Abstract
Asthma is a chronic inflammatory disorder of the airways associated with airway hyper-responsiveness and airflow limitation in response to specific triggers. Whereas inflammation is important for tissue regeneration and wound healing, the profound and sustained inflammatory response associated with asthma may result in airway remodeling that involves smooth muscle hypertrophy, epithelial goblet-cell hyperplasia, and permanent deposition of airway extracellular matrix proteins. Although the specific mechanisms responsible for asthma are still being unraveled, free radicals such as reactive oxygen species and reactive nitrogen species are important mediators of airway tissue damage that are increased in subjects with asthma. There is also a growing body of literature implicating disturbances in oxidation/reduction (redox) reactions and impaired antioxidant defenses as a risk factor for asthma development and asthma severity. Ultimately, these redox-related perturbations result in a vicious cycle of airway inflammation and injury that is not always amenable to current asthma therapy, particularly in cases of severe asthma. This review will discuss disruptions of redox signaling and control in asthma with a focus on the thiol, glutathione, and reduced (thiol) form (GSH). First, GSH synthesis, GSH distribution, and GSH function and homeostasis are discussed. We then review the literature related to GSH redox balance in health and asthma, with an emphasis on human studies. Finally, therapeutic opportunities to restore the GSH redox balance in subjects with asthma are discussed. Antioxid. Redox Signal. 17, 375–408.
I. Introduction and Conceptual Framework
Asthma is a complicated disorder characterized by variable and recurrent episodes of airway inflammation and airflow limitation in response to specific triggers. In both adults and children, asthma is the most common chronic lung disease and affects between 8% and 10% of the entire U.S. population and ∼300 million people worldwide (3, 310). While asthma symptoms are intermittent in some individuals, a significant number of subjects with asthma have persistent symptoms that necessitate daily treatment with anti-inflammatory inhaled corticosteroids (3). Among these subjects requiring daily anti-inflammatory therapy, a small subset (∼10%) has severe asthma that is refractory to treatment with very high doses of inhaled corticosteroids and in some cases, oral corticosteroids as well (1). These individuals with severe asthma suffer from an excessive burden of respiratory symptoms and have extreme morbidity, including an increased risk of exacerbations and hospitalization (85, 229). However, severe exacerbations can occur in all asthmatics regardless of baseline symptoms and underlying disease severity. Thus, despite the heterogeneity of asthma within the larger population (89, 230), all affected individuals with asthma, regardless of their baseline severity, share the risk for adverse outcomes, including asthma-related death (310).
Despite considerable advances in the understanding and treatment of asthma over the past decade, the etiology of asthma and the specific mechanisms underlying the variable severity of the disorder are not understood. Inflammation is the hallmark of asthma and is associated with airway hyperresponsiveness and airflow limitation in response to specific triggers, including allergens and respiratory viruses. Whereas inflammation is important for tissue regeneration and wound healing, the profound and sustained inflammatory response associated with asthma may result in airway injury through complex interactions between cells and inflammatory mediators (42). This process is commonly referred to as airway remodeling and involves smooth muscle hypertrophy, epithelial goblet-cell hyperplasia, and permanent deposition of airway extracellular matrix proteins that may increase airflow obstruction and the respiratory symptom burden of the disorder (61).
While the specific mechanisms responsible for asthma have yet to be unraveled, oxidative stress from free radicals such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) is likely to play a key role in the development and pathogenesis of the disorder. ROS and RNS contribute to airway inflammation and increased concentrations of both ROS and RNS have been observed in the airways of asthmatic individuals (54). Importantly, these ROS and RNS can lead to oxidation and nitration of proteins important for the resolution of inflammation. This frequently occurs through modification of critical cysteine residues present on the surface of proteins, resulting in S-sulfhydration, S-nitrosylation, S-cysteinylation, S-glutathionylation, and other covalent post-translational modifications, which may or may not be reversible (158). Ultimately, these modifications can alter the function and vital role of the proteins in the inflammatory response. One example involves catalase, the enzyme that catalyzes the breakdown of hydrogen peroxide (H2O2), which undergoes oxidative modification (nitration and chlorination) after airway allergen challenge and loses its enzymatic activity as a result (108). The activities of manganese superoxide dismutase and glutathione-S-transferase, which detoxify superoxide and other electrophilic substances, respectively, are also inhibited by nitration (203, 346). Another example involves S-glutathionylation of the p50 subunit of the transcription factor, nuclear factor kappa B (NF-κB), which has been associated with repressed DNA-binding activity (256).
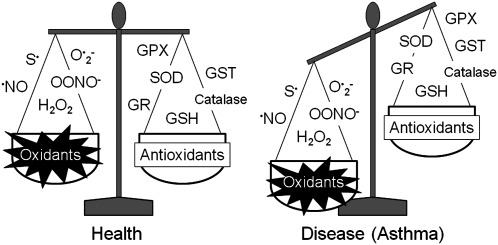
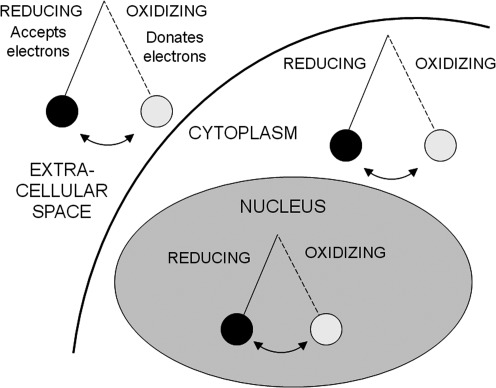
Although oxidative stress has been increasingly implicated in aging (187) and a number of chronic diseases such as cancer (247), diabetes (297), and cardiovascular disease (114), the concept of oxidative stress has evolved considerably since its inception >25 years ago. In the traditional view, oxidative stress was thought to result from a global disruption in the pro-oxidant and antioxidant balance (296). In this view, disease was due to an excessive burden of ROS and RNS with an accompanying deficiency of free radical-scavenging antioxidants (Fig. 1) (296). This view of oxidative stress ultimately led to several large antioxidant interventional trials that were undertaken in a variety of chronic disease states, but many of these failed to demonstrate clear benefits with regard to clinically relevant health outcomes (33–35, 75, 193, 292). These disappointing findings are likely related to a number of factors, including (i) insufficient knowledge of the clinical pharmacology of antioxidants, (ii) insufficient dosing or duration of the antioxidant therapy due to limited dose–response studies, (iii) the method of antioxidant delivery (i.e., oral vs. inhaled), (iv) the composition of the antioxidant agent itself (i.e., solubility, adjuvants, and additives), (v) selection of un-standardized, un-validated, or otherwise soft primary outcome indicators, including outcomes of limited relevance to the mechanism of antioxidant action, and (vi) lack of surrogate markers of oxidative stress to accompany functional outcomes. However, as knowledge of oxidation/reduction (redox)-dependent signaling and molecular mechanisms of antioxidant therapies has increased, many are now questioning the traditional view of oxidative stress as a pro-oxidant/anti-oxidant imbalance and instead are focusing on oxidative stress as “a disruption of redox signaling and control” (156). This alternative view of oxidative stress assumes that (i) oxidative stress occurs through discrete redox pathways within cells, and (ii) oxidative stress involving a disruption of redox circuitry can occur without a global pro-oxidant/anti-oxidant imbalance (156, 157) (Fig. 2). Thus, oxidative stress can lead to both organ-specific and pathway-specific toxicity without macromolecular damage (157). This re-conceptualization of oxidative stress as a disruption of redox signaling and control may explain why some of the previously conducted antioxidant supplementation trials focused solely on the mitigation of global oxidant/antioxidant imbalance have failed to yield significant results. Furthermore, re-conceptualization of asthma as a disorder characterized by disruptions of redox signaling and control may also help to unravel unanswered questions regarding the pathogenesis and severity of the disorder. Because single genes and proteins responsible for asthma susceptibility and severity have yet to be identified (17), asthma remains a complex disorder (or perhaps even a clinical syndrome) associated with significant clinical heterogeneity of symptoms, inflammatory patterns, and airway physiology. Indeed, there are a number of phenotypes or endotypes of asthma associated with unique clinical and biological features that likely require individualized therapeutic interventions (89, 196, 230). While this broad clinical spectrum of asthma may preclude the widespread use of antioxidant therapies in all affected patients, greater understanding of oxidative stress in terms of redox signaling and control may enhance the development of therapeutic strategies to prevent or alleviate asthma morbidity within selected, highly defined asthma subpopulations.
FIG. 1.
The traditional concept of oxidative stress in health and disease. Originally, oxidative stress was thought to result from a global disruption in the pro-oxidant and antioxidant balance. In this view, disease was attributed to an increased burden of free radicals (thiyl radical [S•], nitric oxide radical [NO•], superoxide [O2•-], peroxynitrite [OONO–], and hydrogen peroxide) and a deficiency of endogenous antioxidants (glutathione, reduced [thiol] form [GSH], catalase, glutathione-S-transferase [GST], glutathione reductase [GR], superoxide dismutase [SOD], and glutathione peroxidase [GPX]).
FIG. 2.
A modified concept of oxidative stress. As opposed to a simple disruption in the pro-oxidant and antioxidant balance, oxidative stress is better conceptualized as a disruption of redox signaling and control. In this view, oxidative stress is a dynamic process in constant flux, similar to a pendulum. These oxidative shifts are highly compartmentalized and are not necessarily in equilibrium between the extracellular space, the cytoplasm, and the organelles.
This review will focus on disruptions of redox signaling and control in asthma with a focus on glutathione. Although there are a number of redox-related proteins relevant to asthma, glutathione, in its thiol-reduced form (GSH), is the most abundant antioxidant in the airway epithelial lining fluid, with concentrations 100-fold higher than what is observed in the plasma (46). GSH also serves a number of important functions relevant to asthma, including (i) detoxification of electrophilic compounds such as xenobiotics, (ii) scavenging of free radicals, and (iii) modulation of cellular processes such as DNA synthesis and repair, differentiation, apoptosis, and immune function (198, 212, 334, 337). This review will discuss regulation and homeostasis of GSH in asthma as well as the potential therapeutic uses of GSH and other thiol-derived compounds in the clinical setting.
II. Glutathione Synthesis
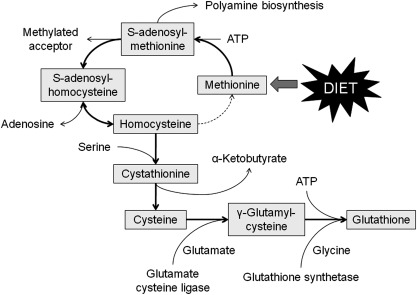
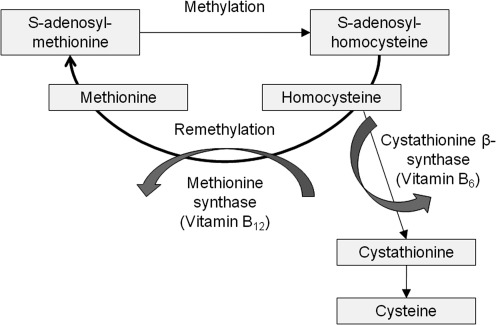
GSH is an organosulfur tri-peptide (γ-glutamyl-cysteinyl-glycine) produced from metabolism of the essential sulfur amino acid, methionine, which is not synthesized de novo in mammals (Fig. 3). After ingestion, dietary methionine is absorbed across the intestinal epithelium and enters the plasma as a free amino acid. Although the majority of methionine is removed by the liver from the portal circulation, some metabolism of methionine also occurs directly in the intestine (19, 295). Once inside the cell, methionine forms S-adenosylmethionine through an ATP-dependent reaction catalyzed by methionine adenosyltransferases. S-adenosylmethionine functions as the primary methyl donor in mammalian cells (215). The co-product of S-adenosylmethionine transmethylation, S-adenosylhomocysteine, is then hydrolyzed by S-adenosylhomocysteine hydrolase to form adenosine and homocysteine. Homocysteine is either methylated back to methionine through the remethylation pathway or is converted to cysteine through the trans-sulfuration pathway.
FIG. 3.
Methionine metabolism. Methionine is metabolized to homocysteine, which is either (i) methylated back to methionine through the remethylation pathway or (ii) converted to cysteine through the trans-sulfuration pathway. The cysteine that is formed may be used for protein synthesis or formation of glutathione.
For trans-sulfuration, homocysteine condenses with serine to form cystathionine through a reaction catalyzed by cystathionine β-synthetase. Cystathionine is then hydrolyzed by cystathionine γ-lyase to form α-ketobutyrate and free cysteine, which may be used for GSH formation (56, 119, 306). The synthesis of GSH from glutamate, cysteine, and glycine involves two steps, catalyzed by glutamate cysteine ligase (also referred to as γ-glutamylcysteine synthetase) and GSH synthetase, respectively, as shown below:
1. Glutamate+cysteine+ATP→γ-glutamylcysteine+ADP+Pi, and
2. γ-glutamylcysteine+glycine+ATP→GSH+ADP+Pi.
The first step of GSH synthesis is primarily controlled by negative feedback from GSH. However, when GSH is consumed and feedback inhibition is impaired, the availability of cysteine becomes the major limiting factor for GSH synthesis (198, 199, 334).
III. Distribution of Glutathione in the Body
GSH is present in all tissues and fluids throughout the body. Although the GSH content of bodily fluids is significantly lower (up to 1000-fold) than that found in tissues, all cells release GSH, suggesting that it is essential for cellular function and defense (231). The total amount of GSH in the body is therefore ∼15 g, of which 5 g is cysteine. This bodily GSH is distributed in all the major organ systems, including the lungs. However, the liver has the highest GSH content (about 4 g or 20% of all bodily GSH), although liver GSH concentrations fluctuate somewhat according to the time of day, the composition of the diet, and bodily demands (36). The cysteine content of these liver GSH stores is nearly equal to the daily recommended dietary intake of total sulfur amino acids (methionine plus cysteine), which is ∼1.1 g/day for a 60-kg woman (equal to 2.7 g/day of GSH) and ∼1.4 g/day for a 75-kg adult male (equal to 3.3 g/day of GSH) (93). Thus, the GSH content of the liver can be quickly depleted in ∼1 day with fasting and starvation. In otherwise healthy individuals, homeostatic mechanisms prevent hepatic GSH stores from becoming too low during these conditions and GSH is instead derived from muscle and other tissues (210). However, the entire body has only a 4-day GSH reserve. Although the majority of adult males and females in the United States consume more than the daily recommended intake of sulfur amino acids (90), these GSH losses can be of importance during prolonged states of protein/energy insufficiency (including catabolic illness) or in individuals with chronic inflammatory illnesses such as alcoholism where GSH stores are otherwise depleted (94, 352).
IV. Glutathione Function and Homeostasis
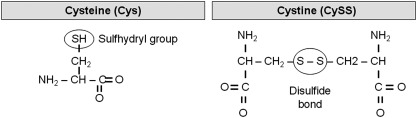
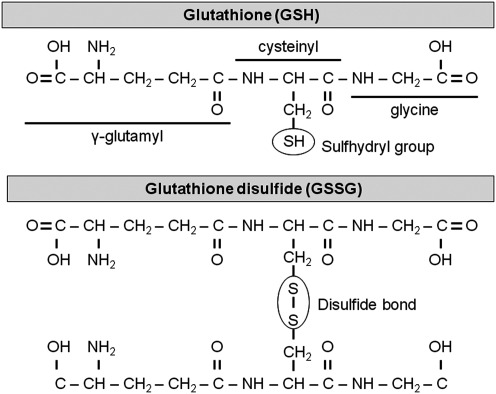
The biological activity of GSH is derived from cysteine. Cysteine contains a carbon-bonded sulfhydryl (–SH) group that is highly nucleophilic and easily oxidized, particularly in the extracellular space (161) (Fig. 4). With oxidation, two cysteine residues covalently link and form a disulfide bond (–SS), yielding the oxidized moiety cystine (CySS). Within a protein, these disulfide bonds contribute to a protein's tertiary structure during the course of protein folding. Disulfide bonding between cysteine residues further contributes to multi-unit protein quarternary structure through the formation of covalent bonds between peptide chains. In the case of GSH, which is not a protein, the disulfide bonding of the –SH group contributes to thiol-disulfide equilibrium and many other vital GSH functions.
FIG. 4.
Chemical structures of cysteine (Cys) and cystine (CySS).
A. Glutathione as a cysteine reservoir
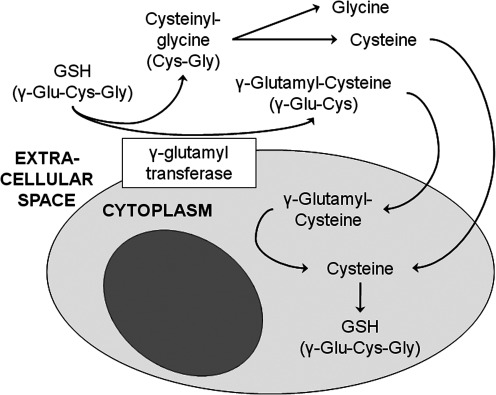
Because cysteine is relatively unstable and rapidly oxidizes to CySS, GSH serves as the primary cysteine reservoir (Fig. 5). The γ-glutamyl cycle allows GSH to serve as a continuous cysteine source for the body when cysteine reserves are not depleted (199, 245) (Fig. 6). Through this cycle, γ-glutamyl transferase, which resides in the cell membrane, transfers the γ-glutamyl moiety of extracellular GSH to cysteine, forming γ-glutamyl-cysteine and cysteinyl-glycine. The cysteinyl-glycine is broken down by dipeptidase in the extracellular space to generate glycine and cysteine, which are readily taken up by the cell. The γ-glutamyl-cysteine that is formed in the extracellular space may also be transferred back into the cell, where it undergoes further metabolism to cysteine, which can be used for additional GSH synthesis or protein incorporation.
FIG. 5.
Chemical structures of GSH and glutathione disulfide (GSSG).
FIG. 6.
The γ-glutamyl transferase cycle. The γ-glutamyl cycle allows glutathione to serve as a continuous source of cysteine. The γ-glutamyl-cysteine and cysteinyl-glycine are broken down to form cysteine, which can be used for glutathione synthesis or protein incorporation.
B. Xenobiotic conjugation and detoxification
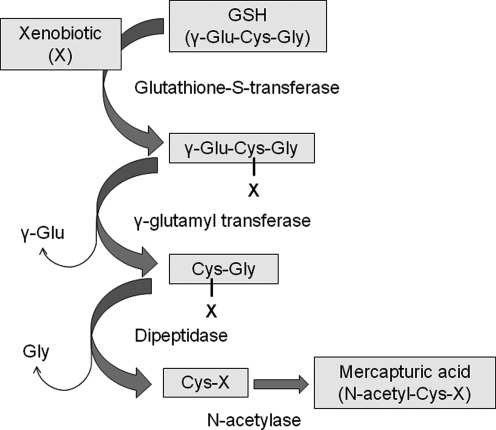
GSH is also crucial for detoxification of xenobiotics (95, 199, 334). Xenobiotics are electron-loving substances that are otherwise highly reactive. Xenobiotics form conjugates with GSH either spontaneously or enzymatically through reactions catalyzed by glutathione-S-transferases (Fig. 7). These conjugates are then excreted from the cell, where they undergo cleavage by γ-glutamyl transferase and further breakdown by dipeptidase, resulting in a cysteinyl conjugate. The cysteinyl conjugate then undergoes N-acetylation, forming a mercapturic acid, which is then excreted by the kidney. While this process is important for detoxification of the parent compound, which is often highly reactive, GSH conjugation also irreversibly consumes intracellular GSH (199).
FIG. 7.
The role of glutathione in xenobiotic conjugation and detoxification. Glutathione conjugates xenobiotics to a less reactive form, yielding a mercapturic acid that is excreted by the kidney.
C. Free radical scavenging
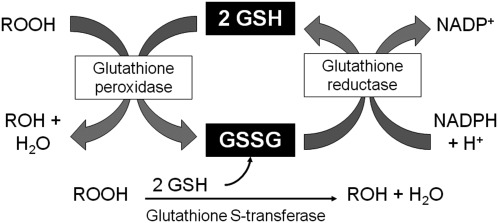
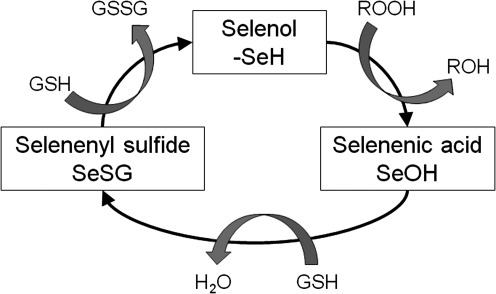
Free radicals are produced through a number of physiologic processes, such as mitochondrial respiration, and can also be encountered exogenously though environmental exposures. While some free radicals are important for physiologic function, intermediates such as superoxide anion (O•2−) and organic hydroperoxides such as H2O2 can ultimately result in lipid peroxidation. GSH provides the primary defense against lipid peroxidation through donation of a reducing equivalent (H++e−) to organic hydroperoxides by means of a glutathione peroxidase or a glutathione-S-transferase-dependent reaction. However, in doing so, it becomes reactive (SG–) and reacts readily with another reactive glutathione (SG–) to form glutathione disulfide (GSSG). GSSG is then reduced back to GSH by glutathione reductase through an NADPH-dependent reaction, forming a redox cycle (Fig. 8). GSSG can also be actively exported from the cell or react with a protein –SH group, forming a protein mixed disulfide.
FIG. 8.
The glutathione redox cycle. In the presence of organic hydroperoxides (ROOH), GSH is converted to GSSG, yielding a less reactive product (ROH). GSSG is converted back to GSH by glutathione reductase through an NADPH-dependent reaction.
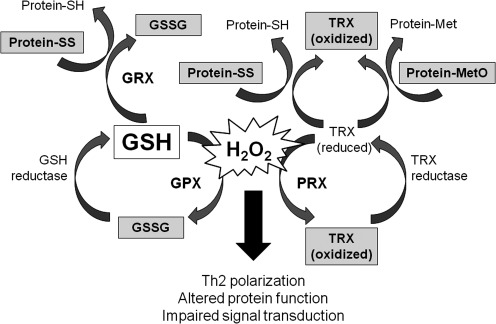
D. Maintenance of thiol equilibrium
Because GSH is the predominant intracellular thiol, GSH also plays a key role in maintaining the essential thiol status of proteins. GSH participates in thiol-disulfide exchange through a reversible reaction catalyzed by thiol-transferases as shown below, where Protein-SSG is a mixed disulfide and Protein-SH is reduced protein:
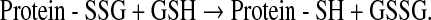 |
The thiol-disulfide equilibrium is determined by the redox state of the cell, which is based on GSH and GSSG concentrations. Typically, GSSG concentrations are low and proportional to the log of [GSH]2/[GSSG] such that protein-mixed disulfide formation is limited. However, with increased formation of GSSG, thiol-disulfide equilibrium is disrupted and protein mixed disulfide formation is increased. While these shifts in the GSH/GSSG balance are essential for redox signaling and the maintenance of cellular metabolism and homeostatic function, sustained disequilibrium from excess GSSG formation ultimately increases protein mixed disulfide formation.
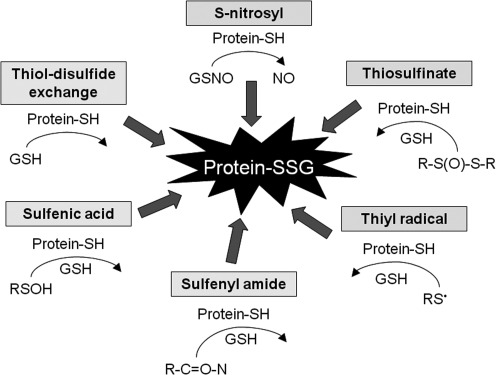
E. Protein S-glutathionylation
Proteins contain a number of cysteine-sulfhydryl (–SH) residues that undergo post-translational modifications as part of their normal function. Protein S-glutathionylation (S-SG) is one example that occurs when a protein –SH moiety forms a disulfide bond with GSH (59, 219). This can occur through a number of mechanisms (Fig. 9). While protein S-glutathionylation occurs more frequently in response to ROS or RNS, it is also important for redox signaling and regulation of protein activity and is therefore present to some extent during basal physiologic conditions (333). For example, the cellular functions of actin are mediated by reversible S-glutathionylation (58) such that impairment of actin S-glutathionylation alters stress fiber formation and cytoskeleton organization (83).
FIG. 9.
Mechanisms of protein S-glutathionylation. Proteins contain a number of cysteine-sulfhydryl (-SH) residues that may form a disulfide bond with GSH, resulting in protein S-glutathionylation (protein-SSG). “R” represents an organic substituent.
Although protein-GSH bonding is usually transient and reversible under reducing conditions, during chronic oxidizing conditions the protein-GSH bond can persist indefinitely (22, 109). Under these oxidizing conditions, S-glutathionylation may serve as a reservoir for GSH inside the cell and may also protect from irreversible oxidation to sulfinic and sulfonic acid, which leads to proteasomal degradation of the protein (209). However, if the cysteine residue is essential, then S-glutathionylation may disrupt critical protein functions and lead to compromised cellular activities (59), such as is the case with the transcription factor, NF-κB. S-glutathionylation of the p65 and p50 subunits of NF-κB inhibits NF-κB binding to the DNA (256, 260), while S-glutathionylation of the inhibitory kappa B kinase beta subunit represses NF-κB transactivation (219, 275).
F. Regulation of cell surface proteins
Thiols such as GSH are also present in the extracellular space, where they play an important role in cell surface protein regulation and signal transduction. The effects of the extracellular thiol redox balance on cellular functions have been studied previously. Whereas reducing states promote proliferation of epithelial cells (153, 241), oxidation of the extracellular thiol pools promotes epithelial cell apoptosis (151). Similarly, oxidation of the extracellular thiol redox potential stimulates proliferation and pro-fibrotic signaling of transforming growth factor beta-1 (TGFβ1) within fibroblasts (269) and also activates NF-κB and stimulates monocyte adhesion to vascular endothelial cells (111). These findings suggest that direct uptake of GSH and related thiols by cells is not essential for cellular function. Rather, beneficial cellular functions can be derived from preservation of cell signaling functions through maintenance of the extracellular GSH and thiol redox balance.
G. Protection against nitrosative stress from RNS
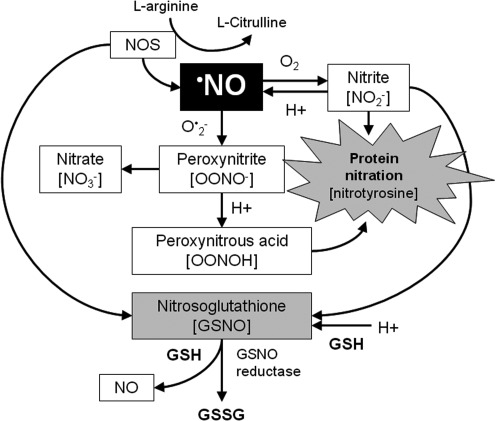
Nitric oxide (•NO) is the primary RNS in the lung. •NO is produced from nitric oxide synthases, which convert L-arginine to •NO and L-citrulline. •NO is then metabolized to the nitric oxide oxidation products nitrite and nitrate through a series of reactions involving O•2− and oxygen (Fig. 10). The peroxynitrite (OONO–) that is generated is then protonated to peroxynitrous acid (ONOOH), which can nitrate tyrosine residues, leading to either a gain or loss of protein function (14, 214). ONOOH may also react with GSH and other thiol residues to form S-nitrosothiols such as nitrosoglutathione (GSNO), a beneficial endogenous bronchodilator (261) that may function as an important signaling mechanism in the presence of nitrosative stress (97, 105, 134).
FIG. 10.
Reactive nitrogen species biochemistry. •NO is produced from nitric oxide synthases and is then metabolized to the nitric oxide oxidation products nitrite (NO2−) and nitrate (NO3−) through a series of reactions involving superoxide anion (O•2−) and oxygen. The OONO– that is generated is then protonated to peroxynitrous acid (ONOOH). ONOOH may also react with GSH and other thiol residues to form S-nitrosothiols such as nitrosoglutathione (GSNO). •NO, nitric oxide radical
V. Glutathione Redox Balance in Health
The redox state of GSH and GSSG in healthy individuals has been well described (154, 156, 157, 231, 288). Because GSSG and GSH concentrations are not directly proportional, the redox state of the GSH/GSSG pool is best expressed with the Nernst equation, rather than a simple ratio of GSH to GSSG. The Nernst equation incorporates the correct stoichiometry of 2 GSH units oxidized per GSSG unit formed and is expressed as follows:
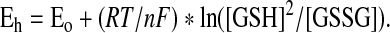 |
In this equation, Eh represents the redox potential at a given pH and is defined as the electromotive force in millivolts relative to a standard hydrogen electrode (1 atm H2, 1 M H+). The Eo term represents the standard potential for the redox couple at a given pH, R is the gas constant, T is the absolute temperature, F is Faraday's constant, and n is the number of electrons transferred. The Nernst equation is not limited to the GSH/GSSG pool but can also be applied to other redox-active biomolecules and therefore provides a means to assess the tendency of the redox couple to donate or accept electrons. Whereas couples with more positive Eh values are more oxidized, couples with more negative Eh values are more reduced (154, 156, 157, 231, 288). Given the compartmentalization of the redox state within the body (156, 157), intracellular and extracellular GSH/GSSG redox states are discussed separately below.
A. Intracellular glutathione redox status
GSH is the predominant intracellular thiol. Within cells, the majority of GSH is found in the cytosol, the primary site of GSH synthesis, with concentrations ranging from 1 to 11 mM (298). While GSSG concentrations are typically very low, GSH and GSSG concentrations vary somewhat as the cell undergoes proliferation, differentiation, and apoptosis, leading to shifts in the GSH/GSSG redox state (154, 170, 231). During cellular proliferation, the GSH/GSSG redox state is most reduced and ranges from −230 to −260 mV. With the onset of differentiation and growth arrest, the redox state becomes more oxidized and ranges from −220 to −190 mV, possibly due to contact inhibition (142, 170). Further oxidation of the GSH/GSSG couple by 40 mV to the range of −170 to −150 mV results in cellular apoptosis (154, 231). These changes are relatively small and suggest that the GSH/GSSG pool within the cells is tightly regulated.
Although total cellular GSH concentrations typically reflect the cytosolic pool, organelles also contain GSH. For instance, cells such as hepatocytes have a higher volume of mitochondria that can comprise up to 15% of the total GSH pool (217). Within the mitochondria, GSH concentrations are similar to those in the cytoplasm and range from 5 to 11 mM (103, 120, 330). However, the GSH/GSSG redox state of the mitochondria (−330 mV) is significantly more reduced (112, 113, 126). Similarly, the GSH content in nucleus comprises 5%–10% of the total cellular GSH pool and 10%–20% of this GSH content is independent from that found in the cytosol (30, 298, 301). Like the mitochondria, the GSH/GSSG redox state of the nucleus (−280 mV) is also more reduced than the cytoplasm (113, 127, 155, 338). By contrast, the GSH/GSSG redox state of the endoplasmic reticulum is more oxidized compared to the cytosol, with a redox potential of approximately −150 mV (112, 113, 143). Within the endoplasmic reticulum, the ratio of GSH to GSSG ranges from 1:1 to 3:1, compared to >30:1 for the overall GSH to GSSG ratio of the cell (288). Although the specific roles of GSH and the GSH/GSSG redox balance in these organelles are still being unraveled, the oxidizing environment of the endoplasmic reticulum may be essential for the production of proteins with disulfide bonds (143, 288). Likewise, the reducing environment of the mitochondria and nuclear compartments may promote redox-sensitive transcriptional regulation and inhibit oxidative DNA damage (113).
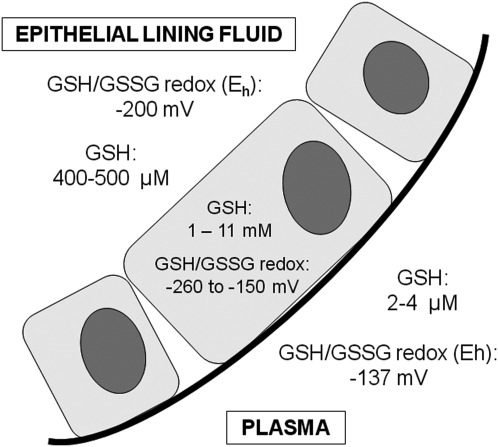
B. Plasma glutathione redox status
Although GSH and GSSG are present outside of the cell, their concentrations are usually 100 to 1000 times less than what is observed inside the cell (288). In the plasma of healthy adults, GSH concentrations range from 2 to 4 μM, with a GSH/GSSG redox potential of approximately −137±9 mV (159, 160). Thus, the redox state of the plasma is considerably more oxidized than that of cells and tissues.
Whereas GSH is the predominant intracellular thiol, cysteine is the most abundant thiol in the plasma. However, given the more oxidizing environment, the majority of cysteine in the plasma exists in its disulfide form, as CySS. In healthy adults, plasma cysteine concentrations range from 8 to 10 μM, whereas plasma CySS concentrations are at least 40 μM (159). Thus, the cysteine/CySS redox potential is significantly more oxidized than the GSH/GSSG redox potential, with normal values approximating −80±9 mV (159). The difference of 57 mV between the redox potentials of cysteine/CySS and GSH/GSSG suggests that they are not in redox equilibrium and likely play distinct roles in redox signaling and control (161). For that reason it has been suggested that plasma GSH/GSSG and plasma cysteine/CySS redox states be used together as a measure of oxidative stress in vivo in humans, since the plasma GSH/GSSG redox potential may less sensitive to acute oxidative disturbances (156).
Although the cysteine/CySS pool is more abundant than the GSH/GSSG pool in the plasma, plasma GSH and GSSG concentrations do yield useful information about the oxidative burden of an otherwise healthy individual. Plasma GSH/GSSG redox may also be highly informative in cases of disease. In a previous study of age-related macular degeneration (285), individuals >60 years of age had oxidation of the plasma GSH/GSSG redox couple by about 25 mV compared to individuals <43 years of age. In a related study, the plasma GSH/GSSG redox potential grew steadily more oxidized in otherwise healthy individuals after the age of 45 years, with an average rate of oxidation of ∼0.7 mV/year (162). Similarly, in the case of cigarette smoking, plasma GSH concentrations were significantly lower in smokers (1.8±1.3 μM) than in nonsmokers (2.4±1.0) and the resulting GSH/GSSG redox potential was significantly more oxidized (−128±18 mV vs.−137±17 mV), with a difference of ∼9 mV between groups (232). This difference has important implications for the function of thiol-containing proteins, since a 9 mV change is sufficient to induce a twofold change in the ratio of reduced to oxidized proteins (154).
C. Epithelial lining fluid glutathione redox status
In contrast to the plasma where cysteine (as CySS) is the predominant thiol pool, in the epithelial lining fluid, GSH is the most abundant thiol (Fig. 11). In healthy nonsmoking adults, total glutathione concentrations in the epithelial lining fluid, measured as the sum total of the GSH and GSSG pools, are ∼400–500 μM and therefore approximate the values found in some cell types (46, 233). Furthermore, in healthy adults, <5% of the total GSH/GSSG pool exists in the oxidized (GSSG) form (46, 233), resulting in a relatively reduced GSH/GSSG redox potential of approximately −200 mV (352). Why GSH (as opposed to cysteine) predominates in the epithelial lining fluid is unclear but may be related to barrier defense against inhaled oxidants and microorganisms. In isolated type II alveolar epithelial cells, extracellular GSH inhibits hyperoxia-induced injury and pro-inflammatory cytokine release and promotes cell growth (273, 282, 345). GSH is also essential for airway innate immune defense and regulates alveolar macrophage apoptosis and phagocytosis of infectious particles (43, 140). GSH depletion further leads to activation of NF-κB and increased pro-inflammatory gene transcription and cytokine release from histone deacetylase suppression in epithelial cells (268, 351).
FIG. 11.
Glutathione distribution in the plasma, cell, and epithelial lining fluid of healthy adults. The GSH/GSSG redox potential within the cell varies as a function of differentiation, proliferation/growth arrest, and apoptosis.
Human studies support these observations and confirm the important role of GSH in maintaining lung health. Although acute insults such as smoking and diesel exhaust exposure initially increase GSH concentrations in the epithelial lining fluid (23, 46, 352), chronic insults such as long-term smoking over many years lead to increased airway GSSG formation and protein carbonylation (238). Likewise, in cases of severe lung injury such as acute respiratory distress syndrome (40, 44, 246), pulmonary fibrosis (21, 25), and hypersensitivity pneumonitis (24), total and reduced GSH concentrations are significantly lower than values observed in healthy adults, with differences up to 10-fold. GSSG further accounts for up to 30% of the total GSH pool in these patients (44), suggesting that the GSH/GSSG redox balance in the epithelial lining fluid may be responsible for some of the respiratory morbidity associated with pulmonary disease.
VI. Glutathione Redox Balance in Asthma
Glutathione was first characterized in subjects with asthma in the late 1970s. In the earliest available publication, 22 adults with asthma across a wide range of severities were exposed to ozone or filtered air (sham) for 2 h in a cross-over design (191). With ozone exposure, blood GSH levels decreased and asthma symptoms worsened, although no changes in pulmonary function were noted (191). Whether the reduction in blood GSH content was related to increased airway oxidative stress is not clear. However, in a similar study where 400 ppb of ozone was administered to healthy subjects for 2 h, increased 8-isoprostane concentrations were noted and were associated with decreased pulmonary function (225). Oxidative stress reflected by 8-isoprostane levels is also apparent after allergen challenge (224).
While the specific mechanisms linking GSH disturbances to asthma are still being unraveled, several studies characterizing GSH and related factors in the airways and systemic circulation of subjects with asthma have been undertaken. These findings, as well as their potential physiological significance, are discussed below. A summary table of the major findings related to GSH/GSSG redox balance in healthy controls and subjects with mild-to-moderate and severe asthma is provided in Table 1.
Table 1.
Summary Table of Glutathione, Reduced (Thiol) Form, and Glutathione, Reduced (Thiol) Form–Redox Balance in Asthma
| Reference | Assay method | Subjects | Total GSH+GSSG | GSH | GSSG |
|---|---|---|---|---|---|
| Whole blood/erythrocytes | |||||
| Hasbal et al. (129) | DNTB reduction assay | 19 pediatric controls | — | 2.52 μmol/g Hb | — |
| 30 children with MMA, before treatment | 2.72 μmol/g Hb | ||||
| 30 children with MMA, after treatmenta | 2.40 μmol/g Hb | ||||
| Mak et al. (206) | DNTB reduction assay | 135 controls | — | — | 158 μM |
| 26 mild asthmaticsa | 162 μM | ||||
| 50 moderate asthmaticsa | 172 μM | ||||
| 28 moderate-to-severe asthmaticsa | |||||
| Nadeem et al. (236) | DNTB reduction assay | 23 controls | 830 μM | — | — |
| 38 symptomatic mild-to-severe asthmaticsa | 610 μM | ||||
| Nadeem et al. (237) | DNTB reduction assay | 97 stable MMAsa | 710 μM | — | — |
| 32 MMAs with acute exacerbationa | 700 μM | ||||
| Pennings et al. (253) | DNTB reduction assay | 40 stable MMAs, before treatment | 7.0 μmol/g Hb | — | — |
| 40 stable MMAs, after treatmenta | 6.6 μmol/g Hb | ||||
| Vural and Uzun (329) | DNTB reduction assay | 43 controls | 0.49 μmol/g Hb | — | — |
| 40 mild asthmatics | 0.59 μmol/g Hb | ||||
| Wood et al. (348) | DNTB reduction assay | 31 controls | 908 μM | 836 μM | 24.8 μM |
| 44 stable mild-to-severe asthmaticsa | 834 μM | 832 μM | 5.7 μM | ||
| Plasma | |||||
| Ercan et al. (74) | DTNB reduction assay | 68 controls | — | ∼6 μM | — |
| 187 controls (separate sample) | ∼6 μM | ||||
| 196 children with mild asthma | ∼4.8 μM | ||||
| 116 children with moderate-to-severe asthmaa | ∼4.2 μM | ||||
| Fitzpatrick et al. (86) | HPLC | 38 children with MMAa | 1.3 μM | ∼0.8 μM | 0.3 μM |
| 51 children with SAa | 0.6 μM | ∼0.4 μM | 0.5 μM | ||
| Sackesen et al. (284) | DTNB reduction assay | 173 pediatric controls | — | ∼5.9 μM | — |
| 164 children with mild asthma | ∼4.7 μM | ||||
| Shanmugasundaram et al. (293) | DTNB reduction assay | 94 preadolescent controls | — | 58 μM | — |
| 86 adolescent controls | 56 μM | ||||
| 118 preadolescent MMAs | 41 μM | ||||
| 92 adolescent MMAs | 40 μM | ||||
| 39 preadolescent MMAs with exacerbation | 38 μM | ||||
| 26 adolescent MMAs with exacerbation | 35 μM | ||||
| Bronchoalveolar lavage and epithelial lining fluid | |||||
| Comhair et al. (52) | HPLC | 5 controls, at baseline | — | 268 μM | 9 μM |
| 5 controls, after allergen challenge (10 min) | 192 μM | 3 μM | |||
| 5 controls, after allergen challenge (48 h) | 231 μM | 11 μM | |||
| 7 mild asthmatics, at baseline | 282 μM | 8 μM | |||
| 7 mild asthmatics, after allergen (10 min) | 165 μM | 15 μM | |||
| 7 mild asthmatics, after allergen (48 h) | 284 μM | 9 μM | |||
| Fitzpatrick et al. (86) | HPLC | 38 children with MMAa | 1.2 μM | ∼0.6 μM | 0.6 μM |
| 51 children with SAa | 0.7 μM | ∼0.35 μM | 0.4 μM | ||
| Fitzpatrick et al. (87) | HPLC | 21 children with moderate asthma (BAL)a | — | 0.33 μM | 0.13 nM |
| 21 children with moderate asthma (ELF)a | 37 μM | 13 μM | |||
| 43 children with SA (BAL)a | 0.26 nM | 0.45 μM | |||
| 43 children with SA (ELF)a | 24 μM | 32 μM | |||
| Fitzpatrick et al. (88) | HPLC | 35 adult controls (ELF) | 436 μM | ∼390 μM | ∼10 μM |
| 6 symptomatic pediatric controls (ELF)a | 260 μM | ∼225 μM | ∼30 μM | ||
| 35 children with SA without airflow limitation (ELF)a | 134 μM | ∼100 μM | ∼40 μM | ||
| 30 children with SA with airflow limitation (ELF)a | 129 μM | ∼50 μM | ∼75 μM | ||
| Kelly et al. (166) | DTNB reduction assay | 20 controls (bronchial wash) | — | 0.30 μM | 0.09 μM |
| 20 controls (BAL fluid) | 0.41 μM | 0.08 μM | |||
| 20 mild asthmatics (bronchial wash) | 0.41 μM | 0.52 μM | |||
| 20 mild asthmatics (BAL fluid) | 0.30 μM | 0.28 μM | |||
| Mudway et al. (234) | DTNB reduction assay | 15 controls, baseline (bronchial wash) | 0.46 μM | — | 0.11 μM |
| 15 controls, baseline (BAL fluid) | 0.88 μM | 0.07 μM | |||
| 15 controls, after ozone (bronchial) | 0.98 μM | 0.31 μM | |||
| 15 controls, after ozone (BAL fluid) | 0.94 μM | 0.30 μM | |||
| 15 mild asthmatics, baseline (bronchial) | 1.08 μM | 0.27 μM | |||
| 15 mild asthmatics, baseline (BAL fluid) | 0.98 μM | 0.24 μM | |||
| 15 mild asthmatics, after ozone (bronchial) | 1.20 μM | 0.35 μM | |||
| 15 mild asthmatics, after ozone (BAL fluid) | 1.40 μM | 0.43 μM | |||
| Smith et al. (299) | DTNB reduction assay | 17 controls (bronchial) | 13 nM/mg protein | — | — |
| 17 controls (alveolar) | 23 nM/mg protein | ||||
| 10 mild asthmatics (bronchial) | 24 nM/mg protein | ||||
| 10 mild asthmatics (alveolar) | 37 nM/mg protein | ||||
| Induced sputum | |||||
| Beier et al. (27) | DTNB reduction assay | 31 controls, baseline | 12.1 μM | — | — |
| 31 controls, 72 h (no intervention) | 9.8 μM | ||||
| 12 mild asthmatics, baseline | 20.2 μM | ||||
| 12 mild asthmatics, 72 h (no intervention) | 18.1 μM | ||||
| Beier et al. (28) | DTNB reduction assay | 15 controls | 9.2 μM | — | ∼1.08 μM |
| 20 mild asthmatics | 8.7 μM | ∼1.29 μM | |||
| 19 moderate asthmaticsa | 4 μM | ∼1.20 μM | |||
| Beier et al. (29) | DTNB reduction assay | 12 mild asthmatics, early allergen responders, baseline | — | 3.3 μM | 4.5 μM |
| 12 mild asthmatics, early allergen responders, postallergen challenge | 5.9 μM | 2.9 μM | |||
| 12 mild asthmatics, late allergen responders, baseline | 3.5 μM | 3.7 μM | |||
| 12 mild asthmatics, late allergen responders, postallergen challenge | 2.8 μM | 3.2 μM | |||
| Dauletbaev et al. (60) | DTNB reduction assay | 10 controls (saliva) | 1.2 μM | — | 0 μM |
| 10 controls (sputum) | 3.9 μM | 50.9 μM | |||
| 10 MMAs (saliva)a | 0.9 μM | 0 μM | |||
| 10 MMAs (sputum)a | 6.4 μM | 72.3 μM | |||
| Deveci et al. (66) | DTNB reduction assay | 11 controls | — | 0.34 μM | — |
| 11 stable MMAsa | 8.53 μM | ||||
| 10 MMAs with acute exacerbationa | 2.85 μM | ||||
| Kongerud et al. (178) | DTNB reduction assay | 18 controls (sputum cells) | 628 ng/106 cells | — | — |
| 16 mild asthmatics (sputum cells) | 1016 ng/106 cells | ||||
| Wood et al. (348) | DTNB reduction assay | 6 controls (whole sputum) | 10.4 μM | 2.7 μM | 3.5 μM |
| 20 controls (sputum supernatant) | 7.0 μM | 1.2 μM | 2.6 μM | ||
| 10 mild-to-severe asthmatics (whole sputum)a | 13.6 μM | 2.2 μM | 3.5 μM | ||
| 37 mild-to-severe asthmatics (sputum supernatant)a | 15.3 μM | 4.1 μM | 5.9 μ | ||
| Exhaled breath condensate | |||||
| Corradi et al. (57) | HPLC | 10 pediatric controls | — | 14.1 nM | |
| 12 children with MMA, with exacerbationa | 5.96 nM | ||||
| 12 children with MMA, after exacerbationa | 8.44 nM | ||||
| Dut et al. (70) | HPLC | 191 pediatric controls | — | ∼17 nM | — |
| 110 children with mild asthma | ∼8 nM | ||||
| 30 children with moderate asthma | ∼8 nM | ||||
Values represent the mean.
Treated with inhaled corticosteroids.
MMA, mild-to-moderate asthma; SA, severe asthma; BAL, bronchoalveolar lavage; ELF, epithelial lining fluid; GSSG, glutathione disulfide; GSH, glutathione, reduced (thiol) form; DNTB, (5,5′-dithiobis-(2-nitrobenzoic acid)); HPLC, high-performance liquid chromatography.
A. Airway glutathione concentrations in asthma, measured invasively
Sampling of the airway epithelial lining fluid is difficult and therefore only seven studies to date have directly examined GSH and GSSG content in the airways of asthmatic subjects. Given ethical and practical considerations, the majority of these studies were limited to inhaled corticosteroid-naive asthmatic adults with mild asthma and good symptom control (52, 166, 234, 299, 300). These subjects underwent invasive bronchoscopy with bronchoalveolar lavage for sampling of the epithelial lining fluid constituents.
In the first studies conducted on the subject, total glutathione concentrations in the bronchoalveolar lavage cells (measured as the sum total of the GSH and GSSG pools) did not differentiate between asthmatics and healthy controls (300). However, the subjects with asthma had nearly twofold higher baseline concentrations of total glutathione in the airway lavage fluid (299). Total glutathione concentrations were highest in the bronchial versus the alveolar space (299), consistent with the bronchial features of asthma (such as airway hyperresponsiveness) and the nature of the inflammation in these subjects, which is primarily localized to the airways. While GSH and GSSG were not measured, the increased total glutathione concentrations may reflect increased airway oxidative stress from increased GSSG formation, as opposed to increased airway reducing capacity from increased GSH synthesis. In keeping with this notion, in similar studies, increased total glutathione concentrations were associated with decreased basal expression of the antioxidants superoxide dismutase, ascorbate, and alpha-tocopherol in asthmatics versus controls (234, 300). Other studies in which both GSH and GSSG were assessed further revealed significant increases in GSSG between asthmatics and controls, particularly in the bronchial versus the alveolar airspaces (166, 234), with no differences in GSH concentrations between groups (52, 166). Furthermore, while acute antigen challenge did not lead to further increases in bronchoalveolar GSSG content in the asthmatic subjects, it did significantly decrease GSH concentrations (52). These findings are of relevance and suggest that the baseline perturbations in airway GSH and GSSG homeostasis in subjects with asthma may increase the risk of airway injury from secondary insults such as antigen exposure.
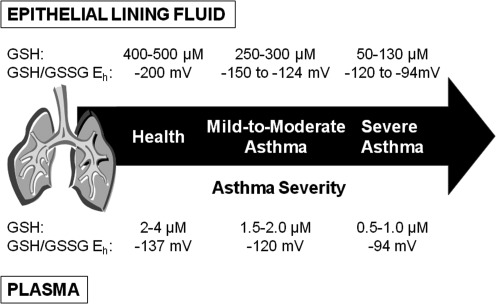
More recently, we characterized the airway GSH/GSSG redox state in controls and children with mild-to-moderate and severe asthma treated with inhaled corticosteroids who underwent bronchoscopy with bronchoalveolar lavage for clinical indications (86–88). Whereas the total glutathione (GSH+GSSG) concentrations in the epithelial lining fluid of healthy adult controls were similar to other published reference values (436 μM) (46, 233), these concentrations were reduced by more than threefold in children with severe asthma (129–134 μM) (88). Whether age influences the airway GSH/GSSG pool is not clear since children have not been previously studied due to the invasive nature of bronchoscopy and a number of ethical and practical concerns. However, total airway glutathione concentrations remained more than twofold lower in children with severe asthma than in nonasthmatic children with current respiratory symptoms (260 μM) (88). Total airway glutathione concentrations in children with severe asthma were also nearly half the levels observed in age-matched children with mild-to-moderate asthma (86). Within the severe asthmatics, these lower total airway glutathione concentrations were further characterized by near-total depletion of GSH and a two- to fourfold increase in GSSG compared to mild-to-moderate asthmatics (86, 87) and controls (88). These findings were also accompanied by up to a 75-mV shift in the epithelial lining fluid GSH/GSSG redox potential (Eh) toward the more oxidized state in children with severe asthma (adult control, −172 mV; pediatric control, −146 mV; mild-to-moderate asthma, −124 mV; severe asthma, −94 to −120 mV [Note: the values for mild-to-moderate asthma are expressed here per mL of epithelial lining fluid and do not correspond directly to reference (86)]) (86–88).
These observations suggest that profound disequilibrium of the airway GSH/GSSG redox balance accompanies severe versus milder forms of asthma. This GSH/GSSG disequilibrium may account for some of the morbidity associated with the disorder. In otherwise stable subjects with severe asthma refractory to inhaled corticosteroid treatment, the perturbations in the epithelial lining fluid GSH/GSSG redox potential (i.e., −75 mV difference between severe asthmatics vs. controls) were most striking in subjects with airflow limitation and were directly associated with lipid peroxidation and DNA oxidation byproducts, potentially indicative of airway structural damage (87, 88). The GSH/GSSG perturbations in severe asthma were further associated with cysteine depletion and increased oxidation of the cysteine/CySS redox potential in the epithelial lining fluid, suggestive of global airway thiol imbalance and possibly decreased cysteine reserves for additional GSH synthesis (86). These observations may account for the refractory nature of severe asthma, in which airway inflammation persists despite intensive corticosteroid therapy (1).
B. Airway glutathione concentrations in asthma, measured noninvasively
Given the invasive nature of bronchoscopy and the practical and ethical considerations prohibiting its use in most research studies, a few investigators have attempted to characterize airway GSH concentrations in induced sputum from asthmatic subjects. However, the results have been conflicting, most likely due to a number of methodological challenges associated with sputum GSH and GSSG analysis (26, 27). Whereas some studies have failed to demonstrate differences in sputum total glutathione concentrations (GSH+GSSG) between mild asthmatics and controls (27, 60, 178), others have shown increased basal total glutathione concentrations in induced sputum from asthmatics at baseline (28, 66, 348) and after allergen challenge (29). Although sputum GSSG concentrations were not consistently elevated in subjects with mild asthma in these studies (28, 60, 348), others have observed significant associations between increased GSSG concentrations, increased eosinophil counts (29), and decreased pulmonary function reflected by the ratio of the forced expiratory volume in one second to the forced vital capacity (348). While these findings may reflect a greater airway oxidative burden, they may also reflect differences in the sample processing methodology. Dithiothreitol, which is commonly used to minimize the viscosity of sputum samples, is a strong reducing agent and concentrations above 0.001% artificially increase total glutathione concentrations while decreasing GSSG (27). Sputum induction time (27) and sample storage conditions (26) may also alter GSH content. Thus, additional studies using standardized methodology are necessary before conclusions about the sputum GSH/GSSG redox state can be reached. Studies comparing GSH concentrations in sputum versus the epithelial lining fluid would also be of benefit.
Exhaled breath condensate, though extremely dilute given the high concentration of exhaled water, is another potential source of airway constituents. Previous studies have shown that GSH/GSSG redox balance can be measured in the exhaled breath with sensitive methods such as high performance liquid chromatography (353). Only two studies to date have assessed GSH in the exhaled breath condensate of subjects with asthma. In these studies, children with mild-to-moderate asthma had significantly lower concentrations of exhaled GSH than healthy controls who were further associated with increased malondialdehyde formation, a marker of lipid peroxidation (57, 70). Within the asthmatics, a 5-day course of systemic corticosteroids increased GSH and decreased malondialdehyde concentrations to levels observed in controls (57). These observations are similar to what we have previously observed in the epithelial lining fluid of asthmatic children (88) and highlight the potential utility of exhaled breath condensate in the assessment of airway GSH/GSSG redox status. Given the noninvasive nature of exhaled breath condensate, which is particularly advantageous for field studies and studies of subject populations (such as children) in whom bronchoscopy is not feasible, additional validation studies are needed. Although there are a number of methodological issues associated with exhaled breath condensate measurement, including the significant dilution of the sample and lack of normalization standards, the exhaled breath does contain leukotrienes, lipid peroxidation byproducts, prostaglandins, H2O2, nitric oxide oxidation products, and ionic constituents (116, 223). Therefore, it would be worth studying the effects of pharmacological asthma treatments on exhaled breath condensate concentrations of GSH/GSSG and 8-isoprostanes (226), a specific marker of lipid peroxidation, which has been associated with airway hyperreactivity (18) and increased asthma severity (286) in affected patients.
C. Systemic glutathione concentrations in asthma
Although several studies have examined systemic glutathione in subjects with asthma, these studies have yielded conflicting results given differences in the type of sample analyzed (i.e., whole blood, erythrocytes, and plasma) and the clinical characteristics of the subjects enrolled. For instance, in two studies, whole blood and erythrocyte total glutathione concentrations (measured as the sum total of the GSH and GSSG pools) were not significantly different between asthmatics and healthy controls at baseline (348) or during acute exacerbations (237). However, in both of these studies, the subjects enrolled were mild asthmatics who had achieved good symptom control on low doses of inhaled corticosteroids (237, 348). By contrast, two other studies of treatment-naïve asthmatics with mild airflow limitation (329) and asthmatics with current symptoms on inhaled corticosteroids (236) demonstrated increased whole blood and erythrocyte total glutathione concentrations in asthmatics versus controls. While previous findings of increased total glutathione in subjects with asthma may reflect an adaptive response whereby synthesis and mobilization of GSH is increased to combat acute insults, these previous findings are more likely due to increased GSSG formation (206) since protein –SHs are also reduced in these subjects (15, 236). Furthermore, in other studies, asthmatics with higher total glutathione concentrations also had decreased pulmonary function evidenced by reductions in the forced expiratory volume in one second (237, 243) and higher concentrations of systemic protein carbonylation and lipid peroxidation byproducts (236). Given the important role of GSH/GSSG redox in free radical scavenging, these findings likely represent increased GSSG formation or relative GSH depletion. However, GSH and GSSG were not measured in the majority of these studies, so the GSH/GSSG redox state cannot be estimated.
Although few studies have studied systemic GSH and GSSG in asthma, the few studies that have been conducted do reveal important insight on the systemic GSH/GSSG redox balance of these subjects. In a recent study, plasma GSH concentrations were decreased by nearly 1.5-fold in inhaled corticosteroid-naïve asymptomatic asthmatics compared to healthy controls (284). The decreased plasma GSH concentrations in these subjects were further associated with decreased expression of superoxide dismutase and increased lipid peroxidation byproducts (284). Although the asthmatics enrolled in this study did not have an obvious burden of respiratory symptoms, these findings suggest that even well-controlled asthmatics may have a significant oxidative burden, which may be a risk factor for future exacerbations or other respiratory complications. Other studies of poorly controlled asthmatics with obvious airway inflammation have likewise revealed lower systemic GSH concentrations (74, 293) and higher GSSG concentrations (206) in these subjects than in healthy controls. With acute asthma exacerbations, these GSH concentrations decreased further and were associated with an increased burden of free radicals and lipid peroxidation byproducts in the plasma and circulating cells (293). While the specific mechanisms underlying these GSH/GSSG redox disturbances are not clear, these findings highlight the role of GSH/GSSG redox in asthma control and suggest that systemic measures of GSH and GSSG, as opposed to total glutathione alone, may be particular relevance in these subjects. Given that GSH and GSSG are also differentially regulated between the plasma and circulating cells (156, 157), these findings also underscore the need for compartmentalized (i.e., plasma or erythrocyte vs. whole blood) assessment of both GSH and GSSG in asthma to approximate the underlying systemic GSH/GSSG redox state.
We recently published the first report of the GSH/GSSG redox state in children with mild-to-moderate asthma and severe asthma refractory to inhaled corticosteroids that included assessment of the GSH/GSSG redox potential (86). In this study, children with severe asthma had lower total glutathione (GSH+GSSG) concentrations in the plasma (0.6 μM) than children with mild-to-moderate asthma (1.7 μM) (86). Although nonasthmatic controls were not enrolled, the total glutathione concentrations observed in both groups of asthmatics were significantly less than the range of 2–4 μM previously reported for healthy adults (159, 160). Whereas plasma GSSG concentrations did not differentiate children with severe and mild-to-moderate asthma, children with severe asthma had twofold lower concentrations of GSH and a shift in the GSH/GSSG redox potential (Eh) by 30 mV to the more oxidized state (−94 vs.−120 mV for severe vs. mild-to-moderate asthma, respectively) (86). The total plasma cysteine pool (cysteine+CySS) was also significantly lower in children with severe (3.1 μM) versus mild-to-moderate asthma (4.5 μM) and was likewise associated with cysteine depletion and a shift by nearly 30 mV in the cysteine/CySS redox potential (Eh) (−62 vs.−89 mV for severe vs. mild-to-moderate asthma, respectively) (86). These findings were mirrored in the airway lavage fluid from these same children (86) (Fig. 12). Given that the extracellular GSH/GSSG and cysteine/CySS redox pools are under tight regulation during physiologic health (156, 157), these observations reflect a global disturbance in both thiol availability and thiol redox signaling in individuals with severe asthma that likely has important clinical and therapeutic implications.
FIG. 12.
Hypothesized continuum of glutathione redox disturbances in asthma. Values for GSH and the GSH/GSSG redox potential (Eh) were estimated from existing literature.
D. Glutathione redox balance in asthma: Effect of corticosteroids
Because inhaled corticosteroids are the cornerstone of treatment for persistent asthma (3), clinical studies of GSH/GSSG redox balance in subjects with asthma are frequently confounded by corticosteroid exposure since both GSH and corticosteroids exert broad anti-inflammatory effects. Therefore, it is not entirely clear how inhaled corticosteroids affect GSH/GSSG redox balance per se. In studies of corticosteroid-naïve children (129) and adults (253) with elevated systemic total glutathione levels (irrespective of GSH or GSSG content), treatment with inhaled corticosteroids for 6 to 8 weeks resulted in a significant reduction of total glutathione concentrations. These reductions mirrored improvements in asthma symptom control and were further accompanied by decreased blood eosinophils, decreased systemic markers of DNA oxidation, and increased systemic catalase activity, suggesting an attenuation of systemic oxidative stress with inhaled corticosteroid treatment (129, 253). Similarly, in vitro studies involving treatment of human airway epithelial cells with hydrocortisone resulted in a reduction in basal and tumor necrosis alpha-stimulated intracellular total glutathione content, although no detectable changes in GSSG were noted (266, 332). Whether corticosteroids decrease GSH synthesis in asthmatic patients is not entirely clear (265). Given previous observations of GSH depletion in severe asthma (86–88), a disorder necessitating treatment with high doses of corticosteroids, additional investigations of the effect of corticosteroids on GSH/GSSG redox balance are needed.
VII. Other Glutathione-Related Proteins and Redox Systems in Asthma
A number of other GSH-related proteins and redox systems are also present in the body and are important for antioxidant defense. These include glutathione peroxidases, glutathione reductases, glutathione-S-transferases, GSNO, thioredoxins, glutaredoxins, and peroxiredoxins. The relevance of these proteins and redox systems to asthma is discussed below.
A. Glutathione peroxidases
Glutathione peroxidases catalyze the reduction of hydroperoxides by GSH and are therefore essential for maintaining GSH/GSSG redox balance. Glutathione peroxidases have been extensively studied in the peripheral blood of subjects with asthma. Although some studies have found no differences in systemic glutathione peroxidase activity or protein expression between asthmatics and healthy controls (55, 207, 257, 312, 347), a few have noted increased glutathione peroxidase expression and activity in asthma subjects (130, 131, 148, 315). In these subjects, many of whom had milder forms of asthma, increases in glutathione peroxidase may reflect a beneficial physiological response to an increased hydroperoxide burden. However, when this burden is severe or chronically sustained, the functional utility of this enzyme can be exhausted as a function of GSH/GSSG redox disturbances (267). This likely explains the results of many studies that have shown decreased glutathione peroxidase activity in the circulating erythrocytes (165, 206, 254, 258, 284), leukocytes (236, 328), platelets (132, 222, 250), and whole blood (91) and plasma (263) of asthmatics versus controls. In keeping with this notion, systemic glutathione peroxidase activities have been shown to decrease as function of asthma severity (255) and during acute asthma exacerbations (31, 123, 293). Decreased glutathione peroxidase activities in subjects with asthma have also been associated with increased systemic lipid peroxidation and malondialdehyde formation (76, 123, 236) and lower pulmonary function (122). While treatment with inhaled corticosteroids alleviates airway inflammation, it has not been shown to increase systemic glutathione peroxidase activity in subjects with asthma (82, 253).
Similar to the systemic circulation, within the lung, investigators have not been able to consistently demonstrate alterations of glutathione peroxidase in asthmatic subjects. In three studies of mild asthmatics and healthy controls, no differences in glutathione peroxidase activity in airway cells or bronchoalveolar lavage fluid were observed at baseline (52, 62, 300) or after allergen challenge (52) despite significant differences in superoxide dismutase activity between groups. By contrast, Comhair et al. found increased expression of glutathione peroxidase in the epithelial lining fluid and bronchial epithelial cells of subjects with mild asthma compared to controls (53). In that study, increased expression and activity of glutathione peroxidase was attributed to increased gene expression from ROS exposure and GSH/GSSG redox alterations (53). While more studies are needed, these findings suggest that the airway GSH/GSSG redox balance may lead to induction of glutathione peroxidase transcription, protein expression, and secretion and activation in the airway. However, this response is likely to be overwhelmed by chronic and sustained GSH/GSSG airway redox alterations and GSH depletion. This might explain why we previously failed to observe differences in epithelial lining fluid glutathione peroxide activities between children with severe asthma and controls despite an increased oxidative burden in the severe asthmatic group (88).
B. Glutathione reductases
Glutathione reductase is a flavoprotein that catalyzes the reduction of GSSG to GSH. Few studies have assessed glutathione reductase in asthma. While three studies found no differences in glutathione reductase activity in the airway lavage cells (300), epithelial lining fluid (88), and erythrocytes (254) of asthmatics versus healthy controls, others have observed decreased glutathione reductase activity in erythrocytes from asymptomatic asthmatics as compared to controls (124, 208). In a separate study, erythrocyte glutathione reductase activity declined significantly in the asthmatics during acute exacerbations as a function of increased lipid peroxidation (123). Similar reductions in basal glutathione reductase activity have also been observed in subjects with chronic obstructive pulmonary disease (179) and chronic inhalation exposures (194). These findings may explain previous observations of GSH deficiency in asthmatics, particularly in those with severe disease. More studies are needed to understand the relationship of glutathione reductase activity to GSH/GSSG redox signaling in these individuals.
C. Glutathione-S-transferases
The glutathione-S-transferase supergene family, which contains enzymes of the mu (GSTM1), theta (GST1), and pi (GSTP1) classes, catalyzes the conjugation of GSH and is therefore critical for detoxification of endogenous compounds and xenobiotics. Much of the interest in glutathione-S-transferases in asthma stems from genetic polymorphism studies. In the original study, the frequency of the GSTP1 Val(105)/Val(105) genotype, the form with greater activity, was significantly lower in asthmatics (99). Furthermore, the GSTP1 Val(105)/Val(105) homozygotes had a reduced asthma risk of six- to ninefold and a decreased severity of airway dysfunction compared to GSTP1 Ile(105)/Ile(105) homozygotes (99). Other studies have also demonstrated associations between asthma and the GSTM1 null genotype (which lacks the GSTM1 gene) and the GSTT1 null genotype (163, 164, 280, 311).
Because these glutathione-S-transferase polymorphism studies included relatively small populations, a systematic review and meta-analysis were recently undertaken to better understand the associations between GSTM1, GSTT1, and GSTP1 polymorphisms and asthma in adults and children (221). These analyses did not identify a substantial role of glutathione-S-transferase genes in asthma risk given significant heterogeneity that existed between these studies (221). However, these analyses were limited to asthma risk and did not focus on the role of these genes in the severity of the disorder. Another important knowledge gap is how glutathione-S-transferase polymorphisms affect protein expression or activity of the glutathione-S-transferase enzymes or related proteins. Whereas one study observed decreased airway neutrophil flux in response to ozone exposure in GSTM1 null versus GSTM1 wild-type asthmatics (7), another failed to demonstrate differences in glutathione-S-transferase enzymatic activity in subjects with asthma stratified by genotype (205). That same study also observed higher total glutathione-S-transferase activity in asthmatics versus healthy controls (205), while others found no difference in glutathione-S-transferase activity between groups (84, 300). Further studies linking glutathione-S-transferase genotypes to protein expression and functional activity of the enzyme and related proteins are needed.
D. Nitrosoglutathione
Nitrosative stress, evidenced by increased formation of •NO and nitric oxide oxidation products, is a characteristic feature of the asthmatic airway and is particularly pronounced in subjects with severe versus mild-to-moderate asthma (71, 84, 204). This process may ultimately contribute to protein dysfunction and cellular destruction. In the airways, nitrosative stress has been associated with cellular apoptosis and necrosis as well as eosinophil recruitment, microvascular leakage, and airway hyperresponsiveness (10, 279). The physiological influence of nitric oxide (•NO) in the airways is largely due to post-translational modification of cysteine residues on proteins through S-nitrosylation (97). This process is primarily regulated by transfer of NO groups between proteins and GSH, which results in the formation of GSNO. Whereas excessive •NO and nitrosative stress may result in airway injury, GSNO is an endogenous bronchodilator associated with airway smooth muscle relaxation (79) that protects against methacholine-induced airway hyperresponsiveness (98, 261) and NF-κB activation during allergic airway inflammation (244). GSNO also suppresses 5-lipoxygenase, the rate limiting enzyme for the formation of the inflammatory and bronchoconstricting cysteinyl leukotrienes, in airway epithelial cells (357).
Although airway GSNO concentrations are relatively stable and range from 200 to 500 nM in healthy subjects (106, 107), in subjects with asthma, GSNO concentrations are significantly decreased (∼60 nM) (262) and are virtually undetectable during severe acute exacerbations resulting in respiratory failure (107). These GSNO reductions are associated with increased activity of the GSH-dependent formaldehyde dehydrogenase, class III alcohol dehydrogenase (also referred to as GSNO reductase) (262), which catalyzes the reduction of GSNO (192). Although the mechanisms underlying increased GSNO reductase activity in asthma are not clear, recent studies have demonstrated increased GSNO reductase activity in response to formaldehyde (303, 316), which is increased in the airways of asthmatics versus controls (as formate) and is associated with airway hyperreactivity and asthma severity in a concentration-dependent manner (116). Formaldehyde-induced GSNO depletion ultimately leads to the formation of glutathione sulfonamide and glutathione sulfinic acid, which inhibit glutathione-S-transferase activity (303). Alternatively, increased GSNO reductase activity in asthma may be related to underlying genetic polymorphisms in the GSNOR gene, which have been associated with an increased risk of asthma in children (349) and decreased bronchodilator responsiveness (48, 228). However, the functional relevance of these polymorphisms is not presently known.
E. Thioredoxins
Thioredoxins, including thioredoxin 1 and thioredoxin 2, are oxidoreductase enzymes that contain a dithiol (–SH HS–) group that forms a disulfide bridge (–S–S–) (139). Whereas thioredoxin 1 is found in the cytoplasm, thioredoxin 2 is located in the mitochondria (11). Both thioredoxins scavenge ROS and inhibit apoptosis (127, 139). Thioredoxins also play a key role in the reduction of methionine residues in proteins. Similar to protein –SH groups, protein methionine residues undergo oxidation to methionine sulfoxide (MetO) in efforts to protect critical residues at the active protein site from oxidative modification and inactivation (80, 201). MetO reductases catalyze the reduction of MetO back to methionine (339) and then undergo reduction by thioredoxins, thus readying the MetO reductase enzyme for another catalytic cycle. Thioredoxin reductases in the cytoplasm and mitochondria also maintain thioredoxins in a reduced state and function similarly to glutathione reductases.
Studies of thioredoxins in asthma are limited and the majority of work that has been conducted in focused on thioredoxin 1. In animal models of allergic airway inflammation, thioredoxin suppresses eosinophil influx and goblet cell hyperplasia and mucin production (144, 319) and further inhibits airway smooth muscle hyperplasia and airway hyperresponsiveness (144). Thioredoxin 1 also inhibits eotaxin and regulated on activation, normal T-cell expressed, and secreted (RANTES)-induced chemotaxis of eosinophils from human subjects (175). While studies in asthma are limited, one study noted increased serum concentrations of thioredoxin 1 in subjects with acute asthma exacerbations compared to asthmatics who were well controlled that were inversely correlated with lung function (350). A separate study of isolated T-lymphocytes from subjects with asthma further observed increased expression of thioredoxin 2 compared to healthy controls (150). However, the role of thioredoxins in the protection against oxidative stress in these subjects is not clear and warrants further study.
F. Glutaredoxins
Glutaredoxins are thiol-disulfide oxidoreductases that reduce protein mixed disulfides (protein-SSG) with glutathione through a process called deglutathionylation (219). Thus, the activity of glutaredoxins is entirely dependent upon GSH availability and the GSH/GSSG redox balance of the cell. Although there are four known glutaredoxins, the dithiols glutaredoxin 1 and glutaredoxin 2 are most commonly studied. Glutaredoxin 1 is a cytosolic protein, whereas glutaredoxin 2 is found in both the mitochondria and the nucleus. Both glutaredoxins exhibit deglutathionylating activity for peptide and protein substrates, but the activity of glutaredoxin 2 is ∼10-fold lower than that of glutaredoxin 2 (101, 200).
In mice, glutaredoxin 1 is associated with suppression of airway neutrophil influx and pro-inflammatory cytokine release as well as decreased activation of NF-κB in the lung (6, 49). Suppression of glutaredoxin 1 is further associated with impaired terminal differentiation of airway macrophages resulting in impaired phagocytic function and altered inflammatory mediator release (5). In experimental models of asthma induced by ovalbumin sensitization, glutaredoxin 1 is increased after allergen challenge while glutaredoxin 2 is not altered (276). However, glutaredoxin 1 decreased significantly in response to TGFβ1 (276), which is commonly found in the asthmatic airway (145). While human studies of asthma are not available, these findings suggest that glutaredoxin1 inhibition may play an important role in asthma-related oxidative stress and airway structural changes given its suppression by TGFβ1. Other studies of adults with fibrotic airway disorders (251) and chronic obstructive pulmonary disease (252) have demonstrated reduced glutaredoxin 1 expression in bronchial epithelium and airway macrophages that associated with increased airflow limitation. Additional studies in asthma are needed.
G. Peroxiredoxins
Peroxiredoxins are nonseleno peroxidases with a wide spectrum of activity against organic hydroperoxides and OONO–. There are six mammalian peroxiredoxins, which are located throughout the cytosol, mitochondria, and peroxisomes (278) and protect the lung from oxidative injury and oxidant-mediated cell death (190, 335). Within the human lung, peroxiredoxins are expressed in abundance in critical areas of oxidant protection, including alveolar macrophages, bronchial epithelial cells, and the alveolar epithelium (169). Although expression of peroxiredoxins initially increases with oxidative stress (167, 186), severe and sustained oxidative stress can lead to over-oxidation and inactivation of the enzyme (186, 278). However, the role of peroxiredoxins in asthma and other lung disorders is not understood. In murine models of allergic airway inflammation, peroxiredoxin 1 and peroxiredoxin 2 are increased in airway and lung tissue of sensitized mice (78, 146, 227). Ablation of peroxiredoxin 1 and 2 is further associated with increased T helper type 2 (Th2)-mediated cytokine release, airway hyperresponsiveness to methacholine, B-cell activation, and T-cell proliferation (146, 227), suggesting that peroxiredoxins are central to the airway antigenic response. Further studies in asthma are needed.
VIII. Physiological and Biological Implications of Altered Glutathione Redox Balance in Asthma
Despite accumulating evidence that the GSH/GSSG redox balance is altered in the airways and possibly even the systemic circulation of subjects with asthma, the functional and ultimately clinical relevance of the altered GSH/GSSG redox balance in asthma is not entirely clear. Several in vitro studies have demonstrated that physiologic concentrations of airway GSH (∼500 μM) are important for maintaining the structure, function, and defenses of airway epithelial cells. Upon exposure to endogenous or environmental toxins or respiratory viruses, GSH content decreases in alveolar epithelial cells in both a dose-dependent manner and a time-dependent manner (152, 189, 235) and is associated with increased apoptosis (344), increased pro-inflammatory cytokine and chemokine release (152), and increased superoxide production (248). In otherwise healthy cells, other defenses help restore airway GSH pools to minimize these adverse effects. These include increased expression of glutamate cysteine ligase (also referred to as γ-glutamylcysteine synthetase) to promote GSH synthesis (272), upregulation of glutaredoxins that catalyze the reduction of S-glutathionylated proteins in the mitochondrial membrane (22), and increased expression and activation of the transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which regulates the expression of several genes essential for GSH synthesis and homeostasis (188). However, these mechanisms may be overwhelmed in asthma, a disorder characterized by chronic airway inflammation. For instance, glutamate cysteine ligase activity in airway epithelial cells may be sensitive to airway thiol deficiency (271), which is particularly pronounced in subjects with severe asthma (86). Nrf2 activity is also altered in cases of severe asthma (86), potentially resulting in increased expression of Th2-related cytokines and other inflammatory features that accompany the disorder (4, 270, 343).
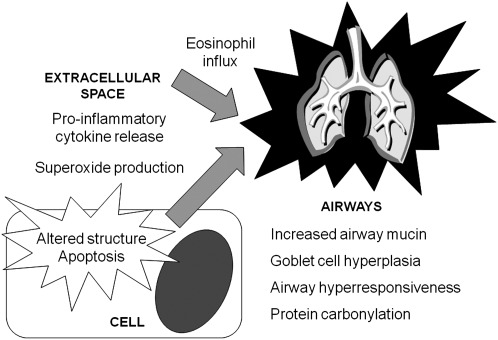
Because human studies are lacking, the functional consequences of the GSH/GSSG redox imbalance in the asthmatic airway have been studied through the use of several animal models. In these studies, guinea pigs and BALB/c mice with allergic airway disease induced by ovalbumin sensitization had significantly lower total lung thiol content, with up to 40% lower GSH concentrations and threefold higher concentrations of GSSG in the lung tissue homogenate (172, 177). The decreased whole lung GSH levels in these animals were further accompanied by a Th2-mediated inflammatory response evidenced by increased airway eosinophil influx; higher concentrations of interleukin (IL)-4, IL-5, IL-10, eotaxin, and RANTES; and decreased concentrations of IL-12 and interferon gamma in the bronchoalveolar lavage (177). In a similar study of BALB/c mice sensitized to ovalbumin, reductions in the GSH/GSSG ratio in lung tissue after ovalbumin challenge were also associated with increased airway mucin production and airway hyperresponsiveness, which persisted for several days after the initial exposure (249). The ability of GSH/GSSG to regulate airway tone was further suggested in a recent study where γ-glutamyl transferase-deficient mice (with high extracellular GSH concentrations) were protected from methacholine-induced airway hyperreactivity (197). In that study, the increased airway GSH content was also associated with decreased airway mucin production, decreased mucous cell hyperplasia, and decreased protein carbonylation after a secondary insult with IL-13 (197). These findings suggest that the airway GSH/GSSG redox balance may play an important role in asthma independent of Th2-mediated inflammation (Fig. 13). Additional functional studies of GSH and airway structure and function are needed.
FIG. 13.
Consequences of altered GSH/GSSG redox balance in asthma.
IX. Altered Glutathione Redox Balance in Asthma: Therapeutic Opportunities
Current treatment paradigms for asthma are focused on relaxation of airway smooth muscle and suppression of airway inflammation. These effects are achieved primary with bronchodilators and inhaled corticosteroids, which are the cornerstone of asthma therapy given their widespread effectiveness in most asthma populations (3). However, in patients with severe asthma that is refractory to inhaled corticosteroid treatment, or in other subpopulations of asthmatics such as smokers, obese asthmatics, or asthmatics with nutrient deficiencies, other interventions may also be warranted to restore the GSH/GSSG redox balance. To this end, several studies have attempted to increase the GSH pool in subjects with asthma through direct delivery of GSH or GSH-ethyl esters. Because cysteine is the functional unit of GSH, studies have also attempted to increase cysteine pools through delivery of cysteine precursors. Others have focused on dietary interventions for subjects with asthma to increase sulfur amino acid and micronutrient availability to restore GSH/GSSG redox balance. These are discussed below.
A. Glutathione and glutathione-ethyl esters
GSH-ethyl esters are efficiently transported into the cell, where they are hydrolyzed to GSH (288). For that reason several studies have used GSH-ethyl esters as opposed to GSH to restore the airway GSH/GSSG redox balance. In one such study, significant reductions in airway inflammatory cell infiltration (including eosinophil chemotaxis) and airway pro-inflammatory cytokine synthesis were observed in ovalbumin-sensitized mice with intraperitoneal GSH-ethyl ester administration (177). GSH-ethyl esters administered to the airways through aerosolization or direct instillation (after isolation) also decreased ovalbumin-induced airway methacholine hyperresponsiveness, thus blunting the airway obstruction that is typically observed during an acute asthma response (172, 173). In another study, direct GSH treatment of isolated bronchi from ovalbumin-sensitized guinea pigs in vitro resulted in similar decreases in smooth muscle contraction and bronchodilation (47). These observations suggest a possible therapeutic role for GSH and GSH donors in the management of airway inflammation and obstruction.
In light of these observations, two studies have attempted to administer GSH via inhalation to subjects with asthma. In the first study, a 600-mg glutathione solution (composition unclear) was administered by nebulization to 12 young adults with mild-to-moderate asthma in a double-blind, placebo-controlled, cross-over study (16). Subjects with asthma were pretreated with the glutathione solution or placebo and then immediately underwent challenge with ultrasonically nebulized distilled water. Compared to placebo, the glutathione solution attenuated the fall in the forced expiratory volume in one second resulting from the challenge (6.04 L decline vs. 20.41 L decline for glutathione solution vs. placebo) (16). However, in another study involving eight adults with mild asthma, a similar glutathione solution (600 mg, composition unknown) administered by nebulization resulted in increased airway resistance, decreased pulmonary function measured by the forced expiratory volume in one second, and increased asthma symptoms, including severe wheezing and breathlessness (n=1), breathlessness (n=2), and cough (n=4) (213). The reasons underlying the bronchoconstricting effect of the glutathione solution are not clear but may be related to sulfite sensitivity since the hypertonic acid solution that served as the placebo resulted in cough in only one subject (213). These findings are in contrast with those from other studies, which did not reveal toxic or adverse effects with similar inhaled glutathione solutions (300–600 mg) administered to subjects with cystic fibrosis (32, 118, 128, 283), chronic otitis media (314), idiopathic pulmonary fibrosis (39), human immunodeficiency virus (138), and chronic rhinitis (313). In those studies, nebulization of the glutathione solution resulted in lower airway GSH deposition (118) in treated subjects with a significant increase in epithelial lining fluid GSH concentrations (39, 118, 138, 283). Increased airway GSH availability in those studies was further associated with decreased O•2− release by airway alveolar macrophages (39, 283), decreased airway prostaglandin E2 synthesis (128), and increased circulating T cells in the bronchoalveolar lavage of treated subjects (128). A small randomized, double-blind, placebo-controlled pilot study of glutathione inhalation in children with cystic fibrosis also demonstrated greater peak expiratory flows and improved respiratory symptoms in the glutathione-treated group (32). While meta-analyses have found no evidence to recommend the routine clinical use of nebulized glutathione or thiol derivatives (240), these analyses were limited by the quality of the trials conducted. Given the profound GSH/GSSG balance that accompanies asthma, further research on the safety and efficacy of inhaled glutathione solutions for asthma may be warranted.
B. Cysteine precursors
N-acetylcysteine and L-2 oxothiazolidine-4-carboxylic acid are examples of cysteine precursors that have been used in animal models of allergic asthma. These studies have shown that N-acetylcysteine, when administered before ovalbumin sensitization, maintains lung GSH/GSSG homeostasis and attenuates lung protein carbonylation and lipid peroxidation after a secondary insult from diesel exhaust or antigen exposure (37, 289, 342). In sensitized animals, N-acetylcysteine further attenuates many of the characteristic features of asthma, including airway cellular infiltration, airway mucin and pro-inflammatory cytokine expression, and airway hyperresponsiveness (37, 249). These beneficial effects on airway inflammation and tone have also been noted after administration of N-acetylcysteine amide and N-acetyl cysteine proline cysteine amide, which have increased membrane permeability and greater reducing activity than N-acetylcysteine (168, 183). Pretreatment of sensitized animals with L-2-oxothiazolidine-4-carboxylic acid has also yielded similar results (184, 185), suggesting that repletion of cysteine pools may be of benefit in asthma.
Although the clinical efficacy of cysteine precursors has not been explored in asthma, several studies have focused on the role N-acetylcysteine in subjects with chronic obstructive pulmonary disease. Although chronic obstructive pulmonary disease is distinct from asthma, it is increasingly recognized that cigarette smoking is not the sole factor underlying the epidemiology and natural history of the disorder (73). Indeed, asthma and chronic obstructive pulmonary disease share a number of clinical features, including airway inflammation, airflow limitation, and respiratory exacerbations (110). In a systematic review of eleven placebo-controlled trials involving subjects with chronic obstructive pulmonary disease, oral N-acetylcysteine at a dose of 400–600 mg was more efficacious than placebo in reducing respiratory symptoms, with no significant adverse effects (305). A similar meta-analysis also showed a 23% reduction with N-acetylcysteine versus placebo in the number of exacerbations over 6 months (115). However, in contrast to these findings, a more recent large-scale randomized trial found no differences in the number of chronic obstructive pulmonary disease exacerbations per year or the yearly rate of decline in the forced expiratory volume in one second when 600 mg of N-acetylcysteine was taken orally once daily (63). This finding may reflect confounding by inhaled corticosteroids since in secondary analyses, the number of exacerbations was significantly lower with N-acetylcysteine treatment in subjects not taking inhaled corticosteroids (risk ratio 0.79 vs. 0.99 overall) (63). Alternatively, this finding may reflect inadequate dosing of N-acetylcysteine, since up to 1800 mg daily may be necessary to increase plasma GSH levels by 50% (41) due to its limited systemic bioavailability (64). Indeed, in a smaller study of critically ill subjects with chronic obstructive pulmonary disease exacerbations who were treated with supplemental oxygen, daily oral administration of 1200 to 1800 mg of N-acetylcysteine preserved erythrocyte GSH concentrations and attenuated systemic levels of oxidized protein as compared to placebo (96). A separate double-blind, cross-over study also found less air trapping and greater exercise performance in subjects with chronic obstructive pulmonary disease when N-acetylcysteine was administered at a daily 1200-mg dose (304).
The mechanisms underlying the benefits of N-acetylcysteine in subjects with chronic obstructive pulmonary disease are not completely understood. It is certainly possible that N-acetylcysteine may not be functioning as a GSH precursor, but rather as a mucolytic in these subjects. It is also possible that the benefits are only present in selected subpopulations. For example, in critically ill subjects or subjects with protein-energy malnutrition from catabolic disease (with increased energy expenditure), N-acetylcysteine supplementation may simply restore sulfur amino acid availability from decreased sulfur amino acid intake. However, 99% of adult males and females in the United States consume greater than the recommended daily intake for sulfur amino acids, while 50% consume more than twice the recommended intake (90), suggesting that additional approaches may be needed to address a functional need for GSH in most Americans.
C. Dietary interventions
The specific role of the diet in maintaining or restoring GSH/GSSG redox balance has not been studied in asthma. However, given that GSH is synthesized from sulfur amino acid metabolism, increased dietary intake of methionine and cysteine may be of benefit in subjects with asthma, particularly those with severe disease in whom GSH stores are low. Other dietary components relevant to GSH synthesis and function, such as selenium, may also be beneficial. The potential role of the diet as a complementary therapy for modification of the GSH/GSSG redox balance in asthma is discussed below.
1. Selenium
Selenium is an essential trace element that plays a vital role in glutathione peroxidase activity. The mechanism by which the glutathione peroxidases catalyzes the reduction of hydroperoxides by GSH involves it selenocysteine (selenol) residue. The selenol is oxidized to selenenic acid by hydroperoxides, which then undergoes a reaction with GSH to form a selenenyl sulfide adduct. The selenenyl adduct reacts with another GSH molecule to regenerate the selenol, releasing GSSG as a byproduct (Fig. 14).
FIG. 14.
Role of selenium in glutathione peroxidase activity. The selenol residue (–SeH) of glutathione peroxidase is converted to a selenenic acid in the presence of hydroperoxides (ROOH). GSSG is formed from the reduction of selenenyl sulfide.
Given the important role of selenium in maintaining GSH/GSSG redox balance, dietary selenium requirements are based on the minimum intakes thought to be necessary for the maximization of glutathione peroxidase activities (317). Although true selenium deficiency is rare, low selenium intakes have been associated with a number of chronic diseases, including asthma (77). Several studies have demonstrated lower selenium concentrations in the plasma, whole blood, and erythrocytes of asthmatics compared to healthy controls (91, 165, 307). In these studies, subjects with the lowest selenium concentrations had a twofold increase in the relative risk of asthma (91) and lower activities of the enzymes glutathione peroxidase (124, 222), glutathione reductase (124), and catalase (124) associated with a significant reduction in total antioxidant defense (176). Furthermore, in mild-to-moderate asthmatics with airflow limitation, low plasma selenium concentrations were also associated with greater lipid peroxidation byproducts, increased concentrations of high-sensitivity C-reactive protein, and an increased ratio of CD4+/CD8+ lymphocytes, suggesting a shift in Th2 polarization (124). These findings were further accompanied by increased concentrations of the trace element, copper (124). Because copper may complex with GSH, resulting in a complex that reacts with oxygen and generates O•2− (302), these findings merit further investigation and may account for decreased copper and zinc superoxide dismutase activities in asthmatics versus controls (62).
Similar associations between selenium and asthma have been observed in mice with allergic airway sensitization induced by ovalbumin sensitization (137, 149). In these studies, mice fed a low-selenium diet for 8 weeks before ovalbumin exposure had significantly lower erythrocyte glutathione peroxidase enzymatic activities than mice fed a high-selenium diet (137). Supplementation with high doses of selenium before ovalbumin sensitization further inhibited airway eosinophil recruitment and the expression of inflammatory proteins in these animals (137, 149), suggesting a potential therapeutic role for selenium in asthma.
In keeping with the animal and human observational findings, one small placebo-controlled trial involving 24 subjects with asthma noted increased serum selenium concentrations in asthmatics treated with daily oral supplementation of 100 mcg of sodium selenite versus placebo for 14 weeks (133). Glutathione peroxidase activities were also increased in the selenium-treated versus the placebo-treated group and were associated with subjective measures of clinical improvement (133). However, the direct benefit of selenium treatment in asthmatic subjects could not be validated in a larger meta-analysis of these results since other objective parameters of lung function and airway hyperresponsiveness were unchanged (8). Furthermore, in a larger randomized, double-blind, placebo-controlled trial of selenium supplementation in 197 participants with asthma, 100 mcg of selenium per day for a treatment period of 24 weeks increased plasma selenium concentrations but failed to improve quality of life or other secondary measures of lung function, airway hyperreactivity, and symptoms when compared to the placebo-treated group (291). These results are in keeping with those of a more recent case–case control study and meta-analysis that found no overall associations between asthma and plasma selenium in a large sample of subjects (45, 242). While these findings may suggest a limited role for selenium supplementation in asthma, confounding from asthma therapy or other exposures such as cigarette smoking may be responsible. It is also possible that the benefit of selenium supplementation is additive and is only observed when taken in combination with other antioxidant or dietary therapies (81). Alternatively, the outcome indicators may not have been of sufficient sensitivity to reflect changes in GSH/GSSG redox balance.
2. Whey protein
Because oral GSH preparations may not effectively increase GSH tissue concentrations due to limited tissue availability (9), some studies have used whey protein preparations rich in cysteine and γ-glutamyl-glycine to increase GSH levels in vivo. These whey-based products have been shown to increase concentrations of GSH in the liver of supplemented animals (171). In humans, whey products also increase circulating lymphocyte and plasma GSH concentrations in healthy subjects (180, 358) and GSH-deficient subjects with cystic fibrosis (117) and human immunodeficiency virus (218). However, studies in asthma are limited and have shown mixed results. While a small study in atopic children with asthma failed to discern significant differences in systemic GSH concentrations with whey protein supplementation (195), a separate study of subjects with exercise induced bronchospasm noted an attenuation of lung function decline after exercise with whey supplementation as compared to placebo (20). A similar study also found increased endurance and decreased exercise-related dyspnea and fatigue with whey protein versus placebo in subjects with chronic obstructive pulmonary disease (181). Because these products contained multiple ingredients, the exact source of the derived benefit is unclear. However, the findings are intriguing and suggest a possible role for whey-based supplemental therapies in asthma. Further studies are needed.
3. Sulfur amino acids
Cysteine is a vital component of GSH. While cysteine is primarily produced from metabolism of the essential sulfur amino acid, methionine, it can also exist independently. Cysteine is released from the breakdown of endogenous proteins (100, 202, 264) and is also ingested from the diet along with methionine. Thus, although methionine serves as the primary source of sulfur for cysteine, dietary cysteine requirements are calculated with the assumption that methionine cannot supply the entire bodily cysteine demand. For healthy adults, the recommended dietary allowance for methionine plus cysteine is 19 mg/kg per day (1140 mg/day for a 60 kg adult) (93). Animal proteins are the richest dietary source (Table 2) (322). These sulfur amino acid requirements are based on classical experiments focused on the minimal intake of methionine for nitrogen balance (68, 281, 355). While total daily sulfur amino acid intake requirements are higher in infants and young children (67, 220), the requirements for older children are similar to adults (321).
Table 2.
Selected Dietary Sources of Sulfur Amino Acids (Methionine and Cysteine)
| Food | Serving | Protein (g) | Methionine and Cysteine (mg)a |
|---|---|---|---|
| Tomato | 1 medium (123 g) | 1.1 | 18 |
| Banana | 1 medium (118 g) | 1.3 | 20 |
| Orange | 1 medium (131 g) | 1.2 | 39 |
| Carrot | 1 small (50 g) | 0.5 | 52 |
| Kale, cooked, shredded | 1 cup (130 g) | 2.5 | 56 |
| Broccoli, cooked, chopped | 1 cup (156 g) | 3.6 | 88 |
| Baked potato | 1 medium (173 g) | 4.3 | 121 |
| Bread, whole wheat | 2 slices (56 g) | 7.3 | 130 |
| Kidney beans, cooked | ½ cup (89 g) | 7.7 | 198 |
| Peanuts, dry roasted | 1/3 cup (48 g) | 11.4 | 286 |
| Oatmeal, boiled | 1 cup (234 g) | 5.9 | 335 |
| Egg, hard boiled | 1 large (50 g) | 6.3 | 342 |
| Milk, 2% fat | 1 cup (244 g) | 8.1 | 464 |
| Chicken, roasted | 1 leg (52 g) | 14.1 | 562 |
| Ground beef, 15% fat, pan-broiled | 3 ounces (85 g) | 20.9 | 745 |
| Tuna | 3 ounces (85 g) | 21.7 | 874 |
Although sulfur amino demands are greater in critically ill subjects or subjects with metabolic stress or cancer-related cachexia (325), whether these demands are similar in subjects with chronic diseases such as asthma is not clear. With low sulfur amino acid intake, cellular methionine is conserved and cysteine stores are utilized to maintain protein synthesis (121, 281). This accumulation of methionine is pro-inflammatory and is associated with inactivation of proteins by homocysteinylation, which is harmful to cardiovascular health (216, 290). Furthermore, in cases of severe protein-energy malnutrition, the reduced sulfur amino acid intake results in decreased GSH synthesis and lower circulating GSH concentrations from decreased cysteine bioavailability (274), which may have detrimental immunomodulatory effects (121). By contrast, consumption of the recommended intake of dietary protein maintains circulating GSH and cysteine pools (147).
Whether the current recommendations for dietary sulfur amino acid intake are sufficient for chronic inflammatory diseases such as asthma is not entirely clear. It is possible that the supply of cysteine from recommended intakes is not sufficient for the maintenance of GSH concentrations and GSH/GSSG redox balance, particularly in cases of severe asthma associated with significant GSH depletion (86, 87). In these individuals and possibly other healthy adults such as athletes with higher protein demands, total sulfur amino acid intakes of up to 25 mg/kg/day may be necessary to maintain nitrogen balance (308, 309, 356). However, excessive intakes should be avoided since high dietary intakes of methionine can also raise plasma homocysteine concentrations (69, 327, 336) and alter immune function (121, 325).
Surprisingly few studies have focused on the dietary composition of subjects with asthma. Given that GSH levels are low in asthma, particularly in cases of severe disease, insufficient methionine intake or alterations of sulfur amino acid metabolism could be responsible. According to the most recent Youth Risk Behavior Surveillance Report (72), 78% of high school students (including students with asthma) had not eaten fruits or vegetables five or more times per day and 82% were not physically active. Furthermore, 48% of children 10 to 12 years of age had salty snack consumption at least one time per week that was primarily associated with television or video game viewing (13). In these children, consumption of salty snacks more than three times per week was associated with a nearly fivefold increased likelihood of asthma symptoms and increased sedentary behavior (13). By contrast, children more adherent to a Mediterranean-style diet characterized by a high intake of fruit, vegetables, and lean proteins had a decreased likelihood of asthma symptoms (13). The benefits of the Mediterranean diet in asthma have also been supported in other large cross-sectional studies spanning several countries (102, 239, 326) and in a recent meta-analysis focused on asthma prevention (242). While it is difficult to determine whether single or multiple nutrients are responsible for these results, a recent study observed fewer asthma symptoms in children with increased fish and meat intake (12), suggesting that dietary sulfur amino acids may play an important role. Studies focused on the role dietary sulfur amino acids in asthma are ultimately needed.
4. B vitamins
Of the B vitamins, vitamin B2 (riboflavin), B6, and B12 are relevant to GSH synthesis and function. Riboflavin is an essential cofactor of flavin adenine dinucleotide (FAD), which carries high-energy electrons for oxidative phosphorylation. The glutathione reductase enzyme, which is necessary for reduction of GSSG to GSH, forms a FAD-bound heterodimer. Glutathione reductase functions through reduction of FAD by NAPDH. Thus, glutathione reductase activity reflected by the erythrocyte glutathione reductase activity coefficient is frequently used as a measure of riboflavin status (135, 287).
Although riboflavin deficiency is rare in Western societies, riboflavin status may be suboptimal in certain populations, such as the elderly (38, 104). Riboflavin supplementation improves riboflavin status in these individuals and others with marginal deficiency (104, 141, 259). While the functional relevance of suboptimal riboflavin status in otherwise healthy individuals is not clear, in vitro studies have shown increased protein carbonylation, DNA strand breaks, and cellular stress and apoptosis with riboflavin deficiency associated with little to no glutathione reductase activity (211, 340). These effects are similar to findings observed in subjects with severe asthma and GSH depletion (87). Whether riboflavin status is similarly altered in subjects with asthma is not clear.
Vitamin B6 and vitamin B12 intake may also be relevant to GSH/GSSG redox balance, particularly in terms of methionine metabolism and cysteine synthesis. Vitamin B6 functions as a cofactor for cystathionine β-synthase and is therefore essential for the conversion of homocysteine to cystathionine. Vitamin B12 likewise functions as a cofactor for methionine synthase (also referred to as 5-methyltetrahydrofolate-homocysteine methyltransferase), which is essential for remethylation of homocysteine to methionine (Fig. 15). Deficiencies of either vitamin B6 or vitamin B12 result in altered homocysteine metabolism and increased homocysteine concentrations (136, 323), which are detrimental to cardiovascular health (2, 216, 331).
FIG. 15.
Role of vitamin B6 and vitamin B12 in maintaining homeocysteine balance and cysteine availability. Vitamin B6 is essential for the conversion of homocysteine to cystathionine. Vitamin B12 likewise functions as a cofactor for the re-methylation of homocysteine to methionine.
Vitamin B6 and vitamin B12 have not been well studied in asthma. Although vitamin B12 levels can vary by genotype, in a recent population-based study, neither vitamin B12 serum levels nor genotype was associated with lung function or asthma (318). However, folate deficiency was associated with an increased prevalence of physician-diagnosed asthma and asthma symptoms, suggesting a possible role of B vitamins in the disorder (318). In keeping with this notion, older studies of children with asthma have observed alternations in tryptophan metabolism that were reversed by vitamin B6 supplementation (50, 51). While one study found no evidence of obvious vitamin B6 deficiency in asthmatic children (125), others have shown decreased plasma and erythrocyte vitamin B6 levels in asthmatics versus controls that were not entirely normalized with vitamin B6 supplementation (65, 277). However, the subjects in these studies were likely treated with the bronchodilator, theophylline, which is a vitamin B6 antagonist and results in decreased circulating vitamin B6 levels in treated subjects (294, 324). Because theophylline is no longer widely used or recommended for the treatment of asthma given its potential for toxicity, additional studies in asthma are needed. In a more recent study of otherwise healthy volunteers, administration of a cereal fortified with recommended intakes of folic acid, vitamin B6, and vitamin B12 led to significant increases in plasma vitamin B6 and vitamin B12 concentrations and beneficial reductions in homocysteine (320). These findings suggest that vitamin B6 and vitamin B12 status may have important implications for inflammation and health even in absence of obvious deficiency.
5. Glutamine and glycine
Like cysteine, glycine and glutamate are also essential for GSH synthesis and ultimately GSH/GSSG redox balance. While few studies have examined glycine and glutamate in subjects with asthma, a case–control study of adult subjects 18–65 years of age found significantly lower concentrations of glycine in the plasma of asthmatics versus controls (92). Furthermore, the odds of asthma diagnosis were significantly reduced for subjects with the highest plasma glycine concentrations (odds ratio 0.30) (92). A trend toward asthma protection was also noted for subjects with higher concentrations of glutamine (odds ratio 0.44), which is synthesized from glutamate and ammonia (92). These observations were supported by a separate study in children, which also found significantly lower plasma concentrations of glycine and glutamate in mild asthmatics than in healthy controls (284). These findings may have important clinical ramifications. In vitro studies have shown that glycine inhibits production of superoxide and tumor necrosis alpha from alveolar macrophages (341) and decreases the contraction of airway smooth muscle (354). Other studies have also demonstrated inhibition of Th2-mediated pro-inflammatory cytokine expression and airway hyperresponsiveness in murine models of allergic asthma induced by ovalbumin sensitization (174, 182). Taken together, these findings suggest a beneficial role of glutamate and glycine in asthma and further study of these amino acids is warranted.
X. Clinical Implications and Future Directions
Despite advances in the understanding and treatment of asthma over the past decade, asthma remains a challenging disorder associated with a significant degree of respiratory morbidity. While the specific mechanisms underlying asthma have yet to be identified, there is accumulating evidence that oxidative stress from altered GSH/GSSG redox status may play an important role in the modulation and severity of the disorder. In more severe patients in whom airway GSH reserves are depleted, this may result in a detrimental cascade with a shift toward GSSG formation and oxidation of thioredoxins, leading to post-translational protein modifications through oxidation of protein-methionine residues and formation of protein disulfides from glutathionylation or other mechanisms (Fig. 16). Ultimately, these disturbances may render the functional activity of other glutathione-related enzymes insufficient and increase susceptibility to oxidant-induced airway injury. However, although GSH plays an essential role of GSH in redox signaling, detoxification, and other vital cellular processes, the functional and clinical ramifications of GSH/GSSG redox alterations in subjects with asthma are not clear and additional studies are clearly warranted in this population. There is a particular need for human studies focused on the following: (i) validation of airway and systemic GSH/GSSG redox balance as a biomarker of asthma susceptibility, (ii) the natural history of GSH/GSSG redox balance in asthma across the lifespan, (iii) the specific role of GSH/GSSG redox balance in clinical manifestations of the disorder, including asthma severity and the response to secondary insults, (iv) the utility of GSH/GSSG redox balance as a predictor and indictor of asthma treatment responsiveness, and (v) therapeutic approaches to restore the GSH/GSSG redox balance in asthma and modify the course of the disorder. The latter may be particularly relevant in subpopulations of asthmatics given the marked clinical heterogeneity of the disorder.
FIG. 16.
Molecular modulation of airway thiol redox balance and possible role in asthma. In subjects with asthma, decreased airway-reduced GSH reserves may result in a detrimental cascade whereby concentrations of GSSG and oxidized thioredoxin (TRX) are increased and significant post-translational modification of proteins occurs (with excessive oxidation of protein-methionine residues [protein-MetO] and protein disulfide [protein-SS] formation from glutathionylation or other mechanisms). These effects may ultimately overwhelm the functional activities of GPX, glutathione reductases, peroxiredoxins (PRX), thioredoxin reductases, and glutaredoxins (GRX), resulting in insufficient oxidant defenses and characteristic features of asthma.
Abbreviations Used
- BAL
bronchoalveolar lavage
- CySS
cystine
- DNTB
5,5′-dithiobis-(2-nitrobenzoic acid)
- ELF
epithelial lining fluid
- FAD
flavin adenine dinucleotide
- GPX
glutathione peroxidases
- GR
glutathione reductase
- GRX
glutaredoxins
- GSH
glutathione, reduced (thiol) form
- GSNO
nitrosoglutathione
- GSSG
glutathione disulfide
- GST
glutathione-S-transferase
- H2O2
hydrogen peroxide
- HPLC
high-performance liquid chromatography
- IL
interleukin
- MetO
methionine sulfoxide
- MMA
mild-to-moderate asthma
- NF-κB
nuclear factor kappa B
- •NO
nitric oxide radical
- NO2−
nitrite
- NO3−
nitrate
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- O•2−
superoxide anion
- ONOOH
peroxynitrous acid
- OONO-
peroxynitrite
- PRX
peroxiredoxins
- RANTES
regulated on activation, normal T-cell expressed and secreted
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SA
severe asthma
- -SeH
selenocysteine (selenol) residue
- SeOH
selenenic acid
- SeSG
selenenyl sulfide adduct
- -SG
reactive glutathione
- -SH
sulfhydryl
- SOD
superoxide dismutase
- TGFβ1
transforming growth factor beta-1
- Th2
T helper type 2
- TRX
thioredoxin
Acknowledgment
This work was supported in part by RO1 NR012021.
References
- 1.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 2.Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Adenuga D. Caito S. Yao H. Sundar IK. Hwang JW. Chung S. Rahman I. Nrf2 deficiency influences susceptibility to steroid resistance via HDAC2 reduction. Biochem Biophys Res Commun. 2010;403:452–456. doi: 10.1016/j.bbrc.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aesif SW. Anathy V. Kuipers I. Guala AS. Reiss JN. Ho YS. Janssen-Heininger YM. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol. 2011;44:491–499. doi: 10.1165/rcmb.2009-0136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aesif SW. Kuipers I. van der Velden J. Tully JE. Guala AS. Anathy V. Sheely JI. Reynaert NL. Wouters EF. van der Vliet A. Janssen-Heininger YM. Activation of the glutaredoxin-1 gene by nuclear factor kappaB enhances signaling. Free Radic Biol Med. 2011;51:1249–1257. doi: 10.1016/j.freeradbiomed.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexis NE. Zhou H. Lay JC. Harris B. Hernandez ML. Lu TS. Bromberg PA. Diaz-Sanchez D. Devlin RB. Kleeberger SR. Peden DB. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. J Allergy Clin Immunol. 2009;124:1222–1228. doi: 10.1016/j.jaci.2009.07.036. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allam MF. Lucane RA. Selenium supplementation for asthma. Cochrane Database Syst Rev. 2004;2:CD003538. doi: 10.1002/14651858.CD003538.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson ME. Glutathione and glutathione delivery compounds. Adv Pharmacol. 1997;38:65–78. doi: 10.1016/s1054-3589(08)60979-5. [DOI] [PubMed] [Google Scholar]
- 10.Andreadis AA. Hazen SL. Comhair SA. Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 11.Arner ES. Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 12.Arvaniti F. Priftis KN. Papadimitriou A. Papadopoulos M. Roma E. Kapsokefalou M. Anthracopoulos MB. Panagiotakos DB. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: the PANACEA study. Pediatr Allergy Immunol. 2011;22:283–289. doi: 10.1111/j.1399-3038.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Arvaniti F. Priftis KN. Papadimitriou A. Yiallouros P. Kapsokefalou M. Anthracopoulos MB. Panagiotakos DB. Salty-snack eating, television or video-game viewing, and asthma symptoms among 10- to 12-year-old children: the PANACEA study. J Am Diet Assoc. 2011;111:251–257. doi: 10.1016/j.jada.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Aulak KS. Miyagi M. Yan L. West KA. Massillon D. Crabb JW. Stuehr DJ. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci U S A. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babusikova E. Jesenak M. Kirschnerova R. Banovcin P. Dobrota D. Association of oxidative stress and GST-T1 gene with childhood bronchial asthma. J Physiol Pharmacol 60 Suppl. 2009;5:27–30. [PubMed] [Google Scholar]
- 16.Bagnato GF. Gulli S. De Pasquale R. Giacobbe O. Spatari G. Purello D'Ambrosio F. Effect of inhaled glutathione on airway response to “Fog” challenge in asthmatic patients. Respiration. 1999;66:518–521. doi: 10.1159/000029428. [DOI] [PubMed] [Google Scholar]
- 17.Barnes KC. Genetic studies of the etiology of asthma. Proc Am Thorac Soc. 2011;8:143–148. doi: 10.1513/pats.201103-030MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barreto M. Villa MP. Olita C. Martella S. Ciabattoni G. Montuschi P. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009;135:66–73. doi: 10.1378/chest.08-0722. [DOI] [PubMed] [Google Scholar]
- 19.Bauchart-Thevret C. Stoll B. Burrin DG. Intestinal metabolism of sulfur amino acids. Nutr Res Rev. 2009;22:175–187. doi: 10.1017/S0954422409990138. [DOI] [PubMed] [Google Scholar]
- 20.Baumann JM. Rundell KW. Evans TM. Levine AM. Effects of cysteine donor supplementation on exercise-induced bronchoconstriction. Med Sci Sports Exerc. 2005;37:1468–1473. doi: 10.1249/01.mss.0000177479.57468.15. [DOI] [PubMed] [Google Scholar]
- 21.Beeh KM. Beier J. Haas IC. Kornmann O. Micke P. Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:1119–1123. doi: 10.1183/09031936.02.00262402. [DOI] [PubMed] [Google Scholar]
- 22.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 23.Behndig AF. Mudway IS. Brown JL. Stenfors N. Helleday R. Duggan ST. Wilson SJ. Boman C. Cassee FR. Frew AJ. Kelly FJ. Sandstrom T. Blomberg A. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J. 2006;27:359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- 24.Behr J. Degenkolb B. Beinert T. Krombach F. Vogelmeier C. Pulmonary glutathione levels in acute episodes of Farmer's lung. Am J Respir Crit Care Med. 2000;161:1968–1971. doi: 10.1164/ajrccm.161.6.9907112. [DOI] [PubMed] [Google Scholar]
- 25.Behr J. Degenkolb B. Maier K. Braun B. Beinert T. Krombach F. Vogelmeier C. Fruhmann G. Increased oxidation of extracellular glutathione by bronchoalveolar inflammatory cells in diffuse fibrosing alveolitis. Eur Respir J. 1995;8:1286–1292. doi: 10.1183/09031936.95.08081286. [DOI] [PubMed] [Google Scholar]
- 26.Beier J. Beeh KM. Kornmann O. Buhl R. Stability of glutathione in induced sputum: impact of freezing. Respiration. 2003;70:523–527. doi: 10.1159/000074211. [DOI] [PubMed] [Google Scholar]
- 27.Beier J. Beeh KM. Kornmann O. Buhl R. Induced sputum methodology: validity and reproducibility of total glutathione measurement in supernatant of healthy and asthmatic individuals. J Lab Clin Med. 2004;144:38–44. doi: 10.1016/j.lab.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Beier J. Beeh KM. Semmler D. Beike N. Buhl R. Increased concentrations of glutathione in induced sputum of patients with mild or moderate allergic asthma. Ann Allergy Asthma Immunol. 2004;92:459–463. doi: 10.1016/S1081-1206(10)61783-8. [DOI] [PubMed] [Google Scholar]
- 29.Beier J. Beeh KM. Semmler D. Buhl R. Sputum levels of reduced glutathione increase 24 hours after allergen challenge in isolated early, but not dual asthmatic responders. Int Arch Allergy Immunol. 2004;135:30–35. doi: 10.1159/000080040. [DOI] [PubMed] [Google Scholar]
- 30.Bellomo G. Vairetti M. Stivala L. Mirabelli F. Richelmi P. Orrenius S. Demonstration of nuclear compartmentalization of glutathione in hepatocytes. Proc Natl Acad Sci U S A. 1992;89:4412–4416. doi: 10.1073/pnas.89.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bibi H. Schlesinger M. Tabachnik E. Schwartz Y. Iscovitz H. Iaina A. Erythrocyte glutathione peroxidase activity in asthmatic children. Ann Allergy. 1988;61:339–340. [PubMed] [Google Scholar]
- 32.Bishop C. Hudson VM. Hilton SC. Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- 33.Bjelakovic G. Gluud LL. Nikolova D. Bjelakovic M. Nagorni A. Gluud C. Antioxidant supplements for liver diseases. Cochrane Database Syst Rev. 2011;3:CD007749. doi: 10.1002/14651858.CD007749.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Bjelakovic G. Nikolova D. Gluud LL. Simonetti RG. Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2008;2:CD007176. doi: 10.1002/14651858.CD007176. [DOI] [PubMed] [Google Scholar]
- 35.Bjelakovic G. Nikolova D. Simonetti RG. Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst Rev. 2008;3:CD004183. doi: 10.1002/14651858.CD004183.pub3. [DOI] [PubMed] [Google Scholar]
- 36.Blanco RA. Ziegler TR. Carlson BA. Cheng PY. Park Y. Cotsonis GA. Accardi CJ. Jones DP. Diurnal variation in glutathione and cysteine redox states in human plasma. Am J Clin Nutr. 2007;86:1016–1023. doi: 10.1093/ajcn/86.4.1016. [DOI] [PubMed] [Google Scholar]
- 37.Blesa S. Cortijo J. Mata M. Serrano A. Closa D. Santangelo F. Estrela JM. Suchankova J. Morcillo EJ. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur Respir J. 2003;21:394–400. doi: 10.1183/09031936.03.00039602. [DOI] [PubMed] [Google Scholar]
- 38.Boisvert WA. Castaneda C. Mendoza I. Langeloh G. Solomons NW. Gershoff SN. Russell RM. Prevalence of riboflavin deficiency among Guatemalan elderly people and its relationship to milk intake. Am J Clin Nutr. 1993;58:85–90. doi: 10.1093/ajcn/58.1.85. [DOI] [PubMed] [Google Scholar]
- 39.Borok Z. Buhl R. Grimes GJ. Bokser AD. Hubbard RC. Holroyd KJ. Roum JH. Czerski DB. Cantin AM. Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–216. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 40.Bowler RP. Velsor LW. Duda B. Chan ED. Abraham E. Ware LB. Matthay MA. Day BJ. Pulmonary edema fluid antioxidants are depressed in acute lung injury. Crit Care Med. 2003;31:2309–2315. doi: 10.1097/01.CCM.0000085090.06078.8C. [DOI] [PubMed] [Google Scholar]
- 41.Bridgeman MM. Marsden M. Selby C. Morrison D. MacNee W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax. 1994;49:670–675. doi: 10.1136/thx.49.7.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broide DH. Finkelman F. Bochner BS. Rothenberg ME. Advances in mechanisms of asthma, allergy, and immunology in 2010. J Allergy Clin Immunol. 2011;127:689–695. doi: 10.1016/j.jaci.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Brown LA. Ping XD. Harris FL. Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:L824–L832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- 44.Bunnell E. Pacht ER. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148:1174–1178. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- 45.Burney P. Potts J. Makowska J. Kowalski M. Phillips J. Gnatiuc L. Shaheen S. Joos G. Van Cauwenberge P. van Zele T. Verbruggen K. van Durme Y. Derudder I. Wohrl S. Godnic-Cvar J. Salameh B. Skadhauge L. Thomsen G. Zuberbier T. Bergmann KC. Heinzerling L. Renz H. Al-Fakhri N. Kosche B. Hildenberg A. Papadopoulos NG. Xepapadaki P. Zannikos K. Gjomarkaj M. Bruno A. Pace E. Bonini S. Bresciani M. Gramiccioni C. Fokkens W. Weersink EJ. Carlsen KH. Bakkeheim E. Loureiro C. Villanueva CM. Sanjuas C. Zock JP. Lundback B. Janson C. A case-control study of the relation between plasma selenium and asthma in European populations: a GAL2EN project. Allergy. 2008;63:865–871. doi: 10.1111/j.1398-9995.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 46.Cantin AM. North SL. Hubbard RC. Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 47.Casoni GL. Chitano P. Pinamonti S. Chicca M. Ciaccia A. Fabbri L. Papi A. Reducing agents inhibit the contractile response of isolated guinea-pig main bronchi. Clin Exp Allergy. 2003;33:999–1004. doi: 10.1046/j.1365-2222.2003.01710.x. [DOI] [PubMed] [Google Scholar]
- 48.Choudhry S. Que LG. Yang Z. Liu L. Eng C. Kim SO. Kumar G. Thyne S. Chapela R. Rodriguez-Santana JR. Rodriguez-Cintron W. Avila PC. Stamler JS. Burchard EG. GSNO reductase and beta2-adrenergic receptor gene-gene interaction: bronchodilator responsiveness to albuterol. Pharmacogenet Genomics. 2010;20:351–358. doi: 10.1097/FPC.0b013e328337f992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung S. Sundar IK. Yao H. Ho YS. Rahman I. Glutaredoxin 1 regulates cigarette smoke-mediated lung inflammation through differential modulation of I [kappa] B kinases in mice: impact on histone acetylation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L192–L203. doi: 10.1152/ajplung.00426.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collipp PJ. Chen SY. Sharma RK. Balachandar V. Maddaiah VT. Tryptophane metabolism in bronchial asthma. Ann Allergy. 1975;35:153–158. [PubMed] [Google Scholar]
- 51.Collipp PJ. Goldzier S., 3rd Weiss N. Soleymani Y. Snyder R. Pyridoxine treatment of childhood bronchial asthma. Ann Allergy. 1975;35:93–97. [PubMed] [Google Scholar]
- 52.Comhair SA. Bhathena PR. Dweik RA. Kavuru M. Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 53.Comhair SA. Bhathena PR. Farver C. Thunnissen FB. Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J. 2001;15:70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- 54.Comhair SA. Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comhair SA. Ricci KS. Arroliga M. Lara AR. Dweik RA. Song W. Hazen SL. Bleecker ER. Busse WW. Chung KF. Gaston B. Hastie A. Hew M. Jarjour N. Moore W. Peters S. Teague WG. Wenzel SE. Erzurum SC. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172:306–313. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper AJ. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- 57.Corradi M. Folesani G. Andreoli R. Manini P. Bodini A. Piacentini G. Carraro S. Zanconato S. Baraldi E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Respir Crit Care Med. 2003;167:395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 58.Dalle-Donne I. Giustarini D. Rossi R. Colombo R. Milzani A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med. 2003;34:23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- 59.Dalle-Donne I. Rossi R. Giustarini D. Colombo R. Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Dauletbaev N. Rickmann J. Viel K. Buhl R. Wagner TO. Bargon J. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax. 2001;56:13–18. doi: 10.1136/thorax.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009;6:678–682. doi: 10.1513/pats.200907-067DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Raeve HR. Thunnissen FB. Kaneko FT. Guo FH. Lewis M. Kavuru MS. Secic M. Thomassen MJ. Erzurum SC. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: correction by inhaled corticosteroid in vivo. Am J Physiol. 1997;272:L148–L154. doi: 10.1152/ajplung.1997.272.1.L148. [DOI] [PubMed] [Google Scholar]
- 63.Decramer M. Rutten-van Molken M. Dekhuijzen PN. Troosters T. van Herwaarden C. Pellegrino R. van Schayck CP. Olivieri D. Del Donno M. De Backer W. Lankhorst I. Ardia A. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 64.Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–636. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 65.Delport R. Ubbink JB. Serfontein WJ. Becker PJ. Walters L. Vitamin B6 nutritional status in asthma: the effect of theophylline therapy on plasma pyridoxal-5'-phosphate and pyridoxal levels. Int J Vitam Nutr Res. 1988;58:67–72. [PubMed] [Google Scholar]
- 66.Deveci F. Ilhan N. Turgut T. Akpolat N. Kirkil G. Muz MH. Glutathione and nitrite in induced sputum from patients with stable and acute asthma compared with controls. Ann Allergy Asthma Immunol. 2004;93:91–97. doi: 10.1016/S1081-1206(10)61452-4. [DOI] [PubMed] [Google Scholar]
- 67.Dewey KG. Beaton G. Fjeld C. Lonnerdal B. Reeds P. Protein requirements of infants and children. Eur J Clin Nutr. 1996;50(Suppl 1):S119–S147. discussion S147–S150. [PubMed] [Google Scholar]
- 68.Di Buono M. Wykes LJ. Ball RO. Pencharz PB. Total sulfur amino acid requirement in young men as determined by indicator amino acid oxidation with L-[1-13C]phenylalanine. Am J Clin Nutr. 2001;74:756–760. doi: 10.1093/ajcn/74.6.756. [DOI] [PubMed] [Google Scholar]
- 69.Ditscheid B. Funfstuck R. Busch M. Schubert R. Gerth J. Jahreis G. Effect of L-methionine supplementation on plasma homocysteine and other free amino acids: a placebo-controlled double-blind cross-over study. Eur J Clin Nutr. 2005;59:768–775. doi: 10.1038/sj.ejcn.1602138. [DOI] [PubMed] [Google Scholar]
- 70.Dut R. Dizdar EA. Birben E. Sackesen C. Soyer OU. Besler T. Kalayci O. Oxidative stress and its determinants in the airways of children with asthma. Allergy. 2008;63:1605–1609. doi: 10.1111/j.1398-9995.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- 71.Dweik RA. Comhair SA. Gaston B. Thunnissen FB. Farver C. Thomassen MJ. Kavuru M. Hammel J. Abu-Soud HM. Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci U S A. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eaton DK. Kann L. Kinchen S. Shanklin S. Ross J. Hawkins J. Harris WA. Lowry R. McManus T. Chyen D. Lim C. Whittle L. Brener ND. Wechsler H. Youth risk behavior surveillance—United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- 73.Eisner MD. Anthonisen N. Coultas D. Kuenzli N. Perez-Padilla R. Postma D. Romieu I. Silverman EK. Balmes JR. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 74.Ercan H. Birben E. Dizdar EA. Keskin O. Karaaslan C. Soyer OU. Dut R. Sackesen C. Besler T. Kalayci O. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J Allergy Clin Immunol. 2006;118:1097–1104. doi: 10.1016/j.jaci.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Evans JR. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2006;2:CD000254. doi: 10.1002/14651858.CD000254.pub2. [DOI] [PubMed] [Google Scholar]
- 76.Fabian E. Poloskey P. Kosa L. Elmadfa I. Rethy LA. Activities of antioxidant enzymes in relation to oxidative and nitrosative challenges in childhood asthma. J Asthma. 2011;48:351–357. doi: 10.3109/02770903.2011.560319. [DOI] [PubMed] [Google Scholar]
- 77.Fairweather-Tait SJ. Bao Y. Broadley MR. Collings R. Ford D. Hesketh JE. Hurst R. Selenium in human health and disease. Antioxid Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 78.Fajardo I. Svensson L. Bucht A. Pejler G. Increased levels of hypoxia-sensitive proteins in allergic airway inflammation. Am J Respir Crit Care Med. 2004;170:477–484. doi: 10.1164/rccm.200402-178OC. [DOI] [PubMed] [Google Scholar]
- 79.Fang K. Johns R. Macdonald T. Kinter M. Gaston B. S-nitrosoglutathione breakdown prevents airway smooth muscle relaxation in the guinea pig. Am J Physiol Lung Cell Mol Physiol. 2000;279:L716–L721. doi: 10.1152/ajplung.2000.279.4.L716. [DOI] [PubMed] [Google Scholar]
- 80.Farber JM. Levine RL. Sequence of a peptide susceptible to mixed-function oxidation. Probable cation binding site in glutamine synthetase. J Biol Chem. 1986;261:4574–4578. [PubMed] [Google Scholar]
- 81.Feary J. Britton J. Dietary supplements and asthma: another one bites the dust. Thorax. 2007;62:466–468. doi: 10.1136/thx.2006.073866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fenech AG. Ellul-Micallef R. Selenium, glutathione peroxidase and superoxide dismutase in maltese asthmatic patients: effect of glucocorticoid administration. Pulm Pharmacol Ther. 1998;11:301–308. doi: 10.1006/pupt.1998.0122. [DOI] [PubMed] [Google Scholar]
- 83.Fiaschi T. Cozzi G. Raugei G. Formigli L. Ramponi G. Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281:22983–22991. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
- 84.Fitzpatrick AM. Brown LA. Holguin F. Teague WG. Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. J Allergy Clin Immunol. 2009;124:990–996. doi: 10.1016/j.jaci.2009.08.039. e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fitzpatrick AM. Gaston BM. Erzurum SC. Teague WG. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118:1218–1225. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitzpatrick AM. Stephenson ST. Hadley GR. Burwell L. Penugonda M. Simon DM. Hansen J. Jones DP. Brown LA. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J Allergy Clin Immunol. 2011;127:1604–1611. doi: 10.1016/j.jaci.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fitzpatrick AM. Teague WG. Burwell L. Brown MS. Brown LA. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69:154–159. doi: 10.1203/PDR.0b013e3182026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzpatrick AM. Teague WG. Holguin F. Yeh M. Brown LA. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. doi: 10.1016/j.jaci.2008.10.047. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fitzpatrick AM. Teague WG. Meyers DA. Peters SP. Li X. Li H. Wenzel SE. Aujla S. Castro M. Bacharier LB. Gaston BM. Bleecker ER. Moore WC. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389. doi: 10.1016/j.jaci.2010.11.015. e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flagg EW. Coates RJ. Eley JW. Jones DP. Gunter EW. Byers TE. Block GS. Greenberg RS. Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level. Nutr Cancer. 1994;21:33–46. doi: 10.1080/01635589409514302. [DOI] [PubMed] [Google Scholar]
- 91.Flatt A. Pearce N. Thomson CD. Sears MR. Robinson MF. Beasley R. Reduced selenium in asthmatic subjects in New Zealand. Thorax. 1990;45:95–99. doi: 10.1136/thx.45.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fogarty A. Broadfield E. Lewis S. Lawson N. Britton J. Amino acids and asthma: a case-control study. Eur Respir J. 2004;23:565–568. doi: 10.1183/09031936.04.00090404. [DOI] [PubMed] [Google Scholar]
- 93.Food, and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acid (Macronutrients) Washington, DC: National Academies Press; 2005. Protein and amino acids; pp. 589–768. [Google Scholar]
- 94.Foreman MG. Hoor TT. Brown LA. Moss M. Effects of chronic hepatic dysfunction on pulmonary glutathione homeostasis. Alcohol Clin Exp Res. 2002;26:1840–1845. doi: 10.1097/01.ALC.0000042149.71731.B7. [DOI] [PubMed] [Google Scholar]
- 95.Forman HJ. Zhang H. Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foschino Barbaro MP. Serviddio G. Resta O. Rollo T. Tamborra R. Elisiana Carpagnano G. Vendemiale G. Altomare E. Oxygen therapy at low flow causes oxidative stress in chronic obstructive pulmonary disease: prevention by N-acetyl cysteine. Free Radic Res. 2005;39:1111–1118. doi: 10.1080/10715760500250257. [DOI] [PubMed] [Google Scholar]
- 97.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foster MW. Yang Z. Potts EN. Michael Foster W. Que LG. S-nitrosoglutathione supplementation to ovalbumin-sensitized and -challenged mice ameliorates methacholine-induced bronchoconstriction. Am J Physiol Lung Cell Mol Physiol. 2011;301:L739–L744. doi: 10.1152/ajplung.00134.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Fryer AA. Bianco A. Hepple M. Jones PW. Strange RC. Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus. A new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med. 2000;161:1437–1442. doi: 10.1164/ajrccm.161.5.9903006. [DOI] [PubMed] [Google Scholar]
- 100.Fukagawa NK. Yu YM. Young VR. Methionine and cysteine kinetics at different intakes of methionine and cysteine in elderly men and women. Am J Clin Nutr. 1998;68:380–388. doi: 10.1093/ajcn/68.2.380. [DOI] [PubMed] [Google Scholar]
- 101.Gallogly MM. Starke DW. Leonberg AK. Ospina SM. Mieyal JJ. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles. Biochemistry. 2008;47:11144–11157. doi: 10.1021/bi800966v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garcia-Marcos L. Canflanca IM. Garrido JB. Varela AL. Garcia-Hernandez G. Guillen Grima F. Gonzalez-Diaz C. Carvajal-Uruena I. Arnedo-Pena A. Busquets-Monge RM. Morales Suarez-Varela M. Blanco-Quiros A. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax. 2007;62:503–508. doi: 10.1136/thx.2006.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garcia-Ruiz C. Morales A. Ballesta A. Rodes J. Kaplowitz N. Fernandez-Checa JC. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J Clin Invest. 1994;94:193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gariballa S. Forster S. Powers H. Riboflavin status in acutely ill patients and response to dietary supplements. JPEN J Parenter Enteral Nutr. 2009;33:656–661. doi: 10.1177/0148607109336602. [DOI] [PubMed] [Google Scholar]
- 105.Gaston B. Drazen JM. Loscalzo J. Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 106.Gaston B. Reilly J. Drazen JM. Fackler J. Ramdev P. Arnelle D. Mullins ME. Sugarbaker DJ. Chee C. Singel DJ, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaston B. Sears S. Woods J. Hunt J. Ponaman M. McMahon T. Stamler JS. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351:1317–1319. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 108.Ghosh S. Janocha AJ. Aronica MA. Swaidani S. Comhair SA. Xu W. Zheng L. Kaveti S. Kinter M. Hazen SL. Erzurum SC. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 109.Gilbert HF. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods Enzymol. 1995;251:8–28. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- 110.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2010. www.goldcopd.org www.goldcopd.org
- 111.Go YM. Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–2980. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 112.Go YM. Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Go YM. Jones DP. Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal. 2010;13:489–509. doi: 10.1089/ars.2009.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Go YM. Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509. doi: 10.1016/j.freeradbiomed.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grandjean EM. Berthet P. Ruffmann R. Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22:209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 116.Greenwald R. Fitzpatrick AM. Gaston B. Marozkina NV. Erzurum S. Teague WG. Breath formate is a marker of airway S-nitrosothiol depletion in severe asthma. PLoS One. 2010;5:e11919. doi: 10.1371/journal.pone.0011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grey V. Mohammed SR. Smountas AA. Bahlool R. Lands LC. Improved glutathione status in young adult patients with cystic fibrosis supplemented with whey protein. J Cyst Fibros. 2003;2:195–198. doi: 10.1016/S1569-1993(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 118.Griese M. Ramakers J. Krasselt A. Starosta V. Van Koningsbruggen S. Fischer R. Ratjen F. Mullinger B. Huber RM. Maier K. Rietschel E. Scheuch G. Improvement of alveolar glutathione and lung function but not oxidative state in cystic fibrosis. Am J Respir Crit Care Med. 2004;169:822–828. doi: 10.1164/rccm.200308-1104OC. [DOI] [PubMed] [Google Scholar]
- 119.Griffith OW. Mammalian sulfur amino acid metabolism: an overview. Methods Enzymol. 1987;143:366–376. doi: 10.1016/0076-6879(87)43065-6. [DOI] [PubMed] [Google Scholar]
- 120.Griffith OW. Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grimble RF. The effects of sulfur amino acid intake on immune function in humans. J Nutr. 2006;136:1660S–1665S. doi: 10.1093/jn/136.6.1660S. [DOI] [PubMed] [Google Scholar]
- 122.Gumral N. Caliskan S. Ozguner F. Kaleli S. Akkaya A. Yilmaz H. Sen S. Melatonin levels and enzymatic antioxidant defense system decrease in blood of patients with bronchial asthma. Toxicol Ind Health. 2009;25:411–416. doi: 10.1177/0748233709106625. [DOI] [PubMed] [Google Scholar]
- 123.Gumral N. Naziroglu M. Ongel K. Beydilli ED. Ozguner F. Sutcu R. Caliskan S. Akkaya A. Antioxidant enzymes and melatonin levels in patients with bronchial asthma and chronic obstructive pulmonary disease during stable and exacerbation periods. Cell Biochem Funct. 2009;27:276–283. doi: 10.1002/cbf.1569. [DOI] [PubMed] [Google Scholar]
- 124.Guo CH. Liu PJ. Hsia S. Chuang CJ. Chen PC. Role of certain trace minerals in oxidative stress, inflammation, CD4/CD8 lymphocyte ratios and lung function in asthmatic patients. Ann Clin Biochem 48(Pt. 2011;4):344–351. doi: 10.1258/acb.2011.010266. [DOI] [PubMed] [Google Scholar]
- 125.Hall MA. Thom H. Russell G. Erythrocyte aspartate amino transferase activity in asthmatic and non-asthmatic children and its enhancement by vitamin B6. Ann Allergy. 1981;47:464–466. [PubMed] [Google Scholar]
- 126.Hansen JM. Go YM. Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 127.Hansen JM. Zhang H. Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 128.Hartl D. Starosta V. Maier K. Beck-Speier I. Rebhan C. Becker BF. Latzin P. Fischer R. Ratjen F. Huber RM. Rietschel E. Krauss-Etschmann S. Griese M. Inhaled glutathione decreases PGE2 and increases lymphocytes in cystic fibrosis lungs. Free Radic Biol Med. 2005;39:463–472. doi: 10.1016/j.freeradbiomed.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 129.Hasbal C. Aksu BY. Himmetoglu S. Dincer Y. Koc EE. Hatipoglu S. Akcay T. DNA damage and glutathione level in children with asthma bronchiale: effect of antiasthmatic therapy. Pediatr Allergy Immunol. 2010;21:e674–e678. doi: 10.1111/j.1399-3038.2009.00959.x. [DOI] [PubMed] [Google Scholar]
- 130.Hassan AM. Glutathione peroxidase activity in blood cells from aspirin-induced asthma patients. Ann Clin Biochem. 2003;40:369–373. doi: 10.1258/000456303766477002. [DOI] [PubMed] [Google Scholar]
- 131.Hassan AM. Selenium status in patients with aspirin-induced asthma. Ann Clin Biochem. 2008;45:508–512. doi: 10.1258/acb.2008.008030. [DOI] [PubMed] [Google Scholar]
- 132.Hasselmark L. Malmgren R. Unge G. Zetterstrom O. Lowered platelet glutathione peroxidase activity in patients with intrinsic asthma. Allergy. 1990;45:523–527. doi: 10.1111/j.1398-9995.1990.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 133.Hasselmark L. Malmgren R. Zetterstrom O. Unge G. Selenium supplementation in intrinsic asthma. Allergy. 1993;48:30–36. [PubMed] [Google Scholar]
- 134.Hess DT. Matsumoto A. Kim SO. Marshall HE. Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 135.Hoey L. McNulty H. Strain JJ. Studies of biomarker responses to intervention with riboflavin: a systematic review. Am J Clin Nutr. 2009;89:1960S–1980S. doi: 10.3945/ajcn.2009.27230B. [DOI] [PubMed] [Google Scholar]
- 136.Hoey L. Strain JJ. McNulty H. Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials. Am J Clin Nutr. 2009;89:1981S–1996S. doi: 10.3945/ajcn.2009.27230C. [DOI] [PubMed] [Google Scholar]
- 137.Hoffmann PR. Jourdan-Le Saux C. Hoffmann FW. Chang PS. Bollt O. He Q. Tam EK. Berry MJ. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007;179:3258–3267. doi: 10.4049/jimmunol.179.5.3258. [DOI] [PubMed] [Google Scholar]
- 138.Holroyd KJ. Buhl R. Borok Z. Roum JH. Bokser AD. Grimes GJ. Czerski D. Cantin AM. Crystal RG. Correction of glutathione deficiency in the lower respiratory tract of HIV seropositive individuals by glutathione aerosol treatment. Thorax. 1993;48:985–989. doi: 10.1136/thx.48.10.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoshino T. Okamoto M. Takei S. Sakazaki Y. Iwanaga T. Aizawa H. Redox-regulated mechanisms in asthma. Antioxid Redox Signal. 2008;10:769–783. doi: 10.1089/ars.2007.1936. [DOI] [PubMed] [Google Scholar]
- 140.Hu S. Zhao H. Al-Humadi NH. Yin XJ. Ma JK. Silica-induced apoptosis in alveolar macrophages: evidence of in vivo thiol depletion and the activation of mitochondrial pathway. J Toxicol Environ Health A. 2006;69:1261–1284. doi: 10.1080/15287390500361875. [DOI] [PubMed] [Google Scholar]
- 141.Hustad S. McKinley MC. McNulty H. Schneede J. Strain JJ. Scott JM. Ueland PM. Riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma and erythrocytes at baseline and after low-dose riboflavin supplementation. Clin Chem. 2002;48:1571–1577. [PubMed] [Google Scholar]
- 142.Hutter DE. Till BG. Greene JJ. Redox state changes in density-dependent regulation of proliferation. Exp Cell Res. 1997;232:435–438. doi: 10.1006/excr.1997.3527. [DOI] [PubMed] [Google Scholar]
- 143.Hwang C. Sinskey AJ. Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 144.Imaoka H. Hoshino T. Okamoto M. Sakazaki Y. Sawada M. Takei S. Kinoshita T. Kawayama T. Kato S. Aizawa H. Endogenous and exogenous thioredoxin 1 prevents goblet cell hyperplasia in a chronic antigen exposure asthma model. Allergol Int. 2009;58:403–410. doi: 10.2332/allergolint.09-OA-0086. [DOI] [PubMed] [Google Scholar]
- 145.Ingram JL. Huggins MJ. Church TD. Li Y. Francisco DC. Degan S. Firszt R. Beaver DM. Lugogo NL. Wang Y. Sunday ME. Noble PW. Kraft M. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med. 2011;183:1625–1632. doi: 10.1164/rccm.201009-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Inoue K. Takano H. Koike E. Warabi E. Yanagawa T. Yanagisawa R. Ishii T. Peroxiredoxin I is a negative regulator of Th2-dominant allergic asthma. Int Immunopharmacol. 2009;9:1281–1288. doi: 10.1016/j.intimp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Jackson AA. Gibson NR. Lu Y. Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am J Clin Nutr. 2004;80:101–107. doi: 10.1093/ajcn/80.1.101. [DOI] [PubMed] [Google Scholar]
- 148.Jacobson GA. Yee KC. Ng CH. Elevated plasma glutathione peroxidase concentration in acute severe asthma: comparison with plasma glutathione peroxidase activity, selenium and malondialdehyde. Scand J Clin Lab Invest. 2007;67:423–430. doi: 10.1080/00365510601153353. [DOI] [PubMed] [Google Scholar]
- 149.Jeong DW. Yoo MH. Kim TS. Kim JH. Kim IY. Protection of mice from allergen-induced asthma by selenite: prevention of eosinophil infiltration by inhibition of NF-kappa B activation. J Biol Chem. 2002;277:17871–17876. doi: 10.1074/jbc.M200808200. [DOI] [PubMed] [Google Scholar]
- 150.Jeong HC. Lee SY. Lee EJ. Jung KH. Kang EH. Kim JH. Park EK. Lee SH. Uhm CS. Cho Y. Shin C. Shim JJ. Kim HK. In KH. Kang KH. Yoo SH. Proteomic analysis of peripheral T-lymphocytes in patients with asthma. Chest. 2007;132:489–496. doi: 10.1378/chest.06-2980. [DOI] [PubMed] [Google Scholar]
- 151.Jiang S. Moriarty-Craige SE. Orr M. Cai J. Sternberg P., Jr. Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- 152.Johannessen LN. Nilsen AM. Lovik M. Mycotoxin-induced depletion of intracellular glutathione and altered cytokine production in the human alveolar epithelial cell line A549. Toxicol Lett. 2007;168:103–112. doi: 10.1016/j.toxlet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 153.Jonas CR. Gu LH. Nkabyo YS. Mannery YO. Avissar NE. Sax HC. Jones DP. Ziegler TR. Glutamine and KGF each regulate extracellular thiol/disulfide redox and enhance proliferation in Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1421–R1429. doi: 10.1152/ajpregu.00702.2002. [DOI] [PubMed] [Google Scholar]
- 154.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 155.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 156.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 157.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones DP. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J Intern Med. 2010;268:432–448. doi: 10.1111/j.1365-2796.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jones DP. Carlson JL. Mody VC. Cai J. Lynn MJ. Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 160.Jones DP. Carlson JL. Samiec PS. Sternberg P., Jr. Mody VC., Jr. Reed RL. Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 161.Jones DP. Go YM. Anderson CL. Ziegler TR. Kinkade JM., Jr. Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 162.Jones DP. Mody VC., Jr. Carlson JL. Lynn MJ. Sternberg P., Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 163.Joubert BR. Reif DM. Edwards SW. Leiner KA. Hudgens EE. Egeghy P. Gallagher JE. Hubal EC. Evaluation of genetic susceptibility to childhood allergy and asthma in an African American urban population. BMC Med Genet. 2011;12:25. doi: 10.1186/1471-2350-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kabesch M. Hoefler C. Carr D. Leupold W. Weiland SK. von Mutius E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax. 2004;59:569–573. doi: 10.1136/thx.2003.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kadrabova J. Mad'aric A. Kovacikova Z. Podivinsky F. Ginter E. Gazdik F. Selenium status is decreased in patients with intrinsic asthma. Biol Trace Elem Res. 1996;52:241–248. doi: 10.1007/BF02789165. [DOI] [PubMed] [Google Scholar]
- 166.Kelly FJ. Mudway I. Blomberg A. Frew A. Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 167.Kim HS. Manevich Y. Feinstein SI. Pak JH. Ho YS. Fisher AB. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L363–L369. doi: 10.1152/ajplung.00078.2003. [DOI] [PubMed] [Google Scholar]
- 168.Kim SR. Lee KS. Park SJ. Min KH. Lee MH. Lee KA. Bartov O. Atlas D. Lee YC. A novel dithiol amide CB3 attenuates allergic airway disease through negative regulation of p38 mitogen-activated protein kinase. Am J Respir Crit Care Med. 2011;183:1015–1024. doi: 10.1164/rccm.200906-0902OC. [DOI] [PubMed] [Google Scholar]
- 169.Kinnula VL. Lehtonen S. Kaarteenaho-Wiik R. Lakari E. Paakko P. Kang SW. Rhee SG. Soini Y. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax. 2002;57:157–164. doi: 10.1136/thorax.57.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kirlin WG. Cai J. Thompson SA. Diaz D. Kavanagh TJ. Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999;27:1208–1218. doi: 10.1016/s0891-5849(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 171.Kloek J. Mortaz E. Van Ark I. Bloksma N. Garssen J. Nijkamp FP. Folkerts G. A whey-based glutathione-enhancing diet decreases allergen-induced airway contraction in a guinea-pig model of asthma. Br J Nutr. 2011;105:1465–1470. doi: 10.1017/S0007114510005337. [DOI] [PubMed] [Google Scholar]
- 172.Kloek J. Mortaz E. van Ark I. Lilly CM. Nijkamp FP. Folkerts G. Glutathione prevents the early asthmatic reaction and airway hyperresponsiveness in guinea pigs. J Physiol Pharmacol. 2010;61:67–72. [PubMed] [Google Scholar]
- 173.Kloek J. Van Ark I. De Clerck F. Bloksma N. Nijkamp FP. Folkerts G. Modulation of airway hyperresponsiveness by thiols in a murine in vivo model of allergic asthma. Inflamm Res. 2003;52:126–131. doi: 10.1007/s000110300025. [DOI] [PubMed] [Google Scholar]
- 174.Ko HM. Kang NI. Kim YS. Lee YM. Jin ZW. Jung YJ. Im SY. Kim JH. Shin YH. Cho BH. Lee HK. Glutamine preferentially inhibits T-helper type 2 cell-mediated airway inflammation and late airway hyperresponsiveness through the inhibition of cytosolic phospholipase A(2) activity in a murine asthma model. Clin Exp Allergy. 2008;38:357–364. doi: 10.1111/j.1365-2222.2007.02900.x. [DOI] [PubMed] [Google Scholar]
- 175.Kobayashi N. Yamada Y. Ito W. Ueki S. Kayaba H. Nakamura H. Yodoi J. Chihara J. Thioredoxin reduces C-C chemokine-induced chemotaxis of human eosinophils. Allergy. 2009;64:1130–1135. doi: 10.1111/j.1398-9995.2009.01969.x. [DOI] [PubMed] [Google Scholar]
- 176.Kocyigit A. Armutcu F. Gurel A. Ermis B. Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biol Trace Elem Res. 2004;97:31–41. doi: 10.1385/BTER:97:1:31. [DOI] [PubMed] [Google Scholar]
- 177.Koike Y. Hisada T. Utsugi M. Ishizuka T. Shimizu Y. Ono A. Murata Y. Hamuro J. Mori M. Dobashi K. Glutathione redox regulates airway hyperresponsiveness and airway inflammation in mice. Am J Respir Cell Mol Biol. 2007;37:322–329. doi: 10.1165/rcmb.2006-0423OC. [DOI] [PubMed] [Google Scholar]
- 178.Kongerud J. Crissman K. Hatch G. Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhal Toxicol. 2003;15:101–109. doi: 10.1080/08958370304477. [DOI] [PubMed] [Google Scholar]
- 179.Lakhdar R. Denden S. Mouhamed MH. Chalgoum A. Leban N. Knani J. Lefranc G. Miled A. Ben Chibani J. Khelil AH. Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic polymorphisms with antioxidative stress markers in chronic obstructive pulmonary disease. Exp Lung Res. 2011;37:195–204. doi: 10.3109/01902148.2010.535093. [DOI] [PubMed] [Google Scholar]
- 180.Lands LC. Grey VL. Smountas AA. Effect of supplementation with a cysteine donor on muscular performance. J Appl Physiol. 1999;87:1381–1385. doi: 10.1152/jappl.1999.87.4.1381. [DOI] [PubMed] [Google Scholar]
- 181.Laviolette L. Lands LC. Dauletbaev N. Saey D. Milot J. Provencher S. LeBlanc P. Maltais F. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food. 2010;13:589–598. doi: 10.1089/jmf.2009.0142. [DOI] [PubMed] [Google Scholar]
- 182.Lee K. Kim SH. Yoon HJ. Paik DJ. Kim JM. Youn J. Bacillus-derived poly-gamma-glutamic acid attenuates allergic airway inflammation through a Toll-like receptor-4-dependent pathway in a murine model of asthma. Clin Exp Allergy. 2011;41:1143–1156. doi: 10.1111/j.1365-2222.2011.03792.x. [DOI] [PubMed] [Google Scholar]
- 183.Lee KS. Kim SR. Park HS. Park SJ. Min KH. Lee KY. Choe YH. Hong SH. Han HJ. Lee YR. Kim JS. Atlas D. Lee YC. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007;39:756–768. doi: 10.1038/emm.2007.82. [DOI] [PubMed] [Google Scholar]
- 184.Lee KS. Kim SR. Park SJ. Min KH. Lee KY. Jin SM. Yoo WH. Lee YC. Antioxidant down-regulates interleukin-18 expression in asthma. Mol Pharmacol. 2006;70:1184–1193. doi: 10.1124/mol.106.024737. [DOI] [PubMed] [Google Scholar]
- 185.Lee YC. Lee KS. Park SJ. Park HS. Lim JS. Park KH. Im MJ. Choi IW. Lee HK. Kim UH. Blockade of airway hyperresponsiveness and inflammation in a murine model of asthma by a prodrug of cysteine, L-2-oxothiazolidine-4-carboxylic acid. FASEB J. 2004;18:1917–1919. doi: 10.1096/fj.04-2212fje. [DOI] [PubMed] [Google Scholar]
- 186.Lehtonen ST. Markkanen PM. Peltoniemi M. Kang SW. Kinnula VL. Variable overoxidation of peroxiredoxins in human lung cells in severe oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2005;288:L997–L1001. doi: 10.1152/ajplung.00432.2004. [DOI] [PubMed] [Google Scholar]
- 187.Li M. Fukagawa NK. Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li N. Alam J. Venkatesan MI. Eiguren-Fernandez A. Schmitz D. Di Stefano E. Slaughter N. Killeen E. Wang X. Huang A. Wang M. Miguel AH. Cho A. Sioutas C. Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 189.Li XY. Gilmour PS. Donaldson K. MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lim SK. Kim JC. Moon CJ. Kim GY. Han HJ. Park SH. Formaldehyde induces apoptosis through decreased Prx 2 via p38 MAPK in lung epithelial cells. Toxicology. 2010;271:100–106. doi: 10.1016/j.tox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 191.Linn WS. Buckley RD. Spier CE. Blessey RL. Jones MP. Fischer DA. Hackney JD. Health effects of ozone exposure in asthmatics. Am Rev Respir Dis. 1978;117:835–843. doi: 10.1164/arrd.1978.117.5.835. [DOI] [PubMed] [Google Scholar]
- 192.Liu L. Hausladen A. Zeng M. Que L. Heitman J. Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 193.Lopes de Jesus CC. Atallah AN. Valente O. Moca Trevisani VF. Vitamin C and superoxide dismutase (SOD) for diabetic retinopathy. Cochrane Database Syst Rev. 2008;1:CD006695. doi: 10.1002/14651858.CD006695.pub2. [DOI] [PubMed] [Google Scholar]
- 194.Lopez O. Hernandez AF. Rodrigo L. Gil F. Pena G. Serrano JL. Parron T. Villanueva E. Pla A. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicol Lett. 2007;171:146–153. doi: 10.1016/j.toxlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 195.Lothian JB. Grey V. Lands LC. Effect of whey protein to modulate immune response in children with atopic asthma. Int J Food Sci Nutr. 2006;57:204–211. doi: 10.1080/09637480600738294. [DOI] [PubMed] [Google Scholar]
- 196.Lotvall J. Akdis CA. Bacharier LB. Bjermer L. Casale TB. Custovic A. Lemanske RF., Jr. Wardlaw AJ. Wenzel SE. Greenberger PA. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 197.Lowry MH. McAllister BP. Jean JC. Brown LA. Hughey RP. Cruikshank WW. Amar S. Lucey EC. Braun K. Johnson P. Wight TN. Joyce-Brady M. Lung lining fluid glutathione attenuates IL-13-induced asthma. Am J Respir Cell Mol Biol. 2008;38:509–516. doi: 10.1165/rcmb.2007-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 199.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lundberg M. Johansson C. Chandra J. Enoksson M. Jacobsson G. Ljung J. Johansson M. Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 201.Luo S. Levine RL. Methionine in proteins defends against oxidative stress. FASEB J. 2009;23:464–472. doi: 10.1096/fj.08-118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.MacCoss MJ. Fukagawa NK. Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am J Physiol Endocrinol Metab. 2001;280:E947–E955. doi: 10.1152/ajpendo.2001.280.6.E947. [DOI] [PubMed] [Google Scholar]
- 203.MacMillan-Crow LA. Crow JP. Kerby JD. Beckman JS. Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.MacPherson JC. Comhair SA. Erzurum SC. Klein DF. Lipscomb MF. Kavuru MS. Samoszuk MK. Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 205.Mak JC. Ho SP. Leung HC. Cheung AH. Law BK. So LK. Chan JW. Chau CH. Lam WK. Ip MS. Chan-Yeung M. Relationship between glutathione S-transferase gene polymorphisms and enzyme activity in Hong Kong Chinese asthmatics. Clin Exp Allergy. 2007;37:1150–1157. doi: 10.1111/j.1365-2222.2007.02704.x. [DOI] [PubMed] [Google Scholar]
- 206.Mak JC. Leung HC. Ho SP. Law BK. Lam WK. Tsang KW. Ip MS. Chan-Yeung M. Systemic oxidative and antioxidative status in Chinese patients with asthma. J Allergy Clin Immunol. 2004;114:260–264. doi: 10.1016/j.jaci.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 207.Malling TH. Sigsgaard T. Andersen HR. Frischknecht L. Deguchi Y. Skadhauge L. Sherson D. Thomsen G. Baelum J. Pedersen JK. Omland O. Sex determines the influence of smoking and gene polymorphism on glutathione peroxidase activity in erythrocytes. Scand J Clin Lab Invest. 2009;69:295–302. doi: 10.1080/00365510802632155. [DOI] [PubMed] [Google Scholar]
- 208.Malling TH. Sigsgaard T. Brasch-Andersen C. Frischknecht L. Andersen HR. Kruse TA. Sherson D. Skadhauge LR. Thomsen G. Baelum J. Omland O. Genetic polymorphisms in antioxidative enzymes are associated to FEV(1) in smokers independently of asthma. Clin Respir J. 2012;6:46–55. doi: 10.1111/j.1752-699X.2011.00245.x. [DOI] [PubMed] [Google Scholar]
- 209.Mallis RJ. Hamann MJ. Zhao W. Zhang T. Hendrich S. Thomas JA. Irreversible thiol oxidation in carbonic anhydrase III: protection by S-glutathiolation and detection in aging rats. Biol Chem. 2002;383:649–662. doi: 10.1515/BC.2002.067. [DOI] [PubMed] [Google Scholar]
- 210.Mannery YO. Ziegler TR. Park Y. Jones DP. Oxidation of plasma cysteine/cystine and GSH/GSSG redox potentials by acetaminophen and sulfur amino acid insufficiency in humans. J Pharmacol Exp Ther. 2010;333:939–947. doi: 10.1124/jpet.110.166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Manthey KC. Rodriguez-Melendez R. Hoi JT. Zempleni J. Riboflavin deficiency causes protein and DNA damage in HepG2 cells, triggering arrest in G1 phase of the cell cycle. J Nutr Biochem. 2006;17:250–256. doi: 10.1016/j.jnutbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Markovic J. Garcia-Gimenez JL. Gimeno A. Vina J. Pallardo FV. Role of glutathione in cell nucleus. Free Radic Res. 2010;44:721–733. doi: 10.3109/10715762.2010.485989. [DOI] [PubMed] [Google Scholar]
- 213.Marrades RM. Roca J. Barbera JA. de Jover L. MacNee W. Rodriguez-Roisin R. Nebulized glutathione induces bronchoconstriction in patients with mild asthma. Am J Respir Crit Care Med. 1997;156:425–430. doi: 10.1164/ajrccm.156.2.9611001. [DOI] [PubMed] [Google Scholar]
- 214.Masri FA. Comhair SA. Koeck T. Xu W. Janocha A. Ghosh S. Dweik RA. Golish J. Kinter M. Stuehr DJ. Erzurum SC. Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005;172:597–605. doi: 10.1164/rccm.200411-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mato JM. Corrales FJ. Lu SC. Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 216.McNulty H. Pentieva K. Hoey L. Ward M. Homocysteine, B-vitamins and CVD. Proc Nutr Soc. 2008;67:232–237. doi: 10.1017/S0029665108007076. [DOI] [PubMed] [Google Scholar]
- 217.Meredith MJ. Reed DJ. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem. 1982;257:3747–3753. [PubMed] [Google Scholar]
- 218.Micke P. Beeh KM. Schlaak JF. Buhl R. Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest. 2001;31:171–178. doi: 10.1046/j.1365-2362.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- 219.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Millward DJ. Fereday A. Gibson N. Pacy PJ. Aging, protein requirements, and protein turnover. Am J Clin Nutr. 1997;66:774–786. doi: 10.1093/ajcn/66.4.774. [DOI] [PubMed] [Google Scholar]
- 221.Minelli C. Granell R. Newson R. Rose-Zerilli MJ. Torrent M. Ring SM. Holloway JW. Shaheen SO. Henderson JA. Glutathione-S-transferase genes and asthma phenotypes: a Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Int J Epidemiol. 2010;39:539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Misso NL. Powers KA. Gillon RL. Stewart GA. Thompson PJ. Reduced platelet glutathione peroxidase activity and serum selenium concentration in atopic asthmatic patients. Clin Exp Allergy. 1996;26:838–847. [PubMed] [Google Scholar]
- 223.Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Ther Adv Respir Dis. 2007;1:5–23. doi: 10.1177/1753465807082373. [DOI] [PubMed] [Google Scholar]
- 224.Montuschi P. Curro D. Ragazzoni E. Preziosi P. Ciabattoni G. Anaphylaxis increases 8-iso-prostaglandin F2alpha release from guinea-pig lung in vitro. Eur J Pharmacol. 1999;365:59–64. doi: 10.1016/s0014-2999(98)00859-0. [DOI] [PubMed] [Google Scholar]
- 225.Montuschi P. Nightingale JA. Kharitonov SA. Barnes PJ. Ozone-induced increase in exhaled 8-isoprostane in healthy subjects is resistant to inhaled budesonide. Free Radic Biol Med. 2002;33:1403–1408. doi: 10.1016/s0891-5849(02)01084-5. [DOI] [PubMed] [Google Scholar]
- 226.Montuschi P. Ragazzoni E. Valente S. Corbo G. Mondino C. Ciappi G. Ciabattoni G. Validation of 8-isoprostane and prostaglandin E(2) measurements in exhaled breath condensate. Inflamm Res. 2003;52:502–507. doi: 10.1007/s00011-003-1212-6. [DOI] [PubMed] [Google Scholar]
- 227.Moon EY. Kang JS. Han SH. Yang KH. Pyo S. Lee MY. Lee HK. Yu DY. Differential role of peroxiredoxin II (PrxII) on the expression of toll-like receptor 4 (TLR4) and B-cell activating factor (BAFF) in ovalbumin (OVA)-induced mouse asthma. Int Immunopharmacol. 2008;8:935–944. doi: 10.1016/j.intimp.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 228.Moore PE. Ryckman KK. Williams SM. Patel N. Summar ML. Sheller JR. Genetic variants of GSNOR and ADRB2 influence response to albuterol in African-American children with severe asthma. Pediatr Pulmonol. 2009;44:649–654. doi: 10.1002/ppul.21033. [DOI] [PubMed] [Google Scholar]
- 229.Moore WC. Bleecker ER. Curran-Everett D. Erzurum SC. Ameredes BT. Bacharier L. Calhoun WJ. Castro M. Chung KF. Clark MP. Dweik RA. Fitzpatrick AM. Gaston B. Hew M. Hussain I. Jarjour NN. Israel E. Levy BD. Murphy JR. Peters SP. Teague WG. Meyers DA. Busse WW. Wenzel SE. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Moore WC. Meyers DA. Wenzel SE. Teague WG. Li H. Li X. D'Agostino R., Jr. Castro M. Curran-Everett D. Fitzpatrick AM. Gaston B. Jarjour NN. Sorkness R. Calhoun WJ. Chung KF. Comhair SA. Dweik RA. Israel E. Peters SP. Busse WW. Erzurum SC. Bleecker ER. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Moriarty-Craige SE. Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 232.Moriarty SE. Shah JH. Lynn M. Jiang S. Openo K. Jones DP. Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 233.Moss M. Guidot DM. Wong-Lambertina M. Ten Hoor T. Perez RL. Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 234.Mudway IS. Stenfors N. Blomberg A. Helleday R. Dunster C. Marklund SL. Frew AJ. Sandstrom T. Kelly FJ. Differences in basal airway antioxidant concentrations are not predictive of individual responsiveness to ozone: a comparison of healthy and mild asthmatic subjects. Free Radic Biol Med. 2001;31:962–974. doi: 10.1016/s0891-5849(01)00671-2. [DOI] [PubMed] [Google Scholar]
- 235.Mulier B. Rahman I. Watchorn T. Donaldson K. MacNee W. Jeffery PK. Hydrogen peroxide-induced epithelial injury: the protective role of intracellular nonprotein thiols (NPSH) Eur Respir J. 1998;11:384–391. doi: 10.1183/09031936.98.11020384. [DOI] [PubMed] [Google Scholar]
- 236.Nadeem A. Chhabra SK. Masood A. Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 237.Nadeem A. Raj HG. Chhabra SK. Increased oxidative stress in acute exacerbations of asthma. J Asthma. 2005;42:45–50. doi: 10.1081/jas-200044774. [DOI] [PubMed] [Google Scholar]
- 238.Nagai K. Betsuyaku T. Kondo T. Nasuhara Y. Nishimura M. Long term smoking with age builds up excessive oxidative stress in bronchoalveolar lavage fluid. Thorax. 2006;61:496–502. doi: 10.1136/thx.2005.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nagel G. Weinmayr G. Kleiner A. Garcia-Marcos L. Strachan DP. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010;65:516–522. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 240.Nash EF. Stephenson A. Ratjen F. Tullis E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2009;1:CD007168. doi: 10.1002/14651858.CD007168.pub2. [DOI] [PubMed] [Google Scholar]
- 241.Nkabyo YS. Go YM. Ziegler TR. Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol. 2005;289:G70–G78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 242.Nurmatov U. Devereux G. Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127:724–733. doi: 10.1016/j.jaci.2010.11.001. e1–30. [DOI] [PubMed] [Google Scholar]
- 243.Ochs-Balcom HM. Grant BJ. Muti P. Sempos CT. Freudenheim JL. Browne RW. McCann SE. Trevisan M. Cassano PA. Iacoviello L. Schunemann HJ. Antioxidants, oxidative stress, and pulmonary function in individuals diagnosed with asthma or COPD. Eur J Clin Nutr. 2006;60:991–999. doi: 10.1038/sj.ejcn.1602410. [DOI] [PubMed] [Google Scholar]
- 244.Olson N. Kasahara DI. Hristova M. Bernstein R. Janssen-Heininger Y. van der Vliet A. Modulation of NF-kappaB and hypoxia-inducible factor—1 by S-nitrosoglutathione does not alter allergic airway inflammation in mice. Am J Respir Cell Mol Biol. 2011;44:813–823. doi: 10.1165/rcmb.2010-0035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Orlowski M. Meister A. The gamma-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci U S A. 1970;67:1248–1255. doi: 10.1073/pnas.67.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pacht ER. Timerman AP. Lykens MG. Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest. 1991;100:1397–1403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- 247.Pani G. Giannoni E. Galeotti T. Chiarugi P. Redox-based escape mechanism from death: the cancer lesson. Antioxid Redox Signal. 2009;11:2791–2806. doi: 10.1089/ars.2009.2739. [DOI] [PubMed] [Google Scholar]
- 248.Papi A. Contoli M. Gasparini P. Bristot L. Edwards MR. Chicca M. Leis M. Ciaccia A. Caramori G. Johnston SL. Pinamonti S. Role of xanthine oxidase activation and reduced glutathione depletion in rhinovirus induction of inflammation in respiratory epithelial cells. J Biol Chem. 2008;283:28595–28606. doi: 10.1074/jbc.M805766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Park CS. Kim TB. Lee KY. Moon KA. Bae YJ. Jang MK. Cho YS. Moon HB. Increased oxidative stress in the airway and development of allergic inflammation in a mouse model of asthma. Ann Allergy Asthma Immunol. 2009;103:238–247. doi: 10.1016/S1081-1206(10)60188-3. [DOI] [PubMed] [Google Scholar]
- 250.Pearson DJ. Suarez-Mendez VJ. Day JP. Miller PF. Selenium status in relation to reduced glutathione peroxidase activity in aspirin-sensitive asthma. Clin Exp Allergy. 1991;21:203–208. doi: 10.1111/j.1365-2222.1991.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 251.Peltoniemi M. Kaarteenaho-Wiik R. Saily M. Sormunen R. Paakko P. Holmgren A. Soini Y. Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol. 2004;35:1000–1007. doi: 10.1016/j.humpath.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 252.Peltoniemi MJ. Rytila PH. Harju TH. Soini YM. Salmenkivi KM. Ruddock LW. Kinnula VL. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res. 2006;7:133. doi: 10.1186/1465-9921-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pennings HJ. Borm PJ. Evelo CT. Wouters EF. Changes in levels of catalase and glutathione in erythrocytes of patients with stable asthma, treated with beclomethasone dipropionate. Eur Respir J. 1999;13:1260–1266. doi: 10.1183/09031936.99.13612679. [DOI] [PubMed] [Google Scholar]
- 254.Petlevski R. Zuntar I. Dodig S. Turkalj M. Cepelak I. Vojvodic J. Sicaja M. Missoni S. Malonaldehyde and erythrocyte antioxidant status in children with controlled asthma. Coll Antropol. 2009;33:1251–1254. [PubMed] [Google Scholar]
- 255.Picado C. Deulofeu R. Lleonart R. Agusti M. Mullol J. Quinto L. Torra M. Dietary micronutrients/antioxidants and their relationship with bronchial asthma severity. Allergy. 2001;56:43–49. doi: 10.1034/j.1398-9995.2001.00793.x. [DOI] [PubMed] [Google Scholar]
- 256.Pineda-Molina E. Klatt P. Vazquez J. Marina A. Garcia de Lacoba M. Perez-Sala D. Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 257.Plaza V. Prat J. Rosello J. Ballester E. Ramis I. Mullol J. Gelpi E. Vives-Corrons JL. Picado C. In vitro release of arachidonic acid metabolites, glutathione peroxidase, and oxygen-free radicals from platelets of asthmatic patients with and without aspirin intolerance. Thorax. 1995;50:490–496. doi: 10.1136/thx.50.5.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Powell CV. Nash AA. Powers HJ. Primhak RA. Antioxidant status in asthma. Pediatr Pulmonol. 1994;18:34–38. doi: 10.1002/ppul.1950180109. [DOI] [PubMed] [Google Scholar]
- 259.Powers HJ. Hill MH. Mushtaq S. Dainty JR. Majsak-Newman G. Williams EA. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM) Am J Clin Nutr. 2011;93:1274–1284. doi: 10.3945/ajcn.110.008409. [DOI] [PubMed] [Google Scholar]
- 260.Qanungo S. Starke DW. Pai HV. Mieyal JJ. Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 261.Que LG. Liu L. Yan Y. Whitehead GS. Gavett SH. Schwartz DA. Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Que LG. Yang Z. Stamler JS. Lugogo NL. Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med. 2009;180:226–231. doi: 10.1164/rccm.200901-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Qujeq D. Hidari B. Bijani K. Shirdel H. Glutathione peroxidase activity and serum selenium concentration in intrinsic asthmatic patients. Clin Chem Lab Med. 2003;41:200–202. doi: 10.1515/CCLM.2003.032. [DOI] [PubMed] [Google Scholar]
- 264.Raguso CA. Regan MM. Young VR. Cysteine kinetics and oxidation at different intakes of methionine and cystine in young adults. Am J Clin Nutr. 2000;71:491–499. doi: 10.1093/ajcn/71.2.491. [DOI] [PubMed] [Google Scholar]
- 265.Rahman I. Antonicelli F. MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-alpha and dexamethasone in human alveolar epithelial cells. J Biol Chem. 1999;274:5088–5096. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- 266.Rahman I. Bel A. Mulier B. Donaldson K. MacNee W. Differential regulation of glutathione by oxidants and dexamethasone in alveolar epithelial cells. Am J Physiol. 1998;275:L80–L86. doi: 10.1152/ajplung.1998.275.1.L80. [DOI] [PubMed] [Google Scholar]
- 267.Rahman I. Biswas SK. Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 268.Rahman I. Gilmour PS. Jimenez LA. MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem. 2002;234–235:239–248. [PubMed] [Google Scholar]
- 269.Ramirez A. Ramadan B. Ritzenthaler JD. Rivera HN. Jones DP. Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L972–L981. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- 270.Rangasamy T. Guo J. Mitzner WA. Roman J. Singh A. Fryer AD. Yamamoto M. Kensler TW. Tuder RM. Georas SN. Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ray S. Misso NL. Lenzo JC. Robinson C. Thompson PJ. Gamma-glutamylcysteine synthetase activity in human lung epithelial (A549) cells: factors influencing its measurement. Free Radic Biol Med. 1999;27:1346–1356. doi: 10.1016/s0891-5849(99)00182-3. [DOI] [PubMed] [Google Scholar]
- 272.Ray S. Watkins DN. Misso NL. Thompson PJ. Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin Exp Allergy. 2002;32:571–577. doi: 10.1046/j.0954-7894.2002.01294.x. [DOI] [PubMed] [Google Scholar]
- 273.Reddy NM. Kleeberger SR. Cho HY. Yamamoto M. Kensler TW. Biswal S. Reddy SP. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am J Respir Cell Mol Biol. 2007;37:3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Reid M. Badaloo A. Forrester T. Morlese JF. Frazer M. Heird WC. Jahoor F. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab. 2000;278:E405–E412. doi: 10.1152/ajpendo.2000.278.3.E405. [DOI] [PubMed] [Google Scholar]
- 275.Reynaert NL. van der Vliet A. Guala AS. McGovern T. Hristova M. Pantano C. Heintz NH. Heim J. Ho YS. Matthews DE. Wouters EF. Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Reynaert NL. Wouters EF. Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol. 2007;36:147–151. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Reynolds RD. Natta CL. Depressed plasma pyridoxal phosphate concentrations in adult asthmatics. Am J Clin Nutr. 1985;41:684–688. doi: 10.1093/ajcn/41.4.684. [DOI] [PubMed] [Google Scholar]
- 278.Rhee SG. Chae HZ. Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 279.Ricciardolo FL. Di Stefano A. Sabatini F. Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol. 2006;533:240–252. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 280.Rogers AJ. Brasch-Andersen C. Ionita-Laza I. Murphy A. Sharma S. Klanderman BJ. Raby BA. The interaction of glutathione S-transferase M1-null variants with tobacco smoke exposure and the development of childhood asthma. Clin Exp Allergy. 2009;39:1721–1729. doi: 10.1111/j.1365-2222.2009.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rose WC. Nutrition Classics. Federation Proceedings 8(2)546–52, June 1949. Amino acid requirements of man. William C. Rose. Nutr Rev. 1976;34:307–309. doi: 10.1111/j.1753-4887.1976.tb05679.x. [DOI] [PubMed] [Google Scholar]
- 282.Roum JH. Aledia AS. Carungcong LA. Kim KJ. Borok Z. Extracellular glutathione inhibits oxygen-induced permeability changes in alveolar epithelial monolayers. J Appl Physiol. 2001;91:748–754. doi: 10.1152/jappl.2001.91.2.748. [DOI] [PubMed] [Google Scholar]
- 283.Roum JH. Borok Z. McElvaney NG. Grimes GJ. Bokser AD. Buhl R. Crystal RG. Glutathione aerosol suppresses lung epithelial surface inflammatory cell-derived oxidants in cystic fibrosis. J Appl Physiol. 1999;87:438–443. doi: 10.1152/jappl.1999.87.1.438. [DOI] [PubMed] [Google Scholar]
- 284.Sackesen C. Ercan H. Dizdar E. Soyer O. Gumus P. Tosun BN. Buyuktuncer Z. Karabulut E. Besler T. Kalayci O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol. 2008;122:78–85. doi: 10.1016/j.jaci.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 285.Samiec PS. Drews-Botsch C. Flagg EW. Kurtz JC. Sternberg P., Jr. Reed RL. Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 286.Samitas K. Chorianopoulos D. Vittorakis S. Zervas E. Economidou E. Papatheodorou G. Loukides S. Gaga M. Exhaled cysteinyl-leukotrienes and 8-isoprostane in patients with asthma and their relation to clinical severity. Respir Med. 2009;103:750–756. doi: 10.1016/j.rmed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 287.Sauberlich HE. Judd JH., Jr. Nichoalds GE. Broquist HP. Darby WJ. Application of the erythrocyte glutathione reductase assay in evaluating riboflavin nutritional status in a high school student population. Am J Clin Nutr. 1972;25:756–762. doi: 10.1093/ajcn/25.8.756. [DOI] [PubMed] [Google Scholar]
- 288.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 289.Schutze N. Lehmann I. Bonisch U. Simon JC. Polte T. Exposure to mycotoxins increases the allergic immune response in a murine asthma model. Am J Respir Crit Care Med. 2010;181:1188–1199. doi: 10.1164/rccm.200909-1350OC. [DOI] [PubMed] [Google Scholar]
- 290.Sen U. Mishra PK. Tyagi N. Tyagi SC. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem Biophys. 2010;57:49–58. doi: 10.1007/s12013-010-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shaheen SO. Newson RB. Rayman MP. Wong AP. Tumilty MK. Phillips JM. Potts JF. Kelly FJ. White PT. Burney PG. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 2007;62:483–490. doi: 10.1136/thx.2006.071563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shamseer L. Adams D. Brown N. Johnson JA. Vohra S. Antioxidant micronutrients for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2010;12:CD007020. doi: 10.1002/14651858.CD007020.pub2. [DOI] [PubMed] [Google Scholar]
- 293.Shanmugasundaram KR. Kumar SS. Rajajee S. Excessive free radical generation in the blood of children suffering from asthma. Clin Chim Acta. 2001;305:107–114. doi: 10.1016/s0009-8981(00)00425-3. [DOI] [PubMed] [Google Scholar]
- 294.Shimizu T. Maeda S. Arakawa H. Mochizuki H. Tokuyama K. Morikawa A. Relation between theophylline and circulating vitamin levels in children with asthma. Pharmacology. 1996;53:384–389. doi: 10.1159/000139454. [DOI] [PubMed] [Google Scholar]
- 295.Shoveller AK. Stoll B. Ball RO. Burrin DG. Nutritional and functional importance of intestinal sulfur amino acid metabolism. J Nutr. 2005;135:1609–1612. doi: 10.1093/jn/135.7.1609. [DOI] [PubMed] [Google Scholar]
- 296.Sies H. Oxidative Stress. London: Orlando: Academic Press; 1985. pp. xv–507. [Google Scholar]
- 297.Sivitz WI. Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Smith CV. Jones DP. Guenthner TM. Lash LH. Lauterburg BH. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 299.Smith LJ. Houston M. Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis. 1993;147:1461–1464. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- 300.Smith LJ. Shamsuddin M. Sporn PH. Denenberg M. Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med. 1997;22:1301–1307. doi: 10.1016/s0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- 301.Soboll S. Grundel S. Harris J. Kolb-Bachofen V. Ketterer B. Sies H. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of cell fractionation. Biochem J. 1995;311(Pt 3):889–894. doi: 10.1042/bj3110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Speisky H. Gomez M. Carrasco-Pozo C. Pastene E. Lopez-Alarcon C. Olea-Azar C. Cu(I)-glutathione complex: a potential source of superoxide radicals generation. Bioorg Med Chem. 2008;16:6568–6574. doi: 10.1016/j.bmc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 303.Staab CA. Alander J. Morgenstern R. Grafstrom RC. Hoog JO. The Janus face of alcohol dehydrogenase 3. Chem Biol Interact. 2009;178:29–35. doi: 10.1016/j.cbi.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 304.Stav D. Raz M. Effect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled study. Chest. 2009;136:381–386. doi: 10.1378/chest.09-0421. [DOI] [PubMed] [Google Scholar]
- 305.Stey C. Steurer J. Bachmann S. Medici TC. Tramer MR. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J. 2000;16:253–262. doi: 10.1034/j.1399-3003.2000.16b12.x. [DOI] [PubMed] [Google Scholar]
- 306.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 307.Stone J. Hinks LJ. Beasley R. Holgate ST. Clayton BA. Reduced selenium status of patients with asthma. Clin Sci (Lond) 1989;77:495–500. doi: 10.1042/cs0770495. [DOI] [PubMed] [Google Scholar]
- 308.Storch KJ. Wagner DA. Burke JF. Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol. 1988;255:E322–E331. doi: 10.1152/ajpendo.1988.255.3.E322. [DOI] [PubMed] [Google Scholar]
- 309.Storch KJ. Wagner DA. Young VR. Methionine kinetics in adult men: effects of dietary betaine on L-[2H3-methyl-1-13C]methionine. Am J Clin Nutr. 1991;54:386–394. doi: 10.1093/ajcn/54.2.386. [DOI] [PubMed] [Google Scholar]
- 310.Szefler SJ. Advancing asthma care: the glass is only half full! J Allergy Clin Immunol. 2011;128:485–494. doi: 10.1016/j.jaci.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tamer L. Calikoglu M. Ates NA. Yildirim H. Ercan B. Saritas E. Unlu A. Atik U. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology. 2004;9:493–498. doi: 10.1111/j.1440-1843.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 312.Tekin D. Sin BA. Mungan D. Misirligil Z. Yavuzer S. The antioxidative defense in asthma. J Asthma. 2000;37:59–63. doi: 10.3109/02770900009055428. [DOI] [PubMed] [Google Scholar]
- 313.Testa B. Mesolella M. Testa D. Giuliano A. Costa G. Maione F. Iaccarino F. Glutathione in the upper respiratory tract. Ann Otol Rhinol Laryngol. 1995;104:117–119. doi: 10.1177/000348949510400205. [DOI] [PubMed] [Google Scholar]
- 314.Testa B. Testa D. Mesolella M. D'Errico G. Tricarico D. Motta G. Management of chronic otitis media with effusion: the role of glutathione. Laryngoscope. 2001;111:1486–1489. doi: 10.1097/00005537-200108000-00028. [DOI] [PubMed] [Google Scholar]
- 315.Tho LL. Candlish JK. Superoxide dismutase and glutathione peroxidase activities in erythrocytes as indices of oxygen loading in disease: a survey of one hundred cases. Biochem Med Metab Biol. 1987;38:74–80. doi: 10.1016/0885-4505(87)90064-8. [DOI] [PubMed] [Google Scholar]
- 316.Thompson CM. Grafstrom RC. Mechanistic considerations for formaldehyde-induced bronchoconstriction involving S-nitrosoglutathione reductase. J Toxicol Environ Health A. 2008;71:244–248. doi: 10.1080/15287390701598259. [DOI] [PubMed] [Google Scholar]
- 317.Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004;58:391–402. doi: 10.1038/sj.ejcn.1601800. [DOI] [PubMed] [Google Scholar]
- 318.Thuesen BH. Husemoen LL. Ovesen L. Jorgensen T. Fenger M. Gilderson G. Linneberg A. Atopy, asthma, and lung function in relation to folate and vitamin B(12) in adults. Allergy. 2010;65:1446–1454. doi: 10.1111/j.1398-9995.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 319.Torii M. Wang L. Ma N. Saito K. Hori T. Sato-Ueshima M. Koyama Y. Nishikawa H. Katayama N. Mizoguchi A. Shiku H. Yodoi J. Kuribayashi K. Kato T. Thioredoxin suppresses airway inflammation independently of systemic Th1/Th2 immune modulation. Eur J Immunol. 2010;40:787–796. doi: 10.1002/eji.200939724. [DOI] [PubMed] [Google Scholar]
- 320.Tucker KL. Olson B. Bakun P. Dallal GE. Selhub J. Rosenberg IH. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am J Clin Nutr. 2004;79:805–811. doi: 10.1093/ajcn/79.5.805. [DOI] [PubMed] [Google Scholar]
- 321.Turner JM. Humayun MA. Elango R. Rafii M. Langos V. Ball RO. Pencharz PB. Total sulfur amino acid requirement of healthy school-age children as determined by indicator amino acid oxidation technique. Am J Clin Nutr. 2006;83:619–623. doi: 10.1093/ajcn.83.3.619. [DOI] [PubMed] [Google Scholar]
- 322.Agricultural Research Service, U.S. Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 23. 2010. Nutrient Data Laboratory Homepage. www.ars.usda.gov/ba/bhnrc/ndl www.ars.usda.gov/ba/bhnrc/ndl
- 323.Ubbink JB. van der Merwe A. Delport R. Allen RH. Stabler SP. Riezler R. Vermaak WJ. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest. 1996;98:177–184. doi: 10.1172/JCI118763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ubbink JB. Vermaak WJ. Delport R. Serfontein WJ. Bartel P. The relationship between vitamin B6 metabolism, asthma, and theophylline therapy. Ann N Y Acad Sci. 1990;585:285–294. doi: 10.1111/j.1749-6632.1990.tb28061.x. [DOI] [PubMed] [Google Scholar]
- 325.van de Poll MC. Dejong CH. Soeters PB. Adequate range for sulfur-containing amino acids and biomarkers for their excess: lessons from enteral and parenteral nutrition. J Nutr. 2006;136:1694S–1700S. doi: 10.1093/jn/136.6.1694S. [DOI] [PubMed] [Google Scholar]
- 326.Varraso R. Kauffmann F. Leynaert B. Le Moual N. Boutron-Ruault MC. Clavel-Chapelon F. Romieu I. Dietary patterns and asthma in the E3N study. Eur Respir J. 2009;33:33–41. doi: 10.1183/09031936.00130807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Verhoef P. van Vliet T. Olthof MR. Katan MB. A high-protein diet increases postprandial but not fasting plasma total homocysteine concentrations: a dietary controlled, crossover trial in healthy volunteers. Am J Clin Nutr. 2005;82:553–558. doi: 10.1093/ajcn.82.3.553. [DOI] [PubMed] [Google Scholar]
- 328.Vural H. Aksoy N. Ceylan E. Gencer M. Ozguner F. Leukocyte oxidant and antioxidant status in asthmatic patients. Arch Med Res. 2005;36:502–506. doi: 10.1016/j.arcmed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 329.Vural H. Uzun K. Serum and red blood cell antioxidant status in patients with bronchial asthma. Can Respir J. 2000;7:476–480. doi: 10.1155/2000/907478. [DOI] [PubMed] [Google Scholar]
- 330.Wahllander A. Soboll S. Sies H. Linke I. Muller M. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979;97:138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- 331.Wald DS. Law M. Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Walther UI. Changes in the glutathione system of lung cell lines after treatment with hydrocortisone. Arch Toxicol. 2004;78:402–409. doi: 10.1007/s00204-004-0557-0. [DOI] [PubMed] [Google Scholar]
- 333.Wang J. Boja ES. Tan W. Tekle E. Fales HM. English S. Mieyal JJ. Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 334.Wang W. Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–356. [PubMed] [Google Scholar]
- 335.Wang Y. Manevich Y. Feinstein SI. Fisher AB. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1188–L1193. doi: 10.1152/ajplung.00288.2003. [DOI] [PubMed] [Google Scholar]
- 336.Ward M. McNulty H. McPartlin J. Strain JJ. Weir DG. Scott JM. Effect of supplemental methionine on plasma homocysteine concentrations in healthy men: a preliminary study. Int J Vitam Nutr Res. 2001;71:82–86. doi: 10.1024/0300-9831.71.1.82. [DOI] [PubMed] [Google Scholar]
- 337.Watson WH. Chen Y. Jones DP. Redox state of glutathione and thioredoxin in differentiation and apoptosis. Biofactors. 2003;17:307–314. doi: 10.1002/biof.5520170130. [DOI] [PubMed] [Google Scholar]
- 338.Watson WH. Jones DP. Oxidation of nuclear thioredoxin during oxidative stress. FEBS Lett. 2003;543:144–147. doi: 10.1016/s0014-5793(03)00430-7. [DOI] [PubMed] [Google Scholar]
- 339.Weissbach H. Resnick L. Brot N. Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta. 2005;1703:203–212. doi: 10.1016/j.bbapap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 340.Werner R. Manthey KC. Griffin JB. Zempleni J. HepG2 cells develop signs of riboflavin deficiency within 4 days of culture in riboflavin-deficient medium. J Nutr Biochem. 2005;16:617–624. doi: 10.1016/j.jnutbio.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wheeler MD. Thurman RG. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol. 1999;277:L952–L959. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- 342.Whitekus MJ. Li N. Zhang M. Wang M. Horwitz MA. Nelson SK. Horwitz LD. Brechun N. Diaz-Sanchez D. Nel AE. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168:2560–2567. doi: 10.4049/jimmunol.168.5.2560. [DOI] [PubMed] [Google Scholar]
- 343.Williams MA. Rangasamy T. Bauer SM. Killedar S. Karp M. Kensler TW. Yamamoto M. Breysse P. Biswal S. Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–4559. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wisnewski AV. Liu Q. Liu J. Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35:352–357. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- 345.Witherden IR. Vanden Bon EJ. Goldstraw P. Ratcliffe C. Pastorino U. Tetley TD. Primary human alveolar type II epithelial cell chemokine release: effects of cigarette smoke and neutrophil elastase. Am J Respir Cell Mol Biol. 2004;30:500–509. doi: 10.1165/rcmb.4890. [DOI] [PubMed] [Google Scholar]
- 346.Wong PS. Eiserich JP. Reddy S. Lopez CL. Cross CE. van der Vliet A. Inactivation of glutathione S-transferases by nitric oxide-derived oxidants: exploring a role for tyrosine nitration. Arch Biochem Biophys. 2001;394:216–228. doi: 10.1006/abbi.2001.2532. [DOI] [PubMed] [Google Scholar]
- 347.Wood LG. Fitzgerald DA. Gibson PG. Cooper DM. Garg ML. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids. 2000;35:967–974. doi: 10.1007/s11745-000-0607-x. [DOI] [PubMed] [Google Scholar]
- 348.Wood LG. Garg ML. Blake RJ. Simpson JL. Gibson PG. Oxidized vitamin E and glutathione as markers of clinical status in asthma. Clin Nutr. 2008;27:579–586. doi: 10.1016/j.clnu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 349.Wu H. Romieu I. Sienra-Monge JJ. Estela Del Rio-Navarro B. Anderson DM. Jenchura CA. Li H. Ramirez-Aguilar M. Del Carmen Lara-Sanchez I. London SJ. Genetic variation in S-nitrosoglutathione reductase (GSNOR) and childhood asthma. J Allergy Clin Immunol. 2007;120:322–328. doi: 10.1016/j.jaci.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yamada Y. Nakamura H. Adachi T. Sannohe S. Oyamada H. Kayaba H. Yodoi J. Chihara J. Elevated serum levels of thioredoxin in patients with acute exacerbation of asthma. Immunol Lett. 2003;86:199–205. doi: 10.1016/s0165-2478(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 351.Yang SR. Chida AS. Bauter MR. Shafiq N. Seweryniak K. Maggirwar SB. Kilty I. Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 352.Yeh MY. Burnham EL. Moss M. Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med. 2007;176:270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yeh MY. Burnham EL. Moss M. Brown LA. Non-invasive evaluation of pulmonary glutathione in the exhaled breath condensate of otherwise healthy alcoholics. Respir Med. 2008;102:248–255. doi: 10.1016/j.rmed.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yim PD. Gallos G. Xu D. Zhang Y. Emala CW. Novel expression of a functional glycine receptor chloride channel that attenuates contraction in airway smooth muscle. FASEB J. 2011;25:1706–1717. doi: 10.1096/fj.10-170530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Young VR. Borgonha S. Nitrogen and amino acid requirements: the Massachusetts Institute of Technology amino acid requirement pattern. J Nutr. 2000;130:1841S–1849S. doi: 10.1093/jn/130.7.1841S. [DOI] [PubMed] [Google Scholar]
- 356.Young VR. Wagner DA. Burini R. Storch KJ. Methionine kinetics and balance at the 1985 FAO/WHO/UNU intake requirement in adult men studied with L-[2H3-methyl-1-13C]methionine as a tracer. Am J Clin Nutr. 1991;54:377–385. doi: 10.1093/ajcn/54.2.377. [DOI] [PubMed] [Google Scholar]
- 357.Zaman K. Hanigan MH. Smith A. Vaughan J. Macdonald T. Jones DR. Hunt JF. Gaston B. Endogenous S-nitrosoglutathione modifies 5-lipoxygenase expression in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:387–393. doi: 10.1165/rcmb.2005-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zavorsky GS. Kubow S. Grey V. Riverin V. Lands LC. An open-label dose-response study of lymphocyte glutathione levels in healthy men and women receiving pressurized whey protein isolate supplements. Int J Food Sci Nutr. 2007;58:429–436. doi: 10.1080/09637480701253581. [DOI] [PubMed] [Google Scholar]