Abstract
Purpose of Review
Tubulointerstitial injury in the kidney is complex, involving a number of independent and overlapping cellular and molecular pathways, with renal interstitial fibrosis and tubular atrophy (IF/TA) as the final common pathway. Furthermore, there are multiple ways to assess IFTA.
Recent findings
Cells involved include tubular epithelial cells, fibroblasts, fibrocytes, myofibroblasts, monocyte/macrophages, and mast cells with complex and still incompletely characterized cell-molecular interactions. Molecular mediators involved are numerous and involve pathways such as transforming growth factor (TGF-β), bone morphogenic protein (BMP), platelet-derived growth factor (PDGF), and hepatocyte growth factor (HGF). Recent genomic approaches have shed insight into some of these cellular and molecular pathways. Pathologic evaluation of IFTA is central in assessing the severity of chronic disease; however, there are a variety of methods used to assess IFTA. Most assessment of IFTA relies on pathologist assessment of special stains such as trichrome, Sirius Red, and collagen III immunohistochemistry. Visual pathologist assessment can be prone to inter- and interobserver variability, but some methods employ computerized morphometery, without a clear consensus as to the best method.
Summary
IFTA results from on orchestration of cell types and molecular pathways. Opinions vary on the optimal qualitative and quantitative assessment of IFTA.
Keywords: kidney/renal fibrosis, epithelial/mesenchymal transition, myofibroblast, morphometry
Introduction
Interstitial extracellular matrix (ECM) accumulation, common to many chronic kidney diseases, contributes to functional loss. Kidney interstitial fibrosis (IF) can be defined as the accumulation of collagen and related molecules in the interstitium. Interstitial collagen is normally present in the kidney, particularly type I and III, which serve as structural scaffolding.[1, 2] This review addresses mechanisms by which IF arises, shown through animal experimentation and analysis of human kidneys. In addition, approaches to assess IF are considered.
IF patterns differ and probably do not have identical causes or consequences. Broad scars with tubular loss are the sequelae of severe focal injury and parenchymal destruction, such as in pyelonephritis and infarcts.[3] This pattern is no doubt a “wound healing” response to repair integrity loss and prima facie beneficial to renal function. In contrast, a second pattern (far more common in renal biopsies) is diffuse or patchy fine IF, surrounding either atrophic or normal tubules and associated with either diffuse or focal disease of glomeruli, tubules, or vessels.[4, 5] Many studies show a reciprocal correlation between kidney function and the IF extent.[6–14]
Tubular atrophy (TA) is defined as loss of specialized transport and metabolic capacity and typically manifested by small tubules with cells with pale cytoplasm or dilated, thin tubules. TA is usually associated with IF (often abbreviated IFTA); but probably has distinct mechanisms related to blood flow, glomerular filtration rate (GFR) or tubular continuity loss. However, IF and TA are separable, as shown by the profound TA in renal artery stenosis, which characteristically has little or no fibrosis (or inflammation).[3]
Cellular and Molecular Mediators
IFTA results from an orchestration of multiple cell types, as detailed below.
Fibroblasts and Myofibroblasts
Fibroblasts constitute a large proportion of renal interstitial cells and are the major cell maintaining constituent ECM, which can be considered the kidney “skeleton”. Fibroblasts lack a cell type-specific marker, making their study difficult.[1] Fibroblasts are distinguished from other interstitial cells by their abundance of rough endoplasmic reticulum, prominent F-actin cytoskeleton, and by ecto-5′-nucleotidase expression in their plasma membrane. Fibroblasts interact with other cells, such as dendritic cells, through cell processes.[15] Fibroblasts may acquire a myofibroblastic phenotype under paracrine signals after attaching to injured tubular basement membranes (TBM),[16–19] eventually producing collagen type III.[20] This hypothesized phenomenon is likely a crucial event in IF.[1, 21, 22]
Myofibroblasts express smooth muscle actin (SMA), contain microfilaments with focal densities (stress fibers), peripheral myofilament condensations known as fibronexi connecting actin microfilaments with extracellular fibronectin, round nuclei, and frequent attachments to basement membranes.[15, 23, 24] Myofibroblasts also contain vimentin, fibronectin with the splice variant containing ectodomain A [15, 18], and S100A4 [also known as FSP-1];[15, 20] S100A4, once considered myofibroblast specific, also colocalizes with some leukocytes.[15, 20] Interstitial myofibroblasts have multiple potential origins with candidates being fibroblasts, pericytes, perivascular cells.[20, 25–30], and endothelial cells.[31, 32]
Fibrocytes
Fibrocytes, thought to be distinct from fibroblasts, are spindle-shaped, ECM-producing cells derived from peripheral blood leukocytes.[33] Both hematopoietic (e.g., CD45) and stromal cell (e.g., type I collagen) markers can be detected on fibrocytes; and furthermore, these cells also express chemokine receptors. Fibrocytes are found in injured kidneys, possibly through in situ differentiation or infiltration through chemokine gradients.[34–36] T-helper-2-type (TH2) cytokines appear to be profibrotic, inducing differentiation of human fibrocytes; and in contrast, T-helper-1-type (TH1) cytokines can inhibit differentiation of fibrocytes.[34, 37, 38] Fibrocytes may be affected by drugs such as cyclosporine which induces type I collagen, possibly explaining IFTA attributed to chronic calcineurin inhibitor toxicity.[34] Fibrocytes are present in systemic nephrogenic fibrosis, related to gadolinium administration.[39]
Extracellular matrix
Multiple ECM components besides collagen have a crucial role in IF.[40–44] Tissue transglutaminase (tTG) expression is increased in animal and human renal disease models, correlating with IF severity; and tTG crosslinks proteins, stabilizing ECM and conferring resistance to protease degradation.[45–47] Matrix metalloproteinase (MMP) enzymes are comprised of proteolytic enzymes that can degrade all matrix protein components.[48] Tissue plasminogen activator (tPA), although proteolytic, increases IF development by inducing MMP-9 gene expression, leading to TBM disruption and EMP promotion.[49] Mice without the MMP-9 gene have less IFTA in obstructive nephropathy.[48] Plasmin, a serine protease, can activate MMPs, leading to ECM degradation and reduced IF.[50–52] Decreased laminin, a component of glomerular and PTC basement membranes, leads to more IFTA.[53, 54] ECM production is also affected by the renin/angiotensin system.[55–62]
Tubular Epithelial Cells
Tubular epithelial cells (TECs) are postulated to contribute to increased ECM through the process of epithelial-to-mesenchymal transition (EMT), defined as the stepwise loss of epithelial markers, such as E-cadherin, and the acquisition of mesenchymal markers, such as vimentin and SMA.[1, 63, 64] As an ultimate step in EMT, cells acquire increased motility and traverse basement membranes into the interstitium.[65] Convincing experimental evidence for epithelial cell migration into the interstitium has been acquired in IF in mouse kidneys, where the origin of the cells can be followed with indelible genetic markers.[2, 65] A similar in situ change in tubular epithelial phenotype occurs in humans, but emigration of TECs into the interstitium has not been demonstrated. Therefore, many question the migration feature of EMT.[66–68] A recent Banff Conference symposium on EMT concluded that the in situ epithelial response exists but needs a name that does not imply emigration of tubular cells to the interstitium,[69] which we will here term “epithelial mesenchymal phenotype” (EMP).
TECs clearly undergo marked phenotypic changes in acute injury[70] and appear to provide key signals to provide IF.[1] Intratubular stretch, fluid shear stress, and biomechanical forces modify intracellular signaling and gene expression, contributing to IF.[16] EMP is supported by the finding of increased intermediate filaments (e.g., vimentin and nestin) in injured tubular epithelium,[71, 72] an association with increased collagen type I and III expression in TECs,[73] and altered E-Cadherin expression.[74] Transcription factors such as the zinc-finger transcription factor snail homolog 1 (Snai1)[75], which interacts with the notch signaling pathway[76], appear to be important to EMP. An important component in the regulation of genes in EMP and IF may include micro(mi)RNAs[77–86], some antifibrotic[87] and others profibrotic.[88] Autophagy, the process whereby cells undergo “self digestion”,[1, 89, 90] and endoplasmic reticulum stress are important to IFTA.[91–97] TECs may undergo damage through the increased action of lipids in a “lipid nephrotoxicity” process, mediated in part by peroxisome proliferator-activated receptor (PPAR) expression.[98–104]
VEGF-A overexpression in tubular cells can result in increased serum VEGF levels, leading to increased capillary number and size, type IV collagen deposition, and fibroblast and myofibroblast numbers.[105] However, other studies show that VEGF administration may decrease IF.[106] Hypoxia promotes fibrosis through multiple mediators including hypoxia-induced factor-1α (HIF-1α).[107–110]
Epigenetic modification through methyltransferase Dnmt1 hypermethylation of the Ras oncogene inhibitor RASAL1 decreases IF.[1, 111, 112] Another chromatin structure modifier, histone deacetylase (HDAC), modulates proinflammatory and fibrotic changes in tubulointerstitial injury,[113, 114] and histone methylation may also be important in fibrotic gene expression.[115] Growth arrest may lead to renal injury through increased fibrosis, characterized by an increased proportion of TECs in phase G2/M, which gives the cells a profibrotic phenotype, in large part mediated by JNK signaling. Pharmacologic induction of growth arrest can promote fibrosis.[116]
Inflammatory cells
Numerous inflammatory cell types contribute to IFTA, as discussed below.
Lymphocytes
Lymphocytes appear to have important roles in the genesis of IFTA.[117–119] CD4+ T cells are considered particularly crucial to this process, since CD4+ but not CD8+ lymphocyte reconstitution increased IF in RAG knockout mice and CD4+ depletion decreased IF.[117] A recent microarray analysis of renal allografts showed increased T-cell and natural killer gene sets in IFTA development.[120] High T cell and macrophage but not B cell infiltration is associated with low IL-10 expression, which conferred susceptibility to IFTA.[121]
Monocyte/Macrophages
Monocyte/macrophages are heterogeneous, and some are profibrotic,[122, 123] particularly CD11b+ cells.[124, 125] Galectin-3 (Gal-3) is a profibrotic mediator comprised of a β-galactosidase-binding lectin released from macrophages.[125] Gal-3 may protect renal tubules from chronic injury by enhancing ECM remodeling and attenuating fibrosis.[126] The macrophage growth factor, CSF-1, is released by renal tubular cells, leading to repair and reduced IF.[127] Models constructed to investigate the role of macrophages in IF include: adriamycin-induced nephropathy,[128] cyclophosphamide depletion of macrophages,[129] and adoptive transfer of bone marrow-derived macrophages.[129, 130] The role of macrophages in IF is clearly complex, since some subsets of bone marrow derived monocytes may actually attenuate fibrosis.[130]
Dendritic cells
Dendritic cells are present in substantial numbers in the renal interstitium,[15] and recent studies have shown their importance in IF. Dendritic cell depletion through injection of diphtheria toxin in transgenic mice with a CD11c/diptheria toxin receptor may ameliorate IF.[131, 132] Other studies show that dendritic cells act indirectly, activating T cells to produce fibrosis.[119]
Mast cells
Mast cells are a component of the primary innate immune system and are typically infrequent in normal kidneys, often congregating around vessels and epithelium. Increased mast cell numbers have been associated with profibrotic roles.[133–137], inversely correlating with renal function.[135, 137, 138] Mast cell deficiency has been associated with decreased fibrosis.[135] Mast cells have also been associated with antifibrotic actions.[139]
Endothelial cells, Peritubular Capillaries, and Vascular Supply
Experimental evidence supports the view that endothelial cells contribute to interstitial fibroblasts, possibly through a process of endothelial-to-mesenchymal transition.[21, 29, 31, 33, 140–143] PTCs decrease with time in allografts and are inversely related to renal function; decreased PTC density at 3 months predicts later loss of function at one year.[144]
Kidney lymphatic vessels are important in facilitating inflammatory cell emigration. IF is associated with increased lymphangiogenesis, partly driven by VEGF-C.[145] Angiogenesis and inflammation inhibition with sirolimus can prevent IF.[146] Newly formed lymphatics may be found close to glomeruli with tuft adhesions,[145] perhaps participating in the misdirection of urine filtrates in these areas.[147] Other studies have found a connection between lymphangiogenesis, tissue remodeling, and differential proteoglycan expression.[148] Lymphangiogenesis occurs as early as 72 hours after transplantation and tends to correlate with inflammation.[149]
Molecular Mediators and Signaling Pathways(Table 1)
Table 1.
Mediators of Interstitial Fibrosis and Selected Interaction Partners
| Mediator | Primary role | Interaction |
|---|---|---|
| Fibrinogen | Profibrotic | Acts as a fibroblast mitogen |
| G2/M arrest | Profibrotic | Increase of cells in the G2/M phase gives the cells a profibrotic phenotype |
| Galectin-3 | Profibrotic | Released from macrophages |
| Integrins | Profibrotic | TGF-β-inducible integrins (e.g. αVβ6) act through integrin-linked kinases to produce collagen |
| Jagged/notch | Profibrotic | Downstream of TGF-β; may be inhibited to decrease fibrosis |
| JAK/STAT | Profibrotic | May be a useful therapeutic target to decrease IF |
| MMP | Profibrotic | Can degrade ECM but also disrupts basement membranes |
| PDGF | Profibrotic | Induces fibroblast proliferation and leukocyte infiltration; inhibited by Crim1 |
| Smad | Profibrotic | Downstream of TGF-β |
| TGF-β | Profibrotic | Downstream: Smad, jagged/notch; Smad antagonist corepressors such as SnoN and Ski; latency-associated peptide (LAP) binds TGF-β1, inhibiting binding to the TGF-β receptor; latent TGF-β binding protein (LTBP) binds the complex and inhibits binding to the ECM; Inhibited by Crim1 |
| TLRs | Profibrotic | Acts through BAMBI (BMP and Activin Membrane Bound Inhibitor) [negative regulator of TGF-β] to attenuate tubular injury but promote IF |
| tPA | Profibrotic | Induces MMP-9 gene expression, leading to TBM disruption and increased IF |
| tTG | Profibrotic | Crosslinks proteins, Stabilizes ECM |
| VEGF | Profibrotic | Increased capillaries and lymphatics, fibroblasts/myofibroblasts, and collagen deposition; Inhibited by Crim1 |
| miRNA | Antifibrotic/Profibrotic | Some are antifibrotic and some are profibrotic; some may be therapeutic targets |
| BMP | Antifibrotic | Antagonist of TGF-β; inhibited by sclerostin domain-containing protein 1 (also known as uterine sensitization-associated gene 1 [USAG-1]) |
| Crim1 | Antifibrotic | Binds TGF-β, VEGF, and PDGF-â to decrease fibrosis |
| CSF-1 | Antifibrotic | Macrophage growth factor released by renal tubular cells leads to repair and reduced IF |
| HGF | Antifibrotic | blocks Smad (e.g., Smad 2/3) |
| Plasmin | Antifibrotic | Activates MMPs, leading to matrix degradation and reduced IF |
| RASAL1 | Antifibrotic | Ras oncogene inhibitor hypermethylated by methyltransferase Dnmt1 decreases IF |
BMP: Bone morphogenic protein, ECM: extracellular matrix, HGF: hepatocyte growth factor, IF: interstitial fibrosis, JAK/STAT: janus kinase/signal transducer and activator of transcription, miRNAs: microRNA, MMP: Matrix metalloproteinase, PPAR: peroxisome proliferator-activated receptor, TBM: tubular basement membrane, tPA: tissue plasminogen activator, TGF: Transforming growth factor, TLRs: Toll-like receptors, tTG: tissue transglutaminase, VEGF: vascular endothelial growth factor
Transforming growth factor-β (TGF β) is regarded as a central mediator of IF.[1, 45, 150–155] TGF-β upregulation occurs in nearly every chronic kidney disease [both human and animal]. TGF-β, possibly one of the most widely studied regulators of ECM production, is produced in a latent form, TGF-β1, bound to latency-associated peptide (LAP), which inhibits TGF-β receptor binding. Latent TGF-β binding protein (LTBP) binds this complex and inhibits binding to the ECM.[156, 157]
TGF-β acts through Smad for downstream signaling,[158–160] which is amplified if Smad antagonists [e.g., SnoN and Ski corepressors] are lost.[161–163] Resulting fibrogenic signals stimulate fibroblasts, presumably initiating tubular EMP. TGF-β also works through the jagged/notch pathway, which may be inhibited to decrease fibrosis,[164–166] and Crim1, which binds and regulates TGFβ-2, VEGF, and PDGF-β, to decrease fibrosis. [167]
Bone morphogenic protein (BMP), particularly BMP-7, acts as a natural TGF-β antagonist; and due to this, BMP may have renoprotective effects and may possibly reverse IF.[161, 168–170] Sclerostin domain-containing protein 1 (also known as uterine sensitization-associated gene 1 [USAG-1]) is an endogenous inhibitor of BMP-7[1, 45, 171, 172]. Inhibiting the circulating proteolytic enzyme BMP1–3 [a tolloid-like proteinase] reduces IF.[173, 174]
Toll-like receptors (TLRs) participate in IFTA.[175, 176] TLR4 modulates IFTA susceptibility through the BAMBI (BMP and Activin Membrane Bound Inhibitor), a negative regulator of TGF-β, attenuating tubular injury but promoting IF;[176] however, other studies fail to show TLR influence.[177, 178]
TGF-β activation blockade can be accomplished with decorin antisense TGF-β expression inhibition, neutralizing TGF-β antibodies and soluble TGF-β receptors.[179] Small molecule inhibitors to TGF-β are being developed.[180] However, it is difficult to ascertain whether TGF-β inhibition will be universally useful in inhibiting fibrosis. TGF-β also has anti-inflammatory properties; and there is concern that TGF-β inhibition could lead to increased inflammation and thus fibrosis.[180] TGF-β action may also be mediated by reactive oxygen species and oxidative damage.[181]
The TGF-β-inducible integrin αVβ6 appears to be restricted to epithelial cells where it is normally expressed in renal tubules at low levels and elevated during injury, development, and neoplasia. This integrin appears to have a role in increasing IF and inflammation.[182] Integrin-linked kinases have associated with increased IFTA, corresponding with increases in collagen IV and TGF-β.[183]
Hepatocyte growth factor (HGF) is considered to be an antifibrotic factor with effects opposite TGF-β, blocking Smad2/3 nuclear translocation in interstitial fibroblasts, inhibiting tubular EMP. Administration of HGF or its gene can decrease IF observed in animal models.[184–186] However, long-term proteinuria has been demonstrated with HGF administration.[187]
Platelet-Derived Growth Factor (PDGF), comprising four isoforms (PDGF-A,-B,-C, and -D) and two receptor chains (PDGFR-α and -β), appears to have an important role in IF.[23, 188] PDGF-C,-α, and -CC are noteworthy contributors to the renal cortical interstitium.[1, 23, 189] Data indicates that PDGF-CC directly induces fibroblast proliferation and enhances leukocyte infiltration;[189] but some studies have only demonstrated PDGF-CC in the peritubular capillary (PTC) endothelium.[190]
The janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway appears to be important in IFTA development, and STAT inhibition may be useful therapeutically.[191] Fibrinogen acts as a fibroblast mitogen, promoting IFTA.[192]
Complement inhibition or lack of complement components appears to be antifibrotic.[193, 194] Proteomic data indicates contributions from the alternative rather than the classic complement pathway to IFTA.[195]
Evaluation of Interstitial Fibrosis
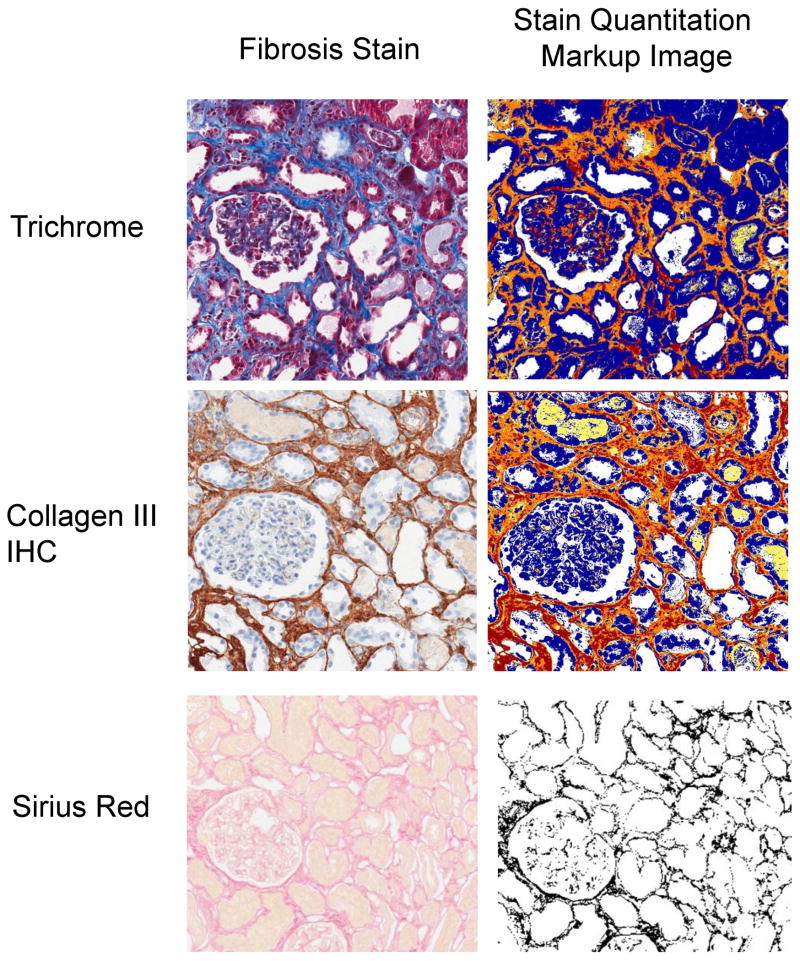
IF extent is predictive of renal allograft outcome and may be considered a surrogate marker.[196–200] Several applications require accurate IF measurement (Table 2)[6–14, 24, 169, 200–211] including research focused on therapeutic inhibition of IF and comparison of renal allograft protocol biopsies.[45, 180, 212, 213] Visual assessment of trichrome-stained slides is often standard institutional practice,[214] but studies have shown this approach may have poor reproducibility.[215, 216] Several morphometry techniques are used to assess IF (Figure 3), including morphometry of slides stained with trichrome;[10, 202] Sirius Red, specific for collagen types I and III under polarized light;[24, 206, 217] and collagen immunohistochemistry, particularly type III collagen.[12, 218, 219] Computer-assisted morphometry has shown utility in the analysis of studies employing trichrome, Sirius Red, and collagen III immunohistochemistry; and analysis in some of these studies have shown correlation with GFR.[6, 8, 11, 12, 24, 169, 202, 206, 217–222] (Table 2)
Table 2.
Interstitial Fibrosis Evaluation
| Method | Description | Measure | Ref.(s) |
|---|---|---|---|
| VA; “routine” | IF, inflammation, and glomerulopathy correlated with poor allograft outcome | By multivariate analysis, IF and inflammation lead to poorer survival (HR = 8.5, p < 0.0001); IF alone had less effect (HR = 4.8, p = not significant) | [200] |
| IA; TC (Masson), SR, and SMA IHC | IA and semi-quantitative assessments (VA) were performed on late allograft biopsies | IF by VA predicted Banff ’97 ci scores (p < 0.0001) and correlated with GFR, Cr, and urine total protein (r = − 0.48, p = 0.0007; r = 0.46, p = 0.0009; r= 0.51, 0.0009, respectively). Of IA methods, only SR- nonpolarized score correlated with GFR and urine total protein (r = −0.29, p = 0.05; r = 0.29, p = 0.05, respectively) | [14] |
| IA; TC (Masson) | IF IA correlates with serum Cr in IgA nephropathy and MPGN | IF occupied > 10% of the interstitium in all 10 cases and > 20% in 6 and IF IA correlated with serum Cr | [201] |
| IA; TC (Masson) | IA of IF in patients receiving cyclosporine | IF grade by IA correlated with worsened Cr clearance between 1 and 3 years | [10] |
| IA; TC (Light Green) | IF IA in patients randomized to cyclosporine or conversion to sirolimus | No difference in groups with respect to fibrosis but GFR improved significantly in the conversion group | [202] |
| IA; TC (Light green) | Quantitative IF in sequential renal biopsies | IF evolution correlated with eGFR | [11] |
| IA; SR and collagen | Renal IF correlates with presence of TGF-β, decorin, SMA, and interstitial collagens | In all samples with IF, TGF-β up-regulation was observed in combination with reduced decorin expression | [169, 203– 205] |
| IA; SR | SR IA predicted long-term renal allograft function | Cortical IF correlated with time to graft failure (r = 0.64, P < 0.001) at 6 months post transplant | [24] |
| IA; SR | SR IA predicted long-term renal allograft function | Positive correlation (r = 0.62, P<0.001) between SR fibrosis and decreased GFR | [7] |
| IA; SR | SR IA corresponded to light microscopic semiquantitative measurements (r = 0.439, P = 0.0003 overall and r = 0.704, P < 0.0001 for just baseline specimens) in kidney allografts | Semiquantitative methods correlated best with long-term graft function (serum Cr at 8 – 10 years (P = 0.010) and late graft loss (P = 0.0445) | [206] |
| IA; SR | IF in non-heart-beating donor kidneys and conventional heart-beating donor kidneys | No significant difference in IF between the two groups | [207] |
| IA; SR | IF scoring predicts survival in lupus nephritis | Fibrillary collagen was predictive of Cr doubling (P = 0.01) and relapse (P = 0.06) | [208] |
| IA; SR | IA-based application (Fibrosis HR) for IF and glomerular morphometry | Intra- and interoperator variability was present in manual segmentation of IF, mesangial matrix, and glomerular areas but interactive identification didn’t have this variability | [209] |
| IA; SR | IF measurements using digital imaging coupled with point counting correlated with GFR | Direct relationship between interstitial volume fraction and renal function (r2 = 0.54) | [8] |
| IA; SR | SR IF measurement combined with ultrasound measurements of renal artery resistance index helped predict “chronic allograft nephropathy” | Positive correlation (r = 0.62, P <0.001) between picroSR-stained cortical fractional IF volume and decreased GFR | [9] |
| IA; CIII IHC | IF measurements by a semiautomatic system correlate with GFR in protocol renal transplant biopsy specimens | Area fraction of collagen III IHC of > 40% @ 6 months associated with decreased GFR @ 24 months compared with ≤ 40% (r=−0.32, P=0.03) | [12] |
| IA; CIII IHC | IF measurements by a semiautomatic system correlate with GFR in protocol renal transplant biopsies | GFR correlated negatively with interstitial volume fraction @ 6 months (P = 0.05) | [13] |
| IA and VA; TC (Masson) | Cyclosporine (CsA) therapy effects on fibrosis IA | IF measured by IA was significantly higher in the CsA group only in renal allografts 6 months posttransplant (P < 0.04) | [210, 211] |
| IA and VA; CII IHC, TC, and SR | Comparison of CII IHC, TC, and SR IA | Collagen III IHC and VA of TC-stained slides correlated best with each other and with GFR | [6] |
CIII: Collagen III, Cr: creatinine, eGFR: estimated GFR, GFR: glomerular filtration rate, IHC: immunohistochemistry, IF: interstitial fibrosis, IA: Image analysis, MPGN: membranoproliferative glomerulonephritis, SMA: smooth muscle actin, SR: Sirius red, Ref(s): References, TC: Trichrome, TGF-β: transforming growth factor, VA: visual analysis.
Figure 3. Fibrosis morphometry.
Stains used to assess fibrosis are shown, including: Trichrome, Collagen III immunohistochemistry, and Sirius Red [on the left] with their corresponding quantitation markup images shown [on right].
There are intrinsic limitations in the measurement of IF, some of which are due to sampling. For example, one study estimated that repeat biopsies show a decrease in the measured level of fibrosis, presumably due to sampling, in 12% of cases.[223] In addition, not all fibrosis is “equal” or the “same” in quality and thus aggregate quantity. For example, “active” or “young” IF may have greater potential for remodeling. Broad scars may have different consequences than diffuse, fine IF. Inflammation in areas of IF has also been noted in several studies to be an adverse risk factor for progression of renal disease.[18, 200, 223–227]
Overall, there is no consensus regarding the best way to assess IF. Efforts to reach a consensus or at least provide recommendations are currently underway under the auspices of the Banff Conference of Allograft Pathology.[228]
Conclusion
Molecular mechanisms leading to IFTA are complex and typically interrelated with the primary processes leading to renal injury. Further elucidation of these mechanisms could lead to targeted inhibitors to alleviate terminal scarring. Furthermore, there are number of ways to assess fibrosis; and efforts are underway to improve these methods.
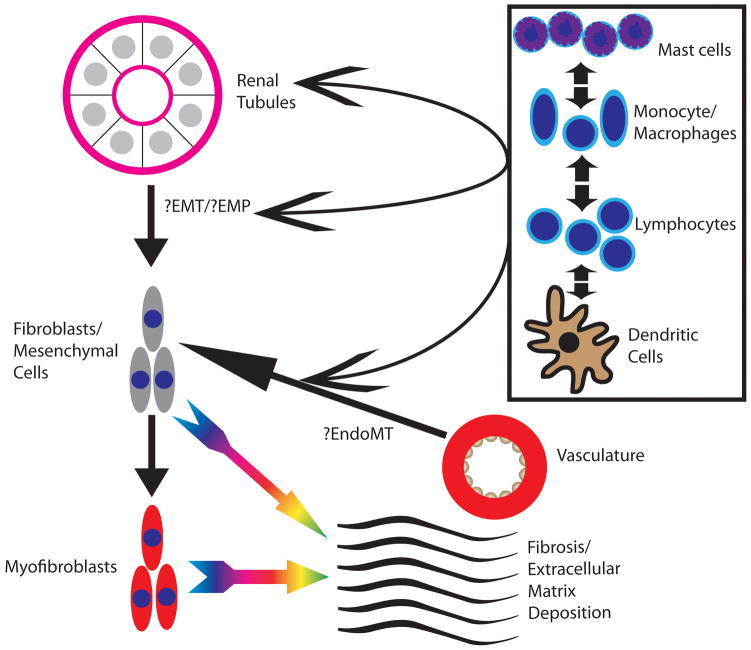
Figure 1. Cellular mediators of fibrosis.
Cells involved in fibrosis include the renal tubules, the renal vasculature, and inflammatory cells, including lymphocytes, monocyte/macrophages, mast cells, and dendritic cells. The renal tubules at least undergo changes that impart them with a epithelial-mesenchymal phenotype (EMP) and are possibly involved in a process of epithelial-mesenchymal transition (EMT). The endothelium is possibly involved in a process of endothelial-mesenchymal transition (EndoMT). Evidence shows that the inflammatory cells participate in both the process of EMT/EMP and EndoMT. Fibroblasts/mesenchymal cells mediate the production of fibrosis and extracellular matrix (ECM) deposition and also may undergo a transition to a myofibroblastic phenotype, further leading to the production of fibrosis and ECM deposition.
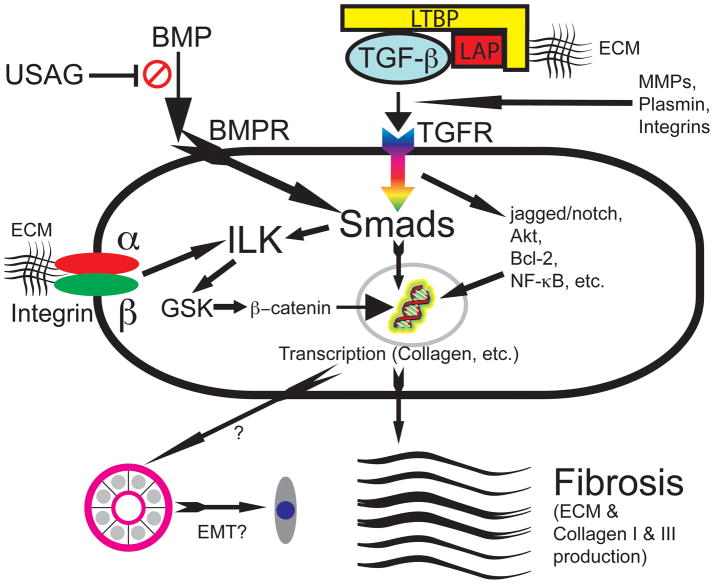
Figure 2. Important molecular mediators of fibrosis.
Transforming growth factor (TGF-β) is released through interactions with the extracellular matrix (ECM) and matrix metalloproteinases (MMPs), plasmin, and integrin; and when released from inhibition by latent TGF-β binding protein (LTBP) and latency-associated peptide (LAP), TGF-β binds the transforming growth factor receptor (TGFR), activating intracellular signals such as the Smad, jagged/notch, Akt, Bcl-2, and NF-κB pathways. These lead to nuclear transcription, ultimately culminating in collagen and ECM production and possibly leading to epithelial to mesenchymal transition (EMT). Smads also act on the integrin-linked kinase (ILK), which acts through glycogen synthase kinase (GSK) to produce β-catenin, which traverses into the nucleus to also induce transcription. The integrins (typically with α and β components [e.g., α5β6 integrin]) also act through ILK in a similar manner. Bone morphogenic protein (BMP), when binding to the BMP receptor (BMPR) also works through Smad, a process inhibited by sclerostin domain-containing protein 1 (also known as uterine sensitization-associated gene 1 [USAG-1]).[Figure adapted from [1, 229, 230].]
Bullet Point Summary.
Interstitial fibrosis and tubular atrophy formation results from a complex cellular and molecular milieu participating in extracellular matrix formation.
Molecular mediators involved are numerous and involve pathways such as transforming growth factor (TGF-β), bone morphogenic protein (BMP), platelet-derived growth factor (PDGF), and hepatocyte growth factor (HGF); and important cells include epithelial cells, fibroblasts, myofibroblasts, fibrocytes, endothelial cells, lymphocytes, monocyte/macrophages, dendritic cells, and mast cells.
Epithelial and endothelial cells may undergo transitional to mesenchymal cells; however, this research may simply indicate transition to a phenotype rather than an actual cell type transition.
Recent genomic approaches have revealed the interplay of molecular and cellular factors, including the role of lymphocytes, in fibrosis formation
The assessment of fibrosis involves a number of visual and morphometric methods, many of which correlate with renal function; but there is no clear consensus regarding the best method.
Acknowledgments
Prior work by the authors related to this publication has been supported by grants from the National Institutes of Health (U01-AI-63623, U01-AI-077816, U01-AI-070107).
Abbreviations
- ACE
angiotensin converting enzyme
- AT1/2R[B]
angiotensin type 1/2 receptor [blockade]
- BMP
Bone morphogenic protein
- CAV1
caveolin-1
- CTI
chronic tubulointerstitial injury
- CYP
cytochrome P450
- ECM
extracellular matrix
- EMP
epithelial to mesenchymal phenotype
- EMT
epithelial to mesenchymal transition
- Gal-3
Galectin-3
- GFP
green fluorescent protein
- GFR
glomerular filtration rate
- HGF
hepatocyte growth factor
- HIF
hypoxia-induced factor
- IF/TA
interstitial fibrosis/tubular atrophy
- ILK
integrin-linked kinase
- JAK/STAT
janus kinase/signal transducer and activator of transcription
- LTBP
latent TGF-β binding protein
- miRNAs
microRNA
- MMP
matrix metalloproteinase
- PPAR
peroxisome proliferator-activated receptor
- PDGF
platelet-derived growth factor
- PTC
peritubular capillary
- RAG
recombinase activator gene
- SMA
smooth muscle actin
- tPA
tissue plasminogen activator
- TEC
tubular epithelial cell
- TGF
Transforming growth factor
- TLR
Toll-like receptor
- UUO
unilateral ureteral obstruction
- USAG-1
uterine sensitization-associated gene 1 [also known as sclerostin domain-containing protein 1]
- VEGF
vascular endothelial growth factor
Footnotes
Statement of Competing Financial Interests
The authors have no relevant competing financial interests to disclose.
References
(*) for special interest,
(**) for outstanding interest
- 1*.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. Review on mechanisms and potential therapeutic targets in renal fibrosis. [DOI] [PubMed] [Google Scholar]
- 2*.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Journal of the American Society of Nephrology: JASN. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. Recent review on IF/TA mechanisms. [DOI] [PubMed] [Google Scholar]
- 3.Colvin RB, Chang A, Farris AB, et al. Diagnostic Pathology: Kidney Diseases. Salt Lake City, UT: Amirsys; 2011. [Google Scholar]
- 4.Racusen LC, Solez K, Colvin R. Fibrosis and atrophy in the renal allograft: interim report and new directions. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2002;2:203–206. doi: 10.1034/j.1600-6143.2002.20303.x. [DOI] [PubMed] [Google Scholar]
- 5.Solez K, Colvin RB, Racusen LC, et al. Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 6*.Farris AB, Adams CD, Brousaides N, et al. Morphometric and Visual Evaluation of Fibrosis in Renal Biopsies. J Am Soc Nephrol. 2010 doi: 10.1681/ASN.2009091005. Recent study comparing Sirius Red, Collagen III, Trichrome, visual analysis, and image analysis showing particular effectiveness of collagen III morphometry and visual assessment of trichrome-stained slides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pape L, Henne T, Offner G, et al. Computer-assisted quantification of fibrosis in chronic allograft nephropaty by picosirius red-staining: a new tool for predicting long-term graft function. Transplantation. 2003;76:955–958. doi: 10.1097/01.TP.0000078899.62040.E5. [DOI] [PubMed] [Google Scholar]
- 8.Moreso F, Seron D, Vitria J, et al. Quantification of interstitial chronic renal damage by means of texture analysis. Kidney international. 1994;46:1721–1727. doi: 10.1038/ki.1994.474. [DOI] [PubMed] [Google Scholar]
- 9.Pape L, Mengel M, Offner G, et al. Renal arterial resistance index and computerized quantification of fibrosis as a combined predictive tool in chronic allograft nephropathy. Pediatr Transplant. 2004;8:565–570. doi: 10.1111/j.1399-3046.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Servais A, Meas-Yedid V, Buchler M, et al. Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation. 2007;84:1595–1601. doi: 10.1097/01.tp.0000295749.50525.bd. [DOI] [PubMed] [Google Scholar]
- 11*.Servais A, Meas-Yedid V, Noel LH, et al. Interstitial Fibrosis Evolution on Early Sequential Screening Renal Allograft Biopsies Using Quantitative Image Analysis. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1456–1463. doi: 10.1111/j.1600-6143.2011.03594.x. Recent paper detailing an customized image analysis algorithm approach measuring fibrosis in sequential renal allograft biopsies. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson ML, Bailey E, Williams S, et al. Computerized histomorphometric assessment of protocol renal transplant biopsy specimens for surrogate markers of chronic rejection. Transplantation. 1999;68:236–241. doi: 10.1097/00007890-199907270-00013. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson ML, McCulloch TA, Harper SJ, et al. Early measurement of interstitial fibrosis predicts long-term renal function and graft survival in renal transplantation. Br J Surg. 1996;83:1082–1085. doi: 10.1002/bjs.1800830813. [DOI] [PubMed] [Google Scholar]
- 14.Diaz Encarnacion MM, Griffin MD, Slezak JM, et al. Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am J Transplant. 2004;4:248–256. doi: 10.1046/j.1600-6143.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochemistry and cell biology. 2008;130:247–262. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohatgi R, Flores D. Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Current opinion in nephrology and hypertension. 2010;19:65–71. doi: 10.1097/MNH.0b013e32833327f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Zepeda-Orozco D, Patel V, et al. Aberrant planar cell polarity induced by urinary tract obstruction. American journal of physiology Renal physiology. 2009;297:F1526–1533. doi: 10.1152/ajprenal.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Fujigaki Y, Muranaka Y, Sun D, et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Archiv: an international journal of pathology. 2005;446:164–176. doi: 10.1007/s00428-004-1155-5. [DOI] [PubMed] [Google Scholar]
- 20.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dussaule JC, Guerrot D, Huby AC, et al. The role of cell plasticity in progression and reversal of renal fibrosis. Int J Exp Pathol. 2011;92:151–157. doi: 10.1111/j.1365-2613.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. Journal of the American Society of Nephrology: JASN. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 24.Grimm PC, Nickerson P, Gough J, et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol. 2003;14:1662–1668. doi: 10.1097/01.asn.0000066143.02832.5e. [DOI] [PubMed] [Google Scholar]
- 25.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Picard N, Baum O, Vogetseder A, et al. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochemistry and cell biology. 2008;130:141–155. doi: 10.1007/s00418-008-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Kida Y, Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol. 2011;38:417–423. doi: 10.1111/j.1440-1681.2011.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 31.Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. The American journal of pathology. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wada T, Sakai N, Sakai Y, et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin Exp Nephrol. 2011;15:8–13. doi: 10.1007/s10157-010-0372-2. [DOI] [PubMed] [Google Scholar]
- 34.Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai N, Furuichi K, Shinozaki Y, et al. Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Human pathology. 2010;41:672–678. doi: 10.1016/j.humpath.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Pilling D, Fan T, Huang D, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao DD, Suresh R, Vakil V, et al. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bucala R. Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39. doi: 10.1016/j.jacr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Takakuta K, Fujimori A, Chikanishi T, et al. Renoprotective properties of pirfenidone in subtotally nephrectomized rats. Eur J Pharmacol. 2010;629:118–124. doi: 10.1016/j.ejphar.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 41.RamachandraRao SP, Zhu Y, Ravasi T, et al. Pirfenidone is renoprotective in diabetic kidney disease. Journal of the American Society of Nephrology: JASN. 2009;20:1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho ME, Kopp JB. Pirfenidone: an anti-fibrotic therapy for progressive kidney disease. Expert Opin Investig Drugs. 2010;19:275–283. doi: 10.1517/13543780903501539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho ME, Smith DC, Branton MH, et al. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clinical journal of the American Society of Nephrology: CJASN. 2007;2:906–913. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- 44.Macias-Barragan J, Sandoval-Rodriguez A, Navarro-Partida J, Armendariz-Borunda J. The multifaceted role of pirfenidone and its novel targets. Fibrogenesis Tissue Repair. 2010;3:16. doi: 10.1186/1755-1536-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boor P, Sebekova K, Ostendorf T, Floege J. Treatment targets in renal fibrosis. Nephrol Dial Transplant. 2007;22:3391–3407. doi: 10.1093/ndt/gfm393. [DOI] [PubMed] [Google Scholar]
- 46.Shweke N, Boulos N, Jouanneau C, et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. The American journal of pathology. 2008;173:631–642. doi: 10.2353/ajpath.2008.080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L, Haylor JL, Hau Z, et al. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney international. 2009;76:383–394. doi: 10.1038/ki.2009.230. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Zhou Y, Tan R, et al. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299:F973–982. doi: 10.1152/ajprenal.00216.2010. [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Shultz RW, Mars WM, et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. The Journal of clinical investigation. 2002;110:1525–1538. doi: 10.1172/JCI16219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgtton KL, Gow RM, Kelly DJ, et al. Plasmin is not protective in experimental renal interstitial fibrosis. Kidney international. 2004;66:68–76. doi: 10.1111/j.1523-1755.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 51.Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. The American journal of pathology. 2003;162:1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrass CK, Hansen KM, Patton BL. Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor. The American journal of pathology. 2010;176:839–849. doi: 10.2353/ajpath.2010.090570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie P, Sun L, Nayak B, et al. C/EBP-beta modulates transcription of tubulointerstitial nephritis antigen in obstructive uropathy. Journal of the American Society of Nephrology: JASN. 2009;20:807–819. doi: 10.1681/ASN.2008091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong J, Guo D, Chen CB, et al. Prevention of angiotensin II-mediated renal oxidative stress, inflammation, and fibrosis by angiotensin-converting enzyme 2. Hypertension. 2011;57:314–322. doi: 10.1161/HYPERTENSIONAHA.110.164244. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Xue H, Yuan P, et al. Angiotensin AT1 receptor activation mediates high glucose-induced epithelial-mesenchymal transition in renal proximal tubular cells. Clin Exp Pharmacol Physiol. 2010;37:e152–157. doi: 10.1111/j.1440-1681.2010.05421.x. [DOI] [PubMed] [Google Scholar]
- 57.Naito T, Ma LJ, Yang H, et al. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298:F683–691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda Y, Nishikimi T, Akimoto K, et al. Beneficial effects of a combination of Rho-kinase inhibitor and ACE inhibitor on tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Hypertens Res. 2010;33:965–973. doi: 10.1038/hr.2010.112. [DOI] [PubMed] [Google Scholar]
- 59.Narasimhan KL, Madhu K, Balpinder K, et al. Association of angiotensin converting enzyme and angiotensin type 2 receptor gene polymorphisms with renal damage in posterior urethral valves. J Pediatr Urol. 2010;6:560–566. doi: 10.1016/j.jpurol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Schulman IH, Zhou MS, Treuer AV, et al. Altered renal expression of angiotensin II receptors, renin receptor, and ACE-2 precede the development of renal fibrosis in aging rats. Am J Nephrol. 2010;32:249–261. doi: 10.1159/000318607. [DOI] [PubMed] [Google Scholar]
- 61.Rodrigues Diez R, Rodrigues-Diez R, Lavoz C, et al. Statins inhibit angiotensin II/Smad pathway and related vascular fibrosis, by a TGF-beta-independent process. PLoS One. 2010;5:e14145. doi: 10.1371/journal.pone.0014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scruggs BS, Zuo Y, Donnert E, et al. Increased capillary branching contributes to angiotensin type 1 receptor blocker (ARB)-induced regression of sclerosis. Am J Pathol. 2011;178:1891–1898. doi: 10.1016/j.ajpath.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang R, Yang C, Tao JL, et al. Epithelial-mesenchymal transdifferentiation of renal tubular epithelial cells induced by urinary proteins requires the activation of PKC-alpha and betaI isozymes. Cell Biol Int. 2011;35:953–959. doi: 10.1042/CBI20100668. [DOI] [PubMed] [Google Scholar]
- 64.Wiwanitkit V. Fibrosis and evidence for epithelial-mesenchymal transition in the kidneys of patients with staghorn calculi. BJU Int. 2011;107:1847. doi: 10.1111/j.1464-410X.2011.10350.x. [DOI] [PubMed] [Google Scholar]
- 65.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. The Journal of clinical investigation. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? The Journal of clinical investigation. 2011;121:468–474. doi: 10.1172/JCI44595. Recent perspective paper questioning the existence of EMT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int J Exp Pathol. 2011;92:143–150. doi: 10.1111/j.1365-2613.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney international. 2011;80:41–50. doi: 10.1038/ki.2011.77. [DOI] [PubMed] [Google Scholar]
- 69.Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting Report: New Concepts in Antibody-Mediated Rejection. Am J Transplant. 2012 doi: 10.1111/j.1600-6143.2011.03926.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nature reviews Nephrology. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 71.Sakairi T, Hiromura K, Yamashita S, et al. Nestin expression in the kidney with an obstructed ureter. Kidney international. 2007;72:307–318. doi: 10.1038/sj.ki.5002277. [DOI] [PubMed] [Google Scholar]
- 72.Hertig A, Anglicheau D, Verine J, et al. Early epithelial phenotypic changes predict graft fibrosis. Journal of the American Society of Nephrology: JASN. 2008;19:1584–1591. doi: 10.1681/ASN.2007101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rastaldi MP, Ferrario F, Giardino L, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney international. 2002;62:137–146. doi: 10.1046/j.1523-1755.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 74.Docherty NG, Calvo IF, Quinlan MR, et al. Increased E-cadherin expression in the ligated kidney following unilateral ureteric obstruction. Kidney international. 2009;75:205–213. doi: 10.1038/ki.2008.482. [DOI] [PubMed] [Google Scholar]
- 75.Ohnuki K, Umezono T, Abe M, et al. Expression of transcription factor Snai1 and tubulointerstitial fibrosis in progressive nephropathy. J Nephrol. 2011:0. doi: 10.5301/JN.2011.8449. [DOI] [PubMed] [Google Scholar]
- 76.Saad S, Stanners SR, Yong R, et al. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol. 2010;42:1115–1122. doi: 10.1016/j.biocel.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Saal S, Harvey SJ. MicroRNAs and the kidney: coming of age. Current opinion in nephrology and hypertension. 2009;18:317–323. doi: 10.1097/MNH.0b013e32832c9da2. [DOI] [PubMed] [Google Scholar]
- 78.Scian MJ, Maluf DG, David KG, et al. MicroRNA profiles in allograft tissues and paired urines associate with chronic allograft dysfunction with IF/TA. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:2110–2122. doi: 10.1111/j.1600-6143.2011.03666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang B, Herman-Edelstein M, Koh P, et al. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes. 2010;59:1794–1802. doi: 10.2337/db09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong M, Jiang L, Zhou Y, et al. MiR-200 family regulates TGF-{beta}1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol. 2011 doi: 10.1152/ajprenal.00268.2011. [DOI] [PubMed] [Google Scholar]
- 81.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zarjou A, Yang S, Abraham E, et al. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol. 2011;301:F793–801. doi: 10.1152/ajprenal.00273.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin J, Jenkins RH, Bennagi R, et al. Post-transcriptional regulation of Transforming Growth Factor Beta-1 by microRNA-744. PLoS One. 2011;6:e25044. doi: 10.1371/journal.pone.0025044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qin W, Chung AC, Huang XR, et al. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. Journal of the American Society of Nephrology: JASN. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong X, Chung AC, Chen HY, et al. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. Journal of the American Society of Nephrology: JASN. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Q, Wang Y, Minto AW, et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. Faseb J. 2008;22:4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oba S, Kumano S, Suzuki E, et al. miR-200b precursor can ameliorate renal tubulointerstitial fibrosis. PLoS One. 2010;5:e13614. doi: 10.1371/journal.pone.0013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88*.Lorenzen JM, Haller H, Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nature reviews. Nephrology. 2011;7:286–294. doi: 10.1038/nrneph.2011.26. Recent review on the potential role for microRNAs in chronic kidney disease. [DOI] [PubMed] [Google Scholar]
- 89.Yuen DA, Connelly KA, Advani A, et al. Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLoS One. 2010;5:e9543. doi: 10.1371/journal.pone.0009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li L, Zepeda-Orozco D, Black R, Lin F. Autophagy is a component of epithelial cell fate in obstructive uropathy. The American journal of pathology. 2010;176:1767–1778. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cybulsky AV. Endoplasmic reticulum stress in proteinuric kidney disease. Kidney international. 2010;77:187–193. doi: 10.1038/ki.2009.389. [DOI] [PubMed] [Google Scholar]
- 92.Kawakami T, Inagi R, Takano H, et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:2665–2672. doi: 10.1093/ndt/gfp215. [DOI] [PubMed] [Google Scholar]
- 93.Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Experimental nephrology. 2009;112:e1–9. doi: 10.1159/000210573. [DOI] [PubMed] [Google Scholar]
- 94.Periyasamy-Thandavan S, Jiang M, Schoenlein P, Dong Z. Autophagy: molecular machinery, regulation, and implications for renal pathophysiology. American journal of physiology Renal physiology. 2009;297:F244–256. doi: 10.1152/ajprenal.00033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pallet N, Bouvier N, Legendre C, et al. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy. 2008;4:783–791. doi: 10.4161/auto.6477. [DOI] [PubMed] [Google Scholar]
- 96.Gozuacik D, Bialik S, Raveh T, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–1886. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 97.Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 98.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nature reviews Nephrology. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 99.Cho KH, Kim HJ, Kamanna VS, Vaziri ND. Niacin improves renal lipid metabolism and slows progression in chronic kidney disease. Biochim Biophys Acta. 2010;1800:6–15. doi: 10.1016/j.bbagen.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 100.Kim HJ, Moradi H, Yuan J, et al. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. American journal of physiology Renal physiology. 2009;296:F1297–1306. doi: 10.1152/ajprenal.90761.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toblli JE, Ferrini MG, Cao G, et al. Antifibrotic effects of pioglitazone on the kidney in a rat model of type 2 diabetes mellitus. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:2384–2391. doi: 10.1093/ndt/gfp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawai T, Masaki T, Doi S, et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Laboratory investigation; a journal of technical methods and pathology. 2009;89:47–58. doi: 10.1038/labinvest.2008.104. [DOI] [PubMed] [Google Scholar]
- 103.Toblli JE, Cao G, Giani JF, et al. Antifibrotic effects of pioglitazone at low doses on the diabetic rat kidney are associated with the improvement of markers of cell turnover, tubular and endothelial integrity, and angiogenesis. Kidney Blood Press Res. 2011;34:20–33. doi: 10.1159/000320380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka Y, Kume S, Araki S, et al. Fenofibrate, a PPARalpha agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney international. 2011;79:871–882. doi: 10.1038/ki.2010.530. [DOI] [PubMed] [Google Scholar]
- 105.Hakroush S, Moeller MJ, Theilig F, et al. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. The American journal of pathology. 2009;175:1883–1895. doi: 10.2353/ajpath.2009.080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lian YG, Zhou QG, Zhang YJ, Zheng FL. VEGF ameliorates tubulointerstitial fibrosis in unilateral ureteral obstruction mice via inhibition of epithelial-mesenchymal transition. Acta Pharmacol Sin. 2011 doi: 10.1038/aps.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kimura K, Iwano M, Higgins DF, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. American journal of physiology Renal physiology. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. The Journal of clinical investigation. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Z, Tang L, Zhu Q, et al. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney international. 2011;79:300–310. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bechtel W, McGoohan S, Zeisberg EM, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nature medicine. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dwivedi RS, Herman JG, McCaffrey TA, Raj DS. Beyond genetics: epigenetic code in chronic kidney disease. Kidney international. 2011;79:23–32. doi: 10.1038/ki.2010.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marumo T, Hishikawa K, Yoshikawa M, et al. Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am J Physiol Renal Physiol. 2010;298:F133–141. doi: 10.1152/ajprenal.00400.2009. [DOI] [PubMed] [Google Scholar]
- 114.Pang M, Zhuang S. Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther. 2010;335:266–272. doi: 10.1124/jpet.110.168385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun G, Reddy MA, Yuan H, et al. Epigenetic histone methylation modulates fibrotic gene expression. Journal of the American Society of Nephrology: JASN. 2010;21:2069–2080. doi: 10.1681/ASN.2010060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nature medicine. 2010;16:535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tapmeier TT, Fearn A, Brown K, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney international. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 118.Nikolic-Paterson DJ. CD4+ T cells: a potential player in renal fibrosis. Kidney international. 2010;78:333–335. doi: 10.1038/ki.2010.182. [DOI] [PubMed] [Google Scholar]
- 119.Snelgrove SL, Kausman JY, Lo C, et al. Renal Dendritic Cells Adopt a Pro-Inflammatory Phenotype in Obstructive Uropathy to Activate T Cells, but Do Not Directly Contribute to Fibrosis. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 120*.Scian MJ, Maluf DG, Archer KJ, et al. Gene expression changes are associated with loss of kidney graft function and interstitial fibrosis and tubular atrophy: diagnosis versus prediction. Transplantation. 2011;91:657–665. doi: 10.1097/TP.0b013e3182094a5a. Genes up-regulated in IF/TA include pathways related to T-cell activation, natural killer cell-mediated cytotoxicity, and programmed cell death based on microarray analysis of renal allografts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khan F, Sar A, Gonul I, et al. Graft inflammation and histologic indicators of kidney chronic allograft failure: low-expressing interleukin-10 genotypes cannot be ignored. Transplantation. 2010;90:630–638. doi: 10.1097/TP.0b013e3181ea391e. [DOI] [PubMed] [Google Scholar]
- 122.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney international. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- 123.Vernon MA, Mylonas KJ, Hughes J. Macrophages and renal fibrosis. Semin Nephrol. 2010;30:302–317. doi: 10.1016/j.semnephrol.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Lin SL, Castano AP, Nowlin BT, et al. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. Journal of immunology. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 125.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. The American journal of pathology. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Okamura DM, Pasichnyk K, Lopez-Guisa JM, et al. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am J Physiol Renal Physiol. 2011;300:F245–253. doi: 10.1152/ajprenal.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Menke J, Iwata Y, Rabacal WA, et al. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. The Journal of clinical investigation. 2009;119:2330–2342. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y, Cao Q, Zheng G, et al. By homing to the kidney, activated macrophages potently exacerbate renal injury. The American journal of pathology. 2008;172:1491–1499. doi: 10.2353/ajpath.2008.070825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nishida M, Okumura Y, Fujimoto S, et al. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun. 2005;332:11–16. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 130.Semedo P, Donizetti-Oliveira C, Burgos-Silva M, et al. Bone marrow mononuclear cells attenuate fibrosis development after severe acute kidney injury. Lab Invest. 2010;90:685–695. doi: 10.1038/labinvest.2010.45. [DOI] [PubMed] [Google Scholar]
- 131.Macconi D, Chiabrando C, Schiarea S, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. Journal of the American Society of Nephrology: JASN. 2009;20:123–130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. The Journal of clinical investigation. 2009;119:1286–1297. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kanamaru Y, Scandiuzzi L, Essig M, et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. Journal of immunology. 2006;176:5607–5615. doi: 10.4049/jimmunol.176.9.5607. [DOI] [PubMed] [Google Scholar]
- 134.Fan YY, Nishiyama A, Fujisawa Y, et al. Contribution of chymase-dependent angiotensin II formation to the progression of tubulointerstitial fibrosis in obstructed kidneys in hamsters. J Pharmacol Sci. 2009;111:82–90. doi: 10.1254/jphs.09152fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Timoshanko JR, Kitching AR, Semple TJ, et al. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. Journal of the American Society of Nephrology: JASN. 2006;17:150–159. doi: 10.1681/ASN.2005080799. [DOI] [PubMed] [Google Scholar]
- 136.Scandiuzzi L, Beghdadi W, Daugas E, et al. Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol. 2010;185:624–633. doi: 10.4049/jimmunol.0902129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veerappan A, Reid AC, O’Connor N, et al. Mast Cells are Required for the Development of Renal Fibrosis in the Rodent Unilateral Ureteral Obstruction Model. Am J Physiol Renal Physiol. 2011 doi: 10.1152/ajprenal.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Y, Zhou L, Liu F, et al. Mast Cell Infiltration Is Involved in Renal Interstitial Fibrosis in a Rat Model of Protein-Overload Nephropathy. Kidney Blood Press Res. 2010;33:240–248. doi: 10.1159/000317102. [DOI] [PubMed] [Google Scholar]
- 139.Kim DH, Moon SO, Jung YJ, et al. Mast cells decrease renal fibrosis in unilateral ureteral obstruction. Kidney international. 2009;75:1031–1038. doi: 10.1038/ki.2009.1. [DOI] [PubMed] [Google Scholar]
- 140.Li J, Bertram JF. Review: Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology (Carlton) 2010;15:507–512. doi: 10.1111/j.1440-1797.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 141.Li J, Qu X, Yao J, et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:2612–2624. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Truong LD, Gaber L, Eknoyan G. Obstructive uropathy. Contrib Nephrol. 2011;169:311–326. doi: 10.1159/000314578. [DOI] [PubMed] [Google Scholar]
- 143.van Meeteren LA, Ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144*.Steegh FM, Gelens MA, Nieman FH, et al. Early loss of peritubular capillaries after kidney transplantation. Journal of the American Society of Nephrology: JASN. 2011;22:1024–1029. doi: 10.1681/ASN.2010050531. Paper demonstrating the loss of peritubular capillaries after transplantation, which correlated with IFTA and reduced renal function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sakamoto I, Ito Y, Mizuno M, et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney international. 2009;75:828–838. doi: 10.1038/ki.2008.661. [DOI] [PubMed] [Google Scholar]
- 146.Ozdemir BH, Ozdemir AA, Erdal R, et al. Rapamycin prevents interstitial fibrosis in renal allografts through decreasing angiogenesis and inflammation. Transplant Proc. 2011;43:524–526. doi: 10.1016/j.transproceed.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 147.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney international. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 148.Rienstra H, Katta K, Celie JW, et al. Differential expression of proteoglycans in tissue remodeling and lymphangiogenesis after experimental renal transplantation in rats. PLoS One. 2010;5:e9095. doi: 10.1371/journal.pone.0009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stuht S, Gwinner W, Franz I, et al. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7:377–384. doi: 10.1111/j.1600-6143.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 150.Ortiz A, Ucero AC, Egido J. Unravelling fibrosis: two newcomers and an old foe. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association -European Renal Association. 2010;25:3492–3495. doi: 10.1093/ndt/gfq518. [DOI] [PubMed] [Google Scholar]
- 151.Wang Q, Usinger W, Nichols B, et al. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S, Wilkes MC, Leof EB, Hirschberg R. Noncanonical TGF-beta pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am J Physiol Renal Physiol. 2010;298:F142–149. doi: 10.1152/ajprenal.00320.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yeh YC, Wei WC, Wang YK, et al. Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol. 2010;177:1743–1754. doi: 10.2353/ajpath.2010.091183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng R, Han M, Luo Y, et al. Role of Sema4C in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association -European Renal Association. 2011;26:1149–1156. doi: 10.1093/ndt/gfq619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu B, Wang YJ, Zhu CF, et al. Triptolide inhibits extracellular matrix protein synthesis by suppressing the Smad2 but not the MAPK pathway in TGF-beta1-stimulated NRK-49F cells. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association -European Renal Association. 2010;25:3180–3191. doi: 10.1093/ndt/gfq239. [DOI] [PubMed] [Google Scholar]
- 156.Huang XR, Chung AC, Wang XJ, et al. Mice overexpressing latent TGF-beta1 are protected against renal fibrosis in obstructive kidney disease. American journal of physiology Renal physiology. 2008;295:F118–127. doi: 10.1152/ajprenal.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang XR, Chung AC, Zhou L, et al. Latent TGF-beta1 protects against crescentic glomerulonephritis. Journal of the American Society of Nephrology: JASN. 2008;19:233–242. doi: 10.1681/ASN.2007040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meng XM, Huang XR, Chung AC, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. Journal of the American Society of Nephrology: JASN. 2010;21:1477–1487. doi: 10.1681/ASN.2009121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou Y, Mao H, Li S, et al. HSP72 inhibits Smad3 activation and nuclear translocation in renal epithelial-to-mesenchymal transition. Journal of the American Society of Nephrology: JASN. 2010;21:598–609. doi: 10.1681/ASN.2009050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luo DD, Phillips A, Fraser D. Bone morphogenetic protein-7 inhibits proximal tubular epithelial cell Smad3 signaling via increased SnoN expression. Am J Pathol. 2010;176:1139–1147. doi: 10.2353/ajpath.2010.090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sakairi T, Hiromura K, Takahashi S, et al. Effects of proteasome inhibitors on rat renal fibrosis in vitro and in vivo. Nephrology (Carlton) 2011;16:76–86. doi: 10.1111/j.1440-1797.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- 163.Yang J, Zhang X, Li Y, Liu Y. Downregulation of Smad transcriptional corepressors SnoN and Ski in the fibrotic kidney: an amplification mechanism for TGF-beta1 signaling. Journal of the American Society of Nephrology: JASN. 2003;14:3167–3177. doi: 10.1097/01.asn.0000099373.33259.b2. [DOI] [PubMed] [Google Scholar]
- 164.Leask A. Targeting the jagged/notch pathway: a new treatment for fibrosis? J Cell Commun Signal. 2010;4:197–198. doi: 10.1007/s12079-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sharma S, Sirin Y, Susztak K. The story of Notch and chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:56–61. doi: 10.1097/MNH.0b013e3283414c88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sirin Y, Susztak K. Notch in the kidney: development and disease. J Pathol. 2011 doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wilkinson L, Gilbert T, Sipos A, et al. Loss of renal microvascular integrity in postnatal Crim1 hypomorphic transgenic mice. Kidney international. 2009;76:1161–1171. doi: 10.1038/ki.2009.345. [DOI] [PubMed] [Google Scholar]
- 168.Zeisberg M. Bone morphogenic protein-7 and the kidney: current concepts and open questions. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:568–573. doi: 10.1093/ndt/gfk010. [DOI] [PubMed] [Google Scholar]
- 169.De Heer E, Sijpkens YW, Verkade M, et al. Morphometry of interstitial fibrosis. Nephrol Dial Transplant. 2000;15 (Suppl 6):72–73. doi: 10.1093/ndt/15.suppl_6.72. [DOI] [PubMed] [Google Scholar]
- 170.Maciel TT, Kempf H, Campos AH. Targeting bone morphogenetic protein signaling on renal and vascular diseases. Curr Opin Nephrol Hypertens. 2010;19:26–31. doi: 10.1097/MNH.0b013e328332fc13. [DOI] [PubMed] [Google Scholar]
- 171.Yanagita M. Modulator of bone morphogenetic protein activity in the progression of kidney diseases. Kidney international. 2006;70:989–993. doi: 10.1038/sj.ki.5001731. [DOI] [PubMed] [Google Scholar]
- 172.Tanaka M, Asada M, Higashi AY, et al. Loss of the BMP antagonist USAG-1 ameliorates disease in a mouse model of the progressive hereditary kidney disease Alport syndrome. The Journal of clinical investigation. 2010;120:768–777. doi: 10.1172/JCI39569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Grgurevic L, Macek B, Healy DR, et al. Circulating bone morphogenetic protein 1–3 isoform increases renal fibrosis. Journal of the American Society of Nephrology: JASN. 2011;22:681–692. doi: 10.1681/ASN.2010070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Okada H. Tolloid-like proteinases orchestrate extracellular matrix formation. Journal of the American Society of Nephrology: JASN. 2011;22:588–589. doi: 10.1681/ASN.2011020131. [DOI] [PubMed] [Google Scholar]
- 175.Xin BM, Wang XX, Jin W, et al. Activation of Toll-like receptor 9 attenuates unilateral ureteral obstruction-induced renal fibrosis. Acta Pharmacol Sin. 2010;31:1583–1592. doi: 10.1038/aps.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pulskens WP, Rampanelli E, Teske GJ, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. Journal of the American Society of Nephrology: JASN. 2010;21:1299–1308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Skuginna V, Lech M, Allam R, et al. Toll-like receptor signaling and SIGIRR in renal fibrosis upon unilateral ureteral obstruction. PLoS One. 2011;6:e19204. doi: 10.1371/journal.pone.0019204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xavier S, Gilbert V, Rastaldi MP, et al. BAMBI is expressed in endothelial cells and is regulated by lysosomal/autolysosomal degradation. PLoS One. 2010;5:e12995. doi: 10.1371/journal.pone.0012995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. Journal of the American Society of Nephrology: JASN. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 180.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 181.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney international. 2011;79:944–956. doi: 10.1038/ki.2010.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hahm K, Lukashev ME, Luo Y, et al. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. The American journal of pathology. 2007;170:110–125. doi: 10.2353/ajpath.2007.060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yan Q, Sui W, Xie S, et al. Expression and role of integrin-linked kinase and collagen IV in human renal allografts with interstitial fibrosis and tubular atrophy. Transpl Immunol. 2010;23:1–5. doi: 10.1016/j.trim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 184.Iekushi K, Taniyama Y, Azuma J, et al. Hepatocyte growth factor attenuates renal fibrosis through TGF-beta1 suppression by apoptosis of myofibroblasts. J Hypertens. 2010;28:2454–2461. doi: 10.1097/HJH.0b013e32833e4149. [DOI] [PubMed] [Google Scholar]
- 185.Dai C, Saleem MA, Holzman LB, et al. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney international. 2010;77:962–973. doi: 10.1038/ki.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Iekushi K, Taniyama Y, Kusunoki H, et al. Hepatocyte growth factor attenuates transforming growth factor-beta-angiotensin II crosstalk through inhibition of the PTEN/Akt pathway. Hypertension. 2011;58:190–196. doi: 10.1161/HYPERTENSIONAHA.111.173013. [DOI] [PubMed] [Google Scholar]
- 187.Mizuno S, Ikebuchi F, Fukuta K, et al. Recombinant human HGF, but not rat HGF, elicits glomerular injury and albuminuria in normal rats via an immune complex-dependent mechanism. Clin Exp Pharmacol Physiol. 2011 doi: 10.1111/j.1440-1681.2011.05483.x. [DOI] [PubMed] [Google Scholar]
- 188.Ostendorf T, Eitner F, Floege J. The PDGF family in renal fibrosis. Pediatr Nephrol. 2011 doi: 10.1007/s00467-011-1892-z. [DOI] [PubMed] [Google Scholar]
- 189.Eitner F, Bucher E, van Roeyen C, et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. Journal of the American Society of Nephrology: JASN. 2008;19:281–289. doi: 10.1681/ASN.2007030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taneda S, Hudkins KL, Topouzis S, et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. Journal of the American Society of Nephrology: JASN. 2003;14:2544–2555. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]
- 191.Pang M, Ma L, Gong R, et al. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney international. 2010;78:257–268. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 192*.Sorensen I, Susnik N, Inhester T, et al. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney international. 2011;80:1035–1044. doi: 10.1038/ki.2011.214. Study demonstrating the action of fibrinogen as a mitogen for fibroblasts, leading to increased fibrosis. [DOI] [PubMed] [Google Scholar]
- 193.Boor P, Konieczny A, Villa L, et al. Complement C5 mediates experimental tubulointerstitial fibrosis. Journal of the American Society of Nephrology: JASN. 2007;18:1508–1515. doi: 10.1681/ASN.2006121343. [DOI] [PubMed] [Google Scholar]
- 194.Pan H, Shen Z, Mukhopadhyay P, et al. Anaphylatoxin C5a contributes to the pathogenesis of cisplatin-induced nephrotoxicity. American journal of physiology Renal physiology. 2009;296:F496–504. doi: 10.1152/ajprenal.90443.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nakorchevsky A, Hewel JA, Kurian SM, et al. Molecular mechanisms of chronic kidney transplant rejection via large-scale proteogenomic analysis of tissue biopsies. Journal of the American Society of Nephrology: JASN. 2010;21:362–373. doi: 10.1681/ASN.2009060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grimm PC, Nickerson P, Gough J, et al. Quantitation of allograft fibrosis and chronic allograft nephropathy. Pediatric transplantation. 1999;3:257–270. doi: 10.1034/j.1399-3046.1999.00044.x. [DOI] [PubMed] [Google Scholar]
- 197.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 198.Choi BS, Shin MJ, Shin SJ, et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005;5:1354–1360. doi: 10.1111/j.1600-6143.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 199.Colvin RB, Nickeleit V. Renal transplant pathology. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. pp. 1347–1490. [Google Scholar]
- 200.Cosio FG, Grande JP, Wadei H, et al. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5:2464–2472. doi: 10.1111/j.1600-6143.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- 201.Kostadinova-Kunovska S, Petrusevska G, Jovanovic R, et al. Histomorphometric analysis of fibrosis in the renal interstitial compartment. Prilozi. 2005;26:51–59. [PubMed] [Google Scholar]
- 202.Servais A, Meas-Yedid V, Toupance O, et al. Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. Am J Transplant. 2009;9:2552–2560. doi: 10.1111/j.1600-6143.2009.02803.x. [DOI] [PubMed] [Google Scholar]
- 203.Vleming LJ, Baelde JJ, Westendorp RG, et al. Progression of chronic renal disease in humans is associated with the deposition of basement membrane components and decorin in the interstitial extracellular matrix. Clin Nephrol. 1995;44:211–219. [PubMed] [Google Scholar]
- 204.Abrass CK, Berfield AK, Stehman-Breen C, et al. Unique changes in interstitial extracellular matrix composition are associated with rejection and cyclosporine toxicity in human renal allograft biopsies. Am J Kidney Dis. 1999;33:11–20. doi: 10.1016/s0272-6386(99)70252-0. [DOI] [PubMed] [Google Scholar]
- 205.Verkade MA, Bajema IM, De Heer E, Bruijn JA. Decorin and TGF-beta-1 protein expression in renal disease: a morphometric analysis. J Am Soc Nephrol. 1999;10:584. (abstract) [Google Scholar]
- 206.Sund S, Grimm P, Reisaeter AV, Hovig T. Computerized image analysis vs semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant. 2004;19:2838–2845. doi: 10.1093/ndt/gfh490. [DOI] [PubMed] [Google Scholar]
- 207.Bains JC, Sandford RM, Brook NR, et al. Comparison of renal allograft fibrosis after transplantation from heart-beating and non-heart-beating donors. Br J Surg. 2005;92:113–118. doi: 10.1002/bjs.4777. [DOI] [PubMed] [Google Scholar]
- 208.Hunter MG, Hurwitz S, Bellamy CO, Duffield JS. Quantitative morphometry of lupus nephritis: the significance of collagen, tubular space, and inflammatory infiltrate. Kidney Int. 2005;67:94–102. doi: 10.1111/j.1523-1755.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 209.Masseroli M, O’Valle F, Andujar M, et al. Design and validation of a new image analysis method for automatic quantification of interstitial fibrosis and glomerular morphometry. Lab Invest. 1998;78:511–522. [PubMed] [Google Scholar]
- 210.Ruiz P, Kolbeck PC, Scroggs MW, Sanfilippo F. Associations between cyclosporine therapy and interstitial fibrosis in renal allograft biopsies. Transplantation. 1988;45:91–95. doi: 10.1097/00007890-198801000-00021. [DOI] [PubMed] [Google Scholar]
- 211.Ruiz P, Kolbeck PC, Scroggs MW, et al. Cyclosporine therapy and the development of interstitial fibrosis in renal allografts. Transplant Proc. 1988;20:807–811. [PubMed] [Google Scholar]
- 212.Liu Y, Yang J. Hepatocyte growth factor: new arsenal in the fights against renal fibrosis? Kidney Int. 2006;70:238–240. doi: 10.1038/sj.ki.5001661. [DOI] [PubMed] [Google Scholar]
- 213.Vilayur E, Harris DC. Emerging therapies for chronic kidney disease: what is their role? Nat Rev Nephrol. 2009 doi: 10.1038/nrneph.2009.76. [DOI] [PubMed] [Google Scholar]
- 214.Moreso F, Lopez M, Vallejos A, et al. Serial protocol biopsies to quantify the progression of chronic transplant nephropathy in stable renal allografts. Am J Transplant. 2001;1:82–88. doi: 10.1034/j.1600-6143.2001.010115.x. [DOI] [PubMed] [Google Scholar]
- 215.Marcussen N, Olsen TS, Benediktsson H, et al. Reproducibility of the Banff classification of renal allograft pathology. Inter- and intraobserver variation Transplantation. 1995;60:1083–1089. doi: 10.1097/00007890-199511270-00004. [DOI] [PubMed] [Google Scholar]
- 216.Furness PN, Taub N. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney international. 2001;60:1998–2012. doi: 10.1046/j.1523-1755.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- 217.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 218.Feldman DL, Mogelesky TC, Chou M, Jeng AY. Enhanced expression of renal endothelin-converting enzyme-1 and endothelin-A-receptor mRNA in rats with interstitial fibrosis following ureter ligation. J Cardiovasc Pharmacol. 2000;36:S255–259. doi: 10.1097/00005344-200036051-00075. [DOI] [PubMed] [Google Scholar]
- 219.Satoh M, Kashihara N, Yamasaki Y, et al. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;12:317–325. doi: 10.1681/ASN.V122317. [DOI] [PubMed] [Google Scholar]
- 220.Meas-Yedid V, Servais A, Noel LH, et al. New computerized color image analysis for the quantification of interstitial fibrosis in renal transplantation. Transplantation. 2011;92:890–899. doi: 10.1097/TP.0b013e31822d879a. [DOI] [PubMed] [Google Scholar]
- 221.Miura Y, Satoh S, Saito M, et al. Factors increasing quantitative interstitial fibrosis from 0 hr to 1 year in living kidney transplant patients receiving tacrolimus. Transplantation. 2011;91:78–85. doi: 10.1097/tp.0b013e3181ff4f7f. [DOI] [PubMed] [Google Scholar]
- 222.Servais A, Meas-Yedid V, Noel LH, et al. Interstitial fibrosis evolution on early sequential screening renal allograft biopsies using quantitative image analysis. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1456–1463. doi: 10.1111/j.1600-6143.2011.03594.x. [DOI] [PubMed] [Google Scholar]
- 223.Seron D, Moreso F. Protocol biopsies in renal transplantation: prognostic value of structural monitoring. Kidney international. 2007;72:690–697. doi: 10.1038/sj.ki.5002396. [DOI] [PubMed] [Google Scholar]
- 224.Halloran PF, de Freitas DG, Einecke G, et al. The molecular phenotype of kidney transplants. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:2215–2222. doi: 10.1111/j.1600-6143.2010.03267.x. [DOI] [PubMed] [Google Scholar]
- 225.Shishido S, Asanuma H, Nakai H, et al. The impact of repeated subclinical acute rejection on the progression of chronic allograft nephropathy. J Am Soc Nephrol. 2003;14:1046–1052. doi: 10.1097/01.asn.0000056189.02819.32. [DOI] [PubMed] [Google Scholar]
- 226.Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:2066–2073. doi: 10.1111/j.1600-6143.2010.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Park WD, Griffin MD, Cornell LD, et al. Fibrosis with inflammation at one year predicts transplant functional decline. Journal of the American Society of Nephrology: JASN. 2010;21:1987–1997. doi: 10.1681/ASN.2010010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sis B, Mengel M, Haas M, et al. Banff ‘09 Meeting Report: Antibody Mediated Graft Deterioration and Implementation of Banff Working Groups. Am J Transplant. 2010 doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 229.Blattner SM, Kretzler M. Integrin-linked kinase in renal disease: connecting cell-matrix interaction to the cytoskeleton. Curr Opin Nephrol Hypertens. 2005;14:404–410. doi: 10.1097/01.mnh.0000172730.67746.5b. [DOI] [PubMed] [Google Scholar]
- 230.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. Journal of the American Society of Nephrology: JASN. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]