Abstract
Aims
This study aims to investigate the mechanisms in the apparent preference for mitogen-activated protein kinase /ERK signaling through interleukin (IL)-6R in dermal fibroblasts.
Methods
Dermal fibroblasts isolated from IL-6KO mice were pretreated with specific ERK or STAT3 chemical inhibitors or SOCS3 specific siRNA and treated with rmIL-6. Phosphorylation was monitored via enzyme-linked immunosorbent assay or immunohistology. SOCS3 interaction with p120Ras-Gap was examined by co-immunoprecipitation and Western blot. Expression of MMP2 mRNA was assessed via real-time quantitative polymerase chain reaction.
Results
A dose response phosphorylation of ERK1/2 occurred while no STAT3 activation (p-Tyr705) was induced after IL-6 treatment, despite an increase in Ser727 phosphorylation. Inhibition of STAT3 in fibroblasts potentiated IL-6R induced ERK phosphorylation and vice versa. Phosphorylated SOCS3 and p120 RasGAP co-immunoprecipitated in response to IL-6 treatment. SOCS3 siRNA knockdown allowed STAT3 phosphorylation after rmIL-6 treatment. Chemical inhibition of IL-6R signaling altered the IL-6 modulated mRNA expression of MMP-2.
Conclusions
SOCS3 interaction with p120 Ras-Gap plays a role in determining the preference for IL-6R signaling through ERK in dermal fibroblasts. This study provides insight into the pleiotropic nature of IL-6 and the selective signaling mechanism elicited by the IL-6R system in dermal fibroblasts. It may further indicate a method for manipulation of IL-6R function.
Introduction
Interleukin-6 (IL-6) is a multifunctional cytokine involved with numerous cellular processes (Kamimura and others 2003). Originally discovered as a B-cell growth and differentiation factor, IL-6 was later implicated in the regulation of hematopoiesis and liver-specific process such as regeneration and the acute phase response (Kopf and others 1995), and facilitating the transition between innate and acquired immunity (Jones and Rose-John 2002; Kaplanski and others 2003). Additionally, IL-6 plays a governing role in inflammation, acting as a pro- and anti-inflammatory regulator. IL-6 is released by numerous immune cells, and muscle cells, endothelial cells, keratinocytes, and fibroblasts (Sehgal 1990). Control of IL-6 expression is carefully maintained by necessity as altered levels can contribute to various pathologies such as autoimmune diseases, carcinogenesis, and impaired wound healing (Gallucci and others 2000; Paschoud and others 2006).
IL-6 exerts its effects by signaling through a multi-subunit receptor complex composed of a ligand-binding 80 kDa IL-6 receptor alpha subunit (IL-6R-α) and a 130 kDa signal transducing subunit (gp130) (Heinrich and others 2003). The alpha subunit primarily exists in the transmembrane form, but a soluble form can be produced that allows for so called “trans-signaling” where the IL-6:sIL-6R-α complex can have an effect on any cell expressing gp130 (Kamimura and others 2003). The gp130 subunit is ubiquitously expressed and exists in both a membrane bound and soluble (sgp130) form (Hibi and others 1990), however unlike the sIL-6R-α, sgp130 acts as an IL-6R antagonist (Narazaki and others 1993). The gp130 subunit has no intrinsic kinase activity but instead contains regions required for its association with nonreceptor Janus tyrosine kinases (JAK). Dimerization of the receptor complex allows receptor bound Jaks to phosphorylate and activate one another, triggering three distinct signaling pathways; JAK/STAT, MAPK/ERK, or AKT/PKB (Heinrich and others 2003; Kamimura and others 2003). Each pathway can function independent of one another; however, cross talk between them also occurs (Kamimura and others 2003). This complex interaction that exists between pathways associated with IL-6R signaling has been termed the “Signal Orchestration Model” (Kamimura and others 2003). This model may explain the very pleiotropic nature of IL-6 and suggests that the overall balance of distinct signals could determine the final biological outputs elicited by its receptor (Kamimura and others 2003).
The control of IL-6 activity is maintained through several negative regulators including protein tyrosine phosphatases, protein inhibitors of activated STAT (PIAS), and members of the suppressor of cytokine signaling (SOCS) family (Fischer and others 2004). In particular, SOCS3 can be rapidly induced by IL-6 (Larsen and Ropke 2002) where it usually acts as classical feedback inhibitor by physically interfering with JAK kinase activity (Fischer and others 2004) and STAT3 nuclear transport (Larsen and Ropke 2002). SOCS3 can also be phosphorylated on two key residues (Y204 and Y221) allowing it to modulate the MAPK/ERK pathway by interacting with Ras GTPase activating protein 120 (p120 Ras-GAP) (Cacalano and others 2001) causing sustained MAPK/ERK signaling (Pamonsinlapatham and others 2009).
It is well known that IL-6 plays a key role in the healing of various organs including liver (Cressman and others 1995) and skin (Gallucci and others 2000). While dermal fibroblasts represent a minor source of IL-6 within the skin as compared with inflammatory cells, they play a central role in the proper healing of wounds. Previous studies investigating the IL-6R signaling mechanisms involved in the modulation of wound healing found that dermal fibroblasts in IL-6 deficient mice (IL-6KO) signal primarily through the ERK pathway (Gallucci and others 2006). Since the JAK/STAT pathway has been reported as a major signaling cascade in response to IL-6R activation, this lack of signaling is perplexing. The current study sought to investigate the mechanisms that are involved in the preference for ERK1/2 signaling in dermal fibroblasts in response to IL-6. Herein it was found that SOCS3 phosphorylated after IL-6 treatment and interacted with the p120 RasGAP. Serine phosphorylation of STAT3 (S727) occurred after IL-6 treatment in these cells but did not result in increased STAT3 function. Knockdown of SOCS3 expression via siRNA, or inhibition of ERK signaling utilizing chemical inhibitors potentiated JAK/STAT signaling in these cells. The functional consequences of selective chemical manipulation of IL-6R signaling were also demonstrated, as IL-6 induced transcriptional modulation of MMP2 was profoundly altered in fibroblasts. These data may partially explain the signaling preference toward the MAPK/ERK pathway in dermal fibroblasts, provide further insight into the pleiotropic nature of IL-6 and its association with cross talk of IL-6R signaling pathways, and indicate the possibility of pharmaceutical manipulation of IL-6R function.
Materials and Methods
Fibroblast isolation
Dermis was collected and separated from epidermis as previously described (Gallucci and others 2004a). Briefly, skin was collected from mouse pups (0–48 h old), rinsed in 70% ethanol. Skins were incubated dermis down in culture dishes containing phosphate-buffered saline (PBS)+3.6% dispase (Sigma, St Louis, MO) overnight at 4°C. The dermis was separated from the epidermis and placed in a collagenase solution (1 mg/mL in PBS), incubated at 37°C, vortexed, and sterile filtered. Cells were adjusted to a concentration of 5×106 and frozen in CryoStore media (VWR, West Chester, PA). Fibroblasts were resuspended in dulbecco's modified Eagle's medium (DMEM)+5% fetal bovine serum (Invitrogen, Carlsbad, CA), plated at 8×103 cells cm−2 in 60 or 100 mm culture dishes, and incubated at 37°C and 5% CO2 and 95% room air. The primary C57/BL6 and IL-6KO fibroblasts were cryopreserved at −140°C until further culture.
Fibroblast culture and treatment
Fibroblasts were isolated and cultured as described. Before treatment with rmIL-6, fibroblasts were washed in PBS, and culture media was replaced with serum free DMEM containing 60 IU/mL penicillin, 100 UI/mL streptomycin, and 1 mg/mL Bovine Serum Albumin for 1 h. For wild type (WT) fibroblasts, cells were incubated for 2 h in serum free media, and media was replaced every 30 min. If cultures were exposed to signal transduction inhibitors, LLL-3 (a gift from Dr. Pui-Kai Li, Ohio State University) and PD98059 (EMD Biosciences, San Diego, CA were used at a concentration of 20 μM (Song and others 2005) and 30 μM, respectively. Each inhibitor or dimethyl sulfoxide (DMSO) (vehicle) was added to respective culture wells. The fibroblasts were incubated with the inhibitor or vehicle for 30 min, and various concentrations of IL-6 were then added to the plates for a 1-h incubation or followed a time course. After treatment, fibroblasts were collected for mRNA or protein analysis as described below.
Immunoprecipitation
IL-6KO dermal fibroblasts were prepared as described above and cultured to 90% confluency in 100 mm culture plates. Before treatment, fibroblasts were cultured in serum free DMEM for 2 h. IL-6 KO fibroblasts were then treated for 2 h with the proteosomal inhibitor MG132 (10 μM) to facilitate the detection of phosphorylated SOCS3 (Haan and others 2003). The inhibitors PD98059 and LLL3 were then applied at 30 μM and 20 μM respectively for 30 min, then treated with various rmIL-6 concentrations. At indicated time points, culture plates were rinsed once with ice-cold phosphate buffered saline, and co-immunoprecipitation was carried out essentially as previously described (Kamat and others 2007). Isolated total protein was determined and 100.0 μg of protein from each sample was incubated with the appropriate antibody while rocking at 4°C overnight. Protein-G Sepharose beads (40 μL, Sigma) were precleared with cell lysis buffer containing 1% milk for 1 h before immunoprecipitation. After preclearing, 20 μL of beads were incubated on a rocker with protein sample at 4°C for 1.5 h. Samples were then spun down, and an additional 20 μL of beads were added to the sample for 1.5 h at 4°C. After final incubation, samples were then spun down and washed two times with completed cell lysis buffer (containing protease inhibitors and PMSF). After final wash, beads were resuspended in 25 μL of cell lysis buffer plus 25 μL of 2× Laemmli denaturing sample buffer (Sigma). Samples were electrophoresed on 4%–20% Precise Protein Gradient gels (Thermo Scientific, Rockford, IL). Western blot was performed as previously described (Gallucci and others 2006). Monoclonal anti-phospho-tyrosine (Cell Signaling, Boston, MA), anti-p120 RasGAP (Santa Cruz Biotechnology, Santa Cruz, CA), anti-ERK (Cell Signaling), and anti-phospho-ERK (Cell Signaling) were applied to the blots at a 1:1,000 dilution and fluorescently labeled secondary anti-species polyclonal antibody(s) (Rockland, Gilbertsville, PA) at a 1:10,000 dilution. Blots were imaged via the Molecular Imager ProPlus imaging system (BioRad, Hercules, CA).
Real-time quantitative polymerase chain reaction
Total RNA from mouse skin or dermal IL-6KO fibroblasts was prepared and cDNA synthesized as previously described (Gallucci and others 2004a). Primers for mouse genes were synthesized by Invitrogen. Quantitative polymerase chain reaction was performed on an ABI PRISM 7000 SDS, utilizing SYBR Green Mastermix (Anaspec, Fremont, CA) according to the manufacturer's instructions. Quantitative values of genes of interest were normalized based on 28s rRNA content using the ddCt method (Livak and Schmittgen 2001).
Enzyme-linked immunosorbent assay analysis
Total and phosphorylated ERK or STAT protein from IL-6KO fibroblast culture was measured using cell-based enzyme-linked immunosorbent assay (ELISA). (Raybiotech, Norcross, GA) IL-6KO fibroblast cells (6×104/well) were seeded in a 96-well plate and incubated until cells were confluent in each well. Upon confluency, cells were serum starved for 1 h and then treated with inhibitors and IL-6 as previously described. The ELISA was performed according to the manufacturer's instructions and analyzed using a 96-well plate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis
All experiments were replicated, and representative findings are shown. Statistical significance was determined by one-way analysis of variance. When the F value was significant, the means were compared using Fisher post-hoc analysis. In all statistical comparisons, a P value of <0.05 was used to indicate a significant difference.
Results
ERK and STAT3 pathway interact in dermal fibroblasts during IL-6R signaling
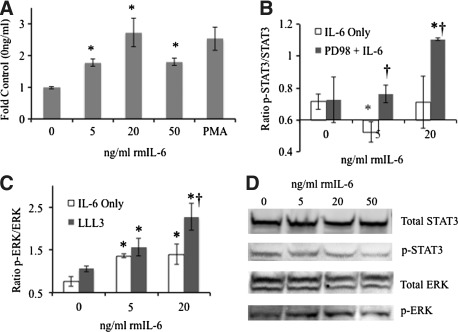
While the JAK/STAT pathway is the most dogmatic of the IL-6R signaling cascades, a previous time course investigation by this laboratory revealed that upon IL-6 treatment, ERK phosphorylation occurred within 1 min, reaching a maximum by 10 min, while minimal alteration in STAT3 phosphorylation was seen in IL-6KO fibroblasts (Gallucci and others 2006). To examine ERK phosphorylation in a dose response to IL-6 treatment, IL-6KO fibroblasts were treated with various concentrations of rmIL-6 for 10 min and phorbol 12-myristate 13-acetate (PMA) as a positive control. After treatment, total and phosphorylated ERK protein were assessed via ELISA. Similar to the previous report, IL-6 induced phosphorylation of ERK after treatment with 5 ng/mL rmIL-6, with maximal phosphorylation at 20 ng/mL with both expression levels similar to that seen with PMA treatment (Fig. 1a).
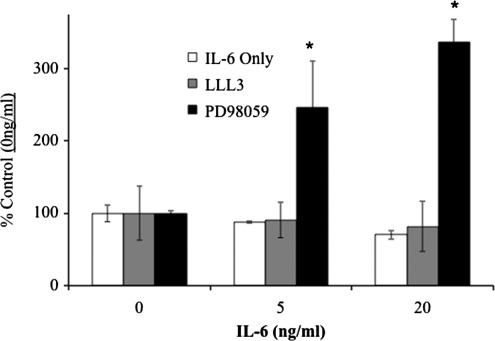
FIG. 1.
Dermal fibroblast IL-6R signaling preference and alteration by chemical inhibitors. Dermal fibroblasts were grown to 80% confluency in 96-well (IL-6KO, A, B, C) or 100 mm culture plates (WT, B). Where indicated, growth media was replaced and cultures were treated with various concentration of PD98059 (ERK inhibitor, B), or LLL-3 (STAT3 Inhibitor, C) 30 min before rmIL-6 treatment as described. Total and Phosphorylated ERK (A, C) or STAT3 (B) was determined via in-cell ELISA according to manufacturer's instructions (RayBiotech, Norcross, GA). Data (A–C) are expressed as mean±SE, (n=6, *Significantly different from 0 ng/mL, †Significantly different from IL-6 alone treatment, P≤0.05). WT fibroblasts (D) grown in 100 mm culture plates were treated with rmIL-6 as described and analyzed via Western blot utilizing anti-STAT3 and anti-p-STAT3-Y705, or total ERK1/2 and p-ERK1/2 antibodies and fluorescently labeled secondary antibodies. Images were obtained utilizing a Molecular Imager ProPlus (BioRad). Representative image shown of three replicate experiments. IL, interleukin; WT, wild type; SE, standard error.
To examine cross talk that may occur between IL-6R signaling pathways, IL-6KO fibroblast cultures were treated with signal transduction inhibitors 30 min before being treated with various concentrations of rmIL-6. Fibroblasts were pretreated with the inhibitors LLL-3 (a novel STAT3 inhibitor (Song and others 2005)) or PD98059 (a MAPK/ERK inhibitor) and total and phosphorylated STAT3 or ERK1/2 was analyzed by ELISA. IL-6 treatment alone either significantly decreased (5 ng/mL) or did not affect (20 ng/mL) STAT3 phosphorylation in these cells, while pretreatment with PD98059 abrogated the inhibition of STAT3 activation at 5 and 20 ng/mL rmIL-6 (Fig. 1b). Conversely, when fibroblasts were treated with the STAT3 inhibitor LLL3, IL-6 induced ERK phosphorylation was significantly augmented compared with IL-6 treatment alone (Fig. 1c).
While these data expand on previous results, it was necessary to determine whether ERK signaling preference also occurs in WT fibroblasts. Thus, fibroblasts from C57BL/6 mice were cultured as described and exposed to rmIL-6 for 10 min. Western blot indicated that indeed STAT3 phosphorylation was not induced by IL-6 treatment, while phosphorylation of ERK was apparent, primarily in the ERK2 band (Fig. 1d) similar to Fig. 1a and previous findings (Gallucci and others 2006).
IL-6 induces phosphorylation of serine727 on STAT3
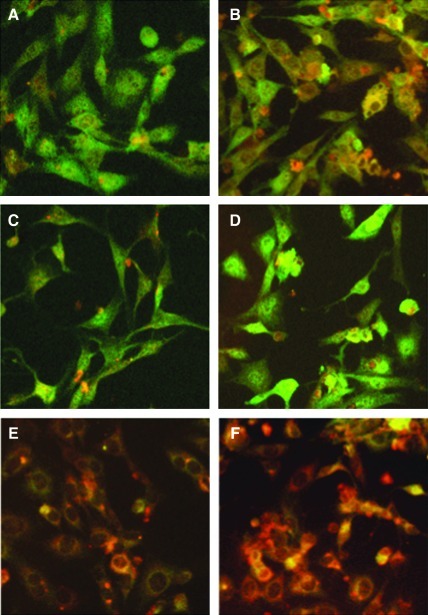
Historically it was thought that only single tyrosine residues on the STAT protein were phosphorylated as a result of cytokine stimulation. However, serine phosphorylation of STATs can occur through MAPK and has been demonstrated to result in positive regulation of its activity (Abe and others 2001; Schuringa and others 2000). To determine if STAT3 serine phosphorylation was modulated as a result of IL-6 treatment, IL-6KO fibroblasts were treated with 0 and 20 ng/mL of rmIL-6 for 30 min, and STAT3 phospho-serine 727 (p-S727) was visualized by immunohistology. Staining for STAT3 was apparent in both cytoplasm and nucleus of untreated cells (green), while p-S727 (red) was very low (Fig. 2a). Upon treatment with 20 ng/mL of rmIL-6, p-S727 of STAT3 is readily detected, but rmIL-6 did not appear to increase nuclear translocation of STAT3 (Fig. 2b). Pretreatment with PD98059 had little effect on either STAT3 or p-S727 staining in untreated cells (Fig. 2c), while IL-6 treatment appeared to increase STAT3 nuclear staining (Fig. 2d). Pretreatment with the STAT3 inhibitor LLL3 appeared to decrease nuclear staining of STAT3 while augmenting phosphorylation of S727 (Fig. 2e). IL-6 treatment further increased the intensity of p-S727 staining, while not affecting STAT3 nuclear staining (Fig. 2f).
FIG. 2.
IL-6 induces STAT3 serine phosphorylation in IL-6KO dermal fibroblasts. IL-6KO fibroblasts were grown to confluency on multi-chamber slides and pretreated with saline control (A, B), PD98059 (C, D), or LLL-3 (E, F). After preincubation, the cells were treated with either 0 ng/mL (A, C, E) or 20 ng/mL (B, D, F) rmIL-6. After paraformaldehyde fixation, slides were incubated with anti-phospho-S727 STAT3 (red), or anti-STAT3 (green) antibody and detected using Alexafluor 546 or 488 conjugated secondary antibodies respectively. Stained slides were visualized using a Leica DM400B fluorescence microscope and 20× objective, photographs are representative of three replicate experiments.
IL-6 induces SOCS3 Phosphorylation and association with p120 RasGAP
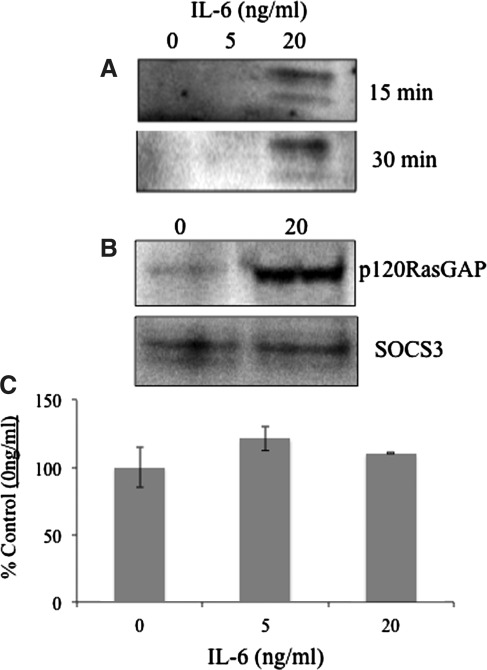
Aside from its negative regulatory functions on STAT3 signaling, SOCS3 can be phosphorylated on key tyrosine residues and interact with the Ras inhibitor p120 RasGAP (Cacalano and others 2001). This ultimately results in disinhibition and potentiation of the MAPK signaling cascade. To investigate whether IL-6 treatment led to phosphorylation of SOCS3 and physical association with p120 RasGAP, fibroblasts were treated with rmIL-6 for various time periods and immunoprecipitation/Western blots (ip) were preformed. Analysis of proteins (100 μg total protein each sample) immunoprecipitated via a SOCS3 specific antibody showed that treatment with 20 ng/mL of IL-6 induced SOCS3 phosphorylation after 15 and 30 min of treatment, as indicated by a lone doublet band revealed by phospho-tyrosine staining (Fig. 3a). It was also found that p120 RasGAP coimmunoprecipitated with phosphorylated SOCS3 after 15 min treatment as well with 20 ng/mL of IL-6 (Fig. 3b). IL-6 treatment (15 min) did not affect SOCS3 protein (Fig. 3b) or mRNA expression (Fig. 3c).
FIG. 3.
IL-6 induces SOCS3 phosphorylation resulting in an association with p120 RasGAP. IL-6KO dermal fibroblasts were cultured as described and treated with the indicated concentrations of rmIL-6 for up to 30 min. Cells were lysed and 100 μg of total protein per sample was immunoprecipitated with anti-SOCS3 antibody. SDS-PAGE gels were transferred to nitrocellulose membranes and blotted with anti-phospho-tyrosine (A), anti-p120 RasGap (B, upper panel), or anti-SOCS3 (B, lower panel). Expression of SOCS3 mRNA was evaluated after IL-6 treatment in fibroblasts (C) as determined by QPCR (n=4). SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; QPCR, quantitative polymerase chain reaction.
Knockdown of SOCS3 expression restores STAT3 phosphorylation in fibroblasts
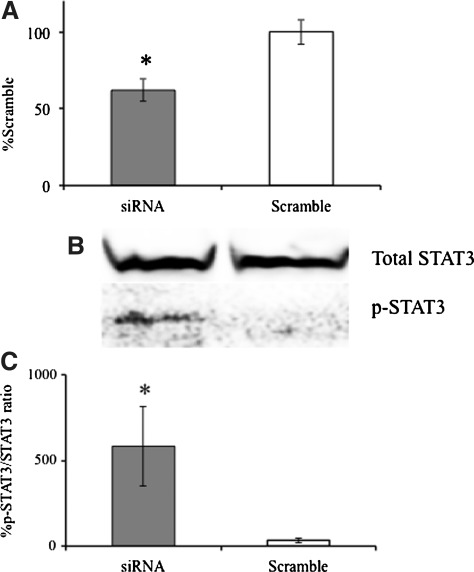
Given that SOCS3 may be a key regulator of IL-6R signal transduction in IL-6KO fibroblasts, it was of interest to determine whether the inhibition of SOCS3 expression would potentiate JAK/STAT activity in IL-6KO fibroblasts. To determine this, IL-6KO fibroblasts were electroporated (Lonza, Walkersville, MD) and transfected with SOCS3 specific siRNA oligonucleotide constructs (Qiagen, Valencia, CA). Twenty-four hours post transfection, fibroblasts were treated with 20 ng/mL rmIL-6 for 30 min. Co-transfection with green fluorescent protein allowed for optimization of >30% transfection with >50% viability in this cell type (not shown). SOCS3 mRNA expression significantly decreased in transfected cells compared with those treated with scrambled siRNA (Fig. 4a). Examination of STAT3 phosphorylation after knockdown of SOCS3 shows that treatment with 20 ng/mL of IL-6 significantly induced STAT3 phosphorylation compared with scrambled control transfected cells (Fig. 4b, c).
FIG. 4.
Knockdown of SOCS3 expression restores STAT3 phosphorylation in IL-6KO fibroblasts. IL-6KO dermal fibroblasts were grown as described and electroporated with SOCS specific siRNA and a scrambled control. Twenty-four hours post transfection, growth media was replaced with serum free media and treated up to 20 ng/mL rmIL-6 for 30 min. Total mRNA and protein were extracted from fibroblasts as described. Expression of SOCS3 (A) was determined via Real-time PCR utilizing an ABI 7000 SDS system and accompanying software. Phospho-STAT3 and total STAT3 was determined by Western blot (representative B) and differences determined by digital image analysis utilizing Image J (C). Data are expressed as a percent of untreated non-transfected control cells (A, C), mean±SE, (n=4, *Significantly different from 0 ng/mL, P≤0.05). SOCS, suppressor of cytokine signaling.
Modulation of IL-6R signaling regulates IL-6 induced gene expression
Matrix-metalloproteinases are important mediators of wound healing and remodeling in skin. The expression of MMP-2 in dermal fibroblasts can be modulated by the IL-6R through the MAPK pathway (Luckett and Gallucci 2007). To examine whether manipulation of either signaling pathways can have an effect on the expression of this gene, IL-6KO fibroblasts were pretreated with the inhibitors LLL3 (STAT3 inhibitor) and PD98059 (MAPK/ERK inhibitor), and IL-6 induced mRNA expression of MMP-2 was assessed. Treatment with LLL3 did not significantly affect the IL-6 mediated suppression of MMP-2 (Fig. 5, gray bars) mRNA expression. However, treatment with PD98059 and rmIL-6 significantly augmented MMP-2 mRNA over basal levels (Fig. 5, dark bars).
FIG. 5.
Signal transduction inhibitors modulate IL-6R induced gene expression. IL-6KO dermal fibroblasts were isolated as described and grown to 80% confluency. Growth media was replaced and cultures were treated with LLL-3 (20 μM, STAT3 Inhibitor), PD98059 (30 μM, ERK inhibitor), or vehicle (0.1% DMSO in culture media) 30 min before rmIL-6 treatment, as described in Methods. Expression of MMP-2 mRNA was determined via Real-time PCR utilizing an ABI 7000 SDS system, and accompanying software. Expression differences were determined relative to 28s rRNA utilizing the ddCt method. Data are expressed as mean±SE, (n=4), *Significantly different from IL-6 only treatment (P<0.05). DMSO, dimethyl sulfoxide.
Discussion
IL-6 can activate three functionally distinct signaling pathways through its receptor, yet these pathways can influence each other to varying degrees (Kamimura and others 2003). Utilizing the IL-6KO fibroblast model it was shown that these cells signal primarily through the MAPK/ERK pathway, with ERK phosphorylation occurring in a time (Gallucci and others 2006) and dose dependent manner (Fig. 1a). However, while this laboratory has previously shown that IL-6R primarily signals through the ERK pathway in these cells, it was not determined whether WT cells responded in a similar manner. Herein, it was found that normal mouse dermal fibroblasts as well appear to only signal through the ERK pathway with no induction of STAT3 phosphorylation up to 50 ng/mL (Fig. 1d). However, these cells require repeated stringent washing with serum free media before rmIL-6 treatment to obtain consistent basal p-ERK activation (not shown), presumably to remove endogenous IL-6. Indeed, the dose response seems to have shifted toward a lower peak concentration of IL-6 compared with IL-6KO fibroblasts, which may reflect residual IL-6 in the culture media or captured on receptor. Thus, studying IL-6R function in fibroblasts is ideal in the IL-6KO model as it offers the advantages of expressing active IL-6R, but does not allow production of IL-6 protein, thus making analysis of cell function less prone to artifacts from receptor feedback inhibition, and allowing consistent and precise determination of dose response relationships of rmIL-6 treatment. It should be taken into account that the preference for ERK signaling and lack of STAT3 activation in fibroblasts reported here occurred at ≤50 ng/mL IL-6, which is thought to be within the physiological range. Indeed, most research on IL-6R function is carried out utilizing approximately 10–20 ng/mL concentrations (for review (Heinrich and others 2003). However, it is possible that quite different signaling might occur at supraphysiological or even pharmacological doses of perhaps greater than 100 ng/mL, and further study will be necessary to determine whether this is the case.
Since IL-6R signaling can activate either JAK/STAT or MAPK/ERK pathways, it was of interest to determine whether the activation of one pathway may result in an interaction that could modulate the activity of the other as explained by the “Signal Orchestration Model” (Kamimura and others 2003). As predicted by the model, chemical inhibition of either IL-6R pathway seemed to augment the IL-6 induction of the other (Fig. 1 and Fig. 2c–f), which fits well with the fact that phorbol ester augmentation of Erk activation has been shown to inhibit IL-6R induced STAT3 activation (Sengupta and others 1998). This indicates that IL-6R induced JAK/STAT3 signaling can occur, and that this pathway is inhibited by the ERK pathway in dermal fibroblasts. Interestingly, ERK can mediate the phosphorylation of STAT3 at S727 which is thought to augment the activation of this transcription factor (Chung and others 1997; Sengupta and others 1998;Decker and Kovarik 2000; O'Rourke and Shepherd 2002). Indeed, IL-6 treatment of fibroblasts increases phosphorylation of STAT3-S727, but this did not result in STAT3 nuclear translocation (Fig. 2). This lack of increased STAT3 translocation may be related to the fact that dual phosphorylation of Y705 and S727 appears to be necessary for STAT3 activation (Schuringa and others 2000; Abe and others 2001). Further, ERK activation can inhibit Y705 phosphorylation and STAT3 transcriptional activity in various cell types (Sengupta and others 1998; Venkatasubbarao and others 2005). Thus, these data seem to indicate that in IL-6 treated fibroblasts, serine phosphorylation of STAT3 does not mediate the translocation/activation of the transcription factor, and in fact may play a role in Erk mediated inhibition of STAT3 signaling in these cells.
There are numerous mechanisms known to be involved in inhibition or termination of IL-6R signaling including receptor internalization, and the actions of protein tyrosine phosphatases, protein inhibitors of activated STAT (PIAS) family, proteasomal degradation of STAT factors and the suppressor of cytokine signaling or SOCS family (Greenhalgh and Hilton 2001; Fischer and others 2004). Each mechanism results in attenuation of the IL-6 signal at different points throughout the cascade, adding redundancy and multiple levels of control to the system. The SOCS family of proteins acts in a classical negative feedback manner in that they are induced by cytokine stimulation yet negatively modulate the same cascade that initiated their production. SOCS proteins can inhibit signal transduction via several proposed mechanisms such as directly binding to the activation loop of JAK and inhibiting its activity, targeting signaling component for proteasomal degradation, and acting as a ubiquitin ligase (Kamura and others 1998; Sasaki and others 1999; Yasukawa and others 1999; Zhang and others 1999; Auernhammer and Melmed 2001; Kile and others 2002; Johnston 2004).
Aside from acting as a negative feedback inhibitor, SOCS3 may play additional roles in cytokine signaling. Cacalano and others (2001) found that in response to IL-2, erythropoetin, EGF, and PDGF, SOCS3 can be phosphorylated at Y204 and Y221 that are located within the SOCS box region of the protein and affect the stability of SOCS3 (Haan and others 2003). Further, it was shown that phosphorylated SOCS3 subsequently binds and inactivates the Ras/GTPase-activating protein p120. Activated GTP-bound Ras acts by regulating the cellular response through distinct Ras effector proteins such as Raf. Activated Raf then phosphorylates MEK-activating the MAPK/ERK cascade (Pamonsinlapatham and others 2009). The interaction of phosphorylated SOCS3 with p120 results in the inhibition of Ras, which leads to a sustained Ras/GTP and MAPK signal (Cacalano and others 2001). Herein, it was indeed found that SOCS3 is tyrosine phosphorylated in fibroblasts (Fig. 3a), and coimmunoprecipitates with p120 RasGap (Fig. 3b). Interestingly, this interaction appears to increase after rmIL-6 treatment (Fig. 3b), possibly directly through the action of JAK as has been shown via insulin receptor in 3T3 fibroblasts (Peraldi and others 2001). Furthermore, after siRNA knockdown, SOCS3 mRNA expression was greatly decreased as compared to the scrambled control (Fig. 4a), and similar to results shown by Lang and others (2003) utilizing SOCS3−/− cells, STAT3 phosphorylation increased as a result of IL-6 treatment (Fig. 4b), confirming the results obtained utilizing chemical inhibitors shown in Fig. 1. Interestingly, SOCS3 itself does not appear to be induced by IL-6 treatment in these cells (Fig. 3b, c), indicating that basal expression is enough to mediate the apparent STAT3 modulation. Overall, these results indicate that the JAK/STAT pathway can indeed be activated in fibroblasts by IL-6 stimulation if the influence of SOCS3 is decreased, indicating its essential role in modulating ERK signaling preference of these cells in response to IL-6.
While it seems apparent that by perturbing the balance of signaling pathway cross talk by utilizing chemical inhibitors could artificially induce one cascade over another, it was not determined whether this might induce functional consequences in these cells. The matrix metalloproteinase family of endopeptidases is very important and tightly regulated within the wound repair process (Madlener and others 1998; Daniels and others 2003). MMP-2 was examined as an indicator of IL-6 function in fibroblasts, as IL-6 treatment is known to decrease MMP-2 expression hepatic stellate cells (Bansal and others 2005) and dermal fibroblasts (Luckett and Gallucci 2007). Indeed, IL-6 treatment with or without STAT3 inhibition resulted a dose dependent decrease in MMP-2 expression when treated with increasing concentrations of rmIL-6, whereas the opposite trend was seen upon cytokine treatment and inhibition of the MAPK/ERK cascade (Fig. 5). These data suggest that the IL-6R system may be manipulated in dermal fibroblasts through the inhibition of either cascade, resulting in an altered outcome as suggested by the “Signal Orchestration Model” (Kamimura and others 2003). These results hint at possible therapeutic applications where an undesirable IL-6R signaling outcome could be manipulated utilizing an appropriate inhibitor, for instance to modulate insulin intolerance in the liver caused by IL-6 overexpression (DiCosmo and others 1994; Kado and others 1999),
When viewed together, the result herein seem to indicate that IL-6R mediated ERK signaling preference in dermal fibroblasts is influenced by several means. In summary, it appears that SOCS3 is tyrosine phosphorylated in response to IL-6 treatment, possibly by JAK (Peraldi and others 2001). This inactivates p120 RasGAP allowing Ras to activate the MAPK/ERK pathway unhindered. Increased ERK activity phosphorylates STAT3, (at least at S727), inhibiting its translocation. This is supported by the fact that if ERK is inhibited by PD98059, STAT3 p-S727 is decreased, and increased translocation is observed (Fig. 2d). Knockdown of SOCS3 (Fig. 4) would allow p120 to inhibit Ras mediated MAPK activation, eliminating STAT3 serine phosphorylation, and directly augmenting JAK/STAT activity as previously shown (Lang and others 2003).
However, despite the fact that induction of STAT3 activation by IL-6 in fibroblasts is not apparent, this transcription factor appears to be activated in untreated fibroblast cultures and is apparent in this (Figs. 1, 2) and a previous study (Gallucci and others 2004b). Thus, this STAT3 activation may be one source for basal levels of SOCS3 expression in these cells that directs the cells toward the ERK pathway. Thus, a question remains as to what causes basal SOCS3 and STAT3 activation in these cells? However, it is well known that IL-6 is not the only cytokine that can activate gp130, and it could be that one of the multiple ligands is inducing the apparent basal phosphorylation of STAT3 and production of SOCS3. Certainly, further study will be necessary to determine the source function of this activation in fibroblasts.
Acknowledgments
The authors wish to thank Dr. Gerard Elberg for his scientific and technical assistance. This work was funded by NIH/NIGMS grant R01 GM067745.
Author Disclosure Statement
No competing financial interests exist.
References
- Abe K. Hirai M. Mizuno K. Higashi N. Sekimoto T. Miki T. Hirano T. Nakajima K. The YXXQ motif in gp 130 is crucial for STAT3 phosphorylation at Ser727 through an H7-sensitive kinase pathway. Oncogene. 2001;20(27):3464–3474. doi: 10.1038/sj.onc.1204461. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ. Melmed S. The central role of SOCS-3 in integrating the neuro-immunoendocrine interface. J Clin Invest. 2001;108(12):1735–1740. doi: 10.1172/JCI14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal MB. Kovalovich K. Gupta R. Li W. Agarwal A. Radbill B. Alvarez CE. Safadi R. Fiel MI. Friedman SL. Taub RA. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol. 2005;42(4):548–556. doi: 10.1016/j.jhep.2004.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano NA. Sanden D. Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3(5):460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- Chung J. Uchida E. Grammer TC. Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17(11):6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman DE. Diamond RH. Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21(5):1443–1449. [PubMed] [Google Scholar]
- Daniels JT. Cambrey AD. Occleston NL. Garrett Q. Tarnuzzer RW. Schultz GS. Khaw PT. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003;44(3):1104–1110. doi: 10.1167/iovs.02-0412. [DOI] [PubMed] [Google Scholar]
- Decker T. Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19(21):2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- DiCosmo BF. Picarella D. Flavell RA. Local production of human IL-6 promotes insulitis but retards the onset of insulin-dependent diabetes mellitus in non-obese diabetic mice. Int Immunol. 1994;6(12):1829–1837. doi: 10.1093/intimm/6.12.1829. [DOI] [PubMed] [Google Scholar]
- Fischer P. Lehmann U. Sobota RM. Schmitz J. Niemand C. Linnemann S. Haan S. Behrmann I. Yoshimura A. Johnston JA. Muller-Newen G. Heinrich PC. Schaper F. The role of the inhibitors of interleukin-6 signal transduction SHP2 and SOCS3 for desensitization of interleukin-6 signalling. Biochem J. 2004;378(Pt 2):449–460. doi: 10.1042/BJ20030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci RM. Lee EG. Tomasek JJ. IL-6 Modulates Alpha-Smooth Muscle Actin Expression in Dermal Fibroblasts from IL-6-Deficient Mice. J Invest Dermatol. 2006;126(3):561–568. doi: 10.1038/sj.jid.5700109. [DOI] [PubMed] [Google Scholar]
- Gallucci RM. Simeonova PP. Matheson JM. Kommineni C. Guriel JL. Sugawara T. Luster MI. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. Faseb J. 2000;14(15):2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- Gallucci RM. Sloan DK. Heck JM. Murray AR. O'Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol. 2004a;122(3):764–772. doi: 10.1111/j.0022-202X.2004.22323.x. [DOI] [PubMed] [Google Scholar]
- Gallucci RM. Sloan DK. O'Dell SJ. Reinke LA. Differential expression of liver interleukin-6 receptor-alpha in female versus male ethanol-consuming rats. Alcohol Clin Exp Res. 2004b;28(3):365–373. doi: 10.1097/01.alc.0000118316.20560.0d. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ. Hilton DJ. Negative regulation of cytokine signaling. J Leukoc Biol. 2001;70(3):348–356. [PubMed] [Google Scholar]
- Haan S. Ferguson P. Sommer U. Hiremath M. McVicar DW. Heinrich PC. Johnston JA. Cacalano NA. Tyrosine phosphorylation disrupts elongin interaction and accelerates SOCS3 degradation. J Biol Chem. 2003;278(34):31972–31979. doi: 10.1074/jbc.M303170200. [DOI] [PubMed] [Google Scholar]
- Heinrich PC. Behrmann I. Haan S. Hermanns HM. Muller-Newen G. Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M. Murakami M. Saito M. Hirano T. Taga T. Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Johnston JA. Are SOCS suppressors, regulators, and degraders? J Leukoc Biol. 2004;75(5):743–748. doi: 10.1189/jlb.1003507. [DOI] [PubMed] [Google Scholar]
- Jones SA. Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta. 2002;1592(3):251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- Kado S. Nagase T. Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36(1–2):67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- Kamat CD. Green DE. Warnke L. Thorpe JE. Ceriello A. Ihnat MA. Mutant p53 facilitates pro-angiogenic, hyperproliferative phenotype in response to chronic relative hypoxia. Cancer letters. 2007;249(2):209–219. doi: 10.1016/j.canlet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Kamimura D. Ishihara K. Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Kamura T. Sato S. Haque D. Liu L. Kaelin WG., Jr. Conaway RC. Conaway JW. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12(24):3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G. Marin V. Montero-Julian F. Mantovani A. Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24(1):25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Kile BT. Schulman BA. Alexander WS. Nicola NA. Martin HM. Hilton DJ. The SOCS box: a tale of destruction and degradation. Trends Biochem Sci. 2002;27(5):235–241. doi: 10.1016/s0968-0004(02)02085-6. [DOI] [PubMed] [Google Scholar]
- Kopf M. Ramsay A. Brombacher F. Baumann H. Freer G. Galanos C. Gutierrez-Ramos JC. Kohler G. Pleiotropic defects of IL-6-deficient mice including early hematopoiesis, T and B cell function, and acute phase responses. Ann N Y Acad Sci. 1995;762:308–318. doi: 10.1111/j.1749-6632.1995.tb32335.x. [DOI] [PubMed] [Google Scholar]
- Lang R. Pauleau AL. Parganas E. Takahashi Y. Mages J. Ihle JN. Rutschman R. Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4(6):546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- Larsen L. Ropke C. Suppressors of cytokine signalling: SOCS. APMIS. 2002;110(12):833–844. doi: 10.1034/j.1600-0463.2002.1101201.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luckett LR. Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol. 2007;156(6):1163–1171. doi: 10.1111/j.1365-2133.2007.07867.x. [DOI] [PubMed] [Google Scholar]
- Madlener M. Parks WC. Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242(1):201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Narazaki M. Yasukawa K. Saito T. Ohsugi Y. Fukui H. Koishihara Y. Yancopoulos GD. Taga T. Kishimoto T. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82(4):1120–1126. [PubMed] [Google Scholar]
- O'Rourke L. Shepherd PR. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. Biochem J. 2002;364(Pt 3):875–879. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamonsinlapatham P. Hadj-Slimane R. Lepelletier Y. Allain B. Toccafondi M. Garbay C. Raynaud F. P120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling. Biochimie. 2009;91(3):320–328. doi: 10.1016/j.biochi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Paschoud S. Dogar AM. Kuntz C. Grisoni-Neupert B. Richman L. Kuhn LC. Destabilization of interleukin-6 mRNA requires a putative RNA stem-loop structure, an AU-rich element, and the RNA-binding protein AUF1. Mol Cell Biol. 2006;26(22):8228–8241. doi: 10.1128/MCB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraldi P. Filloux C. Emanuelli B. Hilton DJ. Van Obberghen E. Insulin induces suppressor of cytokine signaling-3 tyrosine phosphorylation through janus-activated kinase. J Biol Chem. 2001;276(27):24614–24620. doi: 10.1074/jbc.M102209200. [DOI] [PubMed] [Google Scholar]
- Sasaki A. Yasukawa H. Suzuki A. Kamizono S. Syoda T. Kinjyo I. Sasaki M. Johnston JA. Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4(6):339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ. Jonk LJ. Dokter WH. Vellenga E. Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J. 2000;347(Pt 1):89–96. [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB. Interleukin-6: molecular pathophysiology. J Invest Dermatol. 1990;94(6 Suppl):2S–6S. doi: 10.1111/1523-1747.ep12874963. [DOI] [PubMed] [Google Scholar]
- Sengupta TK. Talbot ES. Scherle PA. Ivashkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1998;95(19):11107–11112. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H. Wang R. Wang S. Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102(13):4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubbarao K. Choudary A. Freeman JW. Farnesyl transferase inhibitor (R115777)-induced inhibition of STAT3 (Tyr705) phosphorylation in human pancreatic cancer cell lines require extracellular signal-regulated kinases. Cancer Res. 2005;65(7):2861–2871. doi: 10.1158/0008-5472.CAN-04-2396. [DOI] [PubMed] [Google Scholar]
- Yasukawa H. Misawa H. Sakamoto H. Masuhara M. Sasaki A. Wakioka T. Ohtsuka S. Imaizumi T. Matsuda T. Ihle JN. Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18(5):1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG. Farley A. Nicholson SE. Willson TA. Zugaro LM. Simpson RJ. Moritz RL. Cary D. Richardson R. Hausmann G. Kile BJ. Kent SB. Alexander WS. Metcalf D. Hilton DJ. Nicola NA. Baca M. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci U S A. 1999;96(5):2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]