Abstract
In the United States, serovar Kentucky has become one of the most frequently isolated Salmonella enterica serovars from chickens. The reasons for this prevalence are not well understood. Phenotypic comparisons of poultry Salmonella isolates belonging to various serovars demonstrated that serovar Kentucky isolates differed from those of most other serovars in their response to acid. Microarray and qPCR analyses were performed with aerated exponentially growing poultry isolates, Salmonella enterica serovar Kentucky 3795 and Enteritidis NalR, exposed for 10 min to tryptic soy broth (TSB) adjusted to pH 4.5 with HCl and to pH 5.5 with HCl or acetic acid. Data obtained by microarray analysis indicated that more genes were up- or down-regulated in strain Kentucky 3795 than in Enteritidis NalR under acidic conditions. Acid exposure in general caused up-regulation of energy metabolism genes and down-regulation of protein synthesis genes, particularly of ribosomal protein genes. Both strains appear to similarly utilize the lysine-based pH homeostasis system, as up-regulation of cadB was observed under the acidic conditions. Expression of regulatory genes (rpoS, fur, phoPQ) known to be involved in the acid response showed similar trends in both isolates. Differences between Kentucky 3795 and Enteritidis NalR were observed with respect to the expression of the hdeB-like locus SEN1493 (potentially encoding a chaperone important to acid response), and some differences in the expression of other genes such as those involved in citrate utilization and motility were noted. It appears that the early stages of the transcriptional response to acid by isolates Kentucky 3795 and Enteritidis NalR are similar, but differences exist in the scope and in some facets of the response. Possibly, the quantitative differences observed might lead to differences in protein levels that could explain the observed differences in the acid phenotype of serovar Kentucky and other Salmonella serovars.
Introduction
Salmonella enterica serovar Kentucky is the serovar most frequently isolated from chickens and chicken products in the United States (USDA, 2008; Berrang et al., 2009; Lestari et al., 2009; Melendez et al., 2010). A combination of factors may contribute to the prevalence, including resistance to antibiotics and arsenic encountered in poultry environments (Fricke et al., 2009; Joerger et al. 2009, 2010; Melendez et al., 2010), and plasmids that share virulence factors with avian pathogenic Escherichia coli strains (Fricke et al., 2009; Johnson et al., 2010). A study comparing physiological and genetic traits of different serovars isolated from poultry demonstrated that serovar Kentucky differed from most others in its response to acid, in particular, acetic acid, and in the absence of the cdtB, spvB, spvC, and pefA virulence genes (Joerger et al., 2009). The differences in response to acid is noteworthy since serovar Kentucky, like all other chicken-colonizing isolates, has to confront acidic gastrointestinal environments. A side-by-side comparison of exponentially growing cells of the poultry isolates S. enterica serovar Enteritidis NalR and serovar Kentucky 3795, which are the subject of the present study, demonstrated that both isolates were killed within 40 min when exposed to HCl-induced pH 2.5; however, when first exposed to acetic acid-containing medium, pH 5.5, Enteritidis NalR was up to 1000-times less sensitive to HCl-induced pH 2.5 than Kentucky 3795 (Joerger et al., 2009). This observation suggested differences in the acetic acid-induced acid tolerance response (ATR) of the two isolates.
Growth of Kentucky 3795 and Enteritidis NalR in tryptic soy broth (TSB) adjusted to pH 5.5 with acetic acid was strongly inhibited compared to growth in the same medium adjusted to pH 5.5 with HCl. However, this inhibition was less pronounced for the Kentucky strain (Joerger et al., 2009). The current study was undertaken to examine differences in the transcriptional response of Enteritidis NalR and Kentucky 3795 to exposure to TSB adjusted to pH 4.5 and 5.5 with HCl and to pH 5.5 with acetic acid.
Methods
Culture and acid exposure of Salmonella isolates
Salmonella enterica isolates Enteritidis NalR and Kentucky 3795 (Joerger et al., 2009) were grown in 15-mL tubes containing 4 mL of TSB overnight at 37°C with shaking at 200 rpm. The cultures were diluted 100-fold into pre-warmed (37°C) TSB, and four 40-mL cultures were established in 250-mL flasks for each serovar and shaken at 200 rpm for 2 h at 37°C.
For acid exposure, one culture of each isolate was removed from the shaker, the pH, which was 6.8 at this time, was quickly lowered to 4.5 with 6 N HCl while shaking, flasks were returned to the shaker, and incubation was resumed. The same process was carried out to obtain cultures adjusted to pH 5.5 with HCl and glacial acetic acid. The two cultures that did not receive any acid (controls) were similarly removed from and returned to the shaker. After 10 min, aliquots were quickly removed from the flasks for RNA isolation.
RNA isolation
Treatment of cells with RNA protect (Qiagen, Valencia, CA), RNA isolation using the RNAeasy kit (Qiagen), and on-column DNase treatment were done according to protocols available from Qiagen. Purity and concentration of the RNA was determined on a NanoDrop™ 2000 spectrophotometer (NanoDrop Products, Wilmington, DE).
Microarray analysis
The non-redundant multi-serotype microarray contained 5,660 polymerase chain reaction (PCR) products that cover at least 95% of all genes in the genomes of the Salmonella serovars Typhimurium LT2 and SL1344, Typhi CT18 and Ty2, Paratyphi A SARB42, and Enteritidis PT4 (Porwollik et al., 2001, 2003, 2005).
The cDNA probes were labeled with Cy3- and Cy5- dye-linked dUTP by direct incorporation during reverse transcription from total RNA to cDNA. Probes were purified using the QIAquick PCR purification kit (Qiagen). The cDNA probes from acid-exposed cells (experiment) were compared to cDNA probes from untreated cells (control) for each serotype. Each of these experiments was repeated at least three times (biological replicates) with dye switching. Pre-hybridization, hybridization, and post-hybridization processing was done as described elsewhere (http://www.corning.com/Lifesciences/technical_information/techDocs/gaps_ii_manual_protocol_5_02_cls_gaps_005.pdf). Microarrays were scanned with a ScanArray Lite Laser scanner (PerkinElmer Life and Analytical Sciences, Waltham, MA) using ScanArray Express 3.0 software or with a GenePix 4100A (Molecular Devices, Sunnyvale, CA) and GenePix Pro software as previously described (Porwollik et al., 2001, 2003).
Signal intensities were quantified using QuantArray 3.0 software (Packard BioChip Technologies, Billerica, MA). Statistically relevant differential gene expression was calculated using an eBayes moderated t-test (LIMMA) within the WebArrayDB online microarray data analysis platform (Xia et al., 2009). Array data have been submitted to NCBI GEO (under accession number GSE26597).
qPCR (quantitative polymerase chain reaction) analysis
RNA extraction was performed on three independent cultures for each condition. Reverse transcription was carried out using the SuperScript III First-Strand Synthesis Supermix (Invitrogen, Carlsbad, CA). The primers for qPCR were determined using PrimerPlus software (Untergasser et al., 2007) (see Suppl. Table S1; Supplementary Material is available online at www.liebertonline.com/fpd).
qPCR was performed on triplicate samples of each cDNA. The cDNA was diluted to 20 μg/μL, and 1 μL was used per PCR reaction. Five microliters of 2× SYBR® Green Master Mix (Applied Biosystems, Foster City, CA), 0.1 μL each of 100 μM of forward and reverse primer, and 3.8 μL of water were added to the cDNA. Thermocycling was carried out with one cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The dissociation curve of the final products was checked to ascertain the presence of a single peak.
Selection of a gene for the normalization of qPCR data
Among four candidate reference genes that showed no changes in expression under acid conditions in the microarray experiments (thrA, pepP, aceB, and dnaQ), thrA showed the smallest standard deviation (data not shown) and was selected as reference gene. The thrA qPCR results were assigned an arbitrary value of 100. For normalization, the threshold values for the thrA amplifications (CTthrA) were subtracted from the corresponding threshold values of the samples amplified with other primer pairs (CTexp). mRNA levels in arbitrary units (AU) were calculated with the formula: AU=100 * 2 (CTexp – CTthrA).
Statistical analysis
The resulting values for the three biological replicas were averaged, and the standard error was calculated. Differences between treatments were established using JMP®, Version 7 (SAS Institute Inc., Cary, NC).
Results and Discussion
Overview
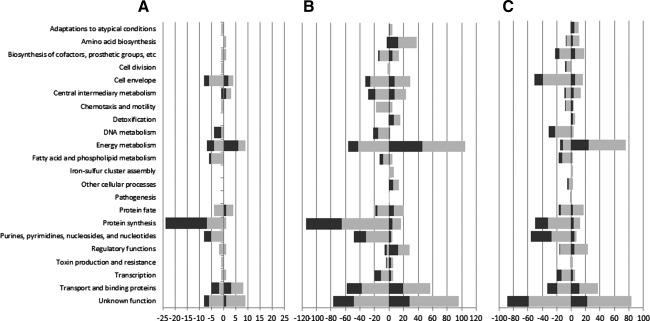
The graphic comparison of log2-fold changes in gene expression of Kentucky 3795 and Enteritidis NalR is shown in Suppl. Fig. S1, and Venn diagrams illustrating differentially expressed loci are presented as Suppl. Fig. S2. Processes affected by acid exposure are outlined in Figure 1. The numbers of genes/loci on the microarrays with signals that significantly (p<0.001) differed for RNA samples obtained from cells exposed to neutral and those obtained from cells exposed to acid conditions are listed in Table 1. The numbers of genes/loci that showed more than twofold changes in expression are also tabulated. For all conditions, the number of sequences for which changes occurred was higher for Kentucky 3795 than for Enteritidis NalR. Supplementary Table S2 displays genes that cleared the arbitrary threshold of highly significant (p<0.001) twofold or higher up- or down-regulation under any of these conditions, and some additional genes of interest; all genes discussed below are illustrated in this table. Results of qPCR analyses performed to corroborate some of the array results are listed in Suppl. Table S3, and a comparative plot is presented as Suppl. Fig. S3.
FIG. 1.
Number of genes/loci belonging to different functional categories (y-axis) up-regulated (positive numbers on x-axis) and down-regulated (negative numbers on x-axis) in Salmonella enterica Enteritidis NalR (black bars) and Salmonella enterica Kentucky 3795 (gray bars) in response to a 10-min exposure to tryptic soy broth (TSB) adjusted to pH 4.5 with HCl (A); pH 5.5 with HCl (B); and pH 5.5 with acetic acid (C).
Table 1.
Number of Genes/Loci Up- or Down-Regulated (p-Value 0.001) in Salmonella enterica Enteritidis NalR and Kentucky 3795 in Response to a 10-min Exposure to Acid
| |
|
Number of sequences with signal ratios |
|||
|---|---|---|---|---|---|
| Strain | pH/acid | >0 | >2 | <0 | <0.5 |
| Salmonella enterica Enteritidis NalR | 4.5/HCl | 59 | 14 | 186 | 35 |
| Salmonella enterica Kentucky 3795 | 4.5/HCl | 107 | 28 | 247 | 49 |
| Salmonella enterica Enteritidis NalR | 5.5/HCl | 302 | 179 | 304 | 179 |
| Salmonella enterica Kentucky 3795 | 5.5/HCl | 495 | 323 | 517 | 367 |
| Salmonella enterica Enteritidis NalR | 5.5/Ac | 218 | 97 | 385 | 148 |
| Salmonella enterica Kentucky 3795 | 5.5/Ac | 617 | 254 | 696 | 314 |
The microarray data have the following limitations: (i) The array was composed of sequences from the Salmonella serovars listed in Methods. Transcripts of genes present in S. enterica Kentucky, but not in these serovars, are not detected by this array. The currently available serovar Kentucky genome sequences indicate that Kentucky has 162 unique genes not present on the microarray (data not shown). (ii) Total RNA was isolated from exponentially growing cells. Stationary cells would likely not be transcriptionally very active, and may already have modified the expression of acid-responsive genes. (iii) Duration of acid exposure was 10 min, and therefore transcriptional changes occurring after longer-term exposure to acid remain unknown. (iv) Cultures were shaken; thus, changes in gene expression specific to anaerobic conditions are not detected. (v) Data on exposure to acetic acid were only collected at pH 5.5 since a 10-min exposure to medium adjusted to pH 4.5 with acetic acid proved lethal to both isolates.
Acid response genes
The microarray study revealed only three genes (cadB, clpA, fpr) that were up- and three genes (dinR, suhB, dacA) that were down-regulated under all conditions in both strains. While changes in the expression of cplA, fpr, dinR, and dacA are likely a sign of general stress conditions, up-regulation of cadB is an acid-specific stress response and underscores the importance of the lysine decarboxylase/lysine-cadaverine antiporter system for pH homeostasis (Park et al., 1996). Expression changes of cadA, the gene located downstream of cadB, paralleled those of cadB; the differences in scope could be the result of mRNA degradation at the 3’ end and/or of transcription termination within the cadBA operon. Putative early termination may serve to modulate CadA levels which negatively influence virulence and adhesion to host cells in Shigella (Maurelli et al., 1998) and E. coli O157:H7 (Vazquez-Juarez et al., 2008). CadBA expression is controlled by cadC, whose transcription has been reported to be acid-inducible in Salmonella (Lee et al., 2007); however, under the conditions of this study, cadC expression did not change.
Salmonella also harbors a system for pH homeostasis requiring the presence of arginine. The genes adi and yjdE did not show the same level of acid-induced expression as the lysine-utilizing counterparts, likely because this pH homeostasis system operates preferentially under anoxic conditions (Kieboom and Abee, 2006).
In exponentially growing E. coli, asr is the most up-regulated gene upon a shift to low pH (Suziedeliene et al., 1999) and is required for growth at pH 4.5 and induction of the acid tolerance response (Šeputiene et al., 2003). Inducibility of STM1485, the asr homologue in S. enterica Typhimurium, by pH 4–4.5 has been demonstrated (Šeputiene et al., 2004). The sequenced S. enterica Enteritidis and Kentucky strains harbor STM1485 homologues (SEN1564A and SeKA_0924, respectively), and qPCR data indicated that both Enteritidis NalR and Kentucky 3795 contained higher levels of STM1485-like mRNA when exposed to pH 4.5 than cells growing at neutral pH. Note that the asr sequence in the Kentucky CVM 29188 genome (SeKA_0924) differs from the Typhimurium LT2 orthologue (STM1485) by six amino acids, perhaps causing some functional differences of the gene product.
The sequenced isolates Enteritidis P125109, and Kentucky CDC 191 and CVM29188 harbor an hdeB-like gene (SEN1493, SeKB_A1706, and SeKA_A1012, respectively). In E. coli, hdeB is part of the hdeAB operon encoding chaperonins important to survival at low pH (Kern et al., 2007). The microarray and qPCR analyses showed up-regulation of SEN1493 in Enteritidis under HCl- but not acetic acid–induced acidic conditions; however, in Kentucky 3795, the hdeB-like gene remained transcriptionally inactive under acidic conditions.
Regulatory genes
Microarray and qPCR data suggest that rpoS is up-regulated similarly in both strains. Expression of rpoN, which has been shown to negatively regulate acid resistance in E. coli O157:H7 (Riordan et al., 2010), was down-regulated in the presence of acetic acid. The qPCR data demonstrated that mRNA levels of this gene were down-regulated in the presence of acid in general. The microarray analysis showed acid-induced down-regulation of fis, a regulatory gene whose product enhances transcription of SPI1 and SPI2 virulence genes and of motility genes in Typhimurium (Kelly et al., 2004). The marAR genes encode proteins involved in regulation of genes responding to chemical stress, and in E. coli, marA is also involved in repression of the acid-resistance genes hdeAB (Ruiz et al., 2008). The marA gene was up-regulated in both strains in response to pH 5.5 generated by HCl, but, curiously, not acetic acid.
Energy metabolism
With some exceptions, energy metabolism gene expression in response to acid followed similar trends in both strains. Most TCA cycle genes were up-regulated, perhaps to satisfy a need for ATP in times of stress, and/or a need for certain intermediates such as citric acid, a compound proposed to be involved in acid resistance (Foster and Hall, 1991; Foster, 1995). Perhaps in response to increased amounts of NADPH generated in a more active tricarboxylic acid cycle and the need to provide NADP+ to maintain the cycle (Sauer et al., 2004), udhA encoding an energy-independent transhydrogenase was up-regulated.
The electron transport chain-encoding genes nuoEFGIJKLMN and cyoACDE were only up-regulated in Kentucky 3795, and the same pattern was found during qPCR analysis of cyoA. Expression of ubiAC, encoding proteins involved in the synthesis of quinones which function as mediators of electron transport between proteins were also up-regulated in the presence of acetate in the Kentucky strain. A number of energy metabolism, electron transport, proton-motive ATPase, and hydrogen metabolism genes (hybABCD and hypDEFO) were down-regulated in both isolates at pH 5.5 induced by HCl, but not by acetic acid. One operon, citCDEFXGT, was down-regulated in the Kentucky strain and essentially unaffected in the Enteritidis strain at pH 5.5. The qPCR analysis showed lower levels of citD and citF mRNA under acidic conditions in both strains, but significant down-regulation only for Kentucky 3795. It is possible that down-regulation of citrate utilization genes is meant to conserve citrate in the cells and/or not to divert citrate from the citric acid cycle.
Iron and copper transport and metabolism
In general, a trend towards increased expression of genes involved in iron transport and metabolisms was noticeable under acidic conditions. FepA, fes, ftnB, and feoB, but not feoA, were up-regulated in both strains under all acid conditions. Possibly the up-regulation of such genes is necessary to supply iron-sulfur cofactors to the respiratory enzymes synthesized perhaps in increased amounts under acidic conditions. Up-regulation of suf genes, particularly sufB encoding part of a complex for assembly and transfer of FeS clusters to other proteins (Outten et al., 2003), also indicates an increased need for such clusters or their maintenance under acidic conditions. Up-regulation of moa and mob genes required for synthesis of molybdenum cofactors that are part of some respiratory enzymes suggests an increased need for this type of cofactor.
Among the most highly up-regulated genes under acidic conditions for both strains was ybaR (copA) encoding a putative copper-transporting ATPase that transports Cu(I) from the cytoplasm to the periplasm (Espariz et al., 2007). Since iron-sulfur clusters are targets of copper toxicity (Macomber and Imlay, 2009) up-regulation of ybaR might support efforts to increase or maintain iron-sulfur clusters. The copper oxidase gene, cueO (cuiD, yacK, SEN0173) required for systemic virulence (Achard et al., 2010) was up-regulated more than twofold only at pH 5.5 induced by HCl.
Cell envelope/motility
Most of the genes involved in envelope synthesis and function showed a decrease in expression in both isolates. The exceptions were lpxO, encoding a dioxygenase involved in lipid synthesis, some osm genes, and tolC. Microarray analysis indicated a general increase in fim gene expression in the presence of acetic acid in Enteritidis NalR, but down-regulation in Kentucky 3795; however, the qPCR analysis showed lower levels of fimC and fimD mRNA in both strains. Paralleling results obtained with Typhimurium (Adams et al., 2001), motility genes were mostly down-regulated in both strains after acid exposure. The qPCR data confirmed significant down-regulation of flgD and fliG in Kentucky 3795 but not Enteritidis NalR.
Stress-related genes
Up-regulation of clpA encoding the protein translocase component of the ClpAP complex that functions in the degradation of denatured proteins (Hoskins et al., 1998) under all conditions suggests that both strains deal with misfolded and aggregated proteins upon exposure to acid. It is not clear why expression of clpP, encoding the proteolytic component of the complex, was not similarly increased. Genes encoding proteins assisting in protein folding generally responded to the acid treatments similarly; the exception was locus SEN1800, corresponding to STM1251 from Typhimurium, encoding a putative chaperone. This gene was up-regulated more than fourfold in Kentucky 3795, but unchanged in Enteritidis NalR in response to acetic acid.
DNA and protein synthesis
Under HCl-induced acid conditions, both strains down-regulated rps and rpl genes. In contrast, yfiA encoding a ribosomal stabilization factor (Ueta et al., 2005) was significantly up-regulated. This gene was also up-regulated upon exposure of Typhimurium and Enteritidis to chlorine (Wang et al., 2010). Acid conditions caused widespread down-regulation of DNA metabolic genes and of genes required for the synthesis of DNA and RNA building blocks (purBEGHKM and pyrCDFGH).
Amino acid/peptide metabolism and transport
Both strains reacted similarly to acid with respect to amino acid metabolism. Most noteworthy is the down-regulation of nine cys genes at HCl-induced pH 5.5. The l-serine dehydratase (l-serine deaminase 2) and the putative serine transporter genes, sdaBC, were also down-regulated. The polyamine synthesis genes, speDE, were down-regulated in both isolates as was the spermidine-preferential uptake system encoded by potABCD. Di- and oligopeptide transporter genes (dppA, oppAD, yjdL) were generally up-regulated by both strains at HCl-induced pH 5.5; at ph 5.5, induced by acetic acid, the opp genes were down-regulated.
Pathogenicity
Transcription of only a few genes in this category changed more than twofold upon a 10-min exposure to acid. The sitABCD operon of SPI-1 encodes a manganese uptake system also capable of iron transport (Kehres et al., 2002). Microarray data showed general up-regulation of this operon in response to acid. Microarray data indicated up-regulation of sip genes in Enteritidis NalR in the presence of acetic acid and no change in expression in Kentucky 3795; however, the qPCR analysis did not show the same trend. The expression of avrA, needed to modulate the effect of other SPI-1-encoded effector proteins on signaling pathways of host cells (Du and Galan, 2009), was increased about fourfold in Enteritidis NalR after exposure to HCl-induced pH 5.5. In addition, in Pathogenicity Island 3, the membrane protein-encoding gene, cigR, was up-regulated under all acid conditions, while slsA increased more than twofold only for the Kentucky strain. Expression of pipA located on SPI-5 was increased twofold in Kentucky 3795, and expression of the pipB homolog, pipB2 (SEN2624) which encodes a protein required for recruitment of kinesin-1 to the membrane of the Salmonella-containing vacuole (Henry et al., 2006), was up-regulated in both isolates.
Summary
Overall, short-term exposure to acid elicited similar global transcriptional responses in Salmonella Enteritidis NalR and Kentucky 3795. The observed differences in growth and survival in acidic media of the two isolates (Joerger et al., 2009) are therefore based on post-translational differences and/or the cumulative effects of perhaps small transcriptional differences that may be difficult to verify by microarray and qPCR analysis.
It has been suggested that the presence of the E. coli-derived ColV plasmid in Kentucky could impact the serovar's acid response phenotype (Johnson et al., 2010); however, like more than a quarter of Kentucky isolates, strain 3795 appears not to harbor this plasmid (data not shown). It is also possible that more obvious transcriptional differences only appear after exposures longer than 10 min. Time-course and proteomic studies would likely help elucidate the molecular basis of the differences in the acid response of serovar Kentucky isolates.
Supplementary Material
Acknowledgments
Funding for the study was provided by the Avian Biotechnology Center at the University of Delaware. C.S. was supported by the USDA (National Needs Graduate Fellowship Competitive grant 2008-38420-18732 from the National Institute of Food and Agriculture). M.M. and S.P. were supported, in part, by the USDA (grant 2009-03579) and the NIH (grants R01AI083646 and R01AI075093).
Disclosure Statement
No competing financial interests exist.
References
- Achard MES. Tree JJ. Holden JA, et al. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun. 2010;78:2312–2319. doi: 10.1128/IAI.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. Fowler R. Kinsella N, et al. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics. 2001;1:597–607. doi: 10.1002/1615-9861(200104)1:4<597::AID-PROT597>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Altschul SF. Madden TL. Schäffer AA, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrang ME. Bailey JS. Altekruse SF, et al. Prevalence, serotype, and antimicrobial resistance of Salmonella on broiler carcasses postpick and postchill in 20 US processing plants. J Food Prot. 2009;72:1610–1615. doi: 10.4315/0362-028x-72.8.1610. [DOI] [PubMed] [Google Scholar]
- Du FY. Galan JE. Selective inhibition of Type III secretion activated signaling by the Salmonella effector AvrA. PLOS Pathogens. 2009;9:e1000595. doi: 10.1371/journal.ppat.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espariz M. Checa SK. Perez Audero ME, et al. 2007. Dissecting the Salmonella response to copper. Microbiology. 2007;153:2989–2997. doi: 10.1099/mic.0.2007/006536-0. [DOI] [PubMed] [Google Scholar]
- Foster JW. Low pH adaptation and the acid tolerance response of Salmonella Typhimurium. Crit Rev Microbiol. 1995;21:215–237. doi: 10.3109/10408419509113541. [DOI] [PubMed] [Google Scholar]
- Foster JW. Hall HK. Adaptice acidification tolerance response of Salmonella Typhimurium. J Bacteriol. 1990;172:771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW. Hall HK. Inducible pH homeostasis and the acid tolerance response of Salmonella Typhimurium. J Bacteriol. 1991;173:5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke WF. McDermott PF. Mammel MK, et al. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar Kentucky isolates from poultry. Appl Environ Microbiol. 2009;75:5963–5971. doi: 10.1128/AEM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. Couillault C. Rockenfeller P, et al. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc Natl Acad Sci USA. 2006;103:13497–13502. doi: 10.1073/pnas.0605443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins JR. Pak M. Maurizi MR, et al. The role of the ClpA chaperone in proteolysis by ClpAP. Proc Natl Acad Sci USA. 1998;95:12135–12140. doi: 10.1073/pnas.95.21.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger RD. Sartori CA. Kniel KE. Comparison of genetic and physiological properties of Salmonella enterica isolates from chickens reveals one major difference between serovar Kentucky and other serovars: Response to acid. Foodborne Pathog Dis. 2009;6:503–512. doi: 10.1089/fpd.2008.0144. [DOI] [PubMed] [Google Scholar]
- Joerger RD. Hanning IB. Ricke SC. Presence of arsenic resistance in Salmonella enterica serovar Kentucky and other serovars isolated from poultry. Avian Dis. 2010;54:1178–1182. doi: 10.1637/9285-022210-Reg.1. [DOI] [PubMed] [Google Scholar]
- Johnson TJ. Thorsness JL. Anderson CP, et al. Horizontal gene transfer of a ColV plasmid has resulted in a dominant avian clonal type of Salmonella enterica serovar Kentucky. PLOS One. 2010;5:e15524. doi: 10.1371/journal.pone.0015524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG. Janakiraman A. Slauch JM, et al. SitABCD is the alkaline Mn2+_transporter of Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:3159–3166. doi: 10.1128/JB.184.12.3159-3166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. Goldberg MD. Carroll RK, et al. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology. 2004;150:2037–2053. doi: 10.1099/mic.0.27209-0. [DOI] [PubMed] [Google Scholar]
- Kern R. Malki A. Abdallah J, et al. Escherichia coli HdeB is an acid stress chaperone. J Bacteriol. 2007;189:603–610. doi: 10.1128/JB.01522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieboom J. Abee T. Arginine-dependent acid resistance in Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188:5650–5653. doi: 10.1128/JB.00323-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH. Kim BH. Kim JH, et al. CadC has a global translational effect during acid adaptation in Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189:2417–2425. doi: 10.1128/JB.01277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestari SI. Han F. Fei W, et al. Prevalence and antimicrobial resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. J Food Prot. 2009;72:1165–1172. doi: 10.4315/0362-028x-72.6.1165. [DOI] [PubMed] [Google Scholar]
- Macomber L. Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli AT. Fernandez RE. Bloch CA, et al. “Black holes” and bacterial pathogenicity: A large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc Natl Acad Sci USA. 1998;95:3943–3948. doi: 10.1073/pnas.95.7.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez SN. Hanning I. Han J, et al. Salmonella enterica isolates from pasture-raised poultry exhibit antimicrobial resistance and class I integrons. J Appl Microbiol. 2010;109:1957–1966. doi: 10.1111/j.1365-2672.2010.04825.x. [DOI] [PubMed] [Google Scholar]
- Outten FW. Wood MJ. Munoz FM, et al. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli. J Biol Chem. 2003;278:45713–45719. doi: 10.1074/jbc.M308004200. [DOI] [PubMed] [Google Scholar]
- Park YK. Bearson B. Bang SH, et al. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella Typhimurium. Mol Microbiol. 1996;20:605–611. doi: 10.1046/j.1365-2958.1996.5441070.x. [DOI] [PubMed] [Google Scholar]
- Porwollik S. Wong RMY. Sims SH, et al. The Delta uvrB mutations in the Ames strains of Salmonella span 15 to 119 genes. Mutation Res. 2001;1-2:1–11. doi: 10.1016/s0027-5107(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Porwollik S. Frye J. Florea LD, et al. A non-redundant microarray of genes for two related bacteria. Nucleic Acids Res. 2003;31:1869–1876. doi: 10.1093/nar/gkg298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwollik S. Santiviago CA. Cheng P, et al. Differences in gene content between Salmonella enterica serovar enteritidis isolates and comparison to closely related serovars Gallinarum and Dublin. J Bacteriol. 2005;187:6545–6555. doi: 10.1128/JB.187.18.6545-6555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JT. Tietjen JA. Walsh CW, et al. Inactivation of alternative sigma factor 54 (RpoN) leads to increased acid resistance, and alters locus of enterocyte effacement (LEE) expression in Escherichia coli O157:H7. Microbiology. 2010;156:719–730. doi: 10.1099/mic.0.032631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz C. McMurry LM. Levy SB. Role of the multidrug resistance regulator MarA in global regulation of the hdeAB acid resistance operon in Escherichia coli. J Bacteriol. 2008;190:1290–1297. doi: 10.1128/JB.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer UF. Canonaco S. Heri A, et al. The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem. 2004;279:6613–6619. doi: 10.1074/jbc.M311657200. [DOI] [PubMed] [Google Scholar]
- Šeputiene V. Motiejûnas D. Sužiedelis K, et al. Molecular characterization of the acid-inducible asr gene of Escherichia coli and its role in acid stress response. J Bacteriol. 2003;185:2475–2484. doi: 10.1128/JB.185.8.2475-2484.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seputiene V. Suziedelis K. Normark S, et al. Transcriptional analysis of the acid-inducible asr gene in enterobacteria. Res Microbiol. 2004;155:535–542. doi: 10.1016/j.resmic.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Suziedeliene E. Suziedelis K. Garbenciute V, et al. The acid-inducible asr gene in Escherichia coli: Transcriptional control by the phoBR operon. J Bacteriol. 1999;181:2084–2093. doi: 10.1128/jb.181.7.2084-2093.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M. Yoshida H. Wada C, et al. Ribosome binding proteins YhbH and YfiA have opposite functions during 100S formation in the stationary phase of Escherichia coli. Genes Cells. 2005;10:1103–1112. doi: 10.1111/j.1365-2443.2005.00903.x. [DOI] [PubMed] [Google Scholar]
- Untergasser A. Nijveen H. Rao X, et al. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [USDA] United States Department of Agriculture–-Food Safety Inspection Services (FSIS) Serotypes profile of Salmonella Isolates from meat and poultry products, January 1998 through December 2007. http://www.fsis.usda.gov/Science/Serotypes_Profile_Salmonella_Isolates/index.asp. [Dec 15;2011 ]. http://www.fsis.usda.gov/Science/Serotypes_Profile_Salmonella_Isolates/index.asp
- Vazquez-Juarez RC. Kuriakose JA. Rasko DA, et al. CadA negatively regulates Escherichia coli O157:H7 adherence and intestinal colonization. Infect Immun. 2008;76:5072–5081. doi: 10.1128/IAI.00677-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Phillippy AM. Deng K, et al. Transcriptomic response of Salmonella enterica serovars Enteritidis and Typhimurium to chlorine-based oxidative stress. Appl Environ Microbiol. 2010;76:5013–5024. doi: 10.1128/AEM.00823-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XQ. McClelland M. Wang YP. WebArray: An online platform for microarray data analysis. BMC Bioinform. 2005;6:306. doi: 10.1186/1471-2105-6-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XQ. McClelland M. Porwollik S, et al. WebArrayDB: Cross-platform microarray data analysis and public data repository. Bioinformatics. 2009;25:2425–2429. doi: 10.1093/bioinformatics/btp430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.