Abstract
Calcitonin gene-related peptide (CGRP) exerts its diverse effects on vasodilation, nociception, secretion, and motor function through a heterodimeric receptor comprising of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1). Despite the importance of CLR•RAMP1 in human disease, little is known about its distribution in the human gastrointestinal (GI) tract, where it participates in inflammation and pain. In this study, we determined that CLR and RAMP1 mRNAs are expressed in normal human stomach, ileum and colon by RT-PCR. We next characterized antibodies that we generated to rat CLR and RAMP1 in transfected HEK cells. Having characterized these antibodies in vitro, we then localized CLR-, RAMP1-, CGRP- and intermedin-immunoreactivity (IMD-IR) in various human GI segments. In the stomach, nerve bundles in the myenteric plexus and nerve fibers throughout the circular and longitudinal muscle had prominent CLR-IR. In the proximal colon and ileum, CLR was found in nerve varicosities of the myenteric plexus and surrounding submucosal neurons. Interestingly, CGRP expressing fibers did not co-localize, but were in close proximity to CLR. However, CLR and RAMP1, the two subunits of a functional CGRP receptor were clearly localized in myenteric plexus, where they may form functional cell-surface receptors. IMD, another member of calcitonin peptide family was also found in close proximity to CLR, and like CGRP, did not co-localize with either CLR or RAMP1 receptors. Thus, CGRP and IMD appear to be released locally, where they can mediate their effect on their receptors regulating diverse functions such as inflammation, pain and motility.
Keywords: CGRP, intermedin, adrenomedullin2, myenteric plexus, primary afferent
1. Introduction
α–alcitonin gene-related peptide (α-CGRP), a 37-amino acid peptide is an alternative splice variant of the calcitonin gene, whereas the β-CGRP isoform is a separate gene product [2]. Besides CGRP, the calcitonin family of peptides also includes adrenomedullin, intermedin/adrenomedullin-2 (IMD/AM2; herein referred to as IMD), and amylin. Existing in α and β isoforms [1, 2, 30], α-CGRP is expressed throughout the central and peripheral nervous systems and is present in up to 80% of substance P containing nerve terminals [9, 29]. Both isoforms are involved in the regulation of diverse physiologic effects, including nociception [34], secretion [23], and feeding [25]. They are both also pro-inflammatory, causing smooth muscle relaxation leading to arteriolar dilation and subsequent increased tissue blood flow [3]. The importance of calcitonin peptide family in human pathophysiology is highlighted by the use of human CGRP receptor antagonists for the treatment of migraines [24].
Receptors for some members of the calcitonin peptide family, such as CGRP and IMD, are known to be heterodimers of the G protein-coupled calcitonin receptor-like receptor (CLR) and receptor activity-modifying proteins (RAMPs). RAMPs are a family of three single transmembrane proteins required to chaperone CLR from intracellular organelles to the cell surface and to effect CLR terminal glycosylation and peptide specificity [17, 21]. Depending on which particular RAMP partners with CLR, the resultant heterodimer is preferentially bound by CGRP, adrenomedullin, or IMD with varying affinities. CGRP serves as a higher affinity ligand than adrenomedullin or IMD when CLR heterodimerizes with RAMP1, whereas adrenomedullin serves as a higher affinity ligand than CGRP when CLR partners with either RAMP2 or RAMP3. When co-expressed with RAMP1, 2, or 3, CLR binds IMD with intermediate affinity relative to adrenomedullin or CGRP [15, 21, 28]. The physiological activity of the calcitonin peptide family is therefore dependent on tissue expression of individual ligands as well as receptor specificity within that tissue.
Differing by 3 out of 37 residues in humans, α–CGRP and β–CGRP have similar biological roles [23, 36]. To characterize these functions, murine localization studies have shown α–CGRP to be localized within spinal afferent Aδ and C fibers of dorsal root ganglia [27], and β–CGRP within the enteric nervous system [32, 33]. Binding sites for radiolabeled CGRP are present within the myenteric plexus of both dogs [8] and rats [25]. Immunolocalization studies in rat intestine show CLR expression in nerve fibers of the muscularis externa and myenteric and submucosal plexuses [6]. Functional studies with rat and mouse intestine demonstrate a role for CGRP in motility [4], as mechanical stimulation of rodent intestine releases CGRP from enterochromaffin cells [10]. Moreover, the CGRP receptor antagonist CGRP8-37 halts the ascending contraction and descending relaxation of the intestine in response to mucosal stimulation [11, 26]. Based on these experiments, it is believed CGRP modulates motility [14] as well as secretion [7, 20] within the gastrointestinal system. IMD is not as widely distributed as AM or CGRP, and has been localized to the GI tract with high expression in the stomach [15, 35]. IMD has also been identified within the human enteric system and has functional activity in rodents by suppressing food intake and gastrointestinal motility in rodent models [28].
Despite prior investigations, the distribution of CLR and RAMP1 has not been fully described in human tissue, especially whether the receptor components are expressed in the same cells or in proximity to their agonists, CGRP and IMD. Here we investigate the cellular and subcellular localization of CLR, RAMP1 and their potential agonists, CGRP and IMD, in the gastrointestinal tract of normal human tissue. Our aims were to (1) characterize antibodies generated in our laboratory to CLR and RAMP1; (2) determine the tissue distribution of CLR and RAMP1 in human GI tract; and (3) determine if CLR and RAMP1 are appropriately localized in the human GI tract to mediate the known biological actions of CGRP and IMD.
2. Materials and Methods
2.1 Human Tissue
Tissues were obtained at University of California, San Francisco from surgical specimens with approval of the Institutional Committee on Human Research (IRB approval # H29148-25283-03A). Tissues were collected from 18 patients undergoing operations for cancers (stomach, small intestinal, colon, or pancreas). All samples were collected early in the operation from an area that was deemed normal tissue and not involved in the tumor, but would have been removed as a routine part of the specimen. This approach provided us with non-ischemic tissue and as healthy and normal as possible. At least two sites of the GI tract were sampled at the time of surgery depending on the nature of their operation and intestinal reconstruction performed.
2.2 Antibodies and Peptides
Antibodies were from the following sources: rabbit anti-rat CLR (RK11) and anti-rat RAMP1 (9891) have been described [6]; mouse anti-rat CGRP (4901) has been described [38]; rabbit anti-human IMD was a gift from Dr. Sheau Yu Teddy Hsu (Stanford University, CA) [28]; mouse anti-c-Myc (Invitrogen, Carlsbad, CA); mouse anti-FLAG (M2) (Sigma; St. Louis, MO) and goat or donkey anti-rabbit, -rat, -mouse or -guinea pig IgG conjugated to fluorescein isothiocyanate, Rhodamine Red-X, Texas Red, Cy5 or horseradish peroxidase, (Jackson ImmunoResearch; West Grove, PA). Human α-CGRP from was Bachem (Weil am Rhein, Germany).
2.3 Vector construction
The cDNAs encoding human CLR (N-terminal FLAG) and RAMP1 were a gift from Pfizer. An N-terminal Myc tag was added to human RAMP1 by PCR using the primers: forward, 5’-gagcagaagctgatctccgaggaagacctgtgccaggaggctaactacgg-3’ and reverse, 5’-atgcggccgcctacacaatgccctcagtgcg-3’. The product was subcloned into pcDNA3.1-Igκ (+) blasticidin [6] to yield the vector pcDNA3.1-IgκmychRAMP1. Identity was confirmed by DNA sequencing.
2.4 Maintenance and Transfection of HEK293 cells
Human embryonic kidney (HEK293) cells containing the Flp-In™ system (HEK-FLP) were from Invitrogen (Carlsbad, CA). Cells were cultured in Advanced Dulbecco’s modified Eagle’s medium supplemented with 2 mM l-glutamine, 2% heat-inactivated fetal bovine serum and zeocin (100 μg/ml). Cells were transiently transfected with human FLAG-CLR and Myc-RAMP1 using Lipofectamine™2000 (Invitrogen) according to the manufacturer’s guidelines. Generation and maintenance of HEK cells stably expressing rat CLR•RAMP1 has been described [5]. For controls, cells were transfected with empty vector.
2.5 RT-PCR
Total RNA from human tissues (stomach, small intestine, colon, n=3-5) and SK-N-MC cells was isolated using Trizol (Invitrogen). RNA (4 μg) was reverse transcribed using standard protocols with oligo(dT)15 and AMV-RT (Promega, Madison, WI). Subsequent PCR reactions used primers specific for human CLR (forward: 5’-tacgcgttctcatcaccaag-3’ and reverse 5’-tcatggatgctttttccattt-3’), and RAMP1 (forward: 5’-ctgccaggaggctaactacg-3’ and reverse: 5’- cctcagtgcgcttgctct-3’). Control reactions omitted reverse transcriptase. The PCR products were separated by electrophoresis (2% agarose gel), detected using ethidium bromide, and sequenced to confirm identity.
2.6 Preparation of Cell Membranes
Cells were scraped into 1x PBS and subjected to 5 cycles of freeze-thawing and centrifuged (100 g, 10 min, 4°C). Supernatants were centrifuged (100,000 g, 1 h, 4°C) and pellets resuspended in 50 mM Tris-HCl, pH 7.4, 1% Triton-X-100. Protein concentrations were determined using the bicinchoninic acid assay [31], with BSA as standard. The method was adapted for use in 96-well microtitre plates [16].
2.7 SDS-PAGE and Western Blotting
Total cell membranes (10 μg protein) were separated by SDS-PAGE (9 and 15% bis-acrylamide). Proteins were transferred to PVDF membranes (Immobilon-P, Millipore) and blocked for 1 h at room temperature (1x PBS, 0.1% Tween20, 5% Milk powder, 2% BSA). Membranes were incubated with antibodies to rat CLR (RK11, 1:10,000), c-Myc (1:5000) or rat RAMP1 (9891, 1:5000) all overnight at 4°C (1x PBS, 0.1% Tween20, 5% Milk powder, 2% BSA). Membranes were washed for 30 min (1x PBS, 0.1% Tween20) and incubated with appropriate secondary antibodies coupled to horseradish peroxidase (1:10,000, 1 h, room temperature). Immunoreactive proteins were detected using enhanced chemiluminescence (Geneflow Ltd., Fradley, UK) using an ImageQuant-RT ECL imaging system (GE Healthcare, Chalfont St Giles, UK). Blots were stripped by incubating membranes in 62.5 mM Tris-HCl, pH 6.8, 2% SDS, 100 mM β-mercaptoethanol (30 min, 60°C).
2.8 Drug treatments
HEK cells transiently transfected human CLR•RAMP1 were stimulated with human α-CGRP (100 nM, 0-30 min) in Dulbecco’s modified Eagle’s medium containing 0.1% bovine serum albumin.
2.9 Immunostaining of cell lines
Following treatments, cells were washed in 100 mm PBS, pH 7.4, and fixed in PBS containing 4% paraformaldehyde, pH 7.4 (20 min, 4°C). Cells were washed with PBS containing 0.1% saponin and 2% normal horse serum for 30 min. Proteins were localized using the primary antibodies CLR (RK11, 1:1000), FLAG (M2, 1:500), c-Myc (1:500); RAMP1 (9891, 1:1000) (overnight, 4°C). Cells were washed and incubated with secondary antibodies coupled to Rhodamine Red-X or Cy5 (1:500, 1 h, room temperature). Cells were observed with a Zeiss laser-scanning confocal microscope (LSM Meta 510) using a Fluar Plan Apochromat ×63 oil immersion objective (numerical aperture 1.4). Images were collected at a zoom of 2 and an iris of <3 μm, and five optical sections were taken at intervals of 0.5 μm. Single sections are shown. Images were processed (colored and merged) using Adobe Photoshop (Adobe Systems, Mountain View, CA, USA).
2.10 Immunostaining of human tissues
Gastrointestinal tissues were obtained from patients undergoing surgery for either benign or malignant disease (n=3-5/GI segment). Only normal or non-diseased tissues were collected and were immersion fixed in 100 mM PBS containing 4% paraformaldehyde (24-48 h, 4°C). For cryostat sections, tissues were incubated in 20-25% sucrose in PBS (24 h, 4°C), embedded in OCT compound (Miles, Elkhart, IN), and sequentially sectioned at 10 μm. Sequential tissue sections were mounted on slides, washed in PBS and incubated with the primary antibodies: CLR (RK11, 1:1000-1:10,000), RAMP1 (9891, 1:250-1:2000), CGRP (1:500-1:1,000) and IMD (1:300) (overnight, 4°C or room temperature). Tissues were washed and incubated with appropriate secondary antibodies. Sections were washed and mounted in Prolong (Invitrogen, Carlsbad, CA). Primary antibodies were preadsorbed, by incubating with the peptide used for immunization (10-5 M, 96 h, 4°C). For confocal microscopy, specimens were observed using laser-scanning confocal microscopes (Zeiss LSM Meta 510), using Plan Apo x40 (NA 1.4), x100 (NA 1.3) objectives. Images were collected at zoom of 1-2 and typically 10-20 optical sections were taken at intervals of 0.5-1.0 μm. Images were processed to adjust contrast and brightness using Adobe Photoshop. Images of stained and control slides were collected and processed (colored and merged) identically.
3. Results
3.1 mRNA transcripts for CLR and RAMP1 are expressed in the human GI tract
To determine if mRNA for CLR and RAMP1 were expressed in the normal human GI tract, we isolated RNA from stomach, ileum and colon biopsies/segments and from SK-N-MC cells (known to express CLR and RAMP1, [37]). Negative control without RT enzyme did not yield a PCR product, whereas, CLR and RAMP1 products were amplified from the human GI segments and SK-N-MC cells (Fig. 1). Thus, RT-PCR data revealed the presence of CLR and RAMP1 throughout the GI tract.
Figure 1. CLR and RAMP1 are expressed in normal human GI tract.
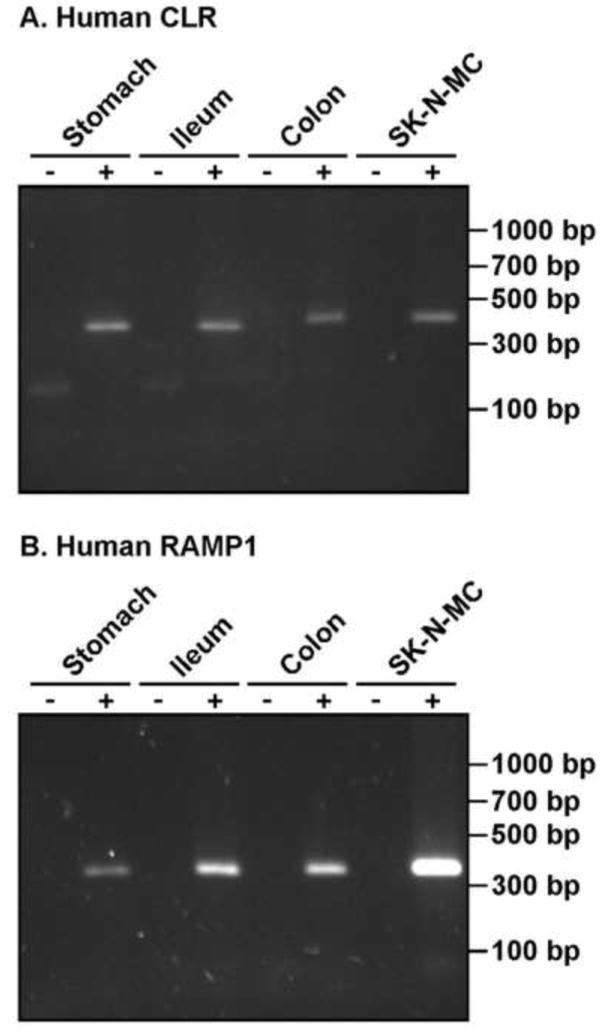
RT-PCR amplification of mRNA for CLR (406 bp) and RAMP1 (359 bp) in human GI tissue (stomach, ileum and colon) and SK-N-MC cells. RT–reverse transcriptase, bp–base pairs.
3.2 Characterization of rat CLR and RAMP1 antibodies to human CLR and RAMP1
We characterized our anti-rat CLR and RAMP1 antibodies with respect to their cross-reactivity with human CLR and RAMP1 using transfected HEK cells. Cell lysates from HEK cells transfected with empty vector (control), human CLR•RAMP1 or rat CLR•RAMP1 were analyzed by Western blotting using the anti-rat CLR and RAMP1 antibodies. The anti-CLR antibody did not detect any proteins in control or human CLR•RAMP1 membranes but readily detected rat CLR (~96 kDa; Fig. 2A). The anti-rat RAMP1 detected immunoreactive proteins of the predicted size (~17 kDa) from both rat and human CLR•RAMP1 membranes, but did not detect any proteins in control membranes indicating specificity of detection (Fig. 2B). The specificity of the immunoreactive bands detected by the anti-rat RAMP1 antibody was confirmed using the extracellular epitope tags (Myc) of human and rat RAMP1. An anti-c-Myc detected the same immunoreactive proteins as the anti-rat RAMP1 antibody (Fig. 2B). Thus, the anti-rat CLR antibody is not suitable for detecting human CLR using Western blotting, but the anti-rat RAMP1 can be used to detect human RAMP1. We next tested the ability of these antibodies to detect human CLR•RAMP1 in human embryonic kidney (HEK) cells by immunofluorescence.
Figure 2. Characterization of human CLR and RAMP1 antibodies by Western blot analysis.
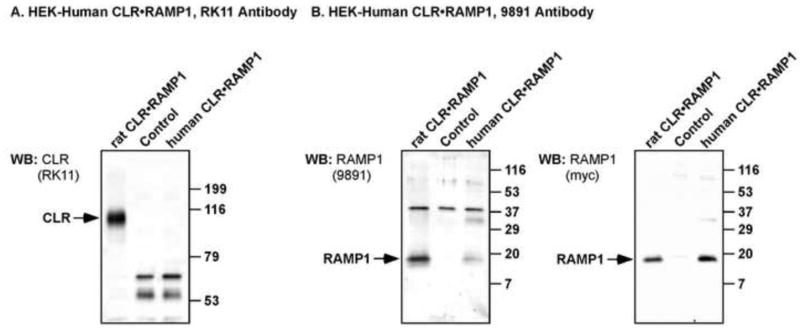
HEK cells were transfected with empty vector (control) or human FLAG-CLR and human myc-RAMP1 and the specificity of anti-rat CLR (RK11) and RAMP1 (9891) antibodies determined by Western blotting. (A) RK11 antibody readily detected rat CLR (positive control, ~96 kDa) but did not cross-react with human CLR. (B) 9891 antibody readily detected RAMP1 from both rat and human (~17 kDa), with no RAMP1 staining detected in vector-transfected control cells. After stripping, blots were re-probed with anti-c-Myc antibody to detect the extracellular epitope tag of rat and human RAMP1. Immunoreactive bands were detected at the same molecular mass for rat and human RAMP1 by anti-c-Myc, indicating specificity of the proteins detected by 9891. As predicted, neither anti-c-Myc nor 9891 detected any immunoreactive proteins of the predicted size of RAMP1 in vector transfected control cells. There were faint signals for human RAMP1 (~35 kDa) with both 9891 and c-Myc antibodies, which we predict to be dimers of RAMP1.
3.3 Rat CLR and RAMP1 antibodies recognize human CLR and RAMP1 in unstimulated and activated states
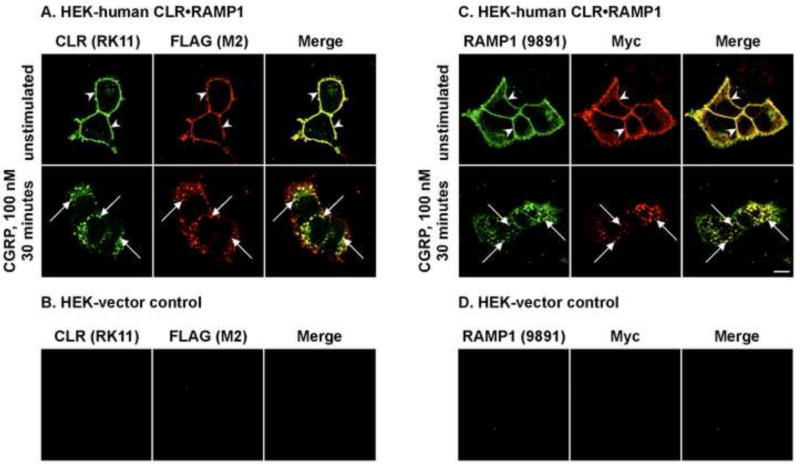
CLR•RAMP1 present in tissues may be modified by phosphorylation or associate with accessory proteins such as β-arrestins, which may interfere with ability of antibodies to recognize the proteins. Therefore, we examined the subcellular localization of CLR and RAMP1, the two subunits of the CGRP receptor before and after stimulation with α-CGRP. HEK cells expressing human CLR and RAMP1 were stimulated with α-CGRP (100 nM, 0-30 min) and CLR and RAMP1 localized by immunofluorescence and confocal microscopy. First, we localized CLR using the anti-rat CLR and anti-FLAG antibodies. In unstimulated cells, human CLR was detected at the cell-surface by both antibodies (Fig. 3A). Following stimulation with α-CGRP, both antibodies detected CLR in intracellular vesicles, presumably endosomes. Neither antibody recognized proteins in HEK cells expressing empty vector (Fig. 3B). Next, we localized human RAMP1 using the anti-rat RAMP1 and anti-c-Myc antibodies. In unstimulated cells, RAMP1 was detected at the cell-surface by both antibodies. Following stimulation with α-CGRP, both antibodies detected RAMP1 in intracellular vesicles. Again, neither detecting antibody recognized proteins in control cells (Fig. 3D). Double-staining with anti-Flag-and anti-Myc to detect CLR and RAMP1, respectively revealed that the both RK11 and 9891 antibodies show staining pattern identical to that seen after staining with the appropriate anti-tag antibodies (Fig. 3A, C, merge). Thus, both anti-rat CLR and RAMP1 antibodies are suitable for detecting unstimulated and activated human CLR and RAMP1 by immunofluorescence.
Figure 3. Detection of human CLR and RAMP1 before and after stimulation with α-CGRP.
HEK cells were transiently transfected with empty vector (control) or human FLAG-CLR and human myc-RAMP1. The specificity of anti-rat CLR (RK11) and RAMP1 (9891) antibodies was determined by immunofluorescence and confocal microscopy. 3A, 3C: In unstimulated HEK-human CLR•RAMP1 cells, CLR (RK11, FLAG (M2)) and RAMP1 (9891, c-Myc) were detected at the cell-surface (upper panel, arrowheads). Incubation with human α-CGRP (100 nM, 30 min) caused internalization of both CLR (RK11, FLAG (M2)) and RAMP1 (9891, c-Myc) to intracellular vesicles (lower panel, arrows). Thus, RK11 and 9891 both cross-react with activated CGRP receptor components. 3B, 23D: In HEK cells transfected with empty vector (control) no signal for CLR (RK11, FLAG (M2)) or RAMP1 (9891, c-Myc) was detected indicating specificity of RK11 and 9891 antibodies. Pre-adsorption with respective peptides for RK11 and 9891 similarly abolished all staining. Scale bar = 10 μm.
3.4 Localization of CLR and RAMP1 in the human GI tract
α-CGRP is an important neurotransmitter in the enteric nervous system and previous studies have localized CLR and RAMP1 in rat GI tissue [4, 6]. However, the localization of CLR and RAMP1 in human GI tissue has not been determined. We obtained normal human GI tissue (stomach, ileum, colon) from surgical specimens and examined expression of CLR and RAMP1 by immunofluorescence and confocal microscopy. As both primary antibodies were raised in the same species, we examined CLR and RAMP1 expression in serial sections of primary human tissue. CLR-IR was clearly visible in the neurons of the myenteric plexus of the stomach, ileum and colon. Prominent staining of large nerve bundles was observed, as well as in individual fibers both in the myenteric plexus and throughout the circular and longitudinal muscles (Fig. 4A, B). CLR-IR was abolished by pre-adsorption of the antibody with the peptide used for immunization, confirming specificity of detection (Fig. 4A).
Figure 4. Localization of CLR and RAMP1 in human stomach, ileum and colon.
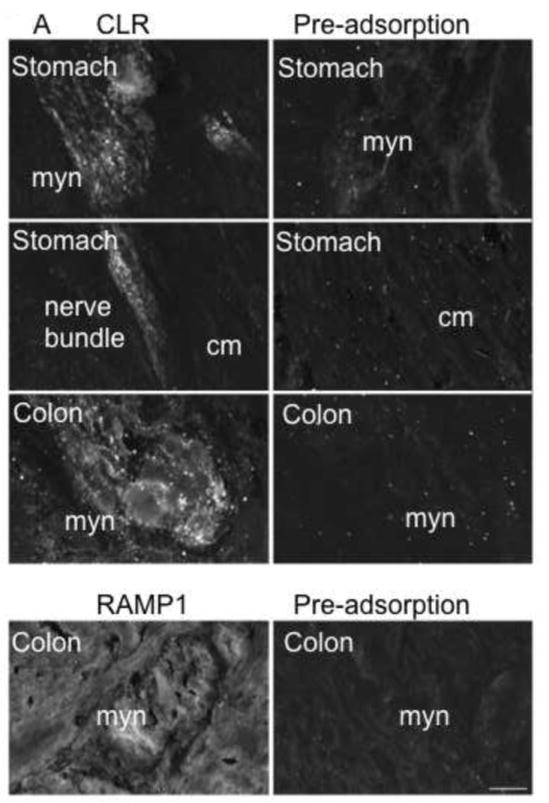
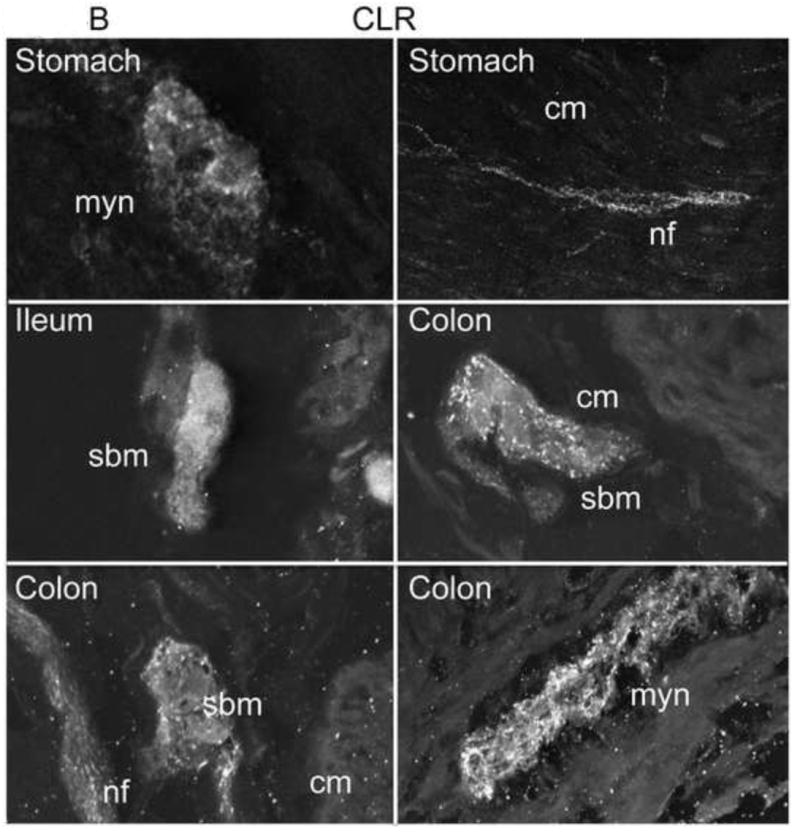
(A) Stomach and colon tissue sections were stained with anti-rat CLR (RK11) or RAMP1 (9891) antibodies (left panels) or with preadsorbed antibodies (right panels). CLR-IR was detected in myenteric neurons and nerve bundles throughout the circular muscle and longitudinal in the stomach. CLR- and RAMP1-IR was observed in myenteric neurons of the colon. Preadsorption of primary antibodies abolished signals for both CLR and RAMP1 indicating specificity of detection. (B). Submucosal neurons were also stained in the ileum and colon, and CLR immunoreactive fibers were more prominent in the myenteric plexus. Myn, myenteric neurons, cm, circular muscle, nf, nerve fiber, sbm, submucosal neurons. Scale bar = 33 μm.
In the stomach and colon, we observed diffuse CLR-IR of the myenteric neurons, interspersed with punctate staining within the nerve bundles. Nerve fibers displayed more prominent and robust staining (Fig. 4A). In the ileum and colon, CLR-IR was detected in submucosal neurons, sometimes surrounded by nerve fibers or in close proximity to larger nerve bundles (Fig. 4B). RAMP1-IR was present in myenteric neurons of the colon, were it could heterodimerize with CLR to form a functional CGRP receptor (Fig. 4A). RAMP1-IR was abolished by pre-adsorption of the antibody with the peptide used for immunization, confirming specificity of detection. Weak RAMP1-IR was also observed in the myocytes of the colon (not shown).
3.5 Localization of CLR and CGRP in human GI tract
To determine if CLR is appropriately localized to mediate biological activity of CGRP, we simultaneously localized CLR and CGRP by immunofluorescence and confocal microscopy. CGRP-IR was detected in close proximity to CLR-IR in myenteric neurons of the stomach, ileum and colon. In the stomach, CLR-IR and CGRP-IR nerve fibers intertwined and within the myenteric plexus, CLR-IR and CGRP-IR colocalized (Fig. 5A). CGRP-IR was localized to nerve fibers throughout the myenteric plexus in the ileum and stomach (Fig. 5A). Similarly, in the colon, we observed colocalization (or close proximity) of CLR-IR and CGRP-IR in the myenteric and submucosal plexus, and CGRP-IR was closely apposed to nerve fibers that were immunoreactive for CLR (Fig. 5B).
Figure 5. CLR co-localizes with CGRP in a subset of neurons.
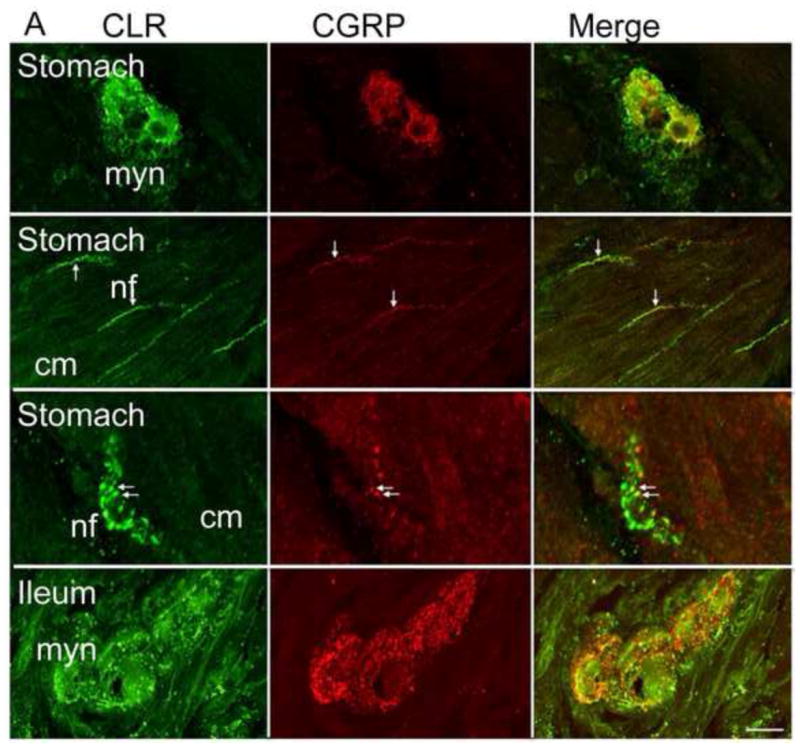
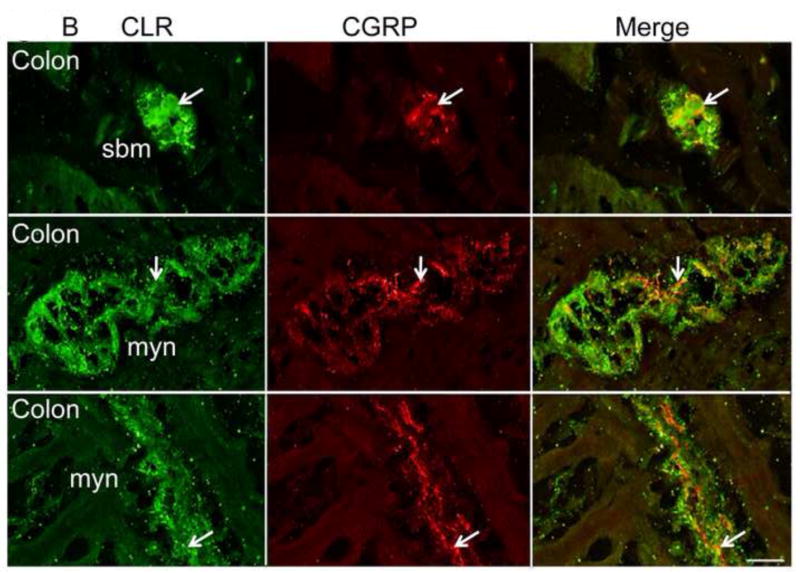
(A, B) CLR and CGRP were simultaneously localized in stomach, ileum and colon tissue sections. CGRP-IR was detected in close proximity to CLR-positive neurons. CGRP- and CLR-positive nerve fibers intertwined, and in a few locations, colocalization seemed apparent in the stomach, ileum (arrows) (A), and colon (B). Thus, CGRP is localized nearby CLR, suggesting it could function as a ligand for CLR•RAMP1. Single optical sections are shown, except where noted. Myn, myenteric neurons, cm, circular muscle, nf, nerve fiber. Scale bar = 33 μm.
3.6 Localization of IMD and CLR in human stomach and colon
IMD is primarily expressed in the pituitary and the GI tract, and in animal models, treatment with IMD results in reduced gastric emptying and food intake [28]. As IMD is a potential CGRP receptor agonist and high levels of IMD are expressed in the stomach [15], we localized CLR and IMD in human stomach and colon. As the anti-rat CLR and anti-human IMD antibodies were raised in the same species, we localized CLR and IMD in serial sections. In the stomach and colon, IMD was prominently expressed in nerve fibers, similar to CGRP. However, unlike CGRP, IMD was weakly expressed in the submucosal and myenteric plexus (Fig. 6). Furthermore, IMD did not co-localize with either CLR in human stomach or colon.
Figure 6. IMD and CLR and expressed within nerve fibers of human gut.
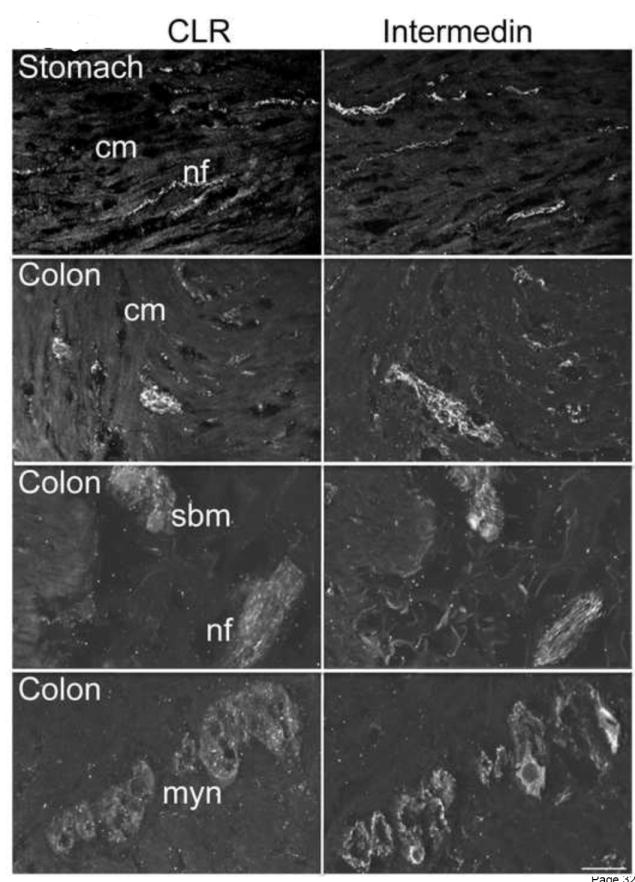
CLR and IMD were localized in serial tissue sections of human stomach and colon sections. Similar to CGRP, IMD-IR was located in close proximity to CLR-IR in nerve fibers in the circular and longitudinal muscle layers of the stomach and colon. Moreover, IMD was present in myenteric and submucosal plexuses, and in nerve fibers in the submucosa in the stomach, ileum, and colon. Cm, circular muscle, sbm, submucosal neurons, nf, nerve fiber, myn, myenteric neurons. Scale bar = 33 μm.
4. Discussion
CGRP and its receptor, CLR•RAMP1, have been previously localized in the central and peripheral nervous systems, and are involved in the regulation of diverse physiologic effects, including nociception, secretion, and smooth muscle relaxation [10, 12, 18, 19]. CGRP has a prominent role in the enteric nervous system as well, and animal studies have suggested a role for the peptide in modulation of intestinal neurotransmission, motility and secretion [12, 18, 19]. Given that the distribution of CLR and RAMP1 in human enteric tissue had yet to be characterized, the primary objectives of this study was to confirm the presence of CLR and RAMP1 within the human GI tract and determine its subcellular and tissue distribution together with its ligands, CGRP and IMD.
We show that CLR and RAMP1 are widely expressed in normal human GI tissue, with mRNA transcripts amplified from stomach, ileum, and colonic tissue by RT-PCR (Fig. 1). Immunohistochemical analysis of human GI tissues using immunofluorescence and confocal microscopy showed that CLR is expressed within the human stomach, ileum, and colon. In agreement with our observation, an earlier study reported presence of CLR in human stomach and also in other peripheral tissues such as the heart, lung, pancreas, and testis [13]. Within the stomach, prominent CLR-IR was observed in the cytoplasm of the fundic chief cells, and sporadic CLR-IR was found within the epithelium and myenteric plexus of enteric tissue [13]. However, no study has localized RAMP1 or co-localized CLR and RAMP1 in human gut. In this study, we found prominent CLR- and RAMP1- IR within nerve bundles in the myenteric plexus, as well as nerve fibers throughout the circular and longitudinal muscle in the stomach (Fig. 5 and Table 1). The differences observed in CLR-IR in the stomach between our study and that previously reported [13], may be due to the different antigenic sequences used to generate the antibodies. Hagner et al [13] raised an antibody to the C-terminus of human CLR (455SIHDIENVLLKPENLYN462), whereas our antibody was raised to the C-terminus of rat CLR (455SIQDIENVALKPEKMDLV463) [6]. These are 6 substitutions between these sequences, which may account for the discrepancies observed in the staining. Another explanation could be the differences in the immunohistochemical staining procedures.
Table 1.
| IR/ GI segment | Stomach | Ileum | Colon |
|---|---|---|---|
| CLR-IR (enteric neurons) | ++ | ++ | +++ |
| RAMP1 (enteric neurons) | +++ | ++ | +++ |
In the ileum and proximal colon, CLR is found in the myenteric plexus and surrounding submucosal neurons (Fig. 5A, B). Similarly, RAMP1-IR is found in neurons in the myenteric plexus (Table 1). The localization of CLR and RAMP1 in the myenteric plexus demonstrates that the two subunits of the receptor are in proximity and can potentially heterodimerize to form a functional CGRP receptor where it may respond to locally secreted CGRP in the human GI tract.
When we localized human tissues for CLR and the potential agonists, CGRP and IMD, we observed no co-localization of CLR with CGRP or IMD. However, we did observe CLR-IR and CGRP- and IMD-IR in very close proximity in nerve fibers in the circular and longitudinal muscle layers of the stomach, ileum, and colon (Fig. 6). The results suggest that CGRP and IMD are appropriately localized with CLR to function as ligands for CLR•RAMP complexes and to exert biologic effects under appropriate stimulation. However, there is no evidence that CLR•RAMP may be autoreceptors for CGRP and IMD in the tissues we examined. It is also possible that other RAMP proteins, namely RAMP2 and 3 may also be present in the human gut and may interact with CLR to form a higher affinity receptor for IMD or indeed adrenomedullin. In support of this, mRNA transcripts for RAMP2 and RAMP3 were amplified from rat duodenum, jejunum, ileum, and proximal and distal colon [6]. Further, a recent study has shown that when co-expressed with RAMP3, CLR interacts with IMD and adrenomedullin with similar affinities, that are up to two orders of magnitude greater than CGRP [15]. Thus, agonists may be secreted in an autocrine manner to act on their receptors to mediate their effects in the gut.
Previous studies have demonstrated the effects of CGRP and IMD on gastrointestinal secretion and motility. Enteric neurons containing CGRP have long been implicated in mediating gut vasodilatation [18] and motility [4, 22]. Rodent studies have identified CGRP secretion after mechanical stimulation of the mucosa, and coordinated ascending contraction and descending relaxation is suppressed after application of CLR-RAMP1 antagonists [10]. Likewise, rodent studies with IMD demonstrated its role in suppressing gastric emptying and food intake [28]. Together, these data corroborate the notion that CGRP and IMD play a prominent role in normal human gut physiology. Functional studies in human tissue are a logical progression of these earlier findings, which is now facilitated by the localization of CLR•RAMP1, CGRP, and IMD within the human gastrointestinal tract. The co-localization of the subunits of the CGRP receptor suggests a functional role in tissue that is punctuated by the receptor’s close tissue (cellular) association with the agonists CGRP and IMD. Future studies will attempt to characterize receptor activity and function in human cells and tissue to ascertain the role of CLR•RAMP1, CGRP and IMD in human enteric physiology and disease.
5. Conclusions
CLR and RAMP1 co-localize in the enteric nervous system of human stomach, ileum, and colon, and are in close proximity to their ligands CGRP and IMD. Autocrine secretion of different ligands and the spatio-temporal expression of different RAMP proteins in combination with CLR, may account for the diverse functions in which the CLR•RAMP system is involved in the human GI tract.
Highlights.
CLR and RAMP1 co-localize in enteric neurons of the human gastrointestinal tract
CLR and RAMP1 are present in close proximity to their ligands CGRP and intermedin
CGRP and intermedin must be secreted in a paracrine manner in human gut to mediate their effects via their receptors
Acknowledgments
This study was supported in part by National Institutes of Health grants: DK026743 (CUC) DK52388 (EFG), DK080787 (AB). Farzad Alemi, a surgery resident was supported by a NIH training grant, T32 DK07573. A British Heart Foundation Fellowship FS/08/017/25027 currently supports GSC. Additional funding support came from the Department of Veterans Affairs (CUC; Washington, DC) and from the Northern California Institute for Research (CUC; San Francisco, CA). The authors thank Dr. N.W. Bunnett for kindly providing the RAMP1 (9891) antibody.
Abbreviations
- AM2
adrenomedullin 2
- CGRP
Calcitonin gene-related peptide
- CLR
calcitonin receptor-like receptor
- GI
gastrointestinal
- IR
immunoreactivity
- IMD
intermedin
- RAMP
receptor activity-modifying protein
Footnotes
Conflict of Interest Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–7. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- 2.Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–4. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- 3.Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–6. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- 4.Clifton MS, Hoy JJ, Chang J, Idumalla PS, Fakhruddin H, Grady EF, et al. Role of calcitonin receptor-like receptor in colonic motility and inflammation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G36–44. doi: 10.1152/ajpgi.00464.2006. [DOI] [PubMed] [Google Scholar]
- 5.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, et al. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. The Journal of biological chemistry. 2007;282:12260–71. doi: 10.1074/jbc.M606338200. [DOI] [PubMed] [Google Scholar]
- 6.Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, et al. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. The Journal of comparative neurology. 2005;490:239–55. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- 7.Esfandyari T, Macnaughton WK, Quirion R, St Pierre S, Junien JL, Sharkey KA. A novel receptor for calcitonin gene-related peptide (CGRP) mediates secretion in the rat colon: implications for secretory function in colitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:1439–46. doi: 10.1096/fj.14.10.1439. [DOI] [PubMed] [Google Scholar]
- 8.Gates TS, Zimmerman RP, Mantyh CR, Vigna SR, Mantyh PW. Calcitonin gene-related peptide-alpha receptor binding sites in the gastrointestinal tract. Neuroscience. 1989;31:757–70. doi: 10.1016/0306-4522(89)90439-9. [DOI] [PubMed] [Google Scholar]
- 9.Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, et al. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1984;4:3101–11. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grider JR. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. The American journal of physiology. 1994;266:G1139–45. doi: 10.1152/ajpgi.1994.266.6.G1139. [DOI] [PubMed] [Google Scholar]
- 11.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther. 2003;307:460–7. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- 12.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:G429–37. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 13.Hagner S, Stahl U, Knoblauch B, McGregor GP, Lang RE. Calcitonin receptor-like receptor: identification and distribution in human peripheral tissues. Cell and tissue research. 2002;310:41–50. doi: 10.1007/s00441-002-0616-x. [DOI] [PubMed] [Google Scholar]
- 14.Holzer P, Bartho L, Matusak O, Bauer V. Calcitonin gene-related peptide action on intestinal circular muscle. The American journal of physiology. 1989;256:G546–52. doi: 10.1152/ajpgi.1989.256.3.G546. [DOI] [PubMed] [Google Scholar]
- 15.Hong Y, Hay DL, Quirion R, Poyner DR. The pharmacology of Adrenomedullin 2/Intermedin. British journal of pharmacology. 2011 doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper NM. Determination of mammalian membrane protein anchorage: Glycosyl-phosphatidylinositol (G-PI) or transmembrane polypeptide anchor. Biochemical Education. 1993;21:212–6. [Google Scholar]
- 17.Husmann K, Born W, Fischer JA, Muff R. Three receptor-activity-modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochemical pharmacology. 2003;66:2107–15. doi: 10.1016/j.bcp.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Kono T, Koseki T, Chiba S, Ebisawa Y, Chisato N, Iwamoto J, et al. Colonic vascular conductance increased by Daikenchuto via calcitonin gene-related peptide and receptor-activity modifying protein 1. The Journal of surgical research. 2008;150:78–84. doi: 10.1016/j.jss.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 19.Maggi CA, Giuliani S, Santicioli P. CGRP potentiates excitatory transmission to the circular muscle of guinea-pig colon. Regul Pept. 1997;69:127–36. doi: 10.1016/s0167-0115(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 20.McCulloch CR, Cooke HJ. Human alpha-calcitonin gene-related peptide influences colonic secretion by acting on myenteric neurons. Regul Pept. 1989;24:87–96. doi: 10.1016/0167-0115(89)90214-0. [DOI] [PubMed] [Google Scholar]
- 21.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 22.Mitsui R. Characterisation of calcitonin gene-related peptide-immunoreactive neurons in the myenteric plexus of rat colon. Cell and tissue research. 2009;337:37–43. doi: 10.1007/s00441-009-0798-6. [DOI] [PubMed] [Google Scholar]
- 23.Muff R, Born W, Lutz TA, Fischer JA. Biological importance of the peptides of the calcitonin family as revealed by disruption and transfer of corresponding genes. Peptides. 2004;25:2027–38. doi: 10.1016/j.peptides.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. The New England journal of medicine. 2004;350:1104–10. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 25.Ozdemir FA, Berghofer P, Goke R, Goke B, McGregor GP. Specific calcitonin gene-related peptide binding sites present throughout rat intestine. Peptides. 1999;20:1361–6. doi: 10.1016/s0196-9781(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 26.Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:3295–309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rethelyi M, Metz CB, Lund PK. Distribution of neurons expressing calcitonin gene-related peptide mRNAs in the brain stem, spinal cord and dorsal root ganglia of rat and guinea-pig. Neuroscience. 1989;29:225–39. doi: 10.1016/0306-4522(89)90345-x. [DOI] [PubMed] [Google Scholar]
- 28.Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. The Journal of biological chemistry. 2004;279:7264–74. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld MG, Amara SG, Evans RM. Alternative RNA processing: determining neuronal phenotype. Science. 1984;225:1315–20. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–35. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 31.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Analytical biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Sternini C. Tachykinin and calcitonin gene-related peptide immunoreactivities and mRNAs in the mammalian enteric nervous system and sensory ganglia. Advances in experimental medicine and biology. 1991;298:39–51. doi: 10.1007/978-1-4899-0744-8_4. [DOI] [PubMed] [Google Scholar]
- 33.Sternini C, Reeve JR, Jr, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987;93:852–62. doi: 10.1016/0016-5085(87)90450-1. [DOI] [PubMed] [Google Scholar]
- 34.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. British journal of pharmacology. 2004;142:1171–81. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 acts within central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R919–27. doi: 10.1152/ajpregu.00744.2004. [DOI] [PubMed] [Google Scholar]
- 36.van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neuroscience and biobehavioral reviews. 1997;21:649–78. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 37.Van Valen F, Piechot G, Jurgens H. Calcitonin gene-related peptide (CGRP) receptors are linked to cyclic adenosine monophosphate production in SK-N-MC human neuroblastoma cells. Neuroscience letters. 1990;119:195–8. doi: 10.1016/0304-3940(90)90832-t. [DOI] [PubMed] [Google Scholar]
- 38.Wong HC, Tache Y, Lloyd KC, Yang H, Sternini C, Holzer P, et al. Monoclonal antibody to rat alpha-CGRP: production, characterization, and in vivo immunoneutralization activity. Hybridoma. 1993;12:93–106. doi: 10.1089/hyb.1993.12.93. [DOI] [PubMed] [Google Scholar]