Abstract
Rationale
Islet1 (Isl1) has been proposed as a marker of cardiac progenitor cells derived from the second heart field and is utilized to identify and purify cardiac progenitors from murine and human specimens for ex vivo expansion. The use of Isl1 as a specific second heart field marker is dependent on its exclusion from other cardiac lineages such as neural crest.
Objective
Determine if Isl1 is expressed by cardiac neural crest.
Methods and Results
We used an intersectional fate-mapping system employing the RC::FrePe allele which reports dual Flpe and Cre recombination. Combining Isl11Cre/+, a SHF driver, and Wnt1::Flpe, a neural crest driver, with Rc::FrePe reveals that some Isl1 derivatives in the cardiac outflow tract derive from Wnt1-expressing neural crest progenitors. In contrast, no overlap was observed between Wnt1-derived neural crest and an alternative second heart field driver, Mef2c-AHF-Cre.
Conclusions
Isl1 is not restricted to second heart field progenitors in the developing heart but also labels cardiac neural crest. The intersection of Isl1 and Wnt1 lineages within the heart provides a caveat to using Isl1 as an exclusive second heart field cardiac progenitor marker and suggests that some Isl1-expressing progenitor cells derived from embryos, ES or iPS cultures may be of neural crest lineage.
Keywords: myocardial lineages, second heart field, neural crest, heart development
Introduction
The recent discovery of the second heart field (SHF) has redefined the understanding of mammalian cardiac development.1 While cells of the first heart field (FHF) form the early cardiac tube, the SHF contributes additional cells to the maturing heart through mid-gestation, ultimately forming large portions of the right ventricle, outflow tract and atria.2–4 SHF precursors have been characterized by expression of the homeobox gene Islet1 (Isl1).2 Isl1 has been used to isolate putative cardiac progenitors from embryonic stem (ES) cell and induced pluripotent stem (iPS) cell cultures for the purpose of expansion and possible therapeutic application.5–10 In vitro studies and in vivo fate-mapping experiments suggest that Isl1 precursors are tri-potential and give rise to endothelium, smooth and cardiac muscle.7, 9 Although SHF-specific enhancer elements from several genes have been identified by the analysis of transgenic mice,3, 11 no other specific markers for SHF precursors have been reported, emphasizing the importance that Isl1 has played as a specific marker of the SHF in cardiac and stem cell biology.
Surprisingly, inactivation of various factors in SHF using Isl1Cre/+ has resulted in mice with congenital heart defects involving the outflow tract (OFT) and aortic arch arteries that are strikingly similar to abnormalities produced by gene manipulation using Wnt1::Cre or Pax3Cre/+, which drive expression in cardiac neural crest.12–14 This has led to the suggestion that SHF signals to neural crest via cell-cell interactions or secreted factors. However, an alternative hypothesis is that Isl1Cre/+ is not restricted to SHF within the developing heart. To address if Isl1Cre/+ may also be expressed in the Wnt1 lineage of cardiac neural crest in addition to lineages of the SHF, we utilized a dual-fluorescent reporter allele that extends previously established recombinase-based intersectional strategies.15–17 This dual fate-mapping approach employs a recently developed reporter mouse, RC::FrePe (Brust, R.D. and Dymecki, S.M., unpublished data and see ref. 18) that activates expression of enhanced green fluorescent protein (eGFP) within cells that have expressed both Flpe and Cre at any time and in any order within their lineage. In cells that have expressed Flpe alone, mCherry is expressed, while Cre activity alone is not reported. Thus, RC::FrePe indicates an intersectional population between expression domains as defined by expression of separate Flpe and Cre drivers.
Methods
A schematic of the RC::FrePe allele can be found within a summary of intersectional fate-mapping strategies and methods.19
Histology and Immunohistochemistry
Samples were harvested, fixed overnight in 2% formaldehyde and subsequently dehydrated through an ethanol series. Samples were then paraffin embedded and sectioned. For whole-mount staining of LacZ expression, samples were lightly fixed with 2% formaldehyde for 30 minutes and then incubated overnight with X-Gal. Antibodies used for immunohistochemistry were anti-dsRed rabbit polyclonal (Clontech), anti-GFP goat polyclonal (Abcam) and anti-αSMA mouse monoclonal 1A4 (Sigma-Aldrich). Quantitation of fluorescence was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Genotyping information can be found in the online data supplement at http://circres.ahajournals.org.
Results
To validate the reporter activity of RC::FrePe in neural crest derivatives, two neural crest drivers, Wnt1::Flpe20, 21 and Pax3Cre/+22 were used to assess recombination and reporter activity (Online Figure I A–O). Direct fluorescence of postnatal (P) day 0 Wnt1::Flpe Pax3Cre/+; RC::FrePe hearts demonstrates eGFP expression within the outflow tract where neural crest derivatives reside (Online Figure I M).22 Subsequent immunofluorescence confirms eGFP protein expression within the tunica media of the mature OFT, as expected for neural crest derivatives (Online Figure I O). Relatively few mCherry-labeled cells remain after dual Flpe/Cre recombination suggesting that most cardiac neural crest cells that expressed Wnt1::Flpe also expressed Pax3Cre/+ (Online Figure I N). An average of fluorescence from nine stained Wnt1::Flpe; Pax3Cre/+; RC::FrePe outflow tract sections revealed 8.4%±3.6% (mean ± SD) of total staining was mCherry positive, while the majority, 91.6%±3.5%, was eGFP positive. Thus, the indicator allele RC::FrePe is sensitive to dual recombination, consistent with previous findings (Brust, R.D. and Dymecki, S.M., unpublished data and see ref. 18). We did not detect any leakiness of eGFP or mCherry expression in these studies (Online Figure I B–E)
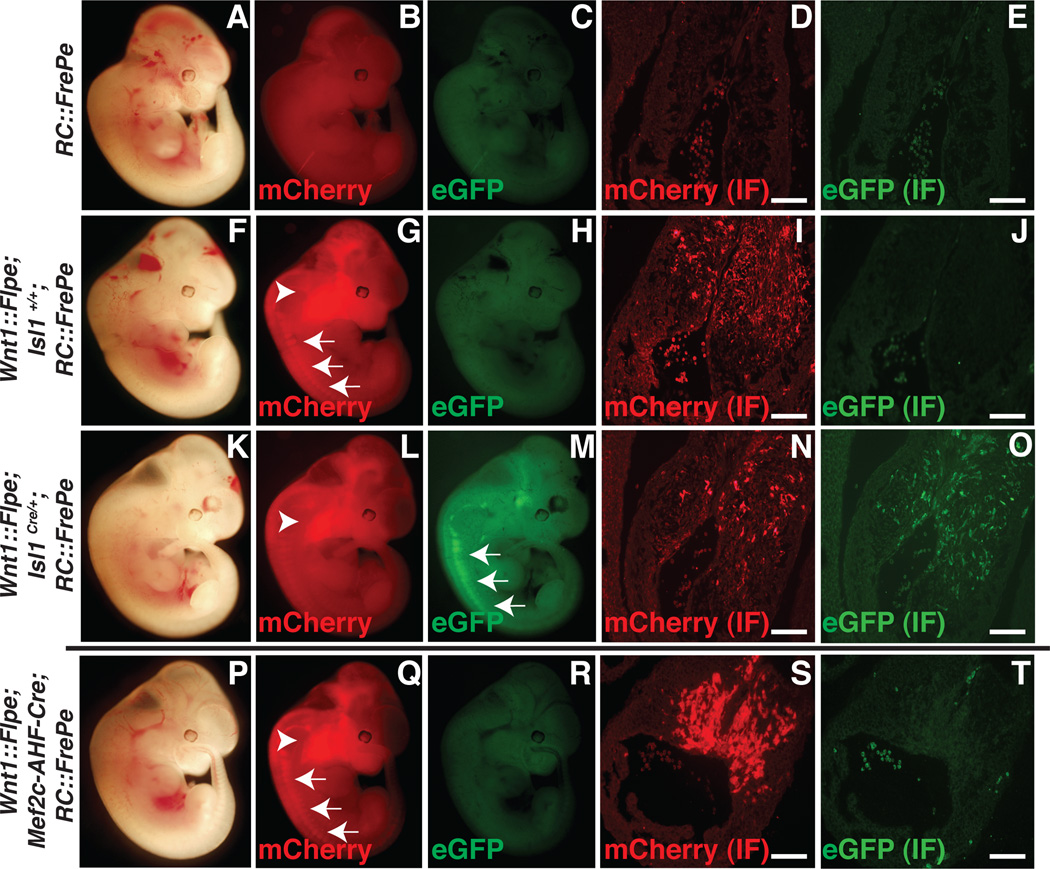
We crossed Isl1Cre/+23, 24 mice with Wnt1::Flpe and RC::FrePe mice to detect overlap in Isl1- and Wnt1-expressing populations (Figure 1A–O). In the absence of Flpe and Cre, only background fluorescence was observed in embryonic (E) day 12.5 embryos (Figure 1A–E). When Wnt1::Flpe was expressed in the presence of RC::FrePe, mCherry expression was observed by direct fluorescence in the craniofacial region populated by neural crest (Figure 1G, arrowhead) and in dorsal root ganglia (DRG, Figure 1G, arrows). eGFP was not detected (Figure 1H). mCherry+ cells were detected by immunofluorescence in sections through the OFT endocardial cushions that are populated by cardiac neural crest by E12.5 (Figure 1I), while eGFP was not detected (Figure 1J). In the presence of both Wnt1::Flpe and Isl1Cre/+, mCherry expression persisted in craniofacial mesenchyme populated by cranial neural crest (Figure 1L, arrowhead) and eGFP fluorescence was observed in DRGs (Figure 1M, arrows), consistent with the known expression of Isl1 in neural crest-derived DRGs.25 Immunofluorescence confirmed expression of both mCherry (Figure 1N) and eGFP (Figure 1O) in sections through the endocardial cushions of the OFT, indicating that at least some cardiac neural crest derivatives in the heart have expressed Isl1Cre/+ at some time in their development.
Figure 1. Dual fate mapping identifies Isl1Cre/+/Wnt1::Flpe-derived cells in the heart at E12.5.
A–E, E12.5 control RC::FrePe embryos (A–C) and immunofluorescence (IF) for mCherry (D) and eGFP (E) of cross sections through cardiac outflow tract (D,E). F–J, Wnt1::Flpe; Isl1+/+; RC::FrePe embryos. Wnt1::Flpe-derived craniofacial neural crest (G, arrowhead) and dorsal root ganglia (G, arrows) express mCherry, which is also detected by IF in the endocardial cushions of the outflow tract (I). K–O, Wnt1::Flpe; Isl1Cre/+; RC::FrePe embryos. mCherry is detected in craniofacial mesenchyme (L, arrowhead) and eGFP is now expressed by dorsal root ganglia (M, arrows). mCherry (N) and eGFP (O) are both detected by IF in the endocardial cushions of the outflow tract. P–T, Wnt1::Flpe; Mef2c-AHF-Cre; RC::FrePe embryos. mCherry is seen in craniofacial mesenchyme (Q, arrowhead) and dorsal root ganglia (Q, arrows) and by IF in the outflow tract (S). eGFP is not detected (R, T). Scale bars: 100µm.
A transgenic mouse with an enhancer element derived from the Mef2c gene directing expression of Cre recombinase, Mef2c-AHF-Cre, is widely used to label SHF derivatives.11 We crossed this mouse to Wnt1::Flpe and RC::FrePe but did not detect any evidence for overlap of expression domains at E12.5 (Figure 1P–T). This result indicates that Mef2c-AHF-Cre may be more restricted to SHF precursors than is Isl1Cre/+ and also serves as an important negative control to minimize the chance that leaky expression explains the presence of eGFP in Wnt1::Flpe ; Isl1Cre/+; RC::FrePe embryos.
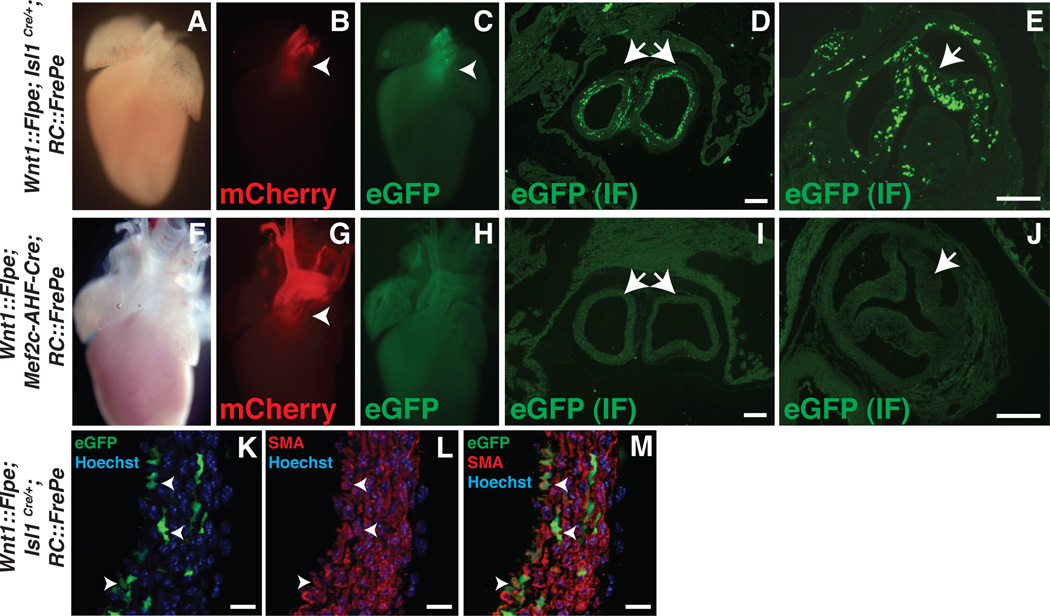
Analysis of P0 hearts of Wnt1::Flpe; Isl1Cre/+; RC::FrePe and Wnt1::Flpe; Mef2c-AHF-Cre; RC::FrePe pups confirms the stark difference between Isl1Cre/+ and Mef2c-AHF-Cre (Figure 2A–J and Online Figure II). Both mCherry and eGFP are evident in the OFT of P0 Wnt1::Flpe; Isl1Cre/+; RC::FrePe hearts (Figure 2B, C) visualized by and eGFP immunofluorescence is evident in the tunica media of the great vessels (Figure 2D) and in the leaflets of the aortic valve (Figure 2E). eGFP expression is not detected in Wnt1::Flpe; Mef2c-AHF-Cre; RC::FrePe hearts (Figure 2H–J). The areas of fluorescence marked by either Isl1Cre/+ or Mef2c-AHF-Cre with Rc::FrePe are within the fate-mapped domains marked by these drivers using R26LacZ/+ (Online Figure II). eGFP expressing derivatives of Isl1Cre/+ and Wnt1-expressing precursors are able to differentiate into smooth muscle, as evidenced by co-expression of eGFP and smooth muscle actin (SMA) in the proximal aorta of Wnt1::Flpe; Isl1Cre/+; RC::FrePe pups (Figure 2K–M, arrowheads). eGFP+ cells are observed near the branchial arches of Wnt1::Flpe; Isl1Cre/+; RC::FrePe embryos as early as day E10.5 and within the aortic sac (Online Figure III).
Figure 2. IslCre/+/Wnt1::Flpe-derived cells are present in the newborn heart.
A–E, Post-natal day 0 (P0) Wnt1::Flpe; Isl1Cre/+; RC::FrePe hearts showing mCherry (B, arrowhead) and eGFP (C, arrowhead) fluorescence in the cardiac outflow tract. D and E, Immunofluorescence (IF) for eGFP in transverse sections through the proximal aorta and pulmonary artery (D) and aortic valve (E) reveals expression in the tunica media of the great vessels (D) and in the valve leaflets (E). F–J, P0 Wnt1::Flpe; Mef2c-AHF-Cre; RC::FrePe hearts showing mCherry fluorescence in the outflow tract (G, arrowhead). eGFP is not detected (H–J). K–M, Cross section of the aortic wall of Wnt1::Flpe; Isl1Cre/+; RC::FrePe P0 embryo stained by IF for eGFP and smooth muscle actin (SMA). Nuclei appear blue after staining with Hoechst dye. Arrowheads denote cells co-expressing eGFP and SMA. Scale bars in panels D, E, I, J: 100µm. Scale bars in panels K–M: 14µm.
Discussion
These results indicate that Isl1Cre/+ is not restricted to SHF precursors of the mature heart. Rather, Isl1Cre/+ labels both SHF precursors and also at least some cardiac neural crest cells. Isl1 is known to be expressed by other neural crest derivatives, including DRG and cardiac ganglia23, 25 but the demonstration of Isl1Cre/+ expression by cardiac neural crest precursors demands re-evaluation of its use as a SHF driver and re-interpretation of some prior studies.
For example, both neural crest and SHF can give rise to smooth muscle. Given our results, it is now unclear if some or all Isl1-derivatives in the OFT that express smooth muscle markers are neural crest or SHF derived.2, 23, 24, 26 Our data suggest that at least some are of neural crest origin. Isolation of Isl1-expressing cells from ES or iPS cultures, or from embryos, for the purpose of expanding SHF precursors (see references listed in supporting information in online data supplement)2, 6, 7, 9, 23 may actually result in the expansion of neural crest cells. Studies in which Isl1Cre/+ has been used to manipulate gene expression in the SHF, and which have resulted in aortic arch and OFT defects, may need to be re-examined for the possibility of cell autonomous neural crest effects. Experimental approaches using multiple or alternative SHF drivers, such as Mef2c-AHF-Cre, and use of dual and intersectional fate-mapping approaches such as the one described here, may define cardiac cell origins and fates more clearly than using the Isl1 marker alone. Our studies emphasize the need for the identification of more specific molecular markers of SHF precursors.
Novelty and Significance.
What is Known?
Within the developing heart field Islet1 is postulated as a selective marker of cardiac progenitor cells derived from the second heart field.
The specificity of Islet1 as a marker for second heart field is critical to lineage tracing, gene inactivation, and differentiation analyses.
Islet1-derivatives include cells populating the outflow tract, an area patterned and formed by derivatives of the cardiac neural crest and second heart field.
What New Information Does This Article Contribute?
A reporter mouse RC::FrePe allows identification of cells undergoing dual Flpe- and Cre-mediated recombination and sensitively indicates intersection between lineages marked by separate Flpe and Cre drivers.
Islet1 is not restricted to second heart derivatives in the heart but is also expressed by a subset of cardiac neural crest cells.
The intersectional population revealed by Wnt1::Flpe ; Islet1Cre ; Rc::FrePe resides in the cardiac outflow tract and includes smooth muscle cells of the tunica media of the aorta and pulmonary artery.
Dual fate mapping using an alternative second heart field driver, a Mef2c enhancer regulating Cre, does not overlap with neural crest.
Islet-1 is postulated as a marker of cardiac progenitor cells from the second heart field. Numerous studies suggest Islet1+ cells represent tri-potential precursors of differentiated cardiac tissues including smooth muscle, cardiac muscle and endothelial cells. Using Flpe- and Cre-mediated dual fate mapping, we show that Islet1 is not restricted to second heart derivatives in the heart but that it is also expressed by a subset of cardiac neural crest cells. Thus, some Islet1 cardiac derivatives may be neural crest-derived rather than a multi-potent second heart field precursor. These findings suggest that results based on Islet1 fate mapping should be interpreted with caution and emphasize the need for additional cardiac lineage tools and markers.
Supplementary Material
Acknowledgements
We thank Min Min Lu and Lan Cheng for their expert histology skills.
Sources of funding:
This work was supported by NIH UO1 HL100405, HL062974, HL095634 to JAE and R01HD051936, R21DA023643-01 and R21MH083613 to SMD.
Non-standard Abbreviations and Acronyms
- Cre
cyclization recombinase
- E
embryonic day
- eGFP
enhanced green fluorescent protein
- ES
embryonic stem
- FHF
first heart field
- Flpe
flippase recombinase enhanced
- IF
immunofluorescence
- iPS
induced pluripotent stem
- Isl1
Islet1
- Mef2c
myocyte-specific enhancer factor 2C
- OFT
outflow tract
- P
post-natal day
- SHF
second heart field
- SMA
smooth muscle actin
- Wnt
Wg (wingless) and Int
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 2.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly R, Brown N, Buckingham M. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 4.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 5.Epstein JA, Franklin H. Epstein Lecture. Cardiac development and implications for heart disease. The New England journal of medicine. 2010;363:1638–1647. doi: 10.1056/NEJMra1003941. [DOI] [PubMed] [Google Scholar]
- 6.Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: A single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- 7.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal Isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretti A, Bellin M, Jung CB, Thies TM, Takashima Y, Bernshausen A, Schiemann M, Fischer S, Moosmang S, Smith AG, Lam JT, Laugwitz KL. Mouse and human induced pluripotent stem cells as a source for multipotent Isl1+ cardiovascular progenitors. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:700–711. doi: 10.1096/fj.09-139477. [DOI] [PubMed] [Google Scholar]
- 9.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic Isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Moretti A, Lam J, Evans SM, Laugwitz KL. Biology of Isl1+ cardiac progenitor cells in development and disease. Cellular and molecular life sciences : CMLS. 2007;64:674–682. doi: 10.1007/s00018-007-6520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 12.High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA. Murine Jagged1/Notch signaling in the second heart field orchestrates FGF8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009;119:1986–1996. doi: 10.1172/JCI38922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest. 2011;121:422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, Camenisch T, Buckingham M, Moon AM. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135:3599–3610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awatramani R, Soriano P, Rodriguez C, Mai J, Dymecki S. Cryptic boundaries in roof plate and choroid plexus revealed by intersectional gene activation. Nature Genetics. 2003;35:70–75. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- 16.Farago AF, Awatramani R, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Jensen P, Farago AF, Awatramani R, Scott MM, Deneris ES, Dymecki SM. Redefining the central serotonergic system by genetic lineage. Nature Neuroscience. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. In: Wassarman PM, Soriano PM, editors. Guide to Techniques in Mouse Development: Mouse Molecular Genetics, Part B. San Diego, CA: Academic Press; 2010. pp. 183–213. [DOI] [PubMed] [Google Scholar]
- 20.Dymecki SM, Tomasiewicz H. Using Flp-recombinase to characterize expansion of Wnt1-expressing neural progenitors in the mouse. Dev Biol. 1998;201:57–65. doi: 10.1006/dbio.1998.8971. [DOI] [PubMed] [Google Scholar]
- 21.Landsberg RL, Awatramani RB, Hunter NL, Farago AF, DiPietrantoio HJ, Dymecki SM. Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron. 2005;48:933–947. doi: 10.1016/j.neuron.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Bio. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Liang X, Najafi N, Cass M, Lin L, Cai CL, Chen J, Evans SM. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev Biol. 2007;304:286–296. doi: 10.1016/j.ydbio.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avivi C, Goldstein RS. Differential expression of Islet-1 in neural crest-derived ganglia: Islet-1+ dorsal root ganglion cells are post-mitotic and Islet-1+ sympathetic ganglion cells are still cycling. Developmental Brain Research. 1999;115:89–92. doi: 10.1016/s0165-3806(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 26.Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2-5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.