Abstract
Burn injuries together with its subsequent complications, mainly bacterial infections originating from gastrointestinal tract, activate the host immune system through stimulation of a series of local and systemic responses, including the release of inflammatory mediators. To gain a more comprehensive understanding of these complex physiological changes and to propose therapeutic approaches to combat the deleterious consequences of burn and septic shocks, it is essential to analyze animal models of burn and sepsis. In this study, we analyzed the long term profiles of cytokines and chemokines in rat models which received burn injury followed two days later by cecal ligation and puncture (CLP) to induce sepsis and were sacrificed at different time points within 10 days (0, 1, 2, 3, 4, 7 and 10 days). It was observed that MCP-1 concentrations were elevated in all animal models following the burn injury or CLP treatment. IP-10 concentration was persistently decreased after CLP or sham-CLP treatments. GRO/KC concentration was also increased following the burn injury and CLP. It was elucidated that, in more severe injury model which received both burn and CLP treatments, GMCSF and MIP-1α (chemokines), IL-1α (a pro-inflammatory cytokine) and IL-6 (exhibiting both pro- and anti-inflammatory behaviors) were upregulated on the 7th and 10th days, which might be to protect the host system from the subsequent complications caused by burn and sepsis. In order to elucidate critical regulatory interactions, putative transcription factors of the inflammatory mediators which have been significantly changed following the injuries were further identified by analyzing the conserved regions of the promoters of cytokines and chemokines. In conclusion, the long term profiles of the inflammatory mediators were profoundly characterized in this study to gain a comprehensive understanding of inflammatory mediators’ behaviors in various injury models.
Keywords: Burn, Cecal ligation and puncture, Cytokines, Chemokines, Long term inflammatory response, Transcription Factors
Introduction
Burn injury leads to denaturation of proteins and loss of membrane integrity of cells around the area of injury, which results in secretion of local mediators and endotoxins such as histamine, nitric oxide, oxygen-free radicals and various cytokines including IL-6, PDGF and TGF-α [1–3]. This eventually recruits different blood cells such as neutrophils, lymphocytes, macro-phages and fibroblasts to the injured area, thus causing a further increase in circulatory proteins and cytokines levels [1]. Although burn wound is an important portal of entry for microbes [4], pathogen originating from gastrointestinal tract is one of the most serious complications, which might be because of physical disruption of the mucosal barrier, intestinal over-growth of bacteria and suppression of the immune defense [5]. Severe burns associated with bacterial infections cause more persistent inflammatory response with an ongoing hypermetabolic and catabolic state. Depending on the severity of the injury and septic complications, hyper-metabolism and other changes associated with the systemic inflammatory response can progress to multiple organ dysfunction syndromes, which can have a mortality rate as high as 90–100% [6].
Animal models of burn injury, cecal ligation and puncture, and endotoxin injection have previously been used to characterize the circulating mediator concentrations [7–14]. However, these studies are limited to one type of insult or a limited number of cytokines’ measurements. In our previous study, we analyzed the short term (24 h) responses of 23 different serum cytokines and chemokines in two types of animal models: rats receiving a “sterile” cutaneous dorsal burn on 20% of the total body surface area (TBSA); rats receiving a cecum ligation and puncture treatment (CLP) to induce infection [15]. We utilized the concept of area under the curve (AUC) method to assess the dynamic responses of cytokines and chemokines considering the temporal variability observed in the baseline corresponding to the control group. It was identified that MCP-1, GROK/KC, IL-12, IL-18 and IL-10 were significantly altered in both burn and CLP groups. While IL-10 concentration was only increased in the burn group, Eotaxin was only elevated in CLP group. Leptin and IP-1 concentrations were found to be decreased in both CLP and sham-CLP groups (control of CLP). We further analyzed the dynamics of the early inflammatory response in double hit injury models of burn and sepsis[16]. The rats received burn injury followed 2 days later by CLP or sham-CLP (SCLP) treatments. A number of cytokines and chemokines, including MCP-1, IP-10, Leptin, TNF-α, MIP-1α, IL-18, GMCSF, RANTES and GCSF were significantly altered in both Burn+CLP and Burn+SCLP groups. IL-10 and IL-6 were significantly up-regulated in the Burn+CLP group when compared to the Burn+SCLP group. Down regulation of Leptin and IP-10 concentrations were found to be related to surgery and/or infection. IL-18 and MCP-1 were elevated in all groups including previously published single injury models receiving similar treatments [15, 16].
The inflammatory response and its consequences are the result of a very complex cascade of events. Quantitative and temporal differences in the secretion of mediators and consequently their signaling patterns result in complex inflammatory responses. We recently identified a number of putative transcription factors of cytokines and chemokines which have been significantly altered following burn injury or sepsis [15]. Some of these transcription factors identified such as NFKB (or NF-κβ), STAT and CEBP have been already well studied in literature and shown to be activated by burn and sepsis in various tissues [13, 17–19]. We further showed that there are also other putative transcription factors including ETS1, SP1, GATA, and VTBP which might play important roles during the inflammation by regulating the production of a number of cytokines and chemokines[15]. Our analysis showed that ETS1 have potential to regulate the MCP-1 and GRO/KC chemokines which had similar concentration profiles following the CLP treatment. CREB was also identified as a putative transcription factor of IL-18. Putative transcription analysis has shown that the network of cytokine-transcription factor interactions is complex and redundant. These complex behaviors mediated by cytokines and chemokines need to be further analyzed to better understand its systems properties and identify multiple targets that could be potentially utilized as medical treatments. Therefore, given our previous studies where short term inflammatory responses in various injury types including single and double injury models of burn and sepsis [15, 16], we further analyzed the long term profiles of cytokines and chemokines in the animal models. Rats received burn injury followed two days later by CLP and they were sacrificed at different time points within 10 days (0, 1, 2, 3, 4, 7 and 10 days) to profile the chemokines/cytokines concentrations. Moreover, the binding sites of promoter regions of the cytokines and chemokines were analyzed to identify putative transcription factors in this study.
Materials and Methods
Animal Models
Male Sprague-Dawley rats (Charles River Labs, Wilmington, MA) weighing between 150 and 200 g were utilized for this study. The animals were housed in a temperature-controlled environment (25°C) with a 12-hour light-dark cycle and provided water and standard chow ad libitum. All experimental procedures were carried out in accordance with National Research Council guidelines and approved by the Rutgers University Animal Care and Facilities Committee.
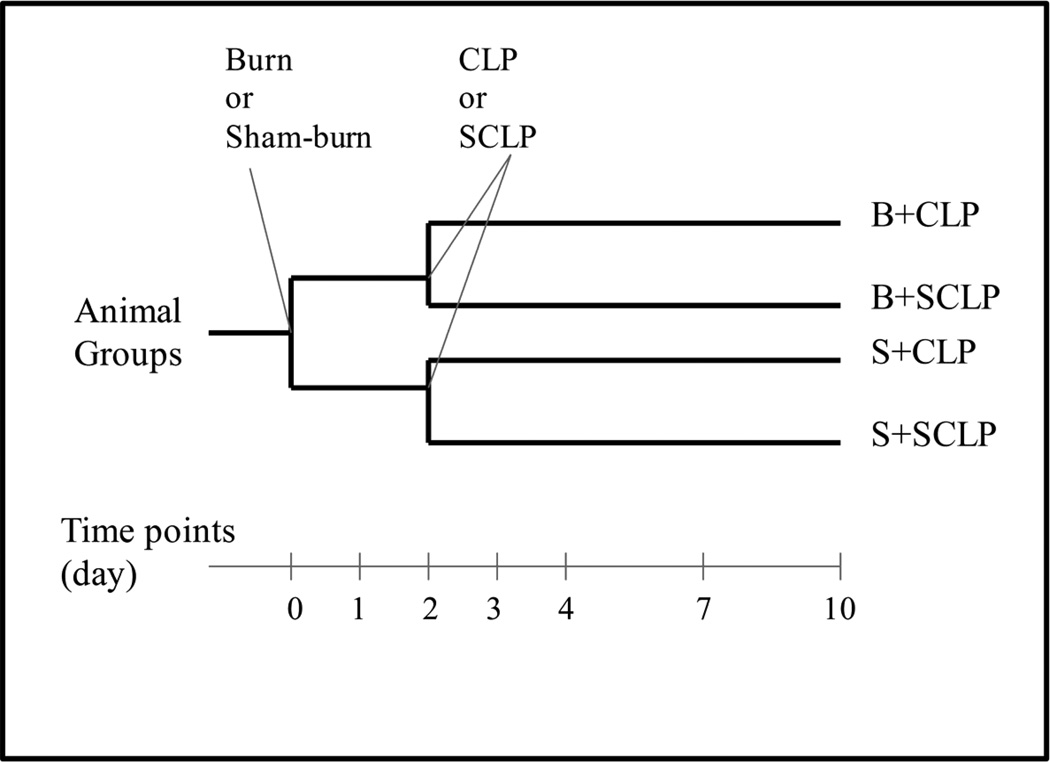
The experimental plan aims to elucidate the changes in serum cytokine concentrations in rats at different time points during the first 10 days after the burn and cecal ligation and puncture (CLP) treatments (Figure 1). A systemic hypermetabolic response is induced in rats by applying a 20 % total body surface area burn followed by (2 days later) CLP to produce sepsis. The control treatments include sham-burn and sham-CLP. Totally four different groups (including control and treatment groups) are analyzed in this study (Figure 1).
Figure 1.
Experimental plan.
A full-thickness burn on the dorsal skin corresponding to 20% of the total body surface area (TBSA), and cecal ligation and puncture treatments were have been explained in great detail in our previous studies [15, 16]. Briefly, rats were first anesthetized by intraperitoneal injection of 80 to 100 mg/kg ketamine + 12 to 10 mg/kg xylazine. After removing all hair from the dorsal abdominal area using electric clippers, the animal's back was immersed in water at 100°C for 10 s to produce a full-thickness scald injury over 20% TBSA. Then, the animals were resuscitated with 50 mL/kg of saline injected intraperitoneally. Sham burn controls consisted of animals treated identically but immersed in warm water at 37 °C. Rats were single caged after burn or sham burn and given standard rat chow and water ad libitum. 2 days after the burn or sham-burn treatments, animals received CLP treatment to induce sepsis. Rats were anesthetized, and then the analgesic buprenorphrine was given subcutaneously at 0.01 to 0.05 mg/kg. Animals were then placed in supine position and hair was shaved on the abdomen. Bupivicaine (0.125% to 0.25%) were applied around the incision site for additional perioperative and postoperative analgesia. The abdominal cavity was cut open by a 2 cm midline incision. The cecum of the rat was exposed and ligated just below the ileocecal valve so that intestinal obstruction was not produced. In order to increase the survival rate and preserve the viability of cecum itself, the cecal branch of the ileocecal artery was not blocked or ligated. The cecum was punctured 4 times (not through and through) with a 20 gauge needle and replaced in the peritoneum. The abdominal incision was then sutured in layers using interrupted monofilament sutures. The animal received 10 mL/kg saline intraperitoneally for resuscitation. Control group (sham-CLP or SCLP) were anesthetized, undergo laparotomy as described above, but no surgical manipulation of the cecum was performed. Rats were single caged after the treatments and given standard rat chow and water ad libitum until sacrifice. Note that all burn, CLP and control treatments as well as sacrificing the animals were carried out in the 8 am – 10 am time period to insure that all animals were at the same stage in their circadian cycle.
Cytokine Analysis
Animals were sacrificed at different time points (0, 1, 2, 3, 4, 7 and 10 day) (n=3 per time point per group) (Figure 1). Blood samples collected from vena cava by heparinized catheters were stored on ice until serum preparation. Serum was separated by centrifugation at 4500 rpm for 3 min at 4 °C and stored at −80 °C until analyzed. MILLIPLEX MAP Rat Cytokine/Chemokine Panel (Millipore) was used for the simultaneous quantification of 23 different cytokines (Eotaxin, G-CSF, GM-CSF, GRO/KC, IFN-γ, IL-10, IL-12 (p70), IL-13, IL-17, IL-18, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IP-10, Leptin, MCP-1, MIP-1α, RANTES, TNF-α, VEGF) using the manufacturer’s manual. One-way ANOVA test was used to identify differentially produced cytokines or chemokines which show significant alterations across the time (P<0.05). Heat maps were generated by the “clustergram” function in MATLAB which was used to cluster the cytokines and chemokines which were significantly changed across the time.
Predicting Putative Transcription Factors
Transcription factors (TFs) analysis has been already described in great detail in our previous study [15]. Briefly, promoters of cytokine genes of rats were extracted from the Genomatix database of promoter information with a default length unless an experimentally defined length has been reported [20]. Each promoter is characterized by a set of orthologous promoters from the same gene of other vertebrate species, if available. DiAlign TF [20] with default parameters was subsequently applied to identify conserved regions on the promoter and then transcription factor binding sites (TFBSs) that are enriched on corresponding conserved regions from the set of orthologous promoters with a common threshold (70% in this study). Transcription factors identified were further refined by using Model Inspector [20]to search for a list of cis-regulatory modules from a library of functional, experimentally-verified, modules (MatBase[20]) that match on the promoter.
Results
Animal Weight Change and Mortality
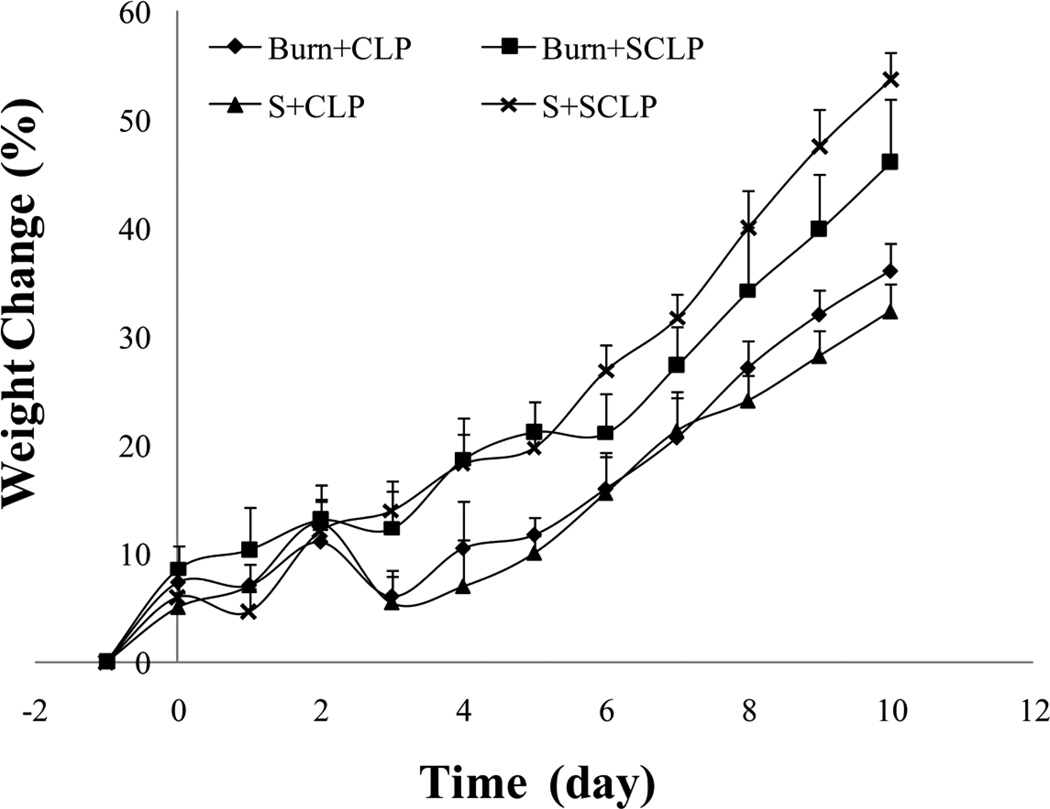
Weight changes of rats were monitored after burn and CLP treatments (Figure 2). Burn and sham-burn treatments (on day 0) did not resulted in any significant weight lost. On the other hand, CLP (on day 2) approximately caused 10 % weight lost on each animal within a day. Animals of double hit injury models recovered from the injuries, and eventually gained weight at the same rate as normal controls (Figure 2). Moreover, all animals survived within 10 days following the treatments.
Figure 2.
Animal weight changes with respect to time (n≥3). Animals receive first burn injury or sham treatment on the 0th day and then CLP or shamCLP (SCLP) on the 2nd day. CLP approximately caused 10 % weight lost on each animal within a day (see S+CLP and B+CLP groups on the figure).
Cytokine Profiles
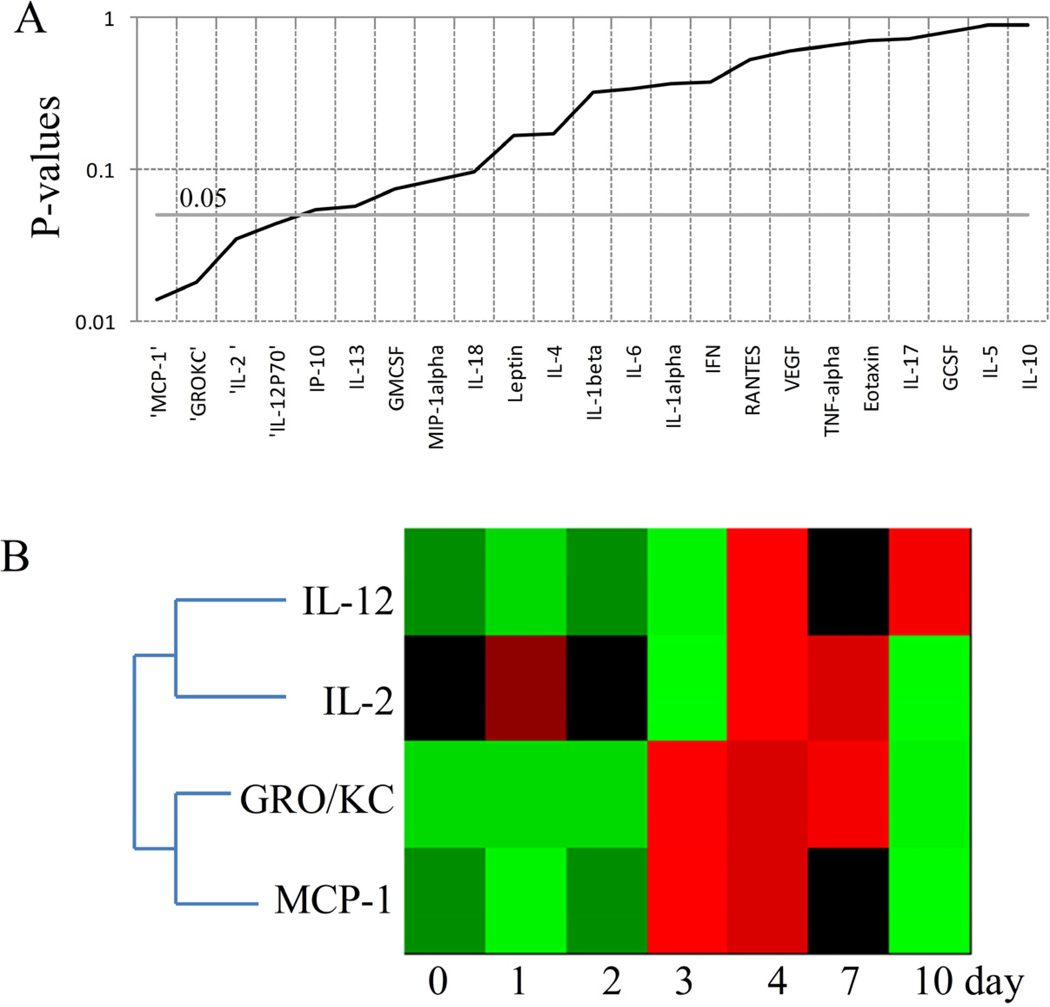
We analyzed the cytokine and chemokine concentration changes at different time points in each group, and Figure 3A shows the P-values of cytokines/chemokines from S+SCLP group obtained by ANOVA test. This group first received sham-burn and then sham-CLP treatment which is a sterile abdominal surgery without surgical manipulation of cecum. It was identified that IL-12, IL-2, GRO/KC and MCP-1 concentrations were significantly altered (Figure 3B). MCP-1 and GRO/KC, the chemokinesrecruiting the white blood cells to the site of damaged tissue and activate the immune cells, were elevated following the sham-CLP treatment until the 7th day. IL-12 which is necessary for the T-cell development, and IL-12 which can behave as both pro-and anti-inflammatory mediator were up-regulated on the 4th day.
Figure 3.
Chemokines and cytokines showing significant changes across the time in S+SCLP group.A. P values of cytokines and chemokines obtained by ANOVA test. B. Heat map of cytokines and chemokines significantly changed across the time (P<0.05). Green indicates the lowest level while red indicates the highest and black average level.
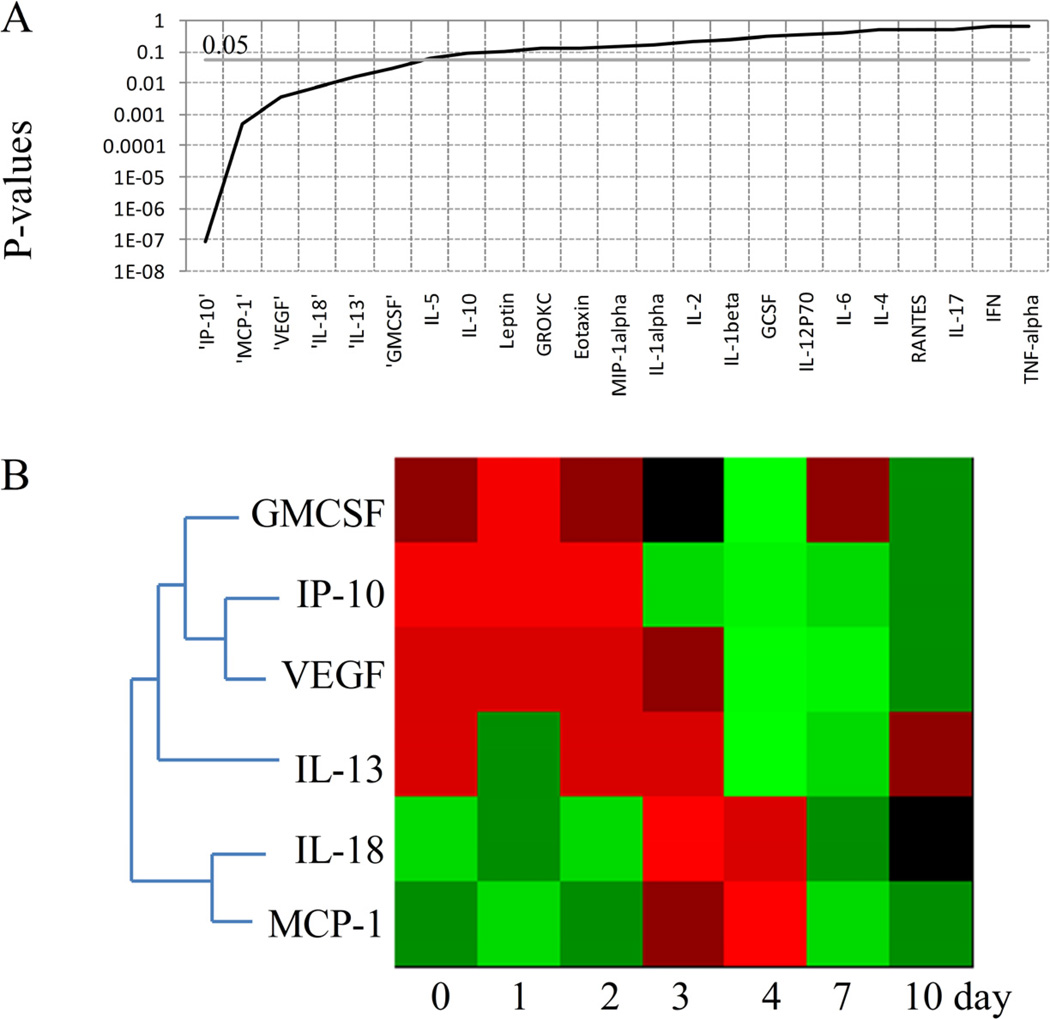
Figure 4A shows the P-values of cytokines/chemokines from S+CLP group obtained by ANOVA test, and Figure 4B illustrates the profiles of circulating mediators which were significantly altered. After receiving CLP treatment on the second day, a number of chemokines (GMCSF, IP-10, and VEGF) exhibiting similar expression pattern, were persistently down-regulated. IL-13 which is an anti-inflammatory cytokine was down-regulated on the 4th and 7th days. On the other hand, an up-regulation was observed in the concentrations of IL-18 and MCP-1 on 3rd and 4th days following the CLP. These two mediators were found to have similar expression pattern.
Figure 4.
Chemokines and cytokines showing significant changes across the time in S+CLP group.A. P values of cytokines and chemokines obtained by ANOVA test. B. Heat map of cytokines and chemokines significantly changed across the time (P<0.05). Green indicates the lowest level while red indicates the highest and black average level.
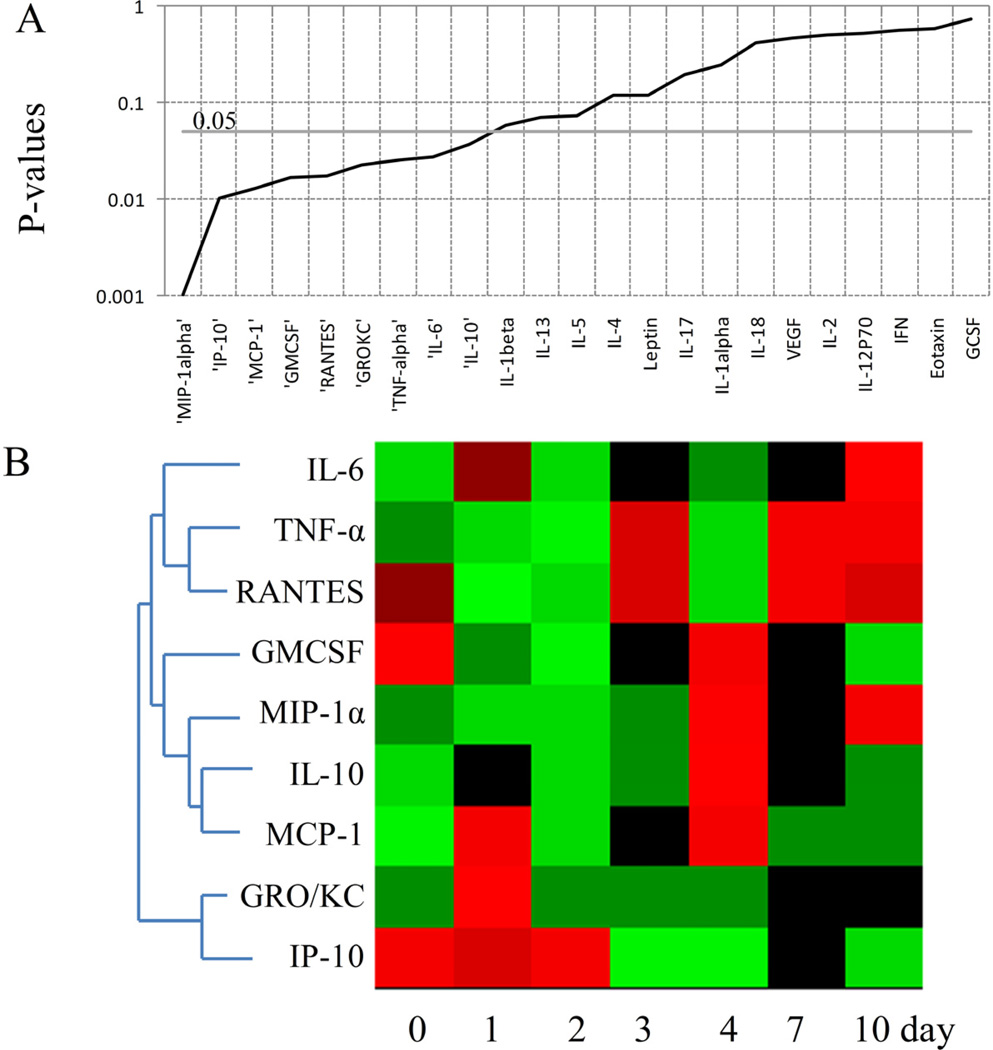
In B+SCLP group, IL-6, TNF-α, RANTES, GMCSF, MIP-1α, IL-10, MCP-1, GRO/KC and IP-10 were found to be significantly changed (Figures 5A and 5B). In this group, animals received burn injury followed two days later by sham-CLP. The time point 0 day represents control group (no injury), whereas 1 and 2 days correspond to animals which have already received burn injury, and 3–10 days correspond to animals which have received sham-CLP treatment in addition to burn injury. On the 1st and 2nd days following the burn injury, RANTES and GMCSF were found to be down-regulated. RANTES concentration was up-regulated after the second injury (sham-CLP) on the 3rd, 7th and 10th days; however GMCSF concentration was increased on the 4th day following the sham-CLP. On the 4th day, MIP-1α, MCP-1 and IL-10 (a well known anti-inflammatory cytokine) concentrations were also increased. Furthermore, MCP-1 and GRO/KC were up-regulated on the 1st day following the burn injury, whereas GRO/KC concentration was not changed after the sham-CLP treatment. Interestingly, the circulatory concentration of IP-10, which is a chemokine, was not affected by burn injury; however its concentration was persistently decreased following the sham-CLP treatment. IL-6, a well known inflammatory mediator, is up-regulated on the 10th day in burn animals after receiving sham-CLP treatment.
Figure 5.
Chemokines and cytokines showing significant changes across the time in B+SCLP group.A. P values of cytokines and chemokines obtained by ANOVA test. B. Heat map of cytokines and chemokines significantly changed across the time (P<0.05). Green indicates the lowest level while red indicates the highest and black average level.
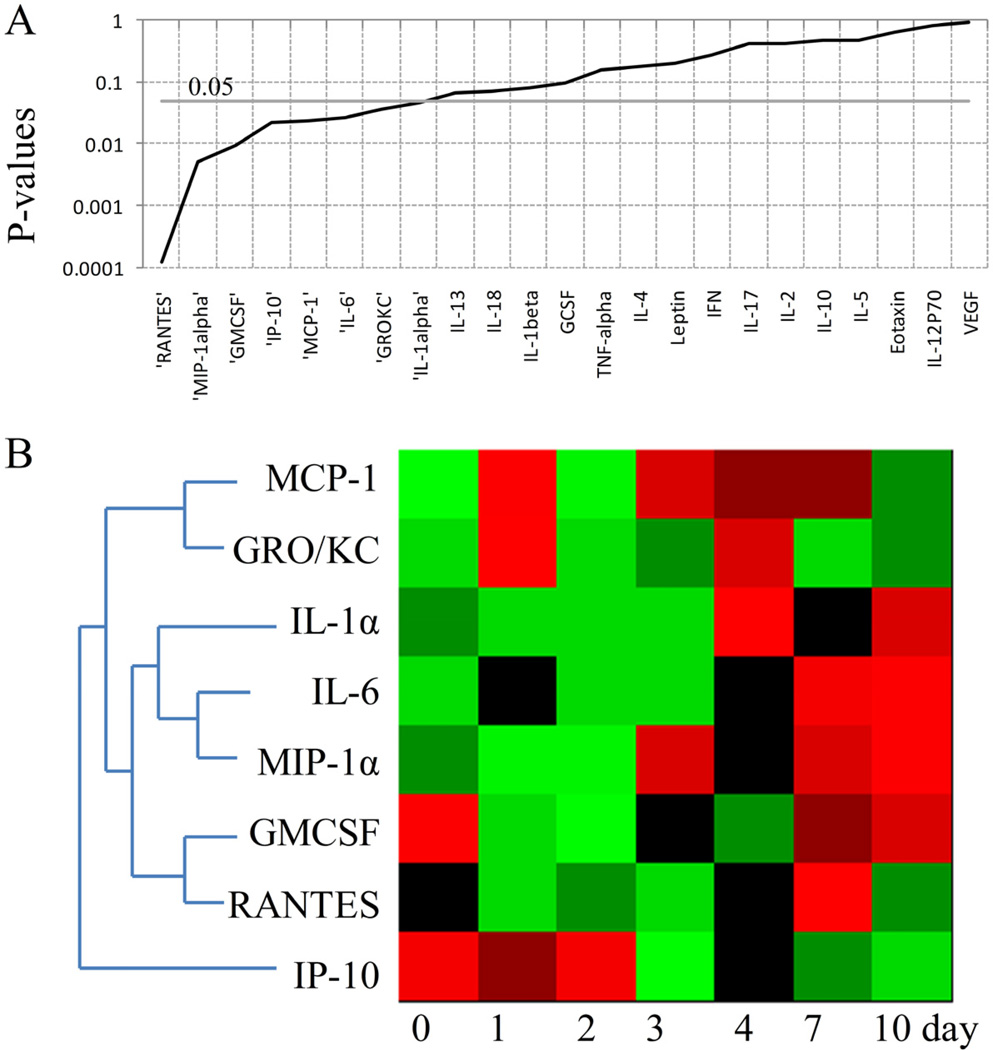
A number of cytokines and chemokines including MCP-1, GRO/KC, IL-1α, IL-6, MIP-1α, GMCSF, RANTES and IP-10 were altered across the time in B+CLP group (Figures 6A and 6B). The chemokines MCP-1 and GRO/KC were identified in the same group. They both were up-regulated following the burn injury on the 1st day. After CLP treatment, MCP-1 showed more persistent up-regulation until the 7th day. On the other hand, GRO/KC was increased on the 4th day and went back to its baseline value corresponding to time point 0 day. IL-1α, IL-6 and MIP-1α exhibited similar concentration profiles. Among these mediators, IL-1α is a member of interleukin-1 family characterized as a pro-inflammatory mediator. The concentrations of IL-1α, IL-6 and MIP-1α started to increase on the 4th day in double hit injury model, and they showed persistent elevation even on the 10th day. GMCSF concentration was decreased following the burn injury and CLP (between 1st and 4th day), and reached to its baseline value on the 7th day. RANTES was only elevated on the 7th day. Similar to the profile observed in B+SCLP group (Figure 5B), IP-10 concentration was also persistently decreased following the CLP treatment (between the 3rd day and 10th day).
Figure 6.
Chemokines and cytokines showing significant changes across the time in B+CLP group.A. P values of cytokines and chemokines obtained by ANOVA test. B. Heat map of cytokines and chemokines significantly changed across the time (P<0.05). Green indicates the lowest level while red indicates the highest and black average level.
Putative Transcription Factors
Putative transcription factors of 15 different mediators which were found to be significantly changed following the injuries have been identified (Table 1). It is obvious that a transcription factor might regulate more than one cytokine, and similarly a cytokine might be regulated by a number of different transcription factors. We found that ETS1 (Table 1) might be involved in the regulation of 10 different mediators including GMCSF, MCP-1, MIP-1α, IL-12, IL-18, IP-10, GRO/KC, RANTES, IL-1α and TNF-α. ETS1 is an important member of ETS family of transcription factors and it plays a key role in the development of immune cells including T lymphocytes, B lymphocytes and natural killer cells[21]. It is known that ETS1 activates the promoter of IL-5 gene whereas it acts as a negative regulator for IL-2 expression [21]. NFAT family (nuclear factor of activated T cells) plying a important role in inducible gene transcription during the immune response [22] was found to be associated with 6 different mediators such as GMCSF, IL-2, IL-6, IL-13, RANTES and TNF-α in this study (Table 1). NFAT family whose activity is regulated by the calcium/calmodulin dependent phosphatase calcineurin has been shown to be involved in the regulation of various cytokines and chemokines including IL-2, IL-3, IL-4, IL-5, IL-8, IL-13, GMCSF and TNF-α in literature [22]. In current study, SP1 was also found to be putative transcription factors of 7 different mediators which are GMCSF, MIP-1α, IL-6, IL-18, TNF-α, IL-1α and VEGF (Table 1). SORY is another transcription factor found to be important for the mediators of GMCSF, MIP-1α, IL-2, GRO/KC, IL-1α and TNF-α (Table 1).
Table 1.
Putative transcription factors of the cytokines and chemokines observed in animal models.
| GMCSF | MCP-1 | MIP-1α | IL-2 | IL-6 | IL-13 | IL-10 | IL-12 | IL-18 | IP-10 | GROKC | RANTES | TNF-α | VEGF | IL-1α | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP2F (Activating protein 2) | √ | ||||||||||||||
| SP1F (GC-box factors SP1/GC) | √ | √ | √ | √ | √ | √ | √ | ||||||||
| KLFS (Krupple like factor family) | √ | √ | |||||||||||||
| STAT (Signal transducer and activator of transcription) | √ | √ | √ | √ | √ | √ | |||||||||
| ETS (Human and murine ETS1 factor-E-twenty-six family) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| GREF (Glucocorticoid responsive and related elements) | √ | ||||||||||||||
| EGRF (EGR/nerve growth factor induced protein C & related factors) | √ | √ | |||||||||||||
| BRNF (Brn POU domain factors) | √ | √ | |||||||||||||
| SORY (SOX/SRY-sex/testis determining and related HMG box factors) | √ | √ | √ | √ | √ | √ | |||||||||
| NFKB (Nuclear factor kappa B/c-rel) | √ | √ | √ | √ | √ | √ | |||||||||
| GATA (GATA binding factors) | √ | √ | √ | √ | |||||||||||
| NFAT (Nuclear factor of activated T-cells) | √ | √ | √ | √ | √ | √ | |||||||||
| VTBP (TATA binding protein factor) | √ | √ | √ | ||||||||||||
| NF1F (Nuclear factor 1) | √ | √ | |||||||||||||
| IRFF (Interferon regulatory factors) | √ | √ | √ | √ | |||||||||||
| CREB (cAMP response element-binding) | √ | √ | √ | ||||||||||||
| AP1F (Activating protein 1) | √ | √ | |||||||||||||
| CEBP (CCAAT-enhancer-binding proteins) | √ | √ | √ | ||||||||||||
| NKXH (NKX homeodomain factors) | √ | ||||||||||||||
| MYOD (Myoblast determining factors) | √ | √ | √ | ||||||||||||
| HAND (Twist subfamily of class B bHLH transcription factors) | √ | ||||||||||||||
| HNF1 (Hepatocyte nuclear factor 1) | √ | ||||||||||||||
| OCT1 (Octamer-binding transcription factor 1) | √ | ||||||||||||||
| CAAT (CCAAT binding factors) | √ | √ | |||||||||||||
| FKHD (Fork head domain factors) | √ | √ | √ | ||||||||||||
| MITF (Microphthalmia transcription factor) | √ | ||||||||||||||
| PBXC (PBX1–MEIS1 complexes) | √ | ||||||||||||||
| PAX6 (PAX-4/PAX-6 paired domain binding sites) | √ |
It is well known that many signaling pathways associated with NFKB (or NF-κB), STAT, and CEBP transcription factors are activated in various tissues due to the burn injury and septic shocks [13, 17–19]. CEBP was identified as a putative transcription factor of IL-6, IL-10 and TNF-α in this study (Table 1). NFKB which can be induced by over 150 different stimuli can control the transcription of over 150 target genes mainly related to immune systems, therefore this protein is assumed to be central mediator of immune response[23]. We identified that NFKB may participate in the control of 6 different inflammatory mediators including GMCSF, IL-6, IL-13, IP-10, GRO/KC and RANTES. STAT family which is mainly activated by anti-inflammatory mediators, such as IL-10, controls the transcription of key proteins inhibiting many inflammatory proteins such as TNF-α, IL-6, and IL-1. Herein, it has been identified that STAT family is putative transcription factors of GMCSF, MCP-1, IL-10, IP-10, IL-1α and VEGF (Table 1). It has been already known that STAT activation up-regulates the IL-10 and VEGF production [24]. IL-2, IL-6 and IL-18 might also be regulated by CREB (Table 1). CREB is known to be inhibiting NF-kβ signaling activating the transcription of pro-inflammatory cytokines [25]. Interestingly, IL-18 concentration was only increased following the CLP treatment (days 3 and 4 on Figure 4) whereas no significant changes in other pro-inflammatory mediators were identified in this group. IL-18 might also be controlled by ETS1 (Table 1). Similar to IL-18 profile, MCP-1 concentration was increased in S+CLP group on the 3rd and 4th days (Figure 4). Moreover, MCP-1 and GRO/KC exhibited the same dynamic profiles in S+SCLP and B+CLP groups (Figures 3 and 6, respectively). ETS1 was identified as a putative transcription factor of these two mediators as well (Table 1).
Discussion
In this study, we investigated long term responses of inflammatory mediators in the double hit injury models of burn and sepsis. All animals were sacrificed in the same time periods so that the concentration changes of inflammatory mediators can be comparable across the time in each group. All animal groups had 100 % survival for at least 10 days following the treatments. CLP caused ~10% weight loss in both burn and sham-burn animals. However, after 24 hours, the animals started to regain weight at a rate similar to that of control group. These observations are consistent with prior studies utilizing the similar models [26].
CLP is an animal model that mimics the physiological changes in human sepsis [27] and clinically relevant since it induces inflammatory response. It has been previously shown that single needle puncture produced systemic bacteremia [28]. Although its application is straightforward, the outcome of CLP is closely associated with several factors during the procedure. Major factors determining the mortality rate is the length of the cecum ligated [27, 29], size of the needle and the number of punctures [30]. Rittirsch et al. [27] observed that all rats died after single through and through puncture and large-grade ligation. However, in our study, the cecal branch of the ileocecal artery was not ligated or blocked. This preserves the viability of the cecum itself, which increases the survival rate as seen in our study or previously published observation by Banta et al. [26].
Cytokines are small signaling proteins which might show structural similarities and are produced by different cell types or tissues. Cytokines are generally classified as pro and anti-inflammatory cytokines, which are required for host defense, healing processes and diminishing excessive inflammatory response which might result in tissue damage. Pro inflammatory cytokines such as IL-1, TNF-α, and IL-18 activate signal transduction pathways including NF-κβ, p38MAPK and JNK, which induce the host defense mechanisms against infection [31], activates the cytokine cascade [32] and promotes the expression or release of coagulation and adhesion molecules [33]. In the current study, long term expression patterns of pro-inflammatory cytokines were found to be diverse considering the all animal models. IL-18 was elevated only in S+CLP group on the 3rd and 4th days following the CLP treatment (Figure 4). On the other hand, TNF-α, a well-known pro-inflammatory cytokine, was significantly increased in burn animals following the sham-CLP treatment (B+SCLP group, Figure 5). IL-1α concentration only increased in B+CLP group starting on the 4th day (Figure 6).
IL-6 is another well studied cytokine which has both pro- and anti-inflammatory properties. In more severe injuries circulatory IL-6 level is generally elevated, which makes it a potential marker in persistent inflammation. In this study, it concentrations were significantly altered in B+SCLP and B+CLP groups (Figure 5 and 6), and more persistently increased in more severe injury model, B+CLP, on 7th and 10th days. IL-6 has different biological effects, including activation of B- and T- lymphocytes, induction of acute phase production in the liver [32]. It has been shown that IL-6 suppresses the TNF-α and IL-1β production in cell cultures and animal models [34–36]. IL-6 affects signal transducers g130, LIF receptor and OSM receptor leading to activation of the JAK/STAT and MAPK cascades [37]. IL-12 which is another important mediator exhibiting both pro- and anti-inflammatory behavior is produced by monocytes, macrophages, dendritic cells, neutrophils and B cells [38]. It is suggested that IL-12 activates JAK/STAT (STAT3, STAT4, STAT5), and MAPK signaling pathways [39–44]. Compared to IL-6, IL-12 might be observed in less severe injuries [15, 16]. In the current study, IL-12 concentration was only increased in S+SCLP model (Figure 3) on the 4th and 7th days.
Among the anti-inflammatory cytokines, in this study it was identified that only IL-10 and IL-13 concentrations were altered. The concentration of IL-13 was found to be decreased in S+CLP groups (Figure 4) on the 4th and 7th days. IL-10 was only increased on the 4th day following the sham-CLP treatment in burn animals (Figure 5). IL-10 is one of the most important anti-inflammatory cytokine which has been profoundly studied to characterize the immune responses in different animal models. IL-10 is capable of decreasing the IL-18 mRNA expression in monocytes [45]. IL-10 induces STAT3 activation which promotes the transcription of Suppressor of Cytokine Signaling 3 (SOCS3), a negative feedback regulator inhibiting many inflammatory cytokines such as TNF-α, IL-6, and IL-1. Although IL-10 concentration increased on the 4th day in B+SCLP group (Figure 5), no significant changes in IL-10 concentration were observed in the long term analysis of B+CLP (Figure 6). The reason might be to eliminate the excessive production of pro-inflammatory cytokines in B+SCLP group which is a less severe injury model compared to B+CLP group. Interestingly, TNF-α concentration which was up-regulated on the 3rd day in B+SCLP group was decreased on the 4th day when the IL-10 concentration was increased (Figure 5). On the other hand, the concentrations of IL-1α (a pro-inflammatory cytokine) and IL-6 mediators started to be increased on the 4th day in B+CLP group (Figure 6) in which IL-10 concentration was not significantly changed.
Chemokines, chemotactic cytokines (such as MIP, RANTES, VEGF, IP-10, MCP and GRO) are mainly involved in recruiting a large variety of white blood cells to injured area. They are structurally similar, and 20 to 70 percent homology in amino acid sequences can be observed among them [46]. Chemokine receptors can be restricted to certain cells; however the same receptor can be induced by different types of chemokines [46]. They interact with G-protein coupled receptors to induce cell migration through binding proteins of Ras and Rho families involved in cell motility [47, 48]. They also activate JAK/STAT signaling pathways [49]. Due to the large degree of overlap in the underlying signaling pathways activated by chemokines, it is difficult to differentiate the cellular responses of different chemokines. In this study, it was identified that some of the chemokine patterns are specific to injury models whereas some do not depend on the type and severity of the injury. For example, in all injury models IP-10 concentration was decreased following the CLP or sham-CLP treatments. MCP-1 concentration generally increased following the burn injury, CLP and sham-CLP treatments in all groups. GRO/KC exhibited insult specific behavior. It was persistently increased after sham-CLP treatment in S+SCLP group (Figure 3) between 3rd and 7th days. Interestingly, GMCSF and VEGF were also found to be decreased persistently in S+CLP group after CLP treatment (Figure 4). On the other hand, GMCSF and MIP-1α concentrations were increased on the 7th and 10th days in B+CLP group (Figure 6) whereas these chemokines were increased on the 4th day in B+SCLP group (Figure 5). RANTES also showed different patterns. It was more persistently increased in B+SCLP group when compared to that of B+CLP group (Figures 5 and 6).
We have previously published short term responses of cytokines and chemokines within 24 h after the insult in single injury models receiving either burn injury or CLP [15], and in double injury models which received burn injury followed 2 days later by CLP treatment [16]. It was identified that most of the cytokines or chemokines up-regulated went back to their baseline values at the end of 24 h post-injury period in all animal groups. MCP-1, GRO/KC, and IL-18 are some important mediators which have been generally increased in all groups just after the treatments within 24 h. When long term responses of these cytokines were analyzed, MCP-1 exhibited more persistent elevation following the CLP treatment between the 4th and 7th days (Figures 4 and 6). Interestingly, IP-10 and Leptin were found to be decreased after CLP or sham-CLP treatment in single or double injury models within 24 h [15, 16]; however decrease in IP-10 concentration was found to be more persistent when its long term response is analyzed (Figures 3, 4, 5 and 6).
Cytokines or chemokines are involved in signaling transduction networks where different pathways are interconnected through various signaling and transcription factors. Although chemokines or cytokines can elicit distinct cellular responses by activating certain pathways depending on the cell type and the pathophysiological state, it is clear that cytokine-receptor interactions result in very comprehensive signaling patterns since there is a large degree of overlap between signaling molecules. In other words, a cytokine might activate its specific transcription factor; however the upstream regulation of this specific transcription factor might also activate or deactivate other transcript factors which might be important regulatory proteins in the expression of other cytokine genes. Therefore, by exploring the conserved regions in the promoters of cytokines/chemokines, we identified the putative transcription factors of these mediators (Table 1). It was identified that ETS, STAT, NFKB, SP1, SORY, and NFAT transcription factors were found to be associated with a significant number of cytokines and chemokines. Although the mammalian regulatory landscape is highly complex and redundant, our preliminary results showed possible interactions between the transcription factors and inflammatory mediators. For instance, the profiles of MCP-1 and GRO/KC concentrations were found to be very similar in S+SCLP (Figure 3), and we have identified that both mediators can be regulated by ETS1 (Table 1). Integrating in vivo responses and bioinformatics tools to formulate critical regulatory interactions also provided possible targets for the experimental researches. A number of cytokines including IL-1α, IL-6, MIP-1α, GMCSF and RANTES were found to be up-regulated at the late stage of inflammatory response (7th and 10th days) of B+SCLP group which is a more severe injury model compared to others (Figure 6). It is noteworthy to mention that most of these mediators might be regulated by ETS1 (Table 1) which might be a central mediator of immune response.
Proposing a therapeutic strategy for burn and septic patients by interfering with circulatory inflammatory mediators is quite challenging due to the complex interactions of cytokines or chemokines through a very redundant and interconnected network. Therefore, a comprehensive understanding of the behaviors of inflammatory mediators following various injuries is essential. In this study, we have observed that MCP-1 concentrations were elevated in all animal models following the burn injury or CLP treatment. IP-10 concentration was persistently decreased after CLP or sham-CLP treatments. GRO/KC concentration was also increased following the burn injury and CLP (Figure 6). Since we used non-lethal animal models which eventually recover from the injuries, the inflammatory response of host body to the injuries should be protective and under control. Therefore, therapeutic effects of the mediators identified herein should be further investigated. In this study, the long term profiles of the inflammatory mediators were also profoundly characterized. In our previous studies [15, 16], we have already showed that a certain number of mediators were up-regulated or down-regulated just after the insult within 24 h in single injury and double injury models; however the majority of these mediators went back to their baseline values at the end of 24 h post-injury period. Herein, we showed that the concentrations of some of those mediators were changed in the late stage of inflammatory response. In double hit injury model (B+CLP, Figure 6), GMCSF and MIP-1α (chemokines), IL-1α (a pro-inflammatory cytokine) and IL-6 (exhibiting both pro- and anti-inflammatory behaviors) were re-up-regulated on the 7th and 10th days, which might be to protect the host systems from the subsequent consequences of burn and sepsis.
In conclusion, we examined the long term responses of serum cytokines and chemokines in the animal models receiving burn injury and CLP. Furthermore, the putative transcription factors were determined by analyzing the promoter regions of the cytokine genes whose protein concentrations were significantly altered in the circulation following the injuries. We tried to show that investigating cytokine expression patterns in different animal models and integrating these responses with bioinformatics tools provide possible regulatory targets, i.e. transcription factor-cytokine interactions, which enable to better characterize the highly complex and redundant inflammatory network.
Highlights.
Double hit injury models of burn (B) and CLP have been investigated.
Long term profiles of 23 different mediators have been characterized up to 10 days.
Important mediators were up-regulated at the later phase in double-injury models.
Acknowledgments
The authors gratefully acknowledge the financial support from NIH grant GM082974.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol. 2010;19:777–783. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 2.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A Study of Cytokines in Burn Blister Fluid Related to Wound-Healing. Burns. 1995;21:352–355. doi: 10.1016/0305-4179(95)00005-4. [DOI] [PubMed] [Google Scholar]
- 4.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403. doi: 10.1128/CMR.19.2.403-434.2006. -+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 6.Beal AL, Cerra FB. Multiple Organ Failure Syndrome in the 1990s - Systemic Inflammatory Response and Organ Dysfunction. JAMA-J Am Med Assoc. 1994;271:226–233. [PubMed] [Google Scholar]
- 7.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, et al. Characterization of the Inflammatory Response During Acute and Post-Acute Phases after Severe Burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine. 2009;45:20–25. doi: 10.1016/j.cyto.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataranovski M, Magic Z, Pejnovic N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol Res. 1999;48:473–482. [PubMed] [Google Scholar]
- 10.Barber RC, Maass DL, White DJ, Horton JW. Increasing percent burn is correlated with increasing inflammation in an adult rodent model. Shock. 2008;30:388–393. doi: 10.1097/SHK.0b013e318164f1cd. [DOI] [PubMed] [Google Scholar]
- 11.Walley ER, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ertel W, Morrison MH, Wang P, Ba ZF, Ayala A, Chaudry IH. The Complex Pattern of Cytokines in Sepsis - Association between Prostaglandins, Cachectin, and Interleukins. Ann Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein D, Einspanier R, Bolder U, Jeschke MG. Differences in the hepatic signal transcription pathway and cytokine expression between thermal injury and sepsis. Shock. 2003;20:536–543. doi: 10.1097/01.shk.0000093345.68755.98. [DOI] [PubMed] [Google Scholar]
- 14.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 15.Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. Comparison of the cytokine and chemokine dynamics of the early inflammatory response in models of burn injury and infection. Cytokine. 2011;55:362–371. doi: 10.1016/j.cyto.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orman MA, Ierapetritou MG, Berthiaume F, Androulakis IP. The dynamics of the early inflammatory response in double-hit burn and sepsis animal models. Cytokine. 2011;56:494–502. doi: 10.1016/j.cyto.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho K, Adamson LK, Jeong J, Crivello SD, Vanhook TG, Palmieri T, et al. CD14-dependent alterations in c-Jun expression in the liver after burn injury. J Surg Res. 2004;122:36–42. doi: 10.1016/j.jss.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Ogle CK, Kong FS, Guo XL, Wells DA, Aosasa S, Noel G, et al. The effect of burn injury on suppressors of cytokine signalling. Shock. 2000;14:392–398. [PubMed] [Google Scholar]
- 19.Andrejko KM, Chen JD, Deutschman CS. Intrahepatic STAT-3 activation and acute phase gene expression predict outcome after CLP sepsis in the rat. Am J Physiol-Gastroint Liver Physiol. 1998;275:G1423–G1429. doi: 10.1152/ajpgi.1998.275.6.G1423. [DOI] [PubMed] [Google Scholar]
- 20.Genomatix. http://www.genomatix.de. [Google Scholar]
- 21.Gallant S, Gilkeson G. ETS transcription factors and regulation of immunity. Archivum Immunologiae et Therapiae Experimentalis. 2006;54:149–163. doi: 10.1007/s00005-006-0017-z. [DOI] [PubMed] [Google Scholar]
- 22.Rao A, Luo C, Hogan PG. TRANSCRIPTION FACTORS OF THE NFAT FAMILY:Regulation and Function. Annual Review of Immunology. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 23.Pahl HL. Activators and target genes of Rel/NF-kappa B transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen AY, Sakamoto KM, Miller LS. The Role of the Transcription Factor CREB in Immune Function. J Immunol. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banta S, Vemula M, Yokoyama T, Jayaraman A, Berthiaume F, Yarmush ML. Contribution of gene expression to metabolic fluxes in hypermetabolic livers induced through burn injury and cecal ligation and puncture in rats. Biotechnol Bioeng. 2007;97:118–137. doi: 10.1002/bit.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otero-Anton E, Gonzalez-Quintela A, Lopez-Soto A, Lopez-Ben S, Llovo J, Perez LF. Cecal ligation and puncture as a model of sepsis in the rat: Influence of the puncture size on mortality, bacteremia, endotoxemia and tumor necrosis factor alpha levels. Eur Surg Res. 2001;33:77–79. doi: 10.1159/000049698. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, et al. Cecal ligation and puncture. Shock. 2005;24:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 30.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 31.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Blackwell TS, Christman JW. Sepsis and cytokines: Current status. Br J Anaesth. 1996;77:110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 33.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–126. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- 34.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 35.Aderka D, Le JM, Vilcek J. Il-6 Inhibits Lipopolysaccharide-Induced Tumor Necrosis Factor Production in Cultured Human-Monocytes, U937 Cells, and in Mice. J Immunol. 1989;143:3517–3523. [PubMed] [Google Scholar]
- 36.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and Interactions in the Production of Interleukin-6 (Il-6), Il-1, and Tumor Necrosis Factor (Tnf) in Human-Blood Mononuclear-Cells - Il-6 Suppresses Il-1 and Tnf. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 37.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paunovic V, Carroll HP, Vandenbroeck K, Gadina M. Signalling, inflammation and arthritis - Crossed signals: the role of interleukin (IL)-12,-17,-23 and-27 in autoimmunity. Rheumatology. 2008;47:771–776. doi: 10.1093/rheumatology/kem352. [DOI] [PubMed] [Google Scholar]
- 39.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O'Shea JJ, Johnston JA. Interleukin-12 (Il-12) Induces Tyrosine Phosphorylation of Jak2 and Tyk2 - Differential Use of Janus Family Tyrosine Kinases by Il-2 and Il-12. J Exp Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn HJ, Tomura M, Yu WG, Iwasaki M, Park WR, Hamaoka T, et al. Requirement for distinct Janus kinases and STAT proteins in T cell proliferation versus IFN-gamma production following IL-12 stimulation. J Immunol. 1998;161:5893–5900. [PubMed] [Google Scholar]
- 41.Jacobson NG, Szabo SJ, Webernordt RM, Zhong Z, Schreiber RD, Darnell JE, et al. Interleukin-12 Signaling in T-Helper Type-1 (Th1) Cells Involves Tyrosine Phosphorylation of Signal Transducer and Activator of Transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visconti R, Gadina M, Chiariello M, Chen EH, Stancato LF, Gutkind JS, et al. Importance of the MKK6/p38 pathway for interleukin-12-induced STAT4 serine phosphorylation and transcriptional activity. Blood. 2000;96:1844–1852. [PubMed] [Google Scholar]
- 43.Gollob JA, Schnipper CP, Murphy EA, Ritz J, Frank DA. The functional synergy between IL-12 and IL-2 involves p38 mitogen-activated protein kinase and is associated with the augmentation of STAT serine phosphorylation. J Immunol. 1999;162:4472–4481. [PubMed] [Google Scholar]
- 44.Zhang SM, Kaplan MH. The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-gamma expression. J Immunol. 2000;165:1374–1380. doi: 10.4049/jimmunol.165.3.1374. [DOI] [PubMed] [Google Scholar]
- 45.Marshall JD, Aste-Amezaga M, Chehimi SS, Murphy M, Olsen H, Trinchieri G. Regulation of human IL-18 mRNA expression. Clin Immunol. 1999;90:15–21. doi: 10.1006/clim.1998.4633. [DOI] [PubMed] [Google Scholar]
- 46.Luster AD. Chemokines - Chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 47.Premack BA, Schall TJ. Chemokine receptors: Gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 48.Murphy PM. The molecular-biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 49.Soriano SF, Serrano A, Hernanz-Falcon P, de Ana AM, Monterrubio M, Martinez C, et al. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. European Journal of Immunology. 2003;33:1328–1333. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]