose of methamphetamine (mg/kg) given per day and at each injection during the 31 d dose regimen
| Week 1 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
| Injection #1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| Week 2 | ||||||
| Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 |
| Injection #1 | 0.25 | 0.35 | 0.45 | 0.55 | 0.65 | |
| Injection #2 | 0.25 | 0.35 | 0.45 | 0.55 | 0.65 | |
| Week 3 | ||||||
| Day 14 | Day 15 | Day 16 | Day 17 | Day 18 | Day 19 | Day 20 |
| Injection #1 | 0.35 | 0.45 | 0.55 | 0.65 | ||
| Injection #2 | 0.45 | 0.55 | 0.65 | 0.75 | ||
| Injection #3 | 0.50 | 0.60 | 0.70 | 0.80 | ||
| Week 4 | ||||||
| Day 21 | Day 22 | Day 23 | Day 24 | Day 25 | Day 26 | Day 27 |
| Injection #1 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| Injection #2 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| Injection #3 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| Injection #4 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |
| Week 5 | ||||||
| Day 28 | Day 29 | Day 30 | Day 31 | |||
| Injection #1 | 1.0 | 1.0 | 1.0 | |||
| Injection #2 | 1.0 | 1.0 | 1.0 | |||
| Injection #3 | 1.0 | 1.0 | 1.0 | |||
| Injection #4 | 1.0 | 1.0 | 1.0 |
Saline-exposed monkeys received injections of saline using the same regimen.
Discrimination acquisition, retention, and reversal learning
Monkeys were first trained to move from their individual cages into a transport cart, and were brought to a quiet testing room where the transport cart was aligned with a Wisconsin General Testing Apparatus. The general procedures used in the current study have been described previously (Groman et al., 2011).
Monkeys were trained to acquire, retain, and reverse novel visual discriminations. Testing sessions began when the experimenter raised an opaque screen, allowing the monkey access to three boxes, each fitted with a novel visual stimulus on top. During each trial, the monkey was allowed to open a single box. A trial ended after a correct choice of the rewarded visual stimulus (reward was a small piece of apple, orange, grape, or raisin), an incorrect choice, or an omission (no response for 2 min), and a 20 s intertrial interval followed. The spatial positions of the different visual stimuli were varied pseudorandomly across trials.
The first session of a discrimination problem was the acquisition phase, during which the monkey was presented with three novel stimuli and had to learn which one was associated with reward on the basis of trial and error. Once a performance criterion was met (seven correct choices in 10 consecutive trials), the session ended and the monkey was returned to its home cage. If the criterion was not met within 80 trials, the session ended and the same discrimination problem was presented the following day until the criterion was met. No monkeys required >2 d of acquisition training.
One day after reaching the acquisition criterion, monkeys were tested in a retention phase, where the stimulus–reward contingencies remained unchanged, and again the monkeys were required to meet a performance criterion (four correct responses in five consecutive trials). Immediately after reaching this criterion, the reversal phase began, with no explicit signal of the transition given to the monkey. In the reversal phase, the stimulus that was rewarded previously was no longer rewarded while one of the previously nonrewarded stimuli became rewarded. Designation of the newly rewarded stimulus was counterbalanced across subjects. The session continued, only for that day, until the monkey met the performance criterion (seven correct choices out of 10 consecutive trials) or 70 trials had been completed, whichever occurred first.
The number of trials required to reach criterion and number of correct responses in the acquisition, retention, and reversal phases were the primary dependent measures. For the reversal phase, the number of responses directed at the previously rewarded stimulus (perseverative responses) and the number of responses directed at the never rewarded stimulus (neutral responses) were also recorded. The frequency of each type of response was also calculated by dividing the number of correct, perseverative, or neutral responses by the total number of trials completed in the reversal phase.
Subjects were trained on several novel discrimination problems before beginning the treatment regimen. The baseline data for 12 of the 14 monkeys used in the current study have been presented in detail previously (Groman et al., 2011). Assignment of monkeys to the saline group (N = 7) or the methamphetamine (N = 7) group was balanced on the basis of average performance in the acquisition, retention, and reversal phases of the last three visual discrimination problems completed immediately before beginning the dosing regimen.
Drug effects on behavioral performance were determined by comparing data at three time-points, each assessment performed using novel stimuli and the same performance criteria: (1) performance derived from the discrimination problem completed immediately before the treatment regimen was started (referred to as the “baseline assessment”); (2) performance during 3 weeks within the course of the treatment regimen (referred to as the “3 week assessment”; to limit the anorectic effects of methamphetamine, this test occurred after at least 36 h had elapsed since the last methamphetamine or saline administration); and (3) performance at 5 d after the last drug administration (referred to as the “5 d postexposure assessment”).
After completion of the dose regimen, two additional behavioral assessments were conducted, but the performance criterion was increased (nine correct choices in 10 completed trials for the acquisition, retention, and reversal phases) to augment the cognitive demands of the task. Specifically, the additional acquisition and retention training was expected to increase the likelihood of perseverative responding; these two “high-difficulty” sessions were conducted at 8 d and 2 weeks after cessation of drug administration (referred to as the “8 d postexposure assessment” and the “2 week postexposure assessment,” respectively).
MRI scanning procedures
One week after the acquisition of baseline PET scans, structural magnetic resonance (MR) images were acquired on a 1.5 T Siemens scanner to allow for anatomically based demarcation of regions of interest (ROIs) using procedures identical to those previously described (Groman et al., 2011). A second MR image was acquired ∼3½ weeks after completion of the dosing regimen; however, only the first MR image was used in the current study for coregistration purposes.
PET scanning procedures
PET scanning was performed using [18F]fallypride and [11C]WIN-35,428 as radioligands for measurements of dopamine D2-like receptors and DAT availability, respectively. Three PET scanning sessions were conducted: (1) ∼2 weeks before initiating drug administration (referred to as the “baseline scan”); (2) ∼2 weeks after cessation of drug administration, to examine the immediate effects of methamphetamine (referred to as the “2 week postexposure scan”); and (3) 7 weeks after cessation of drug administration to examine the stability of neural changes and the potential for recovery (referred to as the “7 week postexposure scan”). All scans were conducted using a MicroPET Model P4 scanner (Concorde Instruments), with procedures previously described (Groman et al., 2011).
Monkeys received an intramuscular injection of ketamine hydrochloride (10 mg/kg) and glycopyrrolate (0.01 mg/kg). After monkeys were sedated, an endotracheal tube was placed to provide inhalation of 2–3% isoflurane (in 100% O2). Vital signs (heart rate, respiratory rate, oxygen saturation, and temperature) were monitored and recorded every 15 min throughout the scan. A tail-vein catheter was placed, and the monkey was positioned on the scanning bed such that the imaging planes were parallel to the orbitomeatal line and the top of the head was at the front of the field of view. A 20 min 68Ge transmission scan was acquired before administration of the radioligand for attenuation correction. All subjects received a bolus injection [11C]WIN-35,428 (1.0 mCi/kg), followed by a 5 ml saline flush, and data were acquired for 90 min. When radioactivity had fallen to baseline levels (∼3 h after [11C]WIN-35,428 administration), a bolus injection of [18F]fallypride was delivered (0.3 mCi/kg), followed by a 5 ml saline flush. Dynamic data were acquired in list mode for 180 min. After the scan, gas anesthesia was removed and the animals were allowed to recover overnight before being returned to their home cages.
Quantification of dopamine and dopamine metabolites
Approximately 2–6 months after completing the dose regimen, monkeys were chemically restrained with ketamine hydrochloride (10 mg/kg, i.m.) and heavily sedated with sodium pentobarbital (30 mg/kg, i.v.). Following loss of the corneal reflex, they were transcardially perfused with ice-cold saline for 10 min; their brains were then removed and regionally dissected on a cold platform. The tissue was immediately frozen on dry ice and stored at −80°C until assayed. Tissue was processed by HPLC, as previously described (Jentsch et al., 1997).
Based on previous studies, we hypothesized that dopamine and dopamine-related metabolites (HVA, homovanilic acid; DOPAC, 3,4-dihydroxyphenylacetic acid; 3-MT, 3-methoxytyramine) would be most affected by exposure to methamphetamine within the striatum (Melega et al., 2008). Therefore, we restricted our analyses to dopamine turnover ratios [tissue content of each metabolite (in ng/mg protein)/tissue content of parent amine (ng/mg protein)] within the following brain regions: caudate nucleus, putamen, nucleus accumbens core and shell. In regions where dopamine turnover differed between methamphetamine- and saline-exposed monkeys, we conducted additional separate analyses on levels of dopamine and the related metabolite to further explore observed differences.
Data processing
Feedback-sensitivity measures.
Feedback sensitivity was determined by examining the choice behavior of subjects on a trial-by-trial basis during the acquisition and reversal phases of the discrimination sessions. First, trials were categorized on the basis of whether the subjects received positive or negative feedback on the preceding trial (i.e., rewarded or not). Following a positive-feedback trial, in which a correct response was made, the subsequent response was classified either as another correct response or an incorrect response. For the reversal-phase data, we further classified an incorrect response as a response directed at the previously rewarded stimulus, or a response to the stimulus that was never rewarded. Following negative feedback, when an incorrect response was made, the subsequent response was classified either as the same incorrect response or a response directed at one of the other two stimuli, regardless of whether the response was correct or incorrect. The total number of these individual types of responses made after positive or negative feedback was determined, and the frequency of being made was calculated by dividing these totals by the total number of positive or negative events, respectively, for the acquisition and reversal phase of each discrimination session.
Reconstruction of PET images.
Three-dimensional sinogram files were created by binning the data into 18 frames (four 60 s frames, three 120 s frames, six 300 s frames, and five 600 s frames) for [11C]WIN-35,428 sinograms, and 33 frames (six 30 s frames, seven 60 s frames, five 120 s frames, four 300 s frames, nine 600 s frames, one 1200 s frame and one 1800 s frame) for [18F]fallypride sinograms. We applied a previously validated algorithm to the transmission scan list-mode data to generate attenuation maps (Vandervoort and Sossi, 2008). This algorithm uses an analytical scatter correction, based upon the Klein–Nishina formula, for singles-mode transmission data. Following construction of the attenuation maps, emission list-mode files were reconstructed using OSEM (Ordered-Subsets Expectation Maximization) algorithm, and corrected for normalization, dead time, scatter, and attenuation using software provided by the scanner manufacturer (microPET Manager version 2.4.1.1, Siemens). The resultant images had voxel dimensions of 0.949 mm × 0.949 mm × 1.212 mm and matrix dimensions of 128 × 128 × 63.
PET data processing.
Using FSL View (FMRIB's Software Library v4.0), the following ROIs were drawn on the structural MR image of each subject by a single experimenter blind to the subject's identity: the caudate nucleus, putamen, ventral striatum, ventral mesencephalon, and cerebellum.
Reconstructed PET images were corrected for motion using the realignment function within Statistical Parametric Mapping 5 (Institute of Neurology, University College London, London, England). Each subject's MR image was then coregistered to the PET image using the Automated Image Registration program (AIR version 5; Woods et al., 1993), and the resultant transformation was applied to the ROIs. ROIs in the native PET space were then used to extract activity from the PET images, and imported into the PMOD kinetic analysis program. Time-activity curves were fit using the Multilinear Reference Tissue Model (MRTM; Ichise et al., 2003) to provide an estimate of k2′, the rate constant of tracer transfer from the reference region (cerebellum) to plasma. The k2′ estimates of the high-activity areas in the caudate nucleus and putamen were averaged, and time-activity curves were refit with MRTM2 using the average fixed k2′ value applied to all brain regions and a fixed starting point of 27.5 min. Binding potentials from the left and right brain structures were averaged to create single measurements for each ROI.
Statistical analyses
Initial inspection of the PET and behavioral measures revealed significant violations of sphericity and non-normal data distributions that violated the general assumptions of general linear models. These violations prevented the use of statistical models, such as a repeated-measures ANOVA or an analysis of covariance for these data, which require sphericity and normally distributed data. All analyses were conducted using generalized estimating equations, a quasi-likelihood-based, population-averaged model that allows for variance of the dependent variable to be a specified function of the mean (to account for non-normal distribution) and for correlated datasets and nonlinear relationships between its mean and the set of linear predictors (Ballinger, 2004). Where applicable, longitudinal data (i.e., PET and behavioral data) were entered into the model as repeated measures, and experimental group was included as a between-subjects factor. For the PET data, measurements calculated for a given ROI at the 3 scans were entered as repeated measures, with experimental group as a between-subjects factor. The covariance structure was determined on the basis of goodness-of-fit estimates (Quasi Likelihood under Independence Model Criterion), and probability distribution and link functions chosen on the basis of known properties of the type of variable or through exploratory analysis of descriptive qualities of data distributions. PET data were analyzed using an unstructured working correlation matrix and an inverse Gaussian distribution with the identity link function. Behavioral data were analyzed using an unstructured working correlation matrix and a negative binomial distribution with a log link function. Finally, for the ex vivo data, dopamine turnover was analyzed using an unstructured working correlation matrix and normal distribution with the identity link function. Dopamine and HVA levels were analyzed using an unstructured correlation matrix and a gamma distribution with the identity link function. Significant interactions were explored using post hoc, pairwise comparisons with a Bonferroni adjustment to correct for multiple comparisons. Because saline-exposed monkeys exhibited changes in the behavioral and neurochemical measures over time, pairwise comparisons were conducted considering the baseline measures for each subject, rather than using simple between-groups contrasts at each time point. Spearman's rank correlation coefficient (Spearman's ρ) was used to assess the statistical dependence of PET and behavioral measures. The number of days since completion of the treatment regimen was included as a covariate in the postmortem analyses.
Results
D2-like receptor availability
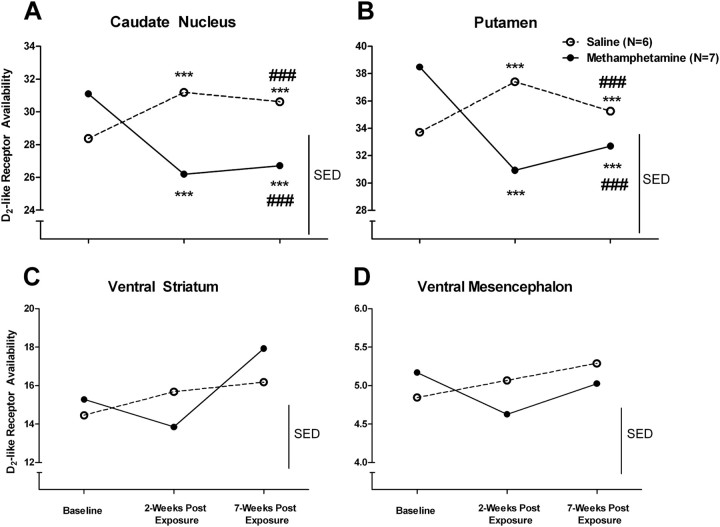
The effects of methamphetamine on D2-like receptor availability revealed no significant group differences (saline vs methamphetamine) before treatment in any of the brain regions examined (all p values >0.35). However, drug exposure significantly affected D2-like receptor availability in the caudate nucleus (group: Wald χ2 with 1 df = 0.030, p = 0.86; scan: Wald χ2 with 2 df = 31.59, p < 0.001; group by scan: Wald χ2 with 2 df = 48.91, p < 0.001; Fig. 1A), putamen (group: Wald χ2 with 1 df = 0.001, p = 0.98; scan: Wald χ2 with 2 df = 37.96, p < 0.001; group by scan: Wald χ2 with 2 df = 54.94, p < 0.001; Fig. 1B), and ventral striatum (group: Wald χ2 with 1 df = 0.012, p = 0.91; scan: Wald χ2 with 2 df = 9.20, p = 0.01; group by scan: Wald χ2 with 2 df = 24.95, p < 0.001; Fig 1C), but not in the ventral mesencephalon (group: Wald χ2 with 1 df = 0.58, p = 0.45; scan: Wald χ2 with 2 df = 10.29, p = 0.006; group by scan: Wald χ2 with 2 df = 1.91, p = 0.38; Fig. 1D). Post hoc analyses confirmed that D2-like receptor availability had decreased from baseline levels in only the caudate nucleus and putamen at the 2 week post-treatment scan in methamphetamine-exposed monkeys (all p values <0.001); however, no significant pairwise comparisons were detected in the ventral striatum (all p values >0.11). The reductions in D2-like receptor availability in both the caudate nucleus and putamen were still present at the 7 week postexposure scan (all p values <0.001), despite a significant increase between the 2 week and 7 week postexposure scans in both regions (all p values <0.001). The saline-exposed group exhibited an unexpected increase in D2-like receptor availability in the caudate and putamen at the 2 week postexposure scan (p < 0.001), an effect that remained at the 7 week postexposure scan (p < 0.001); these effects could reflect the low-level stress associated with daily saline injections.
Figure 1.
D2-like receptor availability in the caudate nucleus (A), putamen (B), ventral striatum (C), and ventral mesencephalon (D) in saline-exposed (open circles) and methamphetamine-exposed (closed circles) monkeys at baseline, 2 weeks post-drug exposure, and 7 weeks post-drug exposure. * indicates a significant change from baseline; # indicates a significant change from 2 weeks post-drug exposure. ***p < 0.001, ###p < 0.001. SED, SE of the mean of differences.
DAT availability
Similar to findings on D2-like receptor availability, no significant group differences (saline vs methamphetamine) in DAT availability were detected before treatment in any of the brain regions examined (all p values >0.30). However, drug exposure altered DAT availability in the caudate nucleus (group: Wald χ2 with 1 df = 4.54, p = 0.03; scan: Wald χ2 with 2 df = 56.75, p < 0.001; group by scan: Wald χ2 with 2 df = 20.67; p < 0.001; Fig. 2A), putamen (group: Wald χ2 with 1 df = 8.16, p = 0.004; scan: Wald χ2 with 2 df = 52.38, p < 0.001; group by scan: Wald χ2 with 2 df = 91.37, p < 0.001; Fig 2B), ventral striatum (group: Wald χ2 with 1 df = 3.62, p = 0.06; scan: Wald χ2 with 2 df = 44.07, p < 0.001; group by scan: Wald χ2 with 2 df = 17.68, p < 0.001; Fig. 2C), and ventral mesencephalon (group: Wald χ2 with 1 df = 1.57, p = 0.21; scan: Wald χ2 with 2 df = 24.29, p < 0.001; group by scan: Wald χ2 with 2 df = 9.62, p = 0.008; Fig. 2D). Post hoc analyses revealed that methamphetamine-exposed monkeys exhibited significant reductions in DAT availability at the 2 week postexposure scan in the caudate nucleus, putamen, and ventral striatum (all p values <0.001), but not in the ventral mesencephalon (p = 0.10). The reduction in DAT availability in methamphetamine-exposed monkeys, relative to baseline, persisted at the 7 week postexposure time-point in the caudate nucleus and putamen (all p values <0.001), although there was a significant increase in DAT availability at the 7 week postexposure scan relative to the 2 week postexposure scan (all p values <0.001). Reductions in DAT availability in the ventral striatum of methamphetamine-exposed monkeys were still present at the 7 week postexposure scan (p < 0.001), with no significant recovery occurring between the 2 week and 7 week postexposure scan (p = 0.38). An unexpected reduction in DAT availability, compared with baseline, at the 2 week postexposure scan was found in saline-exposed monkeys in the ventral striatum (p = 0.03), but not in the caudate nucleus, putamen, or ventral mesencephalon (all p values >0.90). However, no significant changes from baseline in DAT availability were detected in saline-exposed monkeys at the 7 week postexposure scan in any of the brain regions (all p values >0.90).
Figure 2.
DAT availability in the caudate nucleus (A), putamen (B), ventral striatum (C), and ventral mesencephalon (D) in saline-exposed (open circles) and methamphetamine-exposed (closed circles) monkeys at baseline, 2 weeks post-drug exposure, and 7 weeks post-drug exposure. * indicates a significant change from baseline; # indicates a significant change from 2 weeks post-drug exposure. *p < 0.05, **p < 0.01, ***p < 0.001, ###p < 0.001. SED, SE of the mean of differences.
Behavior
Group assignment on the basis of baseline performance achieved balance between the two groups: the average number of trials required to reach criterion in the acquisition, retention, and reversal phase of the last three visual discrimination sessions was not significantly different between groups (all p values >0.30).
Analysis of behavior before, during, and after drug administration revealed no significant differences between groups for the number of trials required to reach criterion in the acquisition phase (group: Wald χ2 with 1 df = 1.38, p = 0.24; discrimination session: Wald χ2 with 2 df = 2.70, p = 0.26; group by discrimination session: Wald χ2 with 2 df = 0.14, p = 0.93; Fig. 3A) or in the retention phase (group: Wald χ2 with 1 df = 0.35, p = 0.56; discrimination session: Wald χ2 with 2 df = 0.80, p = 0.67; group by discrimination session: Wald χ2 with 2 df = 0.17, p = 0.92; Fig. 3B). However, trials required to reach criterion in the reversal phase significantly diverged between groups across subsequent behavioral assessment points (group: Wald χ2 with 1 df = 0.00, p = 0.99; discrimination session: Wald χ2 with 2 df = 13.79, p = 0.001; group by discrimination session: Wald χ2 with 2 df = 67.56, p < 0.001; Fig. 3C). Post hoc analyses confirmed that this was due to an increase in the number of trials required to reach criterion in methamphetamine-exposed monkeys between baseline and the 3 week assessment (p < 0.001); however, no other pairwise comparisons were significant (all p values >0.81), indicating that this behavioral effect was transient. Table 2 presents the number of trials required to reach criterion for each subject during the reversal phase of each discrimination session completed.
Figure 3.

The number of trials required to reach criterion in the acquisition (A), retention (B), and reversal (C) phases between saline-exposed (open circles) and methamphetamine-exposed (closed circles) monkeys at baseline, at the 3 week assessment, at the 5 d post-drug exposure test, and at the two high difficulty sessions (1 week post-drug exposure and 3 weeks post-drug exposure). ***p < 0.001. SED, SE of the mean of differences.
Table 2.
The number of trials required to reach criterion performance in reversal by individual subjects across each ascertainment
| Subject | Exposure group | Baseline | 3 week assessment | 5 d postexposure | 1 week postexposure | 3 weeks postexposure |
|---|---|---|---|---|---|---|
| A9727 | Saline | 50 | 41 | 70 | 32 | 59 |
| B9721 | Saline | 20 | 13 | 13 | 50 | 42 |
| H0271 | Saline | 30 | 37 | 16 | 27 | 16 |
| I0221 | Saline | 55 | 34 | 38 | 47 | 43 |
| L0441 | Saline | 24 | 17 | 8 | 18 | 21 |
| N0406 | Saline | 9 | 10 | 21 | 70 | 22 |
| C9726 | Methamphetamine | 22 | 24 | 17 | 44 | 70 |
| D9802 | Methamphetamine | 49 | 70 | 33 | 70 | 70 |
| E0241 | Methamphetamine | 12 | 48 | 11 | 26 | 19 |
| G0261 | Methamphetamine | 23 | 32 | 27 | 53 | 29 |
| J0247 | Methamphetamine | 17 | 46 | 38 | 27 | 35 |
| K0488 | Methamphetamine | 10 | 14 | 37 | 31 | 32 |
| M0457 | Methamphetamine | 16 | 24 | 25 | 70 | 27 |
Relating changes in D2-like receptor availability with behavior
We previously reported that individual differences in D2-like receptor availability within the dorsal striatum were correlated with reversal-learning performance and sensitivity to positive but not negative feedback (Groman et al., 2011). Therefore, it was hypothesized that the change in D2-like receptor availability within the dorsal striatum across scans would be correlated with the change in positive-feedback sensitivity, regardless of drug exposure.
To test this hypothesis, the relationship between difference scores for D2-like receptor availability in the dorsal striatum (2 weeks postexposure scan, baseline) and feedback-sensitivity measures gathered before and after treatment during the reversal phases (5 d postexposure assessment, baseline) were examined. Consistent with previous results, changes in D2-like receptor availability (in any of the brain regions examined) were not correlated with the change in negative-feedback sensitivity in the reversal phases (all p values >0.23). However, the change in the frequency with which a monkey persisted with a correct response following positive feedback was positively correlated with the change in D2-like receptor availability in the caudate nucleus (Spearman's ρ = 0.587, p = 0.04; Fig. 4A). To examine whether this relationship was present in methamphetamine- and saline-exposed groups, the correlation coefficients for methamphetamine- and saline-exposure groups were compared using the Fisher transformation. The correlation coefficients did not differ statistically between the groups (methamphetamine-exposed monkeys: Spearman's ρ = 0.571; saline-exposed monkeys: Spearman's ρ = 0.60; z = −0.06, p = 0.95), indicating that the change in D2-like receptor was positively correlated with change in behavior, regardless of group.
Figure 4.
The relationship between changes in D2-like receptor availability in the caudate nucleus and in the ability of monkeys to persist with a correct response following positive feedback in saline-exposed monkeys (open circles) and methamphetamine-exposed monkeys (closed circles). A, Compares the change from baseline in D2-like receptor at the 1 week postexposure scan to change from baseline in positive-feedback sensitivity at the 5 d postexposure assessment. B, Compares the change from baseline in D2-like receptor at the 7 week postexposure scan to change from baseline in positive-feedback sensitivity at the 3 week postexposure assessment.
To examine the persistence of this relationship, the same analysis was conducted using difference scores, from baseline, for the 7 week postexposure D2-like receptor availability measurements and the last behavioral assessment conducted. The change in the frequency with which a monkey persisted with a correct response following positive feedback was positively correlated with the change in D2-like receptor availability in the caudate nucleus (Spearman's ρ = 0.60; p = 0.03; Fig. 4B), and the correlation coefficients did not differ between the groups (methamphetamine-exposed monkeys: Spearman's ρ = 0.54; saline-exposed monkeys: Spearman's ρ = 0.54).
Relating changes in DAT availability with behavior
To examine the specificity of the above-mentioned relationships, the same analyses were conducted with the change in DAT availability (2 week postexposure scan, baseline) and the change in positive-feedback sensitivity in the reversal phases (5 d postexposure assessment, baseline). The change in DAT availability within the putamen was negatively correlated with change in the frequency of monkeys choosing the never-rewarded stimulus following positive feedback (Spearman's ρ = −0.65; p = 0.02). No other significant correlations were detected (all p values >0.05).
Quantification of dopamine and dopamine metabolites
Table 3 outlines the results from the ex vivo tissue analysis of the four striatal regions examined in this study. Dopamine turnover with levels of HVA and DOPAC, but not 3-MT, was higher in methamphetamine-exposed monkeys, compared with saline-exposed monkeys, in the caudate nucleus (HVA/dopamine: Wald χ2 with 1 df = 9.05, p = 0.003; DOPAC/dopamine: Wald χ2 with 1 df = 3.16, p = 0.08), putamen (HVA/dopamine: Wald χ2 with 1 df = 10.81, p = 0.001; DOPAC/dopamine: Wald χ2 with 1 df = 6.42, p = 0.01), and nucleus accumbens core (HVA/dopamine: Wald χ2 with 1 df = 9.51, p = 0.002; DOPAC/dopamine: Wald χ2 with 1 df = 10.98, p = 0.001). Further analyses indicated that these effects were the result of lowered dopamine levels in methamphetamine-exposed, compared with saline-exposed, monkeys within the putamen (mean ± SEM saline: 110 ± 17 ng/mg, methamphetamine: 59 ± 4.16 ng/mg; Wald χ2 with 1 df = 9.75, p = 0.002) and nucleus accumbens core (mean ± SEM saline: 77 ± 9.8 ng/mg, methamphetamine: 42 ± 2.87 ng/mg; Wald χ2 with 1 df = 14.81, p < 0.001), rather than differences in DOPAC or HVA levels (all p values >0.14). Dopamine or HVA levels in the caudate did not differ significantly between methamphetamine- and saline-exposed monkeys (all p values >0.39).
Table 3.
Mean levels of dopamine utilization, dopamine and respective metabolites (DOPAC and HVA) measured ex vivo in the caudate, putamen, nucleus accumbens core, and nucleus accumbens shell of saline- and methamphetamine-exposed monkeys
| Caudate |
Putamen |
Nucleus accumbens core |
Nucleus accumbens shell |
|||||
|---|---|---|---|---|---|---|---|---|
| SAL | MA | SAL | MA | SAL | MA | SAL | MA | |
| DOPAC/DA | 0.08 ± 0.01 | 0.11 ± 0.02 | 0.07 ± 0.01 | 0.12 ± 0.02** | 0.14 ± 0.01 | 0.19 ± 0.02** | 0.22 ± 0.02 | 0.25 ± 0.02 |
| HVA/DA | 0.82 ± 0.09 | 1.46 ± 0.23* | 0.99 ± 0.15 | 1.54 ± 0.17* | 1.44 ± 0.17 | 2.29 ± 0.34** | 1.56 ± 0.18 | 1.83 ± 0.24 |
| DA | 87.69 ± 20.98 | 41.66 ± 8.86 | 110.15 ± 18.09 | 59.34 ± 4.30** | 77.02 ± 10 | 42.37 ± 2.87*** | 64.79 ± 13.1 | 46.65 ± 6.35 |
| DOPAC | 6.77 ± 4.11 | 4.85 ± 3.15 | 7.22 ± 1.80 | 7.21 ± 1.22 | 11.41 ± 2.11 | 8.29 ± 0.76 | 14.24 ± 5.42 | 11.92 ± 3.94 |
| HVA | 72.09 ± 16.28 | 60.71 ± 16.04*** | 103.20 ± 16.25 | 91.34 ± 11.86 | 110.39 ± 18.08 | 97.28 ± 14.63 | 99.88 ± 30.2 | 85.56 ± 33.1 |
Mean levels are ng/mg tissue ± SEM. Dopamine utilization, the ratio of levels of the metabolite to levels of dopamine; DA, dopamine; SAL, saline; MA, methamphetamine. Significant group differences are highlighted in bold.
*p < 0.05;
**p < 0.01;
***p < 0.001.
Discussion
The findings reported here provide direct evidence that exposure to an escalating dose regimen of methamphetamine administration produces changes to the dopamine system that mirror those that have been previously observed in human methamphetamine abusers (Volkow et al., 2001b; Lee et al., 2009). By combining a chronic, escalating dose regimen that models human methamphetamine abuse more closely than the acute, large-dose regimens previously used, with a longitudinal study design, we provide evidence that a 31 d escalation of methamphetamine administration significantly reduces striatal D2-like receptor, DAT availability, and dopamine tone. The persistence of these effects following 7 weeks of abstinence indicates that though some recovery occurs, as in humans (Volkow et al., 2001a), it is limited and proceeds slowly. Furthermore, methamphetamine administration produced transient behavioral deficits that were linked to the observed changes in the dopaminergic system.
Escalation of methamphetamine administration results in diminished striatal D2-like receptor availability, DAT availability, and dopamine levels
In line with the current results, previous evidence indicates dysfunction of the dopamine system is a neurochemical consequence of methamphetamine exposure. High doses of methamphetamine rapidly reduce striatal DAT density/availability and dopamine tone in rodents and primates (Fleckenstein et al., 1997; Melega et al., 1998), paralleling the abnormalities found in humans who abuse methamphetamine. However, inconsistent results have been found with respect to the D2-like receptor system. Although research participants who abuse methamphetamine exhibit abnormally low D2-like receptor availability, when compared with controls (Volkow et al., 2001a,b; Lee et al., 2009), there are discrepancies in the literature as to whether this effect is a consequence of methamphetamine (Schmidt et al., 1985; Melega et al., 2008). The data presented here suggest that methamphetamine-induced reductions in D2-like receptors emerge during the course of extended exposure, and that these reductions persist beyond the duration of acute, high-dose administration of the drug (McCabe et al., 1987).
The mechanism by which methamphetamine alters the dopamine system is not fully understood; however, there is evidence that the effects depend on basal dopamine tone (Thomas et al., 2008). Auto-oxidation of dopamine results in the production of reactive oxygen and nitrogen species, and free-radical formation is elevated following methamphetamine administration (Riddle et al., 2006); preventing this oxidation blocks methamphetamine-induced reductions in DAT and tissue-dopamine content (Imam et al., 1999; Fukami et al., 2004; Hashimoto et al., 2004). It is not known, however, whether free-radical formation contributes to the molecular changes elicited by slow, escalating dose administration reported here.
Striatal D2-like receptor availability increased in several of the saline-exposed monkeys. Previous studies have reported greater D2-like receptor levels in rodents exposed to chronic mild stress (Lucas et al., 2007; Yaroslavsky and Tejani-Butt, 2010), but this effect may be a consequence of multiple, daily control injections, or it could be due to the simple passage of time in the study. In either case, these results emphasize the importance of a true control group.
Effects on inhibitory control
Methamphetamine exposure resulted in behavioral deficits that were restricted to the reversal phase of the task: when the contingencies of the task reversed, methamphetamine-treated monkeys required significantly more trials to reach the performance criterion. Similar deficits have been found in human methamphetamine users (Ghahremani et al., 2011) and in rats exposed to multiple, high doses of methamphetamine (Izquierdo et al., 2010). This impairment was most robust during the assessment conducted 3 weeks into the dosing regimen because methamphetamine impaired inhibitory control processes in all monkeys. The lack of group differences in the ability of monkeys to acquire a novel stimulus–reward association suggests that methamphetamine exposure impairs the ability to recruit the additional cognitive resources that are required when contingencies change. Unlike previously reported long-lasting effects of cocaine (Jentsch et al., 2002), the methamphetamine-induced reversal impairment was transient, present only during early withdrawal from methamphetamine. This discrepancy may be due to the extensive pretraining the current subjects received on the task, as the circuitry underlying reversal learning is known to change as a function of experience (Boulougouris and Robbins, 2009; Rygula et al., 2010). Thus, the relatively higher degree of training conducted in this study may have resulted in task performance being governed by an increasingly distributed and/or robust array of neural systems, which in turn may have obscured our ability to detect enduring changes in inhibitory control processes that have been found in cocaine-exposed animals with limited reversal-learning training (Jentsch et al., 2002; Schoenbaum et al., 2004).
Two additional weeks of methamphetamine exposure did not alter reversal-learning performance consistently in all subjects. The reason for the differences in behavioral response to methamphetamine is unknown. However, within methamphetamine-dependent populations there is a high degree of variability in cognitive control capabilities, even when accounting for lifetime methamphetamine use (Cherner et al., 2010). The heterogeneity of the behavioral impact of methamphetamine in the current set of data, in combination with results from studies of methamphetamine-dependent individuals, suggests that pre-existing factors mediate the biobehavioral effects of methamphetamine. Although these factors have yet to be identified, variation in striatal dopamine tone, before drug use, represents one potential candidate. Greater amphetamine-induced dopamine release is associated with the degree of self-reported impulsiveness (Buckholtz et al., 2010), which itself is a risk factor for the development of addictions (Auger et al., 2010; Ersche et al., 2010). Because the effects of methamphetamine on the dopamine system depend on basal dopaminergic tone (Thomas et al., 2008), individual variability in levels of dopamine may mediate the cognitive response to methamphetamine. Further evidence is needed to determine the validity of this hypothesis and to identify other potential factors that can account for the individual variability in long-term behavioral response to methamphetamine.
Deviations in D2-like receptor availability correlate with changes in positive-feedback sensitivity
The change in D2-like receptor availability in the dorsal striatum, but not DAT availability, correlated with the change in positive-feedback sensitivity. These data, taken with previous evidence (Groman et al., 2011), indicate that the D2-like receptor system plays a specific role in modulating sensitivity to positive feedback. The correlations were detected only in the reversal phase, most likely due to the heightened demands this phase of the task places on subjects to rapidly incorporate feedback into subsequent responses. The relationship between D2-like receptor availability and positive-feedback sensitivity was exhibited by both methamphetamine- and saline-exposed groups, indicating that regardless of the influences acting on D2-like receptor function, regulation of D2-like receptor availability is associated with changes in positive-feedback sensitivity.
Individual differences in the biobehavioral response to methamphetamine and implications for addictions
Methamphetamine treatment reduced DAT availability in all monkeys; however, the same was not true regarding the D2-like receptor system. Methamphetamine treatment caused large reductions in D2-like receptor availability for some monkeys (∼30–40%), but had little or no effect in others. In the current study, methamphetamine was administered via an experimenter, rather than by the subject. Although the administration model used in the current study does not capture individual choices in drug use, it does allow for precise control over the dose and timing of drug administration. Even with these controls in place, the results of the current study indicate that there is a substantial degree of variability in the biobehavioral response to methamphetamine. Therefore, biological or environmental factors, outside the patterns or amount of drug intake, affect the biobehavioral effects of drug experience.
Dysfunction of the D2-like receptor has been proposed to be a common biochemical correlate of addiction (Volkow et al., 1993, 2001b; Lee et al., 2009; Johnson and Kenny, 2010), and improving function at these receptors is a treatment strategy of interest (Kosten et al., 2002; Thanos et al., 2004, 2008). Further, deficits in behavioral flexibility are believed to be a core phenotype of addiction (Jentsch and Taylor, 1999) and to be correlated with the retention of cocaine-dependent individuals in a treatment program (Aharonovich et al., 2006), which itself is one of the best predictors of sobriety (Zhang et al., 2003). Indeed, administration of the D2-like receptor agonist pramipexole alleviates impairments of reversal learning in stimulant-dependent individuals (Ersche et al., 2011), indicating that enhancing D2-like receptor function can improve inhibitory control and may, by proxy, improve abstinence rates.
However, D2-agonist-based treatments have been unsuccessful in improving abstinence rates among stimulant-dependent individuals (Handelsman et al., 1997). This failure may be due to the high degree of symptom heterogeneity that exists within addictions, particularly related to inhibitory control. Although some drug-dependent individuals have profound cognitive-control impairments, others perform at levels comparable to that of healthy control subjects (Ersche et al., 2008). Here, we also found heterogeneity in the behavioral impact of methamphetamine. Therefore, D2-like receptor agonists may have the greatest therapeutic benefit in individuals whose primary behavioral deficit is inhibitory control.
Conclusions
By combining neuroimaging and behavioral techniques, the current study provides a mechanistic understanding of the emergence of inhibitory-control deficits in drug-dependent populations. Though recent reviews of the literature have called into question the behavioral and neural toxicity associated with methamphetamine (Hart et al., 2012), the current results provide unambiguous data indicating that a realistic model of methamphetamine experience triggers alterations in dopaminergic transmission that likely culminate in the inhibitory-control deficits associated with the persistent use of stimulants (Ersche et al., 2008; Ghahremani et al., 2011). Specifically, we propose that dysfunction of the D2-like receptor results in reductions in sensitivity to positive feedback, which behaviorally manifests as the habitual and compulsive phenotype exhibited by drug-dependent individuals. These data provide insight into the neural and behavioral consequences of methamphetamine abuse and have broad implications for understanding the biobehavioral interactions that underlie addictions.
Footnotes
This study was supported primarily by Public Health Service (PHS) Grants P20-DA022539, R03-DA020598, T32-DA024635, and F31-DA028812, with additional support from the Consortium for Neuropsychiatric Phenomics at the University of California, Los Angeles. The Consortium is funded by PHS Grants UL1-DE019580 and RL1-MH083270.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Auger N, Lo E, Cantinotti M, O'Loughlin J. Impulsivity and socio-economic status interact to increase the risk of gambling onset among youth. Addiction. 2010;105:2176–2183. doi: 10.1111/j.1360-0443.2010.03100.x. [DOI] [PubMed] [Google Scholar]
- Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organizational Res Methods. 2004;7:127–150. [Google Scholar]
- Boulougouris V, Robbins TW. Pre-surgical training ameliorates orbitofrontal-mediated impairments in spatial reversal learning. Behav Brain Res. 2009;197:469–475. doi: 10.1016/j.bbr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HNRC Group. Cherner M, Suarez P, Casey C, Deiss R, Letendre S, Marcotte T, Vaida F, Atkinson JH, Grant I, Heaton RK. Methamphetamine use parameters do not predict neuropsychological impairment in currently abstinent dependent adults. Drug Alcohol Depend. 2010;106:154–163. doi: 10.1016/j.drugalcdep.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Müller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- Fortier CB, Steffen EM, Lafleche G, Venne JR, Disterhoft JF, McGlinchey RE. Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology. 2008;22:196–208. doi: 10.1037/0894-4105.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-l-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D2-like receptor: A dimensional understanding of addiction. Depression and Anxiety. 2011 doi: 10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E, Paulus MP, Wittmann M, Chung H, Song JM. Hair analysis and self-report of methamphetamine use by methamphetamine dependent individuals. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:541–547. doi: 10.1016/j.jchromb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Rosenblum A, Palij M, Magura S, Foote J, Lovejoy M, Stimmel B. Bromocriptine for cocaine dependence. A controlled clinical trial. Am J Addict. 1997;6:54–64. [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M. Protective effects of N-acetyl-L-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacology. 2004;29:2018–2023. doi: 10.1038/sj.npp.1300512. [DOI] [PubMed] [Google Scholar]
- Herold C. NMDA and D2-like receptors modulate cognitive flexibility in a color discrimination reversal task in pigeons. Behav Neurosci. 2010;124:381–390. doi: 10.1037/a0019504. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Islam F, Slikker W, Jr, Ali SF. Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity. Brain Res. 1999;818:575–578. doi: 10.1016/s0006-8993(98)01311-0. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O'Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, George TP, Kosten TA. The potential of dopamine agonists in drug addiction. Expert Opin Investig Drugs. 2002;11:491–499. doi: 10.1517/13543784.11.4.491. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108–115. doi: 10.1016/j.brainres.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe RT, Hanson GR, Dawson TM, Wamsley JK, Gibb JW. Methamphetamine-induced reduction in D1 and D2 dopamine receptors as evidenced by autoradiography: comparison with tyrosine hydroxylase activity. Neuroscience. 1987;23:253–261. doi: 10.1016/0306-4522(87)90287-9. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Lacan G, Harvey DC, Huang SC, Phelps ME. Dizocilpine and reduced body temperature do not prevent methamphetamine-induced neurotoxicity in the vervet monkey: [11C]WIN 35,428—positron emission tomography studies. Neurosci Lett. 1998;258:17–20. doi: 10.1016/s0304-3940(98)00845-3. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Laæan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33:1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KL, DeSantis SM, Simpson AN, Tolliver BK, McRae-Clark AL, Saladin ME, Baker NL, Wagner MT, Brady KT. The impact of clinical and demographic variables on cognitive performance in methamphetamine-dependent individuals in rural South Carolina. Am J Addict. 2011;20:447–455. doi: 10.1111/j.1521-0391.2011.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Betancourt L, Giannetta JM, Brodsky NL, Farah M, Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Gehlert DR, Peat MA, Sonsalla PK, Hanson GR, Wamsley JK, Gibb JW. Studies on the mechanism of tolerance to methamphetamine. Brain Res. 1985;343:305–313. doi: 10.1016/0006-8993(85)90748-6. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O'Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28:1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–486. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort E, Sossi V. An analytical scatter correction for singles-mode transmission data in PET. IEEE Trans Med Imaging. 2008;27:402–412. doi: 10.1109/TMI.2007.909851. [DOI] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA. Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C]WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci. 1998;18:419–427. doi: 10.1523/JNEUROSCI.18-01-00419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001b;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol Biochem Behav. 2010;94:471–476. doi: 10.1016/j.pbb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Friedmann PD, Gerstein DR. Does retention matter? Treatment duration and improvement in drug use. Addiction. 2003;98:673–684. doi: 10.1046/j.1360-0443.2003.00354.x. [DOI] [PubMed] [Google Scholar]